Abstract
Infection of bone tissue, or osteomyelitis, has become a growing concern in modern healthcare due in no small part to a rise in antibiotic resistance among bacteria, notably Staphylococcus aureus. The current standard of care involves aggressive, prolonged antibiotic therapy combined with surgical debridement of infected tissues. While this treatment may be sufficient for resolving a portion of cases, recurrences of the infection and associated risks including toxicity with long‐term antibiotic usage have been reported. Therefore, there exists a need to produce safer, more efficacious options of treatment for osteomyelitis. In order to test treatment regimens, animal models that closely mimic the clinical condition and allow for accurate evaluation of therapeutics are necessary. Establishing a model that replicates features of osteomyelitis in humans continues to be a challenge to scientists, as there are many variables involved, including choosing an appropriate species and method to establish infection. This review addresses the refinement of animal models of osteomyelitis to reflect the clinical disease and test prospective therapeutics. The aim of this review is to explore studies regarding the use of animals for osteomyelitis therapeutics research and encourage further development of such animal models for the translation of results from the animal experiment to human medicine.
Keywords: animal model, bone infection, orthopedic implant, osteomyelitis, Staphylococcus aureus
This review addresses the refinement of animal models of osteomyelitis to reflect the clinical disease and test prospective therapeutics. The aim of this review is to explore studies regarding the use of animals for osteomyelitis therapeutics research and encourage further development of such animal models for the translation of results from the animal experiment to human medicine. The models discussed in this review utilize three main methods for induction of infection: post‐traumatic, implants, and hematogenous.
1. INTRODUCTION
Osteomyelitis is a disease of bone characterized by the presence of an infectious organism that causes inflammation and the destruction of osseous tissue. 1 , 2 While there are many methods of classifying osteomyelitis, this review focuses on the broader classifications of acute and chronic osteomyelitis. Although no evidence supports an exact time interval dividing them, acute osteomyelitis is generally accepted as a recent infection of several days or weeks that involves purulent debris and local inflammation. With acute osteomyelitis, aggressive and prompt antibiotic therapy can often resolve the infection before it progresses to a chronic state. 2 , 3 Figure 1 illustrates a clinical example of acute osteomyelitis secondary to Staphylococcus intermedius in a dog, following surgical repair of an open traumatic fracture of the left radius and ulna 1 month prior. In contrast, chronic osteomyelitis is a long‐standing, more complex infection characterized by the death of bone tissue. 2 , 3 Treatment for chronic osteomyelitis is typically surgical debridement coupled with irrigation and drainage followed by prolonged antibiotic therapy. Particularly for chronic osteomyelitis, there remain many challenges in devising effective treatment plans, including the decision of whether to remove any colonized orthopedic hardware, which antimicrobial(s) to use, and delivery method for the antimicrobial(s). 1 , 3 Figure 2 provides a clinical example of chronic osteomyelitis in a Quarter Horse stallion with a sequestrum (i.e., a necrotic piece of bone) and its associated draining tract. Although horses are not used for research models of osteomyelitis, this clinical manifestation of post‐traumatic, chronic osteomyelitis exhibits features that many of the models discussed herein attempt to achieve.
FIGURE 1.
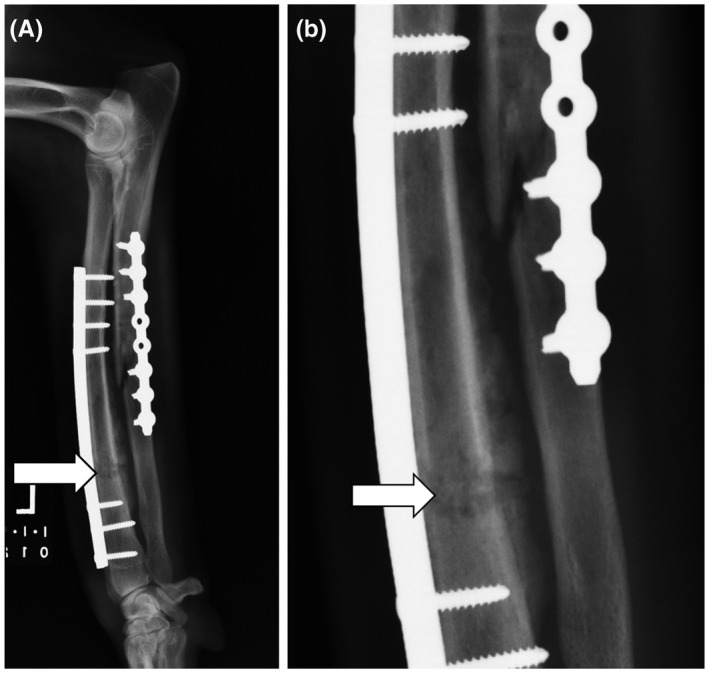
Lateral radiograph (A) and inset (B) of a canine radius and ulna with implant‐associated osteomyelitis 1 month following repair of an open traumatic fracture. Osteolysis of the radial diaphysis (white arrow) is seen as a large, irregularly marginated, rectangular, lucent region with heterogeneous bony sclerosis that obscures the fracture margins. This abnormal region of bone is bordered caudally (between the radius and ulna) by moderate and irregularly marginated periosteal new bone formation. Similar but fainter osteolytic and osteoproliferative changes are seen surrounding the long oblique fracture within the ulna. There is also regional soft tissue swelling characterized by increased soft tissue opacity and undulating cutaneous margins. The infection resolved with prolonged antibiotic therapy based on culture and sensitivity testing and eventual removal of the plates and screws following healing of the fractures
FIGURE 2.
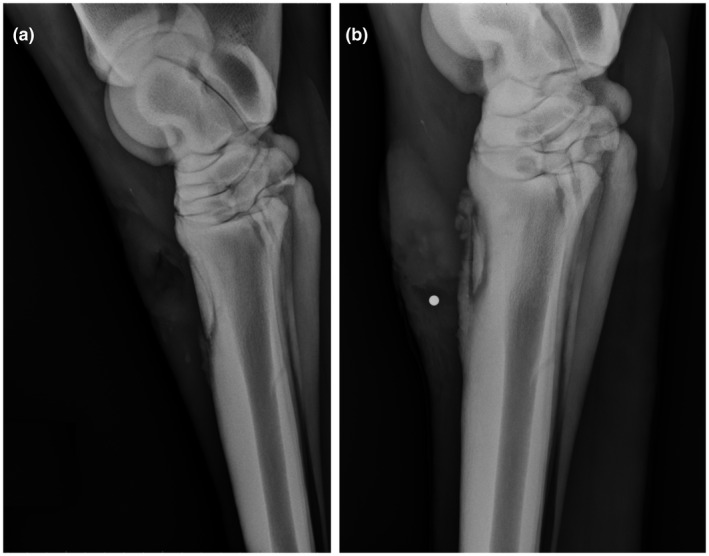
Lateral radiographs of a Quarter Horse stallion depicting an open traumatic chip fracture of the left third metatarsal bone both 2 wk (A) and 5 wk (B) following the initial traumatic injury. After 2 weeks, only soft tissue changes associated with the original open wound and presumptive acute osteomyelitis are seen overlying the fracture dorsally. By 5 wk (B), osseous changes of chronic osteomyelitis with a sequestrum are seen, characterized by irregularly marginated periosteal new bone formation and a sharply demarcated zone of lucency and sclerosis (i.e., involucrum) surrounding the fracture fragment. A round metal opaque radiography marker was placed in a cutaneous draining tract (i.e., cloaca) overlying the sequestrum. The patient fully recovered with surgical removal of the necrotic fracture fragment, debridement, and antibiotic therapy based on culture and sensitivity testing
Staphylococcus aureus is a gram‐positive, coccus bacterium typically arranged in clusters. This infectious agent is the leading cause of osteomyelitis in humans, causing 80% of cases. 4 The bacterium expresses adhesins specific to bone matrix and displays a propensity to bind to plasma proteins and host tissues, including fibrinogen that is known to coat orthopedic hardware after implantation. 5 , 6 Furthermore, S aureus has developed several antimicrobial evasion methods that complicate treatment and allow the infection to persist to a chronic state. Among these are biofilms, a community of bacteria with altered phenotypes capable of evading antibiotics and the host immune system, 7 which secrete enzymes and toxins that damage host cells to allow for nutrient acquisition and the spread of infection. 8 A rise in resistance has been seen in S aureus following extensive antimicrobial use, leading to development of phenotypes such as methicillin‐resistance S aureus (MRSA). 9
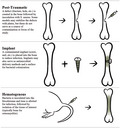
Animal models may provide the most promising outlet for advancing our understanding of the pathogenesis of S aureus osteomyelitis and efficacy of treatments to mitigate infection. While in vitro models are useful for initial testing of therapeutics against different phenotypic states of S aureus (e.g., biofilms), therapeutics may demonstrate high efficacy in vitro but in an infected animal model provide little remedy for infection. 10 , 11 Compared to clinical cases, animal models offer a more reproducible, controlled environment that can be manipulated to reflect different disease presentations. 12 , 13 , 14 Small animal models are often preferable due to lower costs associated with housing and providing adequate care, ease of handling, and ability to evaluate larger sample sizes in a single study. After success in a small animal model, translation of promising therapeutics necessitates demonstration of efficacy in large animal models, as large animals more closely resemble humans in many aspects including bone density, weight, and immune system functions. Large animal models are also particularly useful for the study of orthopedic implants, as minimal if any sizing adjustments are needed to evaluate human‐scale hardware. 15 In this review, we will focus on three main methods used to induce infection in animal models: post‐traumatic, implant, and hematogenous, as illustrated in Figure 3. Several foundational models mentioned briefly in this review are covered more thoroughly in a 2009 review by Patel et al. 16
FIGURE 3.
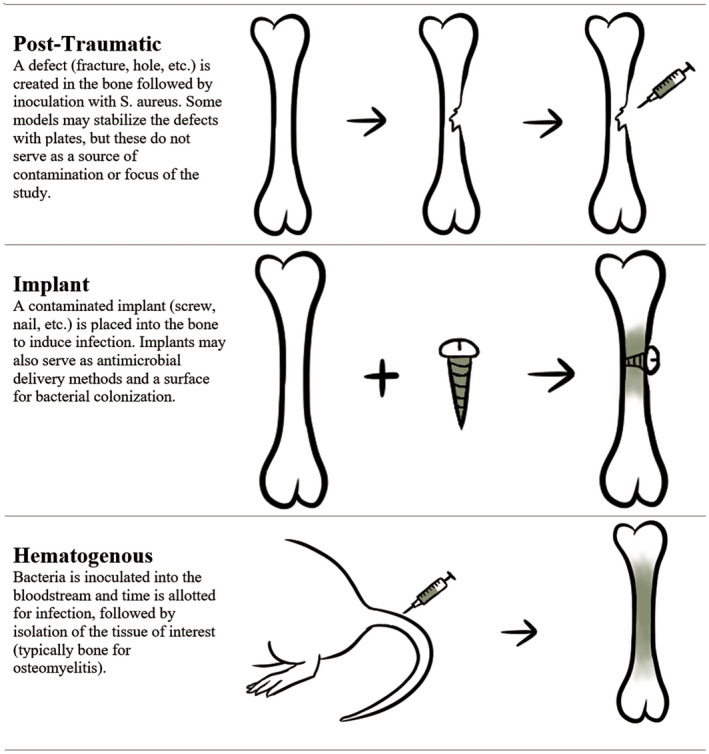
Common methods used to induce osteomyelitis in animal models include post‐traumatic, implant, and hematogenous
2. SMALL ANIMAL MODELS
2.1. Mouse (murine)
Murine models of osteomyelitis are advantageous in their ability to reflect the clinical manifestation of the disease in humans. 17 , 18 Non‐invasive monitoring via bioluminescent community‐associated methicillin‐resistant S aureus strains and whole‐animal bioluminescent imaging has demonstrated utility. 19 One unique aspect of murine models is the ability to evaluate the effect of type I and type II diabetes on osteomyelitis pathophysiology through manipulation of the mouse genome. Examples include the NOD/ShiLtJ mouse, which exhibits symptoms similar to type I diabetes. 20 Diabetic patients are a population at particular risk for osteomyelitis infection, oftentimes arising from foot ulcers, which makes these models particularly appealing. Limitations of murine models include lack of bone integrity and general hardiness, making hardware adjustments or delaying treatment for longer observation intervals challenging. 18 , 21
2.1.1. Post‐traumatic
Despite the limited size of the murine tibia, a post‐traumatic model was created by drilling a 1 mm diameter unicortical hole into the proximal medial tibia, which was then infected with 2 × 103 S aureus bacteria via injection in the medullary cavity. Debridement was performed 2 weeks later using a 20 gauge needle and unsurprisingly, debridement significantly reduced bacterial counts compared to nondebrided tissues. 22 In another study, a fixation plate served to stabilize an 8 mm osteotomy, into which 2 × 103 CFU of S aureus was injected. At days 7 and 14 post‐surgery, debridement, and lavage were performed. There was no evidence of fracture healing by sacrifice on day 28. 23
2.1.2. Implant
A model by Xiao et al used Vicryl suture as the agent of bacterial contamination. The suture, after soaking in 1 × 107 CFU/mL of S aureus for 30 minutes, was inserted into a hole in the proximal tibia. Bioluminescence and radiography were used to confirm localized, stable infection. 24 In another compelling study, a Kirschner wire (K‐wire) was inserted through the femoral canal so 1 mm protruded into the knee joint space. 5 × 102, 5 × 103, or 5 × 104 CFU of S aureus were injected adjacent to the implant where it extended into the surrounding soft tissues. 25 For translational work aimed at improving therapeutics for diabetic patients, NOD/ShiLtJ mice were used for intramedullary nail implantation and injection of 1 × 103 CFU of S aureus into the femoral canal, determining that prostaglandin E1 administered with cephalosporin improved recovery outcomes. 26 In another approach, the implant served as a delivery vehicle for antimicrobials, specifically phosphatidylcholine‐coated K‐wire for release of loaded amikacin, cis‐2‐decenoic acid, or both. 27
For osteotomy implant models in which a fixation plate is needed, the femur is commonly utilized. In one study, a commercially pure titanium fixation plate was contaminated via submerging in a bacterial suspension of 4 × 108 CFU of S aureus, resulting in 9 × 105 CFU delivered per implant, and placed onto the femur, and a 0.44 mm osteotomy was created. In uninfected animals, bridging of the osteotomy gap was observed by 35 days. In contrast, the infected mice exhibited dramatic bone damage, and defects were not bridged at 35 days. Increased expression of transforming growth factor‐β and platelet‐derived growth factor genes, indicative of bone healing, were noted in all uninfected groups compared to all infected groups. 28
2.1.3. Hematogenous
Hematogenous murine models of osteomyelitis are also feasible, typically via S aureus injected into the lateral tail vein. Over the course of one experiment, using 2 × 105, 5 × 105, or 1 × 106 CFU injected into the lateral tail vein, S aureus initially invaded and proliferated not only bone but also other organs, most notably the kidneys. After 60 days, however, these organs were progressively cleared, and S aureus was present only in the tibia. This was attributed to the tropism of the strain (ATCC 6850) for bone, demonstrating the utility of this strain in hematogenous models, and emphasizing the significance of bacterial strain in infection studies. 17
2.2. Rat
First popularized by the publication of Zak et al in 1982, 29 , 30 rat models offer advantages in ease of care, easier surgical manipulation than mice (due to their increased size), as well as greater general hardiness than mice. Furthermore, rats may be appealing to researchers for their lower regulatory burden, relative to larger species for which the USDA has more intensive requirements. Furthermore, rats tolerate long‐term antibiotic therapy, even at high doses. 31 , 32 Similar to diabetic mouse strains, the immune systems of rats have also been manipulated to reflect human conditions, which enables the study of risk factors for osteomyelitis and their impact on pathophysiology of the disease. 30 , 31 Thus, rats serve as effective models for screening of therapeutics, before larger animal models that tend to cost more in regards to housing and care requirements.
2.2.1. Post‐traumatic
The first rat osteomyelitis models largely relied on sclerosing agents to induce infection in post‐traumatic tibial models, likely due to the ability of sclerosing agents to decrease (re)vascularization, thus facilitating bone necrosis and infection development. 31 These agents played a central role in models by Zak et al 29 and Rissing et al, 33 but were later proven unnecessary for infection development in a study by Spagnolo et al 16 , 31 Femoral models with no additives have also been explored. In one study, 100 µL of 104 CFU/mL S aureus was injected directly into a defect created by a needle in the distal femur. After 2 weeks, a bone cement in the femoral cavity was evaluated as an antimicrobial therapeutic. 34
A study by Bonnarens and Einhorn used a drop‐tower apparatus to drop a weight onto the femur, creating a transverse fracture. 35 Prior to fracture, a Steinmann pin was placed into the intramedullary canal of the femur, exiting through the greater trochanter and bent into a 3 mm “handle” buried into the muscle. Although this model did not induce infection, it served as the groundwork for future models involving spontaneous fractures. 35 In one modification, the femur was accessed distally (rather than proximally) for reaming. 104 CFU bacterial suspension was inoculated into the medullary canal, then a pin was inserted and the drop apparatus used. All infected femurs failed to bridge the fracture, whereas a bridging fracture callus was noted in all uninfected controls. 36
Other fracture models utilize fixation plates and induce fracture surgically, rather than via a drop apparatus, and often involve an osteotomy. Typically, a defect 6mm in length is used, as this is accepted as critically sized in rat femora. 37 , 38 , 39 , 40 , 41 , 42 , 43 A popular method of introduction of S aureus into the defect is via contaminated type I bovine collagen. 37 , 38 , 39 , 40 , 41 , 42 , 43 One of the first publications describing a rat model of fixation plate, osteotomy, and osteomyelitis was Chen et al, in which a polyacetyl plate and Kirshner wires served as fixators. 40 A 105 CFU dose of S aureus resulted in osteolysis and loss of fixation plate stability over 2 weeks. This model has been adapted in many research projects in recent years, such as the evaluation of a novel osteogenic bone graft. 38 Other investigations include: biofilm formation in diabetic rats, 30 osteogenic protein‐1 (OP‐1) for inducing bone formation in the presence of S aureus, 40 and debridement optimization. 37 , 43
Although less common, tibial fracture models also exist. In one unique study, an open tibial fracture model, akin to a Gustilo type III wound, was developed. To induce infection, a trough was first created in the tibial medullary cavity by drilling first into the anterior cortex, in a proximal‐to‐distal motion. After the trough was created, a cautery device was used to damage the endosteal blood supply. 10 µL of 1 × 107 CFU/mL S aureus suspension was then placed into the trough, followed by curettage and lavage. This model successfully established acute osteomyelitis, designated as the time within 15 days from initial infection. 44 Another unique study utilized an acute tibial open fracture model to evaluate factors that play a role in infection development and to determine a minimum dose of inoculum for infection. For creation of infection, a 1 cm trough was drilled into the medullary cavity of the craniomedial tibia and contaminated with inoculum ranging from 5 to 6500 CFU of S aureus. After 10 minutes, the troughs lavaged. Twenty‐four hours later, the tibias were removed for bacterial counting. It was found that 72 CFU/g of bone was sufficient to cause infection in 50% of the rats, while 977 CFU/g of bone resulted in a 95% infection rate. This model was then used to evaluate the effect of thermal injury, bimicrobial contamination with the addition of Escherichia coli, and foreign bodies of soil and sand on infection development. Thermal injury contributed to infectivity when challenged with low doses of inoculum, but neither the addition of E coli nor contamination with sand or soil had a significant effect on infectivity. 45
2.2.2. Implant
Perhaps, the most common use of rats in osteomyelitis studies has been for implant‐based models. A popular non‐fractured implant model of osteomyelitis was published by Lucke et al, in which acute osteomyelitis was localized in the tibia with a K‐wire. 16 , 46 In a similar model, the same methodology was used and an extended experimental period was chosen to generate chronic osteomyelitis, characterized by sclerotic bone and a lack of vascularization. Lavage and debridement were performed at days 7 and 14, with sacrifice at day 28. Systemic teicoplanin treatment with or without extracorporeal shockwave therapy was evaluated. 47 Another study placed a K‐wire after a fracture occurred, from the proximal fragment to the distal fragment, until it was partially seated into the epiphysis. This antegrade K‐wire placement resulted in a consistent infection rate (90%‐100%) after 3 weeks with a low dose of 101 or 102 CFU of S aureus. 48
Similar to K‐wires, stainless steel tubing has been used as an implant for infection development. In one study, this tubing was pre‐soaked in 1 × 104 CFU/mL S aureus and inserted into the femur, after which a supplementary 50 CFU S aureus suspension was injected inside the tubing. At days 6 and 45 post‐initial infection, respectively, signs of acute and chronic osteomyelitis were confirmed by biofilm protein isolation and histology. 49
Although not as common as wires, screws 50 and nails 16 , 51 have also been used to simulate implant‐related osteomyelitis in rats. In one study, a polyether ether ketone (PEEK) screw coated in titanium was used as the agent to introduce bacteria into the tibia after soaking in 3.3 × 107 CFU/mL bacterial solution. The animals were monitored through 28 days, during which micro‐computed tomography (µCT) was used to evaluate bone formation and resorption. 50 Bone formation decreased and bone resorption increased in the groups with colonized screw implants. 50 To observe osseointegration and antimicrobial effects of hydroxyapatite‐ and hydroxyapatite‐silver‐coated nails, a unicortical hole 8 mm in depth was drilled into the proximal lateral tibial metaphysis, followed by S aureus injection of 102 or 103 CFU and for the experimental groups, nail placement. 51 In a recent study, a contaminated screw was placed in a solution of ~1 × 108 S aureus for 5‐10 minutes, resulting in approximately 5 × 104 CFU of fluorescent ATCC 6538‐GFP S aureus on the screw, which was placed into the mid‐diaphysis of the femur, establishing infection in the bone and surrounding soft tissue. On day 7 post‐infection, the screw was removed, and treatments were administered: fosfomycin, bacteriophage, fosfomycin, and bacteriophage, or blank alginate hydrogel. On day 8 post‐infection, histology revealed establishment of infection through neutrophilic inflammation as well as fibrosis and the presence of Gram‐positive bacteria. Bone bacterial load was reduced only in the fosfomycin group, while soft tissue bacterial load was lower in all three treatment groups compared to controls. 52 More recently, this model has been used to longitudinally evaluate infection using in vivo radiographic and fluorescent imaging, as shown in Figure 4.
FIGURE 4.
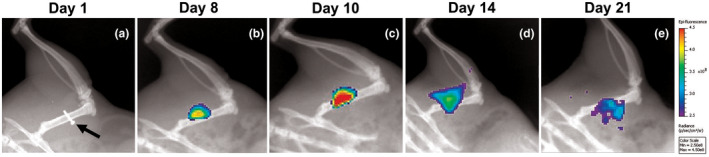
In vivo imaging of an untreated rat in the femoral osteomyelitis model induced by a screw (A, arrow) contaminated with fluorescent Staphylococcus aureus (ATCC 6538‐GFP). A, Radiograph without fluorescence on day 1 post‐infection showing location of the contaminated screw, which is removed on day 7. B‐E, Longitudinal radiographic and fluorescent imaging 8 d (B), 10 d (C), 14 d (D), and 21 d (E) post‐infection
2.2.3. Hematogenous
Although rare, in one hematogenous rat model of osteomyelitis, a medial parapatellar arthrotomy was created, and a cannulated needle was used to clear the medullary canal, into which a K‐wire was inserted. After surgery, S aureus was delivered systemically via catheter in the tail vein. A high dose of S aureus (107 CFU) was necessary to induce infection of both the femur and the implant. Furthermore, after 14 days, the addition of the K‐wire significantly increased the rate of infection. Interestingly, the dose of 107 CFU was not sufficient to induce osteomyelitis in rats without the implant. 53
2.3. Rabbit
Rabbits models are useful for those who need an animal model larger than rodents but do not have the infrastructure to support large animals. Rabbits are also a great choice for studies on implant devices or coatings, as evaluating these products using rodents is limited due to their smaller size. 54 Some products designed for humans can even be evaluated in rabbits with no modifications, and rabbit immune responses can reflect those of humans. 54 , 55 Challenges of rabbits include their hindgut fermenting gastrointestinal system, limiting their utility in evaluation of oral antibiotics due to the disruption of their crucial gut flora, and their tendency to undergo respiratory depression when put under anesthesia. 56
2.3.1. Post‐traumatic
As with rat models, there exist post‐traumatic models of osteomyelitis in the rabbit utilizing sclerosing agents. 57 In one tibial model, microbiological, and histological evidence of chronic osteomyelitis were noted at 4 weeks after inoculation with 1 × 108 CFU of S aureus, at which time the rabbits underwent debridement and either placement of novel bioactive glass implants, or daily intravenous injections of teicoplanin for 4 weeks. 57 These post‐traumatic models using sodium morrhuate are also ideal for mimicking blast wound trauma seen in the battlefield, and resulting bone infection, because sclerosing agents induce necrosis of bone similar to blast wound trauma. One study used this method to evaluate S aureus, Pseudomonas aeruginosa, Klebsiella pneumonia, and Acinetobacter baumannii in monocultures or delivered in combination. All monoculture inoculations (0.15 mL of 107 CFU/mL) as well as the combinations with MRSA (0.15 mL of 105 CFU/mL) were able to reliably induce infection. 58 In a femoral model, a novel 3D printed scaffold composed of poly D,L‐lactic acid powder laced with levofloxacin and tobramycin in concentric, alternating layers was evaluated. 59
Another form of post‐traumatic model by Kishor et al involved the production of a unicortical defect 5 mm in diameter to reflect a more severe situation. To induce infection, 10 µL of 5 × 106 CFU/mL S aureus was injected at the femoral defect site. 60 In another study, a bicortical defect was made in the femoral condyle. Here, a polymethylmethacrylate (PMMA) rod, contaminated by immersion in a S aureus inoculum (concentration not reported), was placed for 7 days, at which point various calcium phosphate cements loaded with doxycycline were implanted for an additional 21 days and evaluated for bacterial clearance. 61 Other femoral models avoid the creation of cortical defects. In one investigation, femoral trepanation was performed, and a suspension of 109 CFU of S aureus was inoculated into the knee cavity through a parapatellar injection. Animals were euthanized at 1, 2, 3, 9, and 14 days for evaluation of bacterial load in bone marrow over time. Histological signs and bacterial counts indicated acute osteomyelitis at all time points, though no significant differences in acute inflammation, intraosseous chronic inflammation, or bone necrosis scores were observed between days 3, 9, or 14. 62
Models involving segmental defects, often involving the radius, are also reported. One major advantage of radial models is that a fixation device is usually not required, as the ulna serves this role. One study, aiming to induce chronic osteomyelitis, employed the removal of a 1 cm segment, where inoculum as small as 2 × 103 CFU of S aureus was injected directly into the medullary canal, and the excised segment was replaced. 63 In another study utilizing a similar methodology, 1 cm of the radius was removed, a K‐wire was placed through the medullary cavity, and intramedullary inoculation of 7.5 × 106 CFU of S aureus was performed on the removed segment, which was then replaced into the defect space. Three weeks later, the site was debrided, the K‐wire was replaced, and a novel biodegradable poly(N‐isopropylacrylamide‐co‐dimethyl‐γ‐butyrolactone acrylate‐co‐Jeffamine® M‐1000 acrylamide) hydrogel was utilized to deliver gentamicin. 64 The segmental defect model has since been adapted for the femur. 65 Notable research involving the humerus utilized custom‐designed intramedullary nails or a seven‐hole locking compression plate to stabilize a 0.45 mm osteotomy, into which S aureus was injected (6 × 103 to 6 × 106 CFU for the plate groups and 6 × 102 to 6 × 106 CFU for the nail groups). 54
2.3.2. Implant
Due to their larger physiology, rabbits enable evaluation of a broader range of implants than mouse and rat models. Implants reported in literature include but are not limited to: nails, 56 , 66 , 67 screws, 68 wires, 64 , 69 rods, 70 , 71 and plates. 54 , 70 , 72 , 73
In one work, a biofilm‐coated stainless steel fixation plate, attached via four screws on the midshaft of the femur, was used to induce osteomyelitis. The plate was contaminated by incubation in 5 mL of 106 CFU/mL of S aureus. A 1 mm defect was created after placement of the plate, and after 21 days, symptoms included: implant failure, callus formation away from the defect site, swelling, pus, and tissue damage. 72
By drilling a defect 4 mm in diameter in the tibia, injecting 3.8 × 105 CFU of S aureus, and placing a titanium nail, one study established an acute osteomyelitis model characterized by early post‐operative infection according to hematological analysis. Interestingly, different calcium‐binding fluorophores were administered at 14, 28, and 41 days to enable longitudinal evaluation of bone formation. 56 Another unique nail‐based study generated a bifocal osteotomy in the tibia to evaluate internal versus external fixation in osteomyelitis. This is of particular interest, as most animal models utilize only a single osteotomy. After this osteotomy was created, the tibial fragment was submerged in a 108 CFU/mL MRSA culture and replaced into the defect, where an intramedullary nail was used for stabilization. Four days later, surgical debridement was performed, nail was removed, and rabbits received either sterile internal nail fixation or bilateral external fixation with a custom device. This study revealed that removal of the internal osteosynthesis device improved recovery outcomes, based on bacterial counts recovered from purulent discharge. 66
In one chronic model, sodium morrhuate was used to induce infection in the tibia over a period of 4 weeks. At this time, debridement was performed and either two Mg‐Cu alloy nails or two pure titanium intramedullary nails were placed in the intramedullary canal. 67 Similarly to nail models, screw models have also been explored. For bacterial contamination, screws of 317L‐copper, 317L‐stainless steel, and titanium alloy (Ti‐6Al‐4V) were submerged in 105 CFU/mL of both S aureus and E coli for 6 minutes prior to implantation into a 2.5 mm cylindrical hole in the femur. The ability of the copper screw to mitigate implant colonization was compared to stainless steel and titanium screw controls, and copper was deemed superior based on ex vivo bacterial loads. 68
Although uncommon, PMMA cylinders have also been used as implants. An 8.5 mm long femoral transcondylar defect was drilled and irrigated. Then, a contaminated (CFU/mL not reported) PMMA cylinder was pressed into the defect space. Four days later, the cylinder was removed, and infected soft tissues surrounding the bony defect were debrided. Rabbits then received either uncoated titanium or polyelectrolyte‐film‐coated titanium implants, for four or seven additional days. 71
3. LARGE ANIMAL MODELS
3.1. Pig (porcine)
One benefit of using a pig model is that pigs are omnivores, and therefore have gut biomes that respond to antibiotics similarly as those of humans, making pigs a good candidate for oral and/or systemic antibiotic treatment evaluation. 74 Furthermore, although the composition of canine bones most closely resembles humans, pigs are the next closest match. Notably, fracture stress is higher in dogs (6.12 MPa) when compared to pigs (2.40 MPa) and humans (1.21 MPa) (Table 1). Studies on fracture and related stress from weight bearing may, thus, be more easily translated for human applications using the pig. 75 One disadvantage of pigs is their rate of bone growth, which is considerably faster than that of humans. 74 Additionally, the porcine tibia and fibula are shorter than those of a human, limiting their utility in the evaluation of implants. 74
TABLE 1.
Species utilized in osteomyelitis models, and characteristics of each that mimic human osteomyelitis
| Species | Similarities to human osteomyelitis |
|---|---|
| Mouse |
|
| Rat | |
| Rabbit |
|
| Pig |
|
| Dog |
|
| Goat/sheep |
3.1.1. Post‐traumatic
For simulation of gunshot wounds, one porcine post‐traumatic osteomyelitis model fired a 200 mg steel fragment into the right tibial metaphysis. After this procedure, approximately 107 CFU of S aureus was inoculated into the defect site on a strip of bovine collagen. Experimentally, pigs received benzylpenicillin and flucloxacillin through intramuscular injection every 6 hours for 7 days. After 14 days, radiographs were taken and bone and soft tissue were collected and processed for histology. All animals in the control group had developed acute osteomyelitis, while the treatment group showed no signs of infection. 76
3.1.2. Implant
An implant‐associated tibial osteomyelitis model has been established in pigs, utilizing a stainless steel implant. Briefly, fluoroscopic guidance was utilized to clear the medullary cavity of the tibia using a K‐wire (4 mm), a low S aureus inoculum of 102, 103, or 104 CFU was delivered into the cavity, and a small steel implant was placed into the medullary cavity. Although animals were observed for only 5 days, signs of localized, acute osteomyelitis were noted to varying degrees on CT scans and implant cavity cultures in all groups. 74
3.1.3. Hematogenous
A benefit of porcine models is their pulmonary intravascular macrophages, which inhibit bacteremia and allow for prolonged survival after hematogenous inoculation of bacteria, in contrast to other species that may have to be euthanized due to septicemia. 77 In one study, a catheter was inserted into the left ear vein of juvenile pigs. Pigs then received either an inoculation of 108 CFU S aureus at the time of surgery, or the initial inoculation followed by another at 12 hours post‐surgery. 78 In groups euthanized at 12, 24, and 48 hours, infection was successfully induced in the long bones and lungs without affecting the vertebrae, with no signs in those euthanized at 6 hours. However, after 48 hours the pulmonary bacterial load decreased, and bacteremia tests were negative, attributed primarily to the pulmonary intravascular macrophages of the pig that can effectively phagocytose S aureus. 77 , 78 This model is promising for evaluating therapeutics for juvenile osteomyelitis, which is often characterized by long bone infection that initiates deep within the metaphysis and spreads to the capillary loops near the growth plate, with the absence of vertebral lesions. 78 , 79 In one revision of this model, the use of the brachial artery rather than the ear vein resulted in 62.5% (5/8) of subjects euthanized for lameness. 80 The use of the right femoral artery was also explored, and found to be superior to other routes of bacterial inoculation in porcine hematogenous models. 81 , 82 Thus, in a subsequent study, Nielsen et al. used the right femoral artery for a diagnostic study. However, three subjects (38% of the experimental group) had to be prematurely euthanized due to complications including elevated C‐reactive protein (CRP), neutrophilia, sepsis, and lameness. Interestingly, one animal from the experimental group was excluded due to lack of infection, leaving only four animals for diagnostic testing. 83 Overall, the current hematogenous porcine models fail to be reliable due to high complication rates, but remain a promising area for future animal model development.
3.2. Dog (canine)
Canine models are some of the more well‐established models for orthopedic research. In a recent study, of all non‐human species tested, canine bones most closely resembled human bones with regards to composition and density. 75 Despite these desirable attributes, few canine models exist, most likely due to the ethical concerns associated with the use of animals commonly adopted as household pets. 84
The first published canine model was in 1976, by Deysine et al. Unique to this study was the use of the nutrient artery of the tibia as the inoculation site of radiopaque barium sulfate (used for radiograph enhancement) and 0.1 mL of a 106 CFU/mL culture of S aureus, somewhat mimicking hematogenous osteomyelitis. 16 , 85 Later, Fitzgerald et al established one of the first canine post‐traumatic tibial models, which was later modified for the femur by Petty et al. 16 , 86 , 87
More recent work deviating from these models, and simulating open fracture, also exist. In one such project, a captive bolt device delivered 6800 N of force to fracture the proximal tibia. Then, intramedullary nails were used to fix the site of fracture. 106 CFU of S aureus was injected into the medullary cavity and allowed to flow freely into the surrounding soft tissue. A transpositional muscle flap from the gastrocnemius muscle was then surgically created on some of the subjects, which displayed increased vascular endothelial growth factor (VEGF) mRNA expression versus the fracture only group at 2 hours post‐surgery, indicating that the type of closure used in surgery should be carefully selected. 88
3.3. Goat (caprine)
Caprine, or goat, osteomyelitis models have not been widely utilized in research, likely due to preference for more well‐established sheep models. Nonetheless, their larger anatomy more closely mimics human long bones. 89 This provides an easier translation of research findings for human applications, avoids the increased economic burden associated with custom‐made, novel devices designed specifically for animals. 90
The only caprine osteomyelitis models that fell within the constraints of this review were tibial models. Salgado et al. developed a popular goat osteomyelitis model through a post‐traumatic tibial study. 16 , 91 Concurrently, a similar defect model was published, utilizing a 12 mm unicortical defect in the metaphysis of the tibia, followed by a 3.14 × 106 CFU bacterial inoculation. With the larger defect size, sclerosing agents were omitted and osteomyelitis was still induced. 92
To compare the infection rate of fractures with external fixation versus intramedullary locking nails, with or without reaming, one study developed two separate surgical protocols. For simulation of external fixation, a chevron osteotomy was created along the tibia, followed by generation of 4 mm drill holes. For intramedullary nail placement, a medial parapatellar incision was performed, followed by use of a 6 mm drill bit for access into the medullary canal. After fixation, 103 CFU of S aureus was introduced to the fracture site on an absorbable gelatin sponge. At 14 days, bacterial growth in the group with reaming and intramedullary nailing was significantly greater than the groups with an external fixation device or no reaming and intramedullary nailing. 90 Intramedullary nails were further analyzed in a using a more recent model, in which a tibial mid‐diaphysial osteotomy was performed with intramedullary nail fixation. Micro‐CT images, histology, and bacterial counts on explanted hardware indicated the successful development of chronic osteomyelitis, but infected soft tissue interference with the antimicrobial silver hybrid coatings of the intramedullary nails make the results inconclusive. 93
3.4. Sheep (ovine)
Sheep are a desirable model of long bone osteomyelitis, as their bones are similar in size to those of humans. Additionally, sheep and humans share a similar rate of osteogenesis. Torsional stiffness of sheep femoral bone has also been shown to closely mimic the torsional stiffness of human bone. 94 However, sheep bone is denser and has fewer Haversian canals than human bone. 94 Sheep models, like all large animal models, come with the burden of increased research costs for appropriate upkeep and housing. It should be noted that goat and sheep bone anatomy have very similar characteristics; largely, these two species could be interchanged depending on availability to research groups.
Kaarsemaker et al pioneered the use of sheep for osteomyelitis models in 1997. 16 , 95 In a similar femoral model, sclerosing agents were not used. A hole was drilled into the medial femoral condyle, and in the infection groups, 4 × 105 CFU of S aureus was inoculated, before the PLGA‐polyethylene glycol scaffold materials were packed in. In both control groups with the scaffold only, and in two treated groups with an antibiotic‐impregnated scaffold, no bacteria were isolated from blood samples, while the group with bacteria and no treatment had bacteria isolated from the bony defect, indicating that localized infection was produced. This provides evidence that sclerosing agents are not necessary in post‐traumatic ovine models. 96
Other efforts have been directed toward implants models. From a 2002 ovine tibial chronic osteomyelitis model using a midshaft chevron osteotomy followed by 3 × 108 CFU of S aureus bacterial inoculation and intramedullary nail placement, it was determined that intramedullary nail fixation may not be appropriate in all models, as it may stimulate virulence, and thus interfere with the efficacy of antibiotic treatment. The use of external fixation was suggested for future studies. 97 Another research group seemingly took this advice when designing their model, in which a titanium locking compression plate was used to stabilize an osteotomy. Another novelty in this protocol was the introduction of 2.5 mL of 106 CFU/mL of S aureus using a catheter at the site of the osteotomy. 98 The reproducibility of this model was further validated in a follow‐up study evaluating a novel N,N‐dodecyl,methyl‐polyethylenimine (PEI) coating on the same titanium locking compression plates. All of the control (untreated) animals successfully developed osteomyelitis, indicating the reliability of this model. 15 Another ovine‐based study utilizing orthopedic plates modeled open fracture type IIIB. 99 To contaminate the stainless steel fixation plates, S aureus was allowed to reach a biofilm state in vitro. Then, it was attached to a polyetheretherketone (PEEK) membrane containing 2.07 × 109 to 5.05 × 109 CFU, which was placed on the stainless steel plate. All five sheep that received the plates contaminated by a biofilm developed infection, while none of the five sheep that received plates contaminated with planktonic bacteria developed infection. 99
4. CONCLUSION
The current treatment regimen of osteomyelitis involves long‐term antibiotic therapy, along with surgical debridement if warranted. However, this regimen is often challenged by a rise in antibiotic resistance, infection recurrence, and difficulty in eradicating the original infection. The use of animal models for osteomyelitis research allows for development and refinement of therapeutics in order to combat this complex disease. A wide variety of reproducible animal models exist across multiple species, including those focusing on post‐traumatic infection, implant‐based infection, and hematogenous seeding of bacteria. As these models are further developed to better reflect the human manifestation of osteomyelitis, the therapeutics and orthopedic hardware tested via these routes will show a higher margin of safety and efficacy when transitioned to human medicine.
ACKNOWLEDGMENTS
The authors acknowledge support from NIH P20GM103646‐07 and 5T35OD010432, and the Mississippi State University Office of Research and Economic Development. We also acknowledge the USDA‐ARS Biophotonics Initiative (58‐6402‐3‐018) and Anna Rourke for providing the in vivo images from the implant‐based model in the rat.
Roux KM, Cobb LH, Seitz MA, et al. Innovations in osteomyelitis research: A review of animal models. Anim Models Exp Med. 2021;4:59–70. 10.1002/ame2.12149
Kylie M. Roux and Leah H. Cobb have equal contribution.
REFERENCES
- 1. Birt MC, Anderson DW, Bruce Toby E, Wang J. Osteomyelitis: recent advances in pathophysiology and therapeutic strategies. J Orthop. 2017;14(1):45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lew PDP, Waldvogel PFA. Osteomyelitis. Lancet. 2004;364(9431):369‐379. [DOI] [PubMed] [Google Scholar]
- 3. Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Jt Surg Ser A. 2004;86:2305‐2318. [DOI] [PubMed] [Google Scholar]
- 4. Ellington JK. Intracellular Staphylococcus aureus. A mechanism for the indolence of osteomyelitis. Artic Bone Jt J. 2003.85‐B 918–921. [PubMed] [Google Scholar]
- 5. Hudson MC, Ramp WK, Frankenburg KP. Staphylococcus aureus adhesion to bone matrix and bone‐associated biomaterials. FEMS Microbiol Lett. 1999;173:279‐284. [DOI] [PubMed] [Google Scholar]
- 6. Darouiche RO, Landon GC, Patti JM, et al. Role of Staphylococcus aureus surface adhesins in orthopaedic device infections: are results model‐dependent? J Med Microbiol. 1997;46:75‐79. [DOI] [PubMed] [Google Scholar]
- 7. Jensen LK, Jensen HE, Koch J, Bjarnsholt T, Eickhardt S, Shirtliff M. Specific antibodies to Staphylococcus aureus biofilm are present in serum from pigs with osteomyelitis. In Vivo (Brooklyn). 2015;29:555‐560. [PubMed] [Google Scholar]
- 8. Brady RA, Leid JG, Costerton JW, Shirtliff ME. Osteomyelitis: clinical overview and mechanisms of infection persistence. Clin Microbiol Newsl. 2006;28:65‐72. [Google Scholar]
- 9. Benner EJ, Kayser FH. Growing clinical significance of methcillin‐resistant Staphylococcus aureus . Lancet. 1968;2:741‐744. [DOI] [PubMed] [Google Scholar]
- 10. Monzón M, García‐Álvare F, Lacleriga A, et al. A simple infection model using pre‐colonized implants to reproduce rat chronic Staphylococcus aureus osteomyelitis and study antibiotic treatment. J Orthop Res. 2001;19:820‐826. [DOI] [PubMed] [Google Scholar]
- 11. Tuchscherr L, Kreis CA, Hoerr V, et al. Staphylococcus aureus develops increased resistance to antibiotics by forming dynamic small colony variants during chronic osteomyelitis. J Antimicrob Chemother. 2016;71:438–448. [DOI] [PubMed] [Google Scholar]
- 12. Norden CW. Lessons learned from animal models of osteomyelitis. Rev Infect Dis. 1988;10:103‐110. [DOI] [PubMed] [Google Scholar]
- 13. Funao H, Ishii K, Nagai S, et al. Establishment of a real‐time, quantitative, and reproducible mouse model of staphylococcus osteomyelitis using bioluminescence imaging. Infect Immun. 2012;80:733‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Emslie KR, Ozanne NR, Nade SML. Acute haematogenous osteomyelitis: an experimental model. J Pathol. 1983;141:157‐167. [DOI] [PubMed] [Google Scholar]
- 15. Schaer TP, Stewart S, Hsu BB, Klibanov AM. Hydrophobic polycationic coatings that inhibit biofilms and support bone healing during infection. Biomaterials. 2012;33:1245‐1254. [DOI] [PubMed] [Google Scholar]
- 16. Patel M, Rojavin Y, Jamali A, Wasielewski S, Salgado C. Animal models for the study of osteomyelitis. Semin Plast Surg. 2009;23:148‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horst SA, Hoerr V, Beineke A, et al. A novel mouse model of Staphylococcus aureus chronic osteomyelitis that closely mimics the human infection: an integrated view of disease pathogenesis. Am J Pathol. 2012;181:1206‐1214. [DOI] [PubMed] [Google Scholar]
- 18. Yokogawa N, Ishikawa M, Nishitani K, et al. Immunotherapy synergizes with debridement and antibiotic therapy in a murine 1‐stage exchange model of MRSA implant‐associated osteomyelitis. J Orthop Res. 2018;36:1590‐1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Cheng LI, Helfer DR, et al. Mouse model of hematogenous implant‐related Staphylococcus aureus biofilm infection reveals therapeutic targets. Proc Natl Acad Sci USA. 2017;114:E5094‐E5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lovati AB, Drago L, Monti L, et al. Diabetic mouse model of orthopaedic implant‐related Staphylococcus aureus infection. PLoS One. 2013;8:e67628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trombetta RP, de Mesy Bentley KL, Schwarz EM, Kate SL, Awad HA. A murine femoral ostectomy model with hardware exchange to assess antibiotic‐impregnated spacers for implant‐associated osteomyelitis. Eur Cell Mater. 2019;37:431‐443. [DOI] [PubMed] [Google Scholar]
- 22. Wagner JM, Zöllner H, Wallner C, et al. Surgical debridement is superior to sole antibiotic therapy in a novel murine posttraumatic osteomyelitis model. PLoS One. 2016;11:e0149389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Windolf CD, Meng W, Lögters TT, MacKenzie CR, Windolf J, Flohé S Implant‐associated localized osteitis in murine femur fracture by biofilm forming Staphylococcus aureus: a novel experimental model. J Orthop Res. 2013;31:2013‐2020. [DOI] [PubMed] [Google Scholar]
- 24. Xiao L, Li T, Ding M, et al. Detecting chronic post‐traumatic osteomyelitis of mouse Tibia via an IL‐13Rα2 targeted metallofullerene magnetic resonance imaging probe. Bioconjug Chem. 2017;28:649‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernthal NM, Stavrakis AI, Billi F, et al. A mouse model of post‐arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One. 2010;5:e12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lovati AB, Romanò CL, Monti L, Vassena C, Previdi S, Drago L. Does PGE1 vasodilator prevent orthopaedic implant‐related infection in diabetes? Preliminary results in a mouse model. PLoS One. 2014;9:e94758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harris MA, Beenken KE, Smeltzer MS, Haggard WO, Jennings JA. Phosphatidylcholine coatings deliver local antimicrobials and reduce infection in a murine model: a preliminary study. Clin Orthop Relat Res. 2017;475:1847‐1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rochford ETJ, Sabaté Brescó M, Zeiter S, et al. Monitoring immune responses in a mouse model of fracture fixation with and without Staphylococcus aureus osteomyelitis. Bone. 2016;83:82‐92. [DOI] [PubMed] [Google Scholar]
- 29. Zak O, Zak F, Rich R, Tosch W, Kradolfer F, Scheld WM. Experimental staphylococcal osteomyelitis in rats: therapy with rifampin and cloxacillin, alone or in combination. In: Perti P, Grassi G, eds. Current chemotherapy and immunotherapy. Washington, DC: American Society for Microbiology; 1982:973‐974. [Google Scholar]
- 30. Brown NL, Rose MB, Blueschke G, et al. Bioburden after Staphylococcus aureus inoculation in type 1 diabetic rats undergoing internal fixation. Plast Reconstr Surg. 2014;134:412e‐419e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spagnolo N, Greco F, Rossi A, Ciolli L, Teti A, Posteraro P. Chronic staphylococcal osteomyelitis: a new experimental rat model. Infect Immunity. 1993;61:5225‐5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mader JT. Animal models of osteomyelitis. Am J Med. 1985;78:213‐217. [DOI] [PubMed] [Google Scholar]
- 33. Rissing JP, Buxton TB, Weinstein RS, Shockley RK. Model of experimental chronic osteomyelitis in rats. Infect Immunity. 1985;47:581‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oh EJ, Oh SH, Lee IS, Kwon OS, Lee JH. Antibiotic‐eluting hydrophilized PMMA bone cement with prolonged bactericidal effect for the treatment of osteomyelitis. J Biomater Appl. 2015;30:1534–1544. [DOI] [PubMed] [Google Scholar]
- 35. Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97‐101. [DOI] [PubMed] [Google Scholar]
- 36. Robinson DA, Bechtold JE, Carlson CS, Evans RB, Conzemius MG. Development of a fracture osteomyelitis model in the rat femur. J Orthop Res. 2011;29:131‐137. [DOI] [PubMed] [Google Scholar]
- 37. Penn‐Barwell JG, Murray CK, Wenke JC. Early antibiotics and debridement independently reduce infection in an open fracture model. Bone Joint J. 2012;94‐B:107‐112. [DOI] [PubMed] [Google Scholar]
- 38. Brown ME, Zou Y, Peyyala R, et al. Testing of a bioactive, moldable bone graft substitute in an infected, critically sized segmental defect model. J Biomed Mater Res Part B Appl Biomater. 2018;106:1878‐1886. [DOI] [PubMed] [Google Scholar]
- 39. Sanchez CJ, Prieto EM, Krueger CA, et al. Effects of local delivery of d‐amino acids from biofilm‐dispersive scaffolds on infection in contaminated rat segmental defects. Biomaterials. 2013;34:7533‐7543. [DOI] [PubMed] [Google Scholar]
- 40. Chen X, Kidder LS, Lew WD. Osteogenic protein‐1 induced bone formation in an infected segmental defect in the rat femur. J Orthop Res. 2002;20:142‐150. [DOI] [PubMed] [Google Scholar]
- 41. Chen X, Tsukayama DT, Kidder LS, Bourgeault CA, Schmidt AH, Lew WD. Characterization of a chronic infection in an internally‐stabilized segmental defect in the rat femur. J Orthop Res. 2005;23:816‐823. [DOI] [PubMed] [Google Scholar]
- 42. Li B, Brown KV, Wenke JC, Guelcher SA. Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. J Controlled Release. 2010;145:221‐230. [DOI] [PubMed] [Google Scholar]
- 43. Penn‐Barwell JG, Rand BCC, Brown KV, Wenke JC. A versatile model of open‐fracture infection: a contaminated segmental rat femur defect. Bone Jt Res. 2014;3:187‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buxton TB, Travis MT, O'shea KJ, et al. Low‐dose infectivity of Staphylococcus aureus (SMH strain) in traumatized rat tibiae provides a model for studying early events in contaminated bone injuries. Comp Med. 2005;55:123‐128. [PubMed] [Google Scholar]
- 45. McPherson JC, Runner RR, Shapiro B, et al. An acute osteomyelitis model in traumatized rat tibiae involving sand as a foreign body, thermal injury, and bimicrobial contamination. Comp Med. 2008;58:369‐374. [PMC free article] [PubMed] [Google Scholar]
- 46. Lucke M, Schmidmaier G, Sadoni S, et al. A new model of implant‐related osteomyelitis in rats. J Biomed Mater Res Part B Appl Biomater. 2003;67B(1):593‐602. [DOI] [PubMed] [Google Scholar]
- 47. Inanmaz ME, Uslu M, Isik C, Kaya E, Tas T, Bayram R. Extracorporeal shockwave increases the effectiveness of systemic antibiotic treatment in implant‐related chronic osteomyelitis: experimental study in a rat model. J Orthop Res. 2014;32:752‐756. [DOI] [PubMed] [Google Scholar]
- 48. Lindsey BA, Clovis NB, Smith ES, Salihu S, Hubbard DF. An animal model for open femur fracture and osteomyelitis: part I. J Orthop Res. 2010;28:43–47. [DOI] [PubMed] [Google Scholar]
- 49. Lei MG, Gupta RK, Lee CY. Proteomics of Staphylococcus aureus biofilm matrix in a rat model of orthopedic implant‐associated infection. PLoS One. 2017;12:e0187981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stadelmann VA, Potapova I, Camenisch K, Nehrbass D, Richards RG, Moriarty TF. In vivo MicroCT monitoring of osteomyelitis in a rat model. Biomed Res Int. 2015;2015:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harrasser N, Gorkotte J, Obermeier A, et al. A new model of implant‐related osteomyelitis in the metaphysis of rat tibiae. BMC Musculoskelet Disord. 2016;17:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cobb LH, Park J, Swanson EA, et al. CRISPR‐Cas9 modified bacteriophage for treatment of Staphylococcus aureus induced osteomyelitis and soft tissue infection. PLOS One. 2019;14:e0220421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shiels SM, Bedigrew KM, Wenke JC. Development of a hematogenous implant‐related infection in a rat model. BMC Musculoskelet Disord. 2015;16:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arens D, Wilke M, Calabro L, et al. A rabbit humerus model of plating and nailing osteosynthesis with and without Staphylococcus aureus osteomyelitis. Eur Cells Mater. 2015;30:148‐162. [DOI] [PubMed] [Google Scholar]
- 55. Nickerson DS, Kazmierowski JA, Kronvall G, Williams RC, Quie PG. Immune response to chronic osteomyelitis in the rabbit. Exp Biol Med. 1970;134:821‐824. [DOI] [PubMed] [Google Scholar]
- 56. Odekerken JCE, Arts JJC, Surtel DAM, Walenkamp GHIM, Welting TJM. A rabbit osteomyelitis model for the longitudinal assessment of early post‐operative implant infections. J Orthop Surg Res. 2013;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang X, Jia W, Gu Y, et al. Teicoplanin‐loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model. Biomaterials. 2010;31:5865‐5874. [DOI] [PubMed] [Google Scholar]
- 58. Yin L‐Y, Manring MM, Calhoun JH. A rabbit osteomyelitis model to simulate multibacterial war wound infections. Mil Med. 2013;178:696‐700. [DOI] [PubMed] [Google Scholar]
- 59. Wu W, Ye C, Zheng Q, Wu G, Cheng Z. A therapeutic delivery system for chronic osteomyelitis via a multi‐drug implant based on three‐dimensional printing technology. J Biomater Appl. 2016;31:250‐260. [DOI] [PubMed] [Google Scholar]
- 60. Kishor C, Mishra RR, Saraf SK, Kumar M, Srivastav AK, Nath Gopal. Phage therapy of staphylococcal chronic osteomyelitis in experimental animal model. Indian J Med Res. 2016;143:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mestres G, Fernandez‐Yague MA, Pastorino D, et al. In vivo efficiency of antimicrobial inorganic bone grafts in osteomyelitis treatments. Mater Sci Eng C. 2019;97:84‐95. [DOI] [PubMed] [Google Scholar]
- 62. Gaudin A, Amador Del Valle G, Hamel A, et al. A new experimental model of acute osteomyelitis due to methicillin‐resistant Staphylococcus aureus in rabbit. Lett Appl Microbiol. 2011;52:253‐257. [DOI] [PubMed] [Google Scholar]
- 63. Smeltzer MS, Thomas JR, Hickraon SG, et al. Characterization of a rabbit model of staphylococcal osteomyelitis. J Orthop Res. 1997;15:414‐421. [DOI] [PubMed] [Google Scholar]
- 64. Overstreet D, McLaren A, Calara F, Vernon B, McLemore R. Local gentamicin delivery from resorbable viscous hydrogels is therapeutically effective. Clin Orthop Relat Res. 2015;473:337‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peng KT, Chen C‐F, Chu I‐M, et al. Treatment of osteomyelitis with teicoplanin‐encapsulated biodegradable thermosensitive hydrogel nanoparticles. Biomaterials. 2010;31:5227‐5236. [DOI] [PubMed] [Google Scholar]
- 66. Hamel A, Caillon J, Jacqueline C, Rogez J‐M, Potel G. Internal device decreases antibiotic’s efficacy on experimental osteomyelitis. J Child Orthop. 2008;2:239‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li Y, Liu L, Wan P, et al. Biodegradable Mg‐Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: In vitro and in vivo evaluations. Biomaterials. 2016;106:250‐263. [DOI] [PubMed] [Google Scholar]
- 68. Chai H, Guo L, Wang X, et al. Antibacterial effect of 317L stainless steel contained copper in prevention of implant‐related infection in vitro and in vivo. J Mater Sci Mater Med. 2011;22:2525‐2535. [DOI] [PubMed] [Google Scholar]
- 69. Jennings JA, Beenken KE, Skinner RA, et al. Antibiotic‐loaded phosphatidylcholine inhibits staphylococcal bone infection. World J Orthop. 2016;7(8):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rosenbaum Chou TG, Petti CA, Szakacs J, Bloebaum RD. Evaluating antimicrobials and implant materials for infection prevention around transcutaneous osseointegrated implants in a rabbit model. J Biomed Mater Res Part A. 2010;92A:942–952. [DOI] [PubMed] [Google Scholar]
- 71. Moskowitz JS, Blaisse MR, Samuel RE, et al. The effectiveness of the controlled release of gentamicin from polyelectrolyte multilayers in the treatment of Staphylococcus aureus infection in a rabbit bone model. Biomaterials. 2010;31:6019‐6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang X, Ma Y‑F, Wang L, et al. A rabbit model of implant‐related osteomyelitis inoculated with biofilm after open femoral fracture. Exp Ther Med. 2017;14:4995–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ter Boo GJA, Schmid T, Zderic I, et al. Local application of a gentamicin‐loaded thermo‐responsive hydrogel allows for fracture healing upon clearance of a high Staphylococcus aureus load in a rabbit model. Eur. Cells Mater. 2018;35:151‐164. [DOI] [PubMed] [Google Scholar]
- 74. Jensen LK, Koch J, Dich‐Jorgensen K, et al. Novel porcine model of implant‐associated osteomyelitis: a comprehensive analysis of local, regional, and systemic response. J Orthop Res. 2017;35:2211‐2221. [DOI] [PubMed] [Google Scholar]
- 75. Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139:663‐670. [DOI] [PubMed] [Google Scholar]
- 76. Hill PF, Watkins PE. The prevention of experimental osteomyelitis in a model of gunshot fracture in the pig. Eur J Orthop Surg Traumatol. 2001;11:237‐241. [Google Scholar]
- 77. Dehring DJ, Crocker SH, Wismar BL, Steinberg SM, Lowery BD, Cloutier CT. Comparison of live bacteria infusions in a porcine model of acute respiratory failure. J Surg Res. 1983;34:151‐158. [DOI] [PubMed] [Google Scholar]
- 78. Jensen HE, Nielsen OL, Agerholm JS, et al. A non‐traumatic Staphylococcus aureus osteomyelitis model in pigs. Vivo (Brooklyn). 2010;24:257‐264. [PubMed] [Google Scholar]
- 79. Dahl LB, Hoyland AL, Dramsdahl H, Kaaresen PI. Acute osteomyelitis in children: a population‐based retrospective study 1965 to 1994. Scand J Infect Dis. 1998;30:573‐577. [DOI] [PubMed] [Google Scholar]
- 80. Johansen LK, Frees D, Aalbaek B, et al. A porcine model of acute, haematogenous, localized osteomyelitis due to Staphylococcus aureus: a pathomorphological study. APMIS. 2011;119:111‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Johansen LK, Koch J, Frees D, et al. Pathology and biofilm formation in a porcine model of staphylococcal osteomyelitis. J Comp Pathol. 2012;147:343‐353. [DOI] [PubMed] [Google Scholar]
- 82. Johansen LK, Svalastoga EL, Frees D, et al. A new technique for modeling of hematogenous osteomyelitis in pigs: inoculation into femoral artery. J Invest Surg. 2013;26:149‐153. [DOI] [PubMed] [Google Scholar]
- 83. Nielsen OL, Afzelius P, Bender D, et al. Comparison of autologous (111)In‐leukocytes, (18)F‐FDG, (11)C‐methionine, (11)C‐PK11195 and (68)Ga‐citrate for diagnostic nuclear imaging in a juvenile porcine haematogenous staphylococcus aureus osteomyelitis model. Am J Nucl Med Mol Imaging; 2015;5:169. [PMC free article] [PubMed] [Google Scholar]
- 84. Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG. Animal models for implant biomaterial research in bone: a review. Eur Cells Mater. 2007;13:1‐10. [DOI] [PubMed] [Google Scholar]
- 85. Deysine M, Rosario E, Isenberg HD. Acute hematogenous osteomyelitis: an experimental model. Surgery. 1976;79:97‐99. [PubMed] [Google Scholar]
- 86. Fitzgerald RH. Experimental osteomyelitis: description of a canine model and the role of depot administration of antibiotics in the prevention and treatment of sepsis. J Bone Jt Surg Am. 1983;65:371‐380. [PubMed] [Google Scholar]
- 87. Petty W, Spanier S, Shuster JJ, Silverthorne C. The influence of skeletal implants on incidence of infection. Experiments in a canine model. J Bone Jt Surg Ser A. 1985;67:1236‐1244. [PubMed] [Google Scholar]
- 88. Khodaparast O, Coberly DM, Mathey J, Rohrich RJ, Levin LS, Brown SA. Effect of a transpositional muscle flap on VEGF mRNA expression in a canine fracture model. Plast Reconstr Surg. 2003;112:171‐176. [DOI] [PubMed] [Google Scholar]
- 89. Dai KR, Xu XL, Tang TT, et al. Repairing of goat tibial bone defects with BMP‐2 gene‐modified tissue‐engineered bone. Calcif Tissue Int. 2005;77:55‐61. [DOI] [PubMed] [Google Scholar]
- 90. Curtis MJ, Brown PR, Dick JD, Jinnah RH. Contaminated fractures of the tibia: a comparison of treatment modalities in an animal model. J Orthop Res. 1995;13:286‐295. [DOI] [PubMed] [Google Scholar]
- 91. Salgado CJ, Jamali AA, Mardini S, Buchanan K, Veit B. A model for chronic osteomyelitis using Staphylococcus aureus in goats. Clin Orthop Relat Res. 2005;436:246–250. [DOI] [PubMed] [Google Scholar]
- 92. Beardmore AA, Brooks DE, Wenke JC, Thomas DB. Effectiveness of local antibiotic delivery with an osteoinductive and osteoconductive bone‐graft substitute. J Bone Jt Surg Ser A. 2005;87:107‐112. [DOI] [PubMed] [Google Scholar]
- 93. Tran N, Tran PA, Jarrell JD, et al. In vivo caprine model for osteomyelitis and evaluation of biofilm‐resistant intramedullary nails. Biomed Res Int. 2013;2013:674478–674489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kieser DC, Kanade S, Waddell NJ, Kieser JA, Theis J‐C, Swain MV. The deer femur‐A morphological and biomechanical animal model of the human femur. Biomed Mater Eng. 2014;24:1693‐1703. [DOI] [PubMed] [Google Scholar]
- 95. Kaarsemaker S, Walenkamp GHIM, Anthony AEJ. New model for chronic osteomyelitis with Staphylococcus aureus in sheep. Clin Orthop Relat Res. 1997;339:246‐252. [DOI] [PubMed] [Google Scholar]
- 96. McLaren JS, White LJ, Cox HC, et al. A biodegradable antibiotic‐impregnated scaffold to prevent osteomyelitis in a contaminated in vivo bone defect model. Eur Cells Mater. 2014;27:332‐349. [DOI] [PubMed] [Google Scholar]
- 97. Hill PF, Clasper JC, Parker SJ, Watkins PE. Early intramedullary nailing in an animal model of a heavily contaminated fracture of the tibia. J Orthop Res. 2002;20:648‐653. [DOI] [PubMed] [Google Scholar]
- 98. Stewart S, Barr S, Engiles J, et al. Vancomycin‐modified implant surface inhibits biofilm formation and supports bone‐healing in an infected osteotomy model in sheep: A proof‐of‐concept study. J Bone Jt Surg Ser A. 2012;94:1406‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Williams DL, Haymond BS, Woodbury KL, et al. Experimental model of biofilm implant‐related osteomyelitis to test combination biomaterials using biofilms as initial inocula. J Biomed Mater Res Part A. 2012;100A:1888‐1900. [DOI] [PMC free article] [PubMed] [Google Scholar]


