Abstract
As an immune-privileged organ, the placenta can tolerate the introduction of antigens without inducing a strong inflammatory response that would lead to abortion. However, for the control of intracellular pathogens, a strong Th1 response characterized by the production of interferon-γ is needed. Thus, invasion of the placenta by intracellular parasites puts the maternal immune system in a quandary: The proinflammatory response needed to eliminate the pathogen can also lead to abortion. Toxoplasma is a highly successful parasite that causes lifelong chronic infections and is a major cause of abortions in humans and livestock. Here, we discuss how Toxoplasma strain type and parasite effectors influence host cell signaling pathways, and we speculate about how this might affect the outcome of gestation.
Toxoplasma, Placenta, and Immune Response
Toxoplasma gondii is an obligate intracellular parasite that infects most warm-blooded animals and is the causative agent of toxoplasmosis (Box 1). To be transmitted, Toxoplasma needs to form orally infectious tissue cysts in intermediate hosts. Hence, it is in the parasite’s best interest to ensure that its host survives infection, which is achieved by the induction of an immune response able to control parasite multiplication. However, to ensure that not all parasites are eliminated by this immune response, Toxoplasma uses a dedicated arsenal of effectors that prevents its killing. This balance between activating the immune response to drive the formation of the chronic tissue cyst stages and the inhibition of the immune response to ensure its survival determines Toxoplasma’s success in a particular animal species. It is unlikely that every Toxoplasma strain can optimally balance activation of the immune response with inhibition of its killing in every animal species. Different Toxoplasma strains (Box 2) likely evolved to perfect this balancing act in particular animal species. Thus, Toxoplasma virulence leading to the death of its host could be seen as a mismatch between host species and Toxoplasma strain. Similarly, in some host genetic backgrounds infected with particular strains, the strong immune response induced by the parasite could lead to immunerelated pathology. Together with other protozoan parasites, such as Neospora caninum or Trypanosoma cruzi, Toxoplasma can invade the placenta, infect the fetus, and cause abortions in animals and humans. Specific Toxoplasma strain–host combinations may lead to detrimental outcomes during pregnancy. A strain that is very efficient in evading the immune response could easily multiply in the placenta and be transmitted to the fetus, causing its death and the subsequent abortion. By contrast, a strain that induces a strong immune response could elicit exacerbated inflammation in the placenta that disrupts the normal course of gestation, resulting in a failure to maintain the fetus. In this opinion article, we cover the different factors that could affect the outcome of gestation during Toxoplasma infection, focusing on parasite effectors and host immune genes.
Box 1. Toxoplasmosis and Toxoplasma Life Cycle.
The protozoan parasite Toxoplasma gondii is the causative agent of toxoplasmosis, a worldwide zoonotic disease that can affect virtually all mammals and birds, including approximately one-third of humans. Although the majority of infected healthy individuals are asymptomatic, Toxoplasma infection can cause neonatal mortality, abortions, and a wide variety of neurological symptoms, especially in immunocompromised or congenitally infected individuals [90]. A range of variables, including individual susceptibility, strain virulence, and infective dose, have been proposed as key factors in the development of one or the other of these clinical forms. Infection can occur after ingestion of oocysts shed in the feces of an infected cat (definitive host) contaminating food or water, or tissue cysts present in meat of infected animals (intermediate hosts). Upon ingestion, excysted parasites infect intestinal epithelial cells, where they differentiate into fast-replicating tachyzoites, the life cycle stage responsible for proliferation, dissemination, and clinical symptoms. After eliciting an efficient immune response, tachyzoites escape by infecting long-lived cells such as neurons and myocytes, where they convert into the slow-dividing bradyzoite stage and form tissue cysts, which normally remain dormant throughout the lifetime of the host [2]. The ingestion of undercooked meat is considered an important source of Toxoplasma infection for humans and a risk factor in pregnant women, representing a significant public health hazard. Likewise, oocysts shed by cats are highly stable in the environment and extremely resistant to inactivation procedures; hence, they are exceptionally infectious (a single oocyst is able to produce the infection), playing a pivotal role in the spread of toxoplasmosis. This is important not only for humans but also for livestock; as a matter of fact, toxoplasmosis causes important economic losses in the livestock sector related to reproductive failure, mainly in sheep and goats [61].
Box 2. Toxoplasma Strain Diversity.
In Europe and North America, strains belonging to four different Toxoplasma clonal lineages (types I, II, III, and XII) are commonly isolated in animals and humans, with most infections caused by type II strains [91]. By contrast, the majority of characterized isolates from South America are genetically distinct from the North American/European strains and have been referred to as atypical strains [92]. Although type II strains have frequently been associated with congenital infections, type II strains are also the prevailing strains in Europe and North America, where most of such studies have been performed [14]. Nevertheless, other studies have observed an association between type XII strains, which are the most dominant non-type II strains in North America, and severe congenital toxoplasmosis cases [93]. Furthermore, nonclonal Toxoplasma strains (i.e., not belonging to type I, II, III, or XII) are associated with disease and abortion in South America (e.g., [94,95]). However, multiple factors might be exerting a strong influence, such as the presence of regionally prevalent strains and a bias against asymptomatic infections from which fewer samples are normally available.
Toxoplasma Dissemination to the Placenta
Toxoplasma resides within a nonfusogenic vacuole called the ‘parasitophorous vacuole’ (PV). The key to Toxoplasma’s successful co-option of the host resides in its three secretory organelles: micronemes, rhoptries, and dense granules. Microneme proteins (MICs) and rhoptry neck proteins mediate the mechanics of host cell invasion, while rhoptry bulb proteins (ROPs) and dense granule proteins (GRAs) are involved in modulating host cell signaling pathways and evasion of the immune response [1]. Once Toxoplasma forms its replicative niche inside the host cell, it multiplies by endodyogeny (i.e., a form of cell division in which two daughter cells are produced within the mother cell) until it egresses, lysing the host cell in the process, and spreads to invade neighboring cells [2].
After crossing the intestinal epithelial barrier, Toxoplasma disseminates throughout its host and reaches immune-privileged sites such as the brain, eyes, or placenta. The Toxoplasma WAVE regulatory complex (WRC)-interacting protein (TgWIP) plays a pivotal role in parasite dissemination [3]. This effector is an ROP secreted upon invasion that interacts with the host WRC and SHP2 phosphatase, both of which regulate actin dynamics [4,5]. It affects the morphology of dendritic cells (DCs) and mediates the dissolution of podosomes (see Glossary), which are used by these cells to adhere to extracellular matrices. Hence, it enhances the motility of DCs, likely providing the parasite with a Trojan horse mechanism to disseminate throughout the host [6]. The Toxoplasma orthologue of the 14-3-3 protein family (Tg14-3-3) can also mediate hypermotility in infected DCs in a γ-amino butyric acid–dependent fashion [7]. Another parasite effector that plays an important role in Toxoplasma dissemination is the secreted kinase ROP17, which promotes the migration of infected monocytes [8]. Although the specific monocyte subsets affected and exact mechanisms involved are not fully understood, the process seems to occur through host Rho-associated protein kinase signaling. Regardless, upon parasite dissemination throughout the organism, Toxoplasma can use a variety of infected cells, including monocytes and natural killer (NK) cells, as carriers [9,10] or use paracellular/transcellular mechanisms allowing extracellular parasites to transmigrate across biological barriers such as ocular, placental, or blood–brain barriers [6,11]. Leukocytes have been described to use both integrin-dependent and -independent mechanisms during interstitial migration, both of which are well suited to promote Toxoplasma dissemination [11]. Although integrins were once thought to be critical for leukocyte migration, recent studies have shown that DCs, neutrophils, and T cells can still migrate in the interstitial tissue in the absence of integrins [12,13]. In this sense, TgWIP would favor this latter mechanism because it promotes the dissolution of podosomes in infected DCs, which are needed for the integrin-dependent transmigration [3]. Likewise, ROP17 supports an integrin-independent interstitial migration by inducing changes in monocyte motility to facilitate Toxoplasma systemic dissemination [8].
After reaching the placenta, it is not completely clear how Toxoplasma crosses the placental barrier. Nevertheless, most published data on transmission from mother to fetus are consistent with Toxoplasma first infecting maternal leukocytes and subsequently using those as Trojan horses to reach the uterine lining forming the maternal part of the placenta (decidua) [14]. Thereafter, Toxoplasma invades trophoblasts and subsequently the villous core and fetal vasculature [15,16]. Because TgWIP plays a pivotal role in parasite dissemination [3], it is tempting to hypothesize that this effector is also critical for the parasite to reach the placenta and be transmitted to the fetus. It would also be interesting to assess the importance of this gene in the closely related parasite N. caninum, as well as in TgWIP polymorphisms among different Toxoplasma strains that could affect its function.
Immune Response in the Placenta
The maternal–fetal relationship is not simply maternal tolerance of a foreign tissue, but a series of intricate cytokine interactions shaping an appropriate immune regulation. A successful gestation depends on multiple immunologic mechanisms designed to suppress antifetal immunity within the placenta [17]. The expansion of the regulatory T-cell (Treg) subset and a maternal shift toward a Th2 immune response creates a ‘tolerant’ microenvironment during pregnancy by preventing harmful effects from Th1 and Th17 cells [18-20]. The latter are involved in the recruitment of neutrophils to infected tissues, which are good at destroying extracellular parasites but can also cause tissue damage, thus promoting immunopathology [21]. The so-called Th1/Th2 paradigm seemed to be a convincing explanation for the challenging question why the fetus is not rejected by the maternal immune system despite the presence of paternal antigens in contact with maternal cells. However, this paradigm appears to be outdated and insufficient to explain the causes of pregnancy loss, because it has been shown that some Th1 cytokines, such as interferon (IFN)-γ or interleukin (IL)-12, may have some protective role during pregnancy, and neither maternal nor fetal production of the anti-inflammatory or regulatory cytokines IL-4 and IL-10 are crucial for the completion of successful allogeneic pregnancies in mice [22,23]. These conflicting findings may be explained by the incorrect consideration of pregnancy as a single homogeneous event that resembles a host-graft model. Rather, gestation should be considered as a dynamic developmental process with different immunological stages, having more similarities to the immune processes taking place during tumor progression and cell metastasis [24]. Indeed, metastatic cell progression resembles the events taking place during gestation: attachment, invasion of tissues, gradual antigen presentation, inflammation, and tolerance induction. Similarly, in the first stages of pregnancy, there is a proinflammatory environment that ensues from implantation and placentation, then there is a shift to an anti-inflammatory state that allows fetal growth, and finally a shift back to a proinflammatory stage that promotes labor and delivery (Box 3). Most studies investigating the gestational immune response have been conducted during the second stage, which is the longest period; hence the generalization that pregnancy is characterized by a Th2 environment [25]. However, pregnancy is both a proinflammatory and anti-inflammatory condition. Thus, its success depends on the ability of the maternal immune response to establish an effective barrier to protect the mother and the fetus against external harms while being permissive at the same time with the nourishment of the developing fetus [24,26]. A failure of this equilibrium could lead to alterations in the placenta causing fetal damage and abortion without the actual transmission of an invading pathogen [27-29].
Box 3. Variations in the Inflammatory Profile during Gestation.
During the early stages of gestation when implantation and placentation take place, there is a mixture of invading, dying, and repairing cells, and thus a strong proinflammatory response is required to secure the adequate repair of the uterine epithelium and the removal of cellular debris [26]. This response at the implantation site is characterized by the presence of IL-6, IL-8, granulocyte-macrophage colony-stimulating factor, CXCL1, CCL4, osteopontin, and TNF- [96]. Following implantation and placentation, the longest period of pregnancy ensues when fetal development takes place. During this period, any proinflammatory response triggered by insults, such as infections, can lead to miscarriage [24]. Several immune cells, including macrophages, NK cells, and Treg cells, contribute to the establishment of an anti-inflammatory microenvironment [97]. Among them, Treg cells promote immune tolerance by secreting IL-10 and TGF-β1 by dampening IFN-γ and other Th1/Th17 inflammatory cytokines, and by acting as an IL-2 sponge to deprive Th1 cells from their activation/proliferation [29,98]. One of the key cytokines in the maintenance of a balanced Th1/Th2 environment in the placenta is TGF-β [38,99]. However, when exposed to both IL-6 and TGF-β, CD4+ T cells develop instead into Th17, the proinflammatory IL-17–producing cells [33,100]. Treg and Th17 cells have opposed effects on inflammation and thus immunologic tolerance. In fact, a low Treg/Th17 rate has been associated with unexplained miscarriage, pre-eclampsia, and recurrent spontaneous abortions in humans [101-103]. However, because they are proinflammatory in nature, these Th17 cells contribute to protecting the maternal–fetal interface from external pathogens. Like T cells, macrophages have also been described to contribute to normal and pathological pregnancies. Most of the decidual macrophages are characterized by an immunosuppressive phenotype (M2), supporting fetal-maternal immune tolerance [104]. However, the macrophage profile varies along gestation: There is a slight inflammatory profile (M1) for implantation, then it switches primarily to anti-inflammatory to avoid rejection and finally at parturition switches again to proinflammatory [105]. Moreover, an M1/M2 dysregulation has been observed during adverse pregnancy outcomes, and upon contact with pathogens, pattern recognition receptors, such as TLRs, may alter decidual macrophage polarity, shifting the immunosuppressive M2 dominancy to an inflammatory M1 phenotype [104]. Another important factor, particularly in Toxoplasma infection, could be the role of indoleamine 2,3-dioxygenase (IDO). IDO is an immunosuppressive enzyme produced primarily by Tregs and alternatively activated macrophages (M2) that locally depletes L-tryptophan needed for T-cell proliferation and promotes more Treg activity [105]. IDO is also expressed by maternal uterine mucosa and fetal syncytiotrophoblast, thereby controlling local inflammation. However, because Toxoplasma is a natural auxotroph for L-tryptophan, it acts as a barrier for parasite growth. To prevent this, Toxoplasma secretes TgIST, an effector that can effectively block IDO induction and promote parasite growth [85], potentially leading to abortion.
In the case of Toxoplasma infection, the alteration of the Th1/Th17 vs. Th2/Treg balance in the placenta might play a pivotal role in the occurrence of abortion. Indeed, Tregs are critical in Toxoplasma infections during pregnancy because their reduction has been associated with fetal loss, regardless of vertical transmission [30,31]. Toxoplasma causes a decrease in Tregs and transforming growth factor (TGF)-β levels at the maternal–fetal interface, thus preventing the control of an exacerbated immune response [32-34]. This decrease in Tregs may be multifactorial, likely caused by apoptosis [30], limitation of proliferation [33], and/or differentiation into Th17 cells in the presence of IL-6 [33,36]. The critical role of TGF-β in Toxoplasma infection during gestation has been confirmed in pregnant mouse models, where the treatment with TGF-β improves adverse effects by increasing Treg differentiation but not proliferation [37,38]. It is noteworthy that most of these studies were carried out in mice and with the type I RH strain that naturally does not promote an exacerbated inflammatory response. Thus, it is possible that other proinflammatory strains, such as type II, may have a different effect. For instance, type II strains induce IL-6 expression through GRA15, while types I and III strains inhibit IL-6 through ROP16 [39,40].
Regardless, it seems clear that Toxoplasma infection during pregnancy puts the maternal immune system in a quandary. The strong Th1 response associated with the production of IFN-γ and tumor necrosis factor (TNF)-α after infection with Toxoplasma is a double-edged sword. Although these cytokines are needed for the control of the pathogen, they can also be deleterious for the maintenance of gestation, leading to enhanced transmission and fetal damage [32,41]. For example, IFN-γ together with either TNF-α or IL-1β upregulates adhesion receptors such as intercellular adhesion molecule 1 (ICAM1) on trophoblasts, leading to increased adhesion of Toxoplasma-infected maternal monocytes to these cells and thus facilitating crossing of the placental barrier by the parasite [40]. In addition, the macrophage migration inhibitory factor (MIF) can induce the expression of ICAM1 on the surface of trophoblasts [43,44]. Toxoplasma also produces a homologue of this cytokine (TgMIF) that facilitates immune evasion and intracellular parasite multiplication. This factor stimulates antiapoptotic pathways through phosphorylation of extracellular signal-regulated kinases, induction of IL-8 secretion, and increase of host cell proliferation and differentiation, globally favoring intracellular parasite multiplication [45,46]. In fact, a recent study described an association between high IL-8 production and parasite transmission to the fetus in pregnant women [41]. This could be explained by an increased immune cell attraction to the placenta, which would in turn favor parasite replication within trophoblastic cells and dissemination to the fetus. IFN-γ and other Th1-produced cytokines can also induce vascular damage (congestion, thrombosis, and/or hemorrhage), leading to a reduced nutrient/oxygen transport in the placenta [47] that may cause embryo resorption or fetal damage without actual vertical transmission. Furthermore, although the precise role of the Treg chemoattractant CC chemokine ligand 22 (CCL22) during Toxoplasma infection is not well understood, it has been shown to be highly upregulated in infected human syncytiotrophoblasts in a manner that is dependent on the Toxoplasma c-Myc regulation (MYR) protein 1 (MYR1) [48]. This secreted protein is localized at the PV membrane and participates in the translocation of Toxoplasma GRA effector molecules to the host cell cytoplasm [49]. Furthermore, very recently, it has been shown that GRA28, a secreted Toxoplasma effector, through MYR1, regulates the secretion of CCL22 in murine and human cells, including syncytiotrophoblasts [50] Because CCL22 is known to be constitutively expressed during pregnancy and has been found to be increased in miscarriage cases [51], it is possible that the increased secretion of this chemokine by infected trophoblasts may contribute to the abortion in Toxoplasma infections.
Outcome of Toxoplasma Infection during Gestation
T. gondii can be vertically transmitted from mother to fetus, leading to miscarriages, stillbirths, and other adverse outcomes, depending on the stage of gestation at which infection takes place [20]. There is an inverse relation between gestation period and fetal death rate. Vertical transmission in the first trimester is infrequent, although it usually triggers fetal death and abortion because the immune system of the fetus is still underdeveloped. In the second trimester, the rate of vertical transmission increases, and fetuses are commonly born prematurely or with severe conditions, such as hydrocephalus, microcephaly, or mental retardation. In the third period of gestation, fetal infection is likely to occur, and although most babies are asymptomatic at birth, they may develop neurological, ophthalmological, or auditory diseases months or years later [14,52]. These observations were reported mainly from pregnant women, and although similar outcomes have been described in other animals, it is possible that a different scenario might occur in more susceptible or resistant animals. In pregnant ewes, two different clinical presentations of toxoplasmosis have been observed: late, classical abortion produced approximately 1 month after infection and early abortion produced during the acute phase of the disease (within the first 2 weeks after infection). Moreover, apart from the time of gestation, the outcome of infection during pregnancy greatly depends on the parasite strain and the host species. In the following sections, these aspects are covered individually.
Late Classical vs. Early Abortion
Typically, pregnant ewes abort 1 month after Toxoplasma infection. The underlying mechanisms are attributed to the tissue damage caused by the multiplication of the parasite in the placenta and, after crossing the placental barrier, in the fetus, ultimately leading to fetal death [15,28]. However, aside from this late ‘classical’ abortion, another type, named ‘early abortion,’ has also been described [53,54]. The mechanism of early abortions during the acute phase of the disease is not well understood but has been associated with hypoxic damage to the fetus without the direct implication of the parasite; hence, these abortions were also described as ‘sterile.’ These early abortions occurred within the first 2 weeks after oral infection with M4 type II oocysts (Box 1), regardless of the time of gestation and surprisingly even with doses as low as 50 oocysts [53-55]. Nevertheless, early abortions were more frequently found in ewes infected in the second trimester of gestation, whereas late abortions were more common after infection with lower doses and at day 40 (first trimester) or 120 (third trimester) of gestation. In addition, placentas from ewes suffering early abortions showed lesions compatible with ischemic necrosis and thrombosis and had higher expression levels of the proinflammatory cytokine TNF-α and the adhesion molecules vascular cell adhesion molecule 1 and ICAM1. Furthermore, ischemic lesions (leukomalacia) were found in the brain of some fetuses, and Toxoplasma DNA was detected in only half of the placentas and in none of the fetuses from the early abortions. Therefore, it seems likely that the acute inflammatory response and vascular lesions found in the placenta caused a disruption in nutrient or oxygen transport, consequently producing lethal hypoxic damage to the fetus [56].
Toxoplasma infections have been associated with spontaneous abortions in pregnant women; hence, it is likely that the abortions early after infection observed in sheep can also happen in humans. However, very few case–control studies have compared Toxoplasma seropositivity between women who had spontaneous abortions and women with normal pregnancies [57,58]. Likewise, the cause of most abortions in sheep and goats is unknown, although it is possible that early abortions during the acute phase of the disease are underdiagnosed in the field because there are no detectable antibodies/parasites when it occurs [53]. Thus, further studies are needed to shed light on these early abortions.
Influence of the Host
Very little is known about the correlation between Toxoplasma strain virulence in mice compared with other animal species and humans. In fact, some studies have shown that the virulence observed in mice by a certain strain cannot always be extrapolated to other animals. For instance, in a recent study, the type II ME49 strain was compared in pregnant mouse and sheep models, being more virulent in the former [59]. This discrepancy is probably due to differences in the physiology of gestation, placentation, or immune response, among others. This inconsistency between murine and ruminant models is also observed with N. caninum, the closely related apicomplexan parasite causing abortion in cattle [60]. In humans, limited data have been obtained so far from clinical studies on pregnant women infected with Toxoplasma [58]. This is mostly due to the relatively low number of human cases and the limitations of the study of processes taking place in the placenta, because these can only be assessed at the end of pregnancy, and the kinetics are difficult to elucidate [20]. In this section, we focus on different animal models that have been used to study abortion and congenital toxoplasmosis. Although animal models are highly valuable to gain further knowledge of Toxoplasma-induced abortion in humans, in veterinary research, the results are also directly relevant to the disease in the host of interest. In general, most farm animals, including pigs, horses, sheep, and goats, are susceptible to abortion at different degrees, although sheep and goats are especially vulnerable [61]. By contrast, cows are very resistant to Toxoplasma infection, and abortions caused by this parasite are considered a rare event [61]; instead, another apicomplexan parasite, N. caninum, is responsible for a considerable part of cattle reproductive failure, similar to the effect that Toxoplasma has in sheep [62].
Despite the marked differences in the placenta and gestation, the mouse is the animal model most frequently used to study congenital toxoplasmosis. These differences include the time of gestation and the morphology and histological structure of the placenta, among others. For instance, rodents possess a discoidal hemochorial placenta, and the gestation period is 3 weeks, while ruminants have a cotyledonary synepitheliochorial placenta and longer gestations (5 months in small ruminants such as sheep or goat and 9 months in cattle) [63]. The availability of numerous transgenic and knockout mouse strains offers a valuable advantage to address specific questions [20,29]. While there are indeed many advantages in using this experimental model, care should be taken in extrapolating results from mice to other species, because their immune response to Toxoplasma is different from that of other hosts. Rats, like humans and other primates, do not display clinical signs after Toxoplasma infection; hence, they could serve as a better model of congenital toxoplasmosis [64]. However, although the placenta of rodents and humans have in common their hemochorial structure [63], the short duration of murine gestation restricts the value of this model [27]. Halfway between the rat and mouse, guinea pig sensitivity to Toxoplasma is medium, and it has a gestation time of approximately 2 months, long enough to enable comparative studies with different inoculation times. Thus, the pregnant guinea pig model could be a good alternative, although experiments based on this model are very scarce [65,66]. Moreover, in murine species, Toxoplasma is primarily detected by endosomal Toll-like receptors (TLRs)11/12, which bind to the parasite actin-binding protein profilin [67]. Given that infection-induced inflammation is likely a major influence on parasite transmission to the fetus or spontaneous abortion, investigating the role of TLRs in abortion seems important. However, inflammation in rodents is mainly dominated by TLR11/12 activation, while larger animals, including humans, lack TLR11/12 [68]. Therefore, other TLRs, such as TLRs 2, 4, and 9, which have been shown to play an indirect role in immunity to oral Toxoplasma infection in rodents [69], could also be involved in abortion. Sheep have been used to investigate congenital toxoplasmosis because, similarly to humans, they do not develop clinical signs beyond transitory fever and are susceptible to Toxoplasma-induced abortion. In addition, sheep do not possess TLR11/12 [68]; therefore, this model provides a useful tool to study Toxoplasma congenital infection and abortion in close relation to humans. Indeed, as mentioned in the previous text, early abortions described in sheep might also happen in humans; thus, the sheep pregnant model could be used to help understand the pathogenesis of these abortions. Moreover, sheep are of interest to study in their own right because abortion due to congenital toxoplasmosis poses a major economic loss to the sheep industry worldwide [70].
Influence of the Strain
Several epidemiological studies have sought a connection between parasite strain and congenital infection in humans, but definitive conclusions have been difficult to reach (Box 2). Likewise, very little information is available in other animal species [61]. Hence, it is unknown whether infections with certain strains induce more abortion or differ in transplacental transmission, although it seems likely that the Toxoplasma strain type is an important factor in the outcome of gestation. Nonetheless, heterogeneous results have been described in pregnant models even within the same type II strain. For example, the Pru strain induced a decrease in fertility if infection occurred during the last two periods of gestation, regardless of host species [27,66,71,72]. In addition, apart from the genetic background, the in vitro passage number has also been proposed to exert an important influence in the parasite phenotype and biological characteristics, typically enhancing virulence in mice. This fact has been widely studied in more virulent type I strains such as RH and GT1 [73] and recently in the less virulent type II ME49 strain [59]. Furthermore, inhibition of host cell death is another important trait that varies between strains, which could determine the outcome of infection. For instance, it is possible that more virulent strains are better at inhibiting apoptosis, because it has been shown that BeWo choriocarcinoma cells are more likely to undergo apoptosis upon infection with ME49 than with RH [74]. Finally, the ability to subvert the host immune response is one of the best studied strain-specific Toxoplasma traits. For example, in mice, ROP5/ROP18 prevents the IFN-γ–induced immunity-related GTPase (IRG) coating of the PV membrane, thereby limiting parasite clearance [75]. However, what determines parasite survival and avoidance of the immune response in other species that do not possess IRGs is currently unclear. Herein, we focus on genes that are directly implicated in the induction of a potentially damaging proinflammatory response that might influence the outcome of infection.
It is well known that type II strains induce a much stronger proinflammatory response than type I and type III strains [1]. Quantitative trait locus analysis performed in F1 progenies identified the Toxoplasma genes that determine most of the strain differences in the modulation of the inflammatory response: the secreted proteins ROP16 [76] and GRA15 [77]. On the one hand, through the nuclear factor-κB (NF-κB) pathway, type II GRA15 (GRA15II) induces the production of inflammatory cytokines, such as IL-12 and IL-1β, and elicits classically activated (M1) macrophages that are responsible for Th1-dominant immunity [39,78,79]. On the other hand, type I/III ROP16 (ROP6I/III) phosphorylates signal transducer and activator of transcription (STAT) 3/6, driving macrophages toward an alternatively activated (M2) state that is associated with increased IL-10 production and a Th2 polarized response [40,80]. In addition, ROP16 can inhibit GRA15-mediated activation of NF-κB [40], providing a safe niche for parasite replication. Moreover, although all three clonal lineages express an active form of GRA24, the GRA24-mediated activation of p38 mitogen-activated protein kinase only has a significant effect on the classical activation of macrophages (M1) when combined with type II GRA15-induced NF-κB activation, resulting in a potent inflammatory response [81,82]. Besides type II strains, we have shown that Cougar (type XI) and other closely related type XII strains (Ari, WTD3, or B41) induce a stronger proinflammatory response than other clonal strains by stimulating a strong secretion of IFN-β1 in both murine bone marrow–derived macrophages and human fibroblasts [83].
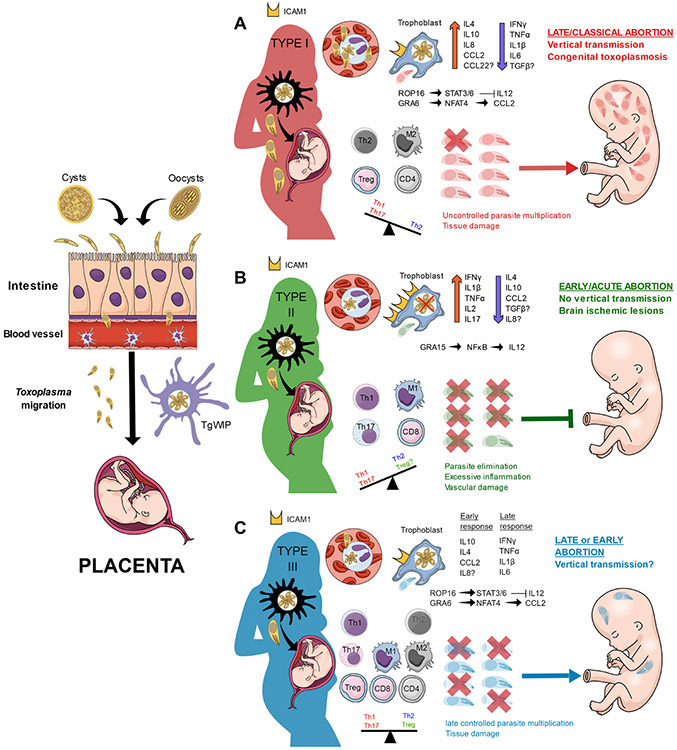
Because Toxoplasma-induced placental inflammation is associated with early abortions, it is likely that Toxoplasma effectors inducing a proinflammatory response play an important role in the pathology of such cases (Figure 1, Key Figure). In this sense, some studies have tested the effect of different type Chinese 1 (ToxoDB 9) strains on adverse pregnancy outcomes in mouse models [29,34]. These strains carry a mixture of the GRA15II and ROP16I/III effectors that is different from those of the archetypal types commonly found in Europe and North America [84], therefore having an opposing inflammation-inducing feature. It is possible that other parasite effectors might be responsible for the biased immunity in the placenta; however, these studies support the notion that GRA15 and ROP16 may play an important role in the pathogenesis of abortion. Indeed, an ROP16-knockout version of the WH3 strain (GRA15II/Δrop16I/III) induced increased apoptosis in vitro when infecting trophoblasts and higher resorption rates in vivo than the wild-type strain. Moreover, the increased IFN-γ, TNF-α, IL-17, and IL-12, together with the decreased IL-4, TGF-β, and IL-10 levels observed in the WH3 Δrop16 strain mimicked the typical type II proinflammatory response and confirmed the critical role of GRA15 in the induction of immune-induced pathogenicity in the placenta [29]. Finally, an RH Δrop16 strain, which lacks an active form of GRA15, was assessed in pregnant mice. Compared with the wild-type strain, infection with the RH Δrop16 strain produced higher resorption rates and promoted an M1 phenotype in macrophages from placental homogenates, with increased TNF-α and IL-12 levels and decreased TGF-β [85].
Figure 1. Key Figure. Immune Response to Toxoplasma Infection during Gestation.
After oral infection, Toxoplasma disseminates either as extracellular parasites or by a Trojan horse mechanism and reaches the placenta. (A) In type I strains, rhoptry bulb protein ROP16I induces sustained activation of signal transducer and activator of transcription (STAT3/6), dampening the production of interleukin (IL)-12, IL-1β, and IL-6, while dense granule protein GRA6I/III induces the secretion of CC chemokine ligand 2 (CCL2). In the absence of IL-12, an anti-inflammatory profile predominates with M2 macrophages and Th2, CD4, and regulatory T cells (Treg). As a consequence, the uncontrolled parasite multiplication produces tissue damage, which in turn facilitates the invasion of the fetus. The most likely outcomes are late abortion and/or congenital infection during early or late gestation, respectively. (B) Type II strains produce a strong inflammatory environment in the placenta due to the early secretion of IL-12 induced by GRA15. This stimulates the secretion of proinflammatory cytokines [interferon {IFN)-γ, IL-1β, and tumor necrosis factor (TNF)-α], which in turn upregulates the expression of intercellular adhesion molecule 1 (ICAM1) on trophoblasts, facilitating the adhesion of infected cells. The intense proinflammatory response eliminates most of the parasites but produces extensive tissue damage that compromises nutrient/oxygen transport to the fetus. Hence, the most likely outcome is an early, ‘sterile’ abortion without vertical transmission. (C)Similar to type I, ROP16III limits the initial production of IL-12, delaying the induction of antiparasitic mechanisms and establishing an initial anti-inflammatory response. However, type III strains are not effective at avoiding intracellular killing, and a proinflammatory immune response is eventually mounted when IL-12 is produced by dendritic cells. However, because the control of the parasite is delayed, some parasites can cross the placental barrier and reach the fetus. Therefore, both early and late abortion with or without vertical transmission are possible, depending on the stage of gestation.
Taken together, these studies strongly suggest that in a dominant GRA15II background, as it naturally happens in type II strains, a subversion of the immune tolerance, attributed to an M1/Th1/Th17 biased response, takes place at the maternal–fetal interface and leads to fetal death. Nevertheless, because these studies were only conducted in mouse models, further studies in more relevant animals, such as pregnant ewes, are needed to confirm its importance in humans. Furthermore, it is likely that, in addition to GRA15 and ROP16, other Toxoplasma genes may contribute to the pathology of the adverse immunity imbalance in pregnant animals. For instance, only type I or III, but not type II, GRA6 induces the expression of the host transcription factor nuclear factor of activated T cells 4 [86], a factor that recruits immune cells through the local induction of chemokines such as CXC chemokine ligand 2 (CXCL2) and CCL2 [87], while only type II GRA25 is able to dampen the expression of the chemokines CCL2 and CXCL1 [88].
Concluding Remarks
Although recent advancements have contributed to a better understanding of the role that certain Toxoplasma effectors exert on the outcome of infection during gestation, many questions remain to be addressed (see Outstanding Questions). It is likely that different Toxoplasma genes can predispose toward early vs. late abortions. For example, on the one hand, Toxoplasma gene products that induce host inflammation could lead to the elimination of the parasite but with the risk of early abortions. On the other hand, Toxoplasma virulence factors involved in evasion of the host immune response and dissemination will likely predispose toward late (classical) abortions caused by the vertical transmission and fetal damage caused by replicating parasites. Different polymorphisms or combinations of active forms of such factors could give each particular strain a specific phenotype that could predict the most likely outcome of gestation. In this sense, serotyping techniques may assist researchers to perform further investigations to associate particular strains with abortions [89]. Likewise, because unexplained abortions are often not further investigated, serotyping in these cases would allow investigators to determine if specific Toxoplasma strains are involved. Finally, further studies are needed to elucidate the pathological mechanisms involved in the outcome of gestation and the effect that particular parasite factors and strains have in abortion and vertical transmission.
Outstanding Questions.
What molecular mechanisms are implicated in the induction of early acute abortions?
What parasite factors are critical in the production of late classical abortions?
Is TgWIP a critical effector in parasite dissemination to the placenta and vertical transmission?
Which Toxoplasma strain types predispose to early/acute and late/classical abortions?
Are Toxoplasma-induced early acute abortions underdiagnosed in livestock and pregnant women on account of the absence of anti-Toxoplasma antibodies and parasite detection in the placenta/fetus at the time of abortion?
What components of the immune response make cows much more resistant than sheep/goats to Toxoplasma-induced abortions?
Why are these components not efficient in controlling Neospora caninum?
Why is abortion in successive gestations common in Neospora but not in Toxoplasma infections?
Highlights.
Different Toxoplasma strains may predispose livestock and humans to early (acute) or late (classical) abortions, depending on the induction of inflammation vs. evasion of the immune response, respectively.
Dense granule proteins and rhoptry bulb proteins, parasite effectors implicated in the balance between evasion of the immune response and induction of a proinflammatory response, likely play a critical role in abortion.
Uncontrolled multiplication of the parasite in the placenta and fetus is associated with late classical abortions.
Severe inflammation at the maternal–fetal interface is associated with acute abortions without direct implication of the parasite. Instead, fetal death is produced by a disruption in nutrient or oxygen transport due to the vascular lesions formed in the placenta, causing lethal hypoxic damage to the fetus.
Acknowledgments
D.A.-S. and D.M. were partially supported by grants from the National Institutes of Health (R21AI139387 and R01AI080621,398 respectively) awarded to J.J.P.S.
Glossary
- Alternatively activated (M2)
M2 macrophages decrease inflammation by secreting anti-inflammatory and regulatory cytokines, allowing tissue repair. These macrophages are less efficient in killing intracellular pathogens.
- Apoptosis
a form of programmed cell death characterized by changes such as blebbing, cell shrinkage, or nuclear fragmentation, among others.
- Classically activated (M1)
M1 macrophages are generally good at killing intracellular pathogens, but their secretion of proinflammatory cytokines can cause pathology through an excessive activation of NK and Th17 cells and induction of proapoptotic pathways.
- Cotyledonary synepitheliochorial
type of placenta observed in ruminants that consists in multiple discrete areas of attachment to the endometrium (placentomes) formed by a fetal portion (cotyledon) and a maternal contact site (caruncle).
- Decidua
modified endometrial layer forming the maternal part of the placenta in close contact with trophoblasts. It protects the embryo from external insults and provides nutritional support.
- Hemochorial
type of placenta present in rodents, high-order primates, and humans where maternal blood is in direct contact with the fetal chorion. It is characterized by a limited cellular barrier and extensive intrauterine trophoblast invasion.
- Immunity-related GTPase (IRG)
family of IFN-inducible GTPases that have been implicated in resistance to intracellular pathogens. In mice infected with Toxoplasma, IRGs mediate the destruction of the intracellular PV.
- Integrin
transmembrane receptor that, after binding to diverse ligands, facilitates cell adhesive interactions with other cells and with the extracellular matrix by cytoskeleton reorganization.
- Intercellular adhesion molecule 1 (ICAM1)
also known as CD54, ICAM1 is a cell surface glycoprotein expressed on endothelial and immune cells that plays an important role in transendothelial migration of immune cells. It is a ligand for Toxoplasma MIC2.
- Nuclear factor-κB (NF-κB)
a family of inducible transcription factors that play a key role in regulating genes implicated in the immune response. Some important members are RelA (p65), RelB, c-Rel, NFκB1 (p50), and NF-κB2 (p52).
- Podosomes
actin-rich conical structures present on the plasma membrane of many cells. Through recruited matrix metalloproteinases, podosomes degrade the extracellular matrix by proteolysis, playing an important role in cell migration.
- Signal transducer and activator of transcription (STAT)
these transcription factors regulate several cellular processes, including immune activation and cell growth/differentiation.
- Syncytiotrophoblasts
multinucleated cells that form the outermost layer of the hemochorial placenta, comprising the primary barrier to avoid vertical transmission. They are extremely resistant to Toxoplasma infection.
- Synepitheliochorial
type of epitheliochorial placenta present in ruminants where the uterine epithelium is invaded by multinucleated cell masses (syncytia).
- Th1/Th2 paradigm
This paradigm supports the idea that the fetus acts as an allograft to the mother and that a predominant anti-inflammatory Th2 response is needed to maintain pregnancy, with increases in proinflammatory Th1 cells/cytokines resulting in abortion.
- Toll-like receptors (TLRs)
type of pattern recognition receptors that, upon recognition of pathogen-associated molecular patterns, activate signal transduction pathways culminating in the expression of cytokines.
- Trojan horse
Mechanism used by intracellular pathogens whereby infected cells act as carriers or vehicles to help the pathogen disseminate and cross biological barriers without being detected.
References
- 1.Melo MB et al. (2011) Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol. 27, 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blader IJ et al. (2015) Lytic cycle of Toxoplasma gondii: 15 years later. Annu. Rev. Microbiol 69, 463–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sangaré LO et al. (2019) In vivo CRISPR screen identifies TgWIP as a Toxoplasma modulator of dendritic cell migration. Cell Host Microbe 26, 478–492.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan Y-R et al. (2013) Protein tyrosine phosphatase SHP2 suppresses podosome rosette formation in Src-transformed fibroblasts. J. Cell Sci 126, 657–666 [DOI] [PubMed] [Google Scholar]
- 5.Chen B et al. (2014) The WAVE regulatory complex links diverse receptors to the actin cytoskeleton. Cell 156, 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert H and Barragan A (2010) Modelling parasite dissemination: host cell subversion and immune evasion by Toxoplasma gondii. Cell. Microbiol 12, 292–300 [DOI] [PubMed] [Google Scholar]
- 7.Weidner JM et al. (2016) Migratory activation of parasitized dendritic cells by the protozoan Toxoplasma gondii 14-3-3 protein. Cell. Microbiol 18, 1537–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drewry LL et al. (2019) The secreted kinase ROP17 promotes Toxoplasma gondii dissemination by hijacking monocyte tissue migration. Nat. Microbiol 4, 1951–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook JH et al. (2018) Toxoplasma gondii disrupts β1 integrin signaling and focal adhesion formation during monocyte hypermotility. J. Biol. Chem 293, 3374–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueno N et al. (2015) Toxoplasma gondii-infected natural killer cells display a hypermotility phenotype in vivo. Immunol. Cell Biol 93, 508–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drewry LL and Sibley LD (2019) The hitchhiker’s guide to parasite dissemination. Cell. Microbiol 21, e13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolf E et al. (2007) Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat. Immunol 8, 1076–1085 [DOI] [PubMed] [Google Scholar]
- 13.Lämmermann T et al. (2008) Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 [DOI] [PubMed] [Google Scholar]
- 14.Robbins JR et al. (2012) Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect. Immun 80, 418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buxton D and Finlayson J (1986) Experimental infection of pregnant sheep with Toxoplasma gondii: pathological and immunological observations on the placenta and foetus. J. Comp. Pathol 96, 319–333 [DOI] [PubMed] [Google Scholar]
- 16.Ferro EAV et al. (2002) Effect of Toxoplasma gondii infection kinetics on trophoblast cell population in Calomys callosus, a model of congenital toxoplasmosis. Infect. Immun 70, 7089–7094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moffett A and Loke YW (2004) The immunological paradox of pregnancy: a reappraisal. Placenta 25, 1–8 [DOI] [PubMed] [Google Scholar]
- 18.Krishnan L et al. (2013) From mice to women: the conundrum of immunity to infection during pregnancy. J. Reprod. Immunol 97, 62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aluvihare VR et al. (2004) Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol 5, 266–271 [DOI] [PubMed] [Google Scholar]
- 20.Pfaff AW et al. (2007) Cellular and molecular physiopathology of congenital toxoplasmosis: the dual role of IFN-gamma. Parasitology 134, 1895–1902 [DOI] [PubMed] [Google Scholar]
- 21.Gaddi PJ and Yap GS (2007) Cytokine regulation of immunopathology in toxoplasmosis. Immunol. Cell Biol 85, 155–159 [DOI] [PubMed] [Google Scholar]
- 22.Svensson L et al. (2001)The Th2 cytokines IL-4 and IL-10 are not crucial for the completion of allogeneic pregnancy in mice. J. Reprod. Immunol 51, 3–7 [DOI] [PubMed] [Google Scholar]
- 23.Zenclussen AC et al. (2006) Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur. J. Immunol 36, 82–94 [DOI] [PubMed] [Google Scholar]
- 24.Mor G et al. (2017) The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol 17, 469–482 [DOI] [PubMed] [Google Scholar]
- 25.Saito S et al. (2010) Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol 63, 601–610 [DOI] [PubMed] [Google Scholar]
- 26.Mor G and Cardenas I (2010) The immune system in pregnancy: a unique complexity. Am. J. Reprod. Immunol 63, 425–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vargas-Villavicencio JA et al. (2016) Mouse model of congenital infection with a non-virulent Toxoplasma gondii strain: vertical transmission, ‘sterile’ fetal damage, or both? Exp. Parasitol 166, 116–123 [DOI] [PubMed] [Google Scholar]
- 28.Benavides J et al. (2017) Ovine toxoplasmosis: a new look at its pathogenesis. J. Comp. Pathol 157, 34–38 [DOI] [PubMed] [Google Scholar]
- 29.Wang C et al. (2018) Toxoplasma Chinese 1 strain of WH3Δrop16I/III/gra15II genetic background contributes to abnormal pregnant outcomes in murine model. Front. Immunol 9, 1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge YY et al. (2008) In pregnant mice, the infection of Toxoplasma gondii causes the decrease of CD4+CD25+-regulatory T cells. Parasite Immunol. 30, 471–481 [DOI] [PubMed] [Google Scholar]
- 31.Chen J-L et al. (2013) The dysfunction of CD4+CD25+ regulatory T cells contributes to the abortion of mice caused by Toxoplasma gondii excreted-secreted antigens in early pregnancy. PLoS One 8, e69012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gómez-Chávez F et al. (2019) Maternal immune response during pregnancy and vertical transmission in human toxoplasmosis. Front. Immunol 10, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H et al. (2012) The Treg/Th17 imbalance in Toxoplasma gondii-infected pregnant mice. Am. J. Reprod. Immunol 67, 112–121 [DOI] [PubMed] [Google Scholar]
- 34.Liu T et al. (2013) Trophoblast apoptosis through polarization of macrophages induced by Chinese Toxoplasma gondii isolates with different virulence in pregnant mice. Parasitol. Res 112, 3019–3027 [DOI] [PubMed] [Google Scholar]
- 35.Oldenhove G et al. (2009) Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31, 772–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangan PR et al. (2006) Transforming growth factor-beta induces development of the TH17 lineage. Nature 441, 231–234 [DOI] [PubMed] [Google Scholar]
- 37.Xu X et al. (2017) TGF-β1 improving abnormal pregnancy outcomes induced by Toxoplasma gondii infection: regulating NKG2D/DAP10 and killer subset of decidual NK cells. Cell. Immunol 317, 9–17 [DOI] [PubMed] [Google Scholar]
- 38.Zhao M et al. (2017) The effect of TGF-β on Treg cells in adverse pregnancy outcome upon Toxoplasma gondii infection. Front. Microbiol 8, 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen KD et al. (2011) Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe 9, 472–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen KD et al. (2013) Toxoplasma gondii rhoptry 16 kinase promotes host resistance to oral infection and intestinal inflammation only in the context of the dense granule protein GRA15. Infect. Immun. 81, 2156–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gómez-Chávez F et al. (2020) A proinflammatory immune response might determine Toxoplasma gondii vertical transmission and severity of clinical features in congenitally infected newborns. Front. Immunol 11, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaff AW et al. (2005) Toxoplasma gondii regulates ICAM-1 mediated monocyte adhesion to trophoblasts. Immunol. Cell Biol 83, 483–489 [DOI] [PubMed] [Google Scholar]
- 43.Ferro EAV et al. (2008) Macrophage migration inhibitory factor is up-regulated in human first-trimester placenta stimulated by soluble antigen of Toxoplasma gondii, resulting in increased monocyte adhesion on villous explants. Am. J. Pathol 172, 50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Oliveira Gomes A et al. (2011) Effect of macrophage migration inhibitory factor (MIF) in human placental explants infected with Toxoplasma gondii depends on gestational age. Am. J. Pathol 178, 2792–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sommerville C et al. (2013) Biochemical and immunological characterization of Toxoplasma gondii macrophage migration inhibitory factor. J. Biol. Chem 288, 12733–12741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barbosa BF et al. (2014) Susceptibility to Toxoplasma gondii proliferation in BeWo human trophoblast cells is dose-dependent of macrophage migration inhibitory factor (MIF), via ERK1/2 phosphorylation and prostaglandin E2 production. Placenta 35, 152–162 [DOI] [PubMed] [Google Scholar]
- 47.Arck PC et al. (1995) Stress-induced murine abortion associated with substance P-dependent alteration in cytokines in maternal uterine decidua. Biol. Reprod 53, 814–819 [DOI] [PubMed] [Google Scholar]
- 48.Ander SE et al. (2018) Human placental syncytiotrophoblasts restrict Toxoplasma gondii attachment and replication and respond to infection by producing immunomodulatory chemokines. mBio 9, e01678–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franco M et al. (2016) A novel secreted protein, MYR1, is central to Toxoplasma’s manipulation of host cells. mBio 7, e02231–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudzki EN et al. (2020) Toxoplasma gondii GRA28 is required for specific induction of the regulatory chemokine CCL22 in human and mouse cells. bioRxiv Published online October 14, 2020. 10.1101/2020.10.14.335802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freier CP et al. (2015) Expression of CCL22 and infiltration by regulatory T Cells are increased in the decidua of human miscarriage placentas. Am. J. Reprod. Immunol 74, 216–227 [DOI] [PubMed] [Google Scholar]
- 52.Gras L et al. (2005) Association between prenatal treatment and clinical manifestations of congenital toxoplasmosis in infancy: a cohort study in 13 European centres. Acta Paediatr. 94, 1721–1731 [DOI] [PubMed] [Google Scholar]
- 53.Castaño P et al. (2014) Placental thrombosis in acute phase abortions during experimental Toxoplasma gondii infection in sheep. Vet. Res 45, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castaño P et al. (2016) Experimental ovine toxoplasmosis: influence of the gestational stage on the clinical course, lesion development and parasite distribution. Vet. Res 47, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castaño P et al. (2019) Peripheral and placental immune responses in sheep after experimental infection with Toxoplasma gondii at the three terms of gestation. Vet. Res 50, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castaño P et al. (2020) Macrophages and T lymphocytes in the ovine placenta after experimental infection with Toxoplasma gondii. Vet. Pathol 57, 545–549 [DOI] [PubMed] [Google Scholar]
- 57.Giakoumelou S et al. (2016) The role of infection in miscarriage. Hum. Reprod. Update 22, 116–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nayeri T et al. (2020) The global seroprevalence of anti-Toxoplasma gondii antibodies in women who had spontaneous abortion: a systematic review and meta-analysis. PLoS Negl. Trop. Dis 14, e0008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sánchez-Sánchez R et al. (2018) Virulence in mice of a Toxoplasma gondii type II isolate does not correlate with the outcome of experimental infection in pregnant sheep. Front. Cell. Infect. Microbiol 8, 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Regidor-Cerrillo J et al. (2014) Neospora caninum infection during early pregnancy in cattle: how the isolate influences infection dynamics, clinical outcome and peripheral and local immune responses. Vet. Res 45, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stelzer S et al. (2019) Toxoplasma gondii infection and toxoplasmosis in farm animals: risk factors and economic impact. Food Waterborne Parasitol. 15, e00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Innes EA et al. (2007) Comparative host-parasite relationships in ovine toxoplasmosis and bovine neosporosis and strategies for vaccination. Vaccine 25, 5495–5503 [DOI] [PubMed] [Google Scholar]
- 63.Chavatte-Palmer P and Tarrade A (2016) Placentation in different mammalian species. Ann. Endocrinol. (Paris) 77, 67–74 [DOI] [PubMed] [Google Scholar]
- 64.Dubey JP et al. (1997) Toxoplasmosis in rats (Rattus norvegicus): congenital transmission to first and second generation offspring and isolation of Toxoplasma gondii from seronegative rats. Parasitology 115, 9–14 [DOI] [PubMed] [Google Scholar]
- 65.Flori P et al. (2002) Experimental model of congenital toxoplasmosis in guinea-pigs: use of quantitative and qualitative PCR for the study of maternofetal transmission. J. Med. Microbiol 51, 871–878 [DOI] [PubMed] [Google Scholar]
- 66.Flori P et al. (2003) Parasite load in guinea pig foetus with real time PCR after maternofoetal transmission of Toxoplasma gondii. Parasite 10, 133–140 [DOI] [PubMed] [Google Scholar]
- 67.Yarovinsky F et al. (2005) TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308, 1626–1629 [DOI] [PubMed] [Google Scholar]
- 68.Mukhopadhyay D et al. (2020) Influence of the host and parasite strain on the immune response during Toxoplasma infection. Front. Infect. Microbiol. Dis 10, 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benson A et al. (2009) Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell Host Microbe 6, 187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dubey JP (2009) Toxoplasmosis in sheep – the last 20 years. Vet. Parasitol 163, 1–14 [DOI] [PubMed] [Google Scholar]
- 71.Abou-Bacar A et al. (2004) Role of gamma interferon and T cells in congenital Toxoplasma transmission. Parasite Immunol. 26, 315–318 [DOI] [PubMed] [Google Scholar]
- 72.Wang T et al. (2011) Toxoplasma gondii: the effects of infection at different stages of pregnancy on the offspring of mice. Exp. Parasitel 127, 107–112 [DOI] [PubMed] [Google Scholar]
- 73.Khan A et al. (2009) Phenotypic and gene expression changes among clonal type I strains of Toxoplasma gondii. Eukaryot. Cell 8, 1828–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Angeloni MB et al. (2013) Differential apoptosis in BeWo cells after infection with highly (RH) or moderately (ME49) virulent strains of Toxoplasma gondii is related to the cytokine profile secreted, the death receptor Fas expression and phosphorylated ERK1/2 expression. Placenta 34, 973–982 [DOI] [PubMed] [Google Scholar]
- 75.Niedelman W et al. (2012) The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response. PLoS Pathog. 8, e1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saeij JPJ et al. (2007) Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445, 324–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosowski EE et al. (2011) Strain-specific activation of the NF-κB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J. Exp. Med 208, 195–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gov L et al. (2013) Human innate immunity to Toxoplasma gondii is mediated by host caspase-1 and ASC and parasite GRA15. mBio 4, e00255–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mukhopadhyay D et al. (2020) Toxoplasma GRA15 limits parasite growth in IFNγ-activated fibroblasts through TRAF ubiquitin ligases. EMBO J. 39, e103758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L et al. (2020) The Toxoplasma gondii virulence factor ROP16 acts in cis and trans, and suppresses T cell responses. J. Exp. Med 217, e20181757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Braun L et al. (2013) A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J. Exp. Med 210, 2071–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hakimi M-A et al. (2017) Toxoplasma effectors targeting host signaling and transcription. Clin. Microbiol. Rev. 30, 615–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Melo MB et al. (2013) Transcriptional analysis of murine macrophages infected with different Toxoplasma strains identifies novel regulation of host signaling pathways. PLoS Pathog. 9, e1003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng W et al. (2015) Variation detection based on next-generation sequencing of type Chinese 1 strains of Toxoplasma gondii with different virulence from China. BMC Genomics 16, 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cui W et al. (2020) Toxoplasma gondii ROP16I deletion: the exacerbated impact on adverse pregnant outcomes in mice. Front Microbiol. 10, 3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma JS et al. (2014) Selective and strain-specific NFAT4 activation by the Toxoplasma gondii polymorphic dense granule protein GRA6. J. Exp. Med 211, 2013–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zlotnik A and Yoshie O (2000) Chemokines: a new classification system and their role in immunity. Immunity 12, 121–127 [DOI] [PubMed] [Google Scholar]
- 88.Shastri AJ et al. (2014) GRA25 is a novel virulence factor of Toxoplasma gondii and influences the host immune response. Infect. Immun 82, 2595–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arranz-Solís D et al. (2019) Serotyping of Toxoplasma gondii infection using peptide membrane arrays. Front. Cell. Infect. Microbiol 9, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pappas G et al. (2009) Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol 39, 1385–1394 [DOI] [PubMed] [Google Scholar]
- 91.Lorenzi H et al. (2016) Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat. Commun 7, 10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su C et al. (2012) Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc. Natl. Acad. Sci. U. S. A 109, 5844–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McLeod R et al. (2012) Prematurity and severity are associated with Toxoplasma gondii alleles (NCCCTS, 1981-2009). Clin. Infect. Dis 54, 1595–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferreira IMR et al. (2011) Toxoplasma gondii isolates: multilocus RFLP-PCR genotyping from human patients in Sao Paulo State, Brazil identified distinct genotypes. Exp. Parasitol, 129, 190–195 [DOI] [PubMed] [Google Scholar]
- 95.Gallego C et al. (2006) Direct genotyping of animal and human isolates of Toxoplasma gondii from Colombia (South America). Acta Trop. 97, 161–167 [DOI] [PubMed] [Google Scholar]
- 96.Norwitz ER et al. , (2015) Molecular regulation of parturition: the role of the decidual clock. Cold Spring Harb. Perspect, Med 5, a023143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Faas MM and de Vos P (2017) Uterine NK cells and macrophages in pregnancy. Placenta 56, 44–52 [DOI] [PubMed] [Google Scholar]
- 98.Kwak-Kim J et al. (2014) Immunological modes of pregnancy loss: inflammation, immune effectors, and stress. Am. J. Reprod. Immunol 72, 129–140 [DOI] [PubMed] [Google Scholar]
- 99.Takimoto T et al. (2010) Smad2 and Smad3 are redundantly essential for the TGF-β–mediated regulation of regulatory T plasticity and Th1 development. J, Immunol 185, 842–855 [DOI] [PubMed] [Google Scholar]
- 100.Yang X-P et al. (2011) Signal transduction and TH17 cell differentiation. In TH17 Cells in Health and Disease (Jiang S, ed.), pp. 157–182, Springer [Google Scholar]
- 101.Santner-Nanan B et al. (2009) Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J, Immunol 183, 7023–7030 [DOI] [PubMed] [Google Scholar]
- 102.Liu Y-S et al. (2011) Study on the relationship between Th17 cells and unexplained recurrent spontaneous abortion. Am. J. Reprod. Immunol 65, 503–511 [DOI] [PubMed] [Google Scholar]
- 103.Wang W-J et al. (2013) Regulation of the expression of Th17 cells and regulatory T cells by IL-27 in patients with unexplained early recurrent miscarriage. J. Reprod. Immunol 99, 39–45 [DOI] [PubMed] [Google Scholar]
- 104.Nagamatsu T and Schust DJ (2010) Review: the immunomodulatory roles of macrophages at the maternal–fetal interface. Reprod, Sci 17, 209–218 [DOI] [PubMed] [Google Scholar]
- 105.Brown MB et al. (2014) M1/M2 macrophage polarity in normal and complicated pregnancy. Front. Immunol 5, 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Munn DH et al. (2013) Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol, 34, 137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]