Abstract
Behavioral Inhibition (BI) is a temperament characterized in early childhood by distress to novelty and avoidance of unfamiliar people and is one of the best known risk factors for the development of social anxiety. However, nearly 60% of children with BI do not go on to meet criteria for social anxiety disorder. In this review we present an approach to understanding differential developmental trajectories among children with BI. We review research using laboratory-based tasks which isolate specific attention processes that enhance versus mitigate risk for social anxiety among behaviorally inhibited children and studies that suggest BI is associated with heightened detection of novelty or threat. Moreover, stimulus-driven control processes, which we term automatic control, increase the probability that behaviorally inhibited children display socially reticent behavior and develop social anxiety. In contrast, goal-driven control processes, which we term planful control decrease risk for anxiety. We suggest that these three categories of processes (detection, automatic control, and planful control) function together to determine whether behaviorally inhibited children are able to flexibly regulate their initial reactions to novelty, and in turn, decrease risk for social anxiety. Although laboratory-based tasks have identified these processes underlying risk and resilience, the challenge is linking them to the emotions, thoughts, and behaviors of behaviorally inhibited children in real-world contexts.
Keywords: Temperament, Social Anxiety, Planful control, Automatic Control, Response to Detection, Developmental Trajectories
Behavioral Inhibition (BI) is a temperament characterized in early childhood by negative reactions to novelty including freezing, distress and withdrawal in novel situations and avoidance of unfamiliar contexts or people (1,2). During middle childhood, children with a history of BI are more socially reticent in the presence of unfamiliar peers, carefully watching others from the periphery (3,4). BI is the best known risk factor for the later diagnosis of anxiety, particularly social anxiety (5–8). Nevertheless, there is a wide range of variability in developmental outcomes amongst children with BI, with only some children going on to develop clinically significant social anxiety (6). While tracking the development of multiple cohorts of children from early infancy through adolescence, we (and others) have administered laboratory tasks assessing attention and information processing to infants, children, and adolescents with and without a history of BI (8–10). In doing so, we have described how various processes differ amongst those with and without BI as well as how individual differences in these processes shape the developmental pathways from early BI to anxiety (5,11). The purpose of the current review is to synthesize these findings within a heuristic model. Critically, although laboratory tasks allow for precise measures of information processing biases, psychologists and clinicians are ultimately interested in understanding their implications for more tangible and proximal correlates of childhood anxiety including real-world emotions, cognitions, and behaviors. Thus, we also advance hypotheses regarding how these laboratory-based assessments relate to the naturalistic behaviors we have observed across two longitudinal cohorts and propose future directions for basic and applied research in this regard.
Though each aspect of our model (see Figure 1) will be described in detail, we begin with a high-level overview. Briefly, we suggest that BI is associated with heightened physiological and behavioral detection of novel, salient, or threatening information in the environment. The detection of such information (whether the result of lower threshold or heightened response) pulls BI children’s attention away from ongoing goal-directed behaviors (e.g., socializing with peers; see Figure 3). We found that the type of control processes BI children deploy in response to detecting such events moderates the child’s risk for anxiety (see Figures 2 and 4). When heightened detection is accompanied by rapid, stimulus-driven automatic control, attention remains fixated on the source of the detected information (e.g., a peer’s face following the perception of a frown) at the continued expense of actively engaging in the original goal-directed behavior (e.g., the social interaction). We believe this pattern of increased detection and automatic control is what largely drives the avoidance, freezing, and lack of approach behavior that is commonly observed in behaviorally inhibited children at risk for anxiety. In contrast, when behaviorally inhibited children detect novel, threatening or otherwise salient information in the environment but then deploy goal-driven processes known as planful control, they are able to redirect their attention towards ongoing activities such as social interactions, allowing for age-typical and competent social exchanges. We believe that the combination of increased detection and planful control characterizes the behavior of children with a history of BI who do not go on to develop clinically significant anxiety. Thus, our model suggests that increased detection is associated with BI, whereas two distinct forms of control, automatic vs. planful, moderate risk for social anxiety in opposing directions; planful control is protective, whereas automatic control exacerbates risk.
Figure 1.
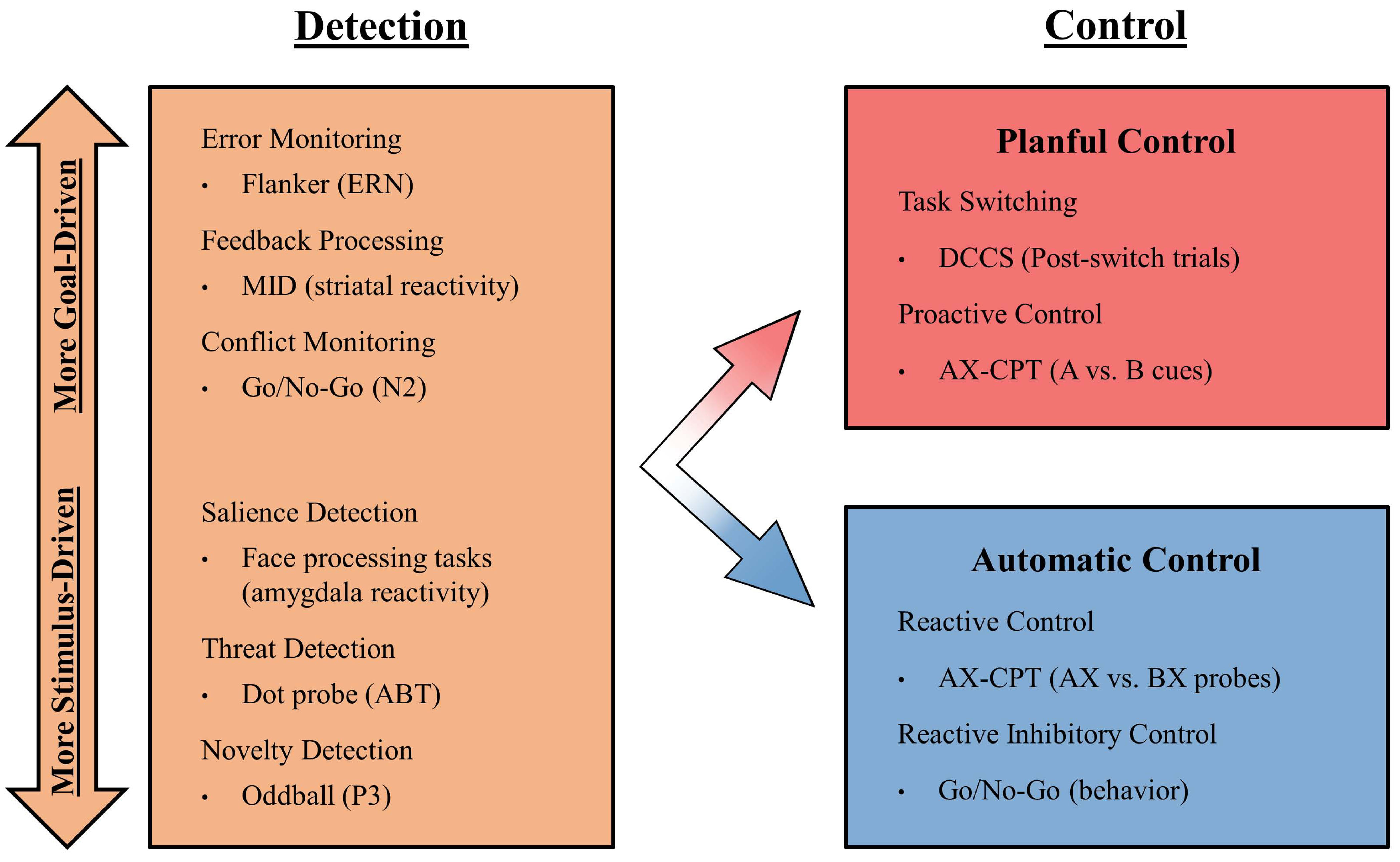
The grouping of attentional and control processes in the current model. Bullets provide an example of a task used in the study of each process, with specific indices from each task presented in parentheses. Note that this list of example is non-comprehensive. Within the leftmost box labelled “Detection”, processes range continuously from “More Stimulus-Driven” at the bottom to “More Goal-Driven”. The inclusion of this continuum reflects the heterogeneity of these processes along this dimension, with their unification in a single box reflecting the equivalency in their relations to BI in the current model. ERN = Error Related Negativity. MID = Monetary Incentive Delay. ABT = Attention Bias to Threat. DCCS = Dimensional Change Card Sort. AX-CPT = AX-Continuous Performance Task.
Figure 3.
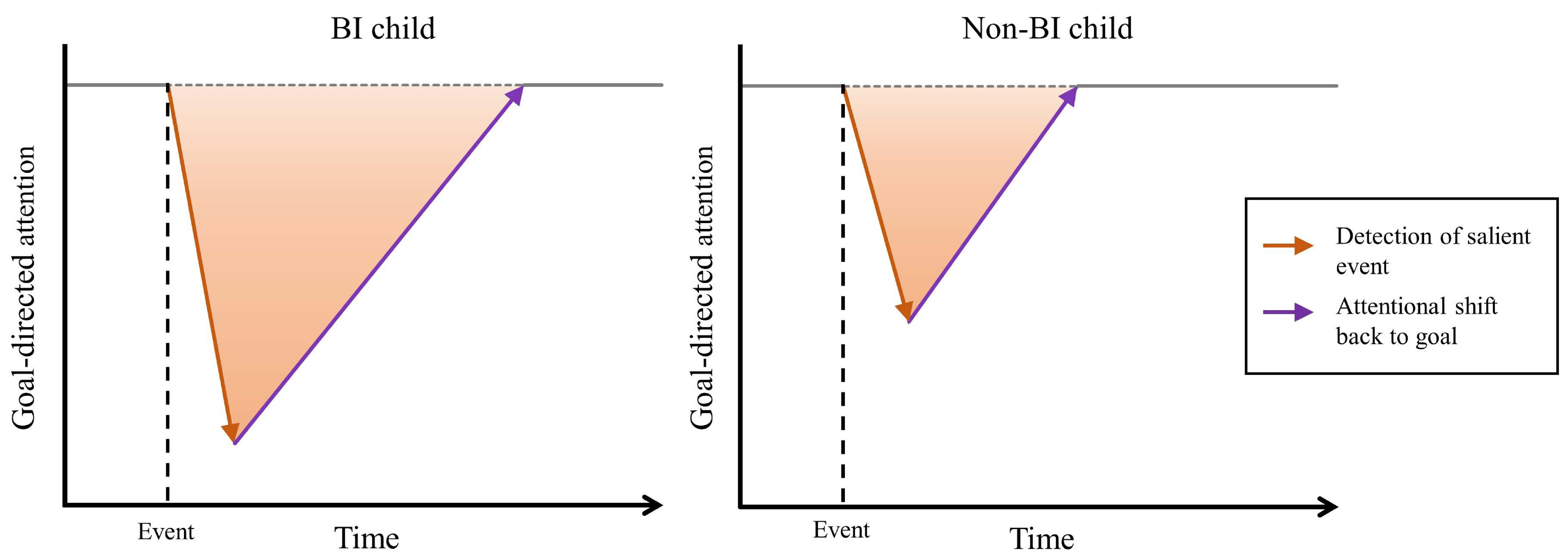
Hypothetical attentional behavior of a) children high in behavioral inhibition (BI) and b) non-BI children in response to a novel or salient stimulus in a goal-directed setting.
Figure 2.
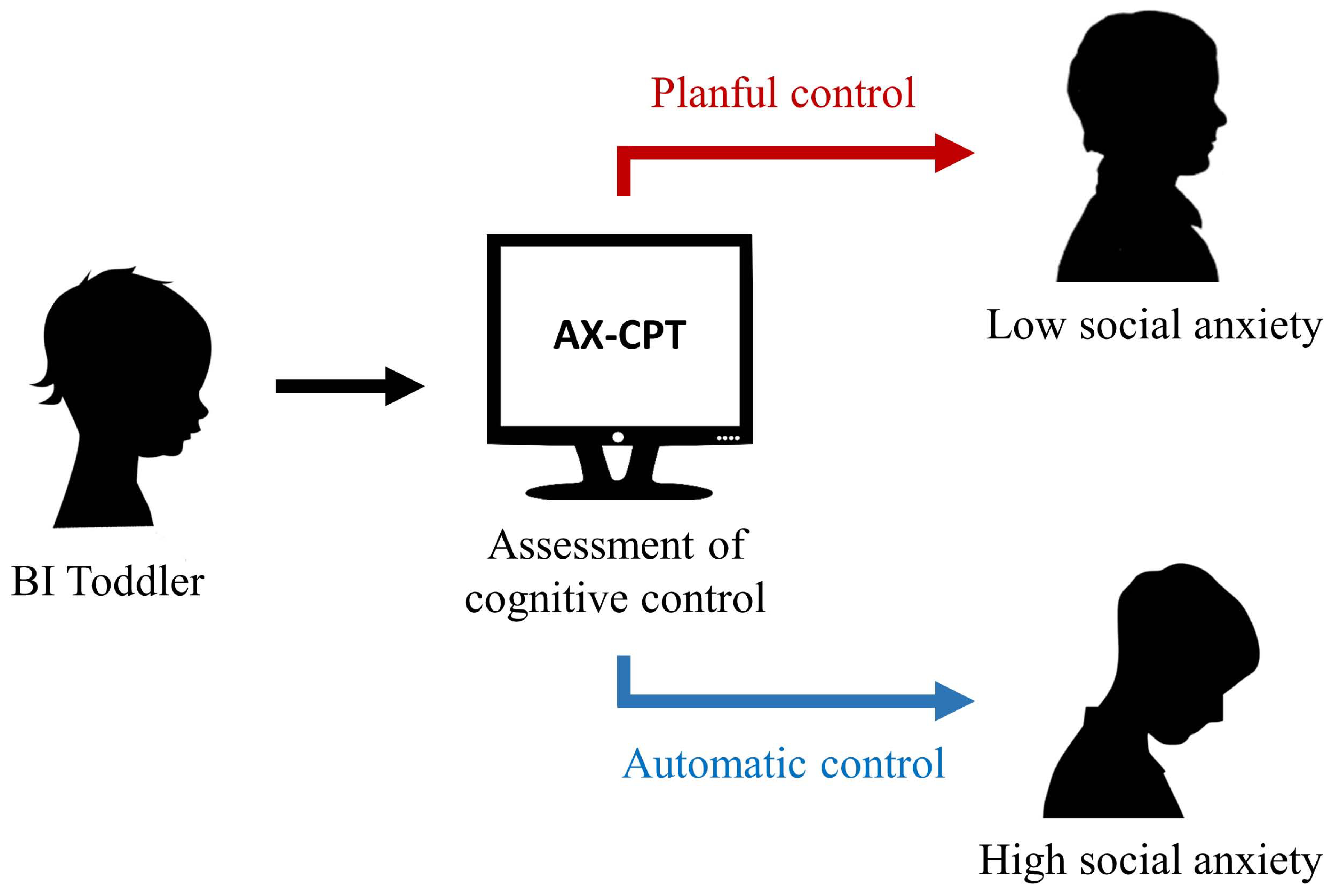
An illustration of the proposed developmental cascade from Behavioral Inhibition in toddlerhood to social behavior in later childhood. Children exhibiting BI in toddlerhood complete an assessment of cognitive control (e.g., AX-CPT) later in childhood. The AX-CPT enables measurement of both automatic and planful control processes through its use of contextual cues (e.g., the letters “A” or “B”) that help signal to the participant how they should respond to an upcoming probe (e.g., the letters “X” or “Y”). Because participants are instructed to only respond when they see an “A” that is followed by an “X,” participants must use a combination of planful control (i.e., upon seeing the cue, anticipating a possible response to the upcoming probe) and automatic control (i.e., upon seeing the probe, choosing the appropriate response given the cue that was seen earlier) in order to maximize task accuracy. Children ending toward planful control are predicted to exhibit lower levels of social anxiety, while those tending toward automatic control are predicted to exhibit higher levels of social anxiety.
Figure 4.
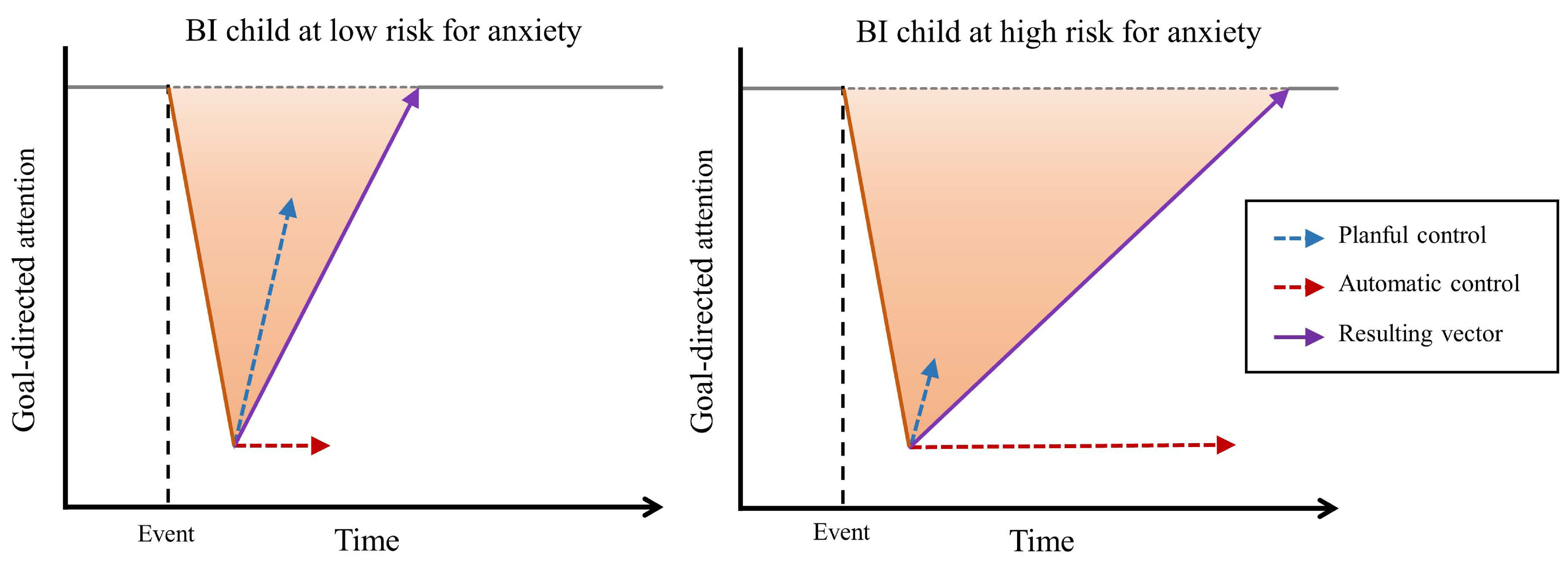
Hypothetical attentional behavior of a) a child high in behavioral inhibition (BI) at low risk of developing anxiety, and b) a high-BI child at higher risk of developing anxiety.
Detection, automatic control, and planful control are not unitarity concepts supported by unique neural networks. Rather, each of these terms refers to a category of related processes that impact information processing at comparable points in time. As will become clear below, grouping these disparate processes into three larger categories provides heuristic value for synthesizing existing data and generating novel hypotheses regarding the mechanisms linking BI to everyday behavior and ultimate risk for later anxiety.
It is important to emphasize the central role of social context in our model. Based on years of observing behaviorally inhibited children interact with adults and other children in the laboratory, we are aware of the importance of context for understanding the link between BI and social reticence and social anxiety (4,5,12). In the remainder of this review, we provide details on the array of tasks and measures that capture specific neurocognitive processes that fall into the three categories introduced above (detection, automatic control, and planful control). However, a limitation of these carefully controlled, lab-based physiological and behavioral assessments is that although they are typically administered within an implicit social context (i.e., in the presence of an experimenter in a laboratory setting) they have largely been carried out without directly manipulating social context (with few exceptions; see (13)). Bridging the gap between measurement of these neurocognitive processes and social behavior is one of the challenges we attempt to address here.
Behavioral inhibition and the detection of novelty, threat, and saliency
BI is defined based on a constellation of behaviors some young children display in response to novelty (1,3). For example, in response to novel environments, objects, and people, behaviorally inhibited toddlers physically withdraw while carefully watching, seek close contact with familiar others (e.g., mother), express negative affect, and are verbally reticent (2). This temperament has been mostly studied as BI, but also as negative affectivity (14), dysregulated fear (15), anxious solitude/withdrawal (16), and in non-human animal models as anxious temperament (17) and neophobia (18). Because all these dimensions are closely related and it is difficult to empirically and conceptually distinguish among them, we use the term BI in the current review to refer to these related constructs (for a more detailed discussion on the similarities and differences of these different theoretical and empirical approaches see (19)). We (and others) hypothesized that reactivity to novel stimuli might originate from either a lower threshold or heightened response to detection of novel, threatening, or salient information in the environment. At the moment, we cannot differentiate among these two possibilities (lower threshold or heightened response). We can examine the hypotheses regarding moderation of BI by either automatic or planful control processes. To do so, we administered a number of highly-controlled laboratory tasks to large cohorts of children with carefully characterized temperaments. Although the tasks are far removed from the naturalistic behavioral observations from which the hypotheses were derived, they allow reliable and precise characterizations of the processes that strengthen or weaken the links between BI and later anxious behaviors.
As a whole, this line of research supports the hypothesis that children with a history of BI display heightened responses to the detection of novel, conflicting, or salient stimuli (either through a lowered threshold or a stronger response). For example, infants who react negatively to novelty and young children with BI are more likely to exhibit heightened physiological responses (ERP amplitudes) to novel auditory stimuli as infants and adolescents (20,21), enhanced physiological (EMG) startle responses to novel auditory stimuli as children and adolescents (22,23), and rapid visual detection of threat (angry faces) in the environment as adolescents (24). By late childhood and adolescence, those with a history of BI exhibit greater striatal responses to reward (25–27) and punishment (28) cues, enhanced N2 ERP amplitudes in response to NoGo stimuli in a Go-No-Go task as children and adolescents (29,30), and more negative ERN ERP amplitudes to their own errors on speeded reaction time tasks like the Flanker task as children and adolescents (31,32). This pattern of enhanced response to the detection of novel, conflicting, and salient stimuli is hypothesized to, at least in part, reflect increased amygdala reactivity to novelty. Although data during infancy and childhood are lacking, individuals characterized as behaviorally inhibited in infancy and childhood display greater amygdala reactivity to novelty as adolescents and adults (33–35). More generally, the measures capturing this enhanced detection and their neural underpinnings are varied and clearly do not reflect the activity of a single, unified neural process or network. For example, the striatum is associated with processing reward and punishment (36) and salient information (37), whereas the amygdala is associated with processing threat (38) and novelty (39). Activity within the cingulate and regions of the medial frontal cortex (MFC) is evoked in response to conflict, errors, or other situations that might require increased attention (40). Despite disparate neural networks, these processes share the common function of registering the presence of potentially important (novel, salient, or threatening) information about the environment. Collectively, these observations suggest that BI is associated with either a lowered threshold for, or a stronger response to, the immediate detection of novel, salient or threatening information.
Figure 1 groups the processes described above within the left-hand box, under the heading “detection.” We present these detection processes along a continuum from lower-level, more stimulus-driven versus more goal-driven (moving from the bottom to the top of the left-hand box), reflecting a developmental progression (i.e., young infants primarily react to external stimuli, as reactions to success or failure in goal-directed behaviors emerge during the first years of life).
Our work and that of others make it strikingly clear that BI and anxiety are not one and the same. Critically, despite the fact that BI remains the best-known predictor of later anxiety, 60% of children with BI do not go on to meet criteria for any anxiety disorder during childhood or adolescence (41,42). The dissociation between BI and anxiety is clear from the results of an early parenting intervention for children with BI that yielded substantial decreases in parent-reported anxiety but no changes in parent-reported BI (43,44). In an attempt to account for this variability in outcomes among BI children, we examined whether individual differences in detection processes impacted developmental trajectories (9). We found that children and adolescents with a history of BI were at particular risk for displaying anxious behaviors (e.g., social withdrawal) and a variety of anxiety symptoms if they also displayed attention biases towards threat faces as assessed on the Dot Probe (45–51), heightened reactivity to novel auditory stimuli (P3) (21), increased startle responses (22,23), or heightened ERN to errors on a Flanker task (13,31,32). These differences in processing parallel findings with clinically anxious individuals, who also display an attention bias towards threat (52), enhanced error processing (53–55), and exaggerated startle responses (56). However, our research also suggests that heterogeneity in developmental outcomes of children with BI is due not only to differences in these quick and early initial detection processes, but to later control processes that either maintain/amplify the heightened response to detection (i.e., reactive control) or moderate the heightened detection response by flexibly shifting attention back towards goal-directed behavior (i.e., planful control) (57,58). These two types of control processes are depicted in the right-hand side of Figure 1 and are the focus of the section below.
Two types of control
Over the course of almost 30 years we have observed infants, children, and adolescents with well characterized early temperaments as they engage in social interactions with unfamiliar adults and peers. These observations of reticent, vigilant, and inflexible behaviors provide the foundation for hypotheses about how various control processes may support or hinder the development of social competence. Behaviorally inhibited preschoolers are more likely to exhibit reticent behavior in a play group of unfamiliar peers (e.g., watching others but remaining unoccupied, not playing with toys or with others) (4,59). When challenged in a novel situation (e.g., having to give a speech or when approached by a stranger), they are more likely to freeze (15,60). They also appear inflexible in their social problem solving and appear to be “spinning their wheels” when it comes to figuring out how to engage with unfamiliar peers especially when they are being excluded (61). Based on these observations, we reasoned that the development of control processes may serve as a critical moderator of developmental outcomes for children with a history of BI. This led to an examination of how various control processes might explain these differences in observed social behavior and risk for social anxiety.
There is a long history of studying control processes across the cognitive, developmental and neuroscience fields, and many taxonomies of control have been proposed (62–66). It is important to distinguish control processes from detection processes: whereas detection involves registering the presence of potentially important information, control governs what one does with this information through changes in attention or sensorimotor inhibition. This distinction between detection and control is reflected in the separation between the left- and right-hand sides of Figure 1. Second, there are at least two types of control processes: stimulus-driven processes that we term “automatic control” (bottom, right-hand box in Figure 1) and a category of goal-driven processes denoted “planful control” (upper, right-hand box in Figure 1). We define automatic control as being associated with immediate, reactive changes in attention or sensorimotor inhibition that are in direct response to a stimulus or event. In contrast, planful control is associated with prolonged and proactive changes in attention or sensorimotor inhibition in support of a specific goal.
The dual mechanisms of control (DMC) framework (67) suggests that control processes can be described as either “proactive” and directed toward events in the future, or “reactive” and directed towards events that have just occurred. Integrating these descriptions, we suggest that measures and tasks tapping “proactive control” fall within the category of planful control (upper, right-hand box in Figure 1), whereas “reactive control” falls within the category of automatic control (lower, right-hand box in Figure 1). In addition to proactive control, task-switching also reflects a form of control that is employed in response to a future goal, and is listed within the planful control category as well. Finally, reactive inhibitory control, which can be assessed via behavioral responses on a go/nogo task, falls within the automatic control category.
Past work suggests that automatic control and planful control differentially impact behaviorally inhibited children’s risk of developing anxiety. Higher levels of planful control (e.g., task switching ability measured via a task or self-reports) reduce the risk of anxiety amongst children with a history of BI (68,69). In contrast, heightened levels of automatic control (e.g., reactive inhibitory control measures) increase BI children’s risk of developing anxiety (58,68,70). For instance, White and colleagues (68) found that the effect of BI in toddlerhood on preschool anxiety was differentially moderated by the levels of planful or automatic control - such that that high levels of attention shifting (planful control) decreased the risk for anxiety problems in children with high levels of BI, whereas high levels of inhibitory control (automatic control) increased this risk for anxiety symptoms. In line with the DMC framework, these two types of control are independent and children (and adults for that matter) regularly engage both types of control. Individuals vary in the balance or extent of bias in which type of control is engaged in certain kinds of situations. Two recent studies employing the AX-Continuous Performance Task (AX-CPT), in which a single bias score is computed to quantify relative amounts of automatic vs. planful control within an individual, demonstrate a contrast in the influence of different control processes in terms of moderating the risk for anxiety in BI (57,71). A visual representation of these relations is presented in Figure 2.
Although each of the categories of control (planful control and automatic control) reflect a number of distinct neurocognitive processes that share similar functions, we do not suggest that these categories are unitary constructs, nor rely on distinct, unitary neural networks. Exemplar brain networks involved in these two categories of control include ventral-lateral prefrontal cortex (72), as well as the dorsal frontal-parietal network (62,66,73). Moreover, both of these control categories involve inputs from and feed back to brain structures associated with detection (e.g., amygdala, cingulate). These processes are reciprocal in nature, meaning that there are most certainly interactions amongst these different brain structures and networks (73). Detection, particularly goal-directed aspects of the detection category (e.g., the processes indexed by the ERN, N2, etc.) and automatic control (e.g., reactive inhibitory control) appear quite similar. Indeed, both have been shown to increase risk for later anxiety amongst behaviorally inhibited individuals. However, most often, only detection exhibits direct relations with BI, whereas automatic control serves as a moderator of later risk for anxiety. Moreover, while detection and automatic control often co-occur, they are separated in time, with detection necessarily preceding automatic control; automatic control is thought to maintain/amplify the information processing initiated by detection through changes in attention or inhibition of sensorimotor cortex. These dynamic interactions between detection, automatic, and planful control are central to understanding which children with BI go on to develop anxiety.
Dynamic interactions between detection, automatic, and planful control
Figure 3 presents hypothetical data highlighting the distinction between behaviorally inhibited children (Figure 3a) and non-behaviorally inhibited children (Figure 3b) with respect to detection. Children’s attention toward a novel social context and their relevant goal within that context is presented on the y-axis. The extent to which attention is captured by a salient stimulus (orange arrow) is dependent upon context and the child’s prior history of interaction. For example, during a social interaction with an unfamiliar peer, when a child perceives a salient stimulus (e.g., a negative social cue), heightened response to detection draws their attention temporarily away from the goal of engaging the peer (represented by the orange arrow). Given heightened levels of detection, the behaviorally inhibited child’s attention is captured to a greater extent than that of the non-behaviorally inhibited child, depicted as a steeper, longer arrow in Figure 3a than Figure 3b. At this point, assuming similar levels of control processing (represented by the purple arrows in each panel of Figure 3), it will take longer for the behaviorally inhibited child to return their attention to the goal of engaging with their social partner. As such, efficient planful control processing is especially important for behaviorally inhibited children as a means of regulating their attention in response to instances of heightened detection, particularly within novel social contexts.
Figure 4 presents hypothetical data from two behaviorally inhibited children: one at lower risk of developing anxiety problems (Figure 4a) and one at higher risk of developing anxiety problems (Figure 4b). As mentioned above, both of these children, when confronted with novelty or unfamiliarity, are likely to exhibit a heightened detection response (steep orange arrows) to salient stimuli in the environment (e.g., a perceived error or a peer’s negative facial expression). However, what differentiates them, according to the present account, is whether they deploy automatic versus planful control in response to the detection of a salient stimulus. The blue and red dashed arrows represent the strength (or the bias) of the child’s planful and automatic control, respectively, whereas the solid purple arrow represents the relative degree, or extent of bias, with which these categories of control are deployed by these children in similar situations. The hypothetical child in Figure 4a is more likely to deploy planful control, reflected in the longer blue arrow pulling the child’s attention back to the goal-directed behavior, which here is the social interaction. As depicted by the steep purple arrow, this child would be predicted to flexibly adapt and quickly redirect attention back to the task at hand. In contrast, the hypothetical child in Figure 4b displays an increased reliance on automatic control and a limited ability to marshal planful control in such contexts. As such, this child would be predicted to freeze, sustain fixation on the attention-grabbing stimulus, or perhaps even engage in ruminative activity, in each case preventing the child from returning his/her attention to the task at hand. The inability to rapidly and fluidly navigate and negotiate these contextual demands may place behaviorally inhibited children at increased risk of developing anxiety.
Developmental implications
This approach has implications for understanding the development of cognitive control processes and the role these processes play in linking early BI to the emergence of anxiety. In normative development, detection and automatic control emerge in infancy, rooted in orienting responses to external factors (i.e., exogenous attention) (74). Automatic control processes are molded over the course of interactions with caregivers, and gradually transition, to more planful, endogenous forms of control (75,76). Planful control processes emerge during early childhood and mature over the preschool and school age period. These processes most likely involve executive skills such as inhibition, working memory, and attention shifting (58,77,78). For example, the ability to flexibly change behavior according to environmental demands (e.g., respond to a peer’s unexpected behavior) shows rapid normative development between 3 and 5 years of age (79–81). These processes continue to be refined with more complex and planful forms of control developing through adolescence (78,82,83), including a shift from more reactive to more proactive control (81, 82).
There is limited evidence on how BI (and associated heightened response to detection) alters normative developmental changes in control processes. One possibility is that BI has no direct influence on the development of control, such that these control processes develop orthogonally to BI and heightened response to detection. Alternatively, BI may directly impact the development of control processes, such that behaviorally inhibited children develop more automatic forms of control (e.g., increased inhibitory control), which sustains their inhibited responses. Recent work suggests that BI predicts higher levels of inhibitory control (automatic control) and lower levels of task switching (planful control) by age 7 (69). Similarly, BI predicted a pattern of behavioral responses indicative of a more reactive (automatic) control and less proactive (planful) control style in adolescence (57). Similar relations with proactive and reactive control have been reported with temperamental shyness, which is conceptually and empirically related to BI (84). While there is some evidence that BI impacts the development of control processes, in all probability, the links between BI and control are both direct and indirect (see for example, (58,68)).
Conclusions and future directions
One of the most pressing questions our model raises is why some children with a history of BI develop biases toward automatic versus planful control. It is likely that critical socialization experiences foster these biases early in development. For example, BI is more strongly associated with social anxiety when mothers engage in highly protective or oversolicitous parenting (59,85,86). It may be that this type of parenting reinforces heightened detection responses and fosters reactive control processes by making children even more aware of potential risks in their environment. In contrast, more sensitive parenting that encourages coping and flexible problem solving may foster children’s abilities to engage planful control processes. Other critical factors such as socialization experiences (e.g., interactions with peers, teachers/caregivers, and the broader cultural context) (87–90) or life stressors (91) may have similar influences on the development of control biases for children with a history of BI.
Future work should examine the development of detection responses, automatic control, and planful control and the functional connectivity amongst brain structures underlying these neurocognitive processes. A number of research groups have started examining the associations between infant temperament and the development of detection and control processes (87,92,93); while others have begun exploring the relations between infant temperament and functional connectivity measures in the first year of life (94–97). Longitudinal studies examining detection and control processes, the brain systems supporting these processes, and temperament will be crucial to understand the developmental cascade from temperament to reticence to anxiety.
It remains unclear at the moment whether/how increased detection or automatic control directly relate to negative emotions. One possibility is that increases in detection and automatic control, even for emotionally neutral stimuli, directly lead to a negative emotional state. Alternatively, increased detection and automatic control may cause problems flexibly engaging in social interactions, which in turn may produce negative emotions. Across development, lowered quality and quantity of social interactions may in turn lead individuals to engage in patterns of thought and behavior that are associated with anxiety, such as ruminative thought and avoidance behavior.
Finally, future studies should focus on ecological validity examining the role of response to detection and control processes in contexts that are relevant to the development of anxiety. New technology (e.g., ambulatory eye-tracking) (98) or ecologically-valid experimental manipulations (13) can examine how these neurocognitive processes support anxious behavior.
Acknowledgements
The authors would like to thank Jerome Kagan for his thoughtful comments on the manuscript. We also wish to thank the many research assistants involved in data collection for the studies of behavioral inhibition in the Child Development Lab and finally great thanks for the families who have participated in our studies.
Financial Disclosures
NAF has received grant support from the National Institute of Mental Health (NIMH), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Science Foundation (NSF), the National Institutes of Health Environmental influences on Child Health Outcomes (NIH ECHO) consortium, the Russell Sage Foundation, and the Lumos Foundation. He has received royalties from Guilford Press and Harvard University Press. He has received honoraria for lectures to professional audiences.
Research and work on this paper was supported in part by NIMH U01MH093349 to NAF and SSHRC Insight Grant 435-201600494 to HAH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
George Buzzell reports no biomedical financial interests or potential conflicts of interest.
Emilio Valadez reports no biomedical financial interests or potential conflicts of interest.
Santiago Morales Pamplona reports no biomedical financial interests or potential conflicts of interest.
McLennon Wilson reports no biomedical financial interests or potential conflicts of interest.
Heather Henderson reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. Behavioral inhibition to the unfamiliar. Child Development. 1984. December;55(6):2212–25. [Google Scholar]
- 2.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral Inhibition: Linking Biology and Behavior within a Developmental Framework. Annual Review of Psychology. 2005;56(1):235–62. [DOI] [PubMed] [Google Scholar]
- 3.Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and Discontinuity of Behavioral Inhibition and Exuberance: Psychophysiological and Behavioral Influences across the First Four Years of Life. Child Development. 2001. January 1;72(1):1–21. [DOI] [PubMed] [Google Scholar]
- 4.Degnan KA, Almas AN, Henderson HA, Hane AA, Walker OL, Fox NA. Longitudinal trajectories of social reticence with unfamiliar peers across early childhood. Developmental Psychology. 2014;50(10):2311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, et al. Stable Early Maternal Report of Behavioral Inhibition Predicts Lifetime Social Anxiety Disorder in Adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2009. September;48(9):928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clauss JA, Blackford JU. Behavioral Inhibition and Risk for Developing Social Anxiety Disorder: A Meta-Analytic Study. Journal of the American Academy of Child and Adolescent Psychiatry. 2012. October;51(10):1066–1075.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandstrom A, Uher R, Pavlova B. Prospective association between childhood behavioral inhibition and anxiety: a meta-analysis. Journal of abnormal child psychology. 2019;1–10. [DOI] [PubMed] [Google Scholar]
- 8.Buss KA, Cho S, Morales S, McDoniel M, Webb AF, Schwartz A, et al. Toddler dysregulated fear predicts continued risk for social anxiety symptoms in early adolescence. Development and psychopathology. 2020;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson HA, Pine DS, Fox NA. Behavioral Inhibition and Developmental Risk: A Dual-Processing Perspective. Neuropsychopharmacology. 2015. January;40(1):207–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein DN, Finsaas MC. The Stony Brook Temperament Study: Early Antecedents and Pathways to Emotional Disorders. Child Dev Perspect. 2017. December;11(4):257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang A, Crawford H, Morales S, Degnan KA, Pine DS, Fox NA. Infant behavioral inhibition predicts personality and social outcomes three decades later. PNAS [Internet]. 2020. [cited 2020 Apr 25]; Available from: https://www.pnas.org/content/early/2020/04/16/1917376117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyson MW, Klein DN, Olino TM, Dougherty LR, Durbin CE. Social and Non-Social Behavioral Inhibition in Preschool-Age Children: Differential Associations with Parent-Reports of Temperament and Anxiety. Child Psychiatry & Human Development. 2011. August;42(4):390–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buzzell GA, Troller-Renfree SV, Barker TV, Bowman LC, Chronis-Tuscano A, Henderson HA, et al. A Neurobehavioral Mechanism Linking Behaviorally Inhibited Temperament and Later Adolescent Social Anxiety. Journal of the American Academy of Child & Adolescent Psychiatry. 2017. December;56(12):1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothbart MK. Becoming Who We Are: Temperament and Personality in Development. Guilford Press; 2011. 337 p. [Google Scholar]
- 15.Buss KA. Which fearful toddlers should we worry about? Context, fear regulation, and anxiety risk. Developmental Psychology. 2011;47(3):804–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gazelle H, Rubin KH. Social anxiety in childhood: Bridging developmental and clinical perspectives. New Directions for Child and Adolescent Development. 2010. December;2010(127):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalin NH, Shelton SE. Nonhuman Primate Models to Study Anxiety, Emotion Regulation, and Psychopathology. Annals of the New York Academy of Sciences. 2003. December;1008(1):189–200. [DOI] [PubMed] [Google Scholar]
- 18.Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proceedings of the National Academy of Sciences of the United States of America. 2003. December 23;100(26):16131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez-Edgar K, Fox NA, editors. Behavioral Inhibition: Integrating Theory, Research, and Clinical Perspectives [Internet]. Springer International Publishing; 2018. [cited 2018 Aug 7]. Available from: //www.springer.com/us/book/9783319980768 [Google Scholar]
- 20.Marshall PJ, Reeb BC, Fox NA. Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Dev Sci. 2009. July;12(4):568–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeb-Sutherland BC, Vanderwert RE, Degnan KA, Marshall PJ, Pérez-Edgar K, Chronis-Tuscano A, et al. Attention to novelty in behaviorally inhibited adolescents moderates risk for anxiety. Journal of Child Psychology and Psychiatry. 2009;50(11):1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker TV, Reeb-Sutherland B, Degnan KA, Walker OL, Chronis-Tuscano A, Henderson HA, et al. Contextual startle responses moderate the relation between behavioral inhibition and anxiety in middle childhood. Psychophysiology. 2015;52(11):1544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeb-Sutherland BC, Helfinstein SM, Degnan KA, Pérez-Edgar K, Henderson HA, Lissek S, et al. Startle response in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(6):610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeb-Sutherland BC, Rankin Williams L, Degnan KA, Pérez-Edgar K, Chronis-Tuscano A, Leibenluft E, et al. Identification of emotional facial expressions among behaviorally inhibited adolescents with lifetime anxiety disorders. Cognition and Emotion. 2015;29(2):372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, et al. Neural Correlates of Reward Processing in Adolescents With a History of Inhibited Temperament. Psychological Science. 2009. August 1;20(8):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, et al. Striatal Functional Alteration in Adolescents Characterized by Early Childhood Behavioral Inhibition. J Neurosci. 2006. June 14;26(24):6399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyer AE, Benson B, Choate VR, Bar-Haim Y, Perez-Edgar K, Jarcho JM, et al. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Development and psychopathology. 2014;26(1):229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helfinstein SM, Benson B, Perez-Edgar K, Bar-Haim Y, Detloff A, Pine DS, et al. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia. 2011. February;49(3):479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarcho JM, Fox NA, Pine DS, Etkin A, Leibenluft E, Shechner T, et al. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biol Psychol. 2013. February;92(2):306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamm C, Walker OL, Degnan KA, Henderson HA, Pine DS, McDermott JM, et al. Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Developmental Science. 2014. September;17(5):667–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA, Fox NA. Early Behavioral Inhibition and Increased Error Monitoring Predict Later Social Phobia Symptoms in Childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2014. April;53(4):447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A History of Childhood Behavioral Inhibition and Enhanced Response Monitoring in Adolescence Are Linked to Clinical Anxiety. Biol Psychiatry. 2009. March 1;65(5):445–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pérez-Edgar KE, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007. May;35(4):1538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and Uninhibited Infants “Grown Up”: Adult Amygdalar Response to Novelty. Science. 2003. June 20;300(5627):1952–3. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz CE, Kunwar PS, Greve DN, Kagan J, Snidman NC, Bloch RB. A phenotype of early infancy predicts reactivity of the amygdala in male adults. Molecular Psychiatry. 2011. September 6;17(10):1042–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the Hemodynamic Responses to Reward and Punishment in the Striatum. Journal of Neurophysiology. 2000. December 1;84(6):3072–7. [DOI] [PubMed] [Google Scholar]
- 37.Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human Striatal Response to Salient Nonrewarding Stimuli. J Neurosci. 2003. September 3;23(22):8092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995. September 1;15(9):5879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blackford JU, Buckholtz JW, Avery SN, Zald DH. A unique role for the human amygdala in novelty detection. NeuroImage. 2010. April 15;50(3):1188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. science. 2004;306(5695):443–447. [DOI] [PubMed] [Google Scholar]
- 41.Biederman J, Rosenbaum JF, Bolduc-murphy EA, Faraone SV, Chaloff J, Hirshfeld DR, et al. A 3-Year Follow-up of Children with and without Behavioral Inhibition. Journal of the American Academy of Child & Adolescent Psychiatry. 1993. July 1;32(4):814–21. [DOI] [PubMed] [Google Scholar]
- 42.Gladstone GL, Parker GB, Mitchell PB, Wilhelm KA, Malhi GS. Relationship between self-reported childhood behavioral inhibition and lifetime anxiety disorders in a clinical sample. Depression and Anxiety [Internet]. 2005. [cited 2020 May 7];22(3):103–13. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/da.20082 [DOI] [PubMed] [Google Scholar]
- 43.Rapee RM, Kennedy SJ, Ingram M, Edwards SL, Sweeney L. Altering the Trajectory of Anxiety in At-Risk Young Children. American Journal of Psychiatry. 2010. December;167(12):1518–25. [DOI] [PubMed] [Google Scholar]
- 44.Rapee RM, Kennedy S, Ingram M, Edwards S, Sweeney L. Prevention and early intervention of anxiety disorders in inhibited preschool children. Journal of consulting and clinical psychology. 2005;73(3):488. [DOI] [PubMed] [Google Scholar]
- 45.Cole CE, Zapp DJ, Fettig NB, Pérez-Edgar KE. Impact of attention biases to threat and effortful control on individual variations in negative affect and social withdrawal in very young children. Journal of Experimental Child Psychology. 2016. January;141:210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morales S, Pérez-Edgar KE, Buss KA. Attention Biases Towards and Away from Threat Mark the Relation between Early Dysregulated Fear and the Later Emergence of Social Withdrawal. Journal of Abnormal Child Psychology. 2015. August;43(6):1067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morales S, Taber-Thomas BC, Pérez-Edgar KE. Patterns of attention to threat across tasks in behaviorally inhibited children at risk for anxiety. Dev Sci [Internet]. 2016. January 1 [cited 2016 Jan 22];20(2). Available from: http://onlinelibrary.wiley.com.ezaccess.libraries.psu.edu/doi/10.1111/desc.12391/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nozadi S, Troller-Renfree S, White L, Frenkel T, Degnan K, Bar-Haim Y, et al. The Moderating Role of Attention Biases to Threat on the Link between Behavioral Inhibition and Anxiety in Children. Journal of Experimental Psychopathology. 2016. June 26;7(3):451–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez-Edgar KE, Bar-Haim Y, McDermott JM, Chronis-Tuscano A, Pine DS, Fox NA. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10(3):349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Edgar KE, Reeb-Sutherland BC, McDermott JM, White LK, Henderson HA, Degnan KA, et al. Attention Biases to Threat Link Behavioral Inhibition to Social Withdrawal over Time in Very Young Children. Journal of Abnormal Child Psychology. 2011. February 12;39(6):885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White LK, Degnan KA, Henderson HA, Pérez-Edgar K, Walker OL, Shechner T, et al. Developmental Relations Among Behavioral Inhibition, Anxiety, and Attention Biases to Threat and Positive Information. Child Dev. 2017. January 1;88(1):141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. [DOI] [PubMed] [Google Scholar]
- 53.Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. Journal of Physiology-Paris. 2015. February;109(1–3):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer A. A biomarker of anxiety in children and adolescents: A review focusing on the error-related negativity (ERN) and anxiety across development. Developmental Cognitive Neuroscience. 2017. October 1;27:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moser J, Moran T, Schroder H, Donnellan B, Yeung N. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Frontiers in human neuroscience. 2013;7:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology. 2008. August 1;199(3):421–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Troller-Renfree SV, Buzzell GA, Pine DS, Henderson HA, Fox NA. Consequences of Not Planning Ahead: Reduced Proactive Control Moderates Longitudinal Relations Between Behavioral Inhibition and Anxiety. Journal of the American Academy of Child & Adolescent Psychiatry. 2019. August 1;58(8):768–775.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Troller-Renfree SV, Buzzell GA, Bowers ME, Salo VC, Forman-Alberti A, Smith E, et al. Development of inhibitory control during childhood and its relations to early temperament and later social anxiety: unique insights provided by latent growth modeling and signal detection theory. Journal of Child Psychology and Psychiatry. 2019;60(6):622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rubin KH, Burgess KB, Hastings PD. Stability and Social–Behavioral Consequences of Toddlers’ Inhibited Temperament and Parenting Behaviors. Child Development. 2002;73(2):483–495. [DOI] [PubMed] [Google Scholar]
- 60.Buss KA, Davis EL, Ram N, Coccia M. Dysregulated fear, social inhibition, and respiratory sinus arrhythmia: A replication and extension. Child Development. 2018;89(3):e214–e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker OL, Henderson HA, Degnan KA, Penela EC, Fox NA. Associations between behavioral inhibition and children’s social problem-solving behavior during social exclusion. Social Development. 2014;23(3):487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petersen SE, Posner MI. The Attention System of the Human Brain: 20 Years After. Annual Review of Neuroscience. 2012. July 21;35(1):73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nigg JT. Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry. 2017. April;58(4):361–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diamond A. Executive Functions. Annual Review of Psychology. 2013;64(1):135–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Development and Psychopathology. 1997. December;null(04):633–652. [DOI] [PubMed] [Google Scholar]
- 66.Miller EK, Cohen JD. An Integrative Theory of Prefrontal Cortex Function. Annual Review of Neuroscience. 2001. March;24(1):167–202. [DOI] [PubMed] [Google Scholar]
- 67.Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Sciences. 2012. February;16(2):106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White LK, McDermott JM, Degnan KA, Henderson HA, Fox NA. Behavioral inhibition and anxiety: The moderating roles of inhibitory control and attention shifting. Journal of abnormal child psychology. 2011;39(5):735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buzzell GA, Morales S, Bowers ME, Troller-Renfree S, Chronis-Tuscano A, Pine DS, et al. A two-pathway model of behavioral inhibition risk for social anxiety: higher inhibition or lower shifting confers risk in children with a behaviorally inhibited temperament. Developmental Science. in press; [Google Scholar]
- 70.Thorell LB, Bohlin G, Rydell A-M. Two types of inhibitory control: Predictive relations to social functioning. International Journal of Behavioral Development. 2004;28(3):193–203. [Google Scholar]
- 71.Valadez EA, Troller-Renfree S, Buzzell GA, Henderson HA, Chronis-Tuscano A, Pine DS, et al. Behavioral inhibition and dual mechanisms of anxiety risk: Disentangling neural correlates of proactive and reactive control. [DOI] [PMC free article] [PubMed]
- 72.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004. April 1;8(4):170–7. [DOI] [PubMed] [Google Scholar]
- 73.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in cognitive sciences. 2008;12(3):99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harman C, Rothbart MK, Posner MI. Distress and attention interactions in early infancy. Motivation and Emotion. 1997;21(1):27–44. [Google Scholar]
- 75.Kopp CB. Regulation of distress and negative emotions: A developmental view. Developmental Psychology. 1989;25(3):343–54. [Google Scholar]
- 76.Morales S, Fox NA. A Neuroscience Perspective on Emotional Development. In: LoBue V, Pérez-Edgar K, Buss KA, editors. Handbook of Emotional Development. Cham, Switzerland: Springer; 2019. p. 57–82. [Google Scholar]
- 77.Joyce AW, Kraybill JH, Chen N, Cuevas K, Deater-Deckard K, Bell MA. A Longitudinal Investigation of Conflict and Delay Inhibitory Control in Toddlers and Preschoolers. Early Educ Dev. 2016;27(6):788–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zelazo PD, Anderson JE, Richler J, Wallner-Allen K, Beaumont JL, Weintraub S II. NIH Toolbox Cognition Battery (CB): Measuring executive function and attention. Monographs of the Society for Research in Child Development. 2013;78(4):16–33. [DOI] [PubMed] [Google Scholar]
- 79.Posner MI, Rothbart MK, Sheese BE, Voelker P. Developing Attention: Behavioral and Brain Mechanisms. Advances in Neuroscience. 2014. May 8;2014:e405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rothbart MK, Sheese BE, Rueda MR, Posner MI. Developing Mechanisms of Self-Regulation in Early Life. Emotion Review. 2011. April 1;3(2):207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zelazo PD. The development of conscious control in childhood. Trends in Cognitive Sciences. 2004. January;8(1):12–7. [DOI] [PubMed] [Google Scholar]
- 82.Doebel S, Zelazo PD. A meta-analysis of the Dimensional Change Card Sort: Implications for developmental theories and the measurement of executive function in children. Developmental Review. 2015. December 1;38:241–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of Cognitive Processes From Late Childhood to Adulthood. Child Development. 2004;75(5):1357–72. [DOI] [PubMed] [Google Scholar]
- 84.Eggum-Wilkens ND, Reichenberg RE, Eisenberg N, Spinrad TL. Components of effortful control and their relations to children’s shyness. International journal of behavioral development. 2016;40(6):544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kiel EJ, Buss KA. Prospective Relations Among Fearful Temperament, Protective Parenting, and Social Withdrawal: The Role of Maternal Accuracy in a Moderated Mediation Framework. J Abnorm Child Psychol. 2011. October 1;39(7):953–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lewis-Morrarty E, Degnan KA, Chronis-Tuscano A, Rubin KH, Cheah CSL, Pine DS, et al. Maternal Over-Control Moderates the Association Between Early Childhood Behavioral Inhibition and Adolescent Social Anxiety Symptoms. J Abnorm Child Psychol. 2012. November 1;40(8):1363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Conejero Á, Rueda MR. Infant temperament and family socio-economic status in relation to the emergence of attention regulation. Scientific reports. 2018;8(1):11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fox NA, Snidman N, Haas SA, Degnan KA, Kagan J. The Relations between Reactivity at 4 Months and Behavioral Inhibition in the Second Year: Replication across Three Independent Samples. Infancy. 2015. January 1;20(1):98–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hane AA, Fox NA. Ordinary Variations in Maternal Caregiving Influence Human Infants’ Stress Reactivity. Psychological Science. 2006. June 1;17(6):550–6. [DOI] [PubMed] [Google Scholar]
- 90.Hane AA, Henderson HA, Reeb-Sutherland BC, Fox NA. Ordinary variations in human maternal caregiving in infancy and biobehavioral development in early childhood: A follow-up study. Dev Psychobiol. 2010. September;52(6):558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kopala-Sibley DC, Danzig AP, Kotov R, Bromet EJ, Carlson GA, Olino TM, et al. Negative emotionality and its facets moderate the effects of exposure to Hurricane Sandy on children’s postdisaster depression and anxiety symptoms. Journal of abnormal psychology. 2016;125(4):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pérez-Edgar K, Morales S, LoBue V, Taber-Thomas BC, Allen EK, Brown KM, et al. The impact of negative affect on attention patterns to threat across the first 2 years of life. Developmental psychology. 2017;53(12):2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morales S, Brown KM, Taber-Thomas BC, LoBue V, Buss KA, Pérez-Edgar KE. Maternal Anxiety Predicts Attentional Bias Towards Threat in Infancy. Emotion. 2017;17(5):874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Graham AM, Buss C, Rasmussen JM, Rudolph MD, Demeter DV, Gilmore JH, et al. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Developmental Cognitive Neuroscience. 2016. April 1;18:12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rogers CE, Sylvester CM, Mintz C, Kenley JK, Shimony JS, Barch DM, et al. Neonatal amygdala functional connectivity at rest in healthy and preterm infants and early internalizing symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 2017;56(2):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sylvester CM, Smyser CD, Smyser T, Kenley J, Ackerman JJ Jr, Shimony JS, et al. Cortical Functional Connectivity Evident After Birth and Behavioral Inhibition at Age 2. American Journal of Psychiatry. 2017;175(2):180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thomas E, Buss C, Rasmussen JM, Entringer S, Ramirez JS, Marr M, et al. Newborn amygdala connectivity and early emerging fear. Developmental cognitive neuroscience. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fu X, Nelson EE, Borge M, Buss KA, Pérez-Edgar K. Stationary and ambulatory attention patterns are differentially associated with early temperamental risk for socioemotional problems: Preliminary evidence from a multimodal eye-tracking investigation. Dev Psychopathol. 2019. August;31(3):971–88. [DOI] [PMC free article] [PubMed] [Google Scholar]


