Abstract
Cytochrome P450 (P450) 11B1 and 11B2 both catalyze the 11β-hydroxylation of 11-deoxycorticosterone and the subsequent 18-hydroxylation of the product. P450 11B2, but not P450 11B1, catalyzes a further C-18 oxidation to yield aldosterone. 11-Oxygenated androgens are of interest, and 11-hydroxy progesterone has been reported to be a precursor of these. Oxidation of progesterone by purified recombinant P450 11B2 yielded a mono-hydroxy derivative as the major product, and co-chromatography with commercial standards and 2-D NMR spectroscopy indicated 11β-hydroxylation. 18Hydroxyprogesterone and a dihydroxyprogesterone were also formed. Similarly, oxidation of androstenedione by P450 11B2 yielded 11β-hydroxyandrostenedione, 18-hydroxyandrostenedione, and a dihydroxyandrostenedione. The steady-state kinetic parameters for androstenedione and progesterone 11β-hydroxylation were similar to those reported for the classic substrate 11-deoxycorticosterone. The source of 11α-hydroxyprogesterone in humans remains unresolved.
Keywords: cytochrome P450 11B2, 11-hydroxy steroids, 18-hydroxy steroids, 11β-hydroxyprogesterone, progesterone, 11β-hydroxyandrostenedione, androstenedione
1. Introduction
11-Oxygenated steroids circulate in the body, with serum levels varying considerably among species. Humans and non-human primates have the highest levels [1]. These compounds include the 11α- and 11β-hydroxy (OH) and 11-keto derivatives of androstenedione, testosterone, and progesterone [2–18]. These compounds have varying biological activities. For instance, 11-keto testosterone is a potent androgen [19]. 11α-OH progesterone has been reported to have anti-androgenic properties and also to potentiate the anti-inflammatory properties of cortisol [15]. 11β-OH androstenedione has a potential role in prostate cancer [7]. 11-Oxygenated steroids are elevated in patients with P450 21A2 deficiency [8, 10, 11]. The 11-oxygenated steroids have been indicated in alternative routes to 5α-dihydro androgens in castration-resistant prostate cancer [7, 14].
The biosynthetic pathways involving 11-oxygenated steroids are still not all clear [15]. The 11α-hydroxylase enzyme has not been identified in human tissue [15]. Strushkevich et al. [20] reported that progesterone, testosterone, and androstenedione were all hydroxylated by human cytochromes P450 (P450, CYP) 11B1 and 11B2 but did not report the identities of any products. Gent et al. [15] reported that 11α-OH progesterone is not a substrate for 11β-OH steroid dehydrogenase (11β-HSD) Type II but acts as a competitive inhibitor of the enzyme. Both 11α- and 11β-OH progesterone have been reported to be inhibitors of both 11β-HSD Types I and II [22]. 11β-OH progesterone is a substrate for 11β-HSD Type II, however [15, 21]. Interestingly, 11-ketoprogesterone was found to be a substrate for reduction by 11β-HSD Type I [13]. 11α-OH and 11β-OH progesterone are both substrates for P450 17A1 and are converted to 11α,17α-diOH- and 11β,17α-diOH- progesterone and on to 11α-OH and 11β-OH androstenedione, respectively [15, 16]. These reactions could be demonstrated in PC3 and LNCaP prostate cancer cells as well as with recombinant enzymes. Human microsomal P450 3A4, generally recognized as a drug-metabolizing enzyme [23], has also been reported to catalyze trace 11β-hydroxylation of testosterone [4].
In the course of our own work on the kinetics and processivity of human P450 11B2 in the 3-step oxidation of deoxycorticosterone to aldosterone [24], we examined the ability of this enzyme to catalyze the oxidation of progesterone and androstenedione (Fig. 1). We identified the major product of progesterone as 11β-OH progesterone using mass spectrometry (MS), NMR, and co-chromatography with commercial standards and another product was identified as 18-OH progesterone. The conversion of androstenedione to 11β-OH androstenedione was established by co-chromatography and mass spectrometry. Steady-state kinetic parameters were also measured, which indicate relatively high rates (~20–30 min−1) for these P450 11B2 11β-hydroxylation reactions.
Fig. 1.
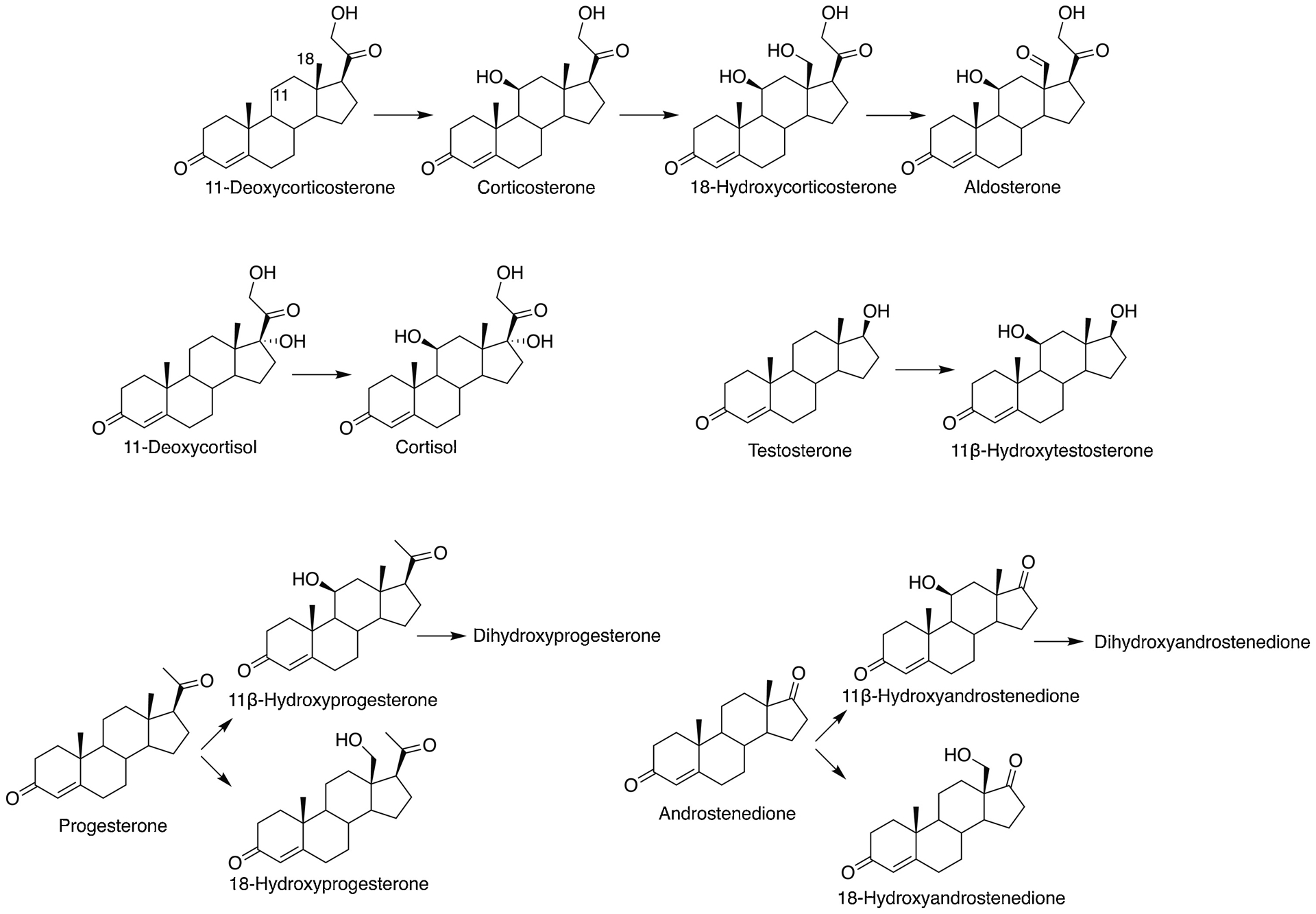
Reactions catalyzed by human P450 11B2 reported earlier [20, 24, 25] and demonstrated in this work.
2. Materials and methods
2.1. Materials
Androstenedione, progesterone, and 11β-OH androstenedione were purchased from SigmaAldrich and used without further purification. 18-OH progesterone and 11-ketoprogesterone were obtained from Steraloids (Newport, RI). 11β-OH progesterone was purchased from both ChemScene (Monmouth Junction, NJ) and from Steraloids. 11α-OH progesterone was obtained from Tokyo Chemical Industry (TCI) America, (Portland, OR).
2.2. Enzymes
A modified recombinant version of human P450 11B2 was expressed in Escherichia coli cells and purified as described previously (first 24 N-terminal amino acids removed, following 6 changed to MATKAAR, C-terminal (His)9 tag) [24]. Bovine adrenodoxin (Adx, lacking the mitochondrial targeting sequence, N-terminal Met modification) and bovine NADPH-Adx reductase (AdR, C-terminal (His)6 tag modification) were expressed in E. coli and purified as described in detail elsewhere [26–29].
2.3. Enzyme reaction conditions
Typical incubations included 5 nM P450 11B2, 1 μM Adx, 0.5 μM AdR, 30 μM L-α−1,2-dilauroyl-sn-glycero-3-phosphocholine, and 0.5–100 μM substrate (androstenedione, progesterone, or 11β-OH androstenedione, dissolved in ethanol and diluted to ≤1% final ethanol concentration in each reaction) in 50 mM potassium phosphate buffer (pH 7.4). After a 5-minute pre-incubation at 37 °C, reactions were initiated by the addition of 1 mM NADPH. Incubations used for LC-MS analysis were prepared as described above, with 10 μM substrate. Additional incubations with OH-steroids were prepared as described above with 11β-OH progesterone, 11α-OH progesterone, 18-OH progesterone (10 μM) and 11β-OH androstenedione (1 μM).
For steady-state kinetic measurements, the final volume was 1 mL and the reaction time was 8 minutes. Incubations with OH-steroids had a reaction time of 20 minutes. Reactions were terminated by the addition of a 4× volume of ethyl acetate (4 mL) and mixing (vortex device). The layers were separated by centrifugation (2000 × g, 5 minutes), and a 3.8 mL aliquot of the organic phase (upper phase) was dried under an N2 stream. The residues were dissolved in 150 μL of 9:1 A:B UPLC mobile phase (v/v, see below) and transferred to autosampler vials with inserts for UPLC.
An aliquot of each sample (20 μL) was analyzed by UPLC, done with a Waters Acquity system using an Acquity BEH octadecylsilane (C18) column (1.7 μm, 2.1 mm × 100 mm) at 35 °C with a flow rate of 0.35 mL min−1. Solvent A was 95% H2O, 5% CH3CN, 0.1% HCO2H and Solvent B was 99% CH3CN, 1% H2O, 0.1% HCO2H (all v/v/v). The solvent gradient used was: 0 min, 0% B; 7.5 min, 62.5% B; 8 min, 62.5% B; 8.25 min, 0% B; 10 min, 0% B.
For preparative reactions with the substrate progesterone, the reaction volume was 12.5 mL and the reaction time was 4 hours, with additional NADPH (200 μM) added every hour. The reaction was terminated by the addition of a 4× volume of CH2Cl2 (50 mL) and mixing (vortex device). An aliquot of the organic phase (lower phase) was reduced to dryness in vacuo and resuspended for preparative HPLC.
Preparative HPLC was done under similar conditions using a Beckman Ultrasphere octadecylsilane (C18) column (5 μm; 4.6 mm × 250 mm) and a linear gradient consisting of increasing CH3CN from 5% to 95% in 0.1% aqueous HCO2H (all v/v) over a period of 20 min. The flow rate was 1.5 mL min−1 and UV detection was at 245 nm. The peak of interest was collected, and the CH3CN was removed under an N2 stream. The product was separated from the remaining aqueous solution by extraction with dichloromethane and concentrated by removal of the solvent under a stream of N2.
2.4. Mass spectrometry
LC-MS analysis was performed on an Acquity UPLC system (Waters) coupled with a Thermo-Finnigan LTQ-Orbitrap or LTQ XL-Orbitrap mass spectrometer (Thermo Scientific, San Jose, CA) equipped with an atmospheric pressure chemical ionization (APCI) source. Samples were separated by LC as described above. MS data were acquired in the positive ion mode using Xcalibur software (Thermo) after tuning with progesterone. Total ion scans were taken in the FTMS mode with 60,000 resolution from m/z 200 to 1500. Settings on the LTQ-Orbitrap were as follows: capillary temperature: 300°C; APCI vaporizer temperature 350 °C; sheath gas flow: 44; auxiliary gas flow 13; sweep gas flow 0; source current 4.5 μV; capillary voltage: 45 V, tube lens voltage 96 V. Settings on the LTQ XL-Orbitrap were as follows: capillary temperature: 275°C; APCI vaporizer temperature 450 °C; sheath gas flow: 59; auxiliary gas flow 10; sweep gas flow 5; source current 5 μV; capillary voltage: 34 V, tube lens voltage 90 V.
2.5. NMR spectroscopy
NMR experiments were acquired using a 14.0 T Bruker magnet equipped with a Bruker AV-III console operating at 600.13 MHz. All spectra were acquired in 3 mm NMR tubes using a Bruker 5 mm TCI cryogenically-cooled NMR probe. Chemical shifts were referenced internally to CDCl3 (7.26 ppm) which also served as the 2H lock solvent. For 1D 1H NMR, typical experimental conditions included 32 K data points, 13 ppm sweep width, a recycle delay of 1.5 s and 64 scans. For 2D 1H-1H COSY experiments, experimental conditions included 2048 × 512 data matrix, 13 ppm sweep width, recycle delay of 1.5 s and 8 scans per increment. The data were processed using a squared sinebell window function, symmetrized, and displayed in magnitude mode. Nuclear Overhauser effect correlated spectroscopy (NOESY) experiments were acquired using a 2048 × 512 data matrix with a 600 ms mixing time, 2 s recycle delay, and 8 scans per increment. The data was processed using a π/2 shifted squared sine window function and displayed in absorption mode. Multiplicity-edited HSQC experiments were acquired using a 1024 × 128 data matrix, a J(C-H) value of 145 Hz which resulted in a multiplicity selection delay of 34 ms, a recycle delay of 1.5 s, and 128 scans per increment along with GARP decoupling on 13C during the acquisition time (150 ms). The data was processed using a π/2 shifted squared sine window function and displayed with CH/CH3 signals phased positive and CH2 signals phased negative. J1(C-H) filtered HMBC experiments were acquired using a 2048 × 128 data matrix, a J(C-H) value of 9 Hz for detection of long range couplings resulting in an evolution delay of 55 ms, J1(C-H) filter delay of 145 Hz (34 ms) for the suppression of one-bond couplings, a recycle delay of 1.5 s, and 128 scans per increment. The HMBC data were processed using a π/2 shifted squared sine window function and displayed in magnitude mode.
2.6. Steady-state kinetic analysis
Rates of product formation were calculated from integration of the A245 peaks in the UPLC chromatograms (all Δ4 steroids have very similar extinction coefficients [30, 31]). The product yields were converted to rates (v) (nmol product formed min−1 (nmol P450)−1). The concentrations of minor products formed from progesterone and androstenedione were calculated based on quantification of the corresponding 11β-OH steroid standard curve. Plots of v for each individual product vs. substrate concentration (S) were fit to hyperbolae (Michaelis-Menten kinetics) in Prism (GraphPad, San Diego, CA), fitting to kcat and ksp (i.e. kcat/Km) as suggested by Johnson [32], then calculating Km from these parameters. The rates from the assay with 11β-OH androstenedione as the starting substrate were fit by linear regression to estimate kcat/Km because the rate of the reaction did not saturate.
3. Results
3.1. Oxidation of androstenedione
HPLC and LC-MS of the reaction products generated by the incubation of P450 11B2 with androstenedione yielded a major peak, judged to be a mono-OH product by MS (Fig. 2, Table 1, Sup Fig. 1) (calc. For C19H26O3H+, 303.1960; found, 303.1964; Δ1.25 ppm). The compound co-eluted with a commercial sample of 11β-OH androstenedione (Sup Fig. 2B). Steady-state kinetic analysis yielded kcat = 24.8 min−1, Km = 1.9 μM, and kcat/Km = 13 μM−1 min−1 (2.2 × 105 M−1 s−1) (Fig. 3, Table 1). The reported values of kcat and kcat/Km are not completely accurate due to the simultaneous formation of other products.
Fig. 2.
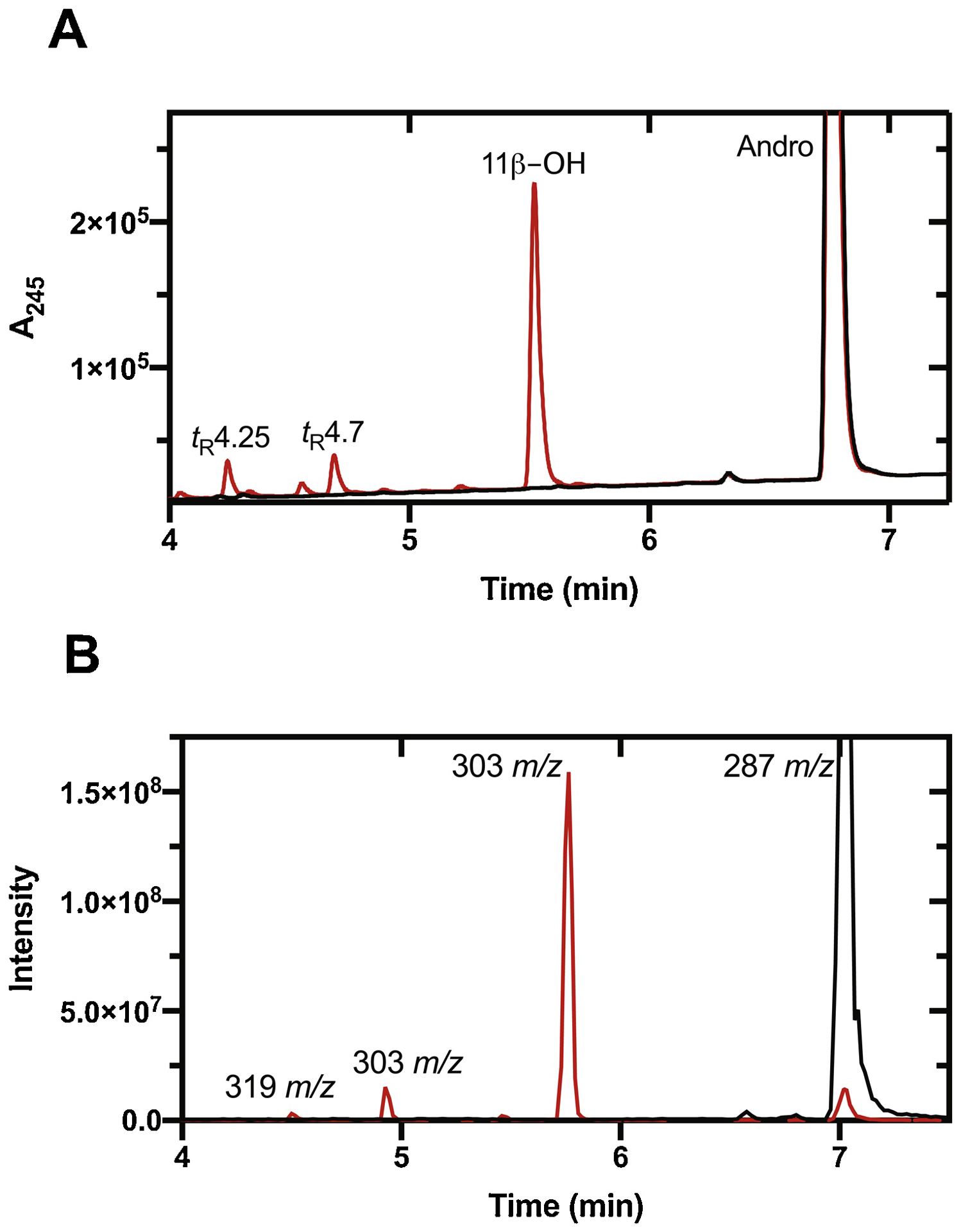
UPLC analysis of products of androstenedione (Andro) oxidation by P450 11B2. (A) LC-UV (A245); (B) LC-MS. Reactions were performed with 10 μM substrate in the presence (red) or absence (black) of NADPH.
Table 1. Steady-state kinetics of P450 11B2 reactions.
See Figs. 3, 8, and Supplemental Fig. 6.
| Substrate | Product | kcat, min−1 | Km, μM | kcat/Km, min−1 μM−1 |
|---|---|---|---|---|
| Progesterone | 11β-OHa (tR 6.6 min, m/z 331.2 [M+H]+) | 31 ± 1 | 5.7 ± 0.5 | 5.4 ± 0.4 |
| 18-OHa (tR 6.3 min, m/z 331.2 [M+H]+) | 5.2 ± 0.1 | 6.8 ± 0.5 | 0.77 ± 0.06 | |
| di-OHa (tR 5.2 min, m/z 347.2 [M+H]+) | 8.2 ± 0.8 | 13 ± 2 | 0.63 ± 0.09 | |
| Androstenedione | 11β-OHa (tR 5.5 min, m/z 303.2 [M+H]+) | 24.8 ± 0.5 | 1.9 ± 0.2 | 13 ± 1 |
| OH-a (tR 4.7 min, m/z 303.2 [M+H]+) | 2.4 ± 0.1 | 1.9 ± 0.3 | 1.3 ± 0.2 | |
| di-OHa (tR 4.25 min, m/z 319.2 [M+H] +) | 5.0 ± 0.4 | 3.8 ± 0.9 | 1.3 ± 0.3 | |
| 11β-OH Androstenedioneb | di-OH | - | - | 0.0083 ± 0.0003 |
| 11-Deoxycorticosteronec | 11β-OH | 33 ± 1 | 2.7 ± 0.3 | 13 ± 1 |
| Corticosteronec | 18-OHa | 14.9 ± 0.4 | 31 ± 1 | 0.49 ± 0.02 |
| Aldosteronea | 2.8 ± 0.1 | 28 ± 2 | 0.10 ± 0.01 |
Multiple products are formed in these reactions. The presented values are not true steady-state parameters because the data are reports of simultaneous multiple reactions.
Only kcat/Km was determined, see details in “Steady-state kinetic analysis” methods.
From [24].
Fig. 3.
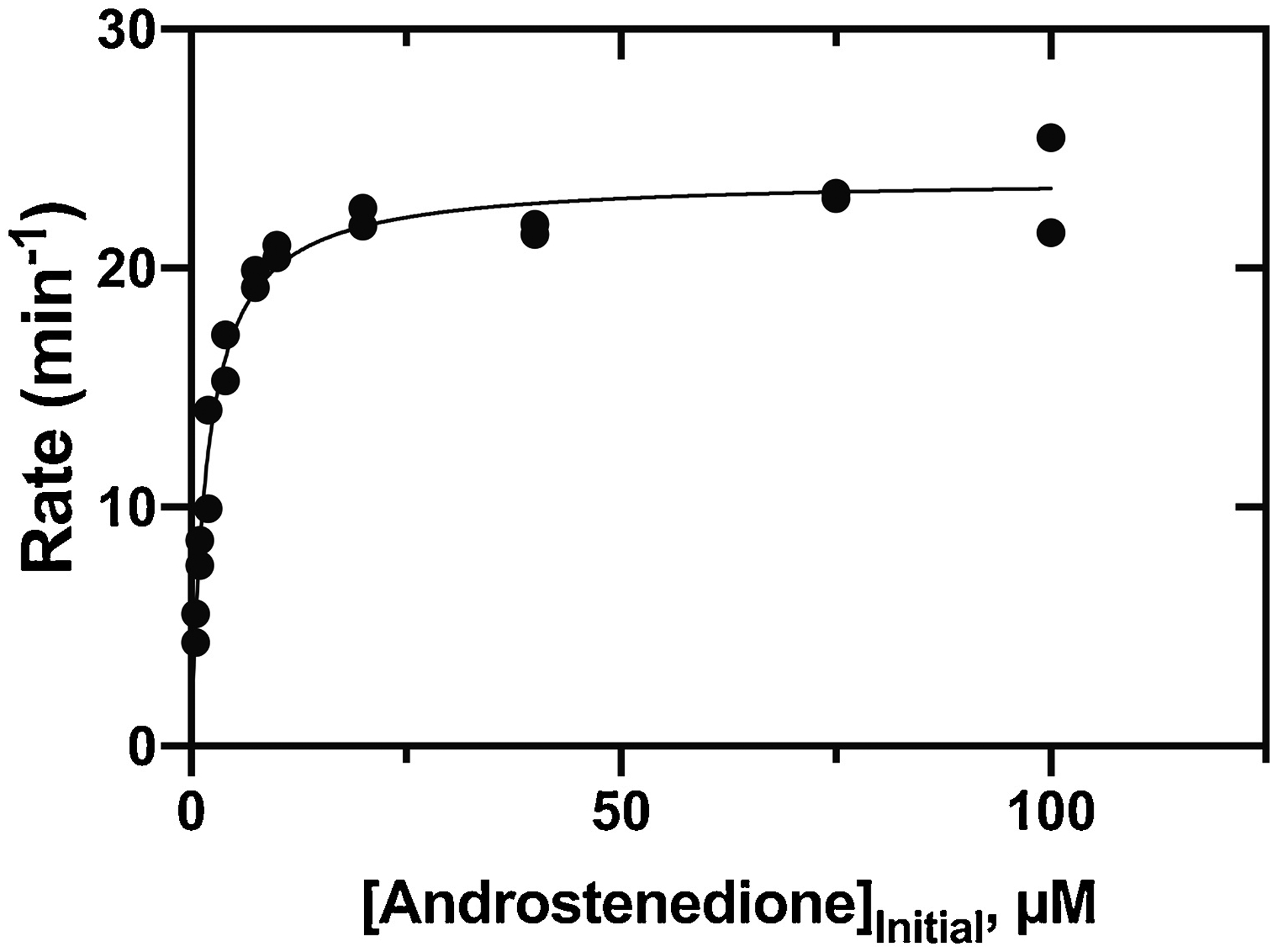
Steady-state kinetics of P450 11B2 11β-hydroxylation of androstenedione. See Table 1. Reactions were completed in duplicate, and both points are shown.
Two other products were formed, at levels an order of magnitude lower (Fig. 2, Table 1). The peaks eluting at tR 4.25 and 4.7 minutes had MH+ ions at m/z 319.2, and 303.2, respectively, indicating that they are putative di-OH (calc. For C19H26O4H+, 319.1909; found, 319.1914; Δ1.5 ppm) and mono-OH products (calc. For C19H26O3H+, 303.1960; found, 303.1965; Δ1.6 ppm) (Sup Fig. 1). The tR 4.7 min peak is assigned as 18-OH androstenedione, which is known to be present in adrenals [33]. Although we did not have a standard reference compound and the amount of product we obtained did not permit NMR analysis, the strong MH+−30 peak in the mass spectrum (Sup Fig. 1A) was not seen in progesterone or other methylene-hydroxylated progesterone derivatives. It appears to be indicative of hydroxylation products of steroid methyl groups, e.g. as seen in 19-OH androstenedione [34] (The MH+−30 peak was confirmed to be due to loss of the elements of HCHO (calc. for C18H25O2+ 273.1855, found 273.1857, Δ 0.9 ppm), and a proposed mechanism for the neutral loss is shown in Fig. S3). We tentatively conclude that the product is 18-OH androstenedione and not 19-OH androstenedione because it lacks the strong MH+−18 peak also seen with that compound [27]. The MH+−30 peak is also observed in the mass spectrum corresponding to di-OH androstenedione (Sup Fig. 1A), suggesting that it may be oxidized in the same position.
Incubation of 11β-OH androstenedione with P450 11B2 yielded a minor product at tR 4.5 (Sup Fig. 4D). Based on the similar tR and mass spectrum (same MH+−30 peak observed, data not shown), this is proposed to be the same di-OH androstenedione product formed by P450 11B2 from androstenedione (Sup Fig. 1). Steady-state kinetic analysis of this reaction yielded a kcat/Km of 0.0083 μM−1 min−1, three orders of magnitude lower than the reaction with androstenedione as the starting substrate.
3.2. Oxidation of progesterone and characterization of products
Incubation of progesterone with P450 11B2 yielded a major peak upon HPLC analysis, with m/z 331.2272 (calc for C21H30O3H+ 331.2273, Δ 0.36 ppm), indicating mono-hydroxylation (Fig. 4, Sup Fig. 5).
Fig. 4.
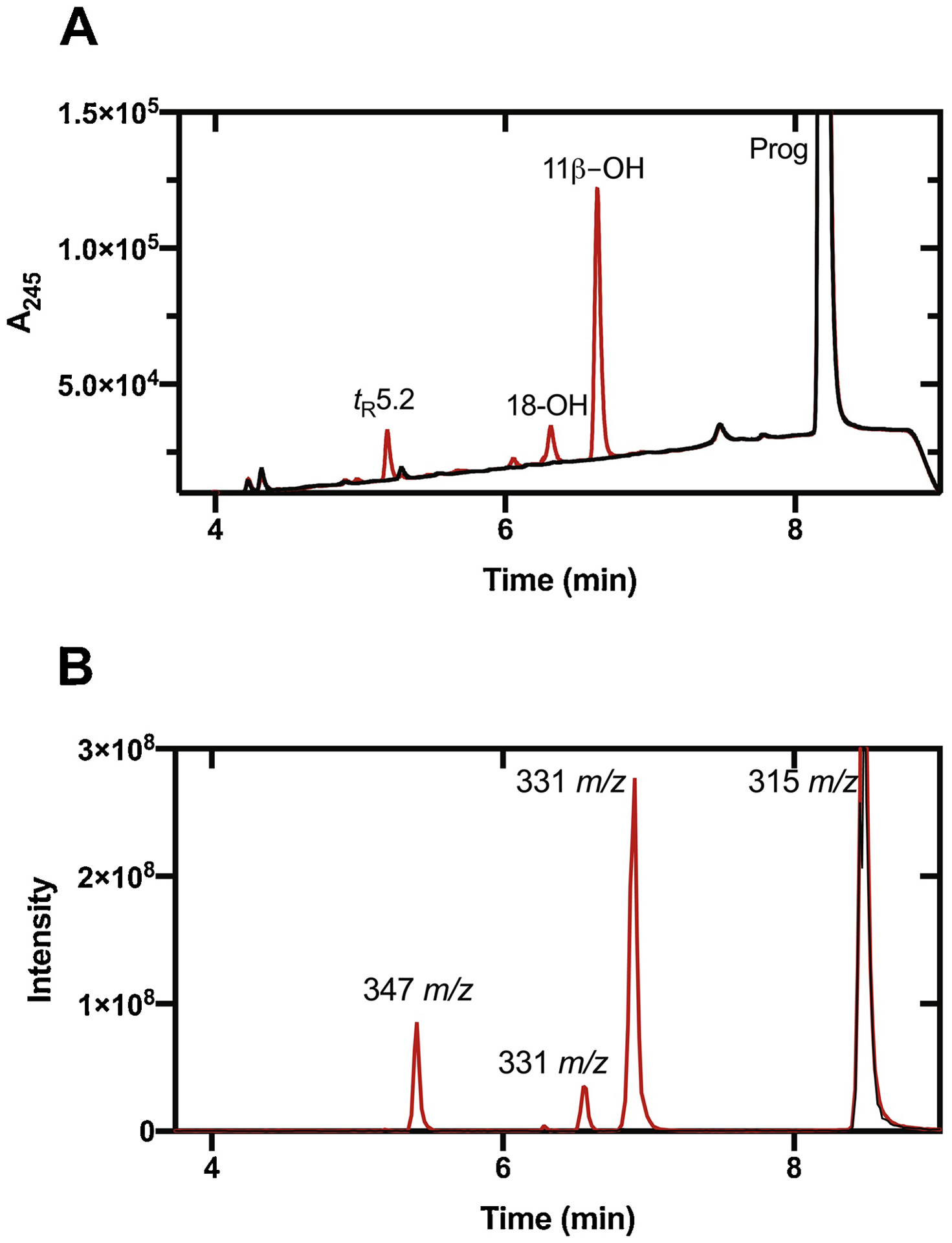
UPLC of products of oxidation of progesterone (Prog) by P450 11B2. (A) UV (A245); (B) LC-MS. Reactions were performed with 10 μM substrate in the presence (red) or absence (black) of NADPH.
Attempts at identification by co-chromatography were ambiguous due to differences between samples of commercial 11β-OH progesterone obtained from two different suppliers. Accordingly, we incubated a larger preparation of progesterone and P450 11B2 and collected the product for NMR analysis (Fig. 5). The NMR data confirmed the hydroxylation of this compound. The position of the hydroxylation at position C-11 is supported by the 2D NMR spectroscopy. 2D 1H-13C HSQC revealed the presence of nine CH/CH3 signals. Since the structure is known to contain three CH3 groups that leaves six peaks that can be assigned to CH groups. In the absence of hydroxylation, the number of observable CH groups should only be five. The additional CH signal is evidence of a hydroxylation. Hydroxylation assigned to H-11 position (4.44 ppm) is supported by 1H-1H COSY correlations to a CH group (H9, 1.009 ppm) and a CH2 group (H12–13, 1.67–2.20 ppm). The assignment for H9 was further confirmed by COSY with correlation to another CH (H8, 1.17 ppm) while the assignment of H12–13 was supported by 2D 1H-13C HMBC which revealed a 3-bond correlation to a CH3 (H-18, 0.91 ppm).
Fig. 5.
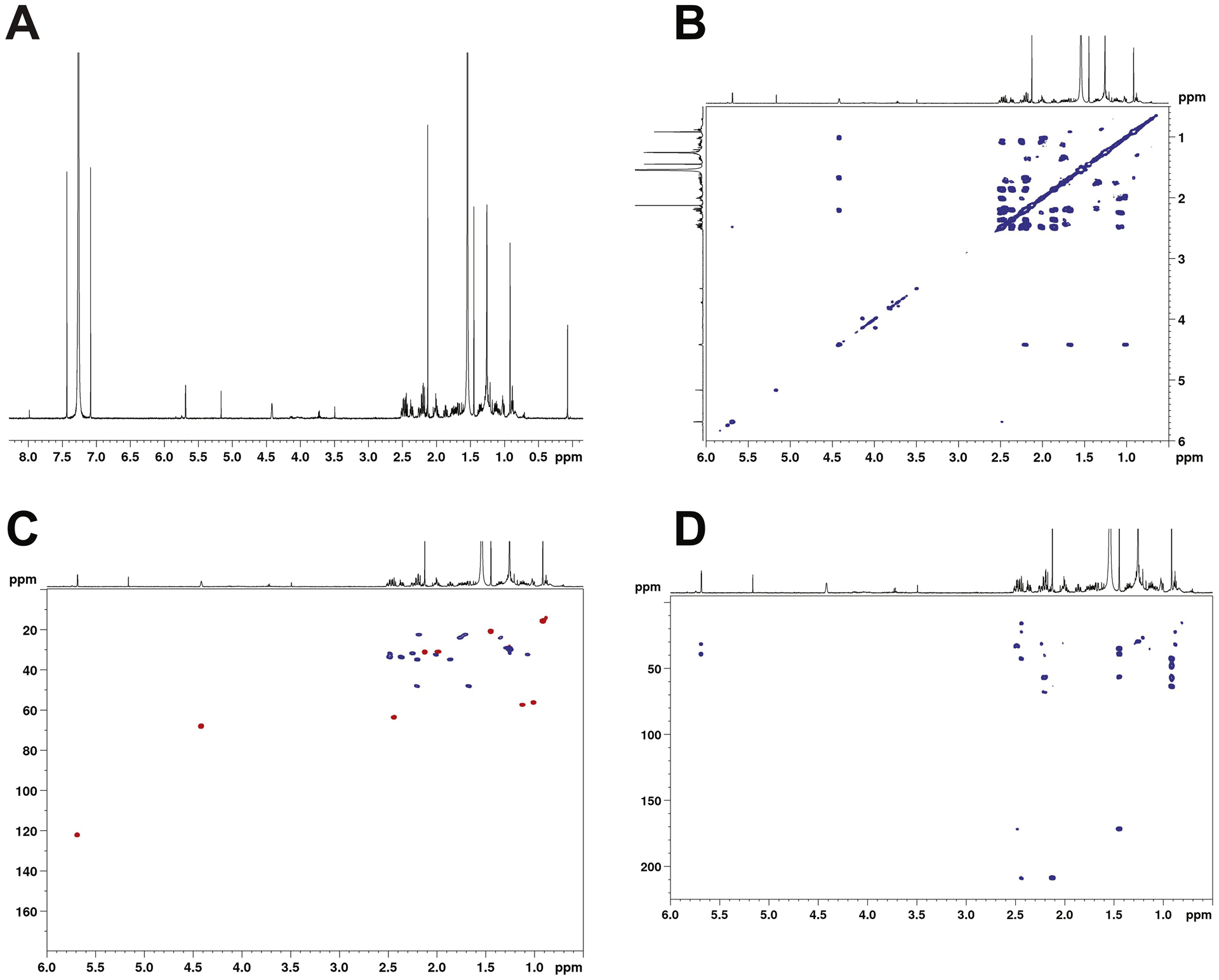
NMR spectra of major product of oxidation of progesterone. Spectra were acquired in CDCl3 at 600 Mz. (A) 1-D 1H spectrum; (B) 2-D COSY spectrum; (C) 2-D HSQC spectrum; (D) 2-D HMBC spectrum.
A sample of commercial 11α-OH progesterone (TCI) did not co-elute with the P450 11B2-generated product (Sup Fig. 2A). Standard 11β-OH progesterone (from Steraloids) co-eluted with the sample (Sup Fig. 2A). Analysis of the NMR spectra of the commercial samples (Fig. 6) indicated that the TCI 11α-OH progesterone and Steraloids 11β-OH progesterone have the correct structures. The assigned stereochemistry of the 11β-OH product (Figure 7B) was supported by 2D Nuclear Overhauser effect (NOE) correlated spectroscopy, which showed correlations for the α-CH (C11, 4.44 pm) to CH (H9, 1.009 ppm), CH (H8, 1.17 ppm), CH2, (H12–13,1.67–2.20 ppm) and CH3 (H19, 1.44ppm). While the stereochemistry for the 11α-OH group (Fig. 7A) was supported by observed NOE correlations from the β-CH (C11, 4.00 ppm) to CH3 (H18, 0.68 ppm), CH3 (H19, 1.31 ppm), and CH2 (H12-H13, 1.51–2.34 ppm).
Fig. 6.
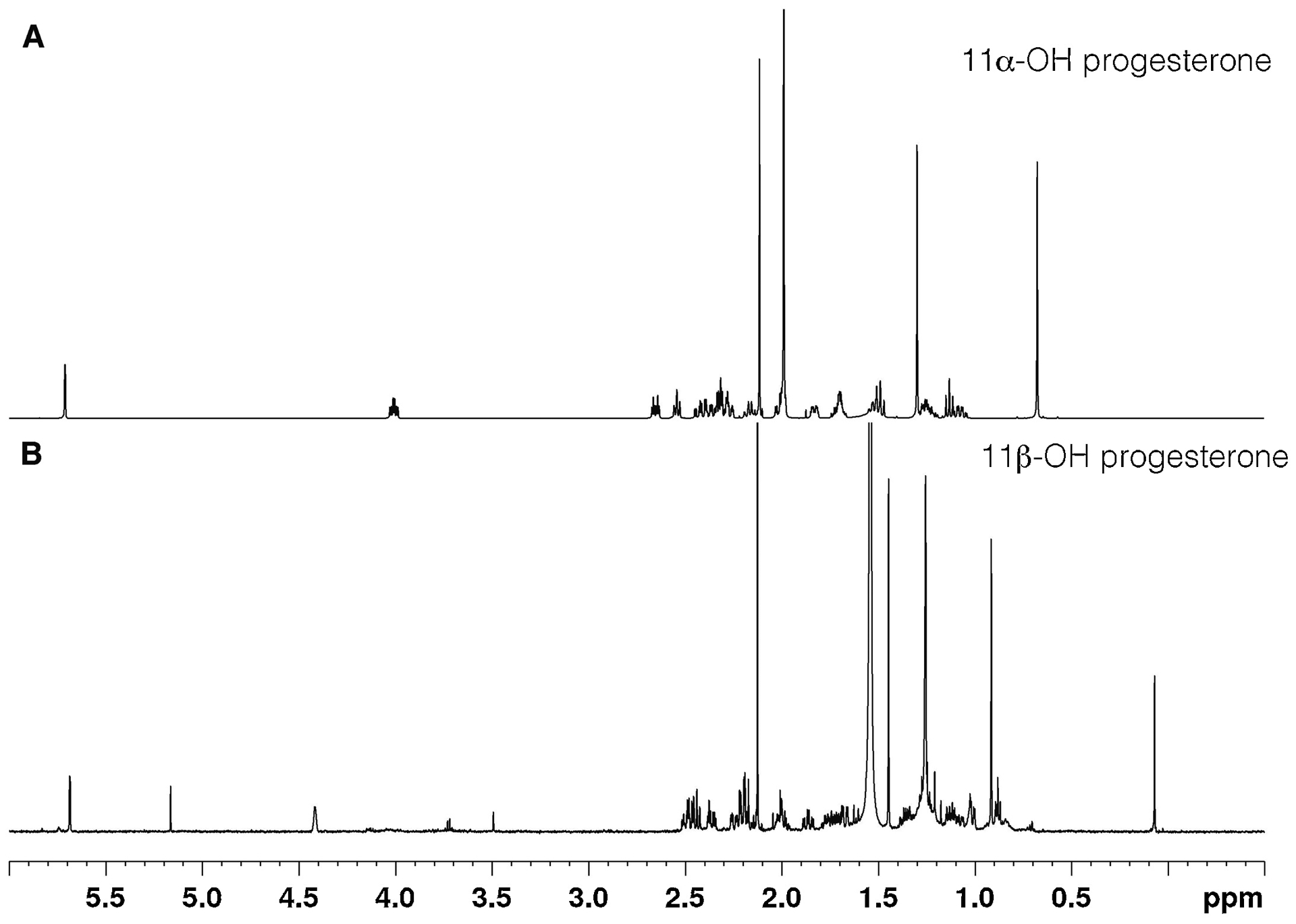
1D NMR spectra of commercial 11-OH progesterone samples. (A) 11α-OH progesterone (TCI); (B) 11β-OH progesterone (Steraloids).
Fig. 7.
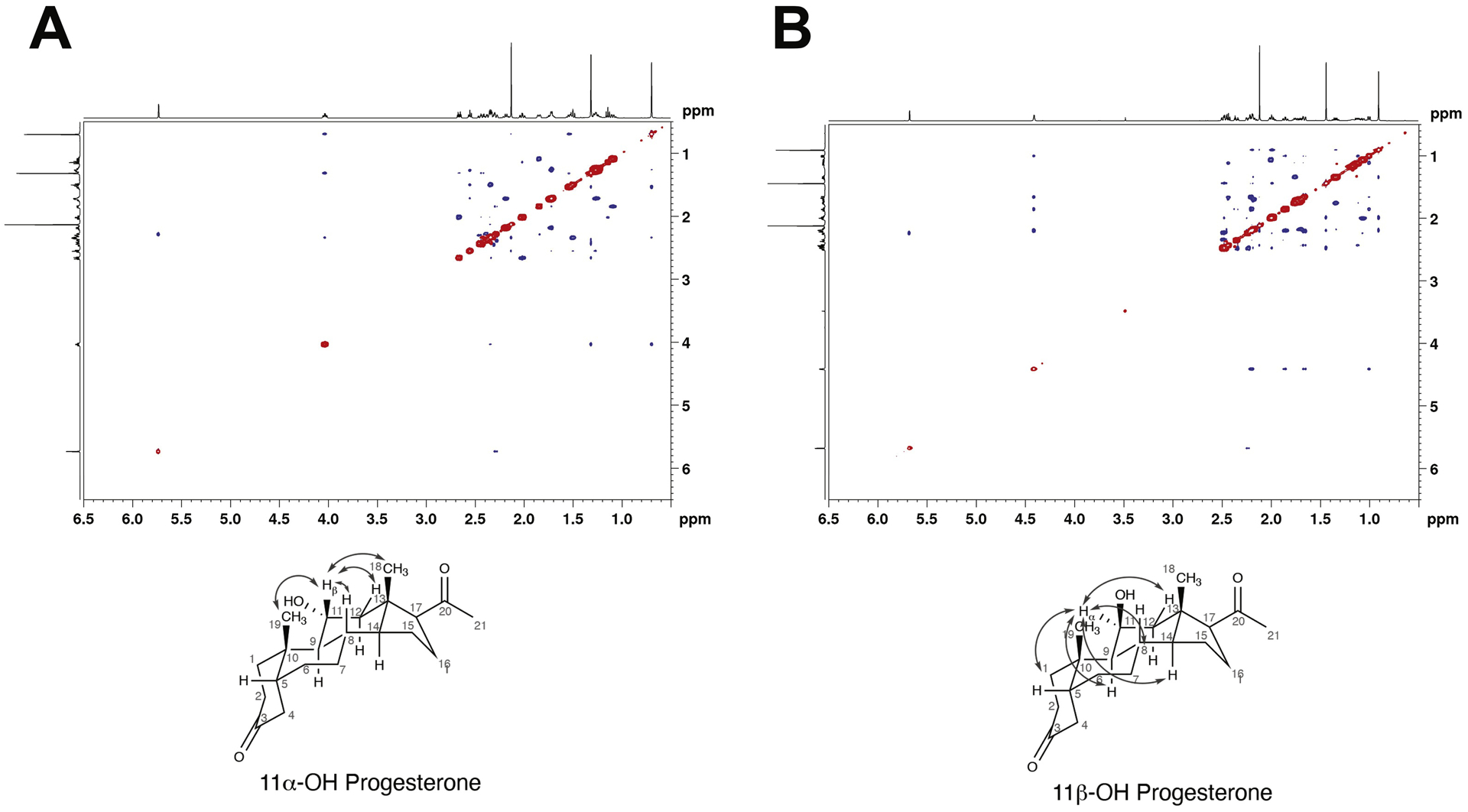
NOESY NMR spectra of commercial 11-OH progesterone samples. (A) 11α-OH progesterone (TCI); (B) 11β-OH progesterone (Steraloids). Assigned connectivity patterns are shown.
The conclusion that P450 11B2 catalyzes 11β-hydroxylation of progesterone is consistent with the published results of van Rooyen et al. [13]. In steady-state kinetics, the kcat was 31 min−1 and Km was 5.7 μM (kcat/Km 5.4 min−1 μM−1, i.e. 9 × 104 M−1 s−1) (Fig. 8). As in the reactions with androstenedione, the reported values of kcat and kcat/Km are not completely accurate due to the formation of other products in the reaction. Two minor products were formed (Fig. 4), at levels an order of magnitude less than the 11β-OH product. The peaks eluting at tR 5.2 and 6.3 minutes had MH+ ions at m/z 347.2227 (calc. for C21H30O4H+ 347.2222, Δ1.3 ppm) and 331.2280 (calc for C21H30O3H+ 331.2273, Δ 2.0 ppm), respectively, indicating that they are putative di-OH and mono-OH products (Sup Fig. S5), similar to what was observed with androstenedione. The other mono-OH product co-eluted with 18-OH progesterone (Sup Fig. 2A). HPLC analysis indicated that the unknown minor hydroxylation product was not 11 keto-, 6β-, 11α-, 16α-, 17α-, or 21-OH progesterone (results not shown).
Fig. 8.
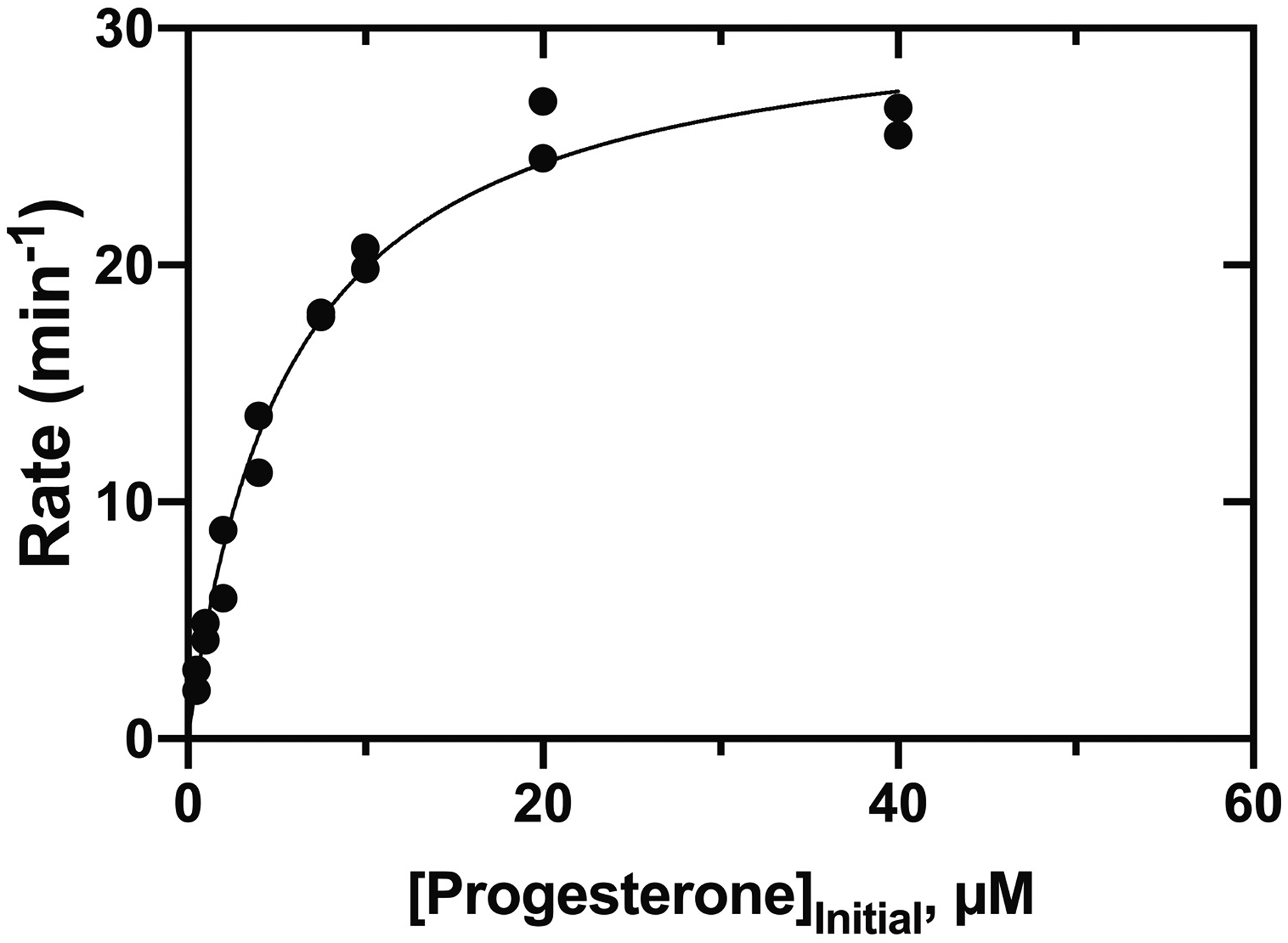
Steady-state kinetics of P450 11B2 11β-hydroxylation of progesterone. See Table 1. Reactions were completed in duplicate, and both points are shown.
Incubation of 11β-OH progesterone with P450 11B2 yielded a minor product (Sup Fig. 4A), which had a tR of 5.48 (Sup Fig. 5) and mass spectrum (data not shown) similar to the di-OH progesterone identified in reactions with progesterone. Additionally, incubation of 11α-OH progesterone with P450 11B2 yielded a minor product with tR 4.96 (Sup Fig. 4B). This product elutes before the proposed di-OH progesterone product that is formed in incubations with 11β-OH progesterone (Sup Fig. 3A). Given the earlier tR of 11α-OH progesterone in comparison with 11β-OH progesterone, the tR 4.96 product may be a di-OH progesterone with an 11α-OH instead of an 11β-OH. Incubation of 18-OH progesterone with a reconstituted system and NADPH did not yield any new products (Sup Fig. 4C).
4. Discussion
11β-OH androstenedione and 11β-OH testosterone have been known for many years, and these and other 11-oxygenated androgens have several biological properties. We confirm that P450 11B2 is one source of 11β-OH androstenedione. Alternatively, 11-OH progesterone can be converted to 11β-OH androstenedione by P450 17A1 via 17α-hydroxylation and lyase activity [16] and androstenedione can be hydroxylated to 11β-OH androstenedione by P450 11B1 in the adrenal [17]. Minor hydroxylation products and further oxidation of 11β-OH androstenedione to a putative di-OH androstenedione were found (Fig. 2, Sup Fig. 4D), but the positions of oxidation were not identified due to the small amounts of material available. Given the mass spectra of the products (Sup. Fig. 1A), we tentatively conclude that the minor hydroxylation product is 18-OH androstenedione and the di-OH product is 11β,18 di-OH androstenedione.
Although the 11β-hydroxylation of progesterone by P450 11B1 and 11B2 has been reported [5, 13], comprehensive characterization of progesterone oxidations by P450 11B2 had not been done. A primary product of progesterone oxidation was obtained, with the m/z corresponding to monooxidation (Fig. 2). Two-dimensional NMR implicated C-11 as the site of hydroxylation (Fig. 5), and we considered the possibility that P450 11B2 might be the source of 11α-OH progesterone but a standard sample did not co-elute. Careful NOESY NMR characterization of all of the commercial 11-OH progesterone samples was done (Fig. 7). The 11-H/19-CH cross peaks were key to resolving the issue (Fig. 7), and we conclude that only the β isomer of 11-OH progesterone is formed. Further oxidation of 11β-OH progesterone and 11α-OH progesterone was minor, though incubations with both produce products with tR of what you would expect for di-OH progesterone.
We note that the specificity constants (kcat/Km) for 11β-hydroxylations of progesterone and androstenedione were nearly the same that we previously reported for 11-deoxycorticosterone (Table 1, Figs. 3, 8) [24]. This finding is important in considering the physiological roles of these reactions, although we are not implying that the reactions with progesterone and androstenedione are necessarily as physiologically important as the 11β-hydroxylation of 11-deoxycorticosterone, which leads to aldosterone. As mammalian P450s go, the specificity constants (~ 105 M−1 s−1) are relatively high [35], and these reactions with progesterone and androstenedione should not be considered artifacts. On the other hand, the specificity constant (kcat/Km) for hydroxylation of 11β-OH androstenedione is much lower than what we previously reported for 18-hydroxylation of corticosterone (Table 1, Sup Fig. 6E). While we did not directly investigate the processivity of the reactions presented in this study as we did with the formation of aldosterone [24], the steady-state kinetic results suggest that the reactions with androstenedione may occur via a processive mechanism (i.e. since the production of di-OH androstenedione is >100 times more favored when androstenedione is the substrate compared with 11β-OH androstenedione).
While we have demonstrated that P450 11B2 can perform these reactions in vitro, the localization of steroids and differential expression of steroid-metabolizing enzymes within the adrenal gland is important to consider when addressing the potential importance in vivo. In normal conditions P450 11B2 is expressed in the zona glomerulosa of the adrenal cortex [36]. Progesterone can be synthesized from pregnenolone in the zona glomerulosa or zona fasciculata by 3β-HSD2. Androgens, including androstenedione, are normally secreted by the zona reticularis (pathways in each adrenal zone reviewed in [37]). Because of this, P450 11B2 may encounter progesterone in normal tissue, but likely not androstenedione. P450 11B2 is also expressed in aldosterone-producing adenomas (APAs) [36] and in some cases, P450 17A1 can be co-expressed [38]. The expression of P450 17A1 could allow for the production of dehydroepiandrosterone (DHEA) within the APA, which could then be converted to androstenedione by 3β-HSD2 (which has also been shown to be strongly expressed in APAs [39]). The co-expression of P450 17A1 and P450 11B2 in APAs has already been proposed to lead to the production of the hybrid steroids 18-OH cortisol and 18-oxocortisol [18]. A similar principle may apply to 11-oxygenated androstenedione.
We did not prepare P450 11B1 and compare kinetic parameters for the 11β-hydroxylation reactions. van Rooyen et al. [13] found a higher conversion of progesterone to 11β-OH progesterone by P450 11B1 than 11B2, but the results are for single time points and are not normalized for levels of expression in the cell culture system (and AdR was not over-expressed in the system). No other products (other than 11β-OH) were reported, while we identified additional minor products. In our work we measured the oxidation of only one androgen, androstenedione, and not testosterone. Swart et al. [5], using a cell system, reported that P450 11B1 hydroxylated androstenedione but P450 11B2 did so only at a very low rate, and both P450 11B1 and 11B2 catalyzed the hydroxylation of testosterone. However, our own rates (Table 1) are as high as for the substrate 11-deoxycorticosterone and higher than for progesterone.
18-Hydroxylation of progesterone and androstenedione was not as efficient as 11β-hydroxylation. Similar to these reactions, the rate of 18-hydroxylation of the classical P450 11B2 substrate, 11-deoxycorticosterone, to 18-hydroxy-11-deoxycoricosterone is also lower than the 11β-hydroxylation to corticosterone [20]. The 18-hydroxylation of C19 and C18 steroids, as well as C21, has been recognized in several systems, for some time [33, 40–44], and may not seem surprising in light of the activity of P450 11B2 on corticosterone. Some 18-oxygenated steroids are biologically active [2, 3], although exactly what their physiological roles are remains unknown.
Finally, the source of 11α-OH steroids remains unknown. It is of interest that the 11α-hydroxylation of progesterone by a fungal (Rhizopus) P450 was one of the first examples of the use of an enzyme in a chemical synthesis in industry [45].To our knowledge the enzyme that forms this in humans has not been identified [15]. 11β-OH progesterone is oxidized to the 11-keto product by 11β-HSD Type 2, and the reverse reaction is catalyzed by 11β-HSD Type 1 but apparently these reactions are stereospecific and do not yield 11α-OH progesterone [15, 21].
Supplementary Material
Highlights.
Human cytochrome P450 11B2 catalyzes the 11β-hydroxylation of progesterone and androstenedione.
The rates and specificity constants are similar to those for 11β-hydroxylation of the classic substrate 11-deoxycorticosterone by the same enzyme.
Minor products of both androstenedione and progesterone were also identified, including 18-hydroxy products.
Acknowledgements.
This work was supported by National Institutes of Health grants R01 GM118122 (F.P.G.), T32 ES007028 (F. P. G., M. J. R., S. M. G.), and F31 AR077386 (S. M. G.). The 600 MHz NMR spectrometer was purchased in part with funding from National Institutes of Health grant S10 RR019022. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare that they have no conflicts of interest with the contents of this article. We thank S. Goyal, Y. Xiao, L. D. Nagy, and J. G. Chapman for their assistance in preparing the accessory enzymes (Adx, AdR) and K. Trisler for assistance in preparation of the manuscript.
Abbreviations:
- Adx
adrenodoxin
- AdR
NADPH-adrenodoxin reductase
- APA
aldosterone-producing adenoma
- APCI
atmospheric pressure chemical ionization
- HSD
hydroxy steroid dehydrogenase
- MS
mass spectrometry
- P450 or CYP
cytochrome P450
- POR
NADPH-cytochrome P450 reductase
- COSY
correlated (NMR) spectroscopy
- HMBC
heteronuclear multiple bond correlation (NMR) spectroscopy
- HSQC
heteronuclear single quantum coherence (NMR) spectroscopy
- NOESY
nuclear Overhauser effect correlated (NMR) spectroscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rege J, Garber S, Conley AJ, Elsey RM, Turcu AF, Auchus RJ, Rainey WE, Circulating 11-oxygenated androgens across species, J. Steroid Biochem. Mol. Biol 190 (2019) 242–249. 10.1016/j.jsbmb.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pappo R, Synthesis of 18-oxygenated progesterones, J. Am. Chem. Soc 81(4) (1959) 1010–1011. 10.1021/ja01513a068 [DOI] [Google Scholar]
- [3].Weet JF, Lenz GR, Mineralocorticoid properties of potential metabolites of 18-hydroxydeoxycorticosterone and 18-hydroxyprogesterone, J. Med. Chem 28(2) (1985) 233–239. 10.1021/jm00380a014 [DOI] [PubMed] [Google Scholar]
- [4].Choi MH, Skipper PL, Wishnok JS, Tannenbaum SR, Characterization of testosterone 11β-hydroxylation catalyzed by human liver microsomal cytochromes P450, Drug Metab. Dispos 33(6) (2005) 714–718. [DOI] [PubMed] [Google Scholar]
- [5].Swart AC, Schloms L, Storbeck KH, Bloem LM, Toit T, Quanson JL, Rainey WE, Swart P, 11β-Hydroxyandrostenedione, the product of androstenedione metabolism in the adrenal, is metabolized in LNCaP cells by 5α-reductase yielding 11β-hydroxy-5α-androstanedione, J. Steroid Biochem. Mol. Biol 138 (2013) 132–142. 10.1016/j.jsbmb.2013.04.010 [DOI] [PubMed] [Google Scholar]
- [6].Bloem LM, Storbeck K-H, Schloms L, Swart AC, 11β-Hydroxyandrostenedione returns to the steroid arena: Biosynthesis, metabolism and function, Molecules (Basel, Switzerland) 18(11) (2013) 13228–13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Storbeck K-H, Bloem LM, Africander D, Schloms L, Swart P, Swart AC, 11β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: A putative role in castration resistant prostate cancer?, Mol. Cell Endocrinol 377(1) (2013) 135–146. 10.1016/j.mce.2013.07.006 [DOI] [PubMed] [Google Scholar]
- [8].Turcu AF, Rege J, Chomic R, Liu J, Nishimoto HK, Else T, Moraitis AG, Palapattu GS, Rainey WE, Auchus RJ, Profiles of 21-carbon steroids in 21-hydroxylase deficiency, J. Clin. Endocrinol. Metab 100(6) (2015) 2283–2290. 10.1210/jc.2015-1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Swart AC, Storbeck K-H, 11β-hydroxyandrostenedione: Downstream metabolism by 11βHSD, 17βHSD and SRD5A produces novel substrates in familiar pathways, Mol. Cell Endocrinol 408 (2015) 114–123. 10.1016/j.mce.2014.12.009 [DOI] [PubMed] [Google Scholar]
- [10].Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, Merke DP, Rainey WE, Auchus RJ, Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency, Eur. J. Endocrinol 174(5) (2016) 601–609. 10.1530/eje-15-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barnard L, Gent R, van Rooyen D, Swart AC, Adrenal C11-oxy C21 steroids contribute to the C11-oxy C19 steroid pool via the backdoor pathway in the biosynthesis and metabolism of 21-deoxycortisol and 21-deoxycortisone, J. Steroid Biochem. Mol. Biol 174 (2017) 86–95. 10.1016/j.jsbmb.2017.07.034 [DOI] [PubMed] [Google Scholar]
- [12].Turcu AF, Nanba AT, Auchus RJ, The rise, fall, and resurrection of 11-oxygenated androgens in human physiology and disease, Hormone Res. Paediatrics 89(5) (2018) 284–291. 10.1159/000486036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].van Rooyen D, Gent R, Barnard L, Swart AC, The in vitro metabolism of 11βhydroxyprogesterone and 11-ketoprogesterone to 11-ketodihydrotestosterone in the backdoor pathway, J. Steroid Biochem. Mol. Biol 178 (2018) 203–212. 10.1016/j.jsbmb.2017.12.014 [DOI] [PubMed] [Google Scholar]
- [14].du Toit T, Swart AC, The 11β-hydroxyandrostenedione pathway and C11-oxy C-21 backdoor pathway are active in benign prostatic hyperplasia yielding 11-ketotestosterone and 11keto-progesterone, J. Steroid Biochem. Mol. Biol 196 (2020). 10.1016/j.jsbmb.2019.105497 [DOI] [PubMed] [Google Scholar]
- [15].Gent R, du Toit T, Swart AC, 11α-Hydroxyprogesterone, a potent 11β-hydroxysteroid dehydrogenase inhibitor, is metabolised by steroid-5α-reductase and cytochrome P450 17α-hydroxylase/17,20-lyase to produce C11α-derivatives of 21-deoxycortisol and 11-hydroxyandrostenedione in vitro, J. Steroid Biochem. Mol. Biol 191 (2019) 105369. 10.1016/j.jsbmb.2019.04.018 [DOI] [PubMed] [Google Scholar]
- [16].van Rooyen D, Yadav R, Scott EE, Swart AC, CYP17A1 exhibits 17α hydroxylase/17,20-lyase activity towards 11β-hydroxyprogesterone and 11-ketoprogesterone metabolites in the C11-oxy backdoor pathway, J. Steroid Biochem. Mol. Biol 199 (2020). 10.1016/j.jsbmb.2020.105614 [DOI] [PubMed] [Google Scholar]
- [17].Schloms L, Storbeck KH, Swart P, Gelderblom WC, Swart AC, The influence of Aspalathus linearis (Rooibos) and dihydrochalcones on adrenal steroidogenesis: quantification of steroid intermediates and end products in H295R cells, J. Steroid Biochem. Mol. Biol 128(3–5) (2012) 128–138. 10.1016/j.jsbmb.2011.11.003 [DOI] [PubMed] [Google Scholar]
- [18].Lenders JWM, Williams TA, Reincke M, Gomez-Sanchez CE, Diagnosis of endocrine disease: 18-Oxocortisol and 18-hydroxycortisol: is there clinical utility of these steroids?, Eur. J. Endocrinol 178(1) (2018) R1–r9. 10.1530/eje-17-0563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rege J, Turcu AF, Else T, Auchus RJ, Rainey WE, Steroid biomarkers in human adrenal disease, J. Steroid Biochem. Mol. Biol 190 (2019) 273–280. 10.1016/j.jsbmb.2019.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Strushkevich N, Gilep AA, Shen L, Arrowsmith CH, Edwards AM, Usanov SA, Park HW, Structural insights into aldosterone synthase substrate specificity and targeted inhibition, Mol. Endocrinol 27(2) (2013) 315–324. 10.1210/me.2012-1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gent R, du Toit T, Bloem LM, Swart AC, The 11β-hydroxysteroid dehydrogenase isoforms: pivotal catalytic activities yield potent C11-oxy C19 steroids with 11βHSD2 favouring 11ketotestosterone, 11-ketoandrostenedione and 11-ketoprogesterone biosynthesis, J. Steroid Biochem. Mol. Biol 189 (2019) 116–126. 10.1016/j.jsbmb.2019.02.013 [DOI] [PubMed] [Google Scholar]
- [22].Souness GW, Latif SA, Laurenzo JL, Morris DJ, 11 alpha- and 11 betahydroxyprogesterone, potent inhibitors of 11β-hydroxysteroid dehydrogenase (isoforms 1 and 2), confer marked mineralocorticoid activity on corticosterone in the ADX rat, Endocrinology 136(4) (1995) 1809–1812. 10.1210/endo.136.4.7895695 [DOI] [PubMed] [Google Scholar]
- [23].Guengerich FP, Martin MV, Beaune PH, Kremers P, Wolff T, Waxman DJ, Characterization of rat and human liver microsomal cytochrome P-450 forms involved in nifedipine oxidation, a prototype for genetic polymorphism in oxidative drug metabolism, J. Biol. Chem 261(11) (1986) 5051–5060. [PubMed] [Google Scholar]
- [24].Reddish MJ, Guengerich FP, Human cytochrome P450 11B2 produces aldosterone by a processive mechanism due to the lactol form of the intermediate 18-hydroxycorticosterone, J. Biol. Chem 294(35) (2019) 12975–12991. 10.1074/jbc.RA119.009830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Auchus RJ, Miller WL, P450 enzymes in steroid processing, in: Ortiz de Montellano PR (Ed.), Cytochrome P450: Structure, Mechanism, and Biochemistry, 4th ed., Springer, New York, 2015. pp. 851–879. [Google Scholar]
- [26].Goyal S, Xiao Y, Porter NA, Xu L, Guengerich FP, Oxidation of 7-dehydrocholesterol and desmosterol by human cytochrome P450 46A1, J. Lipid Res 55(9) (2014) 1933–1943. 10.1194/jlr.M051508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Acimovic J, Goyal S, Kosir R, Golicnik M, Perse M, Belic A, Urlep Z, Guengerich FP, Rozman D, Cytochrome P450 metabolism of the post-lanosterol intermediates explains enigmas of cholesterol synthesis, Sci. Rep 6 (2016) 28462. 10.1038/srep28462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yoshimoto FK, Jung I-J, Goyal S, Gonzalez E, Guengerich FP, Isotope-labeling studies support the electrophilic Compound I iron active species, FeO3+, for the carbon-carbon bond cleavage reaction of the cholesterol side-chain cleavage enzyme, cytochrome P450 11A1, J. Am. Chem. Soc 138(37) (2016) 12124–12141. 10.1021/jacs.6b04437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Enright JM, Toomey MB, Sato SY, Temple SE, Allen JR, Fujiwara R, Kramlinger VM, Nagy LD, Johnson KM, Xiao Y, How MJ, Johnson SL, Roberts NW, Kefalov VJ, Guengerich FP, Corbo JC, Cyp27c1 red-shifts the spectral sensitivity of photoreceptors by converting vitamin A1 into A2, Curr. Biol 25(23) (2015) 3048–3057. 10.1016/j.cub.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dyer JR, Applications of Absorption Spectroscopy of Organic Compounds, Prentice-Hall, Englewood Cliffs, 1965, p 15. [Google Scholar]
- [31].Silverstein RM, Bassler GC, Morrill TC, Spectrometric Identification of Organic Compounds, John Wiley & Sons, New York, 1991, pp 296–304. [Google Scholar]
- [32].Johnson KA, New standards for collecting and fitting steady state kinetic data, Beilstein J. Org. Chem 15(1860–5397 (Print)) (2019) 16–29. 10.3762/bjoc.15.2.eCollection2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ling AM, Loke KH, Metabolism of androstenedione by porcine adrenal homogenates, Steroids 8(5) (1966) 765–775. 10.1016/0039-128x(66)90016-x [DOI] [PubMed] [Google Scholar]
- [34].Yoshimoto FK, Guengerich FP, Mechanism of the third oxidative step in the conversion of androgens to estrogens by cytochrome P450 19A1 steroid aromatase, J. Am. Chem. Soc 136(42) (2014) 15016–15025. 10.1021/ja508185d [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shinkyo R, Guengerich FP, Cytochrome P450 7A1 cholesterol 7α-hydroxylation: individual reaction steps in the catalytic cycle and rate-limiting ferric iron reduction, J. Biol. Chem 286(6) (2011) 4632–4643. 10.1074/jbc.M110.193409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K, Adrenocortical zonation in humans under normal and pathological conditions, J. Clin. Endocrinol. Metab 95(5) (2010) 2296–2305. 10.1210/jc.2009-2010 [DOI] [PubMed] [Google Scholar]
- [37].Miller WL, Auchus RJ, The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders, Endocrin. Rev 32(1) (2011) 81–151. 10.1210/er.2010-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nakamura Y, Kitada M, Satoh F, Maekawa T, Morimoto R, Yamazaki Y, Ise K, Gomez-Sanchez CE, Ito S, Arai Y, Dezawa M, Sasano H, Intratumoral heterogeneity of steroidogenesis in aldosterone-producing adenoma revealed by intensive double- and triple-immunostaining for CYP11B2/B1 and CYP17, Mol. Cell Endocrinol 422 (2016) 57–63. 10.1016/j.mce.2015.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Doi M, Satoh F, Maekawa T, Nakamura Y, Fustin JM, Tainaka M, Hotta Y, Takahashi Y, Morimoto R, Takase K, Ito S, Sasano H, Okamura H, Isoform-specific monoclonal antibodies against 3β-hydroxysteroid dehydrogenase/isomerase family provide markers for subclassification of human primary aldosteronism, J. Clin. Endocrinol. Metab 99(2) (2014) E257–262. 10.1210/jc.2013-3279 [DOI] [PubMed] [Google Scholar]
- [40].Fukushima DK, Bradlow HL, Hellman L, Gallagher TF, Isolation and characterization of 18-hydroxy-17-ketosteroids, J. Biol. Chem 237(11) (1962) 3359–3363. [PubMed] [Google Scholar]
- [41].Gustafsson J-Å, Lisboa BP, Studies on the metabolism of C19 steroids in rat liver. 7. 18-hydroxylation of 17-oxo-C19 steroids in rat liver microsomes, Steroids 15(6) (1970) 723–735. 10.1016/S0039-128X(70)80042-3 [DOI] [PubMed] [Google Scholar]
- [42].Neher R, Wettstein A, Isolierung und Konstitutionsermittlung weiterer Pregnanverbindungen aus Nebennieren. Über Steroide, 144. Mitteilung, Helv. Chim. Acta 39(7) (1956) 2062–2088. 10.1002/hlca.19560390719 [DOI] [Google Scholar]
- [43].Loke KH, Marrian GF, Johnson WS, Meyer WL, Cameron DD, Isolation and identification of 18-hydroxyoestrone from the urine of pregnant women, Biochim. Biophys. Acta 28(1) (1958) 214. 10.1016/0006-3002(58)90457-8 [DOI] [PubMed] [Google Scholar]
- [44].Loke KH, Marrian GF, Watson EJ, The isolation of a sixth Kober chromogen from the urine of pregnant women and its identification as 18-hydroxyoestrone, Biochem. J 71(1) (1959) 43–48. 10.1042/bj0710043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Peterson DH, Microbial transformations of steroids. I. Introduction of oxygen at carbon-11 of progesterone, J. Am. Chem. Soc 74 (1952) 5933–5936. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


