Abstract
Protein localization in endothelial cells is tightly regulated to create distinct signaling domains within their tight spatial restrictions including: luminal membranes, abluminal membranes, and interendothelial junctions, as well as caveolae and calcium signaling domains. Protein localization in endothelial cells is also determined in part by the vascular bed, with differences between arteries and veins and between large and small arteries. Specific protein polarity and localization is essential for endothelial cells in responding to various extracellular stimuli. In this review, we examine protein localization in the endothelium of resistance arteries, with occasional references to other vessels for contrast, and how that polarization contributes to endothelial function and ultimately whole organism physiology. We highlight the protein localization on the luminal surface, discussing important physiological receptors and the glycocalyx. The protein polarization to the abluminal membrane is especially unique in small resistance arteries with the presence of the myoendothelial junction, a signaling microdomain that regulates vasodilation, feedback to smooth muscle cells, and ultimately total peripheral resistance. We also discuss the interendothelial junction, where tight junctions, adherens junctions, and gap junctions all convene and regulate endothelial function. Finally, we address planar cell polarity, or axial polarity, and how this is regulated by mechanosensory signals like blood flow.
Keywords: endothelium, protein localization, signaling domains, arteriole
Introduction
Endothelium of resistance arteries is the key regulator of vasodilation, vascular permeability, inflammation, and immune signaling. The endothelium arguably sees some of the most dynamic stimuli from constant exposure to circulatory pressure and flow on the luminal side, and direct cellular contact with smooth muscle cells on its basal side. Since these perturbations can be chemical, electrical or mechanical in nature, protein localization and polarity must be clearly defined within the cell to manage accurate signal propagation among the diverse and numerous stimuli. This is a challenge in endothelium due to the squamous nature of the cells. Endothelial cell morphology is unique, flattened to be less than 1μm thick with a protruding nuclear bulge.(1) Yet, the endothelium can remarkably polarize along two axes of the blood vessel: (1) apical-basal polarity aligns with the luminal-abluminal axis while (2) planar cell polarity aligns with the longitudinal axis of the vessel. The goal of this review is to highlight the unique protein polarization between the luminal and abluminal membranes as well as the planar cell polarity of the endothelium and to provide insight on how this is regulated to control endothelial function. Although we focus on the endothelium from resistance arteries, comparisons to conduit and venous endothelium are used throughout and sometimes are necessary to highlight or extrapolate concepts.
On the Face of Flow: Luminal Membrane
Endothelial cells are positioned within an asymmetrical extracellular environment, where their apical membrane is exposed to the vessel lumen and in direct contact with the cells and factors of the blood while the abluminal membrane is in close proximity to the overlaying smooth muscle cells and the internal elastic lamina (IEL), a specialized extracellular matrix (shown in Figure 1). It is important to note that due to the “flattened” endothelial morphology, the distance between the luminal and abluminal membranes of an endothelial cell can be as low as 200 nm.(1, 2) Despite this short distance, both the luminal and abluminal surfaces demonstrate unique protein localization and specialized functions. For instance, endothelial proteins involved in the inflammatory response, such as E-selectin, ICAM-1, and VCAM-1, are upregulated and localized to the luminal surface apropos for interacting with leukocytes in the circulation.(3, 4) These proteins facilitate leukocyte adhesion, rolling and transmigration involved in tissue infiltration. One might similarly expect a majority of endothelial signaling receptors and G-coupled proteins receptors (GPCRs) to be localized to their luminal face, to detect signaling ligands in the circulation, however, there is a dearth of evidence of the subcellular distribution of these receptors. In this section, we highlight the polarization of proteins to the luminal surface of endothelium and discuss where explicit localization studies are still needed.
Figure 1. Luminal protein localization in endothelium.
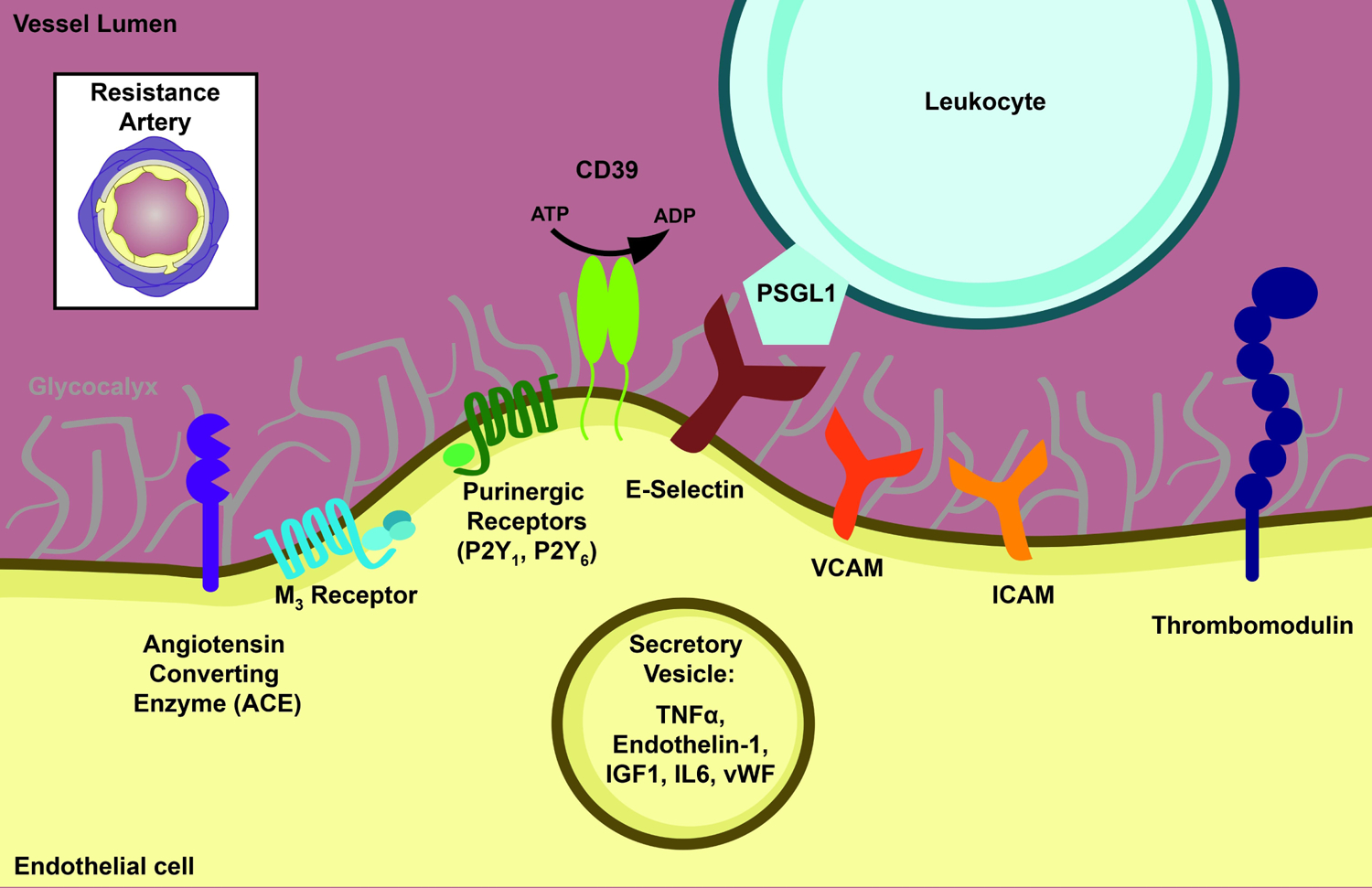
The luminal membrane of the endothelium is in direct contact with the circulation. The glycocalyx, ectonucleases, immune signaling proteins and luminally-secreted proteins localize to the luminal membrane. Transverse view of resistance artery is depicted in the upper left for reference.
Regulators of endothelial function on the luminal surface
In this section, we will highlight some of the major regulators of vasodilation/vasoconstriction, coagulation, and inflammation pathways on the luminal endothelial surface of resistance arteries. Although we limit our discussion to peripheral arterioles, it should be noted that luminal/abluminal endothelial polarity has also been noted in the cerebral vasculature.(5)
Glycocalyx
A hallmark example of this luminal/abluminal polarity is the glycocalyx, which is only present on the luminal side of the endothelium (shown in Figure 1). It is composed of glycosaminoglycans, glycoproteins, and proteoglcycans and is approximately 2–3μm thick in small arteries.(6),(7) The glycocalyx facilitates the formation of luminal microdomains by providing a binding surface for circulating factors. Heparan sulfate proteoglycan (Table 1), a large proportion of the glycocalyx,(8) bears a significant negative charge at physiological pH, which ultimately promotes electrostatic interactions with positively-charged residues on circulating proteins and factors.(9) The electrostatic force is adequate to initiate a stable interaction that can occur with various ligands,(9, 10) and sequences for binding are often noncontiguous positively charged residues.(10) A major physiologically relevant group that binds to luminal heparan sulfate are anticoagulants, including antithrombin III,(11) heparin cofactor II,(12) and tissue factor pathway inhibitor (TFPI).(13) The heparan sulfate-antithrombin interaction is one of the most well studied in the field and involves a rare 3-O-sulfation within a specific pentasaccharide on heparan sulfate, making antithrombin one of its most specific ligands.(14–16) The ability of these anticoagulants to interact with heparan sulfate at injury sites ensures the restoration of homeostasis rather than vessel-occluding thrombi. Circulating growth factors comprise another major group that binds to the glycocalyx, which include fibroblast growth factor (FGF),(17) transforming growth factor beta (TGF-β),(18) platelet-derived growth factor (PDGF),(19) hepatocyte growth factor,(20) and vascular endothelial growth factor (VEGF).(21) Heparan sulfate and heparin act as low affinity receptors for FGF,(22) and their interaction facilitates the dimerization of high affinity receptors (FGFR1–4) leading to activation and induction of signaling.(23, 24) While we have highlighted two major examples of how the glycocalyx facilitates luminal microdomain formation, there are numerous other circulating factors that bind to the glycocalyx including enzymes, chemokines, and cytokines.(9, 25)
Table 1. Summary of protein localization within endothelial signaling domains.
This table summarizes protein localization to one or multiple endothelial signaling compartments that have been described in the text. Although this review focuses on localization within the resistance artery endothelium, there are a limited number of studies that use this model system and for this reason, localization data from other model systems are included (1) to provide a more complete summary of the body of literature on endothelial protein localization, and (2) to highlight the need for more studies in small arteries. Since endothelial cells have unique physiological roles based on vessel type, it is crucial that localization studies be performed in the arteriole endothelium to determine if there are differences compared to large arteries or even veins.
| Full Name | Source: Tissue | Source: Cell Culture | Luminal | Abluminal | Interendothelial | |
|---|---|---|---|---|---|---|
| A kinase anchoring protein 150 | AKAP 150 | Mouse mesenteric artery(128) | (128) | |||
| A kinase anchoring protein 12 | AKAP 12 | HDMEC(202) | (202) | |||
| A kinase anchoring protein 9 | AKAP 9 | HUVEC(276) | (276) | |||
| Alkaline phosphatase | ALP | Rat BCEC(334) | (334) | (334) | ||
| Angiotensin converting enzyme | ACE | Canine vasculature(60) Rabbit vasculature(61) Aorta & pulmonary arteries (bovine, pig & rabbit)(62) Human carotid artery(63) |
(60–63) | |||
| Bradykinin B2 Receptor | Rat aorta(50) | (50) | ||||
| Calnexin | Caln | Third order mesenteric artery(186) | (186) | |||
| Calreticulin | Calr | Third order mesenteric artery(130) | HCAEC(56) | (56, 130) | ||
| Caveolin 1 | Cav1 | Mouse carotid(335) Mouse mesenteric artery(278) |
HCAEC(56) HMVEC(286) BCAEC(287) |
(286, 287, 335) | (278) | |
| Claudin-5 | Cldn5 | Mouse mesenteric artery | Endothelial cells derived from murine ESC(242) | (242) | ||
| Connexin37 | Cx37 | Rat mesenteric artery(159) Mouse mesenteric artery(278) Mouse cremaster arterioles(167) Hamster cheek pouch arteriole(262, 263) |
(159, 167) | (159, 262, 278) | ||
| Connexin40 | Cx40 | Rat mesenteric artery(159, 169) Mouse mesenteric artery(278) Mouse cremaster arteriole(167) Hamster cheek pouch arteriole(262, 263) |
(167, 169) | (159, 262, 263, 278) | ||
| Connexin43 | Cx43 | Rat mesenteric artery(159) Mouse mesenteric artery(278) Mouse cremaster arteriole(167) Hamster cheek pouch arteriole(262, 263) |
(167) | (159, 262, 263, 278) | ||
| E-Selectin | CD62 | Primary HBMEC(4) | (4) | |||
| Endothelial nitric oxide synthase | eNOS | Mouse carotid(336) Mouse thoracodorsal artery(337) |
Primary HCAEC(56) | (56, 336) | (56),(337) | |
| Endothelin B Receptor | ETB | Primary HAoEC(338) | (338) | (338) | ||
| Filamentous actin | F-actin | Mouse cremaster arterioles(167) | HUVEC(274) VCCC(189, 190) |
(167, 189, 190) | (274) | |
| Hemoglobin alpha | Alpha globin | Mouse thoracodorsal artery(124) | HCAEC(125) | (124) | (124, 125) | |
| Heparan sulfate proteoglycan | HSPG | Bovine aorta(217) | Porcine AoEC(339) HAoEC(340) Primary BAoEC(341) HUVEC-pericyte tube co-assembly model(138) |
(340, 341) | (138, 217, 339) | |
| Inositol trisphosphate receptor 1 | IP3R1 | Mouse mesenteric artery(186) | VCCC(132, 133) | (132, 133, 186) | ||
| Integrin α1β1 | HUVEC(211) | (211) | ||||
| Integrin α2β1 | HUVEC(206, 278) | (278) | (206, 278) | |||
| Integrin α5β1 | HUVEC(206, 216, 278) | (216) | (206, 278) | |||
| Integrin αvβ3 | HUVEC (207, 211, 216) | (207, 211, 216) | ||||
| Intermediate conductance | IKCa | Rat cremaster arteriole(294) | (159, 294) | |||
| calcium-activated potassium channel | Rat mesenteric artery(159) | |||||
| Muscarinic M3 Receptor | mAChR | Human cerebral artery(38) | (38) | |||
| N-Cadherin | NCAD | Mouse cremaster arterioles(167) | (167) | |||
| Occludin | Ocln | Rat diaphragm arteriole(237) Rat aorta, mesenteric artery, renal vein, vena cava(238) |
(237, 238) | |||
| Plasminogen activator inhibitor 1 | PAI-1 | VCCC(189–191) | (189–191) | |||
| Platelet endothelial adhesion molecule 1 | PECAM-1 or CD31 | Mouse aorta(272) | HUVEC(271, 273, 274) | (271–274) | ||
| Plakoglobin | γ-catenin | Primary porcine AoEC(305) HDMEC(245) |
(245, 305) | |||
| Purinergic Receptor P2Y1 | P2Y1 | Rat sinus endothelium(114) | (114) | |||
| Purinergic Receptor P2Y6 | P2Y6 | Rat sinus endothelium(114) | (114) | |||
| Sarco/endoplasmic reticulum Ca2+-ATPase | SERCA | Mouse thoracodorsal artery(185) | (185) | |||
| S-endo-1-associated antigen | CD146 | HUVEC(274) | (274) | |||
| Single chain glycoprotein 1 | CD99 | HUVEC(273) | (273) | |||
| Small conductance calcium-activated potassium channel 3 | SK3 | Rat cremaster arteriole(297) Rat mesenteric artery(159) |
(297) | (297) | (159) | |
| Sodium-coupled neutral amino acid transporter 3 | Snat3 | Mouse abdominal aorta(342) | (342) | (342) | ||
| Sphingosine-1- phosphate receptor | S1P | Rat brain capillary endothelium(343) | (343) | (343) | ||
| Thrombomodulin | TM | HUVEC(118, 344) | (118, 344) | (344) | ||
| Transient receptor potential channel subfamily C member 1 | TRPC1 | Human mesenteric artery(345) | HPAEC(346) | (345) | (346) | |
| Transient receptor potential channel subfamily C member 4 | TRPC4 | HMVEC(347) | (347) | (347) | ||
| Transient receptor potential channel subfamily V member 4 | TRPV4 | Mouse 3rd order mesenteric artery(131) Mouse carotid artery(348) Rat cremaster arteriole(294) |
(348) | (131, 294) | ||
| Vascular endothelial cadherin | VE-Cadherin | Mouse carotid artery(249) Mouse diaphragm capillaries(249) Mouse bladder capillaries(249) |
HUVEC(249, 273, 274) | (249, 273, 274) |
Abbreviations: Bovine aortic endothelial cells (BAoEC), Bovine coronary artery endothelial cells (BCAEC), Brain capillary endothelial cells (BCEC), Embryonic stem cells (ESC), Human aortic endothelial cells (HAoEC), Human brain microvascular endothelial cells (HBMEC), Human coronary artery endothelial cells (HCAEC), Human dermal microvascular endothelial cells (HDMEC), Human microvascular endothelial cells (HMVEC), Human pulmonary artery endothelial cells (HPAEC), Human umbilical vein endothelial cells (HUVEC), Vascular cell coculture (VCCC)
The glycocalyx is also instrumental in mechanotransduction. Its presence on the luminal surface regulates endothelial function by relaying changes in shear stress.(26–29) A number of studies have investigated the role of the glycocalyx in nitric oxide (NO) production following exposure to shear stress. Specifically, when the glycocalyx is experimentally degraded, shear stress-induced nitric oxide production is reduced, thereby defining the glycocalyx as a regulator of this process.(26–29) This glycocalyx mediated mechanotransduction may be physiologically more relevant for arterial endothelial cells, which experience higher shear stress compared to the venous endothelium.(30–33) In contrast, glycocalyx shedding may be more physiologically relevant in postcapillary venules than arterioles. The glycocalyx sheds in response to tumor necrosis factor alpha (TNF-α)(34, 35), angiopoeitin-2,(36) and matrix metalloproteinases,(28) factors that are upregulated in cardiovascular disease and inflammation. This shedding helps expose the shorter leukocyte adhesion molecules that are also polarized to the luminal surface, such as P-selectin, in order to facilitate an inflammatory response, which canonically occurs in venules.(37)
Vasodilation/Vasoconstriction
A crucial physiological homeostatic role of endothelial cells is the regulation of peripheral resistance through modulating vasodilation and vasoconstriction pathways. Many vasoactive signaling molecules are expressed on the luminal face of the endothelium, and thus, one could imagine that vasoactive receptors would be preferentially expressed on the luminal face of the endothelium in direct contact with vasoactive ligands in the blood. However, the subcellular localization of many vasoactive receptors has not yet been described in the endothelium. In this section, we discuss luminal localization for the receptors of acetylcholine, bradykinin, angiotensin, and epinephrine.
Muscarinic M3 receptors enriched on the luminal face of the endothelium(38, 39) where the vasoactive neurotransmitter acetylcholine (Ach) can bind and induce vasodilatory signaling in the arterial wall. However, due to the presence of cholinesterases in the plasma, circulating Ach is unlikely to act in an endocrine/paracrine manner and significantly contribute to the activation of muscarinic M3 receptors; rather, it appears the major source of vasoactive Ach is generated from microvascular endothelial cells, which express choline acetyltransferase and can release Ach in an autocrine manner.(40–45) In resistance arteries, the elicited pathway is predominately endothelial derived hyperpolarization (EDH), rather than NO-based signaling.(46–49)
Endothelial cell expression of the bradykinin G-protein coupled receptor B2 is enriched on the luminal face of the endothelium.(50) Bradykinin is a vasoactive substance that binds and activates B2 to promote endothelial dependent dilation,(51–54) by inhibiting adenylyl cyclase, activating PLC, and increasing endothelial intracellular Ca2+, which ultimately causes the release of NO and prostaglandins.(55) Bradykinin binding to B2 can also activate EDH in resistance arteries, although to a lesser extent.(47, 52) In small arterioles, B2 is more highly expressed on SMCs rather than ECs, while larger arteries preferentially express B2 on ECs and not SMCs.(50, 56) This expression pattern aligns with well-described dominance of NO-based vasodilation in conduit arteries compared to resistance arteries.(46) While B2 receptors are widely expressed throughout the healthy endothelium,(50) B1 expression is limited to the endothelium of cerebral(57) and hepatic(58) microvessels. Its expression in homeostasis is minimal, however, it is induced following peripheral tissue injury,(59), in the presence of the proinflammatory cytokine interferon-gamma (IFN-λ), (57) or following infection of Plasmodium chabaudi. (58) The resultant activation increases endothelial calcium activity and promotes inflammatory response.
The renin-angiotensin-aldosterone system is a critical player in regulating blood pressure homeostasis and involve vascular cells. Endothelial cells express angiotensin converting enzyme (ACE) on their luminal surface where it can readily generate the potent vasoconstrictor angiotensin II from circulating angiotensin I.(60–63) ACE is also implicated in the degradation of the circulating factor bradykinin, a process that also favors vasoconstriction and is more highly expressed within arterioles compared to conduit arteries and veins in human samples.(64) However, the polarization of angiotensin II receptors within endothelial cells of resistance arteries is less clear. The receptors for angiotensin II, AT1R and AT2R, are expressed on endothelial cells,(65–67) but expression varies across species and vascular bed,(67) and in resistance vessels, the expression may be regulated by angiotensin II itself.(68) While AT1R is most highly expressed in homeostatic conditions, AT2R is highly expressed during development,(69) and its expression is induced by shear stress(69, 70) or following vascular injury.(71–73) Activation of endothelial AT1R and AT2R counteracts vasoconstriction responses elicited by AT1R activation on SMCs,(67, 74–76) but the endothelial receptors also have roles in proliferation(77) and apoptosis.(77, 78) Many of the functional studies on endothelial AT receptor function focus on conduit arteries, such as the carotid(74, 76) or aorta,(70, 72) while vessel AT receptor function is often studied in small resistance arteries.(79, 80)
Circulating angiotensin II also stimulates the release of endothelin-1,(81, 82) an endogenous 21 amino acid peptide that promotes vasoconstriction and is primarily produced by and stored within endothelial cells.(83–85) Its ETA receptor is found mainly on vascular smooth muscle cells, where binding of endothelin-1 induces the canonical vasoconstriction response; while its ETB receptor is mainly found on endothelial cells (and on VSMCs in large pulmonary arteries)(86) where it can promote nitric oxide and prostacyclin release.(87, 88) Distribution of these receptors has been studied in the specialized vasculature of kidney,(89) liver,(90, 91) and lung(92, 93) via immunohistochemistry methods that demonstrate localization within the endothelium.(94) Despite this, a subcellular electron microscopy study was unable to detect ETB receptors in human coronary endothelial cells;(95) thus, there is limited information about polarization of ETB within endothelial cells (i.e., luminal versus abluminal) or within the endothelium of peripheral resistance arteries.
Functional studies demonstrate that β-adrenergic receptors are expressed by the endothelium, in addition to their well-described role in SMCs. Denudation attenuates the vasodilatory response to increasing doses isoproterenol in both large(96) and small arteries, (97) indicating that endothelial cells respond to β-adrenergic stimuli. However, there is little data available about the localization of endothelial β-adrenergic receptors. The β2-adrenoceptor was reported to be predominantly expressed over the β1-adrenoceptor in the internal mammary artery by autoradiograhy using [125I]-cyanopinodolol binding sites when incubated with ICI 118,551 (β1) or CGP 20712A (β2).(98) However, his assay is insufficient to describe subcellular expression of the adrenoceptors in the endothelium. The β2-adrenoceptor has been reported to localize to the cell surface of rat lung microvascular endothelial cells in vitro under basal conditions and shown to internalize in response depletion of the GTPase Rab5a.(99)
Purinergic Signaling
Purinergic signaling in the arterial vasculature is best known for its vasoconstrictive properties, though it is also associated with vasodilatory signaling.(100) In the kidney, adenosine infusion reduces single nephron glomerular filtration rate due to afferent arteriole vasoconstriction, likely induced by α1-adrenergic receptor activation.(101) Similarly, ATP can be released by perivascular nerves is generally thought to induce α-adrenergic receptor-mediated smooth muscle constriction,(102) though coronary arteries (250–500um diameter) isolated from lamb have been reported to dilate in response to ATP.(103) Recent work from our lab suggests that endothelial ATP, released through pannexin 1 (Panx1) channels, regulates cerebral myogenic tone. Endothelial-specific deletion of Panx1 exhibited diminished myogenic tone while smooth muscle-specific deletion of the channel had no effect on the generation of vessel tone.(104) However, in the systemic vasculature, endothelial purinergic signaling facilitates vasodilation. In cultured endothelial cells, the alpha subunit of ATP synthase localizes to the apical surface,(105) which likely facilitates ATP release by ECs in into the vessel lumen.(106–109) ATP can bind to purinergic receptors on the apical membrane, including P2Y1 and P2Y2.(110, 111), (112) Based on the autocrine signaling pathway and luminal release of ATP, these purinergic receptors may also be localized on the luminal surface of the endothelium.(102, 113) However, immunohistochemistry on P2Y2 receptors in the intact endothelium of murine third order mesenteric arteries demonstrates a punctate-like distribution pattern, but it unclear if these are localized to the luminal face.(110) Furthermore, an electron microscopy study on splenic endothelial cells identified P2Y1 and P2Y6 receptors on the abluminal surface of endothelial cells.(114) Additionally, P2X4 receptors, which bind ATP and its derivatives, expressed more highly in the human saphenous vein compared to human mammary or radial artery.(115) P2X4 deficiency on endothelial cells leads to a reduction in NO production and an increase in blood pressure.(116) The subcellular localization of this receptor has not been described in ECs, and additional studies are needed to determine its polarization in resistance arteries.
Coagulation
A major endothelial function is balancing the injury repair mechanism of clotting to regulate the size of the repair. This requires the localization of anticoagulants to the site of clot formation. These anticoagulants primarily localize to the luminal face of the endothelium, and are able to bind more effectively in lower flow environments like the microcirculation.(117) For instance, thrombomodulin is expressed on the endothelial luminal surface of arteries, veins, and lymphatics(118) and binds directly to thrombin to prevent its interaction with circulating procoagulant factors.(119, 120) CD39, an ectonucleotidase of the E-NTDPase1 family, is localized to the luminal surface of the endothelium and reduces platelet aggregation through converting extracellular ATP to ADP.(121, 122) In pathogenic thrombosis, venous and arterial thrombi exhibit phenotypic differences, where venous thrombi contain more fibrin and arterial thrombi contain more platelets.(123) This suggests a mechanistic difference in the coagulation pathway across vessel type; however, it is likely due to differences in shear stress and flow rather than expression differences of coagulation mediators.
A summary of the polarized protein in luminal endothelium is demonstrated in Figure 1.
Abluminal Membrane
Protein localization on the abluminal face of endothelial cells facilitates cell adhesion and heterocellular communication between ECs and SMCs. Here, we will examine proteins that are polarized to the abluminal compartment of the endothelium and highlight their roles within resistance arteries and impact on whole animal physiology.
Internal Elastic Lamina
In immediate contact with the abluminal endothelial surface is an extracellular matrix termed the internal elastic lamina (IEL), which separates ECs from SMCS. The IEL of intact vessels, when viewed in cross section, is a wavy, dense layer situated between endothelium and smooth muscle.(124, 125) In conduit arteries, laminae are also present between smooth muscle layers.(46) When viewed en face, the IEL of resistance arteries, but not conduit arteries, has a high proportion of fenestrations in the matrix separating the endothelium and SMCs.(46, 124, 126) Although it can vary by vascular bed, the fenestrations generally have a circular shape where extracellular matrix proteins are absent.(46, 124, 126, 127) These fenestrations facilitate the heterocellular communication that is vital to regulating vasodilation and vasoconstriction pathways in resistance arteries, through allowing highly specific signaling microdomains to form called myoendothelial junctions.(46, 56, 124, 128–134) This layer also serves as an adhesion substrate for integrins that are localized to the endothelial abluminal surface, which participate in a number of important processes such as angiogenesis.(135–137)
The IEL layer consists of collagen IV, elastin, lamin-8/10, nidogen, and heparan sulfate proteoglycans;(138–142) however, the composition is not uniform across vascular bed or vessel size.(139, 141, 143, 144) For example, conduit arteries, often referred to as elastic arteries due to their high elastin content, function to absorb the force generated by the heart.(141, 144, 145) Elastin in resistance arteries, although not as prevalent,(144) still demonstrates important functionality in bearing longitudinal stress, rather than circumferential stress (as in conduits).(139, 140) In vitro work demonstrates secretion of other extracellular matrix proteins and suggests a collaborative effort between the endothelial and smooth muscle cells in forming the IEL.(138, 146) For example, endothelial derived extracellular matrix proteins include fibronectin(147) and elastin,(148–150) while collagen I and III are secreted from the smooth muscle cell layer and are regulated by endothelial nitric oxide.(151) In injury or inflammation, additional matrix proteins such as fibrinogen or fibronectin are secreted.(152, 153) However, it is unclear whether these proteins identified in vitro are deposited in the IEL of intact vessels, become part of the adventitia, or are released into the circulation. For example, collagen I is more prevalent in the adventitia rather than the IEL of cremaster arterioles.(139)
Myoendothelial Junctions
Myoendothelial junctions (MEJ), also referred to as myoendothelial projections or myoendothelial gap junctions, are an anatomical hallmark of resistance arteries. The junction, first identified in Rhodin’s electron microscopy ultrastructural analysis of small arteries,(154) is formed through a club-like endothelial projection through the aforementioned IEL fenestrations, in which ECs can directly contact and communicate with the overlaying SMC.(124, 125, 132, 155, 156) Although rare, instances of SMC projections through the IEL have also been reported.(157) The MEJs are considered signaling microdomains due to the localization of many signaling molecules involved in heterocellular communication; thus, a large portion of abluminal protein polarization occurs in these distinct regions. It is hypothesized that the unique protein localization that occurs at MEJs facilitate endothelial derived hyperpolarization (EDH), the dominant vasodilatory signaling mechanism in resistance arterioles.(46–49, 134) (158) Key players of EDH which localize to the MEJ include transient receptor potential vanilloid 4 (TRPV4), calcium-activated intermediate conductance potassium (IKCa) channels, and hemoglobin alpha (shown in Figure 2). TRPV4 channels facilitate calcium influx at the MEJ,(128, 131) leading to IKCa channel activation.(159) The efflux of potassium ions hyperpolarizes SMCs and this results in vasodilation.(160) Inward rectifying channels are also an essential mechanism whereby endothelial cells conduct hyperpolarization and thus, vasodilation in systemic and cerebral arteries.(161–163) While their expression has been implicated in the MEJ, their precise sub-cellular localization in the endothelium is not currently known. Understanding the localization of inwardly rectifying potassium channels could be helpful in deciphering it’s functional relationship to other ion channels or gap junctions.(164)
Figure 2. Abluminal protein localization in endothelium.
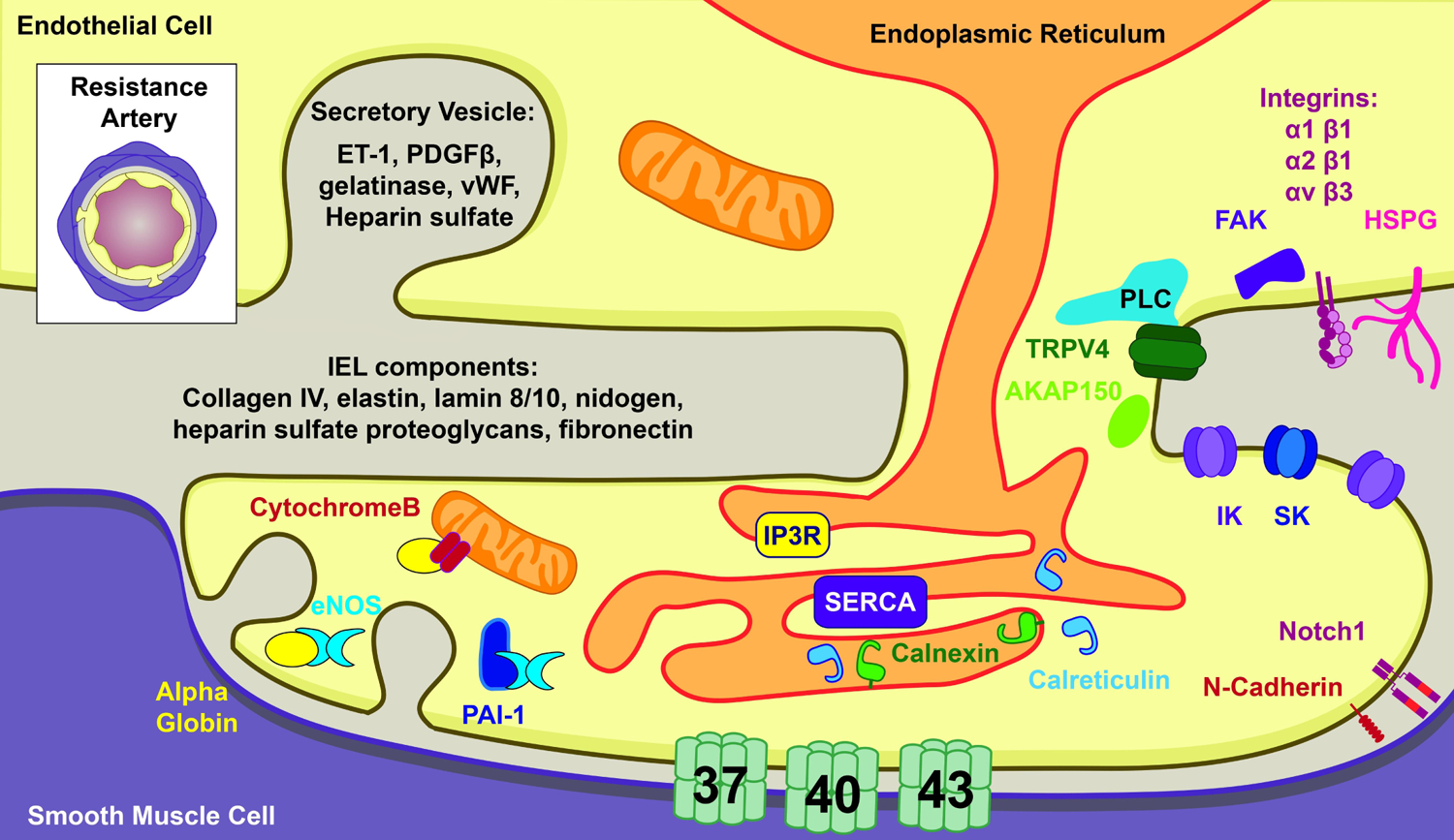
The luminal membrane of the endothelium is in direct contact with the extracellular matrix and sporadically the smooth muscle (but not larger arteries). Transverse view of resistance artery is depicted in the upper left for reference.
Specialized localization to the MEJ can alter how protein activation effects cellular physiology. Calcium-activated small conductance potassium SKCa channels have been observed at the MEJ, though they are primarily localized to interendothelial junctions.(159) The distribution of SKCa in endothelium may indicate the channels participation in distinct signaling pathways. The SKCa localized to the MEJ likely participates in EDH, (165) (166) whereas SKCa channels present at interendothelial junctions may be better positioned to activate eNOS localized in luminal caveolae or on the golgi and generate NO for luminal release. Independent calcium events are likely required to activate both pools of SKCa channels, further illustrating how localization can dictate function.
Connexins are enriched at the MEJ and also regulate vasodilation and vasoconstriction pathways. In small arterioles, connexin 37, 40, and 43 are most prevalent (Cx37, Cx40, Cx43, respectively),(159, 167) with connexin 40 exhibiting arterial specificity in mice.(168) Although connexins are enriched at the MEJ, they are also found at the interendothelial junction (Table 1). When assembled as hexamers, connexins form gap junctions that physically link the cytoplasm of two cells. Heterocellular communication occurs through gap junctions to facilitate EDH (169) and myoendothelial feedback.(132, 170, 171) Myoendothelial feedback is critical in regulating the degree of constriction elicited through α-adrenergic activation on SMCs. This feedback occurs when the inositol 1,4,5-trisphosphate (IP3) generated in VSMC diffuses through myoendothelial gap junctions, where it then activates vasodilatory signaling in ECs as negative feedback regulation.(132, 172–176) Myoendothelial feedback can also occur when calcium from smooth muscle cells transverses the gap junctions to induce vasodilatory signaling in endothelial cells.(132, 177) When Cx40 gap junctions are blocked by loading inhibitory antibodies, this negative regulation is lost, illustrating the functional importance of gap junctions within this microdomain.(169) The localization of gap junctions to the abluminal surface of the endothelium, and within the MEJ signaling microdomains, is essential in modulating vasodilatory signaling.(46)
While EDH-mediated vasodilation is dominant in resistance arteries, NO still contributes to vasodilatory signaling within these vessels.(124, 125, 178, 179) Since NO has such a short half-life,(180) enrichment of eNOS to myoendothelial junction allows for quick diffusion of NO to the VSMC membrane where it activates vasodilation. Our lab has shown that that eNOS activation at MEJ is differentially activated compared to eNOS elsewhere in the endothelium, creating two functionally distinct pools of eNOS (Table 1).(56) This is likely a result of eNOS-lipid interactions at the MEJ microdomain, and another example of how protein localization to microdomains impacts its function.(56) Regulators of eNOS, including hemoglobin alpha, CYB5R3, PAI-1, and caveolin-1 also localize to MEJs.(124, 125, 181–183) Hemoglobin alpha, a protein specifically expressed in the resistance artery endothelium,(125) is also enriched within the MEJ where it regulates endothelial nitric oxide synthase (eNOS),(124, 125) and may influence the dominance of EDH-based vasodilation in these vessels.(46) For an extensive review on the function of nitric oxide in endothelial cells please reference Leo et al. 2020.(184)
As discussed at length in this section, calcium signaling at the MEJ is crucial for regulating the extent of vasodilation and vasoconstriction. It follows that the endoplasmic reticulum (ER), a major source of intracellular calcium, is enriched within MEJ microdomains, as identified via electron microscopy in both hamster mesenteric arteries and mouse cremaster arterioles.(132, 156) Several studies have also demonstrated the presence of ER-associated proteins within these restricted areas.(130, 133, 185) Specifically, inositol trisphosphate receptor (IP3R) 1,(133) calreticulin,(56, 125, 130) SERCA,(185) and calnexin(186) have all been identified within IEL holes. Calreticulin, canonical marker of the ER lumen,(187) localizes to 80% of the IEL holes in resistance arteries, but influences heterocellular communication in an ER-independent manner.(130) Each ER marker appears to have its own level of occupancy within the IEL holes (not all studies cited include quantification).(130) A future study that quantifies each respective ER marker may help us better understand the role of ER localization at the MEJ. Recent work has illustrated that mitochondria are also present in the MEJ and may dicate the localization of calcium events in the MEJ.(188) The presence of these organelles to the abluminally situated signaling microdomain in MEJs is an example of spatial optimization to quickly initiate a vasodilation response downstream of ER calcium release.
The MEJ is a microdomain within resistance arteries where signaling occurs to regulate vasodilation and ultimately total peripheral resistance. In this section, we discussed the following proteins localized MEJ: TRPV4, IKCa, hemoglobin alpha, CYB5R3, caveolin-1, connexin 37, connexin 40, connexin 43, eNOS, inositol trisphosphate receptor 1, calreticulin, SERCA, and calnexin. In the next section, we also discuss a kinase anchoring proteins at the MEJ. Although not discussed at length here, it should be noted that a number of other proteins have also been identified at the MEJ: N-cadherin,(167) F-actin,(167, 189, 190) desmin,(167) plasminogen activator inhibitor 1 (PAI-1),(189–191) serpin binding protein-1 (SERBP1),(190) staufen,(190) nicotinamide phosphoribosyl transferase (NAMPT),(190) Notch 1,(191) Notch 2,(191) Notch 3,(191) and Jagged 1.(191) Although protein localization at the MEJ, and the resulting functional consequences, are well studied, only a few studies have examined their formation mechanism,(46, 189, 190) and much remains to be discovered regarding their temporal dynamics and spatial organization within the arterial wall.
A kinase anchoring proteins
A kinase anchoring proteins (AKAPs) primarily function as scaffolds to create microsignaling complexes within cells that allow for specific, intentional kinase activity while avoiding indiscriminate phosphorylation.(192) This protein family consists of 60 isoforms(193) and is defined by a 14- to 18- residue helix that interacts with protein kinase A (PKA);(194) however, AKAPs have been shown to bind to other enzymes such as protein kinase C (PKC).(195, 196) Within the resistance arteries, AKAPs regulate both vasodilation and vasoconstriction pathways. Murine AKAP150, also known as bovine AKAP5 or human AKAP75,(197) binds and localizes PKC to MEJs within the resistance artery wall; thus demonstrating abluminal localization.(128) This allows PKC-dependent activation of TRPV4 channels and promotes EDH-mediated vasodilation.(128) AKAP1 is expressed on the cytoplasmic face of mitochondria in endothelial cells rather than the MEJ,(198) and resistance arteries from global knockout mice exhibit reduced vasodilation to acetylcholine.(198) These studies demonstrate how AKAPs can regulate vasodilation pathways. While outside the scope of this review, it should be noted that AKAPs also form microsignaling complexes in vascular smooth muscle cells and regulate vasoconstriction.(199–201) Lastly, these anchoring proteins also localize to the interendothelial junction (Table 1) to modulate endothelial barrier function.(202, 203) Upon disrupting PKA’s interaction with AKAP12 or AKAP220, endothelial barrier function is reduced.(202) Further, when Akap12 is depleted from zebrafish embryos, interendothelial junction integrity is reduced resulting in hemorrhage.(203) For an extensive review on AKAPs in the cardiovascular system, please see the following reference.(201)
Integrins on the abluminal face
Integrins are membrane-associated glycoproteins composed of α- and β-subunits and they are most well studied in the context of angiogenesis, development and wound healing when their expression is upregulated.(136, 204, 205) This section of the review will focus on their function in healthy adult arteries wherein they regulate interendothelial adhesion and mechanotransduction.(135, 206) Integrins are capable of binding extracellular matrix components found in the IEL,(135, 136) suggesting localization to the abluminal face of the endothelial cells;(207) however, explicit localization studies of integrins in healthy adult endothelium are limited. While most integrin subunits are thought to be evenly distributed through the arterial and venous endothelium, integrin subunit β4 is expressed predominately in arterioles.(208) Primary human corneal endothelial cells have been shown to express 12 of the 18 α-subtypes (ITGA1, ITGA2, ITGA3, ITGA4, ITGA5, ITGA6, ITGA7, ITGA10, ITGA11, ITGAE, ITGAV, ITGAL) and six of the eight β-subtypes (ITGB1, ITGB3, ITGB4, ITGB5, ITGB7, ITGBL1).(209, 210) While integrin heterodimer protein expression and distribution in endothelial cells is not well understood in vivo, many studies have focused on the contributions of individual heterodimers. Integrins α2β1 and α1β1 facilitate endothelial binding to collagen IV, a major extracellular matrix protein in the IEL.(211) Fibrinogen and fibronectin have similarly been shown to bind αvβ3 and α5β1, respectively.(207, 211) These suggest a localization of these heterodimers to the abluminal face of the endothelium (shown in Fig. 1); however, integrins α2β1 and α5β1 have been described to preferentially localize to interendothelial junctions rather than the abluminal membrane.(211)
Focal adhesion complexes, where integrins convert mechanical stimuli to molecular pathways through outside-in signaling paradigms,(212) are also restricted to the abluminal cell-matrix interface. Focal adhesion kinase (FAK), a nonreceptor tyrosine kinase, is recruited to focal adhesions in ECs under flow conditions and facilitates integrin signaling cascades.(213, 214) The upregulation of fibronectin in the arterial extracellular matrix, which largely occurs during angiogenesis(137) and atherosclerosis,(215) is known to drive the formation of focal adhesion plaques that are enriched with integrins αvβ3 and α5β1.(216) Heparan sulfate proteoglycans also localize to the abluminal membrane (Table 1) and are implicated in focal adhesion formation.(217) While it is known that this interaction is necessary for mechanosensation(218) and directional migration,(219) the individual contribution of each integrin heterodimer had not been understood. Recent work using peptidomimetics designed to specifically and individually antagonize each heterodimer demonstrated that binding of extracellular substrates and subsequent activation of integrin α5β1 lead to rapid recruitment of αvβ3 at focal adhesions.(216) Substrate-induced focal adhesion formation and the resulting cell spreading each require the recruitment, but not activation, of integrin αvβ3.(216) This interaction demonstrates how the dynamic localization of endothelial integrins at the abluminal membrane can dictate cellular function.
Abluminal/luminal polarity of the endothelial secretome
Endothelial protein polarization goes beyond just cell surface expression; it also applies to protein and molecular secretion patterns. The endothelium comprises one of the largest secretory organs(220) and accordingly, it regulates the release of proteins and molecules to its luminal or abluminal face over its thickness of just 200nm (shown in Figure 2).(2, 221) Protein secretion has been studied in the context of varying environmental stimuli, including shear stress,(222, 223) proangiogenic stimuli(224, 225) or proinflammatory(224, 225) stimuli; however, the explicit emphasis on its polarization is more recent.(221) Most secreted factors (90%) from endothelial cells are released to the luminal surface,(221) and are often carried via extracellular vesicles.(221) These include such as TNF-α(221), IL-6(221), IGF-1(221), soluble ACE2(226), and ATP.(106, 107, 109) Extracellular matrix proteins comprise the majority of proteins secreted abluminally, but also included are endothelin-1, platelet derived growth factor-β, cell adhesion molecules, and calcium binding proteins.(221, 227) Surprisingly, extracellular matrix proteins can also be secreted luminally, although these likely represent regulators and turnover.(221) Other proteins and enzymes that are secreted in the abluminal direction include gelatinases,(147) tissue inhibitor of metalloproteinases,(147) and platelet derived growth factor.(221, 227) These secreted factors released abluminally may reach the smooth muscle or stay within the IEL, and the permeability of the IEL may in part their final destination.(228) A number of other factors are described to be secreted in both directions, exhibiting intentional activity in each direction, including Von Willebrand factor,(221, 223, 229) heparan sulfate,(230) and endothelin-1. (221, 231–233) Despite canonical differences in arterial and venous endothelium, the secretome polarization is largely comparable between the arterial and venous endothelial cell cultures.(221) Any secretome differences across vessel type are likely related to individual vessel function, although studies focusing on differential secretion are limited.(221, 223, 234)
In summary, the endothelial luminal face is primed to interact with cells in the blood through secretions or direct interactions and is exposed to hemodynamic forces; while the abluminal face is largely in contact with the IEL, though endothelial cells can come into direct contact with SMC at MEJs in arterioles. Both the luminal and abluminal faces of the endothelium have been specialized by and for the microenvironment they exist in and facilitate directed signaling and function.
A summary of our discussion on endothelial abluminal protein polarization is in Figure 2.
Holding us all together: Interendothelial Junctions
Interendothelial junctions connect adjacent endothelial cells and broadly consist of adherens junctions, tight junctions, and gap junctions.(235, 236) The planar polarity of protein localization to interendothelial junctions allows the endothelium to be an active and selective manager in maintaining circulatory volume and directing stimuli-induced permeability. Thus, the proteins localized to interendothelial junctions are an example of the functional importance of protein polarization in endothelium.
Tight junctions are composed of claudins, occludins, and junctional adhesion molecules (JAMs), (shown in Figure 3) and they are highly organized in arteries compared to veins (e.g., Figure 4).(237),(238) Tight junctions tend to form more towards the luminal surface compared to the adherens junctions, which tend to seal endothelial borders toward the abluminal face.(236) More apical localization allows tight junctions to tightly regulate paracellular transport, and an enrichment of these junctions in cerebral arteries in part explains the strong blood brain barrier.(239) In small arterioles, tight junctions are continuous and highly organized, while small venules have discontinuous tight junctions, consisting of short segments that can be arranged either perpendicularly or parallel to the interendothelial junction, leading to gaps between ECs.(237) This description of venule tight junctions is similar to the organization of adherens junctions described in human umbilical venous endothelial cells under static culture conditions.(240) Lastly, in capillaries, these junctions are also loosely organized, and often times the capillary endothelium is fenestrated, where endothelial cells are not continuously connected via interendothelial junctions.(237, 238) The differential organization of tight junctions across vessel type reflects their respective roles in fluid and solute transport; however, it should be noted that several other factors contribute to vessel permeability including glycocalyx composition and inflammatory cytokines.(241)
Figure 3. Interendothelial junction protein localization in endothelium.
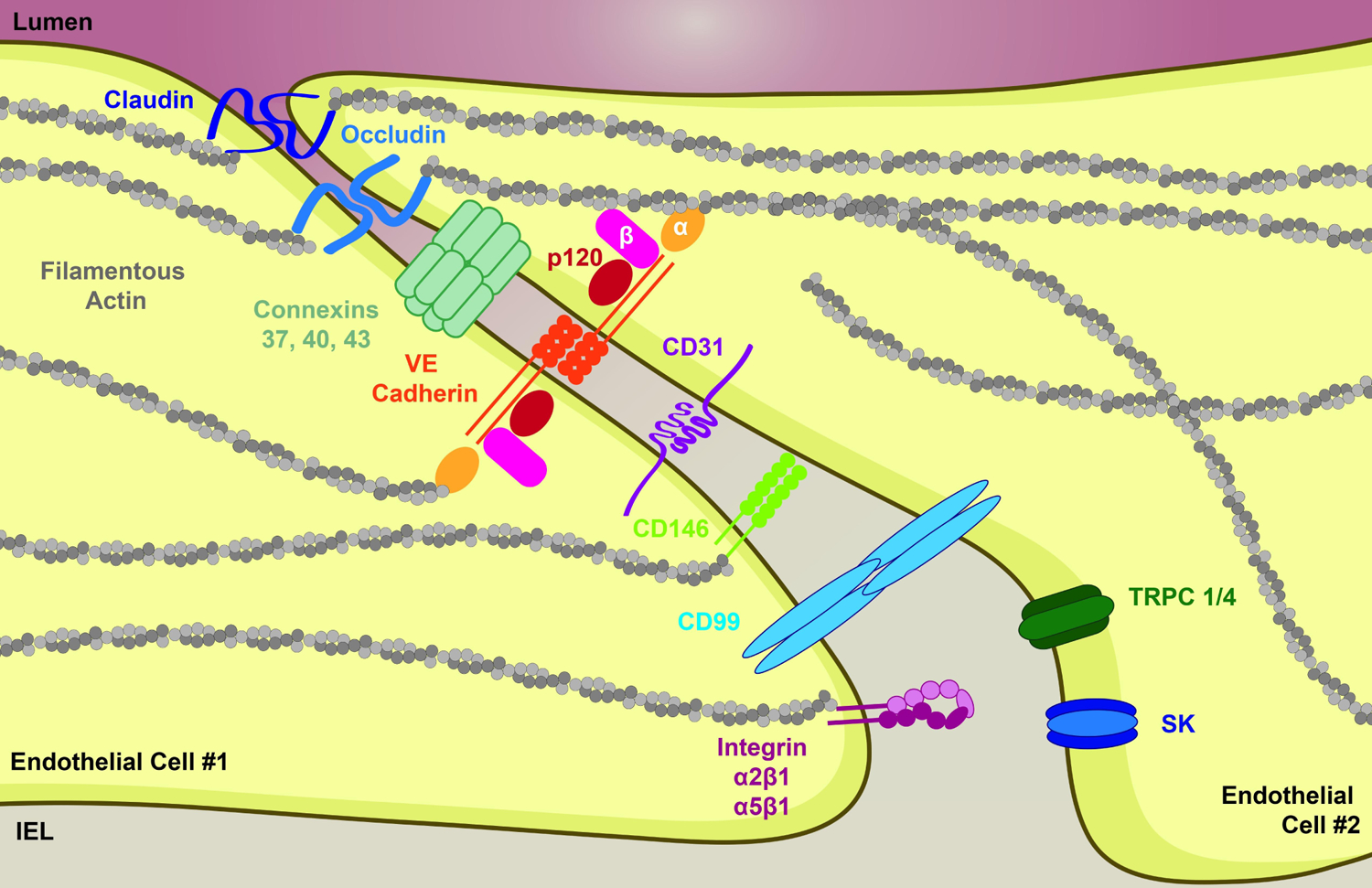
The apposition of endothelium is a key area of protein localization
Figure 4. Polarization of endothelium in response to different flow environments.
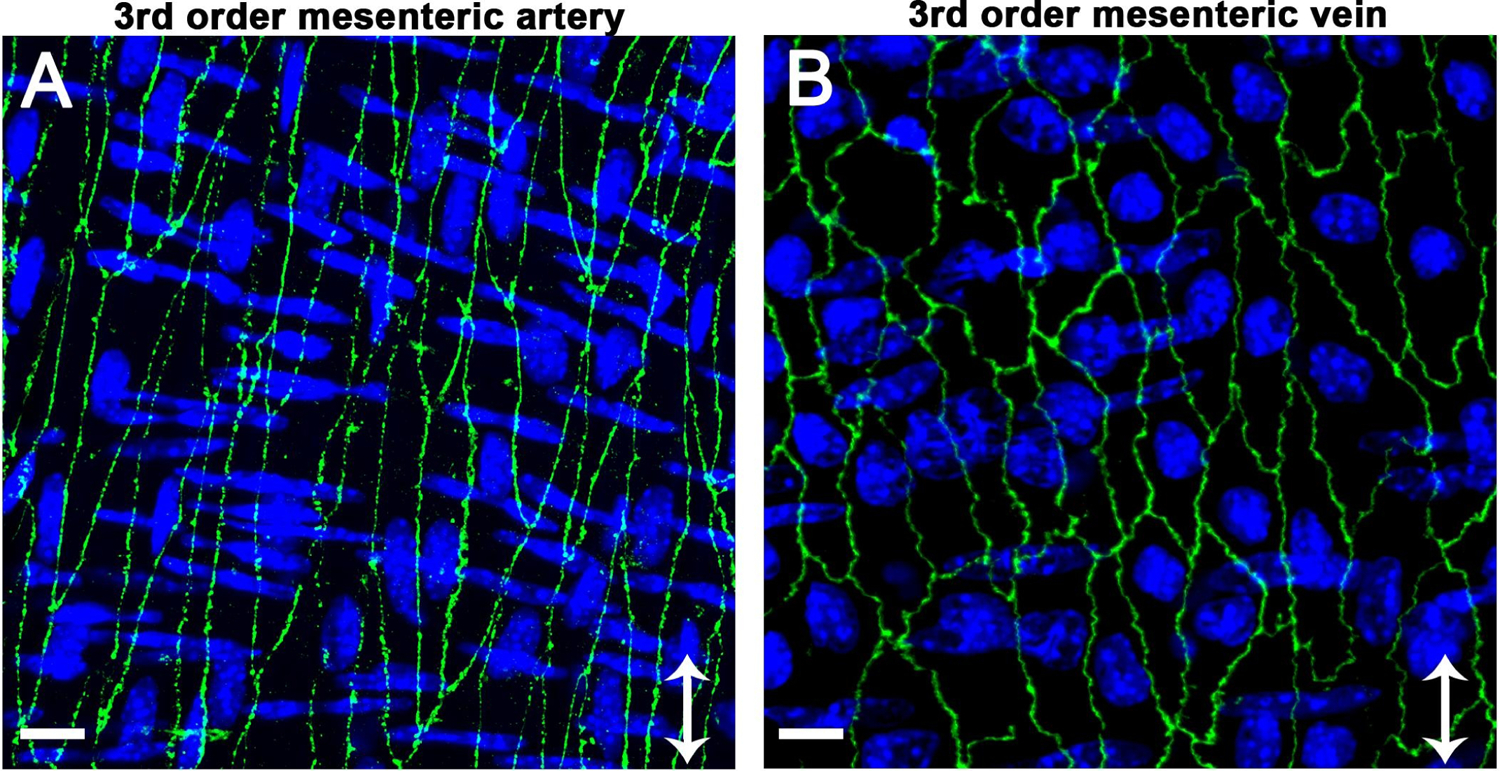
Claudin-5 (green, Thermofisher 34–1600) is used to demonstrate differences in polarization of interendothelial proteins to lateral edges of the cells in a murine third order mesenteric artery with high flow (A) compared with a murine third order mesenteric vein with low flow (B). The images also highlight endothelial morphological differences in arteries versus veins: arterial endothelial cells are elongated in the direction of flow while venous endothelial cells are more rectangular in shape and resemble cultured cells. For each image, arrow indicates direction of flow, scale bar is 10μm, and nuclei are shown in blue, with endothelial nuclei oriented vertically and SMC nuclei oriented horizontally. Images were obtained with a Zeiss 880 LSM Airyscan module (40x oil objective) and are shown as Z-projections.
Adherens junctions also localize to the endothelial cell-cell junctions and can actually direct the organization of tight junctions.(236),(242) The main protein in adherens junctions is cadherin, specifically vascular endothelial (VE)-cadherin in endothelial cells, which is ultimately connected to the actin cytoskeleton through scaffolding proteins such as α-catenin, α-actinin, β-catenin, p120, and plakoglobin (shown in Figure 3).(243–246) While N-cadherin expression is similar to VE-cadherin in endothelial cells, it does not demonstrate polarization to the interendothelial junction.(247, 248) There are no reported differences in the general assembly of adherens junctions between vessel types; however, an important arteriovenous difference is the phosphorylation state of VE-cadherin.(249) In vivo, arterial endothelial cells do not have phosphorylated VE-cadherin, whereas capillary and venous endothelial cells have constitutively phosphorylated VE-cadherin, making their ECs more susceptible to bradykinin-induced permeability.(249) This phosphorylation is regulated by low shear stress which reflects the physiological levels reported for veins.(32, 249, 250) Furthermore, vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) is more enriched within arterial endothelial cells(251, 252) and binds to VE-cadherin.(253) However, to our knowledge, there are no studies that directly demonstrate polarization of VE-PTP to the interendothelial junction with immunohistochemical or electron microscopy experiments. The polarization of VE-cadherin and the associated scaffolding proteins to the interendothelial junction allows the endothelium to dynamically respond to stimuli, such as vascular endothelial growth factor (VEGF).(254, 255)
In addition to tight and adherens junctions, gap junctions demonstrate polarized expression where they have been described to alternate with tight junctions.(237, 256) Gap junctions are formed when two connexons (hexamers of connexin proteins) come together to join the cytoplasm of two neighboring cells. Endothelial connexins that localize to the interendothelial junction include Cx37,(159, 257) Cx40,(168, 258–261) and Cx43.(262) In resistance arteries, Cx43, Cx40, and Cx37 demonstrate localization to the interendothelial junction and MEJs (Table 1).(159, 167, 263) However, connexins are differentially expressed across the vasculature and can vary across species.(257, 264–267) In hamster arterioles, Cx43 is present at the interendothelial junction,(262) while Cx43 is generally not detectable in aortic murine endothelium, except for areas of disturbed flow.(257, 266, 267) In mice, Cx40 is specific for endothelial cells in arteries and not present in smooth muscle cells,(168, 258–260) and regulates vasodilation.(259, 260, 268) At the ultrastructural level, gap junctions have not been detected in small venules;(237) however, valves in small venules demonstrate specific interendothelial expression of Cx37 and Cx43 via immunohistochemistry.(269) These connexins are also differentially expressed throughout the venous vasculature.(269) While outside the scope of this review, it is worth noting that venular valves are an interesting vascular hallmark that exhibits specialized protein localization.(269) The intentional spatial localization of gap junctions within resistance arteries (Table 1) allows for controlled and directed communication within the vascular wall, which is another example of how protein polarization is imperative in maintaining homeostasis. For an extensive review on vascular connexins, we suggest the following reference.(270)
Numerous other proteins also exhibit polarized expression along endothelial cell-cell contacts outside of the well-established tight, adherens, and gap junctions. A great example is platelet endothelial adhesion molecule 1 (PECAM-1, also known as CD31), which localizes to these interendothelial junctions, independent of tight or adherens junctions, and is required for leukocyte transmigration.(271, 272) Similarly, CD99 is also localized to the lateral edges of the endothelial cells and facilitates monocyte transmigration.(273) S-endo-1-associated antigen, or CD146, is expressed at interendothelial junctions and regulates paracellular permeability; however, it is also expressed on the apical membrane of cultured endothelial cells (Table 1).(274) In addition to their abluminal polarization, integrins, specifically α2β1 and α5β1 heterodimers, are highly expressed at the interendothelial junction (Table 1) and regulate endothelial barrier function.(211, 275) Lastly, Akap9 may localize with integrins at the interendothelial junctions to mediate vascular permeability.(276)
Figure 3 summarizes our discussion above.
Other realms of the endothelium
While the localization of many endothelial proteins can be characterized as luminal, abluminal or interendothelial, others are dynamic or occur at multiple sites in the cell. Caveolae create signaling microdomains through invaginations in the membrane which are integral for vasoactive signaling and normal lipid transport. These membrane invaginations have been observed on both faces of the endothelium. Similarly, distinct endothelial calcium signals can occur at ER-plasma membrane contacts and at the MEJ, with very different physiological effects. Lastly, many proteins exhibit variable localization patterns which are dictated by the rate and direction of blood flow.
Caveolae: the Microdomain-Maker
Lipid rafts help facilitate microdomain formation. A subset of lipid rafts is caveolae, which are found in numerous cell types, including epithelial cells, adipocytes, fibroblasts, vascular SMC, and endothelial cells.(277) In endothelial cells, caveolae are enriched within the MEJ,(278) although they also demonstrate luminal localization (Table 1). These membrane microvesicles are uniquely coated with one of the three members of the caveolin protein family, caveolin-1, −2, or −3.(279) Numerous proteins have been shown to localize to endothelial caveolae including several already discussed within this review: bradykinin receptor B2,(280) muscarinic M3 receptor,(281) P2Y1,(114) and eNOS.(282)
Caveolae are required for normal transcytosis of lipoproteins into endothelial cells. In an ApoE−/− background, global deletion of caveolin-1 reduced LDL infiltration of the aortic wall, while EC-specific re-expression of caveolin-1 rescued LDL (283) In human microvascular endothelial cells in culture, immunofluorescent staining has shown that caveolin-1 colocalizes with ALK-1, which has also be implicated in LDL transcytosis.(284) The class B, type I scavenger receptor, SR-BI, a well-described HDL receptor, has been shown to cofractionate and preferentially colocalize with caveolin-1 in CHO cells,(285) further suggesting that caveolae are integral to lipoprotein transport in many cell types, though this observation has yet to be confirmed in endothelial cells.
Proteins that are enriched within the MEJ have been shown to interact with Cav1, such as TRPV4 channels,(286, 287) IP3-Rs,(278) and Cx43.(278) Further, Cav1 may localize AKAP150 to the MEJ.(128) The localization of Cav1 to MEJ may have important functional consequences as Cav1 depletion demonstrates impaired vasodilation.(278, 286, 287) Cav1 negatively regulates eNOS at the MEJ, thereby facilitating two functionally distinct pools of eNOS within the endothelium.(56) Its association with eNOS physically prevents the binding of calmodulin, thereby preventing eNOS activation and nitric oxide production.(282) This interaction may contribute to the dominance of EDH in small arteries, and is discussed more in the following section.(278)
Spot the Spark: Mapping calcium signaling microdomains
As a second messenger with a role in many signaling cascades, the response triggered by a calcium event is determined in part by its subcellular localization. Endothelial calcium events occur in spatially-restricted microdomains, such as caveolae on the plasma membrane and the MEJ. Intracellular calcium signaling is of particular importance due to its role regulating endothelial generation of vasoactive factors either through the production of soluble factors (NO, COX) or through membrane hyperpolarization. This section will focus on the localization of different calcium signaling microdomains, however we recommend the recent review by Ottolini et al(288) for discussion of calcium dynamics in the vasculature.
IP3R-mediated release of stored calcium
Sites of stored calcium release must also be precisely regulated in the endothelium. Inositol 1,4,5-trisphosphate receptors (IP3R) are a family of ER calcium channels which in mammals consists of three subtypes (IP3R1, IP3R2, IP3R3); all of which are expressed in the endothelium.(289–291) IP3R is a tetrameric calcium channel with six transmembrane domains that facilitates stored-calcium release from the ER into the cytosol in response to IP3 synthesis. While IP3R1 has been demonstrated to localize to the ER within MEJ projections in vivo,(132, 133, 292) it exhibits diffuse staining throughout the ER of endothelial cells in culture.(132) However, IP3R2 and IP3R3 are localized in regions of the ER outside of the MEJ.(133, 290) The importance of endothelial IP3R subtypes in systemic blood pressure regulation has been studied in vivo with conflicting results. Deletion of IP3R1 under the Tie2-cre, which is expressed in all endothelial cells and hematopoietic cells throughout development, was initially described to reduced acetylcholine-induced vasodilation and increase basal blood pressure.(293) Deletion of the same subtype under an inducible, endothelial cell-specific Cre recombinase (platelet derived growth factor receptor β (PDGFRβ) exhibited normal vascular reactivity and systemic blood pressure. However, when all three subtypes were genetically deleted in endothelial cells, the mice exhibited reduced plasma NO, vasodilation in response to acetylcholine, and eNOS phosphorylation which all contributed to systemic hypertension.(291) Interestingly, attempts to reproduce the previously reported findings of IP3R1 deletion under the Tie2:Cre(293) were unsuccessful.(291) Taken together, this suggests functional redundancy between the isoforms of IP3R despite their localization differences.
IP3R-mediated calcium events in the endothelium contribute to the generation of myogenic tone. Calcium influx can be triggered by low intraluminal pressure via TRPV4 in the MEJ. This promotes the activation of IP3R, which in turn activates IKCa. (294) Thus, at low intraluminal pressure, hyperpolarization of the EC and SMC membrane reduces myogenic tone, likely acting to stabilize local blood flow in the tissue.
Endothelial barrier function is regulated in part by IP3R clustering within the ER membrane. Clustering amplifies IP3R-mediated Ca2+ events as Ca2+ released following activation of one channel promotes activation of other IP3Rs within close proximity.(295) In human lung microvascular endothelial cells, IP3R clustering via microtubule interactions is required for α-thrombin-induced adhesion disassembly.(296)
SK3, TRPV4 and Caveolae
Caveolae microdomains on the plasma membrane are integral for vasodilation via endothelial-derived hyperpolarization (EDH). EDH involves potassium efflux through calcium-sensitive small and intermediate conductance potassium channels (SKCa and IKCa, respectively). Recent work suggests that TRPV4 channels facilitate local calcium influx required to stimulate SKCa/IKCa activity.(128, 278, 286, 287, 294, 297) TRPV4 is largely localized to the cell periphery,(297) though its expression has also been noted in the MEJ.(128, 294) SK3 exhibits a homogenous distribution on luminal and abluminal membranes.(297) However, both TRPV4 and SK3 are enriched on caveolae, which illustrates a role for caveolae in constructing the microdomain.(278, 286, 287, 297) Here, Cav1 acts as a scaffolding protein, generating 50–100nm membrane invaginations that localize calcium signaling events. In fact, EDH is diminished in endothelium of global Cav1 knockouts but can be rescued by reexpression of Cav1 only in endothelial cells,(298) indicating that TRPV4 is unable to activate SK3 activity without a means to restrict their localization on the membrane. Interestingly, the peripheral localization of TRPV4, which does not colocalize with SK3, appears to be dependent on SK3 expression, as TRPV4 distribution at the periphery is lost in mesenteric arteries collected from endothelial-specific SK3−/− mice.(297) The sum of the data suggests an important role for caveolae microdomains in the recruitment of TRPV4 and SK channels to facilitate efficient stimulation of SK activity and EDH.
Go with the flow: Shear stress regulates planar cell polarity in endothelial cells
Planar cell polarity describes the collective alignment of cell polarity along one axis of a tissue. Early works contended whether endothelial cells exhibited planar cell polarity.(30, 250) The work was confounded by observed differences between arteriolar and venous vascular beds, concluding that endothelial cells polarize due to their position in relation to the heart. Today, we understand that shear stress, the frictional force generated by blood flow, orients endothelial planar cell polarity. Due to the morphology of vascular tissue, the planar cell polarity of the endothelium can also be referenced as axial polarity, in which the longitudinal axis of the vessel can be further segmented into upstream and downstream regions.
The vascular endothelium exhibits a high degree of phenotypic plasticity that is determined in part by shear stress acting as a biomechanical cue. The different types and rates of flow in various vascular beds determines how shear stress regulates endothelial morphology. Large conduit arteries must contend with the volume of blood rapidly ejected from each ventricular contraction of the heart. Thus, the endothelium of conduit arteries is exposed to pulsatile flow patterns at a relatively high speed (~10 dynes/cm2). The elasticity of the vessel wall of conduit arteries helps to convert pulsatile flow in large arteries to continuous laminar flow in smaller resistance arteries. Arterioles and capillaries experience the highest degree of shear stress, at ~50 and 40 dynes/cm2, respectively. This decreases dramatically at the venules (~15 dynes/cm2), with the lowest values of shear stress occurring in the vena cava (~1 dynes/cm2).(299, 300)
This differential shear stress alters the morphology of endothelial cells and can orchestrate planar polarity. Arterial endothelial cells are narrow and elongated in the direction of flow, whereas venous endothelial cells are generally shorter and wider (e.g., with claudin-5 shown in Figure 4). In arteries, the endothelial microtubule organizing center (MTOC) is localized downstream of the nucleus.(30, 31) Large veins exhibit MTOC localization upstream of the nucleus.(30, 31) Similar polarization patterns have been reported in localization of the perinuclear golgi apparatus, which is localized upstream of bovine endothelial cells grown under laminar shear stress in culture.(301) Polarization of the golgi upstream of nuclei has also been observed in live zebrafish embryos using a fluorescent reporter for in endothelial cells, Tg(fli1a:B4GALT1-mCherry). The golgi was localized upstream of endothelial nuclei in the dorsal aorta and branching arteries.(302) In contrast, in the posterior cardinal vein, where shear stress is much lower, endothelial cells do not exhibit golgi polarization.(302)
Upon the initiation of shear stress conditions in vitro, the endothelium undergoes rapid morphological changes as the cells elongate and orient in the direction of flow. Cytoskeletal alterations include MTOC reorganization as well as the formation of actin stress fibers that also align with flow.(31, 303–305) In addition, cell-cell junctional complexes undergo partial disassembly and reorganization. VE-cadherin, α-catenin, and β-catenin form adherens plaques at the end of actin stress fibers that become stabilized by plakoglobin recruitment over the next 48 hours.(305) The reorganization of the cytoskeleton and adherens junctions likely stabilize cell-cell contact and endothelial barrier integrity. Integrins on the abluminal membrane also become activated following exposure to shear stress,(306, 307) which is thought to promote MTOC reorganization.
Mechanosensors of blood flow
Shear forces must be transduced into downstream signaling cascades via endothelial mechanosensors. Understanding and identifying such mechanosensors can help us better understand how planar polarity is regulated. These mechanosensors of shear stress would need to be localized on the luminal membrane such that they receive direct input from the vessel’s lumen. While the identity of the predominate endothelial mechanosensor is not precisely understood, many proteins and organelles have been proposed for the role, including mechanically-gated channels,(308, 309) mechanosensitive receptors,(272, 310) GPCRs,(311–313) mechanosensitive enzymes,(314) primary cilia,(315, 316) the glycocalyx,(317) and the nucleus itself.(318) It is possible that a master regulator of endothelial mechanosensation does not exist and each of the proposed sensors act in concert to confer the morphological and regulatory changes occurring downstream of flow. Thorough reviews of proposed mechanosensors are available for further reading,(319, 320) however this review will highlight mechanosensation via the cation channel Piezo1; the complex composed of PECAM1, VEGFR2/3, and VE-cadherin; and the contributions of the transcription factor yes-associated protein (YAP).
Piezo proteins are mechanically-activated cation channels. Expression of Piezo1 is sufficient to enable HEK293T cells, which are normally unresponsive to shear stress, to exhibit shear stress-induced calcium influx.(308) Piezo1 is expressed in endothelial cells as early as embryonic age 9.5 (E9.5).(321) Endothelial Piezo1 expression is vital as deficiency of Piezo1 is embryonically lethal in both global(308, 321) and endothelial-cell-specific(308) knockouts due to growth retardation and defects in vascular development. This illustrates the essential role of endothelial mechanosensing during vascular development and further demonstrates the importance of Piezo1 activity at this early stage in development. Furthermore, loss of Piezo1 expression was found to reduce shear-evoked calcium influx in endothelial cells isolated from knockout mice(308) as well as cultured cells undergoing RNAi.(322) Piezo1 expression is also required for the morphological changes that endothelial cells undergo in response to shear stress.(308, 321) At the tissue level, this causes endothelial cells in haploinsufficient arteries to fail to align in the direction of flow.(308) While canonically studied for its role in development, the use of an inducible, endothelial-cell specific Piezo1 knockout mouse enabled investigations into the role of Piezo1 in adult endothelial tissue. Flow-induced vasodilation was impaired in mesenteric resistance arteries from mice deficient in endothelial Piezo1, indicating a mechanosensory role in vessel function.(322) While the signaling mechanism downstream of shear-induced Piezo1 activation has yet to be elucidated, the GPCR Gαq/11, and the purinergic receptor P2Y2, and the ATP-release channel Panx1 have all been implicated in regulating flow-mediated dilation.(322) Lastly, the localization of Piezo1 on endothelial cells, while assumed to be present on the luminal surface, still needs to be explicitly studied via immunohistochemistry or electron microscopy experiments.
Endothelial mechanosensors are present within interendothelial junctions. PECAM-1, VEGFR2/3 and VE-cadherin form a mechanosensory complex at interendothelial junctions which is necessary and sufficient for shear-induced integrin activation.(272) PECAM-1 has also been shown to directly transmit mechanical force. Mechanical force transduction experiments using magnetic beads coated in antibody against PECAM-1 were used to demonstrate that PECAM-1 sustains piconewtons of force under shear stress(323) and to show that direct force applied to PECAM-1 is sufficient to activate many shear-induced signaling pathways.(272, 324) In fact, tyrosine phosphorylation of PECAM-1 by Fyn tyrosine kinase is the first cellular response to the onset of shear stress on endothelial cells.(325) Despite its essential role in endothelial mechanosensing, PECAM-1-null mice are healthy and fertile,(326) though not without an impaired shear-induced vasodilatory response.(327) The mild phenotype associated with the PECAM-1 knockout suggests that redundant mechanisms may exist. While previously thought to act simply as an adapter protein in the complex, VE-cadherin has recently been shown to have mechanosensitive properties.(328) Although, it is not yet understood whether mechanosensing through VE-cadherin occurs in vivo. Interestingly, VE-cadherin expression is required for mechanosensation by PECAM-1.(272) Furthermore, the transmembrane domain of VE-cadherin facilitates ligand-independent activation of VEGFR2/3.(310) Activation of VEGFR2/3 initiates the activation of multiple shear-activated signaling pathways including AKT kinase,(272, 310) PI3K,(272) eNOS(272) and integrins,(272, 306) though there is no evidence that VEGFR2 acts as a primary mechanosensor.(329)
The transcriptional co-activator YAP has also been implicated in endothelial mechanosensation. Its canonical role in the Hippo pathway has been well described. Briefly, activated Yap translocates from the cytoplasm into the nucleus where it can interact with TEA domains family of proteins to regulate transcription until it becomes phosphorylated and exported from the nucleus. However, recent work supports a mechanosensing role for YAP in the endothelium. Firstly, YAP-deficient mice exhibit vascular defects in the yolk sac, similar to those observed in the Piezo1 knockout mice.(330) In addition, mechanical perturbations can control the nuclear shuttling of YAP though interactions with F-actin,(331) suggesting that the mechanosensory role that YAP confers is to alter transcription in response to shear stress. Transgenic zebrafish have been used to observe dynamic YAP localization in endothelium, which demonstrated that in vivo shear stress promotes nuclear localization of YAP.(332) Further research is required to determine how mechanical stimuli facilitate the activation of endothelial YAP. The YAP-mediated transcriptomic alterations may promote the maintenance of polarized morphology.
Planar cell polarity adds another dimension to the intricate spatial distribution of the endothelium. The circulation provides a dynamic directional stimulus which is sensed by mechanosensors and transduced to second messengers, phosphorylation cascades, and gene expression differences. Altogether, planar cell polarity facilitates precise spatial regulation in the endothelium. Much of the literature on endothelial mechanosensation and planar cell polarity has focused on large arteries due to the impact of laminar versus disturbed flow in the progression of atherosclerosis. As such, there is an unfortunate dearth of evidence for mechanosensation in the endothelium of small resistance arteries. However, GPR68, a class A rhodopsin-like GPCR, was recently identified in an siRNA screen as a direct mechanosensor and was shown to be expressed exclusively in the endothelium of small diameter arteries in the mesentery, pancreas, liver and bladder. Furthermore, flow-mediated dilation was diminished in the GPR68 knockout mice and murine primary microvascular endothelial cells were unable to generate calcium transients in response to laminar flow following treatment with siRNA.(333) This work may stimulate further investigation into mechanosensation and planar cell polarity in the endothelium of small arteries.
Conclusion
Despite their squamous nature, endothelial cells still demonstrate protein localization and polarity that is crucial for their various physiological functions. As reviewed here, endothelial cells express anticoagulants, receptors, and enzymes among the glycocalyx on their luminal surface to respond to circulating factors. In general, these luminally polarized proteins are found in both arteries and veins and expression differences are not well studied. In contrast, the abluminal protein localization in resistance arterioles is absent from the venous endothelium. Along this surface are myoendothelial junctions, which are endothelial extensions that make heterocellular contact with smooth muscle cells and exhibit specific protein localization. Ultimately, the proteins at the MEJ function to regulate the vasodilatory signals and tightly control total peripheral resistance. Within the MEJ are even smaller microdomain pockets, termed caveolae, which also house factors crucial to vasodilatory pathways. Although caveolae are not unique to endothelial cells, they are enriched within the MEJ in the arteriolar endothelium. Luminal and abluminal polarization go beyond surface expression and is also characteristic of endothelial secretions. Endothelial planar polarity includes interendothelial junctions, where tight and adherens junctions create a selective barrier to the underlying layers of the arterial wall. Gap junctions present at endothelial cell-endothelial cell borders allow the endothelium to function as a coordinated monolayer. Mechanosensory complexes are also polarized along this axis. Their distribution is patterned along the direction of blood flow to communicate cellular signals downstream of shear stress. Overall, endothelial polarity in both the luminal-abluminal and planar axes is critical for arteriolar endothelial cells that are constantly responding to their extracellular environment. Future studies should examine the trafficking of luminal and abluminal proteins in the endothelium, which are likely differentially regulated and may offer novel therapeutic targets for hypertension, diabetes and hyperlipidemia.
Funding Sources
This work was funded by NHLBI 1F31HL149221–01 (AGW), NHLBI 1F31HL149228–01 (CAR), and NIH HL088554 (BEI).
Footnotes
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Florey. The endothelial cell. Br Med J. 1966;2(5512):487–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff JR, Chao TI. Cytoarchitectonics of non-neuronal cells in the central nervous system. Advances in molecular and cell biology. 2003;31:1–51. [Google Scholar]
- 3.Haraldsen G, Kvale D, Lien B, Farstad IN, Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J Immunol. 1996;156(7):2558–65. [PubMed] [Google Scholar]
- 4.Wong D, Dorovini-Zis K. Regualtion by cytokines and lipopolysaccharide of E-selectin expression by human brain microvessel endothelial cells in primary culture. J Neuropathol Exp Neurol. 1996;55(2):225–35. [DOI] [PubMed] [Google Scholar]
- 5.Cornford EM, Hyman S. Localization of brain endothelial luminal and abluminal transporters with immunogold electron microscopy. NeuroRx. 2005;2(1):27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Haaren PM, VanBavel E, Vink H, Spaan JA. Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am J Physiol Heart Circ Physiol. 2003;285(6):H2848–56. [DOI] [PubMed] [Google Scholar]
- 7.van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003;92(6):592–4. [DOI] [PubMed] [Google Scholar]
- 8.Ihrcke NS, Wrenshall LE, Lindman BJ, Platt JL. Role of heparan sulfate in immune system-blood vessel interactions. Immunol Today. 1993;14(10):500–5. [DOI] [PubMed] [Google Scholar]
- 9.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011;3(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HE C Heparin-binding proteins. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 11.Shimada K, Kobayashi M, Kimura S, Nishinaga M, Takeuchi K, Ozawa T. Anticoagulant heparin-like glycosaminoglycans on endothelial cell surface. Jpn Circ J. 1991;55(10):1016–21. [DOI] [PubMed] [Google Scholar]
- 12.Parker KA, Tollefsen DM. The protease specificity of heparin cofactor II. Inhibition of thrombin generated during coagulation. J Biol Chem. 1985;260(6):3501–5. [PubMed] [Google Scholar]
- 13.Kato H Regulation of functions of vascular wall cells by tissue factor pathway inhibitor: basic and clinical aspects. Arterioscler Thromb Vasc Biol. 2002;22(4):539–48. [DOI] [PubMed] [Google Scholar]
- 14.Atha DH, Lormeau JC, Petitou M, Rosenberg RD, Choay J. Contribution of monosaccharide residues in heparin binding to antithrombin III. Biochemistry. 1985;24(23):6723–9. [DOI] [PubMed] [Google Scholar]
- 15.Lindahl U, Backstrom G, Thunberg L, Leder IG. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc Natl Acad Sci U S A. 1980;77(11):6551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosier PD, Krishnasamy C, Kellogg GE, Desai UR. On the specificity of heparin/heparan sulfate binding to proteins. Anion-binding sites on antithrombin and thrombin are fundamentally different. PLoS One. 2012;7(11):e48632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plotnikov AN, Schlessinger J, Hubbard SR, Mohammadi M. Structural basis for FGF receptor dimerization and activation. Cell. 1999;98(5):641–50. [DOI] [PubMed] [Google Scholar]
- 18.Rider CC. Heparin/heparan sulphate binding in the TGF-beta cytokine superfamily. Biochem Soc Trans. 2006;34(Pt 3):458–60. [DOI] [PubMed] [Google Scholar]
- 19.Feyzi E, Lustig F, Fager G, Spillmann D, Lindahl U, Salmivirta M. Characterization of heparin and heparan sulfate domains binding to the long splice variant of platelet-derived growth factor A chain. J Biol Chem. 1997;272(9):5518–24. [DOI] [PubMed] [Google Scholar]
- 20.Lyon M, Deakin JA, Mizuno K, Nakamura T, Gallagher JT. Interaction of hepatocyte growth factor with heparan sulfate. Elucidation of the major heparan sulfate structural determinants. J Biol Chem. 1994;269(15):11216–23. [PubMed] [Google Scholar]
- 21.Robinson CJ, Mulloy B, Gallagher JT, Stringer SE. VEGF165-binding sites within heparan sulfate encompass two highly sulfated domains and can be liberated by K5 lyase. J Biol Chem. 2006;281(3):1731–40. [DOI] [PubMed] [Google Scholar]
- 22.Burgess WH, Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. [DOI] [PubMed] [Google Scholar]
- 23.Ornitz DM, Yayon A, Flanagan JG, Svahn CM, Levi E, Leder P. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992;12(1):240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spivak-Kroizman T, Lemmon MA, Dikic I, Ladbury JE, Pinchasi D, Huang J, et al. Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell. 1994;79(6):1015–24. [DOI] [PubMed] [Google Scholar]
- 25.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454(3):345–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93(10):e136–42. [DOI] [PubMed] [Google Scholar]
- 27.Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun. 2007;355(1):228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper S, Emmott A, McDonald KK, Campeau MA, Leask RL. Increased MMP activity in curved geometries disrupts the endothelial cell glycocalyx creating a proinflammatory environment. PLoS One. 2018;13(8):e0202526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecker M, Mulsch A, Bassenge E, Busse R. Vasoconstriction and increased flow: two principal mechanisms of shear stress-dependent endothelial autacoid release. Am J Physiol. 1993;265(3 Pt 2):H828–33. [DOI] [PubMed] [Google Scholar]
- 30.Rogers KA, McKee NH, Kalnins VI. Preferential orientation of centrioles toward the heart in endothelial cells of major blood vessels is reestablished after reversal of a segment. Proc Natl Acad Sci U S A. 1985;82(10):3272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCue S, Dajnowiec D, Xu F, Zhang M, Jackson MR, Langille BL. Shear stress regulates forward and reverse planar cell polarity of vascular endothelium in vivo and in vitro. Circ Res. 2006;98(7):939–46. [DOI] [PubMed] [Google Scholar]
- 32.Papaioannou TG, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hellenic J Cardiol. 2005;46(1):9–15. [PubMed] [Google Scholar]
- 33.Kamiya A, Bukhari R, Togawa T. Adaptive regulation of wall shear stress optimizing vascular tree function. Bull Math Biol. 1984;46(1):127–37. [DOI] [PubMed] [Google Scholar]
- 34.Chappell D, Hofmann-Kiefer K, Jacob M, Rehm M, Briegel J, Welsch U, et al. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol. 2009;104(1):78–89. [DOI] [PubMed] [Google Scholar]
- 35.Henry CB, Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000;279(6):H2815–23. [DOI] [PubMed] [Google Scholar]
- 36.Lukasz A, Hillgruber C, Oberleithner H, Kusche-Vihrog K, Pavenstadt H, Rovas A, et al. Endothelial glycocalyx breakdown is mediated by angiopoietin-2. Cardiovasc Res. 2017;113(6):671–80. [DOI] [PubMed] [Google Scholar]
- 37.Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol. 2003;23(9):1541–7. [DOI] [PubMed] [Google Scholar]
- 38.Tsukahara T, Kassell NF, Hongo K, Vollmer DG, Ogawa H. Muscarinic cholinergic receptors on the endothelium of human cerebral arteries. J Cereb Blood Flow Metab. 1989;9(6):748–53. [DOI] [PubMed] [Google Scholar]
- 39.Rivers RJ, Duling BR. Arteriolar endothelial cell barrier separates two populations of muscarinic receptors. Am J Physiol. 1992;262(4 Pt 2):H1311–5. [DOI] [PubMed] [Google Scholar]
- 40.Bruning TA, Hendriks MG, Chang PC, Kuypers EA, van Zwieten PA. In vivo characterization of vasodilating muscarinic-receptor subtypes in humans. Circ Res. 1994;74(5):912–9. [DOI] [PubMed] [Google Scholar]
- 41.Milner P, Ralevic V, Hopwood AM, Feher E, Lincoln J, Kirkpatrick KA, et al. Ultrastructural localisation of substance P and choline acetyltransferase in endothelial cells of rat coronary artery and release of substance P and acetylcholine during hypoxia. Experientia. 1989;45(2):121–5. [DOI] [PubMed] [Google Scholar]
- 42.Kirkpatrick CJ, Bittinger F, Nozadze K, Wessler I. Expression and function of the non-neuronal cholinergic system in endothelial cells. Life Sci. 2003;72(18–19):2111–6. [DOI] [PubMed] [Google Scholar]
- 43.Zou Q, Leung SW, Vanhoutte PM. Transient Receptor Potential Channel Opening Releases Endogenous Acetylcholine, which Contributes to Endothelium-Dependent Relaxation Induced by Mild Hypothermia in Spontaneously Hypertensive Rat but Not Wistar-Kyoto Rat Arteries. J Pharmacol Exp Ther. 2015;354(2):121–30. [DOI] [PubMed] [Google Scholar]
- 44.Wilson C, Lee MD, McCarron JG. Acetylcholine released by endothelial cells facilitates flow-mediated dilatation. J Physiol. 2016;594(24):7267–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parnavelas JG, Kelly W, Burnstock G. Ultrastructural localization of choline acetyltransferase in vascular endothelial cells in rat brain. Nature. 1985;316(6030):724–5. [DOI] [PubMed] [Google Scholar]
- 46.Shu X, Ruddiman CA, Keller TCSt, Keller AS, Yang Y, Good ME, et al. Heterocellular Contact Can Dictate Arterial Function. Circ Res. 2019;124(10):1473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kemp BK, Cocks TM. Evidence that mechanisms dependent and independent of nitric oxide mediate endothelium-dependent relaxation to bradykinin in human small resistance-like coronary arteries. Br J Pharmacol. 1997;120(5):757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396(6708):269–72. [DOI] [PubMed] [Google Scholar]
- 49.Garland CJ, Dora KA. EDH: endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol (Oxf). 2017;219(1):152–61. [DOI] [PubMed] [Google Scholar]
- 50.Figueroa CD, Marchant A, Novoa U, Forstermann U, Jarnagin K, Scholkens B, et al. Differential Distribution of Bradykinin B(2) Receptors in the Rat and Human Cardiovascular System. Hypertension. 2001;37(1):110–20. [DOI] [PubMed] [Google Scholar]
- 51.Baydoun AR, Woodward B. Effects of bradykinin in the rat isolated perfused heart: role of kinin receptors and endothelium-derived relaxing factor. Br J Pharmacol. 1991;103(3):1829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohlmann P, Martinez MC, Schneider F, Stoclet JC, Andriantsitohaina R. Characterization of endothelium-derived relaxing factors released by bradykinin in human resistance arteries. Br J Pharmacol. 1997;121(4):657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schini VB, Boulanger C, Regoli D, Vanhoutte PM. Bradykinin stimulates the production of cyclic GMP via activation of B2 kinin receptors in cultured porcine aortic endothelial cells. J Pharmacol Exp Ther. 1990;252(2):581–5. [PubMed] [Google Scholar]
- 54.Pascoal IF, Umans JG. Effect of pregnancy on mechanisms of relaxation in human omental microvessels. Hypertension. 1996;28(2):183–7. [DOI] [PubMed] [Google Scholar]
- 55.Jimenez AH, Tanner MA, Caldwell WM, Myers PR. Effects of oxygen tension on flow-induced vasodilation in porcine coronary resistance arterioles. Microvasc Res. 1996;51(3):365–77. [DOI] [PubMed] [Google Scholar]
- 56.Biwer LA, Taddeo EP, Kenwood BM, Hoehn KL, Straub AC, Isakson BE. Two functionally distinct pools of eNOS in endothelium are facilitated by myoendothelial junction lipid composition. Biochim Biophys Acta. 2016;1861(7):671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prat A, Biernacki K, Pouly S, Nalbantoglu J, Couture R, Antel JP. Kinin B1 receptor expression and function on human brain endothelial cells. J Neuropathol Exp Neurol. 2000;59(10):896–906. [DOI] [PubMed] [Google Scholar]
- 58.Ventura PDS, Carvalho CPF, Barros NMT, Martins-Silva L, Dantas EO, Martinez C, et al. Malaria infection promotes a selective expression of kinin receptors in murine liver. Malar J. 2019;18(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57(1):27–77. [DOI] [PubMed] [Google Scholar]
- 60.Ng KK, Vane JR. Fate of angiotensin I in the circulation. Nature. 1968;218(5137):144–50. [DOI] [PubMed] [Google Scholar]
- 61.Caldwell PR, Seegal BC, Hsu KC, Das M, Soffer RL. Angiotensin-converting enzyme: vascular endothelial localization. Science. 1976;191(4231):1050–1. [DOI] [PubMed] [Google Scholar]
- 62.Ryan US, Ryan JW, Whitaker C, Chiu A. Localization of angiotensin converting enzyme (kininase II). II. Immunocytochemistry and immunofluorescence. Tissue Cell. 1976;8(1):125–45. [DOI] [PubMed] [Google Scholar]
- 63.Fukuhara M, Geary RL, Diz DI, Gallagher PE, Wilson JA, Glazier SS, et al. Angiotensin-converting enzyme expression in human carotid artery atherosclerosis. Hypertension. 2000;35(1 Pt 2):353–9. [DOI] [PubMed] [Google Scholar]
- 64.Metzger R, Franke FE, Bohle RM, Alhenc-Gelas F, Danilov SM. Heterogeneous distribution of angiotensin I-converting enzyme (CD143) in the human and rat vascular systems: vessel, organ and species specificity. Microvasc Res. 2011;81(2):206–15. [DOI] [PubMed] [Google Scholar]
- 65.Nora EH, Munzenmaier DH, Hansen-Smith FM, Lombard JH, Greene AS. Localization of the ANG II type 2 receptor in the microcirculation of skeletal muscle. Am J Physiol. 1998;275(4 Pt 2):H1395–403. [DOI] [PubMed] [Google Scholar]
- 66.Philogene MC, Bagnasco S, Kraus ES, Montgomery RA, Dragun D, Leffell MS, et al. Anti-Angiotensin II Type 1 Receptor and Anti-Endothelial Cell Antibodies: A Cross-Sectional Analysis of Pathological Findings in Allograft Biopsies. Transplantation. 2017;101(3):608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pueyo ME, Michel JB. Angiotensin II receptors in endothelial cells. Gen Pharmacol. 1997;29(5):691–6. [DOI] [PubMed] [Google Scholar]
- 68.Gunther S, Gimbrone MA Jr., Alexander RW. Regulation by angiotensin II of its receptors in resistance blood vessels. Nature. 1980;287(5779):230–2. [DOI] [PubMed] [Google Scholar]
- 69.Pries AR, Kuebler WM. Normal endothelium. Handb Exp Pharmacol. 2006(176 Pt 1):1–40. [DOI] [PubMed] [Google Scholar]
- 70.Ramkhelawon B, Vilar J, Rivas D, Mees B, de Crom R, Tedgui A, et al. Shear stress regulates angiotensin type 1 receptor expression in endothelial cells. Circ Res. 2009;105(9):869–75. [DOI] [PubMed] [Google Scholar]
- 71.Wu JN, Edwards D, Berecek KH. Changes in renal angiotensin II receptors in spontaneously hypertensive rats by early treatment with the angiotensin-converting enzyme inhibitor captopril. Hypertension. 1994;23(6 Pt 2):819–22. [DOI] [PubMed] [Google Scholar]
- 72.Wiemer G, Scholkens BA, Wagner A, Heitsch H, Linz W. The possible role of angiotensin II subtype AT2 receptors in endothelial cells and isolated ischemic rat hearts. J Hypertens Suppl. 1993;11(5):S234–5. [PubMed] [Google Scholar]
- 73.Nio Y, Matsubara H, Murasawa S, Kanasaki M, Inada M. Regulation of gene transcription of angiotensin II receptor subtypes in myocardial infarction. J Clin Invest. 1995;95(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pueyo ME, Arnal JF, Rami J, Michel JB. Angiotensin II stimulates the production of NO and peroxynitrite in endothelial cells. Am J Physiol. 1998;274(1):C214–20. [DOI] [PubMed] [Google Scholar]
- 75.Bayraktutan U, Ulker S. Effects of angiotensin II on nitric oxide generation in proliferating and quiescent rat coronary microvascular endothelial cells. Hypertens Res. 2003;26(9):749–57. [DOI] [PubMed] [Google Scholar]
- 76.Boulanger CM, Caputo L, Levy BI. Endothelial AT1-mediated release of nitric oxide decreases angiotensin II contractions in rat carotid artery. Hypertension. 1995;26(5):752–7. [DOI] [PubMed] [Google Scholar]
- 77.Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 1995;95(2):651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dimmeler S, Rippmann V, Weiland U, Haendeler J, Zeiher AM. Angiotensin II induces apoptosis of human endothelial cells. Protective effect of nitric oxide. Circ Res. 1997;81(6):970–6. [DOI] [PubMed] [Google Scholar]
- 79.Yadav VR, Nayeem MA, Tilley SL, Mustafa SJ. Angiotensin II stimulation alters vasomotor response to adenosine in mouse mesenteric artery: role for A1 and A2B adenosine receptors. Br J Pharmacol. 2015;172(20):4959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gunther S, Gimbrone MA Jr., Alexander RW. Identification and characterization of the high affinity vascular angiotensin II receptor in rat mesenteric artery. Circ Res. 1980;47(2):278–86. [DOI] [PubMed] [Google Scholar]
- 81.Emori T, Hirata Y, Ohta K, Kanno K, Eguchi S, Imai T, et al. Cellular mechanism of endothelin-1 release by angiotensin and vasopressin. Hypertension. 1991;18(2):165–70. [DOI] [PubMed] [Google Scholar]
- 82.Ferri C, Desideri G, Baldoncini R, Bellini C, Valenti M, Santucci A, et al. Angiotensin II increases the release of endothelin-1 from human cultured endothelial cells but does not regulate its circulating levels. Clin Sci (Lond). 1999;96(3):261–70. [PubMed] [Google Scholar]
- 83.Gillespie MN, Owasoyo JO, McMurtry IF, O’Brien RF. Sustained coronary vasoconstriction provoked by a peptidergic substance released from endothelial cells in culture. J Pharmacol Exp Ther. 1986;236(2):339–43. [PubMed] [Google Scholar]
- 84.Hickey KA, Rubanyi G, Paul RJ, Highsmith RF. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am J Physiol. 1985;248(5 Pt 1):C550–6. [DOI] [PubMed] [Google Scholar]
- 85.Loesch A Localisation of endothelin-1 and its receptors in vascular tissue as seen at the electron microscopic level. Curr Vasc Pharmacol. 2005;3(4):381–92. [DOI] [PubMed] [Google Scholar]
- 86.Seo B, Oemar BS, Siebenmann R, von Segesser L, Luscher TF. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation. 1994;89(3):1203–8. [DOI] [PubMed] [Google Scholar]
- 87.Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, et al. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest. 1993;91(4):1367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Warner TD, Mitchell JA, de Nucci G, Vane JR. Endothelin-1 and endothelin-3 release EDRF from isolated perfused arterial vessels of the rat and rabbit. J Cardiovasc Pharmacol. 1989;13 Suppl 5:S85–8; discussion S102. [DOI] [PubMed] [Google Scholar]
- 89.Wendel M, Knels L, Kummer W, Koch T. Distribution of endothelin receptor subtypes ETA and ETB in the rat kidney. J Histochem Cytochem. 2006;54(11):1193–203. [DOI] [PubMed] [Google Scholar]
- 90.Yokomori H, Oda M, Yasogawa Y, Nishi Y, Ogi M, Takahashi M, et al. Enhanced expression of endothelin B receptor at protein and gene levels in human cirrhotic liver. Am J Pathol. 2001;159(4):1353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yokomori H, Oda M, Ogi M, Kamegaya Y, Tsukada N, Nakamura M, et al. Enhanced expression of endothelin receptor subtypes in cirrhotic rat liver. Liver. 2001;21(2):114–22. [DOI] [PubMed] [Google Scholar]
- 92.Davie N, Haleen SJ, Upton PD, Polak JM, Yacoub MH, Morrell NW, et al. ET(A) and ET(B) receptors modulate the proliferation of human pulmonary artery smooth muscle cells. Am J Respir Crit Care Med. 2002;165(3):398–405. [DOI] [PubMed] [Google Scholar]
- 93.Soma S, Takahashi H, Muramatsu M, Oka M, Fukuchi Y. Localization and distribution of endothelin receptor subtypes in pulmonary vasculature of normal and hypoxia-exposed rats. Am J Respir Cell Mol Biol. 1999;20(4):620–30. [DOI] [PubMed] [Google Scholar]
- 94.Filep JG, Battistini B, Cote YP, Beaudoin AR, Sirois P. Endothelin-1 induces prostacyclin release from bovine aortic endothelial cells. Biochem Biophys Res Commun. 1991;177(1):171–6. [DOI] [PubMed] [Google Scholar]
- 95.Russell FD, Skepper JN, Davenport AP. Detection of endothelin receptors in human coronary artery vascular smooth muscle cells but not endothelial cells by using electron microscope autoradiography. J Cardiovasc Pharmacol. 1997;29(6):820–6. [DOI] [PubMed] [Google Scholar]
- 96.Iranami H, Hatano Y, Tsukiyama Y, Maeda H, Mizumoto K. A beta-adrenoceptor agonist evokes a nitric oxide-cGMP relaxation mechanism modulated by adenylyl cyclase in rat aorta. Halothane does not inhibit this mechanism. Anesthesiology. 1996;85(5):1129–38. [DOI] [PubMed] [Google Scholar]
- 97.Briones AM, Daly CJ, Jimenez-Altayo F, Martinez-Revelles S, Gonzalez JM, McGrath JC, et al. Direct demonstration of beta1- and evidence against beta2- and beta3-adrenoceptors, in smooth muscle cells of rat small mesenteric arteries. Br J Pharmacol. 2005;146(5):679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Molenaar P, Malta E, Jones CR, Buxton BF, Summers RJ. Autoradiographic localization and function of beta-adrenoceptors on the human internal mammary artery and saphenous vein. Br J Pharmacol. 1988;95(1):225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang J, Sun H, Zhang J, Hu M, Wang J, Wu G, et al. Regulation of β-adrenergic receptor trafficking and lung microvascular endothelial cell permeability by Rab5 GTPase. Int J Biol Sci. 2015;11(8):868–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Burnstock G Dual control of vascular tone and remodelling by ATP released from nerves and endothelial cells. Pharmacol Rep. 2008;60(1):12–20. [PubMed] [Google Scholar]
- 101.Haas JA, Osswald H. Adenosine induced fall in glomerular capillary pressure. Effect of ureteral obstruction and aortic constriction in the Munich-Wistar rat kidney. Naunyn Schmiedebergs Arch Pharmacol. 1981;317(1):86–9. [DOI] [PubMed] [Google Scholar]
- 102.Lohman AW, Billaud M, Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res. 2012;95(3):269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simonsen U, García-Sacristán A, Prieto D. Involvement of ATP in the non-adrenergic non-cholinergic inhibitory neurotransmission of lamb isolated coronary small arteries. Br J Pharmacol. 1997;120(3):411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Good ME, Eucker SA, Li J, Bacon HM, Lang SM, Butcher JT, et al. Endothelial cell Pannexin1 modulates severity of ischemic stroke by regulating cerebral inflammation and myogenic tone. JCI Insight. 2018;3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, et al. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci U S A. 2001;98(12):6656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bodin P, Burnstock G. ATP-stimulated release of ATP by human endothelial cells. J Cardiovasc Pharmacol. 1996;27(6):872–5. [DOI] [PubMed] [Google Scholar]
- 107.Bodin P, Bailey D, Burnstock G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol. 1991;103(1):1203–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bodin P, Burnstock G. Synergistic effect of acute hypoxia on flow-induced release of ATP from cultured endothelial cells. Experientia. 1995;51(3):256–9. [DOI] [PubMed] [Google Scholar]
- 109.Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS One. 2008;3(7):e2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee MD, Wilson C, Saunter CD, Kennedy C, Girkin JM, McCarron JG. Spatially structured cell populations process multiple sensory signals in parallel in intact vascular endothelium. Sci Signal. 2018;11(561). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moccia F, Baruffi S, Spaggiari S, Coltrini D, Berra-Romani R, Signorelli S, et al. P2y1 and P2y2 receptor-operated Ca2+ signals in primary cultures of cardiac microvascular endothelial cells. Microvasc Res. 2001;61(3):240–52. [DOI] [PubMed] [Google Scholar]
- 112.Raqeeb A, Sheng J, Ao N, Braun AP. Purinergic P2Y2 receptors mediate rapid Ca(2+) mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium. 2011;49(4):240–8. [DOI] [PubMed] [Google Scholar]
- 113.Schwiebert LM, Rice WC, Kudlow BA, Taylor AL, Schwiebert EM. Extracellular ATP signaling and P2X nucleotide receptors in monolayers of primary human vascular endothelial cells. Am J Physiol Cell Physiol. 2002;282(2):C289–301. [DOI] [PubMed] [Google Scholar]
- 114.Uehara K, Uehara A. P2Y1, P2Y6, and P2Y12 receptors in rat splenic sinus endothelial cells: an immunohistochemical and ultrastructural study. Histochem Cell Biol. 2011;136(5):557–67. [DOI] [PubMed] [Google Scholar]
- 115.Ray FR, Huang W, Slater M, Barden JA. Purinergic receptor distribution in endothelial cells in blood vessels: a basis for selection of coronary artery grafts. Atherosclerosis. 2002;162(1):55–61. [DOI] [PubMed] [Google Scholar]
- 116.Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12(1):133–7. [DOI] [PubMed] [Google Scholar]
- 117.dela Paz NG, D’Amore PA. Arterial versus venous endothelial cells. Cell Tissue Res. 2009;335(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maruyama I, Bell CE, Majerus PW. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J Cell Biol. 1985;101(2):363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Light DR, Glaser CB, Betts M, Blasko E, Campbell E, Clarke JH, et al. The interaction of thrombomodulin with Ca2+. Eur J Biochem. 1999;262(2):522–33. [DOI] [PubMed] [Google Scholar]
- 120.Adams TE, Huntington JA. Thrombin-cofactor interactions: structural insights into regulatory mechanisms. Arterioscler Thromb Vasc Biol. 2006;26(8):1738–45. [DOI] [PubMed] [Google Scholar]
- 121.Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Pinsky DJ, Sesti C, et al. Heterologous cell-cell interactions: thromboregulation, cerebroprotection and cardioprotection by CD39 (NTPDase-1). J Thromb Haemost. 2003;1(12):2497–509. [DOI] [PubMed] [Google Scholar]
- 122.Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2(2):409–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Straub AC, Butcher JT, Billaud M, Mutchler SM, Artamonov MV, Nguyen AT, et al. Hemoglobin alpha/eNOS coupling at myoendothelial junctions is required for nitric oxide scavenging during vasoconstriction. Arterioscler Thromb Vasc Biol. 2014;34(12):2594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, et al. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature. 2012;491(7424):473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sandow SL, Gzik DJ, Lee RM. Arterial internal elastic lamina holes: relationship to function? J Anat. 2009;214(2):258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wong LC, Langille BL. Developmental remodeling of the internal elastic lamina of rabbit arteries: effect of blood flow. Circ Res. 1996;78(5):799–805. [DOI] [PubMed] [Google Scholar]
- 128.Sonkusare SK, Dalsgaard T, Bonev AD, Hill-Eubanks DC, Kotlikoff MI, Scott JD, et al. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci Signal. 2014;7(333):ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ottolini M, Hong K, Sonkusare SK. Calcium signals that determine vascular resistance. Wiley Interdiscip Rev Syst Biol Med. 2019:e1448. [DOI] [PMC free article] [PubMed]
- 130.Biwer LA, Good ME, Hong K, Patel RK, Agrawal N, Looft-Wilson R, et al. Non-Endoplasmic Reticulum-Based Calr (Calreticulin) Can Coordinate Heterocellular Calcium Signaling and Vascular Function. Arterioscler Thromb Vasc Biol. 2018;38(1):120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hong K, Cope EL, DeLalio LJ, Marziano C, Isakson BE, Sonkusare SK. TRPV4 (Transient Receptor Potential Vanilloid 4) Channel-Dependent Negative Feedback Mechanism Regulates Gq Protein-Coupled Receptor-Induced Vasoconstriction. Arterioscler Thromb Vasc Biol. 2018;38(3):542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res. 2007;100(2):246–54. [DOI] [PubMed] [Google Scholar]
- 133.Isakson BE. Localized expression of an Ins(1,4,5)P3 receptor at the myoendothelial junction selectively regulates heterocellular Ca2+ communication. J Cell Sci. 2008;121(Pt 21):3664–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, et al. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res. 2003;40(5):480–90. [DOI] [PubMed] [Google Scholar]
- 135.Di Russo J, Luik AL, Yousif L, Budny S, Oberleithner H, Hofschroer V, et al. Endothelial basement membrane laminin 511 is essential for shear stress response. EMBO J. 2017;36(2):183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119(Pt 19):3901–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou X, Rowe RG, Hiraoka N, George JP, Wirtz D, Mosher DF, et al. Fibronectin fibrillogenesis regulates three-dimensional neovessel formation. Genes Dev. 2008;22(9):1231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stratman AN, Davis GE. Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: influence on vascular tube remodeling, maturation, and stabilization. Microsc Microanal. 2012;18(1):68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Clifford PS, Ella SR, Stupica AJ, Nourian Z, Li M, Martinez-Lemus LA, et al. Spatial distribution and mechanical function of elastin in resistance arteries: a role in bearing longitudinal stress. Arterioscler Thromb Vasc Biol. 2011;31(12):2889–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bloksgaard M, Leurgans TM, Nissen I, Jensen PS, Hansen ML, Brewer JR, et al. Elastin organization in pig and cardiovascular disease patients’ pericardial resistance arteries. J Vasc Res. 2015;52(1):1–11. [DOI] [PubMed] [Google Scholar]
- 141.Fischer GM, Llaurado JG. Collagen and elastin content in canine arteries selected from functionally different vascular beds. Circ Res. 1966;19(2):394–9. [DOI] [PubMed] [Google Scholar]
- 142.Schwartz E, Adamany AM, Blumenfeld OO. Isolation and characterization of the internal elastic lamina from calf thoracic aorta. Exp Mol Pathol. 1981;34(3):299–306. [DOI] [PubMed] [Google Scholar]
- 143.Lee RM. Morphology of cerebral arteries. Pharmacol Ther. 1995;66(1):149–73. [DOI] [PubMed] [Google Scholar]
- 144.Harkness ML, Harkness RD, McDonald DA. The collagen and elastin content of the arterial wall in the dog. Proc R Soc Lond B Biol Sci. 1957;146(925):541–51. [DOI] [PubMed] [Google Scholar]
- 145.Dobrin PB. Mechanical properties of arteries. Physiol Rev. 1978;58(2):397–460. [DOI] [PubMed] [Google Scholar]
- 146.Lin CJ, Staiculescu MC, Hawes JZ, Cocciolone AJ, Hunkins BM, Roth RA, et al. Heterogeneous Cellular Contributions to Elastic Laminae Formation in Arterial Wall Development. Circ Res. 2019;125(11):1006–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Unemori EN, Bouhana KS, Werb Z. Vectorial secretion of extracellular matrix proteins, matrix-degrading proteinases, and tissue inhibitor of metalloproteinases by endothelial cells. J Biol Chem. 1990;265(1):445–51. [PubMed] [Google Scholar]
- 148.Carnes WH, Abraham PA, Buonassisi V. Biosynthesis of elastin by an endothelial cell culture. Biochem Biophys Res Commun. 1979;90(4):1393–9. [DOI] [PubMed] [Google Scholar]
- 149.Cantor JO, Keller S, Parshley MS, Darnule TV, Darnule AT, Cerreta JM, et al. Synthesis of crosslinked elastin by an endothelial cell culture. Biochem Biophys Res Commun. 1980;95(4):1381–6. [DOI] [PubMed] [Google Scholar]
- 150.Damiano V, Tsang A, Weinbaum G, Christner P, Rosenbloom J. Secretion of elastin in the embryonic chick aorta as visualized by immunoelectron microscopy. Coll Relat Res. 1984;4(2):153–64. [DOI] [PubMed] [Google Scholar]
- 151.Myers PR, Tanner MA. Vascular endothelial cell regulation of extracellular matrix collagen: role of nitric oxide. Arterioscler Thromb Vasc Biol. 1998;18(5):717–22. [DOI] [PubMed] [Google Scholar]
- 152.Sechler JL, Corbett SA, Wenk MB, Schwarzbauer JE. Modulation of cell-extracellular matrix interactions. Ann N Y Acad Sci. 1998;857:143–54. [DOI] [PubMed] [Google Scholar]
- 153.Rohwedder I, Montanez E, Beckmann K, Bengtsson E, Duner P, Nilsson J, et al. Plasma fibronectin deficiency impedes atherosclerosis progression and fibrous cap formation. EMBO Mol Med. 2012;4(7):564–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967;18(1):181–223. [DOI] [PubMed] [Google Scholar]
- 155.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86(3):341–6. [DOI] [PubMed] [Google Scholar]
- 156.Maarouf N, Sancho M, Furstenhaupt T, Tran CH, Welsh DG. Structural analysis of endothelial projections from mesenteric arteries. Microcirculation. 2017;24(3). [DOI] [PubMed] [Google Scholar]
- 157.Michel RP, Hu F, Meyrick BO. Myoendothelial junctional complexes in postobstructive pulmonary vasculopathy: a quantitative electron microscopic study. Exp Lung Res. 1995;21(3):437–52. [DOI] [PubMed] [Google Scholar]
- 158.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28(5):703–11. [DOI] [PubMed] [Google Scholar]
- 159.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? J Anat. 2006;209(5):689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol. 2003;553(Pt 1):183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Crane GJ, Walker SD, Dora KA, Garland CJ. Evidence for a differential cellular distribution of inward rectifier K channels in the rat isolated mesenteric artery. J Vasc Res. 2003;40(2):159–68. [DOI] [PubMed] [Google Scholar]
- 162.Duling BR. Effects of potassium ion on the microcirculation of the hamster. Circ Res. 1975;37(3):325–32. [DOI] [PubMed] [Google Scholar]
- 163.Knot HJ, Zimmermann PA, Nelson MT. Extracellular K(+)-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K(+) channels. J Physiol. 1996;492 ( Pt 2)(Pt 2):419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Harraz OF, Longden TA, Dabertrand F, Hill-Eubanks D, Nelson MT. Endothelial GqPCR activity controls capillary electrical signaling and brain blood flow through PIP(2) depletion. Proc Natl Acad Sci U S A. 2018;115(15):E3569–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, et al. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol. 2003;138(4):594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gaete PS, Lillo MA, Ardiles NM, Perez FR, Figueroa XF. Ca2+-activated K+ channels of small and intermediate conductance control eNOS activation through NAD(P)H oxidase. Free Radic Biol Med. 2012;52(5):860–70. [DOI] [PubMed] [Google Scholar]
- 167.Isakson BE, Best AK, Duling BR. Incidence of protein on actin bridges between endothelium and smooth muscle in arterioles demonstrates heterogeneous connexin expression and phosphorylation. Am J Physiol Heart Circ Physiol. 2008;294(6):H2898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Beyer S, Kelly RG, Miquerol L. Inducible Cx40-Cre expression in the cardiac conduction system and arterial endothelial cells. Genesis. 2011;49(2):83–91. [DOI] [PubMed] [Google Scholar]
- 169.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid Endothelial Cell–Selective Loading of Connexin 40 Antibody Blocks Endothelium-Derived Hyperpolarizing Factor Dilation in Rat Small Mesenteric Arteries. Circulation Research. 2005;97(4):399–407. [DOI] [PubMed] [Google Scholar]
- 170.Chaytor AT, Evans WH, Griffith TM. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J Physiol. 1998;508 ( Pt 2):561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? Am J Physiol. 1989;256(3 Pt 2):H838–45. [DOI] [PubMed] [Google Scholar]
- 172.Yashiro Y, Duling BR. Integrated Ca(2+) signaling between smooth muscle and endothelium of resistance vessels. Circ Res. 2000;87(11):1048–54. [DOI] [PubMed] [Google Scholar]
- 173.Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci U S A. 1997;94(12):6529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tran CH, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, et al. Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol. 2012;302(8):C1226–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kerr PM, Tam R, Ondrusova K, Mittal R, Narang D, Tran CH, et al. Endothelial feedback and the myoendothelial projection. Microcirculation. 2012;19(5):416–22. [DOI] [PubMed] [Google Scholar]
- 176.Lemmey HAL, Garland CJ, Dora KA. Intrinsic regulation of microvascular tone by myoendothelial feedback circuits. Curr Top Membr. 2020;85:327–55. [DOI] [PubMed] [Google Scholar]
- 177.Garland CJ, Bagher P, Powell C, Ye X, Lemmey HAL, Borysova L, et al. Voltage-dependent Ca(2+) entry into smooth muscle during contraction promotes endothelium-mediated feedback vasodilation in arterioles. Sci Signal. 2017;10(486). [DOI] [PubMed] [Google Scholar]
- 178.Sakai H, Hara H, Tsai AG, Tsuchida E, Intaglietta M. Constriction of resistance arteries determines l-NAME-induced hypertension in a conscious hamster model. Microvasc Res. 2000;60(1):21–7. [DOI] [PubMed] [Google Scholar]
- 179.Budel S, Bartlett IS, Segal SS. Homocellular conduction along endothelium and smooth muscle of arterioles in hamster cheek pouch: unmasking an NO wave. Circ Res. 2003;93(1):61–8. [DOI] [PubMed] [Google Scholar]
- 180.Hakim TS, Sugimori K, Camporesi EM, Anderson G. Half-life of nitric oxide in aqueous solutions with and without haemoglobin. Physiol Meas. 1996;17(4):267–77. [DOI] [PubMed] [Google Scholar]
- 181.Keller TCt, Butcher JT, Broseghini-Filho GB, Marziano C, DeLalio LJ, Rogers S, et al. Modulating Vascular Hemodynamics With an Alpha Globin Mimetic Peptide (HbalphaX). Hypertension. 2016;68(6):1494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Butcher JT, Johnson T, Beers J, Columbus L, Isakson BE. Hemoglobin alpha in the blood vessel wall. Free Radic Biol Med. 2014;73:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Garcia V, Park EJ, Siragusa M, Frohlich F, Mahfuzul Haque M, Pascale JV, et al. Unbiased proteomics identifies plasminogen activator inhibitor-1 as a negative regulator of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2020;117(17):9497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Leo F, Hutzler B, Ruddiman CA, Isakson BE, Cortese-Krott MM. Cellular microdomains for nitric oxide signaling in endothelium and red blood cells. Nitric Oxide. 2020. [DOI] [PMC free article] [PubMed]
- 185.Biwer LA, Isakson BE. Endoplasmic reticulum-mediated signalling in cellular microdomains. Acta Physiol (Oxf). 2017;219(1):162–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, et al. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci U S A. 2008;105(28):9627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Uehara K Localization of TRPC1 channel in the sinus endothelial cells of rat spleen. Histochem Cell Biol. 2005;123(4–5):347–56. [DOI] [PubMed] [Google Scholar]
- 188.Wilson C, Lee MD, Heathcote HR, Zhang X, Buckley C, Girkin JM, et al. Mitochondrial ATP production provides long-range control of endothelial inositol trisphosphate-evoked calcium signaling. J Biol Chem. 2019;294(3):737–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Heberlein KR, Straub AC, Best AK, Greyson MA, Looft-Wilson RC, Sharma PR, et al. Plasminogen activator inhibitor-1 regulates myoendothelial junction formation. Circ Res. 2010;106(6):1092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Heberlein KR, Han J, Straub AC, Best AK, Kaun C, Wojta J, et al. A novel mRNA binding protein complex promotes localized plasminogen activator inhibitor-1 accumulation at the myoendothelial junction. Arterioscler Thromb Vasc Biol. 2012;32(5):1271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.McCallinhart PE, Biwer LA, Clark OE, Isakson BE, Lilly B, Trask AJ. Myoendothelial Junctions of Mature Coronary Vessels Express Notch Signaling Proteins. Front Physiol. 2020;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Carnegie GK, Means CK, Scott JD. A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life. 2009;61(4):394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Langeberg LK, Scott JD. Signalling scaffolds and local organization of cellular behaviour. Nat Rev Mol Cell Biol. 2015;16(4):232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Carr DW, Stofko-Hahn RE, Fraser ID, Bishop SM, Acott TS, Brennan RG, et al. Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J Biol Chem. 1991;266(22):14188–92. [PubMed] [Google Scholar]
- 195.Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271(5255):1589–92. [DOI] [PubMed] [Google Scholar]
- 196.Coghlan VM, Perrino BA, Howard M, Langeberg LK, Hicks JB, Gallatin WM, et al. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267(5194):108–11. [DOI] [PubMed] [Google Scholar]
- 197.Scott JD, Dessauer CW, Tasken K. Creating order from chaos: cellular regulation by kinase anchoring. Annu Rev Pharmacol Toxicol. 2013;53:187–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schiattarella GG, Cattaneo F, Carrizzo A, Paolillo R, Boccella N, Ambrosio M, et al. Akap1 Regulates Vascular Function and Endothelial Cells Behavior. Hypertension. 2018;71(3):507–17. [DOI] [PubMed] [Google Scholar]
- 199.Motawea HK, Blazek AD, Zirwas MJ, Pleister AP, Ahmed AA, McConnell BK, et al. Delocalization of Endogenous A-kinase Antagonizes Rap1-Rho-alpha2C-Adrenoceptor Signaling in Human Microvascular Smooth Muscle Cells. J Cytol Mol Biol. 2014;1(1). [PMC free article] [PubMed] [Google Scholar]
- 200.Mercado J, Baylie R, Navedo MF, Yuan C, Scott JD, Nelson MT, et al. Local control of TRPV4 channels by AKAP150-targeted PKC in arterial smooth muscle. J Gen Physiol. 2014;143(5):559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Diviani D, Reggi E, Arambasic M, Caso S, Maric D. Emerging roles of A-kinase anchoring proteins in cardiovascular pathophysiology. Biochim Biophys Acta. 2016;1863(7 Pt B):1926–36. [DOI] [PubMed] [Google Scholar]
- 202.Radeva MY, Kugelmann D, Spindler V, Waschke J. PKA compartmentalization via AKAP220 and AKAP12 contributes to endothelial barrier regulation. PLoS One. 2014;9(9):e106733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kwon HB, Choi YK, Lim JJ, Kwon SH, Her S, Kim HJ, et al. AKAP12 regulates vascular integrity in zebrafish. Exp Mol Med. 2012;44(3):225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Malinin NL, Pluskota E, Byzova TV. Integrin signaling in vascular function. Curr Opin Hematol. 2012;19(3):206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Akiyama SK. Integrins in cell adhesion and signaling. Hum Cell. 1996;9(3):181–6. [PubMed] [Google Scholar]
- 206.Bhullar IS, Li YS, Miao H, Zandi E, Kim M, Shyy JY, et al. Fluid shear stress activation of IkappaB kinase is integrin-dependent. J Biol Chem. 1998;273(46):30544–9. [DOI] [PubMed] [Google Scholar]
- 207.Dejana ECG , Zanetti A, Lampugnani MG, Marchisio PC Receptors for Extracellular Matrix Proteins in Endothelial Cells. In: Catravas JDGCN, Ryan, editor. Vascular Endothelium Springer, Boston, MA; 1989. p. 141–7. [Google Scholar]
- 208.Welser-Alves JV, Boroujerdi A, Tigges U, Wrabetz L, Feltri ML, Milner R. Endothelial beta4 integrin is predominantly expressed in arterioles, where it promotes vascular remodeling in the hypoxic brain. Arterioscler Thromb Vasc Biol. 2013;33(5):943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Goyer B, Thériault M, Gendron SP, Brunette I, Rochette PJ, Proulx S. Extracellular Matrix and Integrin Expression Profiles in Fuchs Endothelial Corneal Dystrophy Cells and Tissue Model. Tissue Eng Part A. 2018;24(7–8):607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Choi JS, Kim EY, Kim MJ, Giegengack M, Khan FA, Khang G, et al. In vitro evaluation of the interactions between human corneal endothelial cells and extracellular matrix proteins. Biomedical Materials. 2013;8(1):014108. [DOI] [PubMed] [Google Scholar]
- 211.Lampugnani MG, Resnati M, Dejana E, Marchisio PC. The role of integrins in the maintenance of endothelial monolayer integrity. J Cell Biol. 1991;112(3):479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pelletier AJ, Kunicki T, Ruggeri ZM, Quaranta V. The activation state of the integrin alpha IIb beta 3 affects outside-in signals leading to cell spreading and focal adhesion kinase phosphorylation. J Biol Chem. 1995;270(30):18133–40. [DOI] [PubMed] [Google Scholar]
- 213.Petzold T, Orr AW, Hahn C, Jhaveri KA, Parsons JT, Schwartz MA. Focal adhesion kinase modulates activation of NF-kappaB by flow in endothelial cells. Am J Physiol Cell Physiol. 2009;297(4):C814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li S, Kim M, Hu YL, Jalali S, Schlaepfer DD, Hunter T, et al. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J Biol Chem. 1997;272(48):30455–62. [DOI] [PubMed] [Google Scholar]
- 215.Feaver RE, Gelfand BD, Wang C, Schwartz MA, Blackman BR. Atheroprone hemodynamics regulate fibronectin deposition to create positive feedback that sustains endothelial inflammation. Circ Res. 2010;106(11):1703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Diaz C, Neubauer S, Rechenmacher F, Kessler H, Missirlis D. Recruitment of alphanubeta3 integrin to alpha5beta1 integrin-induced clusters enables focal adhesion maturation and cell spreading. J Cell Sci. 2020;133(1). [DOI] [PubMed] [Google Scholar]
- 217.Moon JJ, Matsumoto M, Patel S, Lee L, Guan JL, Li S. Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J Cell Physiol. 2005;203(1):166–76. [DOI] [PubMed] [Google Scholar]
- 218.Schiller HB, Hermann MR, Polleux J, Vignaud T, Zanivan S, Friedel CC, et al. beta1- and alphav-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat Cell Biol. 2013;15(6):625–36. [DOI] [PubMed] [Google Scholar]
- 219.Missirlis D, Haraszti T, Scheele C, Wiegand T, Diaz C, Neubauer S, et al. Substrate engagement of integrins alpha5beta1 and alphavbeta3 is necessary, but not sufficient, for high directional persistence in migration on fibronectin. Sci Rep. 2016;6:23258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–61. [PubMed] [Google Scholar]
- 221.Wei H, Sundararaman A, Dickson E, Rennie-Campbell L, Cross E, Heesom KJ, et al. Characterization of the polarized endothelial secretome. FASEB J. 2019;33(11):12277–87. [DOI] [PubMed] [Google Scholar]
- 222.Burghoff S, Schrader J. Secretome of human endothelial cells under shear stress. J Proteome Res. 2011;10(3):1160–9. [DOI] [PubMed] [Google Scholar]
- 223.Cho JS, Ouriel K. Differential thrombogenicity of artery and vein: the role of von Willebrand factor. Ann Vasc Surg. 1995;9(1):60–70. [DOI] [PubMed] [Google Scholar]
- 224.Mohr T, Haudek-Prinz V, Slany A, Grillari J, Micksche M, Gerner C. Proteome profiling in IL-1beta and VEGF-activated human umbilical vein endothelial cells delineates the interlink between inflammation and angiogenesis. PLoS One. 2017;12(6):e0179065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zanivan S, Maione F, Hein MY, Hernandez-Fernaud JR, Ostasiewicz P, Giraudo E, et al. SILAC-based proteomics of human primary endothelial cell morphogenesis unveils tumor angiogenic markers. Mol Cell Proteomics. 2013;12(12):3599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Epelman S, Tang WH, Chen SY, Van Lente F, Francis GS, Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J Am Coll Cardiol. 2008;52(9):750–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zerwes HG, Risau W. Polarized secretion of a platelet-derived growth factor-like chemotactic factor by endothelial cells in vitro. J Cell Biol. 1987;105(5):2037–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tada S, Tarbell JM. Internal elastic lamina affects the distribution of macromolecules in the arterial wall: a computational study. Am J Physiol Heart Circ Physiol. 2004;287(2):H905–13. [DOI] [PubMed] [Google Scholar]
- 229.Sporn LA, Marder VJ, Wagner DD. Differing polarity of the constitutive and regulated secretory pathways for von Willebrand factor in endothelial cells. J Cell Biol. 1989;108(4):1283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Platt JL, Vercellotti GM, Lindman BJ, Oegema TR Jr., Bach FH, Dalmasso AP. Release of heparan sulfate from endothelial cells. Implications for pathogenesis of hyperacute rejection. J Exp Med. 1990;171(4):1363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wagner OF, Christ G, Wojta J, Vierhapper H, Parzer S, Nowotny PJ, et al. Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem. 1992;267(23):16066–8. [PubMed] [Google Scholar]
- 232.Russell FD, Davenport AP. Secretory pathways in endothelin synthesis. Br J Pharmacol. 1999;126(2):391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Desideri G, Ferri C, Bellini C, De Mattia G, Santucci A. Effects of ACE inhibition on spontaneous and insulin-stimulated endothelin-1 secretion: in vitro and in vivo studies. Diabetes. 1997;46(1):81–6. [DOI] [PubMed] [Google Scholar]
- 234.Upchurch GR Jr., Banes AJ, Wagner WH, Ramadan F, Link GW, Henderson RH, et al. Differences in secretion of prostacyclin by venous and arterial endothelial cells grown in vitro in a static versus a mechanically active environment. J Vasc Surg. 1989;10(3):292–8. [PubMed] [Google Scholar]
- 235.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778(3):794–809. [DOI] [PubMed] [Google Scholar]
- 236.Lampugnani MG. Endothelial cell-to-cell junctions: adhesion and signaling in physiology and pathology. Cold Spring Harb Perspect Med. 2012;2(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol. 1975;67(3):863–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. Arteries and veins. J Cell Biol. 1976;68(3):705–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kniesel U, Risau W, Wolburg H. Development of blood-brain barrier tight junctions in the rat cortex. Brain Res Dev Brain Res. 1996;96(1–2):229–40. [DOI] [PubMed] [Google Scholar]
- 240.Millan J, Cain RJ, Reglero-Real N, Bigarella C, Marcos-Ramiro B, Fernandez-Martin L, et al. Adherens junctions connect stress fibres between adjacent endothelial cells. BMC Biol. 2010;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Curry FR. Microvascular solute and water transport. Microcirculation. 2005;12(1):17–31. [DOI] [PubMed] [Google Scholar]
- 242.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10(8):923–34. [DOI] [PubMed] [Google Scholar]
- 243.Nieset JE, Redfield AR, Jin F, Knudsen KA, Johnson KR, Wheelock MJ. Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin. J Cell Sci. 1997;110 ( Pt 8):1013–22. [DOI] [PubMed] [Google Scholar]
- 244.Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol. 1995;130(1):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Venkiteswaran K, Xiao K, Summers S, Calkins CC, Vincent PA, Pumiglia K, et al. Regulation of endothelial barrier function and growth by VE-cadherin, plakoglobin, and beta-catenin. Am J Physiol Cell Physiol. 2002;283(3):C811–21. [DOI] [PubMed] [Google Scholar]
- 246.Franke WW, Kapprell HP, Cowin P. Immunolocalization of plakoglobin in endothelial junctions: identification as a special type of Zonulae adhaerentes. Biol Cell. 1987;59(3):205–18. [DOI] [PubMed] [Google Scholar]
- 247.Navarro P, Ruco L, Dejana E. Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol. 1998;140(6):1475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Salomon D, Ayalon O, Patel-King R, Hynes RO, Geiger B. Extrajunctional distribution of N-cadherin in cultured human endothelial cells. J Cell Sci. 1992;102 ( Pt 1):7–17. [DOI] [PubMed] [Google Scholar]
- 249.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kiosses WB, McKee NH, Kalnins VI. The distribution of centrosomes in endothelial cells of the rat aorta and inferior vena cava. Artery. 1997;22(5):251–65. [PubMed] [Google Scholar]
- 251.Baumer S, Keller L, Holtmann A, Funke R, August B, Gamp A, et al. Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) activity is required for blood vessel development. Blood. 2006;107(12):4754–62. [DOI] [PubMed] [Google Scholar]
- 252.Dominguez MG, Hughes VC, Pan L, Simmons M, Daly C, Anderson K, et al. Vascular endothelial tyrosine phosphatase (VE-PTP)-null mice undergo vasculogenesis but die embryonically because of defects in angiogenesis. Proc Natl Acad Sci U S A. 2007;104(9):3243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, et al. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21(18):4885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167(4):2323–30. [DOI] [PubMed] [Google Scholar]
- 255.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111 ( Pt 13):1853–65. [DOI] [PubMed] [Google Scholar]
- 256.Wagner R, Kachar B. Linear gap and tight junctional assemblies between capillary endothelial cells in the eel rete mirabile. Anat Rec. 1995;242(4):545–52. [DOI] [PubMed] [Google Scholar]
- 257.Meens MJ, Alonso F, Le Gal L, Kwak BR, Haefliger JA. Endothelial Connexin37 and Connexin40 participate in basal but not agonist-induced NO release. Cell Commun Signal. 2015;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hwan Seul K, Beyer EC. Heterogeneous localization of connexin40 in the renal vasculature. Microvasc Res. 2000;59(1):140–8. [DOI] [PubMed] [Google Scholar]
- 259.Figueroa XF, Paul DL, Simon AM, Goodenough DA, Day KH, Damon DN, et al. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res. 2003;92(7):793–800. [DOI] [PubMed] [Google Scholar]
- 260.de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, et al. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86(6):649–55. [DOI] [PubMed] [Google Scholar]
- 261.Isakson BE, Damon DN, Day KH, Liao Y, Duling BR. Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am J Physiol Heart Circ Physiol. 2006;290(3):H1199–205. [DOI] [PubMed] [Google Scholar]
- 262.Sandow SL, Looft-Wilson R, Doran B, Grayson TH, Segal SS, Hill CE. Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc Res. 2003;60(3):643–53. [DOI] [PubMed] [Google Scholar]
- 263.Hakim CH, Jackson WF, Segal SS. Connexin isoform expression in smooth muscle cells and endothelial cells of hamster cheek pouch arterioles and retractor feed arteries. Microcirculation. 2008;15(6):503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.van Kempen MJ, Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol. 1999;112(6):479–86. [DOI] [PubMed] [Google Scholar]
- 265.Yeh HI, Dupont E, Coppen S, Rothery S, Severs NJ. Gap junction localization and connexin expression in cytochemically identified endothelial cells of arterial tissue. J Histochem Cytochem. 1997;45(4):539–50. [DOI] [PubMed] [Google Scholar]
- 266.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res. 1998;83(6):636–43. [DOI] [PubMed] [Google Scholar]
- 267.Yeh HI, Lu CS, Wu YJ, Chen CC, Hong RC, Ko YS, et al. Reduced expression of endothelial connexin37 and connexin40 in hyperlipidemic mice: recovery of connexin37 after 7-day simvastatin treatment. Arterioscler Thromb Vasc Biol. 2003;23(8):1391–7. [DOI] [PubMed] [Google Scholar]
- 268.Boittin FX, Alonso F, Le Gal L, Allagnat F, Beny JL, Haefliger JA. Connexins and M3 muscarinic receptors contribute to heterogeneous Ca(2+) signaling in mouse aortic endothelium. Cell Physiol Biochem. 2013;31(1):166–78. [DOI] [PubMed] [Google Scholar]
- 269.Munger SJ, Kanady JD, Simon AM. Absence of venous valves in mice lacking Connexin37. Dev Biol. 2013;373(2):338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Johnstone S, Isakson B, Locke D. Biological and biophysical properties of vascular connexin channels. Int Rev Cell Mol Biol. 2009;278:69–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178(2):449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–31. [DOI] [PubMed] [Google Scholar]
- 273.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3(2):143–50. [DOI] [PubMed] [Google Scholar]
- 274.Bardin N, Anfosso F, Masse JM, Cramer E, Sabatier F, Le Bivic A, et al. Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood. 2001;98(13):3677–84. [DOI] [PubMed] [Google Scholar]
- 275.Yamamoto H, Ehling M, Kato K, Kanai K, van Lessen M, Frye M, et al. Integrin beta1 controls VE-cadherin localization and blood vessel stability. Nat Commun. 2015;6:6429. [DOI] [PubMed] [Google Scholar]
- 276.Sehrawat S, Ernandez T, Cullere X, Takahashi M, Ono Y, Komarova Y, et al. AKAP9 regulation of microtubule dynamics promotes Epac1-induced endothelial barrier properties. Blood. 2011;117(2):708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Chidlow JH Jr., Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. 2010;86(2):219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, et al. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation. 2008;117(8):1065–74. [DOI] [PubMed] [Google Scholar]
- 279.Segal SS, Brett SE, Sessa WC. Codistribution of NOS and caveolin throughout peripheral vasculature and skeletal muscle of hamsters. Am J Physiol. 1999;277(3):H1167–77. [DOI] [PubMed] [Google Scholar]
- 280.Calizo RC, Scarlata S. A role for G-proteins in directing G-protein-coupled receptor-caveolae localization. Biochemistry. 2012;51(47):9513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Gosens R, Stelmack GL, Dueck G, Mutawe MM, Hinton M, McNeill KD, et al. Caveolae facilitate muscarinic receptor-mediated intracellular Ca2+ mobilization and contraction in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;293(6):L1406–18. [DOI] [PubMed] [Google Scholar]
- 282.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272(30):18522–5. [DOI] [PubMed] [Google Scholar]
- 283.Fernández-Hernando C, Yu J, Suárez Y, Rahner C, Dávalos A, Lasunción MA, et al. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009;10(1):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Santibanez JF, Blanco FJ, Garrido-Martin EM, Sanz-Rodriguez F, del Pozo MA, Bernabeu C. Caveolin-1 interacts and cooperates with the transforming growth factor-beta type I receptor ALK1 in endothelial caveolae. Cardiovasc Res. 2008;77(4):791–9. [DOI] [PubMed] [Google Scholar]
- 285.Babitt J, Trigatti B, Rigotti A, Smart EJ, Anderson RG, Xu S, et al. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J Biol Chem. 1997;272(20):13242–9. [DOI] [PubMed] [Google Scholar]
- 286.Goedicke-Fritz S, Kaistha A, Kacik M, Markert S, Hofmeister A, Busch C, et al. Evidence for functional and dynamic microcompartmentation of Cav-1/TRPV4/K(Ca) in caveolae of endothelial cells. Eur J Cell Biol. 2015;94(7–9):391–400. [DOI] [PubMed] [Google Scholar]
- 287.Lu T, Wang XL, Chai Q, Sun X, Sieck GC, Katusic ZS, et al. Role of the endothelial caveolae microdomain in shear stress-mediated coronary vasorelaxation. J Biol Chem. 2017;292(46):19013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ottolini M, Hong K, Sonkusare SK. Calcium signals that determine vascular resistance. Wiley Interdiscip Rev Syst Biol Med. 2019;11(5):e1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Mountian I, Manolopoulos VG, De Smedt H, Parys JB, Missiaen L, Wuytack F. Expression patterns of sarco/endoplasmic reticulum Ca(2+)-ATPase and inositol 1,4,5-trisphosphate receptor isoforms in vascular endothelial cells. Cell Calcium. 1999;25(5):371–80. [DOI] [PubMed] [Google Scholar]
- 290.Grayson TH, Haddock RE, Murray TP, Wojcikiewicz RJ, Hill CE. Inositol 1,4,5-trisphosphate receptor subtypes are differentially distributed between smooth muscle and endothelial layers of rat arteries. Cell Calcium. 2004;36(6):447–58. [DOI] [PubMed] [Google Scholar]
- 291.Lin Q, Zhao L, Jing R, Trexler C, Wang H, Li Y, et al. Inositol 1,4,5-Trisphosphate Receptors in Endothelial Cells Play an Essential Role in Vasodilation and Blood Pressure Regulation. J Am Heart Assoc. 2019;8(4):e011704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, et al. Functional architecture of inositol 1, 4, 5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proceedings of the National Academy of Sciences. 2008;105(28):9627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Yuan Q, Yang J, Santulli G, Reiken SR, Wronska A, Kim MM, et al. Maintenance of normal blood pressure is dependent on IP3R1-mediated regulation of eNOS. Proceedings of the National Academy of Sciences. 2016;113(30):8532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci U S A. 2012;109(44):18174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Smith IF, Wiltgen SM, Parker I. Localization of puff sites adjacent to the plasma membrane: functional and spatial characterization of Ca2+ signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium. 2009;45(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Geyer M, Huang F, Sun Y, Vogel SM, Malik AB, Taylor CW, et al. Microtubule-Associated Protein EB3 Regulates IP3 Receptor Clustering and Ca(2+) Signaling in Endothelial Cells. Cell Rep. 2015;12(1):79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Yap FC, Weber DS, Taylor MS, Townsley MI, Comer BS, Maylie J, et al. Endothelial SK3 channel-associated Ca2+ microdomains modulate blood pressure. Am J Physiol Heart Circ Physiol. 2016;310(9):H1151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, et al. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204(10):2373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Lipowsky HH, Kovalcheck S, Zweifach BW. The distribution of blood rheological parameters in the microvasculature of cat mesentery. Circ Res. 1978;43(5):738–49. [DOI] [PubMed] [Google Scholar]
- 300.Lipowsky HH, Usami S, Chien S. In vivo measurements of “apparent viscosity” and microvessel hematocrit in the mesentery of the cat. Microvasc Res. 1980;19(3):297–319. [DOI] [PubMed] [Google Scholar]
- 301.Coan DE, Wechezak AR, Viggers RF, Sauvage LR. Effect of shear stress upon localization of the Golgi apparatus and microtubule organizing center in isolated cultured endothelial cells. Journal of Cell Science. 1993;104(4):1145–53. [DOI] [PubMed] [Google Scholar]
- 302.Kwon H-B, Wang S, Helker CS, Rasouli SJ, Maischein H-M, Offermanns S, et al. In vivo modulation of endothelial polarization by Apelin receptor signalling. Nature communications. 2016;7:11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Simmers MB, Pryor AW, Blackman BR. Arterial shear stress regulates endothelial cell-directed migration, polarity, and morphology in confluent monolayers. American Journal of Physiology-Heart and Circulatory Physiology. 2007;293(3):H1937–H46. [DOI] [PubMed] [Google Scholar]
- 304.Dewey CF Jr., Bussolari SR, Gimbrone MA Jr., Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103(3):177–85. [DOI] [PubMed] [Google Scholar]
- 305.Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res. 1999;85(6):504–14. [DOI] [PubMed] [Google Scholar]
- 306.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. Embo j. 2001;20(17):4639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tzima E, Kiosses WB, del Pozo MA, Schwartz MA. Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. J Biol Chem. 2003;278(33):31020–3. [DOI] [PubMed] [Google Scholar]
- 308.Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515(7526):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Perozo E New structural perspectives on K+ channel gating. Structure. 2002;10(8):1027–9. [DOI] [PubMed] [Google Scholar]
- 310.Coon BG, Baeyens N, Han J, Budatha M, Ross TD, Fang JS, et al. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J Cell Biol. 2015;208(7):975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Jung B, Obinata H, Galvani S, Mendelson K, Ding B-s, Skoura A, et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Developmental cell. 2012;23(3):600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nature cell biology. 2004;6(6):499. [DOI] [PubMed] [Google Scholar]
- 313.Wang S, Iring A, Strilic B, Juárez JA, Kaur H, Troidl K, et al. P2Y 2 and G q/G 11 control blood pressure by mediating endothelial mechanotransduction. The Journal of clinical investigation. 2015;125(8):3077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lehtonen J, Kinnunen P. Phospholipase A2 as a mechanosensor. Biophysical Journal. 1995;68(5):1888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Goetz JG, Steed E, Ferreira RR, Roth S, Ramspacher C, Boselli F, et al. Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell reports. 2014;6(5):799–808. [DOI] [PubMed] [Google Scholar]
- 316.Hierck BP, Van der Heiden K, Alkemade FE, Van de Pas S, Van Thienen JV, Groenendijk BC, et al. Primary cilia sensitize endothelial cells for fluid shear stress. Developmental dynamics: an official publication of the American Association of Anatomists. 2008;237(3):725–35. [DOI] [PubMed] [Google Scholar]
- 317.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A. 2003;100(13):7988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Tkachenko E, Gutierrez E, Saikin SK, Fogelstrand P, Kim C, Groisman A, et al. The nucleus of endothelial cell as a sensor of blood flow direction. Biol Open. 2013;2(10):1007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75(3):519–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Fang Y, Wu D, Birukov KG. Mechanosensing and Mechanoregulation of Endothelial Cell Functions. Compr Physiol. 2019;9(2):873–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A. 2014;111(28):10347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. The Journal of Clinical Investigation. 2016;126(12):4527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013;23(11):1024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158(4):773–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Chiu YJ, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation. J Cell Biol. 2008;182(4):753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, et al. Genetic evidence for functional redundancy of Platelet/Endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999;162(5):3022–30. [PubMed] [Google Scholar]
- 327.Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol. 2005;25(8):1590–5. [DOI] [PubMed] [Google Scholar]
- 328.Barry AK, Wang N, Leckband DE. Local VE-cadherin mechanotransduction triggers long-ranged remodeling of endothelial monolayers. J Cell Sci. 2015;128(7):1341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003;93(4):354–63. [DOI] [PubMed] [Google Scholar]
- 330.Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol. 2006;26(1):77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83. [DOI] [PubMed] [Google Scholar]
- 332.Nakajima H, Yamamoto K, Agarwala S, Terai K, Fukui H, Fukuhara S, et al. Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance. Dev Cell. 2017;40(6):523–36.e6. [DOI] [PubMed] [Google Scholar]
- 333.Xu J, Mathur J, Vessières E, Hammack S, Nonomura K, Favre J, et al. GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell. 2018;173(3):762–75.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Betz AL, Firth JA, Goldstein GW. Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res. 1980;192(1):17–28. [DOI] [PubMed] [Google Scholar]
- 335.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, et al. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116(5):1284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Cheng C, van Haperen R, de Waard M, van Damme LC, Tempel D, Hanemaaijer L, et al. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood. 2005;106(12):3691–8. [DOI] [PubMed] [Google Scholar]
- 337.Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, et al. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler Thromb Vasc Biol. 2011;31(2):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Bkaily G, Choufani S, Sader S, Jacques D, d’Orléans-Juste P, Nader M, et al. Activation of sarcolemma and nuclear membranes ET-1 receptors regulates transcellular calcium levels in heart and vascular smooth muscle cells. Canadian Journal of Physiology and Pharmacology. 2003;81(6):654–62. [DOI] [PubMed] [Google Scholar]
- 339.Garcia J, Patel N, Basehore S, Clyne AM. Fibroblast Growth Factor-2 Binding to Heparan Sulfate Proteoglycans Varies with Shear Stress in Flow-Adapted Cells. Ann Biomed Eng. 2019;47(4):1078–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Mertens G, Cassiman JJ, Van den Berghe H, Vermylen J, David G. Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J Biol Chem. 1992;267(28):20435–43. [PubMed] [Google Scholar]
- 341.Marcum JA, Atha DH, Fritze LM, Nawroth P, Stern D, Rosenberg RD. Cloned bovine aortic endothelial cells synthesize anticoagulantly active heparan sulfate proteoglycan. J Biol Chem. 1986;261(16):7507–17. [PubMed] [Google Scholar]
- 342.Ruderisch N, Virgintino D, Makrides V, Verrey F. Differential axial localization along the mouse brain vascular tree of luminal sodium-dependent glutamine transporters Snat1 and Snat3. J Cereb Blood Flow Metab. 2011;31(7):1637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci U S A. 2012;109(39):15930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Horvat R, Palade GE. Thrombomodulin and thrombin localization on the vascular endothelium; their internalization and transcytosis by plasmalemmal vesicles. Eur J Cell Biol. 1993;61(2):299–313. [PubMed] [Google Scholar]
- 345.Kohler R, Brakemeier S, Kuhn M, Degenhardt C, Buhr H, Pries A, et al. Expression of ryanodine receptor type 3 and TRP channels in endothelial cells: comparison of in situ and cultured human endothelial cells. Cardiovasc Res. 2001;51(1):160–8. [DOI] [PubMed] [Google Scholar]
- 346.Paria BC, Vogel SM, Ahmmed GU, Alamgir S, Shroff J, Malik AB, et al. Tumor necrosis factor-alpha-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1303–13. [DOI] [PubMed] [Google Scholar]
- 347.Graziani A, Poteser M, Heupel WM, Schleifer H, Krenn M, Drenckhahn D, et al. Cell-cell contact formation governs Ca2+ signaling by TRPC4 in the vascular endothelium: evidence for a regulatory TRPC4-beta-catenin interaction. J Biol Chem. 2010;285(6):4213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, et al. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One. 2007;2(9):e827. [DOI] [PMC free article] [PubMed] [Google Scholar]


