As the creator of purinergic signalling [1], Prof. Geoffrey Burnstock (1929–2020) [2] also made important contributions to the science of acupuncture (AP). AP family procedures include mechanical needling, electroacupuncture, moxibustion, cupping, etc. [3]. His insightful hypothesis on the natural scientific basis of AP in 2009 [4] led to an explosion of research worldwide that aimed to explaining how purinergic signalling contributes to AP. The development of our understanding on AP-induced analgesia has been elaborately documented in the past [5–7]; hence, in this article, we summarize Geoff’s distinguished contribution to, and profound impact on, AP and also disclose some personal accounts of his impact on our personal careers in specific, and on the Chinese AP community on a larger scale.
Novel hypothesis on the purinergic mode of action of acupuncture
AP family procedures, originally developed in China, are widely used in over 183 countries and regions all over the world. In 1980, 43 kinds of diseases were recommended by the WHO to be treated by AP, and this number was increased to 64 in 1996. In 1997, an NIH consensus was reached and communicated to the public, stating that AP verifiably works on nausea, vomiting, pain, and other conditions, based on evidence provided by clinical trials and the endorphin theory of its mode of action [8, 9]. However, the endorphin hypothesis still failed to completely explain how AP acts, and therefore, it was a major development when in 2009 Geoff published his hypothesis [4], later updated in 2011 [10] and 2014 [11]. He proposed that insertion and twisting of the needles employed in AP mechanically deform the skin, causing the local release of ATP by keratinocytes (1) (Fig. 1). The ATP binds to and activates P2X3 and P2X2/3 receptors located on sensory nerve endings in the skin (2) to initiate action potentials. The signal is then relayed via the dorsal root ganglia to the spinal cord (3) and subsequently through interneuronal pathways (4) to the brain stem (5), which contains motor neurons that control the activity of the gut, lungs, heart, arteries, and reproductive organs—all major targets for AP. Signals also travel to the pain centers of the cortex, delivering a message to inhibit pain (6) [11].
Fig. 1.
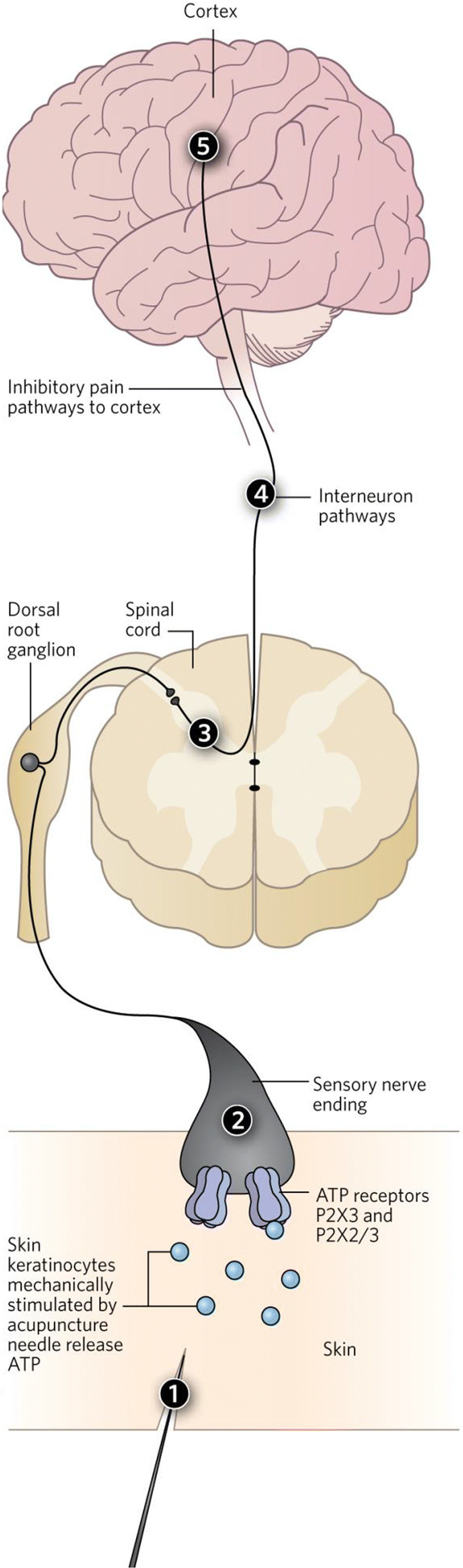
Purinergic hypothesis on acupuncture mechanism. For explanation, see text
Subsequently, AP research in the field of purinergic signalling started to flourish and a large set of data has been accumulated on changes in purine (ATP, adenosine) concentrations, both locally and in the CNS, induced by manual acupuncture, electroacupuncture, or moxibustion [12–17]. The participation of purinergic receptors (P1: A1, A2a, A2b, A3) [18–34], (P2: P2X2, P2X3, P2X7, P2Y1, P2Y13) [35–52], and neuronal pathways bearing these receptors [53, 54] has been confirmed in different AP-sensitive conditions (pain, inflammation, myocardial ischemia, obesity, cerebral ischemia). The functionalized acupuncture needle as a SERS (surface-enhanced Raman spectroscopy)-active platform was developed for rapid and sensitive determination of ATP concentration [55], and led further support to the purinergic basis of AP.
It is worth pointing out that AP science has enormously benefited from two findings confirming purinergic mechanisms as participating in AP. The first is the evidence that local adenosine and adenosine A1 receptors mediate the anti-nociceptive effect of acupuncture in mice, as shown by Maiken Nedergaard and her group, which indicated that the needling site at an AP point is crucial to explain the mode of action of AP analgesia. The second was that the injection of prostatic acid phosphatase (PAP) to an AP point slowed down the degradation of adenosine and caused strong analgesia in mice, a procedure named “PAPupuncture” by Mark Zylka in 2012 [56]. Taken together, the purinergic hypothesis of AP mechanism has opened new vistas to understand what happens at AP points and is expected to uncover more valuable details in future. We still do not know which layer (skin, muscles, vessels, nerve endings, etc.), which cell types (keratinocytes, melanocytes, Merkel cells, Langerhans cells, fibroblasts, myocytes, vascular endothelial cells, etc.) at the AP point are responsible for AP effects. The release of purines in relation to the 14 main meridians of the human body, assumed to be tightly connected with AP according to Traditional Chinese Medicine (TCM), is also unknown. There are many further open questions: (1) Which of the purine degrading enzymes (CD39, CD73) have the largest impact on AP?; (2) Which of the P2 receptors (P2X1, P2X4, P2X5, P2X6, P2X7, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14) are most important for AP?; and (3) Do P2X/Y receptors interact with other transmitter receptors in constituting AP effects? Especially the translational research from bench to bedside should be considered for AP research in the future.
Important ideas to promote our knowledge on the natural scientific basis of acupuncture: Yong Tang (Chengdu, China)
Geoff delivered three inspiring lectures on purinergic signalling and AP in the course of his life, two in Chengdu and one in Leipzig. In the winter of 2011, based on the recommendation of Geoff, Peter Illes (Leipzig University, Germany) and Yong Tang (Chengdu University of TCM, China) worked together to write a proposal to the Sino-German Center for the Promotion of Science and obtained the funding for their common project in the spring of 2012.
Financed by this project, the three co-chairs (Geoff, Peter, and Yong) (Fig. 2), organized the “1st Sino-German Symposium on Purinergic Signalling, Pain and Acupuncture” in Chengdu between October 10 and 15, 2012. Geoff, at the age of 83, flew to China with his wife Nomi at the official invitation of the Chengdu University of TCM and opened this meeting with a 1-h introductory lecture. His excellent talk, focusing on the AP purinergic hypothesis, attracted a number of senior and junior scientists and students to the auditorium. During the conference, Geoff also met a number of AP researchers and students and made concrete suggestions about how to perform experiments in order to test his novel hypothesis. He also initiated personal exchange activities and collaborations between the Chinese and oversea scientists to move forward the purine stories in AP.
Fig. 2.
Geoffrey Burnstock, Peter Illes, and Yong Tang at the 1st Sino-German Symposium on Purinergic Signalling, Pain and Acupuncture in Chengdu, 2012. Reproduced with permission from [57]
In 2017, the “2nd Sino-German Symposium on Purinergic Signalling, Pain and Acupuncture” was held in Leipzig. Geoff, at the age of 88, came to Leipzig with his wife Nomi and again gave the opening talk on purinergic signalling and AP. His lecture greatly encouraged the participating scientists (Fig. 3) to pursue the story of purines in AP. Lectures presented by Chinese participants also inspired the prevailing confidence that purinergic signalling will explain the beneficial effects of AP in pain and inflammation, as well as in immune, vascular, respiratory, gastrointestinal, neurological, and psychological disorders.
Fig. 3.
Geoffrey Burnstock, Peter Illes, and Yong Tang at the 2nd Sino-German Symposium on Purinergic Signalling, Pain and Acupuncture in Leipzig, 2017
In 2018, Geoff at an age of 89, delivered again a stimulating speech, also as an opening lecture at the “1st Chinese Purine Meeting” when the China Purine Club was founded (Fig. 4). His 1-h presentation let us witness the indomitable energy of the creator of purinergic signalling, even at this advanced age.
Fig. 4.

The opening lecture addressed by Geoffrey Burnstock at the 1st Chinese Purine Meeting in Chengdu, 2018
The purine story in acupuncture has experienced an astonishing surge in the last two centuries, from zero at the beginning, to up to over 50 publications presently, and it is still in sustained increase. Prof. Geoffrey Burnstock, the father of purinergic signalling, was appointed as Honorary Professor in the Chengdu University of TCM in 2016 and as Honorary President of the China Purine Club in 2018. During the two meetings in China attended by Geoff and Nomi, they visited the old city center of Chengdu, where Geoff enjoyed local sweets, and also visited the southern Yunnan Province of China, the Chendgu Panda base, and Du Fu Cottage, as well as the nearby Leshan Giant Budda (Fig. 5).
Fig. 5.
Geoffrey Burnstock visited Leshan Giant Buddha in 2012
Geoff, we will miss you badly in the future.
My encounters with Geoff (Peter Illes, Leipzig, Germany)
When looking back on my encounters with Geoff, I remember seeing him the first time in 1976 in Varna, Bulgaria, at a Conference organized on Smooth Muscle Physiology. He spoke of course about purinergic transmission in the taenia coli, eloquent as always, and I was most impressed by his talk. At this time, I was a resident of my hometown, Budapest (Hungary), where I had been working at the Department of Pharmacology of the Semmelweis University of Medicine for 10 years after graduation from Medical School. I was deeply impressed by Geoff’s talk and was able to present to him my poster on sympathetic transmission in the guinea-pig vas deferens, which he criticized in a friendly, but decisive manner, and in the retrospect, quite correctly.
The second time I came into contact with him, I was working in Freiburg (Germany) where I stayed from 1981 onwards, at the Department of Pharmacology of the University, as a naturalized German citizen. In 1983, I published a review on noradrenaline as a neuroeffector transmitter in the vas deferens, and discussed at length the riddle of why α- and β-adrenoceptor antagonists fail to block the excitatory junction potentials known to induce mechanical contractions in this organ [58]. The current idea was that although noradrenaline is released from the sympathetic nerve terminals and acts at postsynaptic α-adrenoceptors located at the smooth muscle, a basement membrane generates an anatomical barrier to block the free diffusion of the abovementioned antagonists to the receptor. Another hypothesis even suggested that a third type of adrenoceptor termed “γ” mediated the noradrenaline effects. Geoff sent me a letter in which he called my attention to the purinergic hypothesis and the strong evidence that the co-transmitter ATP rather than noradrenaline itself is the neuroeffector transmitter. Soon afterwards, I became also an enthusiastic advocate of this idea and joined the purinergic community.
Subsequently, he furthered my career many times, for example by inviting me to join the Editorial Board of the newly founded journal Purinergic Signalling, or supporting the Leipzig application for a Dislocated Research Group in 2007. It was a difficult task to convince the responsible bureaucrats of the German Research Council that such an initiative made sense. Geoff was a member of the Board evaluating our application and enthusiastically supported it. In consequence, the Research Group became established and was very productive over its 6-year period of functioning [59].
I had the opportunity to meet Geoff at many International Purine Congresses and scientific events in diverse countries. Especially memorable were our encounters in Budapest on occasion of him becoming Honorary Member of the Hungarian Pharmacological Society in 2006, and in Leipzig, on occasion of the awarding of an Honorary Doctorate of our University (2011). In both cases, he was accompanied by Nomi and we spent many hours enjoying animated conversation, good food, and tasty wine.
I have co-authored nine publications with Geoff, both original reports and review articles. One of them was especially successful, becoming a “highly cited paper” according to ISI Web of Science [60]. During our accidental co-operation, I learned to admire his sharp intellect and gift to describe complicated scientific processes in understandable and concise wording.
He had probably the greatest influence on my “late” scientific career after having retired as a Chairman of the Department of Pharmacology in Leipzig, by establishing a contact with Prof. Yong Tang (Chengdu, China) [57]. Yong has given an account of our common activities in the “Important ideas to promote our knowledge on the natural scientific basis of acupuncture: Yong Tang (Chengdu, China)” section.
In conclusion, let me state that having lost Geoff, as a benevolent friend, fatherly figure, and illuminating ideal is a huge personal hardship.
Funding
This article received funding from National Key R&D Program of China (2019YFC1709101), the Project First-Class Disciplines Development of Chengdu University of Traditional Chinese Medicine (CZYHW1901) in order to build up the “International Collaborative Centre on Big Science Plan for Purine Signalling,” State Administration of Foreign Experts Affairs to support the stays of P.I. in Chengdu (G20190236012), National Natural Science Foundation of China (81373735, 81873240), and Science and Technology Program of Sichuan Province, China (2019YFH0108).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of the Topical Collection on A Tribute to Professor Geoff Burnstock.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yong Tang, Email: tangyong@cdutcm.edu.cn.
Peter Illes, Email: peter.illes@medizin.uni-leipzig.de.
References
- 1.Abbracchio MP, Jacobson KA, Müller CE, Zimmermann H. Professor Dr. Geoffrey Burnstock (1929–2020) Purinergic Signal. 2020;16:137–149. [Google Scholar]
- 2.Verkhratsky A, Zimmermann H, Abbracchio MP, Illes P, Di Virgilio F. In memoriam Geoffrey Burnstock: creator of purinergic signaling. Function. 2020;1:zqaa006. [Google Scholar]
- 3.NIH Consensus Conference Acupuncture. JAMA. 1998;280:1518–1524. [PubMed] [Google Scholar]
- 4.Burnstock G. Acupuncture: a novel hypothesis for the involvement of purinergic signalling. Med Hypotheses. 2009;73:470–472. doi: 10.1016/j.mehy.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 5.Tang Y, Yin HY, Liu J, Rubini P, Illes P. P2X receptors and acupuncture analgesia. Brain Res Bull. 2019;151:144–152. doi: 10.1016/j.brainresbull.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Tang Y, Yin HY, Rubini P, Illes P. Acupuncture-induced analgesia: a neurobiological basis in purinergic signaling. Neuroscientist. 2016;22:563–578. doi: 10.1177/1073858416654453. [DOI] [PubMed] [Google Scholar]
- 7.He JR, Yu SG, Tang Y, Illes P (2020) Purinergic signaling as a basis of acupuncture-induced analgesia. Purinergic Signal. 10.1007/s11302-020-09708-z [DOI] [PMC free article] [PubMed]
- 8.Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361:258–261. doi: 10.1016/j.neulet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Skrabanek P. Acupuncture and endorphins. Lancet. 1984;28:220. doi: 10.1016/s0140-6736(84)92137-8. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G. Puncturing the myth. Purinergic signaling, not mystical energy, may explain how acupuncture works. Scientist. 2011;25:24–25. [Google Scholar]
- 11.Burnstock G. Purinergic signaling in acupuncture. Science. 2014;346(6216 Suppl):S23–S25. [Google Scholar]
- 12.Takano T, Chen X, Luo F, Fujita T, Ren Z, Goldman N, Zhao Y, Markman JD, Nedergaard M. Traditional acupuncture triggers a local increase in adenosine in human subjects. J Pain. 2012;13:1215–1223. doi: 10.1016/j.jpain.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye TS, Du ZH, Li ZH, Xie WX, Huang KT, Chen Y, Chen ZY, Hu H, Wang JL, Fang JQ. Repeated electroacupuncture persistently elevates adenosine and ameliorates collagen-induced arthritis in rats. Evid Based Complement Alternat Med. 2016;2016:3632168. doi: 10.1155/2016/3632168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinbara H, Nagaoka S, Izutani Y, Okubo M, Kimura K, Mizunuma K, Sumiya E. Contribution of adenosine to the increase in skeletal muscle blood flow caused by manual acupuncture in rats. Acupunct Med. 2017;35:284–288. doi: 10.1136/acupmed-2016-011152. [DOI] [PubMed] [Google Scholar]
- 15.Cheng LL, Wu CY, Sui MH. Effects of electroacupuncture of “Weizhong” (BL40), “Sanyinjiao” (SP 6) and “Yinlingquan” (SP 9) on intravesical pressure and bladder adenosine triphosphate content in rabbits with acute urinary retention. Zhen Ci Yan Jiu. 2012;37:291–295. [PubMed] [Google Scholar]
- 16.Hu L, Wang L, Wei J, Ryszard G, Shen X, Wolfgang S. Heat induces adenosine triphosphate release from mast cells in vitro: a putative mechanism for moxibustion. J Tradit Chin Med. 2015;35:323–328. doi: 10.1016/s0254-6272(15)30105-9. [DOI] [PubMed] [Google Scholar]
- 17.Wang JY, Li H, Zhang L, Ma CM, Wang JL, Lai XS, Zhou SF. Adenosine as a probing tool for the mechanistic study of acupuncture treatment. Clin Exp Pharmacol Physiol. 2014;41:933–939. doi: 10.1111/1440-1681.12304. [DOI] [PubMed] [Google Scholar]
- 18.Huang M, Wang X, Xing B, Yang H, Sa Z, Zhang D, Yao W, Yin N, Xia Y, Ding G. Critical roles of TRPV2 channels, histamine H1 and adenosine A1 receptors in the initiation of acupoint signals for acupuncture analgesia. Sci Rep. 2018;8:6523. doi: 10.1038/s41598-018-24654-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, Chen JF, Schnermann J, Takano T, Bekar L, Tieu K, Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu F, Cui Y, Zeng J, Zhang M, Qiu S, Huang X, Chen A. Acupuncture induces adenosine in fibroblasts through energy metabolism and promotes proliferation by activating MAPK signaling pathway via adenosine 3 receptor. J Cell Physiol. 2020;235:2441–2451. doi: 10.1002/jcp.29148. [DOI] [PubMed] [Google Scholar]
- 21.Dai QF, Wang YY, Liu Q, Xin JJ, Lu FY, Cui JJ, Wu SY, Zhou C, Zhao YX, Gao JH, Yu XC. A potential role of adenosine a 2 b receptor in mediating acupuncture pretreatment induced cardioprotection via influencing intracellular calcium regulator. Zhen Ci Yan Jiu. 2018;43:576–580. doi: 10.13702/j.1000-0607.180089. [DOI] [PubMed] [Google Scholar]
- 22.Hou T, Xiang H, Yu L, Su W, Shu Y, Li H, Zhu H, Lin L, Hu X, Liang S, Zhang H, Li M. Electroacupuncture inhibits visceral pain via adenosine receptors in mice with inflammatory bowel disease. Purinergic Signal. 2019;15:193–204. doi: 10.1007/s11302-019-09655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, Dai Q, Liang D, Li D, Chen S, Chen S, Han K, Huang L, Wang J. Involvement of adenosine A1 receptor in electroacupuncture-mediated inhibition of astrocyte activation during neuropathic pain. Arq Neuropsiquiatr. 2018;76:736–742. doi: 10.1590/0004-282X20180128. [DOI] [PubMed] [Google Scholar]
- 24.Ren W, Tu W, Jiang S, Cheng R, Du Y. Electroacupuncture improves neuropathic pain: adenosine, adenosine 5′-triphosphate disodium and their receptors perhaps change simultaneously. Neural Regen Res. 2012;7:2618–2623. doi: 10.3969/j.issn.1673-5374.2012.33.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du ZH, Zhang CW, Xie WX, Chen Y, Cong WJ, Wang ZD, Wang EP, Wu GY, Ye TS. Adenosine A2A receptor mediates inhibition of synovitis and osteoclastogenesis after electroacupuncture in rats with collagen-induced arthritis. Evid Based Complement Alternat Med. 2019;2019:4617464. doi: 10.1155/2019/4617464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen ZH, Wang R, Xie J, Ren YL. Mechanism of acupoint selection along meridians to improve adenosine receptor of myocardial ischemia based on acupoint specificity. Zhongguo Zhen Jiu. 2019;39:855–860. doi: 10.13703/j.0255-2930.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Lu S, Tang Y, Ding Y, Yu M, Fu S, Zhu B. Effects of electroacupuncture on the expression of adenosine receptors in the heart tissue of myocardial ischemia rats. Zhongguo Zhen Jiu. 2018;38:173–179. doi: 10.13703/j.0255-2930.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Tang YX, Meng JZ, Ding YJ, Hong H, Yu ML, Jing XY, Lu SF, Zhu BM. Effect of electroacupuncture on adenosine receptor expression in white adipose tissue of diet-induced obese mice. Zhen Ci Yan Jiu. 2017;42:39–44. [PubMed] [Google Scholar]
- 29.Dai QF, Gao JH, Xin JJ, Liu Q, Jing XH, Yu XC. The role of adenosine A2b receptor in mediating the cardioprotection of electroacupuncture pretreatment via influencing Ca2+ key regulators. Evid Based Complement Alternat Med. 2019;2019:6721286. doi: 10.1155/2019/6721286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren Y, Chen Z, Wang R, Yu Y, Li D, He Y (2020) Electroacupuncture improves myocardial ischemia injury via activation of adenosine receptors. Purinergic Signal. 10.1007/s11302-020-09704-3 [DOI] [PMC free article] [PubMed]
- 31.Li QH, Xie WX, Li XP, Huang KT, Du ZH, Cong WJ, Zhou LH, Ye TS, Chen JF. Adenosine A2A receptors mediate anti-inflammatory effects of electroacupuncture on synovitis in mice with collagen-induced arthritis. Evid Based Complement Alternat Med. 2015;2015:809560. doi: 10.1155/2015/809560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zylka MJ. Needling adenosine receptors for pain relief. Nat Neurosci. 2010;13:783–784. doi: 10.1038/nn0710-783. [DOI] [PubMed] [Google Scholar]
- 33.Malik S, Samaniego T, Guo ZL. Adenosine receptor A2a, but not A1 in the rVLM participates along with opioids in acupuncture-mediated inhibition of excitatory cardiovascular reflexes. Front Neurosci. 2019;13:1049. doi: 10.3389/fnins.2019.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang DD, Wang HF, Zhang MX, Dai QX, Liu HP, Mo YC, Wang JL. Local adenosine A1 receptors of baihui acupoint mediate cerebral ischemia tolerance induced by electroacupuncture. Zhonghua Yi Xue Za Zhi. 2013;93:537–540. [PubMed] [Google Scholar]
- 35.Weng Z, Wu L, Lu Y, Wang L, Tan L, Dong M, Xin Y. Electroacupuncture diminishes P2X2 and P2X3 purinergic receptor expression in dorsal root ganglia of rats with visceral hypersensitivity. Neural Regen Res. 2013;8:802–808. doi: 10.3969/j.issn.1673-5374.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiang X, Wang S, Shao F, Fang J, Xu Y, Wang W, Sun H, Liu X, Du J, Fang J. Electroacupuncture stimulation alleviates CFA-induced inflammatory pain via suppressing P2X3 expression. Int J Mol Sci. 2019;20:3248. doi: 10.3390/ijms20133248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou YF, Ying XM, He XF, Shou SY, Wei JJ, Tai ZX, Shao XM, Liang Y, Fang F, Fang JQ, Jiang YL. Suppressing PKC-dependent membrane P2X3 receptor upregulation in dorsal root ganglia mediated electroacupuncture analgesia in rat painful diabetic neuropathy. Purinergic Signal. 2018;14:359–369. doi: 10.1007/s11302-018-9617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang JQ, Du JY, Fang JF, Xiao T, Le XQ, Pan NF, Yu J, Liu BY. Parameter-specific analgesic effects of electroacupuncture mediated by degree of regulation TRPV1 and P2X3 in inflammatory pain in rats. Life Sci. 2018;200:69–80. doi: 10.1016/j.lfs.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 39.Weng ZJ, Wu LY, Zhou CL, Dou CZ, Shi Y, Liu HR, Wu HG. Effect of electroacupuncture on P2X3 receptor regulation in the peripheral and central nervous systems of rats with visceral pain caused by irritable bowel syndrome. Purinergic Signal. 2015;11:321–329. doi: 10.1007/s11302-015-9447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Leng C, Gong X, Ma B, Ru Q, Xiong Q, Zhou M, Tian X, Yue K, Li C, Wu Y. 2Hz EA reduces heroin withdrawal-induced hyperalgesia and heroin relapse by downregulating P2X3 receptors in DRG neurons. Biomed Res Int. 2019;2019:1873859. doi: 10.1155/2019/1873859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang Y, Gu Y, Shi R, Li G, Chen Y, Huang LM. Electroacupuncture downregulates P2X3 receptor expression in dorsal root ganglia of the spinal nerve-ligated rat. Mol Pain. 2019;15:1744806919847810. doi: 10.1177/1744806919847810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang WS, Tu WZ, Cheng RD, He R, Ruan LH, Zhang L, Gong YS, Fan XF, Hu J, Cheng B, Lai YP, Zou EM, Jiang SH. Electroacupuncture and A-317491 depress thetransmission of pain on primary afferent mediated by the P2X3 receptor in rats with chronic neuropathic pain states. J Neurosci Res. 2014;92:1703–1713. doi: 10.1002/jnr.23451. [DOI] [PubMed] [Google Scholar]
- 43.Yu J, Zhao C, Luo X. The effects of electroacupuncture on the extracellular signal-regulated kinase 1/2/P2X3 signal pathway in the spinal cord of rats with chronic constriction injury. Anesth Analg. 2013;116:239–246. doi: 10.1213/ANE.0b013e31826f0a4a. [DOI] [PubMed] [Google Scholar]
- 44.Xiao Z, Ou S, He WJ, Zhao YD, Liu XH, Ruan HZ. Role of midbrain periaqueductal gray P2X3 receptors in electroacupuncture-mediated endogenous pain modulatory systems. Brain Res. 2010;1330:31–44. doi: 10.1016/j.brainres.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 45.Cheng RD, Tu WZ, Wang WS, Zou EM, Cao F, Cheng B, Wang JZ, Jiang YX, Jiang SH. Effect of electroacupuncture on the pathomorphology of the sciatic nerve and the sensitization of P2X4 receptors in the dorsal root ganglion in rats with chronic constrictive injury. Chin J Integr Med. 2013;19:374–379. doi: 10.1007/s11655-013-1447-1. [DOI] [PubMed] [Google Scholar]
- 46.Tu WZ, Cheng RD, Cheng B, Lu J, Cao F, Lin HY, Jiang YX, Wang JZ, Chen H, Jiang SH. Analgesic effect of electroacupuncture on chronic neuropathic pain mediated by P2X3 receptors in rat dorsal root ganglion neurons. Neurochem Int. 2012;60:379–386. doi: 10.1016/j.neuint.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Guo X, Chen J, Lu Y, Wu L, Weng Z, Yang L, Xin Y, Lin X, Liang Y, Fang J. Electroacupuncture at He-Mu points reduces P2X4 receptor expression in visceral hypersensitivity. Neural Regen Res. 2013;8:2069–2077. doi: 10.3969/j.issn.1673-5374.2013.22.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen XM, Xu J, Song JG, Zheng BJ, Wang XR. Electroacupuncture inhibits excessive interferon- evoked up-regulation of P2X4 receptor in spinal microglia in a CCI rat model for neuropathic pain. Br J Anaesth. 2015;114:150–157. doi: 10.1093/bja/aeu199. [DOI] [PubMed] [Google Scholar]
- 49.Gao YH, Li CW, Wang JY, Tan LH, Duanmu CL, Jing XH, Chang XR, Liu JL. Effect of electroacupuncture on the cervicospinal P2X7 receptor/fractalkine/CX3CR1 signaling pathway in a rat neck-incision pain model. Purinergic Signal. 2017;13:215–225. doi: 10.1007/s11302-016-9552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu J, Chen XM, Zheng BJ, Wang XR. Electroacupuncture relieves nerve injury-induced pain hypersensitivity via the inhibition of spinal P2X7 receptor-positive microglia. Anesth Analg. 2016;122:882–892. doi: 10.1213/ANE.0000000000001097. [DOI] [PubMed] [Google Scholar]
- 51.Liu S, Shi Q, Zhu Q, Zou T, Li G, Huang A, Wu B, Peng L, Song M, Wu Q, Xie Q, Lin W, Xie W, Wen S, Zhang Z, Lv Q, Zou L, Zhang X, Ying M, Li G, Liang S. P2X7 receptor of rat dorsal root ganglia is involved in the effect of moxibustion on visceral hyperalgesia. Purinergic Signal. 2015;11:161–169. doi: 10.1007/s11302-014-9439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen D, Shen X, Schwarz W, Grygorczyk R, Wang L. P2Y13 and P2X7 receptors modulate mechanically induced adenosine triphosphate release from mast cells. Exp Dermatol. 2020;29:499–508. doi: 10.1111/exd.14093. [DOI] [PubMed] [Google Scholar]
- 53.Liao HY, Hsieh CL, Huang CP, Lin YW. Electroacupuncture attenuates CFA-induced inflammatory pain by suppressing Nav1.8 through S100B, TRPV1, opioid, and adenosine pathways in mice. Sci Rep. 2017;7:42531. doi: 10.1038/srep42531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao HY, Hsieh CL, Huang CP, Lin YW. Electroacupuncture attenuates induction of inflammatory pain by regulating opioid and adenosine pathways in mice. Sci Rep. 2017;7:15679. doi: 10.1038/s41598-017-16031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li P, Ge M, Lin D, Yang L. Functionalized acupuncture needle as a SERS-active platform for rapid and sensitive determination of adenosine triphosphate. Anal Bioanal Chem. 2019;411(22):5669–5679. doi: 10.1007/s00216-019-01945-5. [DOI] [PubMed] [Google Scholar]
- 56.Hurt JK, Zylka MJ. PAPupuncture has localized and long-lasting antinociceptive effects in mouse models of acute and chronic pain. Mol Pain. 2012;8:28. doi: 10.1186/1744-8069-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang Y, Yin HY, Illes P, Burnstock G, Chen JF. From the Sino-German collaboration on purines to the Chinese Purine Club. Purinergic Signal. 2019;15:387–389. doi: 10.1007/s11302-019-09665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Illes P. Electrophysiology of noradrenaline release from smooth muscle organs. Trends Pharmacol Sci. 1983;4:335–338. [Google Scholar]
- 59.Schöneberg T, Illes P, Burnstock G. The German Research Unit “Neuronal and glial P2 receptors; molecular basis and functional significance”. Purinergic Signal. 2010;6:285–287. doi: 10.1007/s11302-009-9176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burnstock G, Krügel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]





