Abstract
Fumarates are successfully used for the treatment of psoriasis and multiple sclerosis. Their antioxidative, immunomodulatory and neuroprotective properties make fumarates attractive therapeutic candidates for other pathologies. The exact working mechanisms of fumarates are, however, not fully understood. Further elucidation of the mechanisms is required if these drugs are to be successfully repurposed for other diseases. Towards this, administration route, dosage, and treatment timing, frequency and duration are important parameters to consider and optimize with clinical paradigms in mind. Here we summarize the rapidly expanding literature on the pharmacokinetics and pharmacodynamics of fumarates, including a discussion on two recently FDA approved fumarates Vumerity™ and Bafiertam™. We review emerging applications of fumarates, focussing on neurological and cardiovascular diseases.
Keywords: fumarates, neuropathic pain, neurodegenerative disease, atherosclerosis, ischemia-reperfusion injury
The history and future potential of fumarates
Fumaric acid salts were originally derived from fungi, lichen, and moss (e.g. Fumaria officinalis). In medieval Europe, Asia and the Middle East, fumaric acid-containing herbal remedies were prescribed for ailments ranging from general blood cleansing, eye diseases, and digestive/hepatobiliary complaints, to rheumatoid arthritis [1]. In the 1950s, Walter Schweckendiek, a German chemist, first showed the therapeutic effects of fumarates on psoriasis after self-treatment [2]. About 30 years later, a mixture of fumarates, primarily consisting of dimethyl fumarate, was approved in Germany under the name Fumaderm® for the treatment of psoriasis. Following a successful exploratory, prospective pilot study in multiple sclerosis patients [3], Biogen repurposed dimethyl fumarate for treatment of relapsing-remitting multiple sclerosis in 2013 under the name Tecfidera®. The clinical success of fumarates stimulated ongoing investigations into the associated molecular mechanisms of action, revealing antioxidant and anti-inflammatory effects. This article reviews the currently available fumarate-based drugs and their applications beyond psoriasis and multiple sclerosis [4], including application in neurological and cardiovascular disease. Furthermore, perspectives on new prodrugs, drug formulations and options for targeted delivery methods will be discussed with an eye on improving bioavailability and reducing side-effects.
Fumarate pharmacokinetics
Four fumarate esters of fumaric acid are approved as human drugs: monomethyl fumarate (MMF) (Bafiertam™), dimethyl fumarate (DMF) (Tecfidera®, Skilarence®, Fumaderm®), diroximel fumarate (DRF) (Vumerity™) and zinc, magnesium and calcium salts of monoethyl fumarate (MEF) (Fumaderm®). Tepilamide fumarate (TPF) is currently under investigation in phase 2 clinical trials (Clinical Trial Number: NCT03421197). These fumarates are approved or under investigation for the treatment of moderate to severe plaque psoriasis or relapsing-remitting multiple sclerosis (MS) (Table 1).
Table 1.
Pharmacokinetic properties of fumarate drugs evaluated in humans.
| Drug | Approval | Indication | Dosing regime | MMF equiv. | MMF T1/2 (h) | MMF Tmax (h) | MMF Cmax (mg/l) | MMF AUC0−∞ (h.mg/l) | MMF Vd (l) | MMF %PPB | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fed | fasted | fed | fasted | fed | fasted | fed | fasted | ||||||||
Fumaderm® (dimethyl fumarate) (salts of monoethyl fumarate) |
German Drug Administration (1994) | Psoriasis | Single dose 2 × 120 mg tablet (total 240 mg)a,d | 216 mg | 0.65 | 3.5 | 1.46 | 2.87 | [7] | ||||||
Tecfidera® (dimethyl fumarate) |
EMA (30st January 2014); FDA (27th March 2013) | MS | Single dose 240 mgb | 216 mg | 1 | 5.0 | 2.0–2.5 | 1.87 | 3.21 (reported as 40% increase from fed) | 8.21e (4.11) | Equivalent to fed | 53–73 | 27–45 | [131] | |
| Single dose 240 mgc | 216 mg | 1.28 | 3.0 | 1.83 | 3.67 | [132] | |||||||||
| 240 mg tablet BIDc | 432 mg | 2.17f | 7.01g | [133] | |||||||||||
Skilarence® (dimethyl fumarate) |
EMA (23rd June 2017) | Psoriasis | 120 mg tablet once dailya | 108 mg | 9.0 | 3.5 | 1.311 | 1.325 | 50 | [134] | |||||
Vumerity™
|
FDA (29th October 2019) | MS | Single dose 462 mgb | 235 mg | 1 | 7.0 | 2.5–3.0 | 1.18 (reported as 44% decrease from fasted) | 2.11 | Equivalent to fasted | 8.32e (4.16) | 72–83 | 27–45 | [135] | |
| Single dose 462 mgc | 235 mg | 0.76 | 2.5 | 1.70 | 3.81 | [132] | |||||||||
Bafiertam™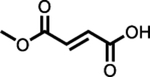 (monometh yl fumarate) |
FDA (28th April 2020) | MS | Single dose 2 × 95 mg tablets (total 190 mg)b | Figure | 0.5 | 11 | 4.03 | Reported as decreased by 20% from fasted | Bioequivalent to Tecfidera 240 mg (216 mg MMF equiv.) | Bioequivalent to Tecfidera 240 mg (216 mg MMF equiv.) | 53–73 | 27–45 | [107] | ||
| Tepilamide
fumarate |
Phase 2 clinical trials | Psoriasis | 200 mg tablet BIDc | 214 mg | 1.49f | 3.67g | [133] | ||||||||
| 400 mg tablet BIDc | 428 mg | 2.20f | 6.91g | [133] | |||||||||||
Moderate to severe plaque psoriasis patients.
Relapsing-remitting multiple sclerosis patients.
Healthy subjects.
Each 120 mg tablet also contains 95 mg of a mixture of MEF salts.
AUC0−∞ after intake twice a day (BID)
Reported as Cmax,ss
Reported as AUC24,ss. Tmax (time of maximal concentration), Cmax (maximal concentration), AUC (area under the curve, drug concentration in blood plasma as a function of time), Vd (volume of distribution), PPB (plasma protein binding).
DMF, DRF and TPF are known precursors to MMF, which is the only detectible metabolite in the blood stream of patients following oral intake [5–10]. It seems likely that MMF elicits the therapeutic action driving amelioration of disease. This has however been debated in the literature up until very recently [7,8,11–14]. MEF, the other monoester fumarate used in humans, is also found in the blood stream after administration. However, it is now well established that MEF has negligible therapeutic value and may increase the risk of adverse events when compared to DMF monotherapy [15,16].
All fumarates used as human drugs are delivered by enteric coated tablets to ensure release in the duodenum and small intestine, preventing the formation of gastric ulcers in patients. Here, MMF is rapidly and bioequivalently generated from diester prodrugs DMF, DRF and TPF through a combination of spontaneous and esterase hydrolysis [6,17] (Fig. 1). Interestingly, direct MMF administration leads to the same systemic MMF exposure compared to all diester prodrugs (Table 1). Once liberated, MMF becomes much less susceptible to esterase hydrolysis and spontaneous hydrolysis, preventing further degradation in the intestine which enables absorption into the presystemic circulatory system [6,17]. This lower susceptibility to hydrolysis is due to the ionizable carboxylic acid moiety of MMF, making it electronically less favorable for spontaneous hydrolysis and a poor substrate for the esterases in the small intestine [17].
Fig. 1. Hydrolysis pathways of fumarates.
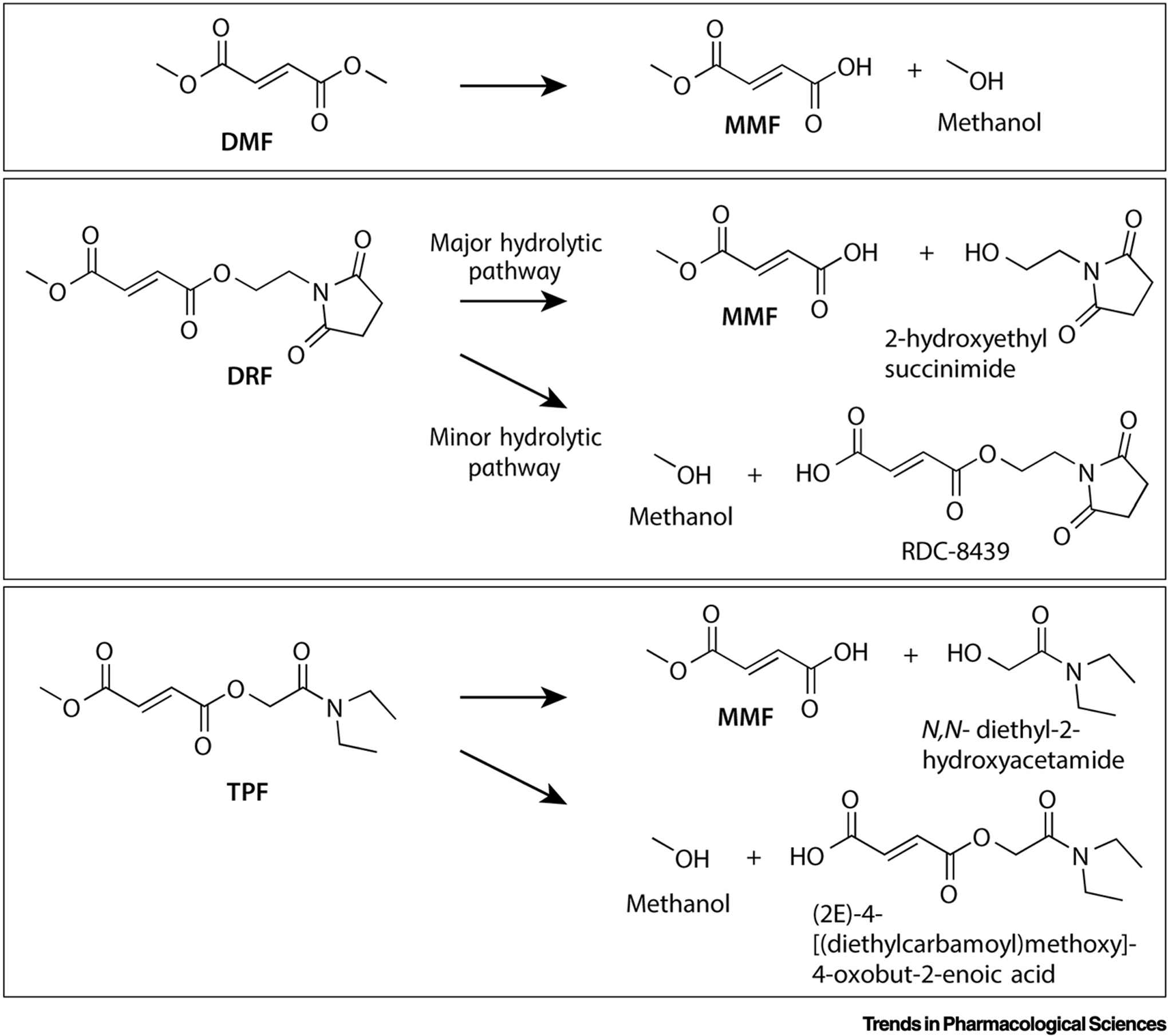
MMF is the major metabolite, along with the cleaved prodrug fragments methanol (DMF), 2-hydroxyethyl succinimide (DRF) and N,N-diethyl-2-hydroxyacetamide (TPF). DMF is a symmetrical diester and leads solely to MMF and methanol. For DRF, 2-hydroxyethyl succinimide and methanol formation occurs asymmetrically in a 9:1 ratio [18]. The biologically inert 2-hydroxyethyl succinimide is eliminated primarily through the renal system (58–63%) and the small amount of RDC-8439 formed is presumably converted to FA and 2-hydroxyethyl succinimide in the liver [17]. It is expected that TPF undergoes similar asymmetric cleavage and clearance, given its bioequivalent MMF production (Table 1), even though its spontaneous hydrolysis is not asymmetric [19].
DMF and MMF have high solubility, with high to medium membrane permeability respectively [17,18]. Based on their chemical characteristics, DRF and TPF are expected to have similar properties. As mentioned, monoesters (MMF, MEF) are absorbed into the pre-systemic circulatory system, along with a small proportion of the highly permeable diesters (DMF, DRF, TPF) that escape hydrolysis in the small intestine [17]. DMF, and likely DRF and TPF, react rapidly, in the order of minutes, with glutathione [14], resulting in undetectable diester fumarate levels in the plasma of patients [5–10]. Glutathione-DMF adducts observed in the portal vein and intestinal mucosa of rats after oral administration [8], and the mercapturic acid derivative of DMF found in the urine of human patients [13] outline this metabolic fate. It has been concluded that in the metabolism of MMF, glutathione adduct formation does not play a big role [14].
Only the monoesters MMF and MEF reach the liver. Cytochrome P450 enzymes are not involved in the metabolism of MMF, nor does MMF interact with P-glycoprotein in the liver [18]. Based on hepatic metabolism studies, MMF and MEF are degraded in the liver to produce methanol and ethanol respectively, along with fumaric acid [17]. Once in the blood, this same clearance process occurs in blood cells, and presumably other tissues as MMF and MEF are distributed systemically [6]. The secondary metabolite, fumaric acid, enters the tricarbocyclic acid cycle where it is metabolized to carbon dioxide and expelled in the breath [12].
MMF has a high apparent volume of distribution and high free fraction in blood plasma (Table 1), providing plenty of exposure for therapeutic action. MMF has been shown to penetrate the central nervous system of MS patients, with levels ranging from 0 – 0.0889 mg/l, significantly lower than in the blood stream [19].
Pre-clinical versus clinical doses of fumarates
One challenge when designing pre-clinical studies is the identification of clinically relevant doses. The recommended human dose of DMF (Tecfidera) for multiple sclerosis is 480 mg/day. Based on an average human weight of 62kg, this equals a dose of 7.74 mg/kg/day. Using a body surface area dose translation from humans to mice or rats [20], the equivalent daily dose is 95 mg/kg/day in mice and 48 mg/kg/day in rats. While this type of isometric scaling represents a starting point, it neglects the pharmacokinetic differences between species [21]. To emphasise this point, mice have a Cmax of 30–40 mg/l at Tmax of 30 minutes after a single 100 mg/kg dose of DMF [22,23]. This is very different from the pharmacokinetic profile in humans where an MMF equivalent dose of 216–235 mg has a Cmax of 1.5–2 mg/l in plasma at Tmax around 3 h (Table 1). Without knowledge of total drug exposure (AUC0−∞), T1/2, protein binding and volume of distribution in mice or rats, it is difficult to determine which factors contribute most to inter-species differences. Reverse translation of fumarates in experimental autoimmune encephalomyelitis models of multiple sclerosis, where the effective dose is 30–100 mg/kg/day DMF [24–26], is currently the best estimate of clinically relevant doses of prodrugs of MMF to treat new indications.
Mechanisms of action of fumarates
Three main mechanisms of action have been described for MMF and its prodrugs: 1) activation of nuclear factor erythroid 2-related factor 2 (NRF2; NFE2L2); 2) activation of the hydroxycarboxylic acid receptor 2 (HCAR2), and 3) direct inhibition of pro-inflammatory signaling and immune cell polarization (Fig. 2). As noted above, the pharmacodynamics of MMF are likely to be most clinically relevant.
Fig. 2. Intracellular mechanisms of action of MMF and fumarate prodrugs.
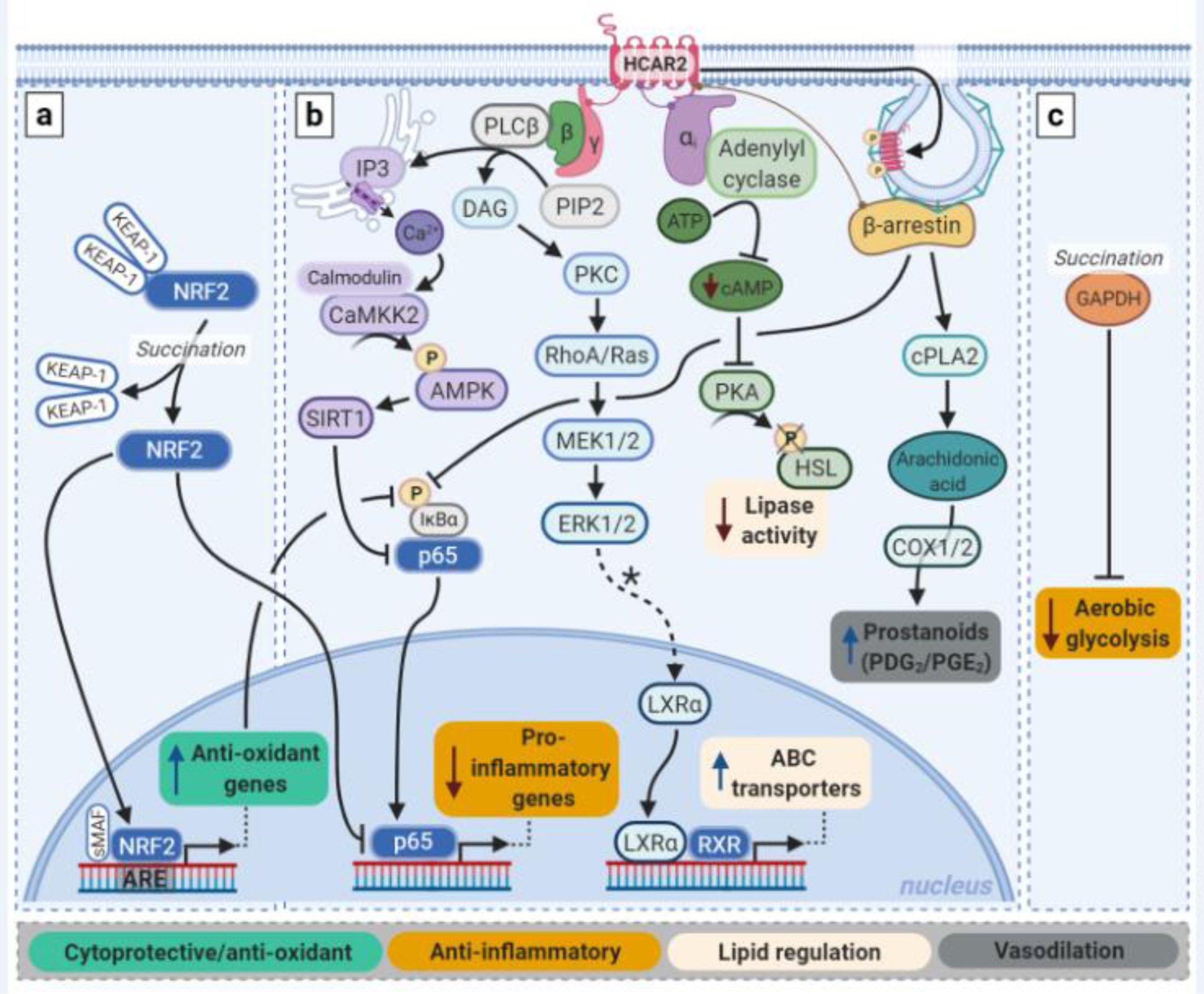
Fumarates impose their effects via three main pathways: a) the NRF2 pathway; b) the HCAR2 pathway; and c) via immunomodulation. a) Both MMF and fumarate prodrugs can induce activation and nuclear translocation of NRF2 by succination of Keap-1. Once inside the nucleus, NRF2 promotes transcription of cytoprotective genes, including those encoding antioxidants. The pro-inflammatory transcription factor NFkB can be inhibited by the NRF2 pathway both via a reduction in ROS levels and via competition with NRF2 over CREB-binding proteins. b) MMF is a full agonist of HCAR2. Activation of this Gi-protein coupled receptor results in release of the Giα and Gβƴ proteins. This leads to inhibition of NFkB activation and lipase activity. Via PKC and ERK1/2, PPARƴ-mediated transcription of LXRα is thought to be upregulated, which in turn promotes transcription of ABC transporters. β-arrestin binding to HCAR2 is important in receptor regulation by promoting desensitization and internalization via clathrin-coated pits, and it can inhibit NFĸB activity. β-arrestin is also involved in prostanoid production mediated by cyclo-oxygenase (COX) 1/2 in keratinocyte and Langerhans cells. c) Independent of the NRF2 and HCAR2 pathways, MMF and prodrugs can reduce inflammation by blocking glycolytic metabolism via succination and thus inactivation of GADPH, which favors differentiation of macrophages and T-cells to an anti-inflammatory phenotype. In general, of note, the pharmacodynamics of MMF are likely to be most clinically relevant. PIP2: phosphatidylinositol biphosphate; DAG: diacylglycerol; ATP: adenosine triphosphate; HSL: hormone-sensitive lipase. *Hypothesized.
Fumarates activate NRF2
Fumarates have widely recognized antioxidant properties, mediated through the transcription factor NRF2 which is ubiquitously expressed [24,26–29]. Under physiological conditions, Kelch-like ECH-associated protein 1 (Keap1) sequesters NRF2 in the cytosol, forming a complex with ubiquitin ligase to tag NRF2 for proteasomal degradation. Electrophiles and oxidants target reactive thiols of Keap1, altering its conformation to release and stabilize NRF2. Following translocation to the nucleus, NRF2 binds to the antioxidant response elements of a suite of cytoprotective genes including those encoding antioxidants. The electrophiles DMF and MMF specifically succinate Cys151 of Keap1 [24–26]. Consequently, fumarates induce nuclear translocation of NRF2 and increase expression of antioxidant genes in vitro and in vivo [24,26–28].
Besides the cyto- and mito-protective effects, the NRF2 pathway is also an intermediate in some anti-inflammatory effects of fumarates [24,26,28,30,31]. For example, NRF2 can attenuate activation of nuclear factor κB (NFκB (see Glossary)) in several ways [32,33]. NRF2-dependent antioxidants catabolize reactive oxygen species (ROS) that otherwise stabilize the NFκB p65 subunit by phosphorylating IκB [34]. NRF2 can also reduce NFκB activity by competing for CREB-binding protein, a transcriptional co-activator required by both factors [35]. Other anti-inflammatory effects include NRF2-dependent induction of heme oxygenase-1, an enzyme with numerous anti-inflammatory effects [36,37].
MMF is an HCAR2 agonist
MMF, but not its prodrugs, has been identified as a full agonist of the Gi/Go-protein coupled receptor HCAR2, also called GPR109A (human) or PUMA-G (mouse) [38]. HCAR2 is highly expressed on adipocytes and neutrophils, and is expressed at lower levels by other immune cells including monocytes, macrophages, microglia, dendritic cells and Langerhans cells, but not lymphocytes, neurons or other glial cells. Epithelial cells like intestinal epithelial cells and keratinocytes also express HCAR2 [38,39].
Under physiological conditions, HCAR2 regulates lipolysis in adipocytes and is only activated upon fasting which increases β-hydroxybutyrate levels, the endogenous ligand of HCAR2 [40]. One of the canonical HCAR2 signaling pathways runs via the G-protein αi subunit which inhibits adenylyl cyclase, that in turn reduces cAMP levels. Subsequent decrease in protein kinase A (PKA) activity can reduce phosphorylation of multiple substrates, including important lipolysis regulators like hormone-sensitive lipase [41]. A second signaling pathway runs via the G-protein Gβƴ subunits which, upon dissociation, can activate protein kinase C, causing ERK1/2 activation. This has implications for important lipid transport mechanisms as ERK1/2 is thought to increase ATP-binding cassette transporter A1 expression (via PPARƴ and LXRα), which mediates cholesterol efflux [42,43] (Fig. 2).
HCAR2 agonism with exogenous ligands like fumarates can impose anti-inflammatory effects. Inflammatory cell migration is thought to be attenuated by competition between HCAR2 and chemokine receptors for the same Gβƴ proteins and/or by ERK1/2 activation [42]. Immune cell activation can be reduced via Gβƴ-regulated phospholipase Cβ (PLC-β) and inositol triphosphate (IP3) which increase intracellular calcium levels, activating calcium/calmodulin-dependent protein kinase 2 (CaMKK2). This leads to phosphorylation and activation of 5’ AMP-activated protein kinase (AMPK) which elicits a range of effects including activation of the protein deacetylase sirtuin-1 (SIRT1). SIRT1 can inhibit NFĸB activity (Fig. 2) [44,45].
HCAR2 activation can also result in recruitment of β-arrestin. Besides its role in receptor desensitization and internalization, β-arrestin, via cytosolic phospolipase A2 (cPLA2), can increase production of prostanoids, which are regarded the main factors responsible for the flushing side effects of fumarates (see ‘Side effects’ section) [46]. Moreover, β-arrestin can interact with IĸBα which prevents NFĸB activation [47].
Fumarates are immunomodulatory
Fumarates possess anti-inflammatory properties that are independent of the NRF2 and HCAR2 pathways. Polarization towards anti-inflammatory immune cell phenotypes [22] is at least in part explained by effects on cell metabolism. Upon pro-inflammatory activation, immune cells switch from oxidative phosphorylation to aerobic glycolysis [48]. Although glycolytic metabolism is less efficient, glycolysis can be rapidly activated to meet the increased energy demands of classically activated (pro-inflammatory) immune cells. Glycolytic metabolism also generates biosynthetic intermediates that support activation, proliferation and effector functions of classically activated immune cells. In contrast, oxidative phosphorylation supports the functions of cells with an alternative (anti-inflammatory) activation phenotype [48]. DMF and MMF block glycolysis by irreversibly inactivating GAPDH through succination of the active thiols Cys150 (mouse) and Cys152 (human) (Fig. 2) [25]. Consequently, DMF and MMF inhibit differentiation and function of classically activated T cells (Th1, Th17) and macrophages (M1), instead favoring differentiation of alternatively activated Th2, Treg and M2 phenotypes [25].
In summary, fumarates have pleiotropic therapeutic effects through NRF2, HCAR2 and immunomodulation. Oxidative stress and inflammation subserve more than 40 human diseases that constitute the major proportion of the world’s current healthcare burden [49,50]. Fumarates therefore have the potential for major therapeutic impact.
Therapeutic applications and future potential of fumarates
Fumarates are FDA- and EMA-approved for treatment of psoriasis and multiple sclerosis [51–53] and have been investigated in clinical trials for the treatment of various other diseases (Box 1). Growing preclinical literature however also supports the use of fumarates for other indications. Here, we summarize this evidence for chronic pain and other neurological disorders like Parkinson’s disease and Alzheimer’s disease, as well as for cardiovascular diseases like myocardial and cerebral infarctions, and atherosclerosis.
Box 1. Clinical application of fumarates in psoriasis, multiple sclerosis, and other indications.
The success of fumarate treatment in psoriasis and multiple sclerosis stimulated research into the working mechanisms of fumarates in these pathologies, which have recently been reviewed elsewhere [105,113]. Accumulating evidence from in vitro, preclinical and clinical studies shows that fumarates exert their antipsoriatic effect via modulation of the antioxidant defenses of cells and of inflammatory pathways which are known to exacerbate psoriasis [4,114,115]. Studies using the experimental autoimmune encephalomyelitis model and clinical samples from patients with multiple sclerosis have revealed that fumarates treat both the inflammatory and neurodegenerative components of the disease through immunomodulatory- and Nrf2-dependent mechanisms [22,25,26,116,117]. Besides psoriasis and multiple sclerosis, fumarates have been investigated in clinical trials for the treatment of rheumatoid arthritis, various lymphomas, obstructive sleep apnea and adult brain glioblastoma [108]. A case study in lupus erythematosus showed beneficial effects in two patients [118].
Chronic pain
Fumarates are emerging as a promising therapeutic in preclinical models of chronic pain. To date, most studies have focused on neuropathic pain. Damage to the nervous system increases the sensitivity and excitability of neurons within the pain neuroaxis—both directly and through intermediate processes like nitro-oxidative stress and neuroinflammation—to cause spontaneous pain and nociceptive hypersensitivity. In rodent models, repeated oral administration of DMF alleviated nociceptive hypersensitivity induced by peripheral nerve injury and the chemotherapy oxaliplatin [54–56].
NRF2 and HCAR2 signaling underlie the therapeutic effects of DMF in these neuropathic pain models. However, these mechanisms may be both sex- and temporally-dependent. DMF treatment induced nuclear translocation of NRF2 and increased levels of antioxidants such as SOD1/2/3 and total glutathione in the dorsal root ganglia of rats [54]. Further, the antinociceptive effects of DMF were abolished in male and female Nrf2−/− mice, or by co-administration of the NRF2 inhibitor trigonelline [54]. These behavioral assessments implicating NRF2 were performed between 2 and 4 h after DMF dosing [54]. When nociceptive hypersensitivity was assessed within 2 h of DMF administration in another study, antinociception was more pronounced in females, compared to males, and completely abolished in female, but not male Hcar2−/− mice [55]. These data suggest that HCAR2 mediates the immediate antinociceptive effects of DMF preferentially in females, whereas the longer-acting therapeutic effects of DMF are mediated through NRF2 in a sex-independent manner.
Fumarates treat several mechanisms that underlie neuropathic pain. Repeated DMF administration completely normalized evoked and spontaneous neuronal hyperexcitability in the rostral ventromedial medulla after peripheral nerve injury [55]. DMF and MMF were also protective of primary sensory neurons when co-treated with several chemotherapies in vitro [57]. This result predicts that fumarates may prevent loss of intraepidermal nerve fibers following chemotherapy, which is believed to underlie symptoms of numbness and dysesthesia. Relatedly, DMF treatment can reverse the deficits in sensory neuron mitochondrial function caused by peripheral nerve injury [54]. While the mechanisms linking neurodegeneration and mitochondrial damage to neuronal hyperexcitability are not yet fully understood, restoring such deficits attenuates evoked and ongoing pain. The extent to which activation of NRF2, HCAR2 and/or immunomodulation underlie the cyto- and mito-protective effects of fumarates still requires study. DMF also attenuated injury-induced increases in reactive oxygen species (ROS) and of pro-inflammatory cytokines in the dorsal root ganglia [54]. These actions are directly antinociceptive: reactive oxygen and nitrogen species induced after nerve injury hyperexcite sensory neurons via transient receptor potential ion channels, while inflammatory mediators enhance neuroexcitatory glutamatergic signaling in the pain neuraxis, among other mechanisms [58–60].
Fumarates also offer potential as therapeutics for other types of chronic pain that involve inflammation and oxidative stress, including arthritis and migraine. In a rat model of arthritis, a single intraperitoneal dose of MMF dose-dependently alleviated pressure hyperalgesia, as well as unevoked vocalizations, a sign of spontaneous pain [61]. In a nitroglycerine-induced migraine model, acute oral administration of DMF attenuated heat hyperalgesia and photophobia [62]. DMF also increased levels of NRF2, SOD2, and HO-1, while decreasing NFκB p65 levels in the whole brain [62]. However, the therapeutic mechanisms of action in both models remain to be defined. In summary, fumarates are antinociceptive in several chronic pain models through suppression of the key mechanisms. These models recapitulate the neurobiology and symptomatic profiles of neuropathic pain, arthritis, and migraine. However, future studies should continue to assess therapeutic efficacy of fumarates in complex and operant pain-related behavioral tasks; such behavioral endpoints may improve predictive validity of preclinical studies [63–67].
Neurodegenerative diseases
Accumulating evidence from preclinical studies supports a therapeutic role for fumarates in a range of neurodegenerative diseases, including Parkinson’s disease and Alzheimer’s disease. Repeated oral administration of DMF prevented the loss of dopaminergic neurons in the substantia nigra pars compacta across several chemically-induced rodent models of Parkinson’s disease [24,68–70]. To date, most studies have focused on the role of NRF2 in the therapeutic effects of DMF in rodent models of Parkinson’s disease. DMF treatment increased transcript and protein levels of NRF2 as well as expression of NRF2 target genes in the striatum and cortex [68–70]. Demonstrating a causal role of NRF2, the neuroprotective effect of DMF was lost in Nrf2−/− mice, but was retained in wild-type controls [24,70]. DMF may also exert neuroprotective actions by reducing astrogliosis and promoting autophagy of α-synuclein in the ventral midbrain [70]. Collectively, fumarates are neuroprotective in chemically-induced models of Parkinson’s disease. Future studies should also test fumarates in models of α-synucleinopathy to test whether they can slow, halt or reverse the disease course [71,72].
DMF also shows some potential for the treatment of Alzheimer’s disease. In a streptozotocin model of Alzheimer’s disease, rats were pre- and post-treated daily with DMF. DMF prevented spatial memory loss and concomitant degeneration of neurons in all regions of the hippocampus [73,74]. These neuroprotective effects may be driven by attendant decreases in nitrotyrosine labelling (peroxynitrite marker) and the number of CD68+ microglia [74]. However, DMF’s mechanisms of action in Alzheimer’s disease still require elucidation, and treatment paradigms should be investigated for maximal clinical relevance. The efficacy of fumarates should also be tested in other models of Alzheimer’s disease, as individual models only partially mimic the spectrum of human pathology [75–77].
Cardiovascular diseases
Cardiovascular disease is a collective term for a range of pathologies of the myocardium and vasculature driven by inflammation and oxidative stress. Fumarates have been tested in preclinical models of stroke, myocardial infarction, fibrotic cardiac disorders, and atherosclerosis.
Stroke
Fumarate treatment for ischemic stroke (Box 2) is an emerging field. Oral DMF treatment has been tested as prophylactic therapy in two studies that utilized a middle cerebral artery occlusion model of permanent cerebral ischemia. The results were promising with a reduced edema volume, decreased cognitive impairment and improved neuronal integrity. However, the unpredictable nature of a stroke limits the clinical application of prophylactic therapies. In both studies, DMF reduced oxidative stress in the infarct region as marked by reduced 4-hydroxynonenal levels, and increased levels of NRF2 and some of its downstream targets like NQO1 [78,79]. Furthermore, anti-inflammatory IL-10 levels were increased in the plasma and infarct zone. Reperfusion post-stroke can also cause extensive tissue damage which might be alleviated by fumarate treatment (Box 2).
Box 2. Ischemia-reperfusion injury – a potential indication as fumarate target.
Ischemia occurs when blood supply to a tissue region does not meet the demand. This imbalance results in a deficiency in metabolically relevant molecules (e.g., oxygen, glucose). During ischemia, mitochondrial anaerobic glycolysis leads to breakdown of glycogen into ATP and lactic acid. Lactic acid reduces tissue pH, which in turn inhibits ATP production. A lack of ATP causes failure of ATP-dependent ion-pumps and loss of the transmembrane gradients, resulting in cellular swelling and tissue edema. Ischemia also activates phospholipases, which degrade membrane lipids. In the brain, these processes also disrupt the integrity of the blood-brain barrier. The hibernating tissue (penumbra) is irreversibly damaged (ischemic core) within hours if perfusion is not restored [119,120].
During ischemia, ATP degradation leads to hypoxanthine production. Upon reperfusion by for example thrombolysis or stenting, oxygen degrades hypoxanthine, liberating and converting superoxide anions to hydrogen peroxide and hydroxyl radicals. These species cause disruption of cell permeability, eventually leading to cell death. ROS activate endothelial cells to facilitate adhesion and transmigration of inflammatory cells, promoting inflammatory damage to the area surrounding the infarct. The disrupted blood-brain barrier allows edema formation in and around the infarct zone in the brain parenchyma [120,121].
Preventing initial ischemic damage is challenging, if not impossible in a clinical setting. However, there is a window of opportunity to treat reperfusion injury. As reperfusion injury involves inflammation and oxidative stress, fumarates are high-potential therapeutic candidates. Preclinical studies of reperfusion injury after stroke showed that fumarates reduced local oxidative stress, inflammation, and cerebral edema by restoring blood-brain barrier integrity. These changes were accompanied by reduced infarct size and improved motor and sensory function [122–125]. In myocardial infarction, DMF treatment consistently reduced infarct volume and improved hemodynamic parameters (e.g., cardiac function) which was found to be dependent of NRF2 signaling [126–128].
Besides the major role of ischemia-reperfusion injury in stroke and myocardial infarctions, it can also play a role following ischemic injury of other organs like the kidneys, intestine, liver and lungs [121]. Future studies could identify the optimal treatment paradigm for each organ and the protective mechanisms of action of fumarates during reperfusion.
Although ischemic stroke comprises the majority of stroke cases in humans, intracerebral hemorrhage is a form of stroke with a poor prognosis and no available therapy. Following initial hematoma-induced mechanical injury to the initial infarct area, edema and cellular damage caused by hemolysis products and inflammation aggravate the damage in the penumbra [80]. In collagenase and autologous blood injection models of hemorrhagic stroke, a single post-surgical dose of DMF attenuated neurological deficits and reduced cerebral edema. The latter was linked to improved integrity of the blood-brain barrier [81]. Moreover, DMF treatment reduced the number of activated microglia and ICAM-1 protein levels, an adhesion molecule that facilitates extravasation of leukocytes. All observed changes could be related to involvement of the NRF2 pathway [81]. In contrast to a single dose of DMF, multi-day dosing of DMF after infarction reduced hematoma volume by stimulating the phagocytic activity of microglia in a model of autologous blood injection-intracerebral hemorrhage [82].
For both ischemic and hemorrhagic stroke, the significant reduction in inflammation and oxidative stress, and the improved blood-brain barrier integrity following fumarate treatment protect the penumbra against secondary damage. It would be instructive to determine whether other mechanisms contribute to the therapeutic effects of fumarates, besides the NRF2 pathway. Although these pre-clinical studies show positive outcomes, there are ongoing discussions on the frequent failure of therapeutics tested in rodent stroke models in human clinical trials [83]. One of the main issues is that rodents have lissencephalic brains, with a very different architecture and blood supply from human brains [84]. Recent efforts in developing larger animal models for stroke like pigs, sheep and nonhuman primates will greatly contribute to assess the effectiveness of fumarates in stroke treatment [84].
Atherosclerosis and other vascular diseases
Untreated atherosclerosis can cause ischemic stroke or myocardial infarction, as rupture of unstable plaques triggers acute thrombotic events. In psoriasis and MS patients, fumarates increase atheroprotective serum adiponectin and high-density lipoprotein levels, while reducing proatherogenic total cholesterol and ApoB levels, indicating potential as anti-atherosclerotic drugs [85–87]. Although clinical outcome data of fumarate-treated atherosclerotic patients are lacking, preclinical studies in balloon-, diabetes- or high fat diet-induced animal models of atherosclerosis showed that DMF treatment reduced neointimal or aortic plaque formation [88–90]. ROS and inflammation markers including NFĸB-p65, TNF and IL-1β were concomitantly reduced in the plaque region, together with an increase in antioxidant markers such as NRF2 and superoxide dismutase [88–90]. Similar to the observations in human patients, DMF treatment reduced total, LDL- and HDL-cholesterol serum levels in hypercholesterolemic rabbits [89], which might further contribute to the observed reduction in plaque development.
Fumarates may also be indicated for other diabetes-related vascular pathologies. In a streptozotocin model of vascular dysfunction, repeated oral DMF treatment improved ex vivo aortic relaxation and contraction responsiveness [91]. This was accompanied by reduced aortic ROS levels, and transcript (Nfĸbiα and Nlrp3) and protein (i.a. IL-1β, TNF and TGFβ) expression of inflammatory mediators in the vessel wall, together with an upregulation of NRF2-regulated antioxidant genes [91]. Another complication of diabetes is vascular calcification. Prophylactic oral DMF treatment dose-dependently reduced vitamin D3-induced vascular calcification in a mouse model [92]. In vitro, DMF attenuated the differentiation of rat vascular smooth muscle cells to osteoblasts which inhibited calcium production [92]. Whether DMF elicits the same effects in diabetes-induced vascular calcification remains to be elucidated.
Overall, the reduction in inflammation and oxidative stress following fumarate treatment is consistent and promising, and has proven effective in prevention of these vascular pathologies. Studies with different treatment paradigms and longer treatment times are now needed to establish whether fumarates can also reverse these pathologies, and whether fumarates prevent the associated clinical events following these often slowly developing diseases. Furthermore, all these studies have been conducted in rodent models which show pronounced differences in (lipid) metabolism, physiology and hemodynamics compared to humans which hampers development of human-like advanced vascular disease [93,94]. For clinical translation, the capacity of fumarates to reduce or stabilize more advanced vascular pathologies will be most interesting to study, which inherently requires the use of larger, older or surgically manipulated animal models to improve the resemblance to human disease.
Cardiac pathologies
Fumarates have been tested as prophylactic treatments in three common cardiac pathologies: ischemia/reperfusion myocardial infarction (Box 2), cardiac hypertrophy and cardiomyopathy. In models of cardiac hypertrophy and diabetes-induced cardiomyopathy, fumarate administration reduced fibrosis and damage of the cardiac muscle which was associated with an improved cardiac function [95,96]. Furthermore, inflammation markers like NFκB-p65, TNF and IL-6 were reduced in the cardiac tissue while protein and mRNA levels of NRF2 and some of its downstream targets were elevated [95,96]. Future studies will have to show whether fumarates are also effective as a therapeutic in the more clinically relevant advanced stages of cardiac hypertrophy/myopathy.
Another increasingly common cardiac pathology is heart failure. At the moment of writing, there are no studies published on the potential benefits of fumarates in this disease. However, the importance of both inflammation and oxidative stress in the development and long-term progression of heart failure [97] warrants future studies to explore the potential therapeutic benefits of fumarates.
Side-effects of fumarates
The common, although often mild, side-effects of DMF include flushing and gastrointestinal complications [98,99]. These side effect frequently peak during the initial start-up period of treatment, despite the recommended dose-titration strategy to mitigate these effects. Flushing is believed to occur via HCAR2-dependent production of prostanoids (Fig. 2) by keratinocytes and Langerhans cells in the skin. Prostanoids act on smooth muscle cells and mast cells, causing vasodilation [46]. Recently, the FDA granted orphan drug designation to Vitalis LLC for DRF or MMF and its VT-Aspirin platform as a combination therapy to alleviate this common complaint occurring in around 35% of patients [53,100,101]. Gastrointestinal adverse effects have been attributed to the local release of damaging levels of methanol in the intestine due to metabolism of the prodrugs and is sited as a reason for reduced adverse GI events when using DRF (Fig. 1) [102]. With DRF (Vumerity®), the gastrointestinal tolerability is much improved (34.8%) compared to DMF (49.0%) [53,102]. This is however still a considerable proportion of patients. In patients with persistent gastrointestinal complaints, drug intake with food is recommended and a variety of additional drugs can be prescribed to alleviate these side effects [103].
Besides these common side effects, fumarate treatment has also been associated with leukopenia, especially of lymphocytes [104]. These effects have been partially attributed to increased apoptosis and decreased proliferation, mainly of the pro-inflammatory CD4+ T-lymphocyte subset as further described in [105]. Guidelines advise monitoring of leukocyte levels during fumarate therapy commencement to prevent long-standing leukopenia, which is associated with an increased infection risk.
The recent FDA approval of MMF (Bafiertam™) [106,107], holds promise for alleviation of some fumarate induced adverse events since adverse event profiles differ between prodrugs Tecfidera® and Vumerity™ [53], attributing these events to the prodrug delivery method (Box 3). It should however be noted that side effects such as leukopenia and HCAR2-mediated flushing are linked to MMF exposure and will not be eliminated by moving to direct administration of MMF [12,46,104].
Box 3. Bafiertam: a different view on prodrug necessity.
The use of esters as prodrug moieties is generally to facilitate absorption of drugs with properties that limit this. Ester prodrug hydrolysis typically occurs through spontaneous hydrolysis in the aqueous environment of the body, or through interaction with non-specific or specific esterase or cytochrome P450 activity [129]. The recent FDA approval of Bafiertam™, an oral MMF monotherapy for the treatment of MS, suggests 1) that prodrugs of MMF are unnecessary for systemic delivery of MMF and 2) that MMF is the therapeutic agent driving amelioration of disease. Bafiertam’s approval was based on a single 5-week phase 1 clinical trial head-to-head with Tecfidera® (NCT04022473). FDA approval specifies that enteric coated delivery of both MMF and diesters prodrugs result in the same plasma levels of MMF in this trial, indicating bioequivalency (Table 1) [106,107]. Bafiertam’s pharmacodynamic, efficacy, safety and tolerability details reported in the FDA’s highlights of prescribing information are taken from studies of Tecfidera®. This assumes MMF is the therapeutic agent. Efficacy and adverse effects for Bafiertam™ are beginning to be investigated, with promising results indicating improved GI tolerability [130]. Further studies will be very informative.
Recent in vitro and pre-clinical studies show that fumarates, via their NRF2-mediated cytoprotective effects, possess promising anti-neoplastic properties for some tumors [4,108]. However, constitutive activation of NRF2 can also promote the development of various cancers and is associated with a poor prognosis via promotion of infinite cell growth, inhibition of apoptosis and enhancement of chemo- and radioresistance [109]. This paradoxical effect of NRF2 could have implications for fumarate treatment in cancer patients and could potentially increase risk in people with undiagnosed cancer or those in remission.
Concluding Remarks and Future Perspectives
Fumarates have had remarkable clinical success with psoriasis and multiple sclerosis, and now show therapeutic promise for numerous neurological and cardiovascular diseases. Several major questions remain to be addressed (see Outstanding Questions) as we strive for the first clinical studies of fumarates for new indications. For the future, repurposing of fumarates has to go hand-in-hand with further exploration of fumarate pharmacodynamics, but could also be accompanied by development of other MMF-releasing drugs or nano-carriers to impart significantly different pharmacokinetic properties to target MMF delivery for specific pathologies.
Outstanding Questions.
What is the role of HCAR2 and immune modulation in the therapeutic effects of fumarates in neurological and cardiovascular diseases, as well as in other diseases not addressed in this review?
What is the optimal administration timing of fumarates in different neuro- and cardiovascular pathologies and other diseases not addressed in this review considering preventive and/or treatment paradigms?
Are there sex-related differences in treatment efficacy, pharmacodynamics and occurrence of side effects of fumarates?
Should researchers work backwards from clinical studies and only design preclinical studies that achieve clinically relevant MMF concentrations?
To establish a reliable inter-species dose-translation: what is the total drug exposure (AUC0−∞), T1/2, protein binding and volume of distribution of MMF in mice and rats?
Will the side-effect profile limit repurposing of fumarates for indications with a less favorable risk-benefit ratio than multiple sclerosis and psoriasis?
Could targeted delivery, or fumarate prodrugs that are activated at the site of disease, improve drug efficacy and reduce side effects?
In vitro and in vivo studies have demonstrated the capacity of fumarates to reduce oxidative stress and inflammation in numerous neurological and cardiovascular disorders, attenuating disease endpoints. Most studies to date have focused on the role of the NRF2 pathway while the role of the HCAR2 pathway in the pharmacodynamics of fumarates in these pathologies is mostly unknown. With unwanted flushing a result of HCAR2 activation in skin cells, development of fumarate drugs with tissue-specific release of MMF could alleviate this side effect. The inhibitory effects of fumarates on aerobic glycolysis that underpins the immunomodulatory properties of fumarates, has only recently been identified and should be considered in future studies.
Administration routes, drug dosage, treatment frequency and treatment duration have varied in different preclinical disease models and in in vitro experiments as described in this review. Thorough investigation and comparison are needed to find the most optimal conditions for fumarate treatment in different pathologies, considering the specific pharmacokinetics of fumarate prodrugs and clinically relevant treatment paradigms. Future studies should also take into account potential sex-related differences since, so far, almost all studies described in this review have been conducted in male animals.
Recent efforts to incorporate fumarates into targeted nanocarriers, e.g. [110–112], offer great potential to further improve the therapeutic efficacy and limit off-target side effects of fumarates. Besides nanocarriers, one could also consider molecules that release MMF via a particular endogenous stimulus as a method to reorient the pharmacokinetic profile of MMF delivery and target specific cells, tissues or compartments within the body.
In conclusion, some large steps have to be made to improve our understanding of the biological effects of fumarates. This will not only aid in optimizing current treatment paradigms and development of novel fumarate-based drugs, it could also support the repurposing of these readily-available and cheap drugs to other pathologies driven by inflammation and nitro-oxidative stress.
Highlights.
Fumarates are attractive candidates for repurposing in pathologies that are driven by oxidative stress and inflammation, beyond psoriasis and multiple sclerosis
The antioxidant, anti-inflammatory and neuroprotective effects of fumarates are regulated via activation of NRF2 and HCAR2, and control of immunometabolism.
Preclinical studies of fumarates for treatment of neuropathic pain, neurodegenerative diseases, stroke, ischemia reperfusion-injury and atherosclerosis show promise.
The pharmacodynamics and optimal treatment paradigms are being elucidated in these preclinical studies and warrant ongoing investigation.
Current efforts to develop new formulations and targeted delivery methods for fumarates may reduce side effects and improve efficacy in specific pathologies.
Acknowledgements
This work is supported by Netherlands Heart Institute Personal Fellowship 2018 (A.H.); Australian Research Council grants CE140100003 (C.B., A.A.) and DP180101581 (A.A.); and, National Institutes of Health grant RF1 NS113840 (P.M.G.).
Disclaimer Statement
Drs. Grace, Abell and Avery receive funding from Biogen Inc. The other authors declare no competing financial interests.
GLOSSARY
- Alzheimer’s disease
characterized by accumulation of plaques and neurofibrillary tangles (aggregates of hyperphosphorylated tau protein) in the brain, resulting in cognitive impairment and social and occupational dysfunction
- Astrogliosis
abnormal molecular, cellular and functional changes in astrocytes that occurs in response to injury and disease of the central nervous system.
- Atherosclerosis
an arterial disease in which dysfunctional (activated) endothelial cells allow low-density lipoproteins to penetrate into the vessel wall where these are oxidized. These oxidized lipoproteins trigger an inflammatory response which, over time, causes the formation of an atherosclerotic plaque.
- Cardiac hypertrophy
abnormal thickening of the heart muscle resulting from enlargement of cardiomyocytes and/or changes in extracellular matrix.
- Cardiomyopathy
reduced contraction or relaxation capacity of the heart which can precede heart failure. Can be either dilated, hypertrophic or restrictive. Frequently results from a genetic mutation, but also longstanding hypertension, myocardial infarction, and diabetes can be underlying pathologies.
- Chronic pain
pain lasting beyond the period normally expected for healing, and serving no adaptive benefit.
- Dysesthesia
an unpleasant abnormal sensation, whether evoked or unevoked.
- Hyperalgesia
increased pain from a stimulus that normally provokes pain.
- Neuropathic pain
chronic pain caused by lesions or diseases of the somatosensory nervous system.
- NFĸB
nuclear factor κB, a pivotal transcription factor that stimulates a host of inflammatory mechanisms predominantly leading to increased expression of cytokines and chemokines.
- Nociceptive hypersensitivity
increased sensitivity to noxious (see hyperalgesia) and non-noxious stimuli.
- Parkinson’s disease
characterised by death of dopaminergic neurons in the substantia nigra. The disease is associated with bradykinesia, resting tremor and postural instability, as well as many non-motor symptoms, such as cognitive impairment, autonomic dysfunction and depression.
- Vascular dysfunction
a common pathology which mainly composes endothelial dysfunction and arterial stiffness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Committee on Herbal Medicinal Products (HMPC) - EMA (2011) Assessment report on Fumaria officinalis L., herba, EMA. [Google Scholar]
- 2.Schweckendiek W (1959) [Treatment of psoriasis vulgaris]. Med. Monatsschr 13, 103–4 [PubMed] [Google Scholar]
- 3.Schimrigk S et al. (2006) Oral fumaric acid esters for the treatment of active multiple sclerosis: An open-label, baseline-controlled pilot study. Eur. J. Neurol 13, 604–610 [DOI] [PubMed] [Google Scholar]
- 4.Meissner M et al. (2012) Dimethyl fumarate - Only an anti-psoriatic medication? JDDG - J. Ger. Soc. Dermatology 10, 793–801 [DOI] [PubMed] [Google Scholar]
- 5.Litjens NHR et al. (2004) Pharmacokinetics of oral fumarates in healthy subjects. Br. J. Clin. Pharmacol 58, 429–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litjens NHR et al. (2004) In vitro pharmacokinetics of anti-psoriatic fumaric acid esters. BMC Pharmacol 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rostami-Yazdi M et al. (2010) Pharmacokinetics of anti-psoriatic fumaric acid esters in psoriasis patients. Arch. Dermatol. Res 302, 531–538 [DOI] [PubMed] [Google Scholar]
- 8.Dibbert S et al. (2013) Detection of fumarate-glutathione adducts in the portal vein blood of rats: Evidence for rapid dimethylfumarate metabolism. Arch. Dermatol. Res 305, 447–451 [DOI] [PubMed] [Google Scholar]
- 9.Hunt TL et al. (2015) Safety, Tolerability, and Pharmacokinetics of ALKS 8700, a Novel Oral Therapy for Relapsing-Remitting Multiple Sclerosis, in Healthy Subjects. 2015 Annu. Meet. C [Google Scholar]
- 10.Lissin D et al. (2013), Favorable Metabolism and Pharmacokinetics of Formulations of XP23829, a Novel Fumaric Acid Ester, in Healthy Subjects., in Neurology, 80, pp. P05.189 [Google Scholar]
- 11.Nibbering PH et al. (1993) Effects of monomethylfumarate on human granulocytes. J. Invest. Dermatol 101, 37–42 [DOI] [PubMed] [Google Scholar]
- 12.Gafson AR et al. (2019) Breaking the cycle: Reversal of flux in the tricarboxylic acid cycle by dimethyl fumarate. Neurol. Neuroimmunol. NeuroInflammation 6, e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rostami-Yazdi M et al. (2009) Detection of metabolites of fumaric acid esters in human urine: implications for their mode of action. J. Invest. Dermatol 129, 231–234 [DOI] [PubMed] [Google Scholar]
- 14.Schmidt TJ et al. (2007) Reactivity of dimethyl fumarate and methylhydrogen fumarate towards glutathione and N-acetyl-l-cysteine-Preparation of S-substituted thiosuccinic acid esters. Bioorganic Med. Chem 15, 333–342 [DOI] [PubMed] [Google Scholar]
- 15.Nieboer C et al. (1990) Fumarie acid therapy in psoriasis: A double-blind comparison between fumarie acid compound therapy and monotherapy with dimethylfumaric acid ester. Dermatology 181, 33–37 [DOI] [PubMed] [Google Scholar]
- 16.Landeck L et al. (2018) Dimethyl fumarate (DMF) vs. monoethyl fumarate (MEF) salts for the treatment of plaque psoriasis: a review of clinical data. Arch. Dermatol. Res 310, 475–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werdenberg D et al. (2003) Presystemic metabolism and intestinal absorption of antipsoriatic fumaric acid esters. Biopharm. Drug Dispos 24, 259–273 [DOI] [PubMed] [Google Scholar]
- 18.Aubets J et al. (2019) No evidence for interactions of dimethylfumarate (DMF) and its main metabolite monomethylfumarate (MMF) with human cytochrome P450 (CYP) enzymes and the P-glycoprotein (P-gp) drug transporter. Pharmacol. Res. Perspect 7, e00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penner N et al. (2018), Cerebrospinal fluid penetration of dimethyl fumarate in patients with multiple sclerosis - Abstract., in ECTRIMS Conference, P904 [Google Scholar]
- 20.Reagan-Shaw S et al. (2008) Dose translation from animal to human studies revisited. FASEB J 22, 659–661 [DOI] [PubMed] [Google Scholar]
- 21.Blanchard OL and Smoliga JM Translating dosages from animal models to human clinical trials-revisiting body surface area scaling., FASEB Journal, 29. 01-May-(2015), FASEB, 1629–1634 [DOI] [PubMed] [Google Scholar]
- 22.Schulze-Topphoff U et al. (2016) Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc. Natl. Acad. Sci. U. S. A 113, 4777–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brennan MS et al. (2016) Pharmacodynamics of Dimethyl Fumarate Are Tissue Specific and Involve NRF2-Dependent and -Independent Mechanisms. Antioxidants Redox Signal 24, 1058–1071 [DOI] [PubMed] [Google Scholar]
- 24.Ahuja M et al. (2016) Distinct Nrf2 signaling mechanisms of fumaric acid esters and their role in neuroprotection against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-induced experimental parkinson’s-like disease. J. Neurosci 36, 6332–6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kornberg MD et al. (2018) Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 360, 449–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linker RA et al. (2011) Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134, 678–92 [DOI] [PubMed] [Google Scholar]
- 27.Lin SX et al. (2011) The anti-inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and haem oxygenase-1. ASN Neuro 3, 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scannevin RH et al. (2012) Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J. Pharmacol. Exp. Ther 341, 274–284 [DOI] [PubMed] [Google Scholar]
- 29.Cuadrado A et al. (2019) Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov 18, 295–317 [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto M et al. (2018) The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev 98, 1169–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paraiso HC et al. (2018) Dimethyl fumarate attenuates reactive microglia and long-term memory deficits following systemic immune challenge. J. Neuroinflammation 15, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W et al. (2008) Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem. Pharmacol 76, 1485–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wardyn JD et al. (2015) Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans 43, 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan MJ and Liu ZG (2011) Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 21, 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S-W et al. (2013) Up-down Regulation of HO-1 and iNOS Gene Expressions by Ethyl Pyruvate via Recruiting p300 to Nrf2 and Depriving It from p65. Free Radic. Biol. Med 65, 468–476 [DOI] [PubMed] [Google Scholar]
- 36.Paine A et al. (2010) Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol 80, 1895–1903 [DOI] [PubMed] [Google Scholar]
- 37.Soares MP and Bach FH (2009) Heme oxygenase-1: from biology to therapeutic potential. Trends Mol. Med 15, 50–58 [DOI] [PubMed] [Google Scholar]
- 38.Tang H et al. (2008) The psoriasis drug monomethylfumarate is a potent nicotinic acid receptor agonist. Biochem. Biophys. Res. Commun 375, 562–565 [DOI] [PubMed] [Google Scholar]
- 39.Tunaru S et al. (2003) PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med 9, 352–355 [DOI] [PubMed] [Google Scholar]
- 40.Offermanns S (2017) Hydroxy-Carboxylic Acid Receptor Actions in Metabolism. Trends Endocrinol. Metab 28, 227–236 [DOI] [PubMed] [Google Scholar]
- 41.Gerlo S et al. (2011) Cyclic AMP: A selective modulator of NF-κB action. Cell. Mol. Life Sci 68, 3823–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi Y et al. (2017) Activated niacin receptor HCA2 inhibits chemoattractant-mediated macrophage migration via Gβγ/PKC/ERK1/2 pathway and heterologous receptor desensitization. Sci. Rep 7, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chai JT et al. (2013) GPR109A and vascular inflammation. Curr. Atheroscler. Rep 15, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parodi B et al. (2015) Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol 130, 279–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiang HC et al. (2019) AMPK activation attenuates inflammatory pain through inhibiting NF-κB activation and IL-1β expression. J. Neuroinflammation 16, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walters RW et al. (2009) β-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J. Clin. Invest 119, 1312–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Witherow DS et al. (2004) β-arrestin inhibits NF-κB activity by means of its interaction with the NF-κB inhibitor IκBα. Proc. Natl. Acad. Sci. U. S. A 101, 8603–8607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Neill LAJ and Pearce EJ (2016) Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med 213, 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.James SL et al. (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Institute for Health Metrics and Evaluation (IHME) (2015), GBD Compare | IHME Viz Hub., University of Washington. [Online]. Available: https://vizhub.healthdata.org/gbd-compare/. [Accessed: 24-Jul-2020] [Google Scholar]
- 51.Gold R et al. (2012) Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis. N. Engl. J. Med 367, 1098–1107 [DOI] [PubMed] [Google Scholar]
- 52.Kappos L et al. (2008) Efficacy and safety of oral fumarate in patients with relapsing remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 372, 1463–1472 [DOI] [PubMed] [Google Scholar]
- 53.Naismith RT et al. (2020) Diroximel Fumarate Demonstrates an Improved Gastrointestinal Tolerability Profile Compared with Dimethyl Fumarate in Patients with Relapsing–Remitting Multiple Sclerosis: Results from the Randomized, Double-Blind, Phase III EVOLVE-MS-2 Study. CNS Drugs 34, 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J et al. (2020) Oral Dimethyl Fumarate Reduces Peripheral Neuropathic Pain in Rodents via NFE2L2 Antioxidant Signaling. Anesthesiology 132, 343–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boccella S et al. (2019) Ketones and pain: Unexplored role of hydroxyl carboxylic acid receptor type 2 in the pathophysiology of neuropathic pain. FASEB J 33, 1062–1073 [DOI] [PubMed] [Google Scholar]
- 56.Miyagi A et al. (2019) Dimethyl fumarate attenuates oxaliplatin-induced peripheral neuropathy without affecting the anti-tumor activity of oxaliplatin in rodents. Biol. Pharm. Bull 42, 638–644 [DOI] [PubMed] [Google Scholar]
- 57.Kawashiri T et al. (2018) Dimethyl fumarate ameliorates chemotherapy agent-induced neurotoxicity in vitro. J. Pharmacol. Sci 137, 202–211 [DOI] [PubMed] [Google Scholar]
- 58.Grace PM et al. (2014) Pathological pain and the neuroimmune interface. Nat. Rev. Immunol 14, 217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grace PM et al. (2016) Nitroxidative Signaling Mechanisms in Pathological Pain. Trends Neurosci 39, 862–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grace PM et al. (2020) The Neuroimmunology of Chronic Pain: From Rodents to Humans. J. Neurosci DOI: 10.1523/jneurosci.1650-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim H et al. (2017) Monomethyl fumarate (MMF) inhibits pain behaviors and amygdala activity in a rat arthritis model. Pain 158, 2376–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casili G et al. (2020) Dimethyl fumarate alleviates the nitroglycerin (NTG)-induced migraine in mice. J. Neuroinflammation 17, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rice ASC et al. Sensory profiling in animal models of neuropathic pain: a call for back-translation. Pain, 159, 819–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Percie Du Sert N and Rice ASC (2014) Improving the translation of analgesic drugs to the clinic: Animal models of neuropathic pain. British Journal of Pharmacology, 171, 2951–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yezierski RP and Hansson P (2018) Inflammatory and Neuropathic Pain From Bench to Bedside: What Went Wrong? Journal of Pain, 19, 571–588 [DOI] [PubMed] [Google Scholar]
- 66.Tappe-Theodor A et al. (2019) Pros and Cons of Clinically Relevant Methods to Assess Pain in Rodents. Neuroscience and Biobehavioral Reviews, 100, 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mogil JS (2009) Animal models of pain: Progress and challenges. Nat. Rev. Neurosci 10, 283–294 [DOI] [PubMed] [Google Scholar]
- 68.Campolo M et al. (2017) The Neuroprotective Effect of Dimethyl Fumarate in an MPTP-Mouse Model of Parkinson’s Disease: Involvement of Reactive Oxygen Species/Nuclear Factor-κB/Nuclear Transcription Factor Related to NF-E2. Antioxidants Redox Signal 27, 453–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jing X et al. (2015) Dimethyl fumarate attenuates 6-OHDA-induced neurotoxicity in SHSY5Y cells and in animal model of Parkinson’s disease by enhancing Nrf2 activity. Neuroscience 286, 131–140 [DOI] [PubMed] [Google Scholar]
- 70.Lastres-Becker I et al. (2016) Repurposing the NRF2 Activator Dimethyl Fumarate as Therapy Against Synucleinopathy in Parkinson’s Disease. Antioxidants Redox Signal 25, 61–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duty S and Jenner P Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. British Journal of Pharmacology 164, 1357–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koprich JB et al. (2017) Animal models of α-synucleinopathy for Parkinson disease drug development. Nature Reviews Neuroscience, 18, 515–529 [DOI] [PubMed] [Google Scholar]
- 73.Majkutewicz I et al. (2016) Dimethyl fumarate attenuates intracerebroventricular streptozotocin-induced spatial memory impairment and hippocampal neurodegeneration in rats. Behav. Brain Res 308, 24–37 [DOI] [PubMed] [Google Scholar]
- 74.Majkutewicz I et al. (2018) Age-dependent effects of dimethyl fumarate on cognitive and neuropathological features in the streptozotocin-induced rat model of Alzheimer’s disease. Brain Res 1686, 19–33 [DOI] [PubMed] [Google Scholar]
- 75.Drummond E and Wisniewski T (2017) Alzheimer’s disease: experimental models and reality., Acta Neuropathologica 133, 155–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.LaFerla FM and Green KN (2012) Animal models of Alzheimer disease. Cold Spring Harb. Perspect. Med 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amram S and Frenkel D (2017) Animal Models of Alzheimer’s Disease. In Neuroprotection in Alzheimer’s Disease pp. 31–58 [Google Scholar]
- 78.Clausen BH et al. (2017) Fumarate decreases edema volume and improves functional outcome after experimental stroke. Exp. Neurol 295, 144–154 [DOI] [PubMed] [Google Scholar]
- 79.Hou X et al. (2020) Neuroprotective effect of dimethyl fumarate on cognitive impairment induced by ischemic stroke. Ann. Transl. Med 8, 375–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keep RF et al. (2012) Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol 11, 720–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iniaghe LO et al. (2015) Dimethyl fumarate confers neuroprotection by casein kinase 2 phosphorylation of Nrf2 in murine intracerebral hemorrhage. Neurobiol. Dis 82, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82., Zhao X et al. (2015) Dimethyl Fumarate Protects Brain from Damage Produced by Intracerebral Hemorrhage by Mechanism Involving Nrf2. Stroke 46, 1923–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pound P and Ram R (2020) Are researchers moving away from animal models as a result of poor clinical translation in the field of stroke? An analysis of opinion papers. BMJ Open Sci 4, e100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sorby-Adams AJ et al. (2018) Large animal models of stroke and traumatic brain injury as translational tools. American Journal of Physiology - Regulatory Integrative and Comparative Physiology, 315, R165–R190 [DOI] [PubMed] [Google Scholar]
- 85.Schmieder A et al. (2015) Impact of fumaric acid esters on cardiovascular risk factors and depression in psoriasis: a prospective pilot study. Arch. Dermatol. Res 307, 413–424 [DOI] [PubMed] [Google Scholar]
- 86.Holzer G et al. (2020) Disparate effects of adalimumab and fumaric acid esters on cardiovascular risk factors in psoriasis patients: Results from a prospective, randomized, observer-blinded head-to-head trial. J. Eur. Acad. Dermatology Venereol DOI: 10.1111/jdv.16635 [DOI] [PubMed] [Google Scholar]
- 87.Blumenfeld Kan S et al. (2019) HDL-cholesterol elevation associated with fingolimod and dimethyl fumarate therapies in multiple sclerosis. Mult. Scler. J. - Exp. Transl. Clin 5, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luo M et al. (2019) The Effects of Dimethyl Fumarate on Atherosclerosis in the Apolipoprotein E-Deficient Mouse Model with Streptozotocin-Induced Hyperglycemia Mediated By the Nuclear Factor Erythroid 2-Related Factor 2/Antioxidant Response Element (Nrf2/ARE) Signaling Pathw. Med. Sci. Monit 25, 7966–7975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nour OA et al. (2017) Antioxidant and anti-inflammatory effects of dimethyl fumarate in hypercholesterolemic rabbits. Egypt. J. Basic Appl. Sci 4, 153–159 [Google Scholar]
- 90.Oh CJ et al. (2014) Dimethylfumarate attenuates restenosis after acute vascular injury by cell-specific and Nrf2-dependent mechanisms. Redox Biol 2, 855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amin FM et al. (2020) Dimethyl fumarate ameliorates diabetes-associated vascular complications through ROS-TXNIP-NLRP3 inflammasome pathway. Life Sci 256, 117887. [DOI] [PubMed] [Google Scholar]
- 92.Ha CM et al. (2014) Activation of Nrf2 by dimethyl fumarate improves vascular calcification. Vascul. Pharmacol 63, 29–36 [DOI] [PubMed] [Google Scholar]
- 93.Getz GS and Reardon CA (2012) Animal models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol 32, 1104–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van der Heiden K et al. (2015) Animal models for plaque rupture: a biomechanical assessment. Thromb. Haemost 115, 501–508 [DOI] [PubMed] [Google Scholar]
- 95.Ahmed AA et al. (2018) Dimethyl fumarate interferes with MyD88-dependent toll-like receptor signalling pathway in isoproterenol-induced cardiac hypertrophy model. J. Pharm. Pharmacol 70, 1521–1530 [DOI] [PubMed] [Google Scholar]
- 96.Hu X et al. (2018) Protection by dimethyl fumarate against diabetic cardiomyopathy in type 1 diabetic mice likely via activation of nuclear factor erythroid-2 related factor 2. Toxicol. Lett 287, 131–141 [DOI] [PubMed] [Google Scholar]
- 97.Aimo A et al. (2020) Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. European Journal of Preventive Cardiology, 27, 494–510 [DOI] [PubMed] [Google Scholar]
- 98.Mrowietz U et al. (2018) Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J. Eur. Acad. Dermatology Venereol 32, 3–14 [DOI] [PubMed] [Google Scholar]
- 99.Fox RJ et al. (2014) BG-12 (dimethyl fumarate): a review of mechanism of action, efficacy, and safety. Curr. Med. Res. Opin 30, 251–262 [DOI] [PubMed] [Google Scholar]
- 100.O’Gorman J et al. (2015) Effect of Aspirin Pretreatment or Slow Dose Titration on Flushing and Gastrointestinal Events in Healthy Volunteers Receiving Delayed-release Dimethyl Fumarate. Clin. Ther 37, 1402–1419.e5 [DOI] [PubMed] [Google Scholar]
- 101.Vitalis Pharmaceuticals (2020), Vitalis Receives Orphan Drug Designation for Diroximel Fumarate and Monomethyl Fumarate, in Combination with VTS-Aspirin, for Multiple Sclerosis Patients Who Experience Fumarate Flush. GlobeNewswire, 1 [Google Scholar]
- 102.Palte MJ et al. (2019) Improving the Gastrointestinal Tolerability of Fumaric Acid Esters: Early Findings on Gastrointestinal Events with Diroximel Fumarate in Patients with Relapsing-Remitting Multiple Sclerosis from the Phase 3, Open-Label EVOLVE-MS-1 Study. Adv. Ther 36, 3154–3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Phillips JT et al. (2014) Managing flushing and gastrointestinal events associated with delayed-release dimethyl fumarate: Experiences of an international panel. Mult. Scler. Relat. Disord 3, 513–519 [DOI] [PubMed] [Google Scholar]
- 104.Fox RJ et al. (2016) Characterizing absolute lymphocyte count profiles in dimethyl fumarate’treated patients with MS Patient management considerations. Neurol. Clin. Pract 6, 220–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mills EA et al. (2018) Emerging understanding of the mechanism of action for dimethyl fumarate in the treatment of multiple sclerosis. Front. Neurol 9, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Biopharma Services and Clinicaltrials.gov (2019) Study to Compare GI Tolerability Following Oral Administration of Bafiertam™ or Tecfidera to Healthy Volunteers,
- 107.FDA (2013) Bafiertram (monomethyl fumarate) - Highlight of prescribing information
- 108.Saidu NEB et al. (2019) Dimethyl fumarate, a two-edged drug: Current status and future directions. Med. Res. Rev 39, 1923–1952 [DOI] [PubMed] [Google Scholar]
- 109.Wu S et al. (2019) Nrf2 in cancers: A double-edged sword. Cancer Med 8, 2252–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kumar P et al. (2017) Enhanced Brain Delivery of Dimethyl Fumarate Employing Tocopherol-Acetate-Based Nanolipidic Carriers: Evidence from Pharmacokinetic, Biodistribution, and Cellular Uptake Studies. ACS Chem. Neurosci 8, 860–865 [DOI] [PubMed] [Google Scholar]
- 111.da Silva PV and de Queiroz AAA (2020) Long term multiple sclerosis drug delivery using dendritic polyglycerol flower-like microspheres. J. Biomater. Sci. Polym. Ed 31, 188–206 [DOI] [PubMed] [Google Scholar]
- 112.Kumar M et al. (2018) Lysine-Based C60-Fullerene Nanoconjugates for Monomethyl Fumarate Delivery: A Novel Nanomedicine for Brain Cancer Cells. ACS Biomater. Sci. Eng 4, 2134–2142 [DOI] [PubMed] [Google Scholar]
- 113.Brück J et al. (2018) A review of the mechanisms of action of dimethylfumarate in the treatment of psoriasis. Exp. Dermatol 27, 611–624 [DOI] [PubMed] [Google Scholar]
- 114.Ghoreschi K et al. (2011) Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J. Exp. Med 208, 2291–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ogawa T et al. (2020) Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Regulates Epidermal Keratinization under Psoriatic Skin Inflammation. Am. J. Pathol 190, 577–585 [DOI] [PubMed] [Google Scholar]
- 116.Carlström KE et al. (2019) Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes. Nat. Commun 10, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lückel C et al. (2019) IL-17+ CD8+ T cell suppression by dimethyl fumarate associates with clinical response in multiple sclerosis. Nat. Commun 10, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Balak DM and Thio HB (2011) Treatment of lupus erythematosus with fumaric acid ester derivatives: two case-reports. J. Transl. Med 9, P15 [Google Scholar]
- 119.Khatri R et al. (2012) Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 79, S52–7 [DOI] [PubMed] [Google Scholar]
- 120.Cowled P and Fitridge R (2011) Pathophysiology of reperfusion injury. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists pp. 331–350, University of Adelaide Press; [PubMed] [Google Scholar]
- 121.Eltzschig HK and Eckle T (2011) Ischemia and reperfusion-from mechanism to translation. Nat. Med 17, 1391–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lin R et al. (2016) Fumarate modulates the immune/inflammatory response and rescues nerve cells and neurological function after stroke in rats. J. Neuroinflammation 13, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Safari A et al. (2019) Neuroprotective effect of dimethyl fumarate in stroke: The role of nuclear factor erythroid 2-related factor 2. Iran. J. Neurol 18, 108–113 [PMC free article] [PubMed] [Google Scholar]
- 124.Yao Y et al. (2016) Dimethyl Fumarate and Monomethyl Fumarate Promote Post-Ischemic Recovery in Mice. Transl. Stroke Res 7, 535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kunze R et al. (2015) Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp. Neurol 266, 99–111 [DOI] [PubMed] [Google Scholar]
- 126.Ding S et al. (2016) Protective Effects of L-Malate against Myocardial Ischemia/Reperfusion Injury in Rats. Evid. Based. Complement. Alternat. Med 2016, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ashrafian H et al. (2012) Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab 15, 361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meili-Butz S et al. (2008) Dimethyl fumarate, a small molecule drug for psoriasis, inhibits Nuclear Factor-κB and reduces myocardial infarct size in rats. Eur. J. Pharmacol 586, 251–258 [DOI] [PubMed] [Google Scholar]
- 129.Beaumont K et al. (2005) Design of Ester Prodrugs to Enhance Oral Absorption of Poorly Permeable Compounds: Challenges to the Discovery Scientist. Curr. Drug Metab 4, 461–485 [DOI] [PubMed] [Google Scholar]
- 130.Wynn D et al. (2020) Monomethyl fumarate has better gastrointestinal tolerability profile compared with dimethyl fumarate. Mult. Scler. Relat. Disord 45, 102335. [DOI] [PubMed] [Google Scholar]
- 131.FDA (2013) Tecfidera (dimethyl fumarate) - Highlights of prescribing information
- 132.Wehr A et al. (2018) Relative Bioavailability of Monomethyl Fumarate after Administration of ALKS 8700 and Dimethyl Fumarate in Healthy Subjects. Neurology, 90, pp. p1.403 [Google Scholar]
- 133.Lissin D et al. (2014) Steady State Pharmacokinetics of Formulations of XP23829, a Novel Prodrug of Monomethyl Fumarate (MMF), in Healthy Subjects. Neurology, 82, pp. p1.188 [Google Scholar]
- 134.European Medicines Agency (2017) Skilarence
- 135.FDA (2013) Vumerity (diroxymel fumarate) - Highlights of prescribing information


