Abstract
Purinergic signalling plays important roles in somatosensory and nociceptive transmission in the dorsal horn of the spinal cord under physiological and pathophysiological conditions. Physiologically, ATP mediates excitatory postsynaptic responses in nociceptive transmission in the superficial dorsal horn, and in transmission of innocuous primary afferent inputs in the deep dorsal horn. Additionally, extracellular conversion of ATP to adenosine mediates inhibitory postsynaptic responses from Pacinian corpuscle afferents, and is implicated in analgesia caused by transcutaneous electrical nerve stimulation in humans. In terms of pathological pain, P2X4 receptors de novo expressed on dorsal horn microglia are implicated in pain hypersensitivity following peripheral nerve injury. There is evidence that involvement of such P2X4 receptors is sexually dimorphic, occurring in males but not in females. Thus, the roles of purinergic signalling in physiological and pathological pain processing are complex and remain an ever-expanding field of research.
Keywords: Pain, Spinal dorsal horn, Neuropathic pain, Primary afferents
Introduction
The story of the development of the purinergic signalling field is intimately and forever linked to the somatosensory system and primary sensory neurons. In the early 1950s, the search was on for chemical substances that act as neurotransmitters in the central nervous system (CNS). Invoking the so-called Dale’s principle [1]—that neurons release the same neurotransmitter from all of their axonal endings—Pamela Holton took advantage of the pseudo-unipolar topology of primary sensory neurons with their long peripheral axons to infer about what neurotransmitters might be released from the experimentally inaccessible central terminals of these neurons in the spinal cord. According to this principle, one could deduce the substances released from central endings by identifying substances released from the peripheral endings. In pioneering studies [2, 3], Holton used a preparation, the ear of the anaesthetized rabbit, and found that electrically stimulating the sensory fibres in the ear caused efflux of adenosine 5′-triphosphate (ATP) into the venous effluent, along with a number of other substances. These other substances were eliminated from consideration because only ATP mimicked the physiological response—vasodilation—that was produced by stimulating the sensory fibres. She reasoned that ATP is the physiological transmitter mediating the response in the periphery and, by Dale’s principle, also the transmitter substance of the central endings of those same sensory afferents [2, 3]. The findings from Holton were critical for the young firebrand Geoffrey Burnstock, whose developing ideas ultimately led to the purinergic signalling hypothesis: that ATP can act as a neurotransmitter molecule [4]. For decades, Burnstock would regularly regale audiences, and anyone else who would listen, concerning the pivotal importance of Holton’s work. No doubt, Burnstock’s interest in sensory neurons, or as he more-rightly called them ‘sensory-motor neurons’, flowed from these early days and led to his lifelong interest in, and influence on, the field of somatosensation and pain. In this review, we will concentrate on purinergic signalling in the sensory processing region of the CNS, the dorsal horn of the spinal cord, with two foci: (i) physiological neurotransmission in the healthy condition and (ii) signalling in pathological pain states. Purinergic signalling is also implicated in innocuous and noxious sensory transduction in the periphery [5, 6].
Physiological roles for purinergic neurotransmission in the spinal dorsal horn
Following on from Holton’s concept of ATP as a transmitter from primary afferent neurons, Krnjevic and colleagues found that ATP excites neurons in the cuneate nucleus [7], which receives direct input from primary afferents. These observations provided evidence that ATP mimics the excitatory action of primary afferents on central neurons, a criterion important for identifying a neurotransmitter, reinforcing the concept from Holton. Investigation of ATP and primary afferents was largely dormant until demonstration by Jessell and Jahr that applying ATP directly depolarizes dorsal horn neurons in primary culture [8]. This finding sparked a series of studies demonstrating excitatory effects in vivo of iontophoretically applying ATP onto neurons in the superficial [9] and deep [10, 11] dorsal horn. The strongest evidence for ATP-mediating excitatory synaptic transmission in the dorsal horn came from MacDermott and colleagues [12]. They found excitatory postsynaptic currents (EPSCs) in neurons in the substantia gelatinosa that were blocked by the P2X receptor inhibitor suramin, but were resistant to antagonists of glutamate receptors or other excitatory receptors. Pharmacological investigation implicated P2X2, P2X4, and P2X6 receptors as potential postsynaptic receptors mediating these EPSCs. As neurons in the substantia gelatinosa and other parts of the superficial dorsal horn receive synaptic inputs from nociceptive primary afferent, the excitatory effects of ATP on these neurons gave rise to the concept that ATP may be a transmitter of primary sensory nociceptors in spinal cord.
During the time studies on effects of ATP in the dorsal horn neurons were being done, abundant evidence was accumulating that primary afferent nociceptors are themselves excited by ATP [6]. This excitation was found to be mediated by P2X2 and P2X3, largely P2X2/3 heteroreceptors [13, 14]. While most studies focused on effects of ATP on the soma of the primary afferents in the dorsal root ganglia, presynaptic actions of ATP in the dorsal horn mediated by P2X2/3 receptors supported the concept these receptors are trafficked to the presynaptic terminals of primary afferent nociceptors [15]. Thus, in the dorsal horn, ATP may act in synaptic transmission presynaptically as well as postsynaptically. We would be remiss to not mention evidence that P2X2/3 receptors are trafficked to the peripheral terminals of primary sensory nociceptors where they may be activated by ATP released from damaged or inflammatory cells [16, 17]. Recently, new roles have been proposed for ATP in transducing non-noxious stimuli in the periphery. Stucky and colleagues found that ATP released by keratinocytes may mediate innocuous mechanosensation [18], as well as cold and heat sensation [19] through P2X4 receptors on primary afferents. It is conceivable that these receptors may also be trafficked to the central terminals of primary afferents, providing an additional substrate for ATP to presynaptically affect synaptic transmission in the spinal dorsal horn.
While the most well-known physiological role of ATP in the spinal dorsal horn is in nociceptive processing in the superficial lamina, ATP does have a prominent effect of exciting neurons in the deep dorsal horn that are driven by non-noxious peripheral stimuli. In vivo, iontophoretically applied ATP in the deep dorsal horn preferentially excites neurons that are synaptically driven by innocuous mechanical stimuli, such as light touch or vibration [10]. In contrast to strict excitation of such non-nociceptive deep dorsal horn neurons, wide dynamic range (WDR) neurons—neurons driven by noxious as well as innocuous stimuli—show excitation followed by profound inhibition in response to iontophoretically applied ATP [10]. Inhibition alone is produced by administering AMP, which does not activate P2X receptors, and inhibition by ATP or AMP is blocked by local applying adenosine receptor blockers. The inhibition is produced postsynaptically through activation of a K+ conductance that appears to be mediated by K-ATP channels. Thus, ATP causes excitation through activation of P2X receptors and inhibition through extracellular degradation of ATP to adenosine which subsequently activates adenosine receptors [10, 11].
The pattern of excitation alone of non-nociceptive neurons and a biphasic excitatory/inhibitory effect on WDR neurons matches the actions of peripheral innocuous stimuli on deep dorsal horn neurons. Selectively activating primary afferents from Pacinian corpuscles, by high-frequency vibratory stimulation [20, 21], not only drives deep dorsal horn neurons in this pattern but also produces an inhibitory postsynaptic response in WDR neurons that is blocked by adenosine receptor antagonists [22] and mediated by K-ATP channels [23]. Thus, it has been proposed that release of ATP from Pacinian corpuscle afferents, and conversion to adenosine, may mediate the physiological synaptic actions of these afferents on WDR neurons in the deep dorsal horn [11].
A prediction from the adenosine-mediated inhibition of nociceptive responses by Pacinian corpuscle afferents is that adenosine mediates the well-known analgesic effects of stimulating these afferents, or other heavily myelinated primary afferents, in humans. This prediction was tested by Marchand and colleagues [24]. In a placebo-controlled, double-blind study, they found that the analgesic effect produced by transcutaneous electrical nerve stimulation (TENS) on heat-induced pain was significantly reduced by intravenous administration of caffeine. This effect of caffeine is consistent with the prediction that the analgesic effect of TENS is mediated by adenosine.
In addition to release of ATP from primary afferents, this nucleotide may also be released from intrinsic neurons in the dorsal horn. In a provocative study by Jo and Schlichter [25], through an elegant series of experiments, they provided evidence for ATP release from subset of dorsal horn neurons that co-release GABA. The postsynaptic actions of these transmitters were excitation and inhibition, respectively, which turned on its head the idea that the sign of the action of all transmitters released at a given synapse must be the same (i.e. excitation or inhibition, but not both) [26].
Thus, there are multiple physiological roles for purinergic signalling in somatosensory processing in the dorsal horn. Through release from differing subtypes of primary sensory afferents, ATP may mediate nociceptive and also non-nociceptive synaptic excitation. Through extracellular conversion of released ATP to adenosine, a second physiological role for purinergic signalling is postsynaptic inhibition of nociceptive processing, which is particularly evident in neurons in the deep dorsal horn.
Purinergic signalling in the spinal dorsal horn in pathological pain
Pathological pain is conceptualized as an alteration of the physiological processes leading to amplification of transmission in nociceptive pathways in the periphery and in the central nervous system [27], as well as perturbation of the local inhibitory control that normally prevents inputs from innocuous stimuli from driving transmission in these pathways [28]. Here we provide an overview on the role of P2X4 receptors in pathological pain states focusing primarily on neuropathic pain—a pathological condition caused by damage or lesion to the peripheral or central nervous system, resulting in hyperalgesia, allodynia, or spontaneous pain.
The role of P2X4 receptors in pain hypersensitivity caused by peripheral nerve injury (PNI) was discovered by Tsuda et al. [29]. Using intrathecal delivery of TNP-ATP (a broad inhibitor of P2X1-4 receptors), they found that a decrease in PNI-induced mechanical hypersensitivity. Conversely, PPADS (a broad P2X receptor antagonist which excludes P2X4 receptors) had no effect on mechanical hypersensitivity, suggesting that the P2X4 receptor may be the critical P2X receptor subtype mediating mechanical hypersensitivity after nerve injury, which was confirmed with intrathecal delivery of antisense oligonucleotides against P2X4 receptors. It was further found that PNI induced upregulation of P2X4 receptor expression in the dorsal horn of the spinal cord. Unexpectedly at the time, the P2X4 receptors were selectively expressed within microglia.
In the naïve state, expression of P2rx4—the gene encoding P2X4 receptors—in microglia is low. Following PNI, P2rx4 expression is turned on by a sequence of transcription factors interferon regulatory factor (IRF) 8 and IRF5 [30, 31]. It was found that PNI upregulates IRF8 expression in microglia, but not astrocytes or neurons, in the spinal cord [31]. Mice lacking IRF8 display reduced mechanical hypersensitivity from PNI and inhibition of IRF8 with small interfering RNA reversed PNI-induced hypersensitivity. Furthermore, intrathecal injection of microglia which overexpress IRF8 is sufficient to induce mechanical hypersensitivity [31]. IRF8 acts through upregulating the transcription of Irf5, and by binding of IRF5 protein to sites in the promoter region of P2rx4, IRF5 drives transcription of P2rx4 and ultimately de novo translation of P2X4 receptors [30].
In pain hypersensitivity induced by PNI, extracellular ATP has been found to be elevated in the spinal dorsal horn due to increased exocytotic release of ATP and upregulated expression of the vesicular nucleotide transporter (VNUT) gene, Slc17a9, whose protein is involved in storing nucleotides within vesicles [32]. VNUT deletion from spinal dorsal horn neurons in mice does not alter basal nociception but does attenuate PNI-induced mechanical hypersensitivity [32, 33]. Altogether, these findings suggest that intrinsic neurons in spinal dorsal horn are the source of ATP acting on microglial P2X4 receptors, leading to hypersensitivity.
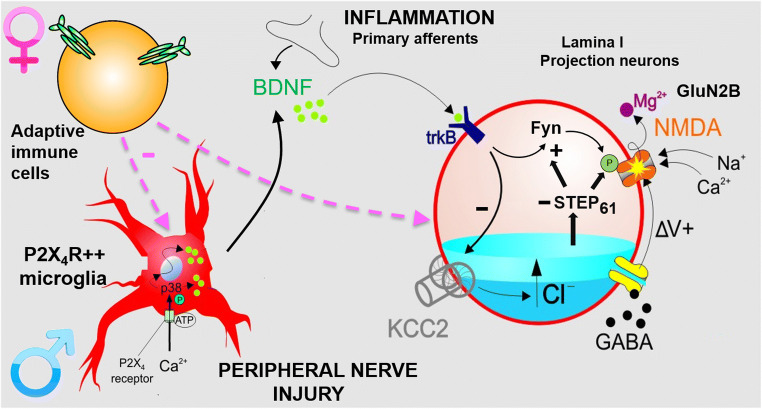
The molecular pathways by which newly expressed and activated P2X4 receptors on microglia alter nociceptive processing by neurons in the dorsal horn has been investigated extensively. Multiple lines of converging evidence implicate brain-derived neurotrophic factor (BDNF) as the mediator released microglia and acting on neurons in spinal lamina I [34–37]. Most directly, cell-type specific deletion of BDNF from microglia both prevents and reverses mechanical hypersensitivity after PNI [38].
BDNF has been found to have two principal actions that drive aberrant output of the lamina I projection neurons, which under normal circumstances only provide nociceptive signals to the brain [39]. First, BDNF causes downregulation of the potassium-chloride cotransporter 2, KCC2, resulting in a depolarizing shift in the anion reversal potential in lamina I dorsal horn neurons by increasing intracellular Cl−, thereby weakening GABA- or glycine-mediated inhibition, i.e., causing disinhibition [35, 40]. Enhancing Cl− extrusion or circumventing the disinhibition by inhibiting efflux of HCO3− reverses hypersensitivity induced by PNI [41–43]. The second action of BDNF is to enhance activation of the N-methyl-d-aspartate receptors (NMDARs) through increasing phosphorylation of the GluN2B subunit, thereby potentiating synaptic NMDAR currents in lamina I neurons [44]. This enhanced GluN2B function is mediated by enhancing phosphorylation of Tyr1472 by the tyrosine kinase Fyn [45] and by reducing dephosphorylation of this residue through suppression of the tyrosine phosphatase STEP61 activity [46].
A key insight into the microglial P2X4 receptor pain pathway has come from the surprising finding that microglial P2X4 receptor-BDNF signalling drives pain hypersensitivity in males, but not in females [38]. Despite the lack of contribution of microglia to pain hypersensitivity in females, microglial proliferation in the dorsal horn still occurs in females after PNI [38, 47]. While PNI upregulates transcriptional factors Irf8 and Irf5 in both sexes, P2rx4 upregulation only occurs in males [38]. IRF5 binding to the P2rx4 promoter after PNI increases in males but not in females [47]. Thus, in females, PNI does not result in increased expression of P2X4 receptors by microglia which prevents expression of BDNF and subsequent downstream signalling. Nevertheless, PNI-induced downregulation of KCC2 occurs in both sexes and enhancing chloride extrusion reverses PNI-induced mechanical hypersensitivity in both sexes [43]. Inhibiting NMDAR also reverses mechanical hypersensitivity in both sexes [38]. ATP-stimulated microglia cultured from male rats injected intrathecally into both male and female rats induced mechanical hypersensitivity, while ATP-stimulated microglia cultured from female rats had no effect when delivered to either male or female rats [47], demonstrating that the sex-dependency of P2X4 receptor-mediated pain occurs within the microglia. Taken together, these results indicate that PNI-induced neuropathic pain hypersensitivity results from convergent mechanisms in the neurons despite the sexually dimorphic signalling in the microglia (Fig. 1).
Fig. 1.
A unified schema for signalling in the superficial spinal dorsal horn in pain hypersensitivity. Peripheral nerve injury drives de novo expression of P2X4Rs in microglia, but only in males, leading to release of BDNF and activation of its cognate receptor, TrkB, on neurons in lamina I. Subsequent intracellular pathways in the neurons downregulate KCC2 and upregulate the function of GluN2B-containing NMDARs by activating Fyn and suppressing STEP61. In females, although microglia proliferation occurs, P2X4R expression does not increase. Nevertheless, KCC2 is downregulated and NMDAR is enhanced in females by signalling molecules and pathways that remain to be identified. Also shown is that BDNF may be released from primary sensory neurons activated by peripheral inflammation
Conclusion
Purinergic signalling is an important pathway in physiological and pathological sensory processing. Burnstock’s early contributions, from the proposal of purinergic neurotransmission [4] to an early unified purinergic hypothesis of pain [48], helped spark an ever-expanding field of research. Under pathological conditions, changes in ATP release and upregulation of purinergic receptors play a role in pain hypersensitivity. Beyond neuron-neuron transmission, purinergic signalling through microglial P2X4 receptors initiate complex signalling cascades that contribute to hyperexcitability of dorsal horn neurons and hypersensitivity to nociceptive stimuli. While the intricacies of these pathways continue to be dissected, it is evident that purinergic signalling is integral in sensory and nociceptive processing.
Personal reminiscence from Mike Salter
To say that Geoff Burnstock was a giant in the field is an understatement of gargantuan proportions—there would be no purinergic field without Geoff and his unbridled passion, originality, intuition, wit, resilience, and courage. His seemingly limitless support for the concept of purinergic signalling and pain sustained and drove the field on over several decades. As one interested in pain mechanisms, as a medical student, I seem to have been destined to study ATP and purinergic signalling. During my interview for a PhD position in the lab, Jim Henry, who was then driving the field of substance P and pain, asked me if I knew anything about ATP as a neurotransmitter because some ‘guy named Geoff Burnstock’ was promoting this idea. I did not—but all that changed a little over a year later with a chance attendance at the 1983 Society for Neuroscience meeting where I listened, spell bound, to work showing that ATP depolarizes dorsal horn neurons in culture. I immediately became a purine-ologist, a badge I proudly wear to this day.
Biographies
Theresa Tam
is a doctoral student at the Hospital for Sick Children and the University of Toronto Department of Physiology. She received her Honours Bachelors of Science from the University of Toronto. Her research focuses on inflammation and cellular mechanisms underlying pain.
Michael Salter
is a Senior Scientist and Chief of Research Emeritus at The Hospital for Sick Children Research Institute and a Professor in the Department of Physiology at the University of Toronto. He received his MD at the University of Western Ontario and his PhD from McGill University. Dr. Salter is determining fundamental molecular and cellular mechanisms of normal and pathological neuroplasticity. His discoveries have broad implications for the control of cell-cell communication throughout the nervous system. He is using his discoveries to design and test new types of treatment for individual suffering from a variety of disorders of the CNS. He is developing molecules that target major cell signalling pathways in neurons and in glial cells involved in pain, stroke, neurodegenerative diseases and schizophrenia.
Funding
Work of the authors is supported by grants to MWS from the Canadian Institutes of Health Research and the Krembil Foundation. MWS held the Northbridge Chair in Paediatric Research at the Hospital for Sick Children. THT is supported by graduate scholarships from the Canadian Institutes of Health Research and the Ontario Ministry of Health and Long-Term Care.
Compliance with ethical standards
Conflict of interest
Theresa H. Tam declares that she has no conflict of interest. Michael W. Salter declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of the Topical Collection on A Tribute to Professor Geoff Burnstock.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Theresa H. Tam, Email: theresa.tam@sickkids.ca
Michael W. Salter, Email: michael.salter@sickkids.ca
References
- 1.Dale H. Pharmacology and nerve-endings (Walter Ernest Dixon Memorial Lecture): (Section of Therapeutics and Pharmacology) Proc R Soc Med. 1935;28(3):319–332. doi: 10.1177/003591573502800330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holton FA, Holton P. The capillary dilator substances in dry powders of spinal roots; a possible role of adenosine triphosphate in chemical transmission from nerve endings. J Physiol. 1954;126(1):124–140. doi: 10.1113/jphysiol.1954.sp005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holton P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J Physiol. 1959;145(3):494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24(3):509–581. [PubMed] [Google Scholar]
- 5.Burnstock G. Purinergic mechanisms and pain. Adv Pharmacol. 2016;75:91–137. doi: 10.1016/bs.apha.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton SG, McMahon SB. ATP as a peripheral mediator of pain. J Auton Nerv Syst. 2000;81(1–3):187–194. doi: 10.1016/s0165-1838(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 7.Galindo A, Krnjević K, Schwartz S. Micro-iontophoretic studies on neurones in the cuneate nucleus. J Physiol. 1967;192(2):359–377. doi: 10.1113/jphysiol.1967.sp008305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jahr CE, Jessell TM. ATP excites a subpopulation of rat dorsal horn neurones. Nature. 1983;304(5928):730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- 9.Fyffe RE, Perl ER. Is ATP a central synaptic mediator for certain primary afferent fibers from mammalian skin? Proc Natl Acad Sci U S A. 1984;81(21):6890–6893. doi: 10.1073/pnas.81.21.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salter MW, Henry JL. Effects of adenosine 5′-monophosphate and adenosine 5′-triphosphate on functionally identified units in the cat spinal dorsal horn. Evidence for a differential effect of adenosine 5′-triphosphate on nociceptive vs non-nociceptive units. Neuroscience. 1985;15(3):815–825. doi: 10.1016/0306-4522(85)90080-6. [DOI] [PubMed] [Google Scholar]
- 11.Salter MW, De Koninck Y, Henry JL. Physiological roles for adenosine and ATP in synaptic transmission in the spinal dorsal horn. Prog Neurobiol. 1993;41(2):125–156. doi: 10.1016/0301-0082(93)90006-e. [DOI] [PubMed] [Google Scholar]
- 12.Bardoni R, Goldstein PA, Lee CJ, Gu JG, MacDermott AB. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J Neurosci. 1997;17(14):5297–5304. doi: 10.1523/jneurosci.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377(6548):428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 14.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377(6548):432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 15.Gu JG, Heft MW. P2X receptor-mediated purinergic sensory pathways to the spinal cord dorsal horn. Purinergic Signal. 2004;1(1):11–16. doi: 10.1007/s11302-004-4743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 17.Burnstock G. Purinergic mechanosensory transduction and visceral pain. Mol Pain. 2009;5:69. doi: 10.1186/1744-8069-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moehring F, Cowie AM, Menzel AD, Weyer AD, Grzybowski M, Arzua T, Geurts AM, Palygin O, Stucky CL (2018) Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. eLife 7. 10.7554/eLife.31684 [DOI] [PMC free article] [PubMed]
- 19.Sadler KE, Moehring F, Stucky CL (2020) Keratinocytes contribute to normal cold and heat sensation. eLife 9. 10.7554/eLife.58625 [DOI] [PMC free article] [PubMed]
- 20.Salter MW, Henry JL. Differential responses of nociceptive vs. non-nociceptive spinal dorsal horn neurones to cutaneously applied vibration in the cat. Pain. 1990;40(3):311–322. doi: 10.1016/0304-3959(90)91128-6. [DOI] [PubMed] [Google Scholar]
- 21.Salter MW, Henry JL. Physiological characteristics of responses of wide dynamic range spinal neurones to cutaneously applied vibration in the cat. Brain Res. 1990;507(1):69–84. doi: 10.1016/0006-8993(90)90524-f. [DOI] [PubMed] [Google Scholar]
- 22.Salter MW, Henry JL. Evidence that adenosine mediates the depression of spinal dorsal horn neurons induced by peripheral vibration in the cat. Neuroscience. 1987;22(2):631–650. doi: 10.1016/0306-4522(87)90359-9. [DOI] [PubMed] [Google Scholar]
- 23.Salter MW, De Koninck Y, Henry JL. ATP-sensitive K+ channels mediate an IPSP in dorsal horn neurones elicited by sensory stimulation. Synapse (New York, NY) 1992;11(3):214–220. doi: 10.1002/syn.890110306. [DOI] [PubMed] [Google Scholar]
- 24.Marchand S, Li J, Charest J. Effects of caffeine on analgesia from transcutaneous electrical nerve stimulation. N Engl J Med. 1995;333(5):325–326. doi: 10.1056/nejm199508033330521. [DOI] [PubMed] [Google Scholar]
- 25.Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci. 1999;2(3):241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- 26.Salter MW, De Koninck Y. An ambiguous fast synapse: a new twist in the tale of two transmitters. Nat Neurosci. 1999;2(3):199–200. doi: 10.1038/6296. [DOI] [PubMed] [Google Scholar]
- 27.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science (New York, NY) 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 28.Prescott SA, Ma Q, De Koninck Y. Normal and abnormal coding of somatosensory stimuli causing pain. Nat Neurosci. 2014;17(2):183–191. doi: 10.1038/nn.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 30.Masuda T, Iwamoto S, Yoshinaga R, Tozaki-Saitoh H, Nishiyama A, Mak TW, Tamura T, Tsuda M, Inoue K. Transcription factor IRF5 drives P2X4R+-reactive microglia gating neuropathic pain. Nat Commun. 2014;5:3771. doi: 10.1038/ncomms4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuda T, Tsuda M, Yoshinaga R, Tozaki-Saitoh H, Ozato K, Tamura T, Inoue K. IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep. 2012;1(4):334–340. doi: 10.1016/j.celrep.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masuda T, Ozono Y, Mikuriya S, Kohro Y, Tozaki-Saitoh H, Iwatsuki K, Uneyama H, Ichikawa R, Salter MW, Tsuda M, Inoue K. Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nat Commun. 2016;7:12529. doi: 10.1038/ncomms12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato Y, Hiasa M, Ichikawa R, Hasuzawa N, Kadowaki A, Iwatsuki K, Shima K, Endo Y, Kitahara Y, Inoue T, Nomura M, Omote H, Moriyama Y, Miyaji T. Identification of a vesicular ATP release inhibitor for the treatment of neuropathic and inflammatory pain. Proc Natl Acad Sci U S A. 2017;114(31):E6297–e6305. doi: 10.1073/pnas.1704847114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beggs S, Trang T, Salter MW. P2X4R+ microglia drive neuropathic pain. Nat Neurosci. 2012;15(8):1068–1073. doi: 10.1038/nn.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 36.Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29(11):3518–3528. doi: 10.1523/jneurosci.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28(44):11263–11268. doi: 10.1523/jneurosci.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keller AF, Beggs S, Salter MW, De Koninck Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424(6951):938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 41.Gagnon M, Bergeron MJ, Lavertu G, Castonguay A, Tripathy S, Bonin RP, Perez-Sanchez J, Boudreau D, Wang B, Dumas L, Valade I, Bachand K, Jacob-Wagner M, Tardif C, Kianicka I, Isenring P, Attardo G, Coull JA, De Koninck Y. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med. 2013;19(11):1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee KY, Prescott SA. Chloride dysregulation and inhibitory receptor blockade yield equivalent disinhibition of spinal neurons yet are differentially reversed by carbonic anhydrase blockade. Pain. 2015;156(12):2431–2437. doi: 10.1097/j.pain.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 43.Mapplebeck JCS, Lorenzo LE, Lee KY, Gauthier C, Muley MM, De Koninck Y, Prescott SA, Salter MW. Chloride dysregulation through downregulation of KCC2 mediates neuropathic pain in both sexes. Cell Rep. 2019;28(3):590-596.e594. doi: 10.1016/j.celrep.2019.06.059. [DOI] [PubMed] [Google Scholar]
- 44.Hildebrand ME, Xu J, Dedek A, Li Y, Sengar AS, Beggs S, Lombroso PJ, Salter MW. Potentiation of synaptic GluN2B NMDAR currents by Fyn kinase is gated through BDNF-mediated disinhibition in spinal pain processing. Cell Rep. 2016;17(10):2753–2765. doi: 10.1016/j.celrep.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 45.Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M, Nakazawa T, Yamamoto T, Mishina M, Nakai Y, Ito S. Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci. 2005;22(6):1445–1454. doi: 10.1111/j.1460-9568.2005.04340.x. [DOI] [PubMed] [Google Scholar]
- 46.Dedek A, Xu J, Kandegedara CM, Lorenzo L, Godin AG, De Koninck Y, Lombroso PJ, Tsai EC, Hildebrand ME. Loss of STEP61 couples disinhibition to N-methyl-d-aspartate receptor potentiation in rodent and human spinal pain processing. Brain. 2019;142(6):1535–1546. doi: 10.1093/brain/awz105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mapplebeck JCS, Dalgarno R, Tu Y, Moriarty O, Beggs S, Kwok CHT, Halievski K, Assi S, Mogil JS, Trang T, Salter MW. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain. 2018;159(9):1752–1763. doi: 10.1097/j.pain.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 48.Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet (London, England) 1996;347(9015):1604–1605. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]