Abstract
Background:
Contrast volume used during percutaneous coronary intervention has a direct relationship with contrast-associated acute kidney injury. While several models estimate the risk of contrast-associated acute kidney injury, only the strategy of limiting contrast volume to 3×estimated glomerular filtration rate (eGFR) gives actionable estimates of safe contrast volume doses. However, this method does not consider other patient characteristics associated with risk, such as age, diabetes or heart failure.
Methods:
Using the National Cardiovascular Data Registry acute kidney injury risk model, we developed a novel strategy to define safe contrast limits by entering a contrast term into the model and using it to meet specific (e.g. 10%) relative risk reductions. We then estimated acute kidney injury rates when our patient-centered model-derived thresholds were and were not exceeded using data from CathPCI version 5 between April 2018 and June 2019. We repeated the same analysis in a sub-set of patients who received ≤ 3 × eGFR contrast.
Results:
After excluding patients on hemodialysis, below average risk (<7%), missing data and multiple percutaneous coronary interventions, our final analytical cohort included 141,133 patients at high risk for acute kidney injury. The rate of acute kidney injury was 10.0% when the contrast thresholds derived from our patient-centered model were met and 18.2% when they were exceeded (p<0.001). In patients who received contrast ≤ 3×eGFR (n=82,318), contrast associated acute kidney injury rate was 9.8% when the contrast thresholds derived from our patient centered model were met and 14.5% when they were exceeded (p <0.001).
Conclusion:
A novel strategy for developing personalized contrast volume thresholds, provides actionable information for providers that could decrease rates of contrast associated acute kidney injury. This strategy needs further prospective testing to assess efficacy in improving patient outcomes.
Keywords: Contrast Associated Acute Kidney Injury, Percutaneous Coronary Intervention, Risk Models
INTRODUCTION
Contrast-associated acute kidney injury (CA AKI), defined as a ≥0.3mg/dL increase in serum creatinine within 48 hours after contrast exposure, occurs in about 7% of patients undergoing percutaneous coronary intervention (PCI) (1) and is associated with death, hemodialysis, longer hospital stays and higher costs (2–5). For example, a study of nearly 1 million patients undergoing PCI at 1,253 US centers demonstrated that in-hospital mortality was 9.7% in those who developed CA AKI and 0.5% in those who did not (6), underscoring the importance of preventing CA AKI. Interventions, such as using intravenous sodium bicarbonate and n-acetyl cysteine do not reduce CA AKI (7,8), leaving hydration and contrast minimization as the key interventions to decrease CA AKI risk. Importantly, there is evidence of wide variability across physicians in the amount of contrast used and little evidence that less contrast is used in higher-risk patients (1).
While several risk models have been developed to estimate CA AKI risk (9–12), they do not give specific, actionable information to help providers decrease the risk of CA AKI. A volume of contrast media to creatinine clearance ratio (V/Cr Cl) of more than 3.7 was previously found to be a significant and independent risk factor for CA AKI (13). Accordingly, a practice of limiting contrast to less than 3 times the estimated glomerular filtration rate (eGFR) has become the most commonly used guide to reduce CA AKI (14). However, this method, although practical, does not consider patient risk factors other than pre-procedural kidney function. Some patients are at a higher risk of CA AKI based on their demographics and comorbidities. For example, an older patient with diabetes mellitus (DM) and congestive heart failure (CHF) has a higher risk of CA AKI, as compared with a younger patient without any comorbidities and comparable renal function. Using the validated National Cardiovascular Data Registry (NCDR) Cath PCI risk prediction model (12), we developed a novel, patient-centered approach to set contrast volume thresholds based on individual patient’s risk and compared CA AKI rates when our thresholds were and were not exceeded.
METHODS
Data Source for derivation cohort and validation cohort:
The NCDR CathPCI registry has been previously described (15,16). It collects data, including patients’ clinical presentation, disease severity, treatments, and outcomes of PCI at 1577 US sites. For the derivation cohort we used data for all patients enrolled in the CathPCI registry from June 2009 to June 2012. For the validation cohort. For both derivation and validation cohorts we excluded patients with low pre-procedural risk of CA AKI (<7%),those undergoing multiple PCIs, those on dialysis, patients missing creatinine values before or after PCI, as well as those discharged on the day of their procedure, were excluded from the final study cohort. Because patients undergoing same-day discharge would not have a post-procedure creatinine available, we compared baseline characteristics and procedural details in patients in our final analytical cohort with patients who were excluded due to missing creatinine. The Saint Luke’s Institutional Review Board approved the analyses with a waiver of informed consent.
Definitions and pre-procedural CA AKI risk assessment:
CA AKI was defined as an absolute increase in serum creatinine of 0.3 mg/dl or ≥50% increase in serum creatinine within 48-hours of contrast exposure, in accordance with the Acute Kidney Injury Network definition (17). eGFR was calculated from patients’ pre-procedural serum creatinine using the modification in diet in renal disease equation (18). Patient’s pre-procedural serum creatinine value was defined as the most recent value within the past 30 days that was available prior to the procedure.
Pre-procedural CA AKI risk was calculated using the NCDR risk model published by Tsai et al (12). Briefly, the model includes the following predictors of CA AKI: age, presence of an intra-aortic balloon pump before PCI, baseline mild [eGFR 45 to 60 mL/min], moderate [eGFR =30 to 45 mL/min], and severe [eGFR<30 mL/min] chronic kidney disease; heart failure, diabetes, cerebrovascular disease, non-ST elevation myocardial infarction/Unstable Angina, ST elevation myocardial infarction and cardiogenic shock.
Patient-Centered Model with Contrast Term Added:
In our derivation cohort, to the NCDR risk model, we added the contrast term create a new model, the patient-centered model.. We then applied this model to validation cohort and examined the predictive ability of our model by calculating the C-statistic. We used this cohort to generate safe contrast thresholds as described below.
NCDR risk model-derived contrast volume threshold:
The contrast-term-enhanced logistic regression model can calculate and estimate the probability of an outcome, given specific values of each model covariate (19). By incorporating a patient’s covariates and the average amount of contrast used in the CathPCI registry, an individual patient’s baseline risk (log-odds or logit) of CA AKI after PCI can be defined as follows:
With the contrast term included in the model, we then back-calculated the logistic regression equation for the contrast volume needed to reduce the risk of CA AKI by a given percentage. From a practical perspective, we envisioned that a hospital would define a targeted goal for CA AKI risk reduction, such as 5, 10 or 15%, for their center. Once a hospital target is established, it is then straightforward to use this patient-centered model to estimate each patient’s expected risk of CA AKI and apply the center-level risk reduction goal to the contrast-enhanced risk model to calculate what amount of contrast that would result in the pre-defined risk reduction target. This allowed a personalized, patient-specific safe contrast volume that would meet the pre-defined risk reduction strategy. For example, if a patient’s individualized, pre-procedural risk of CA AKI was 10% and the center-level goal was a 10% risk reduction, then the calculated contrast volume that would result in 9% CA AKI risk for that patient would be 120ml. Alternatively, if the center-level goal for risk reduction were 15%, then the safe contrast limit for a patient with a pre-procedural risk of 10% would be 83ml to achieve an estimated risk of 8.5%. For this study, we used a center goal of 10% relative risk reduction, with secondary estimates for a 15% relative risk reduction.
Application of the NCDR risk model-derived strategy to patients with above-average pre-procedural AKI risk:
Application of our NCDR risk model-derived strategy on patients with below-average pre-procedural CA AKI risk would not be practical as the absolute risk reduction would be small. For example, limiting contrast in a patient with a pre-procedural risk of CA AKI that is well below the 7% national average (e.g. 2%), even if it could reduce the risk by 25%, would have only a minimal reduction in the absolute risk of CA AKI (number needed to treat of 200 to reduce the patient’s risk to 1.5%). In contrast, a patient with a very high risk of CA AKI (e.g. 20%) could never practically achieve the national average, but a 25% relative risk reduction could lead to a very large absolute benefit (number needed to treat of 20). Thus, we propose applying this proposed approach to patients with higher than average CA AKI risk (i.e. >7%), and all analyses were applied to patients with above-average (i.e. >7%) CA AKI risk. For patients at very high risk of CA AKI, in whom a contrast limit of <40ml was generated, we truncated the contrast limit to 40ml, so as to support achievable contrast limits.
Statistical Analysis:
We described baseline demographics, comorbidities, presentation, lesion and PCI details of patients in our study and compared them among patients in whom our patient-centered model thresholds were met and those in whom this threshold was exceeded. Continuous variables are presented as mean ± Standard Deviation (SD) and categorical variables are presented as number (n), and percentage. Due to large cohort sizes, standardized differences were used for comparison (>10% difference is considered clinically important).
We compared CA AKI rates among patients in whom our patient-centered model thresholds were met and those in whom this threshold was exceeded in patients stratified by pre-procedural CA AKI risk. To demonstrate the potential advantage of this new approach in the subset of patients in our study who received ≤ 3 × eGFR contrast, we compared CA AKI rates when our patient-centered model thresholds were and were not exceeded using the chi-square test. We also repeated this approach in a subset of patients who received ≤ 2 × eGFR contrast. We then constructed histograms of differences between the safe contrast volume estimates given by our patient-centered model and by 3 × eGFR and 2 × eGFR approaches.
To help support the clinical use of our approach we leveraged the integer scoring system for the original NCDR model that had similar predictive accuracy (12). The integer scoring system gives a percentage risk for CA AKI, and by using this risk in our patient-centered model we could calculate safe contrast limits for 10% and 15% risk reductions. Finally, to describe the relationship between contrast volume and risk of CA AKI we plotted the risk of CA AKI as a function of contrast volume/eGFR. Missing data for other covariates in the Cath PCI registry is minimal, with variables of interest having missing rates <1%. All analyses were done using SAS version 9.4 (SAS Institute Inc., Cary NC, USA).
RESULTS
The derivation cohort (patients enrolled in Cath-PCI registry between June 2009 and June 2012) consisted of 1,753,138 patients with above average risk of CA AKI. After excluding patients with below average (<7%) risk (n=936,697), patients on hemodialysis (n=34,780), those who had multiple PCIs during a hospital stay (n=46,066), those with missing creatinine (n=274,673) and same-day discharges (n=63,109), the final analytical cohort included 397,813 patients. Supplemental figure 1 shows a consort diagram from which the final study population was derived. Supplemental table 1 describes the patient characteristics in the derivation cohort. The mean age of the cohort was 70.7 ± 11.4 years, 41.3% of the patients were female and 87.6% were Caucasian. Cardiac and non-cardiac comorbidities were common, with 60.8% having diabetes, 87.4% hypertension, 34.9% a prior myocardial infarction and 20.7% chronic lung disease. The c-statistic for the enhanced CA AKI model that included the contrast term was 0.72.
The validation cohort (patients enrolled in Cath-PCI registry between April 2018 and June 2019), consisted of 196,394 patients with above average risk of CA AKI. After excluding patients on hemodialysis (n=18,820), those who had multiple PCIs during a hospital stay (n=5,555), those with missing creatinine (n=27,619), missing contrast dose (n=1,259) and same-day discharges (n=2,008), the final analytical cohort included 141,113 patients. Figure 1 shows a consort diagram from which the final study population was derived. Supplemental table 2 compares patients in the final analytical cohort with patients with above-average risk who were excluded due to missing creatinine. Patients who were excluded due to missing creatinine were generally similar to patients in our analytical cohort, except that they were at lower risk for CA AKI. Patients who had missing creatinine, had marginally lower baseline eGFR, received less contrast, had higher ejection fractions and were more likely to undergo an elective PCI. The C-statistic for the model in this cohort was 0.70.
Figure 1. Study population selection process.
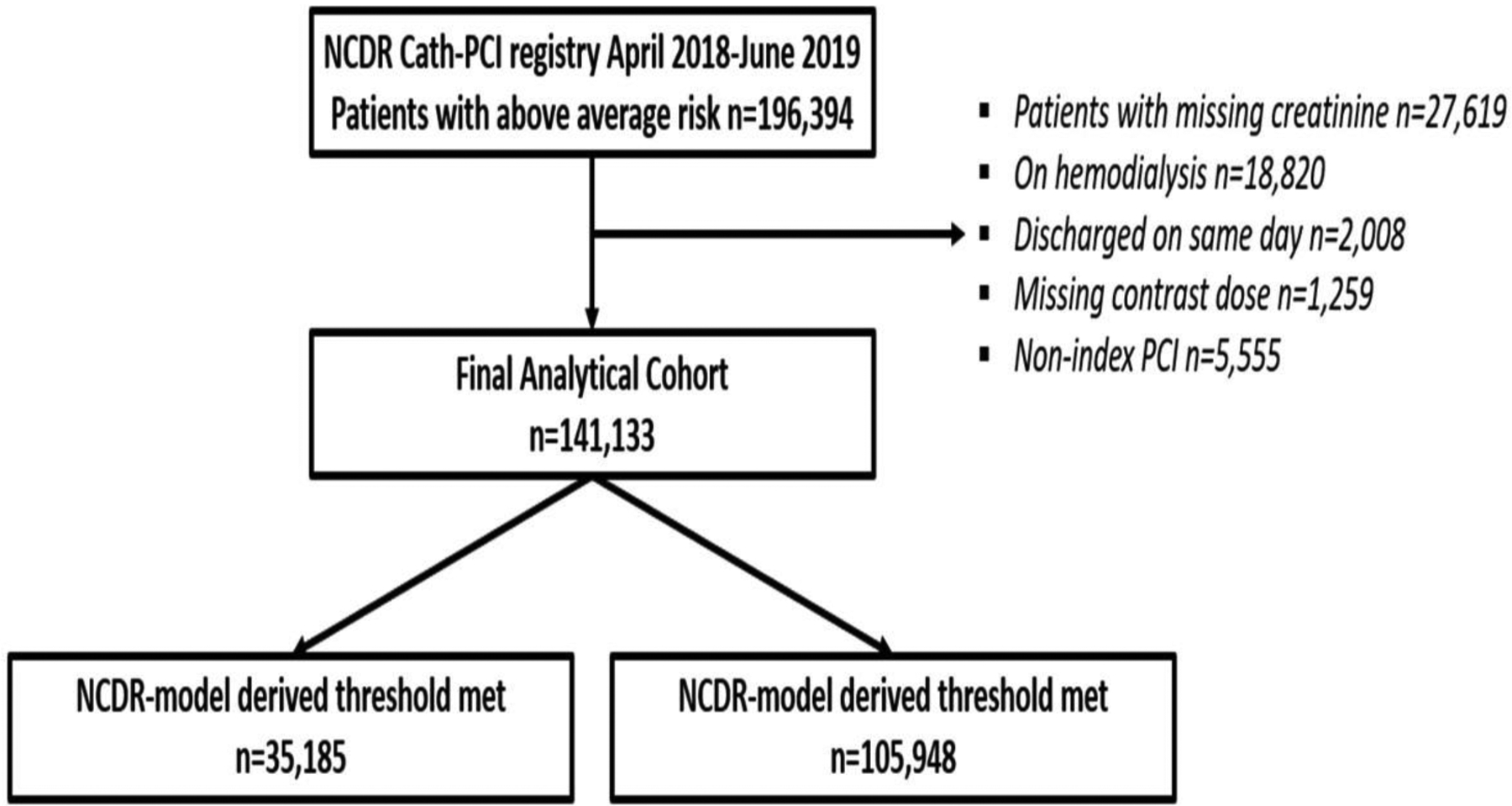
Consort diagram describing study population selection.
The mean age of the cohort was 71.8 ± 11.2 years, 60.5% were male and 87.1% Caucasian. Comorbidities were common, with 60.6% having diabetes, 89.3% hypertension, 33.5% a prior myocardial infarction and 56.8% being active or recent smokers. Less than 25% of patients at high risk for CA AKI (35,185 of 141,113) received contrast doses below our patient-centered model recommended limits. Patients who received contrast within the threshold derived from our patient-centered model were of similar age compared with patients receiving contrast above the threshold, but had a lower rate of ST segment elevation myocardial infarction and a higher rate of elective procedures (Table 1). Moreover, patients who were below the model threshold had similar proportion of chronic total occlusions, and severely calcified lesions, but a lower type C and bifurcate lesions and non-radial access. However, PCI outcomes including guidewire crossing lesion, device deployment, post PCI TIMI 3 flow and complications including coronary perforation, dissection and cardiac tamponade were similar in the two groups (Table 1).
Table 1.
Comparison of baseline demographics, comorbidities, procedure details and outcomes in patients stratified by pre-procedural CA AKI risk.
| Adherence to NCDR model threshold | |||
|---|---|---|---|
| Below n= 35,185 |
Above n= 105,948 |
Standardized difference (%) | |
| Demographics | |||
| Age years (mean ± SD) | 72.1 ± 11.3 | 71.7 ± 11.2 | 3.5 |
| Male Sex (n, %) | 20,489 (58.2%) | 64,920 (61.3%) | 6.2 |
| Caucasian Race (n, %) | 29,722 (86.5%) | 90,269 (87.3%) | 2.5 |
| Comorbidities | |||
| Current/Recent Smoker | 19,734 (56.1%) | 60,363 (57.0%) | 1.8 |
| Diabetes Mellitus | 20,549 (58.4%) | 65,022 (61.4%) | 6.1 |
| Prior MI | 11,962 (34.0%) | 35,310 (33.3%) | 1.4 |
| EF% (mean ± SD) | 45.9 ± 15.3 | 44.3 ± 15.2 | 10.6 |
| Baseline eGFR | 57.3 ± 26.0 | 59.6 ± 27.3 | 8.5 |
| Presentation | |||
| STEMI | 8,194 (23.3%) | 34,581 (32.6%) | 20.9 |
| Elective PCI | 7,750 (22.0%) | 14,690 (13.9%) | 21.4 |
| Cardiogenic Shock | 543 (1.5%) | 11,867 (11.2%) | 40.3 |
| Cardiac Arrest | 1,673 (4.8%) | 12,616 (11.9%) | 26.1 |
| PCI and Lesion Details | |||
| Non-radial amless (%) | 19,742 (56.1%) | 69,180 (65.3%) | 18.9 |
| CTO (%) | 1,010 (2.9%) | 4,418 (4.2%) | 7.1 |
| Multivessel PCI (%) | 3,862 (11.0%) | 21,058 (19.9%) | 24.8 |
| Bifurcate lesion (%) | 3,302 (9.4%) | 15,517 (14.6%) | 16.2 |
| Type C lesion (%) | 21,262 (60.4%) | 73,949 (69.8%) | 19.7 |
| Severely Calcified (%) | 3,464 (9.8%) | 13,681 (12.9%) | 9.7 |
| Contrast Volume ml (mean ± SD) | 83.7 ± 27.1 | 185.9 ± 73.3 | 185 |
| PCI Outcomes | |||
| Guidewire crossed | 34,653 (98.5%) | 104,717 (98.8%) | 3.0 |
| Device deployed | 34,494 (98.1%) | 104,323 (98.5%) | 3.2 |
| Post PCI TIMI 3 flow | 33,658 (98.3%) | 100,657 (97.3%) | 7.5 |
| Coronary perforation | 104 (0.3%) | 510 (0.5%) | 3.0 |
| Coronary dissection | 138 (0.4%) | 998 (0.9%) | 6.8 |
| Tamponade | 39 (0.1%) | 264 (0.3%) | 3.3 |
CA AKI rate in our high-risk study population was 16.1%. The overall CA AKI rate was 10.0% when the patient-centered contrast limits were met and 18.2% when they were exceeded (p<0.001). Table 2 describes the CA AKI rates in patients in whom patient-centered thresholds were and were not exceeded stratified by pre-procedure CA AKI risk. The benefit of lower CA AKI rates when the model derived thresholds were not exceeded, was strongest in patients with the highest risk.
Table 2.
Comparison of CA AKI rates when NCDR model derived threshold was and was not exceeded.
| Risk | CA AKI when Contrast<Model | CA AKI when Contrast>Model | Delta (%) | NNT | P value |
|---|---|---|---|---|---|
| 7–10% | 1,220/17,543 (7.0%) | 3,059/34,388 (8.9%) | 1.9 | 53 | <.001 |
| 10–15% | 1,071/10,645 (10.1%) | 3,715/28,569 (13.0%) | 2.9 | 34 | <.001 |
| >15% | 1,231/6,997 (17.6%) | 12,474/42,991 (29.0%) | 11.4 | 9 | <.001 |
| Overall | 3,522/35,185 (10.0%) | 19,248/105,948 (18.2%) | 8.2 | 12 | <.001 |
Contrast Induced Acute Kidney Injury (CA AKI), Number needed to treat to prevent 1 CA AKI (NNT)
In patients who received ≤3 × eGFR contrast (n=33,567), the CA AKI rate was 9.8% when the patient-centered model threshold was achieved, compared with 14.5% when it was exceeded (p <0.001). Similarly, in patients who received ≤ 2 eGFR contrast (n=25,544) CA AKI rate was 9.7% when the patient-centered model threshold was achieved compared with 14.8% when it was exceeded (p < 0.001).
Figure 2 shows the differences in contrast volume thresholds calculated from the patient-centered model and 3×eGFR approach. The use of the patient-centered safe contrast limits, in general, suggested less contrast than the 3×GFR approach. Mean differences in safe contrast limits was 70.3ml lower by the patient-centered model than the 3 × eGFR estimate, although in about 10% of cases, the safe contrast threshold estimated form the patient-centered model was greater than that estimated from 3×eGFR. To compare the safe contrast limits derived from the patient-centered model with stricter contrast thresholds, we also compared the difference in contrast limits suggested by the NCDR model and by the 2×eGFR approach. Figure 3 describes the differences in safe contrast volume limits from patient-centered model and 2×eGFR strategy with a mean difference in safe contrast being 17.7ml r with the patient-centered model than the 2 × eGFR approach and 45.3% of cases having higher estimates from the patient-centered model than the 2×eGFR estimated limits.
Figure 2. Difference in safe contrast threshold with NCDR derived approach and 3 × eGFR.
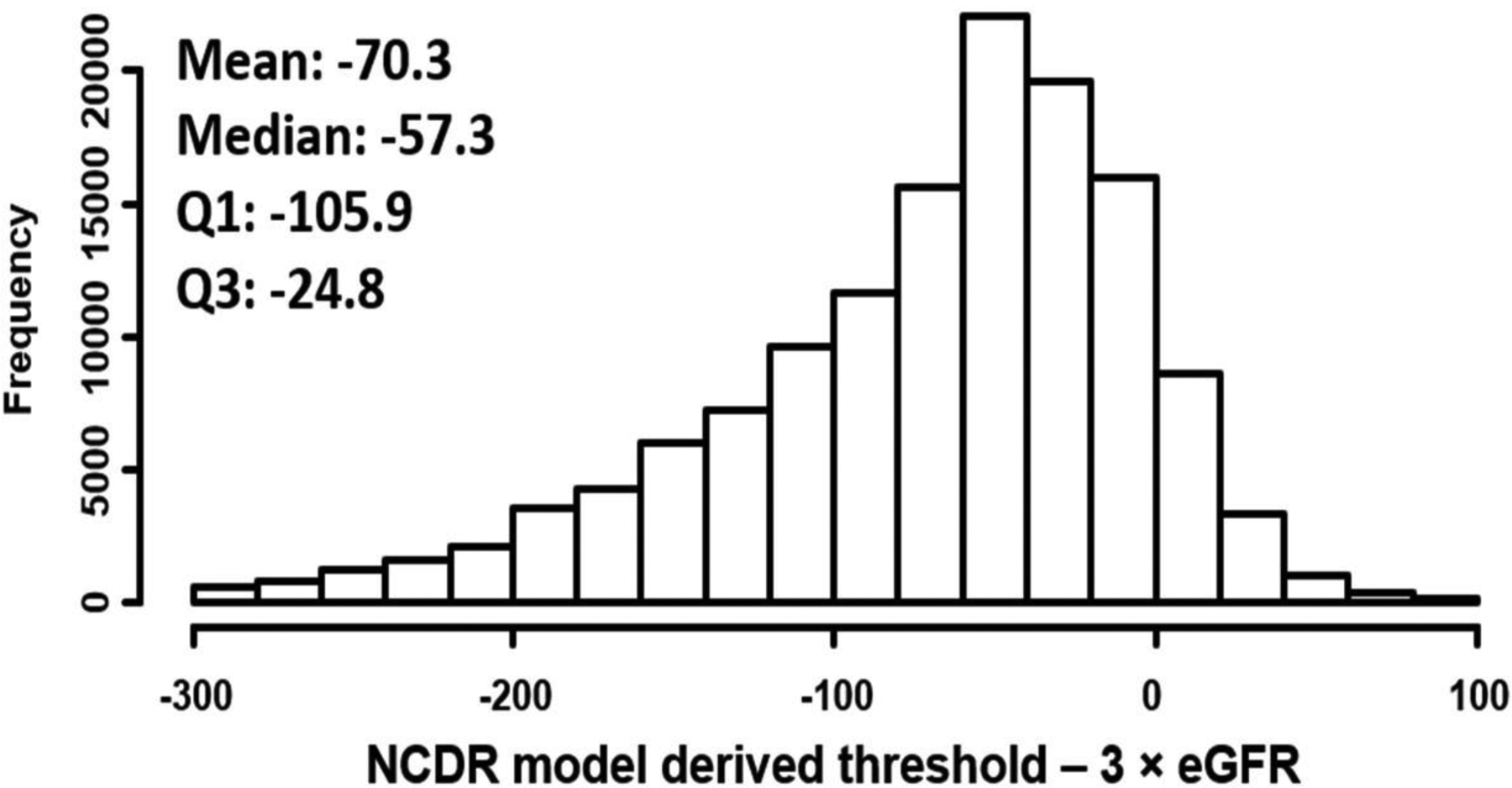
Histogram showing differences in contrast volume thresholds calculated from NCDR model and 3×eGFR approach.
Figure 3. Difference in safe contrast threshold with NCDR derived approach and 2 × eGFR.
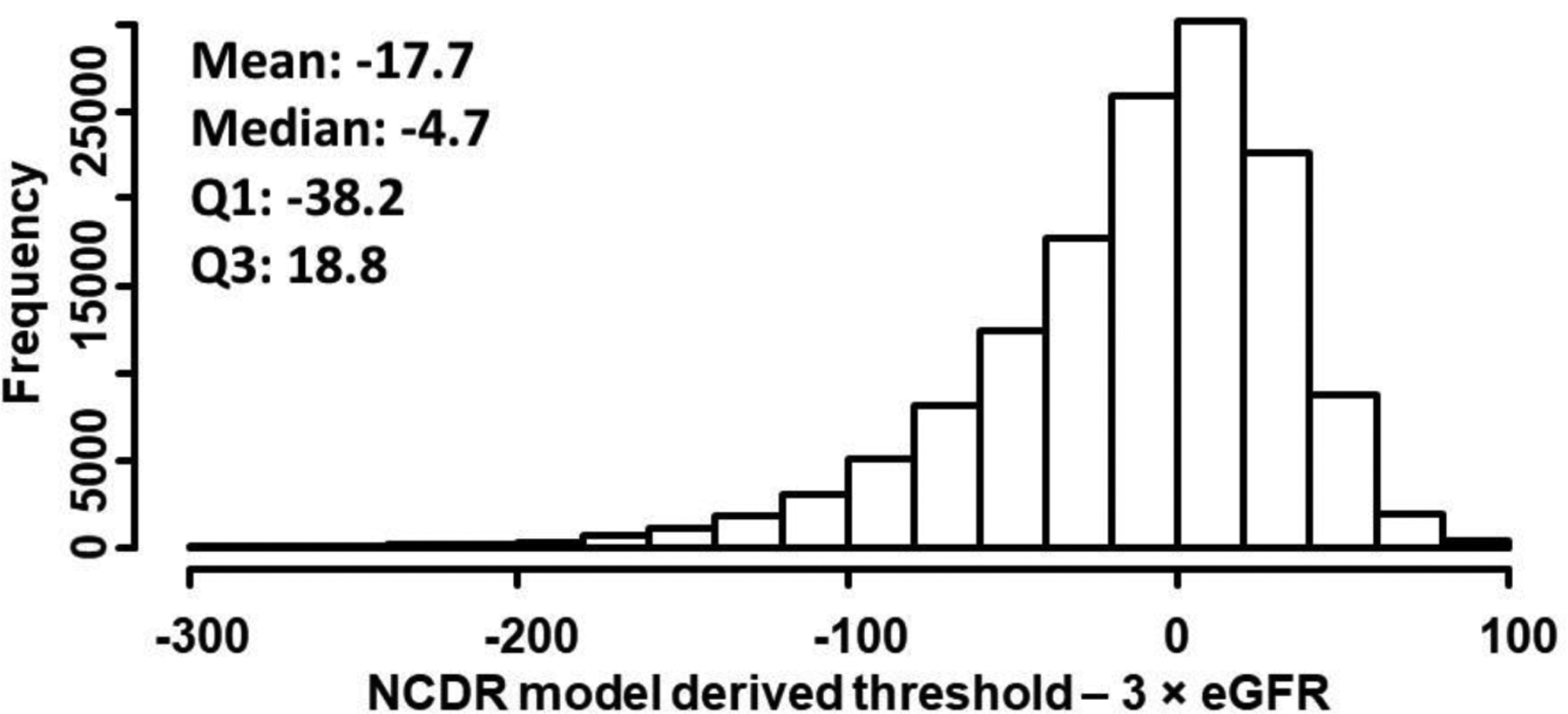
Histogram showing differences in contrast volume thresholds calculated from NCDR model and 2×eGFR approach.
For bedside calculations of safe contrast threshold using our approach the integer scoring system for the original NCDR risk model is provided in Table 3. Safe contrast thresholds for 10% and 15% risk reductions are also provided in Table 3. Supplemental Figure 2 describes the association between contrast volume/eGFR with risk of CA AKI. The risk of CA AKI increased with higher values of contrast volume/eGFR.
Table 3.
Bedside calculation of safe contrast thresholds using the integer scoring system for NCDR risk model.
| Factors in Model | Points | Total Score | AKI Risk | Average Total Contrast Volume Threshold | |
|---|---|---|---|---|---|
| 10% Risk Reduction | 15% Risk Reduction | ||||
| Age (years) | 20–24 | 7–9% | 129ml | 93ml | |
| <50 | 0 | 25–29 | 9–12% | 124ml | 88ml |
| 50–59 | 2 | 30–34 | 12–16% | 116ml | 79ml |
| 60–69 | 4 | 35–39 | 17–22% | 101ml | 62ml |
| 70–79 | 6 | 40–44 | 22–28% | 84ml | 47ml |
| 80–89 | 8 | 45–49 | 28–35% | 65ml | 40ml** |
| >90 | 10 | 50–55 | 35–43% | 48ml | 40ml** |
| Prior 2-weeks HF | 11 | >55 | >43% | 40ml** | 40ml** |
| Estimated GFR (ml/min/1.73m2) | |||||
| Severely Low (<30) | 18 | ||||
| Moderate Low (31–45) | 8 | ||||
| Mildly Low (46–60) | 3 | ||||
| Diabetes | 7 | ||||
| Prior HF | 4 | ||||
| Prior CVD | 4 | ||||
| NSTEMI/UA | 6 | ||||
| STEMI | 15 | ||||
| Cardiogenic Shock | 16 | ||||
| Cardiac Arrest | 8 | ||||
| Anemia (Hb <10g/dL) | 10 | ||||
| IABP | 11 | ||||
Full reduction not possible due to lower bound limit of 40ml
DISCUSSION
Restricting contrast exposure is one of the most powerful interventions to reduce the risk of CA AKI. While eGFR is the strongest predictor of CA AKI, other patient characteristics also influence this risk and it has been shown that pre-procedural patient variables and contrast volume reliably predict risk of CA AKI (20). Importantly, there is evidence of wide variability across physicians in the amount of contrast used and little evidence that less contrast is used in higher-risk patients (1). To provide a more holistic, patient-centered approach to tailoring contrast volume to CA AKI risk we describe a strategy to first identify patients who have average CA AKI risk using the NCDR AKI risk model, and then describe a unique approach of converting the NCDR AKI risk model into a clinically-actionable guide for defining contrast volume thresholds for PCI. This method can be tailored to each provider’s or institution’s goals for CA AKI relative risk reduction and enables clear targets for achieving these goals. In a national cohort of high-risk patients (61% diabetes, 33 % prior MI) undergoing PCI, we found that less than 1 in 4 patients received contrast volume below what the new patient-centered model calculated would be needed to reduce CA AKI risk by a modest 10%. Yet, adherence to the contrast thresholds derived from the patient-centered model was associated with markedly lower CA AKI rates than when it was not achieved, which was more apparent as the pre-procedural risk for CA AKI increased. Contrary to other risk estimation tools that either give a risk of CA AKI for a given contrast volume (9–12) or give contrast thresholds based on creatinine clearance or weight (21–25), this new approach gives a contrast volume threshold based on each patient’s totality of risk.
Every year over half a million PCIs are performed in the US alone (26), and patients who develop CA AKI have significantly higher risks of major adverse events, including death, hemodialysis and longer hospital stays (2–4). The socioeconomic impact of CA AKI in the US is substantial, with an estimated cost of each CA AKI event of over $9000 (27,28). A recent sub analysis of the PRESERVE trial suggested that CA AKI did not mediate the effect of baseline kidney function on adverse outcomes (defined as death, need for dialysis or persistent kidney impairment) (29). While this shows that baseline kidney function is an important factor for determining a patient’s risk of adverse outcome independent of CA AKI, the added risk due to development of CA AKI is still an important consideration. Indeed, the authors reported an adjusted OR for adverse clinical outcome associated with CA AKI of 3.98 (adjusted for age, sex, baseline urine to albumin ratio, diabetes, myocardial infarction). Notably, further adjustment for baseline eGFR did not attenuate but rather amplified this effect (OR=11.4, 95% CI=6.38–20.37), suggesting that if CA AKI is a marker of anything, it does not appear to be a marker of baseline renal function or other risk factors included in the model. Whether CA AKI has a causal effect, or is a marker, of adverse clinical outcomes remains an important area for future work. Regardless preventing CA AKI remains an important goal in clinical practice.
Minimizing contrast use is one of the two most actionable targets to decrease national CA AKI rates. In a study of over 1.3 million patients enrolled in the NCDR CathPCI registry, Amin et al found that there was significant variability among providers for the volume of contrast used during PCI, with little evidence of using less contrast in patients with higher risks of CA AKI (1). A national analysis from the Veterans Affairs hospitals further confirmed these findings (30). Higher doses of contrast volume have been associated with higher risk of CA AKI (31). Most importantly, contrast volume is a modifiable risk factor. Several quality improvement initiatives to reduce contrast volumes in patients undergoing PCI have shown positive results. In fact, using the approach outlined in this paper, a quality improvement program at Barnes Jewish Hospital led to a significant reduction in CA AKI rates and substantial cost savings of $2,000 per PCI procedure (32). A multi-centered quality improvement initiative that focused on standardization of patient care processes for amount of contrast agent used, reduced CA AKI rates significantly in New England region (33). Another State-wide program in Michigan, aimed at reducing contrast volume for PCI procedures was associated with reduction in contrast volume and CA AKI rates (34). These findings are also consistent with several regional studies that have shown the benefit of decreasing CA AKI rates by quality improvement initiatives targeting contrast volume minimization (33,35). What is needed is a better strategy for prospectively identifying the safe contrast limits for each patient, implementing this estimate into routine clinical care, and creating strategies to help clinicians adhere to safe contrast limits. Implementing such an approach is not only feasible but can lead to a rapid improvement in patient care and cost. While models using machine learning approaches can also be used to set contrast volume thresholds for PCI (20), these models are hard to implement in routine clinical practice. Our model had similar discriminative ability and by using the integer scoring system that we provide, can readily be adapted in routing clinical practice.
Estimating safe contrast thresholds based on multiples of eGFR is predicated upon a single, albeit important, risk factor (renal dysfunction). The NCDR risk model method, in contrast, considers multiple risk factors known to increase a patient’s risk of CA AKI and using this model to determine safe contrast limits generally results in lower contrast volumes than commonly used multiples of eGFR, although occasionally it affords higher levels of contrast when the baseline eGFR is very low. This points towards a potential benefit of using more personalized estimates for safe contrast thresholds as some patients might not need extremely strict thresholds when all of their clinical factors are taken into account.
This strategy can practically be implemented by incorporating the NCDR-risk calculator in the patient’s electronic medical record and adding the NCDR-model derived contrast threshold as part of the pre-procedure time out. Moreover, to assist in bedside calculation of safe contrast limits we have leveraged the integer scoring system for the NCDR model to provide a readily available tool. There are a range of strategies that interventionalists can use to achieve these thresholds, including the avoidance of left ventriculograms, smaller catheters, contrast diversion devices, use of intravascular ultrasound and staging multi-vessel PCI (36). By creating clear thresholds, operators can better judge which of these strategies are most appropriate in individual patients to minimize the risk of CA AKI.
Study Limitations:
Our study should be interpreted in context of several potential limitations. AKI is multifactorial and clinical factors such as cholesterol embolization, and other strategies such as, stopping nephrotoxic medications and pre-procedural hydration, could be significant contributory factors. These are not included in the NCDR risk model. Patients who received a contrast dose that exceeded the patient-centered model threshold, were more likely to present with a STEMI and less likely to have an elective PCI. However, the observed CA AKI rates even in this population were lower when the patient-centered contrast threshold were met. Moreover, pre-procedural CA AKI risk was estimated from a patient’s pre-procedural creatinine which could have been lower if patients had received pre-procedural intravenous fluids. Lack of data on each patient’s hydration regimen is an important limitation. Furthermore, 87% of the patients in our study were Caucasian, and our results might not be generalizable to more racially diverse populations. Another limitation is that the NCDR does not collect the type of contrast agent used and the safe contrast limits might differ if iso-osmolar or low-osmolar contrast is used. It is also possible that post-procedure creatinine was not the peak value and our rates of CA AKI could have been underestimated. Moreover, long term outcomes such as 90-day death, persistently low renal function etc., were not assessed. Finally, this was a retrospective analysis and whether the implementation of these contrast volume thresholds can reduce CA AKI, beyond the single-center experience previously reported (32), require further testing.
Conclusions:
In summary, we developed a novel strategy for estimating individual patients’ safe contrast limits based on a number of important clinical risk factors. Implementing this approach could allow physicians to define a targeted reduction in CA AKI risk (5,10 or 15%) and estimate safe contrast thresholds for their risk reduction goal to improve the safety of PCI. While this approach generally resulted in lower contrast volume thresholds than 3×GFR, it was also associated with reduced risk of CA AKI even when 3×GFR threshold was not exceeded. This strategy needs further prospective testing to establish its safety and utility in decreasing CA AKI among patients undergoing PCI, beyond the single-center experience already reported (32).
Supplementary Material
Funding Disclosure:
Dr. Malik is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL110837. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Amin is funded via an AHRQ R18 grant award (grant number R18HS0224181-01A1); and is a consultant to Terumo, and GE HealthCare. Dr. Mehran has received funding from Abbott Laboratories (consultant), Abiomed (consultant spouse), American College of Cardiology (consultant), AstraZeneca (research funding to institution), Bayer (research funding to institution), Beth Israel Deaconess (research funding to institution, BMS (research funding to institution), Boston Scientific (consultant advisory board), Bristol-Myers, Squibb (research funding to institution), CardioKinetix (research consultant), Cardiovascular Research (consultant), Cardiovascular Systems, Inc (consultant) and Claret Medical (medical monitor, equity) Dr. Spertus is the principal investigator of an analytic contract from the American College of Cardiology Foundation to provide analytic services for the National Cardiovascular Data Registries and has an equity interest in Health Outcomes Sciences. The other authors report no conflicts.
Abbreviations
- CA AKI
Contrast Associated Acute Kidney Injury
- PCI
Percutaneous Coronary Intervention
- V/Cr Cl
Contrast Media to Creatinine Clearance ratio
- eGFR
estimated Glomerular Filtration Rate
- DM
Diabetes Mellitus
- CHF
Congestive Heart Failure
- NCDR
National Cardiovascular Data Registry
- ACC
American College of Cardiology
- SD
Standard Deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Amin AP, Bach RG, Caruso ML, Kennedy KF, Spertus JA. Association of Variation in Contrast Volume With Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Intervention. JAMA cardiology 2017;2:1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. Journal of the American Society of Nephrology : JASN 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- 3.Gruberg L, Mehran R, Dangas G et al. Acute renal failure requiring dialysis after percutaneous coronary interventions. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 2001;52:409–16. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Gurm HS, Bhatt DL, Chew DP, Ellis SG. Renal failure after percutaneous coronary intervention is associated with high mortality. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 2005;64:442–8. [DOI] [PubMed] [Google Scholar]
- 5.Subramanian S, Tumlin J, Bapat B, Zyczynski T. Economic burden of contrast-induced nephropathy: implications for prevention strategies. Journal of medical economics 2007;10:119–34. [DOI] [PubMed] [Google Scholar]
- 6.Tsai TT, Patel UD, Chang TI et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovascular interventions 2014;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia S, Bhatt DL, Gallagher M et al. Strategies to Reduce Acute Kidney Injury and Improve Clinical Outcomes Following Percutaneous Coronary Intervention: A Subgroup Analysis of the PRESERVE Trial. JACC Cardiovascular interventions 2018;11:2254–2261. [DOI] [PubMed] [Google Scholar]
- 8.Weisbord SD, Gallagher M, Jneid H et al. Outcomes after Angiography with Sodium Bicarbonate and Acetylcysteine. The New England journal of medicine 2018;378:603–614. [DOI] [PubMed] [Google Scholar]
- 9.Brown JR, DeVries JT, Piper WD et al. Serious renal dysfunction after percutaneous coronary interventions can be predicted. American heart journal 2008;155:260–6. [DOI] [PubMed] [Google Scholar]
- 10.Duan C, Cao Y, Liu Y et al. A New Preprocedure Risk Score for Predicting Contrast-Induced Acute Kidney Injury. The Canadian journal of cardiology 2017;33:714–723. [DOI] [PubMed] [Google Scholar]
- 11.Mehran R, Aymong ED, Nikolsky E et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. Journal of the American College of Cardiology 2004;44:1393–9. [DOI] [PubMed] [Google Scholar]
- 12.Tsai TT, Patel UD, Chang TI et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath-PCI Registry. Journal of the American Heart Association 2014;3:e001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laskey WK, Jenkins C, Selzer F et al. Volume-to-creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. Journal of the American College of Cardiology 2007;50:584–90. [DOI] [PubMed] [Google Scholar]
- 14.Gurm HS, Seth M, Dixon SR et al. Contemporary use of and outcomes associated with ultra-low contrast volume in patients undergoing percutaneous coronary interventions. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 2019;93:222–230. [DOI] [PubMed] [Google Scholar]
- 15.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. Journal of the American College of Cardiology 2001;37:2240–5. [DOI] [PubMed] [Google Scholar]
- 16.Weintraub WS, McKay CR, Riner RN et al. The American College of Cardiology National Database: progress and challenges. American College of Cardiology Database Committee. Journal of the American College of Cardiology 1997;29:459–65. [DOI] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical care (London, England) 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- 19.DaL SH. Applied Logistic Regression. 2nd Edition. New York: Wiley, 2000. [Google Scholar]
- 20.Huang C, Li SX, Mahajan S et al. Development and Validation of a Model for Predicting the Risk of Acute Kidney Injury Associated With Contrast Volume Levels During Percutaneous Coronary Intervention. JAMA network open 2019;2:e1916021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbieri L, Verdoia M, Marino P, Suryapranata H, De Luca G. Contrast volume to creatinine clearance ratio for the prediction of contrast-induced nephropathy in patients undergoing coronary angiography or percutaneous intervention. European journal of preventive cardiology 2016;23:931–7. [DOI] [PubMed] [Google Scholar]
- 22.Brown JR, Robb JF, Block CA et al. Does safe dosing of iodinated contrast prevent contrast-induced acute kidney injury? Circulation Cardiovascular interventions 2010;3:346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capodanno D, Ministeri M, Cumbo S, Dalessandro V, Tamburino C. Volume-to-creatinine clearance ratio in patients undergoing coronary angiography with or without percutaneous coronary intervention: implications of varying definitions of contrast-induced nephropathy. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 2014;83:907–12. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Liu YH, Chen JY et al. Safe contrast volumes for preventing contrast-induced nephropathy in elderly patients with relatively normal renal function during percutaneous coronary intervention. Medicine 2015;94:e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan N, Liu Y, Zhou YL et al. Contrast medium volume to creatinine clearance ratio: a predictor of contrast-induced nephropathy in the first 72 hours following percutaneous coronary intervention. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 2012;79:70–5. [DOI] [PubMed] [Google Scholar]
- 26.Masoudi FA, Ponirakis A, de Lemos JA et al. Executive Summary: Trends in U.S. Cardiovascular Care: 2016 Report From 4 ACC National Cardiovascular Data Registries. Journal of the American College of Cardiology 2017;69:1424–1426. [DOI] [PubMed] [Google Scholar]
- 27.Inohara T, Numasawa Y, Higashi T et al. Predictors of high cost after percutaneous coronary intervention: A review from Japanese multicenter registry overviewing the influence of procedural complications. American heart journal 2017;194:61–72. [DOI] [PubMed] [Google Scholar]
- 28.McNeely C J S, Singh J, Kurz H, House J, Frogge N, Lindner S, Kulkarni H, Masoudi F, Amin A. THE INCREMENTAL COST OF ACUTE KIDNEY INJURY AFTER PERCUTANEOUS CORONARY INTERVENTION: IMPLICATIONS FOR PRACTICE IN THE UNITED STATES. Journal of the American College of Cardiology;Volume 73. [Google Scholar]
- 29.Weisbord SD, Palevsky PM, Kaufman JS et al. Contrast-Associated Acute Kidney Injury and Serious Adverse Outcomes Following Angiography. Journal of the American College of Cardiology 2020;75:1311–1320. [DOI] [PubMed] [Google Scholar]
- 30.Keach JW, Stanislawski MA, Barón AE et al. Variation in contrast-associated acute kidney injury prophylaxis for percutaneous coronary intervention: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking (CART) program. BMC nephrology 2020;21:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurm HS, Dixon SR, Smith DE et al. Renal function-based contrast dosing to define safe limits of radiographic contrast media in patients undergoing percutaneous coronary interventions. Journal of the American College of Cardiology 2011;58:907–14. [DOI] [PubMed] [Google Scholar]
- 32.Amin AP, Crimmins-Reda P, Miller S et al. Reducing Acute Kidney Injury and Costs of Percutaneous Coronary Intervention by Patient-Centered, Evidence-Based Contrast Use. Circulation Cardiovascular quality and outcomes 2019;12:e004961. [DOI] [PubMed] [Google Scholar]
- 33.Brown JR, Solomon RJ, Sarnak MJ et al. Reducing contrast-induced acute kidney injury using a regional multicenter quality improvement intervention. Circulation Cardiovascular quality and outcomes 2014;7:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurm HS, Seth M, Dixon S, Kraft P, Jensen A. Trends in Contrast Volume Use and Incidence of Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Intervention: Insights From Blue Cross Blue Shield of Michigan Cardiovascular Collaborative (BMC2). JACC Cardiovascular interventions 2018;11:509–511. [DOI] [PubMed] [Google Scholar]
- 35.Moscucci M, Rogers EK, Montoye C et al. Association of a continuous quality improvement initiative with practice and outcome variations of contemporary percutaneous coronary interventions. Circulation 2006;113:814–22. [DOI] [PubMed] [Google Scholar]
- 36.Gurm HS, Mavromatis K, Bertolet B et al. Minimizing radiographic contrast administration during coronary angiography using a novel contrast reduction system: A multicenter observational study of the DyeVert plus contrast reduction system. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions 2019;93:1228–1235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


