Abstract
As the most common symptomatic reason to seek medical consultation, pain is a complex experience that has been classified into different categories and stages. In pain processing, noxious stimuli may activate the anterior cingulate cortex (ACC). But the function of ACC in the different pain conditions is not well discussed. In this review, we elaborate the commonalities and differences from accumulated evidence by a variety of pain assays for physiological pain and pathological pain including inflammatory pain, neuropathic pain, and cancer pain in the ACC, and discuss the cellular receptors and signaling molecules from animal studies. We further summarize the ACC as a new central neuromodulation target for invasive and non-invasive stimulation techniques in clinical pain management. The comprehensive understanding of pain processing in the ACC may lead to bridging the gap in translational research between basic and clinical studies and to develop new therapies.
Keywords: Anterior cingulate cortex, Deep brain stimulation, Transcranial magnetic stimulation, Transcranial direct current stimulation, Pathological pain, Inflammatory pain, Neuropathic pain, Cancer pain
Introduction
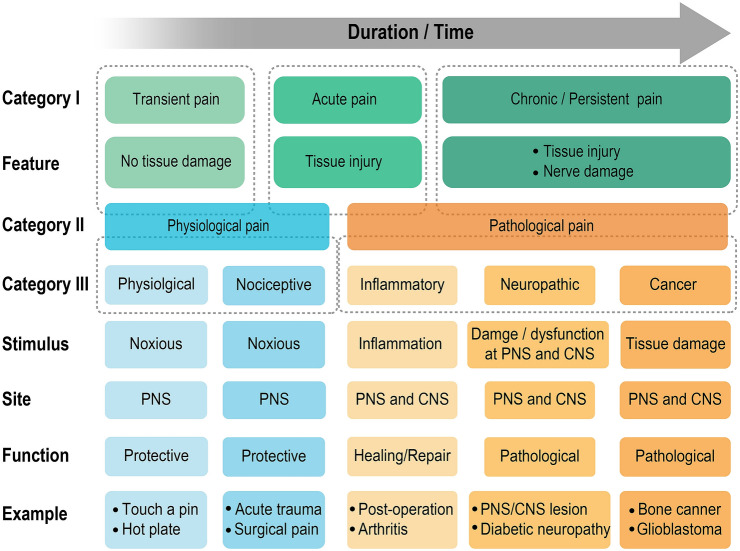
Pain is a multidimensional experience and has been defined and classified into different categories and stages. Based on duration, pain is simply divided into transient, acute, and chronic pain (Fig. 1). Transient pain is elicited when skin and tissues receive nociceptive stimuli without damage. Acute pain lasts a relatively short time and is caused by tissue injury. Chronic or persistent pain is commonly triggered by tissue injury or nerve damage that may exceed the body’s capability to heal. Chronic or persistent pain continues and has long-term changes even after completing curative treatment. Given that the sensation of pain is a result of physiological and/or pathological processes within the nociceptive system, another classification into physiological and pathological pain (Fig. 1) is more widely accepted [1–3]. Physiological pain, which mostly refers to transient pain but sometimes includes acute pain, serves an adaptive function to promote the avoidance of further damage. It is often referred to as a “good pain”, which is a warning system for survival that protects an organism by influencing it to withdraw from harmful stimuli. Pathological pain (also called clinical pain, see below for details), which has a major overlap with chronic pain, happens when intensely or continuously noxious stimuli induce injury of body tissue and occurs when stimuli are excessively intense or prolonged to induce tissue injury and even nerve damage with abnormal sensitivity [4].
Fig. 1.
Classification of pain and its major characteristics. Pain can be divided into three categories based on different characteristics. Category I is characterized by duration: transient, acute, and chronic/persistent pain. Category II is based on the initiating stimulus and function. Physiological pain is mainly associated with noxious stimuli and protective functions, and can be further separated into physiological and nociceptive pain. Pathological pain includes inflammatory, neuropathic, and cancer pain. Neural substrates the peripheral nervous system (PNS) or central nervous system (CNS) are involved in both physiological and pathological pain.
The anterior cingulate cortex (ACC), a key region of the limbic system, is involved in a wide variety of cognitive and emotional processing. Its most basic functions are conflict monitoring, error detection, and attention [5–7]. Related to the error detection and conflict monitory theory, the ACC also acts a part in reward-based learning and anticipation before task performance [8–10] and sexual attraction stimulation-induced pleasure [11]. On the other hand, the ACC is most frequently connected to pain processing in all cortical areas. Related to its higher-level function in cognition and emotion, the ACC is considered to be involved in affective pain [12, 13]. A series of our previous studies have demonstrated that the excitatory activity of ACC neurons is necessary for pain-related negative emotion [14–18]. Based on research from our and other laboratories, we have reviewed the role of the ACC in affective pain [19], but whether and how the ACC decodes and distinguishes sensory pain from affective pain is a long-running controversy.
Human functional imaging studies [20–24] and functional magnetic resonance imaging (fMRI) studies in rat models [25–27] have revealed that the ACC is activated during pain conditions. Clinically, some studies showed a reduction in the sensory component of pain after surgical ablation of the ACC (cingulotomy and cingulectomy) or blockade of the main projection pathway to the ACC [28, 29]. Interestingly, a recent study showed that the ACC is activated normally in both pain-free patients and control individuals without significant difference, which is similar as other pain matrix regions that are commonly activated by painful stimuli [30]. Therefore, how do the cortical regions actually manipulate the feelings of pain? Do they directly decode pain perception, or do they affect sensory pain only when they control the affective pain?
In this topical review we attempt to show the contribution of ACC in pain processing and discuss commonalities and differences using evidence accumulated by a variety of pain assays. We first marshal the preclinical animal studies on the ACC and its functions in different categories of pain for a discussion on mechanisms. Then we discuss the clinical treatments for pain by using invasive and non-invasive ACC stimulation. To summarize these translational studies on the ACC in the different pain conditions, we put forward a better comprehension of the role of the ACC.
Functions of the ACC in preclinical models of pain
Despite significant progress in pain research over the past decades, the complexity of pain phenomena has made them hard to address. The ACC, with its well-documented role in the different states and categories of pain, appears a good entry point. In this section, we review the functions of the ACC in physiological and pathological pain in animal models. Unlike clinical studies in humans, animals are not able to self-report, but it is reliable and objective to score their behaviors under nociceptive stimuli [31]. In preclinical studies, physiological pain generally induces physiological or nociceptive signal inputs to the ACC, generally in the peripheral nervous system with a protective function (Fig. 1). Pathological pain is caused by tissue damage (inflammatory pain) and nerve injury in the peripheral and central nervous systems (neuropathic pain), or by tumors infiltrating and metastasizing (cancer pain) (Fig. 1). The etiologies of inflammatory, neuropathic, and cancer pain are fundamentally distinct (Fig. 1). Injury and inflammation result in an intrinsic immune cascade which may increase the release of active factors from inflammatory cells, injured cells, and blood cells [32]. The induced inflammatory system helps in healing and repair. Neuropathic pain is caused by nerve injury or dysfunction at peripheral terminals and in the central nervous system; it is persistent and includes components of hyperalgesia and allodynia [33]. Cancer pain may involve a complex combination of inflammatory and neuropathic pain with unique characteristics [34].
Even though pain has been separated into different categories, the pain processing is not independent. For example, in some cases, neuropathic pain may be associated with peri- or intraneural inflammation [35]. Hyperactivity of the ACC drives neuropathic pain-induced anxiodepressive behaviors [36]. Especially with cancer pain, an inflammatory response is inevitable in its early phase, while the nervous system is invaded in its later phase [37]. Also, most of the models of pain-related emotion are induced by inflammatory or neuropathic pain and its persistence enhances these emotions over time [19, 38]. Conversely, anxiety and fear of pain significantly alter perceived pain [39–41]. A recent study of a rat model showed convincing evidence that ACC (areas 24a and 24b) contains mirror neurons that respond to both sensory pain and pain-related emotion. The mirror neurons in the ACC respond when a rat experiences pain and witnesses another rat in pain, but these neurons do not respond when the rat experiences shock-induced fear [42]. This interesting study is consistent with the previous research that felt and seen pain evoke the same activity in the ACC and insular cortex [43].
Physiological pain and the ACC
The nociceptors and their central connections, and even the autonomic nervous system, may change after local injury [44]. Physiological pain involves a noxious stimulus-induced nocifensive withdrawal or to other easily-scored behavior. Anatomical studies have demonstrated that the medial/intralaminar thalamic nuclei contain nociceptive neurons that receive noxious input from the spinothalamic tract [45–49], and then send a projection to the ACC [50–55]. Opioid receptors in the ACC are further evidence of nociceptive processes [56–59]. Most of the earliest studies on the ACC in physiological pain were in human subjects. By using painful cutaneous stimulation, Lenz and colleagues showed that the anterior cingulate gyrus in humans receives direct nociceptive input [60]. Davis et al. detected physiological pain in humans from fMRI studies [23, 61]. The ACC is activated by transcutaneous electrical nerve stimulation of the median nerve [61], and either innocuous or noxious thermal stimuli activate different parts of the ACC [23]. Besides somatic pain, visceral pain also presents a significant clinical challenge, and the development of valid behavioral procedures for visceral pain will facilitate the understanding and development of effective treatments of various painful conditions in the viscera [62]. Patients with irritable bowel syndrome (IBS) are hypersensitive to visceral distention. Thirty years ago, both Saper and Neafsey found that electrical stimulation of the rostral ACC elicits autonomic or visceral responses [63, 64]. Magnetic resonance imaging (MRI) studies reported that IBS patients who experience experimental and anticipated rectal pain show activation of the ACC, thalamus, insula, and prefrontal cortex [65–69].
However, some human studies are inconsistent. Positron emission tomography (PET) studies have shown elevated blood flow in the ACC during noxious thermal stimuli in both normal subjects [20–22, 24, 70–76] and in chronic pain patients during the pain state [77]. By using bilateral cingulotomy in patients with schizoaffective disorder [78], chronic depression, or obsessive compulsive disorder [79], Dostrovsky and colleagues found that cingulotomy reduces both the intensity and the affective component of pain, and that noxious thermal or mechanical stimulation, but not electrical stimulation, alter the activity of ACC neurons in awake humans. However, many groups have also reported that cingulotomy strongly inhibits pain-related emotions and avoidance without impact on the subject’s ability to identify the localization and intensity of nociceptive stimuli [80–86].
Besides the controversial human studies, animal studies are more consistent in physiological pain research. Schaffer and colleagues first concluded the ACC function in pain perception in a monkey model in 1888 and showed that a lesion of the cingulate cortex reduces pain sensation [87, 88]. Followed Ivan Pavlov’s classical conditioning theory, behavioral testing in cingulectomized animals demonstrated that the ACC is essential for learning to avoid a noxious footshock [89–94]. To measure the function of the ACC in physiological pain behavior in animals, mechanical stimulation (light touch, pressure, or tap stimulation of the skin are innocuous stimulation; sharp pinching or squeezing is noxious stimulation), thermal stimulation (radiant heat, hot/cold water stimulation), electrical stimulation (electrophysiological activation of noxious input, footshock), and/or chemical stimulation (formalin test) are available. Shyu and colleagues demonstrated that mechanical stimulation-induced nociceptive inputs to medial thalamic afferents (combined with electrical stimulation) causes neuronal responses and synaptic plasticity in the ACC (Table 1) [95–97]. Nociception-specific cells in the ACC have been shown to encode noxious stimulus intensity by using electrodes implanted in the ACC to record electrophysiological signal in the rat [98], rabbit [99] and macaque monkey [100]. Notably, descending facilitation of spinal nociception, such as the tail flick reflex or paw withdrawal latency induced by noxious heating, is modulated by ACC activation via electrical stimulation of excitatory neurons or chemical activation of glutamate N-methyl-D-aspartate (NMDA) receptors or metabotropic glutamate receptors [101–103]. In general, descending facilitation from the ACC modulates neuronal activity in the rostral ventral medulla (RVM) [102], dorsal reticular nucleus [103], and thalamus [104]. Controversially, in a recent study, Chen et al. found descending modulation from the ACC to sensory synaptic transmission in the spinal cord without activation of the RVM [101]. In a rat model of visceral pain, by recording the activity of single neurons in the ACC during colorectal distension (CRD), Li and colleagues reported ACC responses to visceral pain and visceral pain-induced aversion [105, 106].
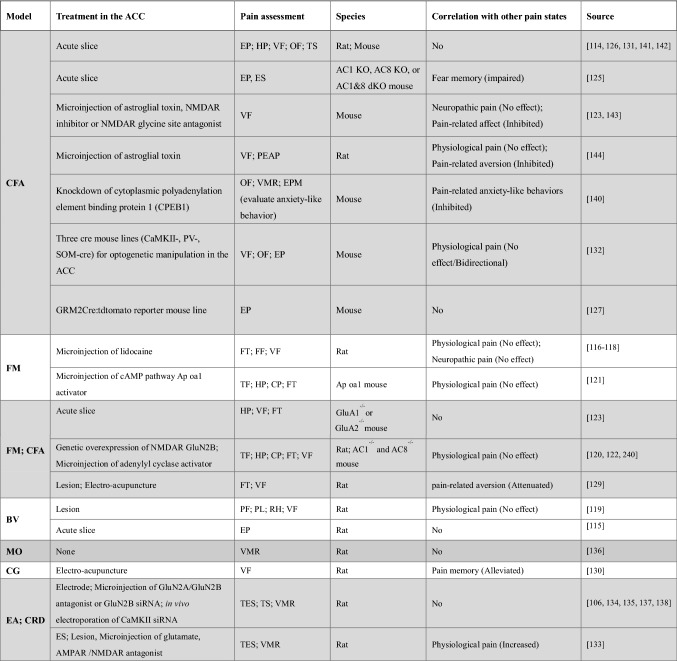
Table 1.
Treatment in the ACC for physiological pain.
ES, electrical stimulation; TES, transcutaneous ES; MS, mechanical stimulation; TS, thermal stimulation; CS, chemical stimulation; FF, foot flick test; PL, paw lifting/licking; OPS, optical stimulation; VMR, visceromotor response; CRD, colorectal distension
In summary, nociceptive neurons in the ACC of differing species respond to physiological pain induced by different types of noxious stimulation. A peripheral noxious stimulus or nociceptive input from the medial thalamus increases neuronal activity and synaptic plasticity in the ACC. Besides the function of receiving noxious signals, activation of the ACC may facilitate spinal nociception and contribute to chronic neuropathic pain [101]. However, a few studies reported that the ACC is involved in descending inhibitory control via the periaqueductal grey (PAG), nucleus raphe magnus, and wide-dynamic-range neurons in the spinal cord [107, 108]. The classic chemical stimulation, the formalin test, is used to measure both physiological and pathological pain. Activation of the ACC significant decreases both the acute physiological phase and the late inflammatory phase of formalin test [109], while the lesion of the ACC inhibits the loss of formalin-evoked nocifensive behavior [108], indicating that the function of the ACC in pain-related nociceptive processing in rodents and primates may rely on different states of pain.
Pathological pain and the ACC
Inflammatory pain
Inflammatory pain is a longer-lasting, inescapable response that involves supraspinal organization. Inflammatory agents are inject into a hindpaw or knee/ankle joint as a routine operation to build rodent models of inflammatory pain [110]. Formalin, complete Freund’s adjuvant (CFA), capsaicin, carrageenan, urate crystals, and zymosan are widely used as inflammatory agents (Table 2). The subcutaneous injection of nociceptor-sensitizing molecules, such as pro-inflammatory cytokines, substance P, serotonin, and prostaglandins, has also been used to induce inflammatory responses. The formalin test (dilute formalin injection into a hindpaw) is commonly used in studies of inflammatory pain. The latency to licking the injected paw is measured in the rodents. Two identified periods of high licking activity occur in response to formalin: an early phase in the first 5 min (as a measure of acute pain) and a late phase persisting from 20 to 30 min after injection (as a measure of inflammatory pain) [111]. Besides the formalin test, the behavioral test paradigms of inflammatory pain measure the withdrawal response to mechanical or thermal stimulation as a reflexive behavior. The injection of adjuvants triggers a stimulus-response function that reflects inflammatory responses, such as allodynia (decreased withdrawal thresholds responding to innocuous stimulation) and hyperalgesia (enhanced withdrawal response to normally noxious stimulation) [112, 113]. Thermal hyperalgesia assays (such as hot plate, tail flick, Hargreaves test), mechanical allodynia assays (such as von Frey filaments, strain gauges) and grip force/strength assays are mostly used in animal behavior testing for inflammatory pain (Table 2).
Table 2.
Treatment in the ACC for inflammatory pain.
Agents: FM, formalin; BV, bee venom; CFA, complete Freund's adjuvant; CP, capsaicin; CG, carrageenan; EA, egg albumin; MO, mustard oil; Pain assessment: FT, formalin test; VF, von Frey filaments; FF, foot flick test; HP, hot plate test; CP, cold plate test; TF, tail flick reflex; RH, radiant heat; PF, paw flinching reflex; PL, paw lifting/licking; ES, electrical stimulation; PEAP, place escape/avoidance paradigm; CPA, conditioned place aversion; F-CPA, formalin-induced CPA; EP, electrophysiological recording; VMR, visceromotor response; CRD, colorectal distension; VH, visceral hypersensitivity; TES, transcutaneous electrical stimulation; EPM, elevated plus maze; OF, open field; TS, thermal stimulation
Similar to physiological pain models, in vitro electrophysiological studies have provided evidence that ACC neurons have increased excitability in the inflammatory pain model [114, 115]. However, differences have been detected between physiological pain and inflammatory pain in behavioral tests. Vaccarino and Melzack reported that microinjection of lidocaine (temporary lesion) into the ACC significant reduces in formalin pain score [116, 117], but does not affect foot-flick latency, indicating distinct functions of the ACC in the modulation of physiological and pathological pain [116]. More solid evidence has demonstrated that electrolytic lesion of the ACC reduce the late phase of the formalin test in inflammatory pain, but does not affect acute pain (early phase of the formalin test) [118]. Moreover, similar results in the model of bee venom (BV)-induced inflammatory pain showed that ACC lesions have no effect on the BV-induced spontaneous paw-flick reflex but decrease inflammatory paw-lifting/licking behavior and thermal/mechanical hypersensitivity [119].
Zhuo and colleagues first reported a series of studies of the cellular mechanism of inflammatory pain in the ACC. They found that peripheral inflammation enhanced forebrain-targeted GluN2B overexpression in mice [120] and in mice heterologously expressing the Aplysia octopamine receptor (Ap oa1) that selectively activates the cAMP pathway [121]. As supportive evidence, adenylyl cyclase 1 (AC1) and AC8 double-knockout mice [122] and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) subunit GluA1-knockout mice [123] show decreased behavioral responses to inflammatory pain via the extracellular signal-regulated kinase (ERK) and cAMP response element binding protein (CREB) signaling pathway. Importantly, none of these transgenic mouse models showed effects on physiological pain assays, such as the hot-plate test, tail-flick reflex test, and mechanical withdrawal threshold [124]. Electrophysiological recordings showed that CFA-induced inflammation in rodents causes the enhancement of neurotransmitter release probability, number of available vesicles, synaptic transmission, AMPAR GluR1 expression, and group II metabotropic glutamate receptor expression in ACC synapses [123, 125–127]. Enhanced presynaptic glutamate release and glutamatergic transmission have been detected in the ACC of Ap oa1 mice [124], whereas in AC1 and AC8 double-knockout mice [125] and GluA1 knockout mice [123] the inflammation-induced enhancement of synaptic transmission is reduced. The neuropeptide calcitonin gene-related peptide (CGRP) also induces excitatory transmission and synaptic plasticity via NMDARs and AC1 in the ACC [128]. These results indicate that the enhanced presynaptic glutamate release and enhanced glutamatergic synaptic transmission including the AMPAR GluA1-ERK-CREB signaling pathway and the CGRP-NMDAR GluN2B-AC1-cAMP pathway contribute to inflammatory pain. To support this, a widely used Chinese medical therapy, electroacupuncture, has been demonstrated to reduce the intensity of formalin-, CFA- or carrageenan-induced inflammatory pain via the NMDAR or CREB pathway in the ACC [129, 130]. Tetrahydroxystilbene glucoside has been found to relieve chronic CFA-induced inflammatory pain by inhibiting microglia activation, neuronal apoptosis, and GluN2B overexpression [131]. Interestingly, ACC neurons also show bidirectional modulation after CFA treatment. Activation of ACC pyramidal neurons decreases the nociceptive mechanical threshold without affecting CFA-induced pain hypersensitivity, while inhibition of these neurons or activation of parvalbumin (PV) interneurons results in an analgesic effect on inflammatory nociception [132].
Visceral inflammation-induced pain also presents a significant clinical challenge. Li and colleagues found sustained application of a heightened tonic visceral afferent nociceptive input increases the responses of ACC neurons in viscerally hypersensitive rats (VH, colorectal anaphylaxis elicited by chicken egg albumin injection, i.p.) [106]. Electrical stimulation of the ACC enhances the visceromotor response (VMR) to CRD in normal rats, while ACC lesions cause a decrease in the VMR in VH rats [133]. Glutamate NMDAR activation is enhanced in the ACC of VH rats [133, 134]. The enhanced GluN2B activity and expression in the ACC contributes to allodynia and hyperalgesia in VH rats [135, 136], and phosphorylated CaMKII postsynaptic binding to GluN2B in postsynaptic sites modulates visceral pain in the VH ACC [137]. Furthermore, VH is correlated with synaptic plasticity, and electrical stimulation-induced medial thalamus input shares a common pathway with visceral pain in the ACC [138]. CRD-induced visceral pain combined with ACC activation is involved in long-term pain-related affect [139] but is independent of an inflammation effect [135]. Recently, by using zymosan injection-induced visceral pain, cytoplasmic polyadenylation element binding protein 1 (CPEB1) expression and GluA1 phosphorylation are increased in the ACC [140].
Interestingly, inflammatory pain with enhancement of synaptic transmission and presynaptic release probability is ACC-specific, and does not occur in the amygdala or spinal cord [123]. However, the ACC corresponds with primary somatosensory (S1) cortex to encode CFA-induced inflammatory pain by modulation of the AMPAR – GABAaR balance [141]. Furthermore, the CFA model also induces GABAergic synaptic plasticity with reduced miniature and spontaneous inhibitory postsynaptic currents and reduced expression of the vesicular GABA transporter [142]. Astrocytes are also involved in inflammatory pain but not neuropathic pain [143]. Peripheral injection of CFA enhances the level of glial fibrillary acidic protein (GFAP) mRNA and protein expression in the ACC [144] and facilitates long-term optical responses via the NMDAR glycine site [143]. Furthermore, formalin- or CFA- elicited inflammatory pain also induces avoidance behavior when combined with mechanical stimuli to the hyperalgesic paw, reflecting the affective components of inflammatory pain in animal studies [145, 146], which we discuss below. In conclusion, inflammatory pain is encoded and correlates with other pain states in the ACC under specific regulation. Once the ACC is activated by inflammatory pain, treatment of the ACC has few effects on physiological pain. But the pain-related emotions or memories, together with inflammatory pain, may be affected by treatment (Table 2).
Neuropathic pain
A feature of neuropathic pain is partial damage to the nervous system. Treede et al. re-defined neuropathic pain as a dysfunction or lesion in the nervous system that initiates pain [147]. In the current preclinical models of neuropathic pain, enhanced responses to stimuli are used as the primary measure, since most patients with neuropathic pain show increased sensitivity to innocuous stimuli such as cold water or touch (allodynia) [4]. In human brain imaging studies, painful neuropathy activates different brain areas in different studies. Moisset and Bouhassira found the ACC and insula are distinctly activated in neuropathic pain patients, while the primary somatosensory (S1) and secondary somatosensory (S2) cortex and the thalamus do not show significant changes [148]. In contrast, brush-evoked allodynia induces activity associated with the lateral thalamus, S1, and S2 rather than the ACC and insula [149–152].
Spared nerve injury (SNI), chronic constriction injury (CCI) by loose ligation of the sciatic nerve, tight ligation of spinal nerves (SNL), and tight ligation of the partial sciatic nerve (PSL) have commonly been used as animal models of neuropathic pain (Table 3) [153]. The paw withdrawal latency and withdrawal duration are commonly used to infer pain and hyperalgesia in neuropathic animals. Using in vivo two-photon imaging and whole-cell patch recording, ACC neurons show enhanced intrinsic excitability and neural activity in the SNI model of neuropathic pain [154, 155]. Furthermore, neurons in the SNI model show increased dendritic complexity and spine density, increased excitatory synaptic transmission by glutamate release and the NMDAR/AMPAR ratio in excitatory postsynaptic currents, activated astrocytes, and decreased inhibitory synaptic transmission by extracellular GABA in the ACC of SNI rats [156–158]. Increased presynaptic release probability and postsynaptic AMPAR-mediated transmission, upregulated GluR1 protein phosphorylation/expression, downregulated ephrin type-A receptor 4-mediated anaphase-promoting complex/cyclosome (APC/C-Cdh1) activity, and the activation of somatostatin (SOM)/PV-positive GABAergic neurons and pro-inflammatory cytokines have been reported in the ACC from mice with nerve injury [159–162]. Notably, chemogenetic activation of GABAergic neurons in the ACC lowers the mechanical pain threshold, while chemogenetic inhibition of GABAergic neurons does not affect mechanical allodynia [162]. In both diabetes-induced neuropathic and peripheral nerve injury-induced pain models, the levels of phosphorylated protein kinase M-ζ (PKMζ) and tumor necrosis factor-alpha (TNF-α) are significantly enhanced in the ACC [163–165]. Interestingly, inhibiting PKMζ decreases the mechanical allodynia and synaptic transmission, and erases synaptic potentiation in the ACC neurons of mice with neuropathic pain, but does not affect memory retrieval or the tail-flick reflex and paw-withdrawal threshold in physiological pain models [163]. Recently, they further showed that blockade of PKMζ using the zeta inhibitory peptide is sufficient to alleviate mechanical allodynia in mice [166]. Besides excitatory and inhibitory imbalance in neuropathic pain, Ca2+ channels and the serotonin system in ACC neurons are also altered in the CCI model [167–169], and the dopaminergic system also interacts with glutamatergic transmission in the ACC, which is associated with neuropathic pain-related behaviors [170, 171]. By using phantom pain as a specific neuropathic pain model, Zhuo and colleagues found digit amputation in a single hindpaw induces the rapid expression of immediate-early genes, enhances sensory responses, and alters synaptic plasticity in the ACC [172, 173]. Furthermore, they found that formalin injection-induced inflammatory pain and digit amputation-induced neuropathic pain activate different patterns of ERK, suggesting that ERK may contribute to different types of pathological pain in different manners (Table 3) [124].
Table 3.
Treatment in the ACC for neuropathic pain.
Neuropathic pain models: SNI, spared nerve injury; BA, brachial plexus avulsion; CCI, chronic constriction injury; CPN, ligation of the common peroneal nerve; SNL, ligation of spinal nerves; DAP, digit amputation; Pain assessment: VF, von Frey filaments; RH, radiant heat; CPA, conditioned place aversion; TF, tail flick reflex; HA, Hargreaves assay; EPM, elevated plus maze; OF, open field; CP, cold plate test; AB, autonomic behavior; CPP, conditioned place preference; CFC, contextual fear conditioning; EP, electrophysiological recording; TS, thermal stimulation
In short, these findings suggest that ACC neuron activation occurs in neuropathic pain, as in the inflammatory pain model. The excitatory/inhibitory imbalance, such as increased presynaptic release probability, upregulated AMPAR GluR1 level, and downregulated extracellular GABA level, are involved in neuropathic pain as well, indicating that different types of pathological pain may share a certain signaling pathway to modulate synaptic transmission and neuronal excitability in the ACC. In the meantime, some pathways, like ERK, are involved in both neuropathic and inflammatory pain but may act in different manners, while others, such as PKMζ, serotonin, or dopamine, may play more specific roles in pathological pain.
Cancer pain
Cancer pain is mostly induced by tumors that metastasize from distant sites such as breast, prostate, lung, and bone. Infiltration of nerves and hollow viscera by tumors leads to the second most common cancer pain. The other forms of cancer pain results from treatment, such as chemotherapy, radiation, or surgery. Cancer pain has become a major clinical problem due to the insufficiency of common analgesics and side-effects such as addiction. In humans, surgical cingulotomy is a successful therapy in chronic cancer patients [82, 174–176]. In a study by Ballintine et al., cingulotomy relieved pain symptoms in >60% of patients with pain from terminal cancer or with non-malignant pain [82]. Animal models of cancer pain have been developed using osteolytic fibrosarcoma cells, mammary gland carcinoma cells, or prostate cancer cells implanted into bones [177]. In a mouse model of cancer pain, decreased NMDAR/AMPAR ratio and the induction of NMDAR-dependent long-term depression occurs at rostral ACC synapses in conjunction with mechanical allodynia in tumor-bearing mice [178]. Xu et al. showed GluN2B in the ACC is a potential target of RNAi for pain treatment in a rat model of pain associated with bone cancer. They found specific siRNA-mediated downregulation of GluN2B in the rACC results in reduced mechanical allodynia and thermal hyperalgesia (Table 4) [179].
Table 4.
Treatment in the ACC for cancer pain.
Neuromodulation of the ACC in clinical pain management
There are a variety of therapies for helping to reduce symptoms and control chronic pain. For example, oral medications are the most widely-used treatment; they include nonsteroidal anti-inflammatory drugs, acetaminophen, and opioids. However, these systemic treatments always have significant side-effects. In the management of acute and terminal pain, opioids are relatively highly efficient, but have become a major driver of the opioid crisis that is associated with addiction, availability, and overprescription [180]. In addition, topical pharmaceuticals such as gabapentin and carbamazepine are used to modulate abnormal function in the peripheral nervous system in neuropathic pain treatment, but sometimes have side-effects such as nausea [181]. Thus, a more efficient and straightforward treatment acting on a specific central nervous system pain circuit is badly needed.
Neurotechnologies based on invasive and non-invasive stimulation, such as deep brain stimulation (DBS), transcranial magnetic stimulation (TMS), and transcranial direct current stimulation (tDCS) have been considered as promising tools to centrally modulate different types of pain syndromes. Here, we collected recent evidence on the ACC as a new potential neuromodulation target using these techniques for pain management.
Deep brain stimulation (DBS) in the ACC
DBS is a neurosurgical procedure that has been used for more than 50 years to modulate the activity of dysfunctional brain circuits. DBS has gradually been shown to have therapeutic effects on alleviating the symptoms of treatment-resistant diseases such as chronic pain [182, 183], Parkinson’s disease [184, 185], tremor [186], and dystonia [187]. The key procedure of DBS surgery is the placement of neurostimulator electrodes that induce electrical activity in specific brain regions [188]. Neuroimaging techniques such as PET and fMRI can be used to measure changes in brain activity after DBS.
In general, the ACC is not a primary target in the use of DBS treatment for pain management. For medically intractable pain (post-stroke, atypical facial pain, phantom limb, malignancy), the sensory thalamus (ventral posterolateral for arm and/or leg pain, ventral posteromedial for facial pain), periventricular gray matter (PVG), PAG and internal capsule are normally used as efficient stimulation sites for DBS (Fig. 2A) [184, 189]. Davis and colleagues first demonstrated that thalamic DBS induces activation of the ACC in patients with chronic pain [28]. In 2007, Spooner et al. reported that high-frequency DBS in the cingulum of patients with severe neuropathic pain results in significant pain relief [190]. Since then, the dorsal ACC (Brodmann areas 24 and 32) has opened up the possibility of alleviating the symptoms of chronic pain [181]. Aziz and colleagues made a remarkable contribution to DBS in the ACC for chronic pain treatment. They first demonstrated that bilateral DBS in the ACC provides effective pain relief from chronic neuropathic pain that is refractory to other treatments [191]. Furthermore, they improved the accuracy of the DBS in the ACC by combining it with diffusion MRI to demonstrate differences between successful and unsuccessful patients due to the volume of ACC tissue activated by DBS electrodes [192]. Pre-surgical planning and fiber tract projection targeting have been effectively improved (Fig. 2A). Recently, Boccard et al. also revealed that DBS in the ACC helps to relieve chronic neuropathic pain in patients who failed in DBS placed in the PVG/PAG and/or sensory thalamus [193].
Fig. 2.
Targeted brain regions for invasive and non-invasive stimulation techniques in pain management. A Deep brain stimulation (DBS) treatment for pain normally uses the sensory thalamus (ventral posterolateral nucleus (VPL), ventral posteromedial nucleus (VPM)), periventricular gray matter (PVG), periaqueductal grey matter (PAG), internal capsule (IC), and ACC as efficient stimulation sites. The dashed enclosure lists the disorders studied for DBS treatment in the ACC: neuropathic pain, anxiety, obsessive-compulsive disorder (OCD), and depression. B Transcranial direct current stimulation (tDCS) for pain management normally uses the prefrontal cortex (PFC), dorsolateral PFC (dlPFC), supplementary motor area (SMA), primary motor cortex (M1), PAG, insular cortex, and ACC as efficient stimulation sites. The dashed enclosure lists the disorders treated. C Transcranial magnetic stimulation (TMS) treatment for pain normally uses the medial frontal cortex (MFC), PFC, dlPFC, SMA, premotor cortex (PM), M1 primary somatosensory cortex (S1), insular cortex, and ACC as efficient stimulation sites. The dashed enclosure lists the disorders treated.
Not surprisingly, due to the function of ACC in the affective components of pain and emotions, 6 months of subcallosal cingulate DBS treatment also contributed to the improvements in anxiety, obsessions, and compulsive disorder as well as affective regulation in 4 patients [194]. Another study showed that intraoperative DBS of the subcallosal cingulate changed the rapid onset of major depressive disorder in 9 patients. This change of transient behavior may be used for refining protocals in surgical implantation guidance in long-term stimulation for treatment-resistant depression [195].
Transcranial direct current stimulation (tDCS) in the ACC
tDCS, also called ‘polarization’, is a non-invasive technology with important advantages including low cost, relative safety, and ease of use [196]. tDCS is placed on the head with sponge electrodes a 1–2 mA currents are delivered [197]. The cathode is placed to reduce cortical activity due to hyperpolarization, whereas the anode is for increasing activity [198]. tDCS was recently used as a possible therapy for depression [199], pain [200] and Parkinson’s disease [201], but the focality of electrode designs is a challenge for clinic treatment.
Similar to DBS treatment, the ACC is not the first choice for tDCS in pain management. The sensorimotor cortex, the dorsolateral prefrontal cortex (dlPFC), and the primary motor cortex are the most frequently chosen areas to target for treating pain (Fig. 2B) [202–204]. The first tDCS study related to the ACC for pain treatment was carried out in 2014: four females with neuropathic pain induced by traumatic spinal cord injury received tDCS in the motor cortex for 10 days, resulting in a significant increase of metabolism in the subgenual anterior cingulate cortex and insula [205]. Magis et al. showed that excitatory tDCS targeting this are of the ACC is effective in preventing refractory chronic cluster headache [206]. A multi-site, double-blinded, randomized placebo-controlled trial of tDCS in the left dorsal ACC revealed significant improvements in not only pain disability but also depression in patients with chronic low back pain [207].
By using refined electrode arrays, tDCS can be converted to high definition-tDCS (HD-tDCS) that has more complex and focal stimulation procedures and targets deeper brain structures [208, 209]. HD-tDCS has been demonstrated to modulate beta frequency band activity in the dorsal ACC [210]. The mechanisms of tDCS have been briefly researched. Activation of μ-opioid receptors is induced by tDCS in the precuneus, PAG, and PFC (Fig. 2B) [211]. Rosa and Lisanby suggested that the long-term effects of tDCS are due to modifications of NMDAR efficacy [197]. Interestingly, effective treatment by tDCS in the ACC to reduce neuropathic pain is associated with increased glutamine combined glutamate (Glx) per creatine (Glx/Cr) and N-acetylaspartate (NAA) per creatine (NAA/Cr), which may be involved in the descending pain modulation system [212].
Transcranial magnetic stimulation (TMS) in the ACC
TMS is a newer technology for non-invasively stimulating the brain; a magnetic pulse is used to deliver stimulation. The magnetic field induces an electrical field which passes through the skull to activate cortical neurons [213]. Application of TMS with repetitive trains causes persistent changes in neuronal excitability [197, 214]. Repetitive TMS (rTMS) at a high frequency (>5 Hz) for excitatory modulation or at a low frequency (≤1 Hz) for inhibition [214] is generally used in the treatment of depression by modulating cortical activity [215].
Normally, rTMS is targeted to the PFC for antidepressant activity [215]. For pain treatment, the choices have been more variable: the precentral (motor) cortex, dorsolateral PFC and parietal cortex [214]. Lefaucheur and colleagues first used chronic motor cortex stimulation for analgesic effects in humans for post-stroke pain [216], thermal pain [217], and neuropathic pain [218]. rTMS in the dorsolateral PFC has been studied in capsaicin-induced pain (inflammatory pain) [219, 220], spinal cord injury-induced neuropathic pain [221, 222], and visceral pain [223, 224].
Central processing of pain perception is facilitated by TMS in the sensorimotor cortex and is suppressed by TMS in the medial frontal cortex (Fig. 2C) [225], which is the closest implant position to the ACC (1.5 cm anterior) [226]. By using PET imaging combined with multi-coil rTMS, the dorsal ACC was preferentially targeted and rTMS treatment alleviated chronic pain in fibromyalgia chronic pain patients [227]. In a mouse model, it has been confirmed that rTMS decreases the excitability of the ACC and suppress neuropathic pain [228]. Interestingly, the analgesic effects of deep rTMS in the posterior superior insular (PSI) cortex and the ACC have been compared. A significant antinociceptive effect was found for the PSI after, while more anxiolytic effects were found for the ACC (Fig. 2C) [229].
Conclusions and implications
As a ubiquitously active brain region [230], the ACC reflects a multiplicity of functions in both human and animals. Convergent evidence shows the crucial role of the ACC in a functional circuit that mediates pain processing [13, 231]. In humans, pain processing is complicated and in comorbidity with a wide range of complex states. Pain involves a complex cascade of signaling in the peripheral and central nervous system, leads to cortical activation, and finally results in a behavioral response. In the pain assays of animal studies, pain perception and processing have been simplified and separated into pathological and physiological pain. Since pathological pain includes inflammatory, neuropathic, and cancer pain, we discussed them separately (Fig. 1). Based on the evidence from preclinical animal studies, the function of the ACC in pain processing at different levels follows its own rhythms and rules. Compared with physiological pain, more cell types are activated and more receptors, proteins and kinases in the ACC are involved in pathological pain. Furthermore, when the ACC contributes to pain-related emotions, more signaling pathways and complex microcircuits are involved. Since the ACC functions in the affective component of pain, we integrated this into the review [19].
The literature demonstrates that ACC nociceptive neurons are activated in physiological pain [95–100]. Once the ACC activated by receiving nociceptive signals, it sends descending signals to other regions such as the nucleus accumbens and RVM [102, 103], and then eventually modulates the pain sensation from spinal nociception. The ACC has also been demonstrated to modulate pathological pain. From different reports, lesions of the ACC reduce inflammatory pain-related behavior [109, 116, 118], while activation of the ACC reduces mechanical allodynia of neuropathic pain [232]. Pathological pain alters synaptic transmission and neural plasticity in the ACC. Both neuropathic and inflammatory pain enhance presynaptic release probability, upregulate postsynaptic glutamate functions, and alter the balance of inhibition and excitation in the ACC [121, 123, 125, 128, 141, 156, 157, 159, 160]. In the ACC, inflammatory pain is specifically associated with the NMDAR GluN2B, NMDAR glycine site, AMPAR GluA1, cAMP, CGRP, AC, ERK, CREB, GABAaR, and GFAP pathways, whereas neuropathic pain specifically is associated with the AMPAR GluR1, NMDA GluN2B, CaMKII, APC/C-Cdh1, and PKMζ pathways.
Besides the mechanisms of pain processing in the ACC from preclinical studies, we also discussed pain management in the ACC. We focused more on non-invasive and invasive treatment in cortical regions, especially in the ACC, rather than systemic analgesic drugs. As an efficient invasive tool, DBS of the ACC significantly alleviates the symptoms of chronic pain that is refractory to other treatments. There are still safety and regulatory concerns about DBS, including the infection risk of surgery, unreliable connections of the stimulator, and accuracy of implant sites. But technological advances might fix this very soon: for instance, computational modelling is currently available for predicting the effectiveness of DBS treatment in targeted regions [233]. Concerning non-invasive treatment, TMS and tDCS have been shown to relieve physiological, inflammatory, and neuropathic pain. The motor cortex and PFC have been targeted more commonly in chronic pain management using TMS, but the ACC has proved to be efficacious in many studies [226–229, 234]. tDCS also is functional in treating chronic pain by targeting the ACC. Compared to TMS, tDCS is easier and less expensive for daily treatment. The analgesic efficacy of TMS and tDCS in pain should be evaluated in the future. More factors, including the frequency and duration of stimulation, the target and affected sites, and the neural circuits activated after treatment should be further studied. Last but not least, the molecular and cellular mechanisms underlying these invasive and non-invasive brain stimulation methods are still unclear. The cortical and thalamic neural oscillations have been associated with neuromodulatory effects of DBS in neuropathic pain [235, 236]. But it is still unknown what kinds of neurons, interneurons, and glial cells are involved in these treatments for analgesia, and how they interaction to affect local field potentials, synaptic plasticity, and neural oscillations. It is necessary to develop more specific animal models with DBS, TMS, and tDCS for studying the mechanisms. Also, we have to understand which neurotransmitters and signaling pathways are crucial in the ACC and other regions when we take advantage of these treatments for pain management. For example, brain stimulation could be combined with drug delivery via nanoparticle transport [237] to specifically target the key transmitter or pathway for the ACC neuromodulation in humans to achieve better curative effects.
Acknowledgements
This review was supported by the National Key R&D Program of China (2019YFA0709504), the National Natural Science Foundation of China (31930042, 31771164, 31900719, and 91630314), the Innovative Research Team of High-level Local Universities in Shanghai, Development Project of Shanghai Peak Disciplines Integrated Chinese and Western Medicine, Shanghai Science and Technology Committee Rising-Star Program (19QA1401400), 111 Project (B18015), Key Project of Shanghai Science & Technology (16JC1420402), Shanghai Municipal Science and Technology Major Project (2018SHZDZX01), and ZJLab.
Conflict of interest
No conflicts of interest were disclosed.
Contributor Information
Xiao Xiao, Email: xiaoxiao@fudan.edu.cn.
Yu-Qiu Zhang, Email: yuqiuzhang@fudan.edu.cn.
References
- 1.Ashburn MA, Staats PS. Management of chronic pain. Lancet. 1999;353:1865–1869. doi: 10.1016/S0140-6736(99)04088-X. [DOI] [PubMed] [Google Scholar]
- 2.Woolf CJ. Recent advances in the pathophysiology of acute pain. Br J Anaesth. 1989;63:139–146. doi: 10.1093/bja/63.2.139. [DOI] [PubMed] [Google Scholar]
- 3.Zhuo M. Neuronal mechanism for neuropathic pain. Mol Pain. 2007;3:14. doi: 10.1186/1744-8069-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 6.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 7.Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 9.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proc Natl Acad Sci U S A. 2005;102:15700–15705. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu JS, Chen QY, Zhou S, Inokuchi K, Zhuo M. Dual roles of anterior cingulate cortex neurons in pain and pleasure in adult mice. Mol Brain. 2018;11:72. doi: 10.1186/s13041-018-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 13.Bliss TV, Collingridge GL, Kaang BK, Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci. 2016;17:485–496. doi: 10.1038/nrn.2016.68. [DOI] [PubMed] [Google Scholar]
- 14.Ren WH, Guo JD, Cao H, Wang H, Wang PF, Sha H, et al. Is endogenous D-serine in the rostral anterior cingulate cortex necessary for pain-related negative affect? J Neurochem. 2006;96:1636–1647. doi: 10.1111/j.1471-4159.2006.03677.x. [DOI] [PubMed] [Google Scholar]
- 15.Li TT, Ren WH, Xiao X, Nan J, Cheng LZ, Zhang XH, et al. NMDA NR2A and NR2B receptors in the rostral anterior cingulate cortex contribute to pain-related aversion in male rats. Pain. 2009;146:183–193. doi: 10.1016/j.pain.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao H, Gao YJ, Ren WH, Li TT, Duan KZ, Cui YH, et al. Activation of extracellular signal-regulated kinase in the anterior cingulate cortex contributes to the induction and expression of affective pain. J Neurosci. 2009;29:3307–3321. doi: 10.1523/JNEUROSCI.4300-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao X, Yang Y, Zhang Y, Zhang XM, Zhao ZQ, Zhang YQ. Estrogen in the Anterior Cingulate Cortex Contributes to Pain-Related Aversion. Cerebral Cortex. 2013;23:2190–2203. doi: 10.1093/cercor/bhs201. [DOI] [PubMed] [Google Scholar]
- 18.Han M, Xiao X, Yang Y, Huang RY, Cao H, Zhao ZQ, et al. SIP30 Is Required for Neuropathic Pain-Evoked Aversion in Rats. J Neurosci. 2014;34:346–355. doi: 10.1523/JNEUROSCI.3160-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao X, Zhang YQ. A new perspective on the anterior cingulate cortex and affective pain. Neurosci Biobehav Rev. 2018;90:200–211. doi: 10.1016/j.neubiorev.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, et al. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14:4095–4108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derbyshire SW, Vogt BA, Jones AK. Pain and Stroop interference tasks activate separate processing modules in anterior cingulate cortex. Exp Brain Res. 1998;118:52–60. doi: 10.1007/s002210050254. [DOI] [PubMed] [Google Scholar]
- 22.Jones AK, Brown WD, Friston KJ, Qi LY, Frackowiak RS. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc Biol Sci. 1991;244:39–44. doi: 10.1098/rspb.1991.0048. [DOI] [PubMed] [Google Scholar]
- 23.Kwan CL, Crawley AP, Mikulis DJ, Davis KD. An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain. 2000;85:359–374. doi: 10.1016/S0304-3959(99)00287-0. [DOI] [PubMed] [Google Scholar]
- 24.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 25.Morris LS, Sprenger C, Koda K, de la Mora DM, Yamada T, Mano H, et al. Anterior cingulate cortex connectivity is associated with suppression of behaviour in a rat model of chronic pain. Brain Neurosci Adv. 2018;2:2398212818779646. doi: 10.1177/2398212818779646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanvanson P, Li Z, Mei L, Kounev V, Kern M, Ward BD, et al. Interplay of spinal and vagal pathways on esophageal acid-related anterior cingulate cortex functional networks in rats. Am J Physiol Gastrointest Liver Physiol. 2019;316:G615–g622. doi: 10.1152/ajpgi.00228.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia Z, Chen X, Tang W, Zhao D, Yu S. Atypical functional connectivity between the anterior cingulate cortex and other brain regions in a rat model of recurrent headache. Mol Pain. 2019;15:1744806919842483. doi: 10.1177/1744806919842483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis KD, Taub E, Duffner F, Lozano AM, Tasker RR, Houle S, et al. Activation of the anterior cingulate cortex by thalamic stimulation in patients with chronic pain: a positron emission tomography study. J Neurosurg. 2000;92:64–69. doi: 10.3171/jns.2000.92.1.0064. [DOI] [PubMed] [Google Scholar]
- 29.Talbot JD, Villemure JG, Bushnell MC, Duncan GH. Evaluation of pain perception after anterior capsulotomy: a case report. Somatosens Mot Res. 1995;12:115–126. doi: 10.3109/08990229509101503. [DOI] [PubMed] [Google Scholar]
- 30.Salomons TV, Iannetti GD, Liang M, Wood JN. The “Pain Matrix” in pain-free individuals. JAMA Neurol. 2016;73:755–756. doi: 10.1001/jamaneurol.2016.0653. [DOI] [PubMed] [Google Scholar]
- 31.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 32.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nature Medicine. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Q, Yaksh TL. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr Opin Anaesthesiol. 2011;24:400–407. doi: 10.1097/ACO.0b013e32834871df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Li H, Li TT, Luo H, Gu XY, Lu N, et al. Delayed activation of spinal microglia contributes to the maintenance of bone cancer pain in female Wistar rats via P2X7 receptor and IL-18. J Neurosci. 2015;35:7950–7963. doi: 10.1523/JNEUROSCI.5250-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui JG, Holmin S, Mathiesen T, Meyerson BA, Linderoth B. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain. 2000;88:239–248. doi: 10.1016/S0304-3959(00)00331-6. [DOI] [PubMed] [Google Scholar]
- 36.Sellmeijer J, Mathis V, Hugel S, Li XH, Song Q, Chen QY, et al. Hyperactivity of anterior cingulate cortex areas 24a/24b drives chronic pain-induced anxiodepressive-like consequences. J Neurosci. 2018;38:3102–3115. doi: 10.1523/JNEUROSCI.3195-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/S0306-4522(00)00110-X. [DOI] [PubMed] [Google Scholar]
- 38.Schulz E, Stankewitz A, Witkovsky V, Winkler AM, Tracey I. Strategy-dependent modulation of cortical pain circuits for the attenuation of pain. Cortex. 2019;113:255–266. doi: 10.1016/j.cortex.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 39.Crombez G, Eccleston C, Baeyens F, van Houdenhove B, van den Broeck A. Attention to chronic pain is dependent upon pain-related fear. J Psychosom Res. 1999;47:403–410. doi: 10.1016/S0022-3999(99)00046-X. [DOI] [PubMed] [Google Scholar]
- 40.McCracken LM, Gauntlett-Gilbert J, Vowles KE. The role of mindfulness in a contextual cognitive-behavioral analysis of chronic pain-related suffering and disability. Pain. 2007;131:63–69. doi: 10.1016/j.pain.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Villemure C, Schweinhardt P. Supraspinal Pain Processing: Distinct Roles of Emotion and Attention. Neuroscientist. 2010;16:276–284. doi: 10.1177/1073858409359200. [DOI] [PubMed] [Google Scholar]
- 42.Carrillo M, Han Y, Migliorati F, Liu M, Gazzola V, Keysers C. Emotional Mirror Neurons in the Rat’s Anterior Cingulate Cortex. Curr Biol. 2019;29(1301–1312):e1306. doi: 10.1016/j.cub.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corradi-Dell’Acqua C, Hofstetter C, Vuilleumier P. Felt and seen pain evoke the same local patterns of cortical activity in insular and cingulate cortex. J Neurosci. 2011;31:17996–18006. doi: 10.1523/JNEUROSCI.2686-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loeser JD. Melzack R. Pain: an overview. Lancet. 1999;353:1607–1609. doi: 10.1016/S0140-6736(99)01311-2. [DOI] [PubMed] [Google Scholar]
- 45.Albe-Fessard D, Berkley KJ, Kruger L, Ralston HJ, 3rd, Willis WD., Jr Diencephalic mechanisms of pain sensation. Brain Res. 1985;356:217–296. doi: 10.1016/0165-0173(85)90013-X. [DOI] [PubMed] [Google Scholar]
- 46.Craig KD. Pain and affectivity in infancy: their interdependence and independence. Cephalalgia. 1987;7(Suppl 6):115–118. doi: 10.1177/03331024870070S636. [DOI] [PubMed] [Google Scholar]
- 47.Apkarian AV, Shi T. Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J Neurosci. 1994;14:6779–6795. doi: 10.1523/JNEUROSCI.14-11-06779.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Craig AD, Serrano LP. Effects of systemic morphine on lamina I spinothalamic tract neurons in the cat. Brain Res. 1994;636:233–244. doi: 10.1016/0006-8993(94)91022-7. [DOI] [PubMed] [Google Scholar]
- 49.Shi T, Apkarian AV. Morphology of thalamocortical neurons projecting to the primary somatosensory cortex and their relationship to spinothalamic terminals in the squirrel monkey. J Comp Neurol. 1995;361:1–24. doi: 10.1002/cne.903610102. [DOI] [PubMed] [Google Scholar]
- 50.Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979;204:205–207. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- 51.Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262:256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- 52.Robertson RT, Kaitz SS. Thalamic connections with limbic cortex. I. Thalamocortical projections. J Comp Neurol. 1981;195:501–525. doi: 10.1002/cne.901950308. [DOI] [PubMed] [Google Scholar]
- 53.Musil SY, Olson CR. Organization of cortical and subcortical projections to anterior cingulate cortex in the cat. J Comp Neurol. 1988;272:203–218. doi: 10.1002/cne.902720205. [DOI] [PubMed] [Google Scholar]
- 54.Yasui Y, Itoh K, Kamiya H, Ino T, Mizuno N. Cingulate gyrus of the cat receives projection fibers from the thalamic region ventral to the ventral border of the ventrobasal complex. J Comp Neurol. 1988;274:91–100. doi: 10.1002/cne.902740109. [DOI] [PubMed] [Google Scholar]
- 55.Marini G, Pianca L, Tredici G. Thalamocortical projection from the parafascicular nucleus to layer V pyramidal cells in frontal and cingulate areas of the rat. Neurosci Lett. 1996;203:81–84. doi: 10.1016/0304-3940(95)12266-4. [DOI] [PubMed] [Google Scholar]
- 56.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 57.Sadzot B, Price JC, Mayberg HS, Douglass KH, Dannals RF, Lever JR, et al. Quantification of human opiate receptor concentration and affinity using high and low specific activity [11C]diprenorphine and positron emission tomography. J Cereb Blood Flow Metab. 1991;11:204–219. doi: 10.1038/jcbfm.1991.52. [DOI] [PubMed] [Google Scholar]
- 58.Jones AK, Qi LY, Fujirawa T, Luthra SK, Ashburner J, Bloomfield P, et al. In vivo distribution of opioid receptors in man in relation to the cortical projections of the medial and lateral pain systems measured with positron emission tomography. Neurosci Lett. 1991;126:25–28. doi: 10.1016/0304-3940(91)90362-W. [DOI] [PubMed] [Google Scholar]
- 59.Vogt BA, Wiley RG, Jensen EL. Localization of Mu and delta opioid receptors to anterior cingulate afferents and projection neurons and input/output model of Mu regulation. Exp Neurol. 1995;135:83–92. doi: 10.1006/exnr.1995.1069. [DOI] [PubMed] [Google Scholar]
- 60.Lenz FA, Rios M, Zirh A, Chau D, Krauss G, Lesser RP. Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus. J Neurophysiol. 1998;79:2231–2234. doi: 10.1152/jn.1998.79.4.2231. [DOI] [PubMed] [Google Scholar]
- 61.Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J Neurophysiol. 1997;77:3370–3380. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- 62.Li JX. The application of conditioning paradigms in the measurement of pain. Eur J Pharmacol. 2013;716:158–168. doi: 10.1016/j.ejphar.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neafsey EJ. Prefrontal cortical control of the autonomic nervous system: anatomical and physiological observations. Prog Brain Res. 1990;85:147–165. doi: 10.1016/S0079-6123(08)62679-5. [DOI] [PubMed] [Google Scholar]
- 64.Yasui Y, Cechetto DF, Saper CB. Evidence for a cholinergic projection from the pedunculopontine tegmental nucleus to the rostral ventrolateral medulla in the rat. Brain Res. 1990;517:19–24. doi: 10.1016/0006-8993(90)91002-X. [DOI] [PubMed] [Google Scholar]
- 65.Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/S0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 66.Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842–848. doi: 10.1016/S0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 67.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 68.Kern M, Shaker R. Further characterization of human brain processing of viscero-sensation: the role of gender and a word of caution. Gastroenterology. 2003;124:1975–1977. doi: 10.1016/S0016-5085(03)00554-7. [DOI] [PubMed] [Google Scholar]
- 69.Xiao Z, Martinez E, Kulkarni PM, Zhang Q, Hou Q, Rosenberg D, et al. Cortical pain processing in the rat anterior cingulate cortex and primary somatosensory cortex. Front Cell Neurosci. 2019;13:165. doi: 10.3389/fncel.2019.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- 71.Casey KL, Minoshima S, Morrow TJ, Koeppe RA. Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. J Neurophysiol. 1996;76:571–581. doi: 10.1152/jn.1996.76.1.571. [DOI] [PubMed] [Google Scholar]
- 72.Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802–807. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- 73.Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- 74.Vogt BA, Derbyshire S, Jones AK. Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR imaging. Eur J Neurosci. 1996;8:1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- 75.Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- 76.Svensson P, Minoshima S, Beydoun A, Morrow TJ, Casey KL. Cerebral processing of acute skin and muscle pain in humans. J Neurophysiol. 1997;78:450–460. doi: 10.1152/jn.1997.78.1.450. [DOI] [PubMed] [Google Scholar]
- 77.Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain. 1995;63:225–236. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- 78.Davis KD, Hutchison WD, Lozano AM, Dostrovsky JO. Altered pain and temperature perception following cingulotomy and capsulotomy in a patient with schizoaffective disorder. Pain. 1994;59:189–199. doi: 10.1016/0304-3959(94)90071-X. [DOI] [PubMed] [Google Scholar]
- 79.Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO. Pain-related neurons in the human cingulate cortex. Nat Neurosci. 1999;2:403–405. doi: 10.1038/8065. [DOI] [PubMed] [Google Scholar]
- 80.Hurt RW, Ballantine HT., Jr Stereotactic anterior cingulate lesions for persistent pain: a report on 68 cases. Clin Neurosurg. 1974;21:334–351. doi: 10.1093/neurosurgery/21.CN_suppl_1.334. [DOI] [PubMed] [Google Scholar]
- 81.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 82.Ballantine HT, Jr, Cassidy WL, Flanagan NB, Marino R., Jr Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg. 1967;26:488–495. doi: 10.3171/jns.1967.26.5.0488. [DOI] [PubMed] [Google Scholar]
- 83.Hassenbusch SJ, Pillay PK, Barnett GH. Radiofrequency cingulotomy for intractable cancer pain using stereotaxis guided by magnetic resonance imaging. Neurosurgery. 1990;27:220–223. doi: 10.1227/00006123-199008000-00008. [DOI] [PubMed] [Google Scholar]
- 84.Santo JL, Arias LM, Barolat G, Schwartzman RJ, Grossman K. Bilateral cingulumotomy in the treatment of reflex sympathetic dystrophy. Pain. 1990;41:55–59. doi: 10.1016/0304-3959(90)91109-V. [DOI] [PubMed] [Google Scholar]
- 85.Wilkinson HA, Davidson KM, Davidson RI. Bilateral anterior cingulotomy for chronic noncancer pain. Neurosurgery. 1999;45:1129–1134. doi: 10.1097/00006123-199911000-00023. [DOI] [PubMed] [Google Scholar]
- 86.Wong ET, Gunes S, Gaughan E, Patt RB, Ginsberg LE, Hassenbusch SJ, et al. Palliation of intractable cancer pain by MRI-guided cingulotomy. Clin J Pain. 1997;13:260–263. doi: 10.1097/00002508-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 87.Brown S, Schafer EA. An investigaion into the functions of the occipital and pemporal lobes of the monkey’s brain. Philos Trans R Soc Lond B Biol Sci 1888, 48–50.
- 88.Horslry V, Schafer EA. A record of experiments upon the functions of the cerebral cortex. Philos Trans R Soc Lond B Biol Sci 1888, 1–7.
- 89.Peretz E. The effects of lesions of the anterior cingulate cortex on the behavior of the rat. J Comp Physiol Psychol. 1960;53:540–548. doi: 10.1037/h0040297. [DOI] [PubMed] [Google Scholar]
- 90.Gabriel M, Kubota Y, Sparenborg S, Straube K, Vogt BA. Effects of cingulate cortical lesions on avoidance learning and training-induced unit activity in rabbits. Exp Brain Res. 1991;86:585–600. doi: 10.1007/BF00230532. [DOI] [PubMed] [Google Scholar]
- 91.Lubar JF, Perachio AA. One-way and two-way learning and transfer of an active avoidance response in normal and cingulectomized cats. J Comp Physiol Psychol. 1965;60:46–52. doi: 10.1037/h0022292. [DOI] [PubMed] [Google Scholar]
- 92.Lubar JF. Effect of Medial Cortical Lesions on the Avoidance Behavior of the Cat. J Comp Physiol Psychol. 1964;58:38–46. doi: 10.1037/h0041014. [DOI] [PubMed] [Google Scholar]
- 93.Thomas GJ, Slotnick BM. Impairment of avoidance responding by lesions in cingulate cortex in rats depends on food drive. J Comp Physiol Psychol. 1963;56:959–964. doi: 10.1037/h0048819. [DOI] [PubMed] [Google Scholar]
- 94.Thomas GJ, Slotnick B. Effects of lesions in the cingulum one maze learning and avoidance conditioning in the rat. J Comp Physiol Psychol. 1962;55:1085–1091. doi: 10.1037/h0046145. [DOI] [PubMed] [Google Scholar]
- 95.Hsu MM, Shyu BC. Electrophysiological study of the connection between medial thalamus and anterior cingulate cortex in the rat. Neuroreport. 1997;8:2701–2707. doi: 10.1097/00001756-199708180-00013. [DOI] [PubMed] [Google Scholar]
- 96.Kung JC, Shyu BC. Potentiation of local field potentials in the anterior cingulate cortex evoked by the stimulation of the medial thalamic nuclei in rats. Brain Res. 2002;953:37–44. doi: 10.1016/S0006-8993(02)03265-1. [DOI] [PubMed] [Google Scholar]
- 97.Sun JJ, Chuang Kung J, Wang CC, Chen SL, Shyu BC. Short-term facilitation in the anterior cingulate cortex following stimulation of the medial thalamus in the rat. Brain Res. 2006;1097:101–115. doi: 10.1016/j.brainres.2006.04.065. [DOI] [PubMed] [Google Scholar]
- 98.Yamamura H, Iwata K, Tsuboi Y, Toda K, Kitajima K, Shimizu N, et al. Morphological and electrophysiological properties of ACCx nociceptive neurons in rats. Brain Res. 1996;735:83–92. doi: 10.1016/0006-8993(96)00561-6. [DOI] [PubMed] [Google Scholar]
- 99.Sikes RW, Vogt BA. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol. 1992;68:1720–1732. doi: 10.1152/jn.1992.68.5.1720. [DOI] [PubMed] [Google Scholar]
- 100.Koyama T, Tanaka YZ, Mikami A. Nociceptive neurons in the macaque anterior cingulate activate during anticipation of pain. Neuroreport. 1998;9:2663–2667. doi: 10.1097/00001756-199808030-00044. [DOI] [PubMed] [Google Scholar]
- 101.Chen T, Taniguchi W, Chen QY, Tozaki-Saitoh H, Song Q, Liu RH, et al. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat Commun. 1886;2018:9. doi: 10.1038/s41467-018-04309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Calejesan AA, Kim SJ, Zhuo M. Descending facilitatory modulation of a behavioral nociceptive response by stimulation in the adult rat anterior cingulate cortex. Eur J Pain. 2000;4:83–96. doi: 10.1053/eujp.1999.0158. [DOI] [PubMed] [Google Scholar]
- 103.Zhang L, Zhang Y, Zhao ZQ. Anterior cingulate cortex contributes to the descending facilitatory modulation of pain via dorsal reticular nucleus. Eur J Neurosci. 2005;22:1141–1148. doi: 10.1111/j.1460-9568.2005.04302.x. [DOI] [PubMed] [Google Scholar]
- 104.Moon HC, Heo WI, Kim YJ, Lee D, Won SY, Kim HR, et al. Optical inactivation of the anterior cingulate cortex modulate descending pain pathway in a rat model of trigeminal neuropathic pain created via chronic constriction injury of the infraorbital nerve. J Pain Res. 2017;10:2355–2364. doi: 10.2147/JPR.S138626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang J, Cao B, Yu TR, Jelfs B, Yan J, Chan RH, et al. Theta-frequency phase-locking of single anterior cingulate cortex neurons and synchronization with the medial thalamus are modulated by visceral noxious stimulation in rats. Neuroscience. 2015;298:200–210. doi: 10.1016/j.neuroscience.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 106.Gao J, Wu X, Owyang C, Li Y. Enhanced responses of the anterior cingulate cortex neurones to colonic distension in viscerally hypersensitive rats. J Physiol. 2006;570:169–183. doi: 10.1113/jphysiol.2005.096073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Ma JH, Xiao TH, Chang CW, Gao L, Wang XL, Gao GD, et al. Activation of anterior cingulate cortex produces inhibitory effects on noxious mechanical and electrical stimuli-evoked responses in rat spinal WDR neurons. Eur J Pain. 2011;15:895–899. doi: 10.1016/j.ejpain.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 108.Ikeda H, Takasu S, Murase K. Contribution of anterior cingulate cortex and descending pain inhibitory system to analgesic effect of lemon odor in mice. Mol Pain. 2014;10:14. doi: 10.1186/1744-8069-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fuchs PN, Balinsky M, Melzack R. Electrical stimulation of the cingulum bundle and surrounding cortical tissue reduces formalin-test pain in the rat. Brain Res. 1996;743:116–123. doi: 10.1016/S0006-8993(96)01035-9. [DOI] [PubMed] [Google Scholar]
- 110.Ren K, Dubner R. Inflammatory Models of Pain and Hyperalgesia. ILAR J. 1999;40:111–118. doi: 10.1093/ilar.40.3.111. [DOI] [PubMed] [Google Scholar]
- 111.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 112.Gao YJ, Xu ZZ, Liu YC, Wen YR, Decosterd I, Ji RR. The c-Jun N-terminal kinase 1 (JNK1) in spinal astrocytes is required for the maintenance of bilateral mechanical allodynia under a persistent inflammatory pain condition. Pain. 2010;148:309–319. doi: 10.1016/j.pain.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 114.Guo JD, Wang H, Zhang YQ, Zhao ZQ. Alterations of membrane properties and effects of D-serine on NMDA-induced current in rat anterior cingulate cortex neurons after monoarthritis. Neurosci Lett. 2005;384:245–249. doi: 10.1016/j.neulet.2005.04.096. [DOI] [PubMed] [Google Scholar]
- 115.Gong KR, Cao FL, He Y, Gao CY, Wang DD, Li H, et al. Enhanced excitatory and reduced inhibitory synaptic transmission contribute to persistent pain-induced neuronal hyper-responsiveness in anterior cingulate cortex. Neuroscience. 2010;171:1314–1325. doi: 10.1016/j.neuroscience.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 116.Vaccarino AL, Melzack R. Analgesia produced by injection of lidocaine into the anterior cingulum bundle of the rat. Pain. 1989;39:213–219. doi: 10.1016/0304-3959(89)90008-0. [DOI] [PubMed] [Google Scholar]
- 117.Vaccarino AL, Melzack R. Temporal processes of formalin pain: differential role of the cingulum bundle, fornix pathway and medial bulboreticular formation. Pain. 1992;49:257–271. doi: 10.1016/0304-3959(92)90150-A. [DOI] [PubMed] [Google Scholar]
- 118.Donahue RR, LaGraize SC, Fuchs PN. Electrolytic lesion of the anterior cingulate cortex decreases inflammatory, but not neuropathic nociceptive behavior in rats. Brain Res. 2001;897:131–138. doi: 10.1016/S0006-8993(01)02103-5. [DOI] [PubMed] [Google Scholar]
- 119.Ren LY, Lu ZM, Liu MG, Yu YQ, Li Z, Shang GW, et al. Distinct roles of the anterior cingulate cortex in spinal and supraspinal bee venom-induced pain behaviors. Neuroscience. 2008;153:268–278. doi: 10.1016/j.neuroscience.2008.01.067. [DOI] [PubMed] [Google Scholar]
- 120.Wu LJ, Toyoda H, Zhao MG, Lee YG, Tang J, Ko SW, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25:11107–11116. doi: 10.1523/JNEUROSCI.1678-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu LJ, Steenland HW, Kim SS, Isiegas C, Abel T, Kaang BK, et al. Enhancement of presynaptic glutamate release and persistent inflammatory pain by increasing neuronal cAMP in the anterior cingulate cortex. Mol Pain. 2008;4:40. doi: 10.1186/1744-8069-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wei F, Qiu CS, Kim SJ, Muglia L, Maas JW, Pineda VV, et al. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron. 2002;36:713–726. doi: 10.1016/S0896-6273(02)01019-X. [DOI] [PubMed] [Google Scholar]
- 123.Toyoda H, Zhao MG, Ulzhofer B, Wu LJ, Xu H, Seeburg PH, et al. Roles of the AMPA receptor subunit GluA1 but not GluA2 in synaptic potentiation and activation of ERK in the anterior cingulate cortex. Mol Pain. 2009;5:46. doi: 10.1186/1744-8069-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wei F, Zhuo M. Activation of Erk in the anterior cingulate cortex during the induction and expression of chronic pain. Mol Pain. 2008;4:28. doi: 10.1186/1744-8069-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao MG, Ko SW, Wu LJ, Toyoda H, Xu H, Quan J, et al. Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. J Neurosci. 2006;26:8923–8930. doi: 10.1523/JNEUROSCI.2103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bie B, Brown DL, Naguib M. Increased synaptic GluR1 subunits in the anterior cingulate cortex of rats with peripheral inflammation. Eur J Pharmacol. 2011;653:26–31. doi: 10.1016/j.ejphar.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 127.Chen S, Kadakia F, Davidson S. Group II metabotropic glutamate receptor expressing neurons in anterior cingulate cortex become sensitized after inflammatory and neuropathic pain. Mol Pain. 2020;16:1744806920915339. doi: 10.1177/1744806920915339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li XH, Matsuura T, Liu RH, Xue M, Zhuo M. Calcitonin gene-related peptide potentiated the excitatory transmission and network propagation in the anterior cingulate cortex of adult mice. Mol Pain. 2019;15:1744806919832718. doi: 10.1177/1744806919832718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yi M, Zhang H, Lao L, Xing GG, Wan Y. Anterior cingulate cortex is crucial for contra- but not ipsi-lateral electro-acupuncture in the formalin-induced inflammatory pain model of rats. Mol Pain. 2011;7:61. doi: 10.1186/1744-8069-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun J, Shao XM, Fang F, Shen Z, Wu YY, Fang JQ. Electroacupuncture alleviates retrieval of pain memory and its effect on phosphorylation of cAMP response element-binding protein in anterior cingulate cortex in rats. Behav Brain Funct. 2015;11:9. doi: 10.1186/s12993-015-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fan YF, Guan SY, Luo L, Li YJ, Yang L, Zhou XX, et al. Tetrahydroxystilbene glucoside relieves the chronic inflammatory pain by inhibiting neuronal apoptosis, microglia activation, and GluN2B overexpression in anterior cingulate cortex. Mol Pain. 2018;14:1744806918814367. doi: 10.1177/1744806918814367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kang SJ, Kwak C, Lee J, Sim SE, Shim J, Choi T, et al. Bidirectional modulation of hyperalgesia via the specific control of excitatory and inhibitory neuronal activity in the ACC. Mol Brain. 2015;8:81. doi: 10.1186/s13041-015-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cao Z, Wu X, Chen S, Fan J, Zhang R, Owyang C, et al. Anterior cingulate cortex modulates visceral pain as measured by visceromotor responses in viscerally hypersensitive rats. Gastroenterology. 2008;134:535–543. doi: 10.1053/j.gastro.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 134.Wu X, Gao J, Yan J, Fan J, Owyang C, Li Y. Role for NMDA receptors in visceral nociceptive transmission in the anterior cingulate cortex of viscerally hypersensitive rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G918–G927. doi: 10.1152/ajpgi.00452.2007. [DOI] [PubMed] [Google Scholar]
- 135.Fan J, Wu X, Cao Z, Chen S, Owyang C, Li Y. Up-regulation of anterior cingulate cortex NR2B receptors contributes to visceral pain responses in rats. Gastroenterology. 2009;136(1732–1740):e1733. doi: 10.1053/j.gastro.2009.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136.Zhou L, Huang J, Gao J, Zhang G, Jiang J. NMDA and AMPA receptors in the anterior cingulate cortex mediates visceral pain in visceral hypersensitivity rats. Cell Immunol. 2014;287:86–90. doi: 10.1016/j.cellimm.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 137.Li Y, Zhang X, Liu H, Cao Z, Chen S, Cao B, et al. Phosphorylated CaMKII post-synaptic binding to NR2B subunits in the anterior cingulate cortex mediates visceral pain in visceral hypersensitive rats. J Neurochem. 2012;121:662–671. doi: 10.1111/j.1471-4159.2012.07717.x. [DOI] [PubMed] [Google Scholar]
- 138.Wang J, Zhang X, Cao B, Liu J, Li Y. Facilitation of synaptic transmission in the anterior cingulate cortex in viscerally hypersensitive rats. Cereb Cortex. 2015;25:859–868. doi: 10.1093/cercor/bht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yan N, Cao B, Xu J, Hao C, Zhang X, Li Y. Glutamatergic activation of anterior cingulate cortex mediates the affective component of visceral pain memory in rats. Neurobiol Learn Mem. 2012;97:156–164. doi: 10.1016/j.nlm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 140.Wang XS, Yue J, Hu LN, Tian Z, Yang LK, Lu L, et al. Effects of CPEB1 in the anterior cingulate cortex on visceral pain in mice. Brain Res. 2019;1712:55–62. doi: 10.1016/j.brainres.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 141.Eto K, Wake H, Watanabe M, Ishibashi H, Noda M, Yanagawa Y, et al. Inter-regional contribution of enhanced activity of the primary somatosensory cortex to the anterior cingulate cortex accelerates chronic pain behavior. J Neurosci. 2011;31:7631–7636. doi: 10.1523/JNEUROSCI.0946-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koga K, Shimoyama S, Yamada A, Furukawa T, Nikaido Y, Furue H, et al. Chronic inflammatory pain induced GABAergic synaptic plasticity in the adult mouse anterior cingulate cortex. Mol Pain. 2018;14:1744806918783478. doi: 10.1177/1744806918783478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ikeda H, Mochizuki K, Murase K. Astrocytes are involved in long-term facilitation of neuronal excitation in the anterior cingulate cortex of mice with inflammatory pain. Pain. 2013;154:2836–2843. doi: 10.1016/j.pain.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 144.Chen FL, Dong YL, Zhang ZJ, Cao DL, Xu J, Hui J, et al. Activation of astrocytes in the anterior cingulate cortex contributes to the affective component of pain in an inflammatory pain model. Brain Res Bull. 2012;87:60–66. doi: 10.1016/j.brainresbull.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 145.LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol. 2000;163:490–494. doi: 10.1006/exnr.2000.7395. [DOI] [PubMed] [Google Scholar]
- 146.Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- 147.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 148.Schweinhardt P, Glynn C, Brooks J, McQuay H, Jack T, Chessell I, et al. An fMRI study of cerebral processing of brush-evoked allodynia in neuropathic pain patients. Neuroimage. 2006;32:256–265. doi: 10.1016/j.neuroimage.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 149.Witting N, Kupers RC, Svensson P, Jensen TS. A PET activation study of brush-evoked allodynia in patients with nerve injury pain. Pain. 2006;120:145–154. doi: 10.1016/j.pain.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 150.Petrovic P, Ingvar M, Stone-Elander S, Petersson KM, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain. 1999;83:459–470. doi: 10.1016/S0304-3959(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 151.Peyron R, Garcia-Larrea L, Gregoire MC, Convers P, Lavenne F, Veyre L, et al. Allodynia after lateral-medullary (Wallenberg) infarct. A PET study. Brain. 1998;121(Pt 2):345–356. doi: 10.1093/brain/121.2.345. [DOI] [PubMed] [Google Scholar]
- 152.Ducreux D, Attal N, Parker F, Bouhassira D. Mechanisms of central neuropathic pain: a combined psychophysical and fMRI study in syringomyelia. Brain. 2006;129:963–976. doi: 10.1093/brain/awl016. [DOI] [PubMed] [Google Scholar]
- 153.Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113:200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- 154.Zhao R, Zhou H, Huang L, Xie Z, Wang J, Gan WB, et al. Neuropathic pain causes pyramidal neuronal hyperactivity in the anterior cingulate cortex. Front Cell Neurosci. 2018;12:107. doi: 10.3389/fncel.2018.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang Z, Tan Q, Cheng D, Zhang L, Zhang J, Gu EW, et al. The changes of intrinsic excitability of pyramidal neurons in anterior cingulate cortex in neuropathic pain. Front Cell Neurosci. 2018;12:436. doi: 10.3389/fncel.2018.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009;106:2423–2428. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Narita M, Niikura K, Nanjo-Niikura K, Narita M, Furuya M, Yamashita A, et al. Sleep disturbances in a neuropathic pain-like condition in the mouse are associated with altered GABAergic transmission in the cingulate cortex. Pain. 2011;152:1358–1372. doi: 10.1016/j.pain.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 158.Yamashita A, Hamada A, Suhara Y, Kawabe R, Yanase M, Kuzumaki N, et al. Astrocytic activation in the anterior cingulate cortex is critical for sleep disorder under neuropathic pain. Synapse. 2014;68:235–247. doi: 10.1002/syn.21733. [DOI] [PubMed] [Google Scholar]
- 159.Tan W, Yao WL, Hu R, Lv YY, Wan L, Zhang CH, et al. Alleviating neuropathic pain mechanical allodynia by increasing Cdh1 in the anterior cingulate cortex. Mol Pain. 2015;11:56. doi: 10.1186/s12990-015-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, et al. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci. 2008;28:7445–7453. doi: 10.1523/JNEUROSCI.1812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meng XL, Fu P, Wang L, Yang X, Hong G, Zhao X, et al. Increased EZH2 levels in anterior cingulate cortex microglia aggravate neuropathic pain by inhibiting autophagy following brachial plexus avulsion in rats. Neurosci Bull. 2020;36:793–805. doi: 10.1007/s12264-020-00502-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li YD, Ge J, Luo YJ, Xu W, Wang J, Lazarus M, et al. High cortical delta power correlates with aggravated allodynia by activating anterior cingulate cortex GABAergic neurons in neuropathic pain mice. Pain. 2020;161:288–299. doi: 10.1097/j.pain.0000000000001725. [DOI] [PubMed] [Google Scholar]
- 163.Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science. 2010;330:1400–1404. doi: 10.1126/science.1191792. [DOI] [PubMed] [Google Scholar]
- 164.Li W, Wang P, Li H. Upregulation of glutamatergic transmission in anterior cingulate cortex in the diabetic rats with neuropathic pain. Neurosci Lett. 2014;568:29–34. doi: 10.1016/j.neulet.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 165.Yao PW, Wang SK, Chen SX, Xin WJ, Liu XG, Zang Y. Upregulation of tumor necrosis factor-alpha in the anterior cingulate cortex contributes to neuropathic pain and pain-associated aversion. Neurobiol Dis. 2019;130:104456. doi: 10.1016/j.nbd.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 166.Ko HG, Ye S, Han DH, Park P, Lim CS, Lee K, et al. Transcription-independent expression of PKMzeta in the anterior cingulate cortex contributes to chronically maintained neuropathic pain. Mol Pain. 2018;14:1744806918783943. doi: 10.1177/1744806918783943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shen FY, Chen ZY, Zhong W, Ma LQ, Chen C, Yang ZJ, et al. Alleviation of neuropathic pain by regulating T-type calcium channels in rat anterior cingulate cortex. Mol Pain. 2015;11:7. doi: 10.1186/s12990-015-0008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Takeda R, Watanabe Y, Ikeda T, Abe H, Ebihara K, Matsuo H, et al. Analgesic effect of milnacipran is associated with c-Fos expression in the anterior cingulate cortex in the rat neuropathic pain model. Neurosci Res. 2009;64:380–384. doi: 10.1016/j.neures.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 169.Chen Z, Shen X, Huang WuH, Zhang M. Membrane potential synchrony of neurons in anterior cingulate cortex plays a pivotal role in generation of neuropathic pain. Sci Rep. 2018;8:1691. doi: 10.1038/s41598-018-20080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lopez-Avila A, Coffeen U, Ortega-Legaspi JM, del Angel R, Pellicer F. Dopamine and NMDA systems modulate long-term nociception in the rat anterior cingulate cortex. Pain. 2004;111:136–143. doi: 10.1016/j.pain.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 171.Ortega-Legaspi JM, de Gortari P, Garduno-Gutierrez R, Amaya MI, Leon-Olea M, Coffeen U, et al. Expression of the dopaminergic D1 and D2 receptors in the anterior cingulate cortex in a model of neuropathic pain. Mol Pain. 2011;7:97. doi: 10.1186/1744-8069-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wei F, Li P, Zhuo M. Loss of synaptic depression in mammalian anterior cingulate cortex after amputation. J Neurosci. 1999;19:9346–9354. doi: 10.1523/JNEUROSCI.19-21-09346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wei F, Zhuo M. Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. J Physiol. 2001;532:823–833. doi: 10.1111/j.1469-7793.2001.0823e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bouckoms AJ, Welch CA, Drop LJ, Dao T, Kolton K. Atropine in electroconvulsive therapy. Convuls Ther. 1989;5:48–55. [PubMed] [Google Scholar]
- 175.Pillay PK, Hassenbusch SJ. Bilateral MRI-guided stereotactic cingulotomy for intractable pain. Stereotact Funct Neurosurg. 1992;59:33–38. doi: 10.1159/000098914. [DOI] [PubMed] [Google Scholar]
- 176.Pereira EA, Paranathala M, Hyam JA, Green AL, Aziz TZ. Anterior cingulotomy improves malignant mesothelioma pain and dyspnoea. Br J Neurosurg. 2014;28:471–474. doi: 10.3109/02688697.2013.857006. [DOI] [PubMed] [Google Scholar]
- 177.Currie GL, Delaney A, Bennett MI, Dickenson AH, Egan KJ, Vesterinen HM, et al. Animal models of bone cancer pain: Systematic review and meta-analyses. Pain. 2013;154:917–926. doi: 10.1016/j.pain.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 178.Chiou CS, Huang CC, Liang YC, Tsai YC, Hsu KS. Impairment of long-term depression in the anterior cingulate cortex of mice with bone cancer pain. Pain. 2012;153:2097–2108. doi: 10.1016/j.pain.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 179.Xu Y, Wang G, Zou X, Yang Z, Wang Q, Feng H, et al. siRNA-mediated downregulation of GluN2B in the rostral anterior cingulate cortex attenuates mechanical allodynia and thermal hyperalgesia in a rat model of pain associated with bone cancer. Exp Ther Med. 2016;11:221–229. doi: 10.3892/etm.2015.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Woolf CJ. Capturing novel non-opioid pain targets. Biol Psychiatry. 2020;87:74–81. doi: 10.1016/j.biopsych.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Russo JF, Sheth SA. Deep brain stimulation of the dorsal anterior cingulate cortex for the treatment of chronic neuropathic pain. Neurosurg Focus. 2015;38:E11. doi: 10.3171/2015.3.FOCUS1543. [DOI] [PubMed] [Google Scholar]
- 182.Owen SL, Green AL, Stein JF, Aziz TZ. Deep brain stimulation for the alleviation of post-stroke neuropathic pain. Pain. 2006;120:202–206. doi: 10.1016/j.pain.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 183.Marchand S, Kupers RC, Bushnell MC, Duncan GH. Analgesic and placebo effects of thalamic stimulation. Pain. 2003;105:481–488. doi: 10.1016/S0304-3959(03)00265-3. [DOI] [PubMed] [Google Scholar]
- 184.Bittar RG, Burn SC, Bain PG, Owen SL, Joint C, Shlugman D, et al. Deep brain stimulation for movement disorders and pain. J Clin Neurosci. 2005;12:457–463. doi: 10.1016/j.jocn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 185.Pereira EA, Aziz TZ. Neuropathic pain and deep brain stimulation. Neurotherapeutics. 2014;11:496–507. doi: 10.1007/s13311-014-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Koller WC, Lyons KE, Wilkinson SB, Pahwa R. Efficacy of unilateral deep brain stimulation of the VIM nucleus of the thalamus for essential head tremor. Mov Disord. 1999;14:847–850. doi: 10.1002/1531-8257(199909)14:5<847::AID-MDS1021>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 187.Ortiz RM, Scheperjans F, Pekkonen E. Deep brain stimulation for dystonia in Finland during 2007–2016. BMC Neurol. 2019;19:137. doi: 10.1186/s12883-019-1370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8:623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- 189.Bittar RG, Kar-Purkayastha I, Owen SL, Bear RE, Green A, Wang S, et al. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12:515–519. doi: 10.1016/j.jocn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 190.Spooner J, Yu H, Kao C, Sillay K, Konrad P. Neuromodulation of the cingulum for neuropathic pain after spinal cord injury. Case report. J Neurosurg. 2007;107:169–172. doi: 10.3171/JNS-07/07/0169. [DOI] [PubMed] [Google Scholar]
- 191.Boccard SG, Pereira EA, Moir L, Van Hartevelt TJ, Kringelbach ML, FitzGerald JJ, et al. Deep brain stimulation of the anterior cingulate cortex: targeting the affective component of chronic pain. Neuroreport. 2014;25:83–88. doi: 10.1097/WNR.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 192.Boccard SG, Fernandes HM, Jbabdi S, Van Hartevelt TJ, Kringelbach ML, Quaghebeur G, et al. Tractography Study of Deep Brain Stimulation of the Anterior Cingulate Cortex in Chronic Pain: Key to Improve the Targeting. World Neurosurg. 2016;86(361–370):e361–e363. doi: 10.1016/j.wneu.2015.08.065. [DOI] [PubMed] [Google Scholar]
- 193.Boccard SGJ, Prangnell SJ, Pycroft L, Cheeran B, Moir L, Pereira EAC, et al. Long-term results of deep brain stimulation of the anterior cingulate cortex for neuropathic pain. World Neurosurgery. 2017;106:625–637. doi: 10.1016/j.wneu.2017.06.173. [DOI] [PubMed] [Google Scholar]
- 194.Lipsman N, Woodside DB, Giacobbe P, Hamani C, Carter JC, Norwood SJ, et al. Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial. The Lancet. 2013;381:1361–1370. doi: 10.1016/S0140-6736(12)62188-6. [DOI] [PubMed] [Google Scholar]
- 195.Choi KS, Riva-Posse P, Gross RE, Mayberg HS. Mapping the “Depression Switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 2015;72:1252–1260. doi: 10.1001/jamaneurol.2015.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Palm U, Keeser D, Schiller C, Fintescu Z, Nitsche M, Reisinger E, et al. Skin lesions after treatment with transcranial direct current stimulation (tDCS) Brain Stimul. 2008;1:386–387. doi: 10.1016/j.brs.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 197.Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37:102–116. doi: 10.1038/npp.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Scholfield CN. Properties of K-currents in unmyelinated presynaptic axons of brain revealed revealed by extracellular polarisation. Brain Res. 1990;507:121–128. doi: 10.1016/0006-8993(90)90530-O. [DOI] [PubMed] [Google Scholar]
- 199.Meron D, Hedger N, Garner M, Baldwin DS. Transcranial direct current stimulation (tDCS) in the treatment of depression: Systematic review and meta-analysis of efficacy and tolerability. Neurosci Biobehav Rev. 2015;57:46–62. doi: 10.1016/j.neubiorev.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 200.Beltran Serrano G, Rodrigues LP, Schein B, Souza A, Torres ILS, da Conceicao Antunes L, et al. Comparison of hypnotic suggestion and transcranial direct-current stimulation effects on pain perception and the descending pain modulating system: A crossover randomized clinical trial. Front Neurosci. 2019;13:662. doi: 10.3389/fnins.2019.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Simpson MW, Mak M. The effect of transcranial direct current stimulation on upper limb motor performance in Parkinson’s disease: A systematic review. J Neurol 2019. [DOI] [PubMed]
- 202.Thibaut A, Zafonte R, Morse LR, Fregni F. Understanding negative results in tDCS research: The importance of neural targeting and cortical engagement. Front Neurosci. 2017;11:707. doi: 10.3389/fnins.2017.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54:3988–3998. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- 204.Antal A, Terney D, Kuhnl S, Paulus W. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manage. 2010;39:890–903. doi: 10.1016/j.jpainsymman.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 205.Yoon EJ, Kim YK, Kim HR, Kim SE, Lee Y, Shin HI. Transcranial direct current stimulation to lessen neuropathic pain after spinal cord injury: a mechanistic PET study. Neurorehabil Neural Repair. 2014;28:250–259. doi: 10.1177/1545968313507632. [DOI] [PubMed] [Google Scholar]
- 206.Magis D, D’Ostilio K, Lisicki M, Lee C, Schoenen J. Anodal frontal tDCS for chronic cluster headache treatment: a proof-of-concept trial targeting the anterior cingulate cortex and searching for nociceptive correlates. J Headache Pain. 2018;19:72. doi: 10.1186/s10194-018-0904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mariano T, Burgess F, Bowker M, Kirschner J, Wout-Frank Mvt, Halladay C, et al. T187. Transcranial direct current stimulation (tDCS) for the affective symptoms of chronic low back pain (CLBP): A double-blinded, randomized, placebo-controlled trial. Biological Psychiatry. 2018;83:S200–S201. doi: 10.1016/j.biopsych.2018.02.524. [DOI] [Google Scholar]
- 208.DaSilva AF, Truong DQ, DosSantos MF, Toback RL, Datta A, Bikson M. State-of-art neuroanatomical target analysis of high-definition and conventional tDCS montages used for migraine and pain control. Front Neuroanat. 2015;9:89. doi: 10.3389/fnana.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2:201–207. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.To WT, Eroh J, Hart J, Vanneste S. Exploring the effects of anodal and cathodal high definition transcranial direct current stimulation targeting the dorsal anterior cingulate cortex. Scientific Reports. 2018;8:1–16. doi: 10.1038/s41598-018-22730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.DosSantos MF, Martikainen IK, Nascimento TD, Love TM, DeBoer MD, Schambra HM, et al. Building up analgesia in humans via the endogenous mu-opioid system by combining placebo and active tDCS: a preliminary report. PLoS One. 2014;9:e102350. doi: 10.1371/journal.pone.0102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Auvichayapat P, Keeratitanont K, Janyachareon T, Auvichayapat N. The effects of transcranial direct current stimulation on metabolite changes at the anterior cingulate cortex in neuropathic pain: a pilot study. J Pain Res. 2018;11:2301–2309. doi: 10.2147/JPR.S172920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.George MS, Lisanby SH, Sackeim HA. Transcranial magnetic stimulation: applications in neuropsychiatry. Arch Gen Psychiatry. 1999;56:300–311. doi: 10.1001/archpsyc.56.4.300. [DOI] [PubMed] [Google Scholar]
- 214.Lefaucheur JP. Use of repetitive transcranial magnetic stimulation in pain relief. Expert Rev Neurother. 2008;8:799–808. doi: 10.1586/14737175.8.5.799. [DOI] [PubMed] [Google Scholar]
- 215.George MS, Nahas Z, Molloy M, Speer AM, Oliver NC, Li XB, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry. 2000;48:962–970. doi: 10.1016/S0006-3223(00)01048-9. [DOI] [PubMed] [Google Scholar]
- 216.Lefaucheur JP, Drouot X, Keravel Y, Nguyen JP. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport. 2001;12:2963–2965. doi: 10.1097/00001756-200109170-00041. [DOI] [PubMed] [Google Scholar]
- 217.Drouot X, Nguyen JP, Peschanski M, Lefaucheur JP. The antalgic efficacy of chronic motor cortex stimulation is related to sensory changes in the painful zone. Brain. 2002;125:1660–1664. doi: 10.1093/brain/awf161. [DOI] [PubMed] [Google Scholar]
- 218.Lefaucheur JP. The use of repetitive transcranial magnetic stimulation (rTMS) in chronic neuropathic pain. Clin Neurophysiol. 2006;36:117–124. doi: 10.1016/j.neucli.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 219.Brighina F, De Tommaso M, Giglia F, Scalia S, Cosentino G, Puma A, et al. Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J Headache Pain. 2011;12:185–191. doi: 10.1007/s10194-011-0322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fierro B, De Tommaso M, Giglia F, Giglia G, Palermo A, Brighina F. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex excitability. Exp Brain Res. 2010;203:31–38. doi: 10.1007/s00221-010-2206-6. [DOI] [PubMed] [Google Scholar]
- 221.Nardone R, Holler Y, Langthaler PB, Lochner P, Golaszewski S, Schwenker K, et al. rTMS of the prefrontal cortex has analgesic effects on neuropathic pain in subjects with spinal cord injury. Spinal Cord. 2017;55:20–25. doi: 10.1038/sc.2016.87. [DOI] [PubMed] [Google Scholar]
- 222.Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122:197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 223.Nizard J, Esnault J, Bouche B, Suarez Moreno A, Lefaucheur JP, Nguyen JP. Long-term relief of painful bladder syndrome by high-intensity, low-frequency repetitive transcranial magnetic stimulation of the right and left dorsolateral prefrontal cortices. Front Neurosci. 2018;12:925. doi: 10.3389/fnins.2018.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fregni F, DaSilva D, Potvin K, Ramos-Estebanez C, Cohen D, Pascual-Leone A, et al. Treatment of chronic visceral pain with brain stimulation. Ann Neurol. 2005;58:971–972. doi: 10.1002/ana.20651. [DOI] [PubMed] [Google Scholar]
- 225.Kanda M, Mima T, Oga T, Matsuhashi M, Toma K, Hara H, et al. Transcranial magnetic stimulation (TMS) of the sensorimotor cortex and medial frontal cortex modifies human pain perception. Clin Neurophysiol. 2003;114:860–866. doi: 10.1016/S1388-2457(03)00034-8. [DOI] [PubMed] [Google Scholar]
- 226.Kanda M, Nagamine T, Ikeda A, Ohara S, Kunieda T, Fujiwara N, et al. Primary somatosensory cortex is actively involved in pain processing in human. Brain Res. 2000;853:282–289. doi: 10.1016/S0006-8993(99)02274-X. [DOI] [PubMed] [Google Scholar]
- 227.Tzabazis A, Aparici CM, Rowbotham MC, Schneider MB, Etkin A, Yeomans DC. Shaped magnetic field pulses by multi-coil repetitive transcranial magnetic stimulation (rTMS) differentially modulate anterior cingulate cortex responses and pain in volunteers and fibromyalgia patients. Mol Pain. 2013;9:33. doi: 10.1186/1744-8069-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Vyshlova I, Karpov S, Starodubtcev A. Non-pharmacological therapy of pain in lumbar region. J NeurolSci. 2017;381:854–855. [Google Scholar]
- 229.Galhardoni R, Aparecida da Silva V, Garcia-Larrea L, Dale C, Baptista AF, Barbosa LM, et al. Insular and anterior cingulate cortex deep stimulation for central neuropathic pain: Disassembling the percept of pain. Neurology. 2019;92:e2165–e2175. doi: 10.1212/WNL.0000000000007396. [DOI] [PubMed] [Google Scholar]
- 230.Luu P, Posner MI. Anterior cingulate cortex regulation of sympathetic activity. Brain. 2003;126:2119–2120. doi: 10.1093/brain/awg257. [DOI] [PubMed] [Google Scholar]
- 231.Weston CS. Another major function of the anterior cingulate cortex: the representation of requirements. Neurosci Biobehav Rev. 2012;36:90–110. doi: 10.1016/j.neubiorev.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 232.Park SI, Oh JH, Hwang YS, Kim SJ, Chang JW. Electrical stimulation of the anterior cingulate cortex in a rat neuropathic pain model. Acta Neurochir Suppl. 2006;99:65–71. doi: 10.1007/978-3-211-35205-2_13. [DOI] [PubMed] [Google Scholar]
- 233.McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol. 2004;115:589–595. doi: 10.1016/j.clinph.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 234.Lefaucheur JP, Antal A, Ahdab R, Ciampi de Andrade D, Fregni F, Khedr EM, et al. The use of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) to relieve pain. Brain Stimul 2008, 1: 337–344. [DOI] [PubMed]
- 235.Green AL, Wang S, Stein JF, Pereira EA, Kringelbach ML, Liu X, et al. Neural signatures in patients with neuropathic pain. Neurology. 2009;72:569–571. doi: 10.1212/01.wnl.0000342122.25498.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.LeBlanc BW, Cross B, Smith KA, Roach C, Xia J, Chao YC, et al. Thalamic bursts down-regulate cortical theta and nociceptive behavior. Sci Rep. 2017;7:2482. doi: 10.1038/s41598-017-02753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Schuerle S, Soleimany AP, Yeh T, Anand GM, Häberli M, Fleming HE, et al. Synthetic and living micropropellers for convection-enhanced nanoparticle transport. Sci Adv. 2019;5:eaav4803. doi: 10.1126/sciadv.aav4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang J, Cao B, Yu TR, Jelfs B, Yan J, Chan RHM, et al. Theta-frequency phase-locking of single anterior cingulate cortex neurons and synchronization with the medial thalamus are modulated by visceral noxious stimulation in rats. Neuroscience. 2015;298:200–210. doi: 10.1016/j.neuroscience.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 239.Matsuura T, Li XH, Tao C, Zhuo M. Effects of matrix metalloproteinase inhibitors on N-methyl-D-aspartate receptor and contribute to long-term potentiation in the anterior cingulate cortex of adult mice. Mol Pain. 2019;15:1744806919842958. doi: 10.1177/1744806919842958. [DOI] [PMC free article] [PubMed] [Google Scholar]