To the editor:
Despite the recent development of targeted therapies, chronic lymphocytic leukemia (CLL) remains incurable. Survival of CLL cells depends on constitutively activated signaling pathways that converge on a small number of transcription factors which mediate the altered gene expression that underlies the pathobiology of CLL. One such oncogenic transcription factor, which is downstream of both B cell receptor signaling and cytokines that drive B cell proliferation and survival, is STAT3 (1). STAT3 regulates the expression of genes controlling central cellular events, including proliferation, survival, and pluripotency. In essentially all patients with CLL, STAT3 is phosphorylated on serine-727 (2), which drives changes in gene expression underlying the pathogenesis of this disease (3).
Through a chemical biology approach, we identified the anti-microbial agent pyrimethamine as an inhibitor of STAT3 transcriptional function (4, 5). To test the hypothesis that an inhibitor of the transcriptional function of STAT3 would have therapeutic benefit in CLL, we first evaluated the effects of pyrimethamine on CLL cells in vitro (Methods found in Supplementary Material). Pyrimethamine caused a dramatic decrease in viable CLL cells, and did so through the induction of apoptosis (Supplementary Figure 1). Peripheral blood mononuclear cells (PBMC) from healthy donors showed little effect from pyrimethamine, consistent with the known excellent safety profile of this drug.
To identify genes regulated by STAT3 in CLL cells, which could serve as biomarkers for STAT3 inhibition, we first started with a set of 361 genes known to be upregulated in CLL cells compared to normal B lymphocytes (6) (Supplementary Figure 2A). We then filtered these genes based on regulation by STAT3 in independent data sets, or STAT3 binding in proximity to the gene by chromatin immunoprecipitation (ChIP). From this analysis, we identified five genes (AIM2, ATXN1, ENPP2, GAB1, and ID3) that showed increased expression in CLL cells compared to healthy B lymphocytes, and which had the criteria of direct STAT3 target genes.
When primary CLL cells were treated ex vivo with pyrimethamine, decreased expression of all five STAT3 signature genes was consistently observed (Supplementary Figure 2B). As expected, lymphocytes purified from the blood of healthy donors showed minimal expression of these genes and no significant change with pyrimethamine treatment. Given that pyrimethamine decreased the expression of STAT3 target genes and the survival of CLL cells in vitro, along with its known excellent safety profile, we designed a clinical trial to assess the efficacy of pyrimethamine as a single agent in patients with relapsed refractory CLL. This trial was initiated prior to the introduction of BTK, PI3K, or BCL-2 inhibitors into clinical use for this disease.
Sixteen heavily pretreated patients, with a median of six prior therapies, enrolled on the phase 1 portion of this study. Patient characteristics are provided in Supplemental Table 1. Three patients each were enrolled on cohorts 1 (12.5 mg daily) and 2 (25 mg daily) with no dose-limiting toxicities (DLTs) and no significant drug-related toxicities (Supplementary Table 2). Cohort 3 (50 mg daily) enrolled 10 patients, also with no DLTs observed. The maximum tolerated dose was not reached at doses up to 50 mg daily.
Plasma levels of pyrimethamine increased progressively in samples obtained during the first two weeks of treatment, with apparent steady state conditions achieved after dosing for 2 weeks. The steady state plasma concentration of pyrimethamine increased linearly with escalation of the daily dose from 12.5 to 50 mg (Supplementary Figure 3 and Supplementary Table 3). The geometric mean steady state concentration of pyrimethamine in plasma was 6.17 μM for the 5 patients with evaluable samples who received the 50 mg daily dose, somewhat less than the target concentration of 10 μM projected for maximal STAT3 inhibition (4). The steady state concentration of pyrimethamine in PBMCs, was also linearly related to the dose (Supplementary Figure 3B) and correlated with the corresponding concentration of the drug in plasma.
No objective responses by IW-CLL 2008 criteria were observed. However, 50% of patients achieved stable disease, with one patient dosed at 50 mg/d on therapy for 12 months, and two at 25 mg/d on for 4 and 6 months. The remaining patients had progressive disease, with all but one patient discontinuing for progressive disease. The median overall survival was 22.2 months (Supplementary Figures 4 and 5), consistent with the heavily pretreated status and limited therapeutic options for these patients.
To determine whether pyrimethamine was inhibiting STAT3 transcriptional function in the CLL cells in vivo, mRNA was harvested from CLL cells from each patient prior to initiating pyrimethamine and while on therapy. Decreased expression of the STAT3 signature could be detected at a minimum of two different time points in eight of the 16 patients within the first two months of treatment (Figure 1A). However, at the time of clinical progression, increasing expression of the STAT3 signature generally occurred (Figure 1B). The expression of the five signature genes was generally highly concordant and was well reflected with an arithmetic mean.
Figure 1. Changes in the STAT3 gene expression signature in patients on therapy.
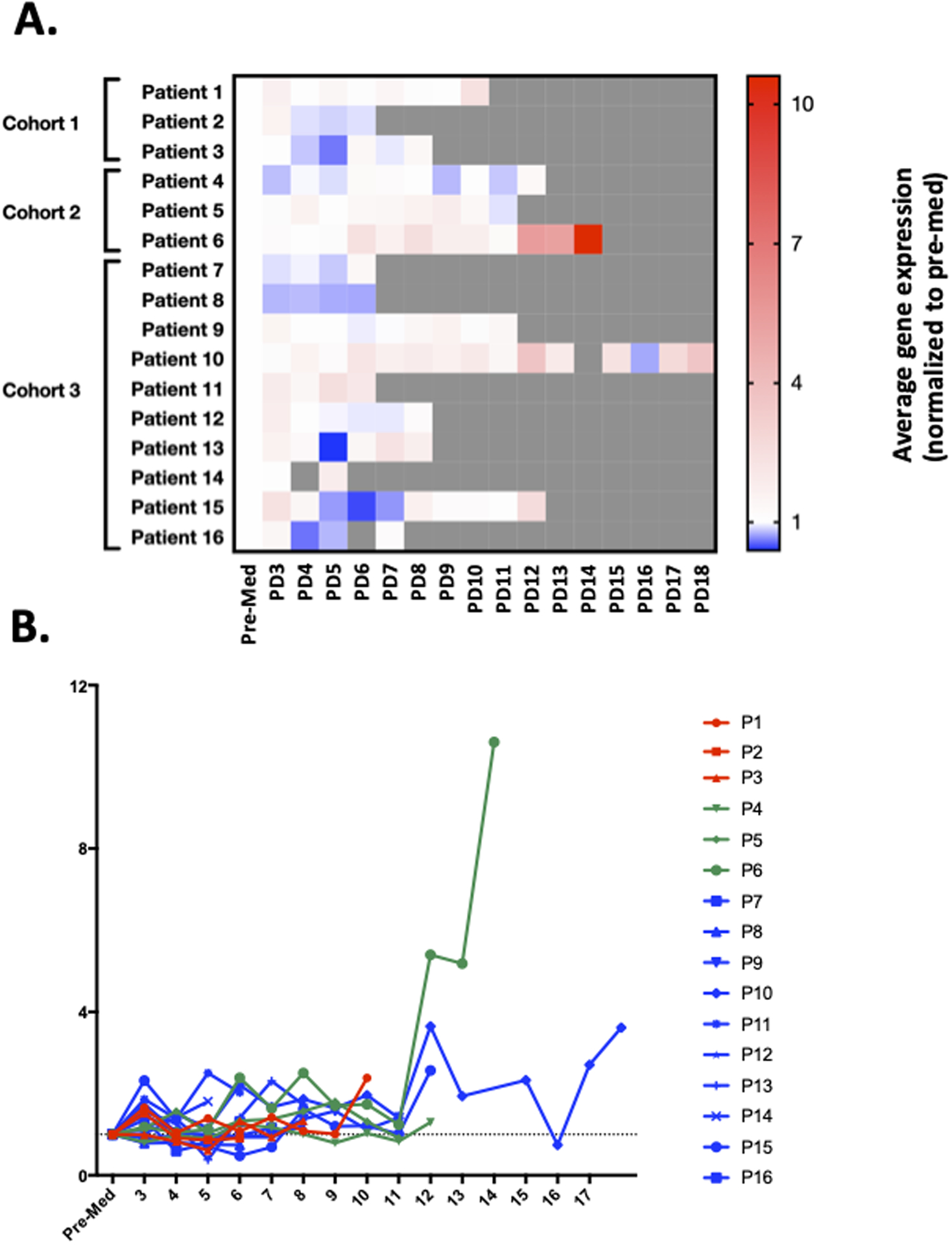
Samples for pharmacodynamic analysis of changes in expression of STAT3 target genes were obtained prior to treatment (Pre-Med), and then two hours post drug on day 1 of cycle 1 (PD3), one day among days 2 through 5 of therapy (optional; PD4), day 8 (PD5), 15 (PD6), and 22 (PD7) of cycle 1, day 1 (PD8) and 15 (PD9) of cycle 2, and day 1 of subsequent cycles (PD10–18). (A) Expression of the five gene signature for each patient normalized to pre-treatment levels. (B) Expression of the STAT3 signature showed increasing expression at the time of progression in most patients.
These analyses also revealed correlations between pharmacokinetic and pharmacodynamic data that suggest mechanistic underpinnings for clinical effects. For example, in patient 6 (Supplementary Figure 6A), expression of the five STAT3 signature genes remained suppressed at the times of most of the pharmacodynamic blood sampling. However, at the last four samplings, the trough level of pyrimethamine decreased significantly in the setting of intermittent drug holds, and this coincided with increased expression of all five of the signature genes and clinical progression. In patient 8, by contrast, pyrimethamine levels were relatively high, and expression of the signature genes remained suppressed (Supplementary Figure 6B). These findings suggest that quantitation of this five gene signature may be an effective way to measure on-target effects of STAT3 inhibition for pyrimethamine (and likely other STAT3 inhibitors) and that loss of suppression of these genes may presage clinical progression.
To determine whether the response of a patient’s CLL cells to pyrimethamine ex vivo would be a predictive marker for a patient’s clinical response, we treated pre-tretment patients’ cells in culture with vehicle or 10 μM pyrimethamine. Samples were available from four patients for analysis (Supplementary Figure 7). All showed varying levels of loss of viability in the presence of pyrimethamine compared to vehicle treatment. Of the two patients from the highest dose cohort who were analyzed in this way (patients 9 and 12), both showed relatively little loss of viability ex vivo (Supplementary Figure 7), and both showed relatively little effect on the STAT3 gene expression signature in vivo (Supplementary Figure 6).
For six patients, we were able to analyze change in expression of the five STAT3 signature genes following 24 hours of treatment with pyrimethamine (10 μM) ex vivo (Supplementary Figure 8). Variable degrees of inhibition of the STAT3 signature genes were observed, though four of the six patients had a majority of the signature genes decrease by at least 25%. Of the three patients who had both gene expression analysis and ex vivo cell survival analysis (patients 4, 5, and 9), only one showed a decrease of viable cell number greater than 50% (patient 4), and this was the only one of these patients in whose cells gene expression was suppressed by greater than 25% in a majority of the STAT3 signature genes. This patient also showed notable suppression of STAT3 target genes in vivo (Supplemental Figure 6A). These findings are suggestive of a correlation between inhibition of STAT3 transcriptional activity and inhibition of CLL cell survival.
Two of the six patients for whom ex vivo gene expression analysis was performed (patients 7 and 9) had been in the highest dose cohort. Patient 7 showed suppression of a majority of STAT3 signature genes when treated ex vivo and also showed suppression of the gene signature at multiple time points in vivo (Supplemental Figure 6). By contrast, patient 9 showed little repression of the STAT3 signature with either ex vivo or in vivo treatment with pyrimethamine. These data raise the possibility that testing for the response to pyrimethamine ex vivo, either based on cell survival or suppression of expression of STAT3 target genes, may be a predictive marker for the response to this STAT3 inhibitor.
We anticipate that single agent activity of pyrimethamine would require dose escalation above 50 mg daily, which should be feasible and safe based on our data. By decreasing expression of pro-survival genes, pyrimethamine can also sensitize cells to undergoing apoptosis. Thus, combinations of pyrimethamine with signaling inhibitors active in CLL, such as the BTK or PI3K inhibitors, might result in enhanced activity. In addition, by decreasing expression of anti-apoptotic genes such as MCL1 and BCL-xl, pyrimethamine may also synergize with a BCL2 inhibitor active in CLL, such as venetoclax. Finally, STAT3 activation leads to modulation of expression of immune-regulatory genes resulting in tumor cells that are more resistant to immune-based killing as well as the establishment of an immunosuppressive microenvironment, and this can be reversed with pyrimethamine. Therefore, combinations of pyrimethamine with immune activating treatments, such as immune checkpoint inhibitors, may be particularly active in CLL, a disease that has not generally been sensitive to single agent immunotherapies.
Supplementary Material
Acknowledgements
This clinical trial was supported by a grant from the Lymphoma Research Foundation (D.A.F.), and the related studies were supported by NIH grant R01-CA160979, the Gabrielle’s Angel Foundation (New York, NY), The Joan Harris Cancer Foundation (Boston, MA), and the Brent Leahey Fund (Dana-Farber Cancer Institute) (D.A.F.). This research was also supported by a generous gift from Stephen P. Koster, Esq.
References
- 1.Frank DA. STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett. 2007;251(2):199–210. [DOI] [PubMed] [Google Scholar]
- 2.Frank DA, Mahajan S, Ritz J. B lymphocytes from patients with chronic lymphocytic leukemia contain signal transducer and activator of transcription (STAT) 1 and STAT3 constitutively phosphorylated on serine residues. J Clin Invest. 1997;100(12):3140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hazan-Halevy I, Harris D, Liu Z, Liu J, Li P, Chen X, et al. STAT3 is constitutively phosphorylated on serine 727 residues, binds DNA, and activates transcription in CLL cells. Blood. 2010;115(14):2852–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takakura A, Nelson EA, Haque N, Humphreys BD, Zandi-Nejad K, Frank DA, et al. Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Human Molecular Genetics. 2011;20(21):4143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson EA, Sharma SV, Settleman J, Frank DA. A chemical biology approach to developing STAT inhibitors: Molecular strategies for accelerating clinical translation. Oncotargets. 2011;2:518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Shalek AK, Lawrence M, Ding R, Gaublomme JT, Pochet N, et al. Somatic mutation as a mechanism of Wnt/β-catenin pathway activation in CLL. Blood. 2014;124(7):1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


