Abstract
Pain is a complex experience with far-reaching organismal influences ranging from biological factors to those that are psychological and social. Such influences can serve as pain-related risk factors that represent susceptibilities to opioid use disorder. This review evaluates various pain-related risk factors to form a consensus on those that facilitate opioid abuse. Epidemiological findings represent a high degree of co-occurrence between chronic pain and opioid use disorder that is, in part, driven by an increase in the availability of opioid analgesics and the diversion of their use in a non-medical context. Brain imaging studies in individuals with chronic pain that use/abuse opioids suggest abuse-related mechanisms that are rooted within mesocorticolimbic processing. Preclinical studies suggest that pain states have a limited impact on increasing the rewarding effects of opioids. Indeed, many findings indicate a reduction in the rewarding and reinforcing effects of opioids during pain states. An increase in opioid use may be facilitated by an increase in the availability of opioids and a decrease in access to non-opioid reinforcers that require mobility or social interaction. Moreover, chronic pain and substance abuse conditions are known to impair cognitive function, resulting in deficits in attention and decision making that may promote opioid abuse. A better understanding of pain-related risk factors can improve our knowledge in the development of OUD in persons with pain conditions and can help identify appropriate treatment strategies.
Keywords: Pain, opioid, abuse, reward, cognition, vulnerability
1. Introduction
Factors that contribute to the formation of substance use disorders can range from biological variables to those that are social and environmental. Certain qualities of these factors constitute vulnerabilities that facilitate substance use and abuse. The impact of these vulnerabilities may be direct, such as ontogenetic effects that lead to an enhanced propensity to acquire nicotine and alcohol use during adolescence (Garofoli, 2020; Lees et al., 2020; O’Dell, 2011). Other factors may be indirect, such as metabolic disorders that consist of variables like hypoinsulinemia, which can prime a neurobiological landscape that is favorable for substance abuse (Könner et al., 2011; O’Dell and Nazarian, 2016; Owens et al., 2005; Zheng et al., 2013). Likewise, pain serves as a significant contributing factor to substance use disorders (SUD), most notably opioid use disorder (OUD). This view is highlighted by the current opioid epidemic in the United States, which was enabled by liberal prescribing practices of health care providers to individuals seeking treatments for various pain conditions as well as inadequate regulatory controls, particularly within some individual states. Such prescribing practices increased opioid availability not only for licit pain management but also for illicit diversion for use in non-medical contexts. A major commonality amongst those suffering from OUD is the present or prior experience with acute or chronic pain. Therefore, pain can be viewed as an indirect vulnerability that allows the convergence of biological, psychological, and social constructs that result in the facilitation of opioid abuse and the formation of OUD. This article provides a brief overview of several variables that may mediate the effects of pain on vulnerability to OUD. Considerations are given to epidemiology and imaging studies that set the groundwork for understanding the inception of the opioid epidemic and the role of pain. These sections are followed by an examination of clinical and preclinical studies that explore the impact of pain on opioid reward and reinforcement, on access to both opioid and non-opioid reinforcers, and on cognitive processes that influence vulnerability to OUD. The review concludes with a consideration of interventions that may be useful in reducing OUD among pain patients. The goal of this review is to demonstrate and consider the multifactorial nature of the role of pain in OUD and encourage further discussion and engagement in better understanding their combined consequences.
2. Epidemiology, imaging and genetic studies in patients with chronic pain and OUD
2.1. Epidemiology
There are several reviews of epidemiological studies that demonstrate the existence of significant pain in a large percentage of patients that present for the treatment of OUD. Likewise, a significant percentage of patients being treated for chronic non-malignant pain display OUD as well (Juurlink and Dhalla, 2012; Kaye et al., 2017a, 2017b). Epidemiological studies from people presenting for OUD treatment report that approximately 60% of OUD patients seeking treatment report chronic pain, defined generally as moderate to severe pain that has been present for 3 months or longer, compared to an estimated prevalence of 20% of adults in the general population of the United States (Cicero et al., 2008; Dahlhamer et al., 2018). Chronic pain is the most prevalent and consistent comorbidity in this population and was listed as the reason for the initial use of an opioid in 80% of the patients (Cicero et al., 2008). Other significant psychological comorbidities found with OUD in patients presenting for treatment include depression (72%), anxiety (55%), and bipolar disorder (28%). It is clear that the presence of chronic pain is a risk factor for OUD, and that the presence of OUD is a risk factor for chronic pain. What is less clear are the underlying mechanisms or additional comorbidities that are potentially responsible for this association.
Access to strong opioids is now considered one significant factor in promoting OUD in patients with significant pain, particularly chronic non-malignant pain (Volkow et al., 2018). The recent surge of OUD can be traced to the liberalization of opioid prescribing practices beginning in the early 1990’s. Several seminal papers from the late 1980’s to 1990 suggested that chronic non-malignant pain could be effectively managed in a modest subset of these patients, provided that effectiveness, side effects, and aberrant behaviors indicative of OUD were monitored closely (Portenoy, 1996; Rosenblum et al., 2008). Two respected societies published a position statement calling for chronic opioid therapy to be considered for the treatment of chronic pain, and the Joint Commission on Health Care called for pain to be considered the “5th vital sign”, requiring assessment in all patients and offer of treatment if found to be significant (Chisholm-Burns et al., 2019; Chou et al., 2009; Kolodny et al., 2015; Medicine and Society, 1997). The result was a clear correlation between access to large quantities of opioids and an increase in OUD, emergency room visits for life-threatening adverse effects, and deaths from overdose (Cicero et al., 2005). Analyzing the dramatic change in the demographics of OUD across these decades is also informative on how drug access strongly influences epidemiological data (Cicero et al., 2015, 2014, 2005). These data show that in the 1960’s, OUD was mostly characterized as a predominantly male phenomenon with heroin as the primary drug of choice. However, with increased access to opioids through legitimate medical practice, OUD became a predominantly gender-neutral phenomenon driven largely by misuse or abuse of hydrocodone and oxycodone, as well as some other commonly prescribed drugs. A majority of patients presenting for treatment for OUD now list pain treatment as the main reason for initiating opioid use and a drug other than heroin as the original opioid taken. This is in stark contrast to the earlier decades, during which pharmaceutical opioids were not as readily available. Determining the role of pain per se in the neurobiology of OUD in humans is therefore confounded by increased access, a known factor for OUD in general.
Another factor that can influence the ability to directly identify the presence of chronic pain as a risk factor for OUD is that some of the same comorbidities serve as risk factors for each separate disorder (Brooner et al., 1997; Cicero et al., 2008; Wiech and Tracey, 2009). These include previous trauma, either physical or psychological, particularly during adolescence (Geisser et al., 1996; Gibson, 2012; Gupta, 2013; Martin et al., 2010; O’Donnell et al., 2009; Pagé et al., 2018; Roth et al., 2008). General anxiety disorder is a risk factor for both chronic pain and OUD, irrespective of their co-occurrence (Kleiman et al., 2011). The same is also true for depression, as patients with pre-existing clinical depression are 5 times more likely to develop fibromyalgia and 6 times more likely to develop chronic pain after injury as those without depression (Caputi et al., 2019; Miller and Cano, 2009; Williams et al., 2004). Depression is also found to be present in patients presenting with OUD at comparable increased rates of occurrence compared to the general population, regardless of the presence of pain (Adan et al., 2017; Davis et al., 2008). Given the co-occurrence of psychiatric disorders with chronic pain or OUD, the role that chronic pain has as a separate risk factor for OUD is unclear (Wiech and Tracey, 2009). This may seem inconsequential from a statistical or epidemiological perspective; however, from a neurobiological or mechanistic point of view, these data suggest alternative hypotheses that may only be resolved in a controlled laboratory setting.
Other significant comorbidities that are present between chronic pain patients and those with OUD are the use, misuse, or abuse of other common drugs of abuse. These include alcohol, tobacco, and cannabis primarily. Alcohol and tobacco use are high in the general population, but disproportionally so in both chronic pain patients and those with OUD (Ditre et al., 2011; Martel et al., 2018; McHugh et al., 2020; Robins et al., 2019; Witkiewitz and Vowles, 2018). Cannabis use and misuse is likewise relatively high in patients with chronic pain (Martel et al., 2018). Past or current use of all three of these substances is a significant risk factor for both the development of chronic pain and OUD. Whether these data represent coincident but not causative factors, separate unrelated factors, or mechanistic neurobiological factors with distinct mechanisms that relate to increased risk of pain and OUD remain to be elucidated.
2.2. Imaging and genetic studies
Beyond the statistical analysis of epidemiological data, other studies that suggest similarities in the neurobiology of chronic pain and OUD are imaging and genetic studies. Human imaging studies in chronic pain patients and those suffering from OUD identify several overlapping brain regions that suggest potential common neurobiological mechanisms. Regions of the frontal cortex, including the anterior cingulate and medial prefrontal cortex, display reduced activity and ultrastructural changes indicative of decreased activity and connectivity changes in both chronic pain patients and those with OUD (McConnell et al., 2020; Wollman et al., 2017). These data suggest that diminished frontal cortical activity induced by chronic pain may be one factor that predisposes such patients to OUD, and likewise may explain the increased occurrence of chronic pain in OUD patients. While these regions are typically associated with effects on cognition and executive function, they also influence limbic regions such as the amygdala and the nucleus accumbens (NAc), which are critical components of the reward system. There are relatively fewer studies on genetic and epigenetic changes that occur across both chronic pain and OUD patients; however, some similar changes have been shown to exist with respect to mu-opioid receptor (MOR) splice variants (Barbierato et al., 2015; Crist et al., 2018; Peciña et al., 2015). Genes associated with both alcohol use disorder and chronic pain include 5HT7 serotonergic receptors, alpha 1a adrenergic receptors, and many others comprising catecholaminergic systems and stress and immune modulators (Yeung et al., 2017). The potential role of these alterations beyond simple correlation will be a topic for research in future controlled laboratory animal studies. A unifying hypothesis has been put forth that pain chronification leads to both a decrease in activation of limbic reward circuitry induced by natural rewards, as well as pain-related increased activity in brain regions and mechanisms that counter activation of reward systems by activating mechanisms associated with stress and depression, so-called “anti-reward” mechanisms (Borsook et al., 2016; Elman and Borsook, 2016). Such hypotheses and precise mechanisms are likely best studied in preclinical animal models with appropriate pain manipulations and behavioral outcomes.
2.3. Summary
The epidemiological data and limited controlled studies indicate clearly that the incidence of chronic pain is high in people presenting with OUD, and vice versa, compared to the general population. Despite several confounding variables, data are available from imaging and genetic studies that suggest potential mechanisms. Evaluating causative mechanisms will likely involve appropriate laboratory animal studies reverse engineered to reflect the clinical data as closely as possible. The status of this effort is described in the following sections.
3. Examination of opioid reward and reinforcement in the presence of pain
One possible mechanism for pain-related increase in OUD risk is a pain-related increase in the reinforcing and rewarding effects of MOR agonists that maintain opioid-taking behavior. This possibility has been extensively studies in preclinical procedures of drug self-administration, intracranial self-stimulation, and place conditioning that are used to examine drug reinforcement and reward.
3.1.1. Drug self-administration
Self-administration is widely considered the gold standard for evaluation of both the abuse potential of drugs across many pharmacological classes as well as mechanisms related to this abuse potential (Ator and Griffiths, 2003; Henningfield et al., 1991). As the name suggests, self-administration procedures involve voluntary drug consumption by the subject under study. This is typically achieved by making intravenous (IV) infusions contingent upon behavioral responses, and reinforcement is evidenced by a greater rate of behavior maintained by drug infusions than by vehicle infusions or no infusion. Implantation of a chronic indwelling venous catheter is required, and several procedures are described for implantation and maintenance of IV catheters in rodents and nonhuman primates (Howell and Fantegrossi, 2009; Weeks, 1962). Voluntary oral consumption has also been documented in rodents and nonhuman primates, principally for ethanol but also for opioids in several studies (Meisch, 2001). Opioids and psychostimulants reliably maintain robust behavior in these models, while the reinforcing effects of alcohol and nicotine are typically modest in comparison (Goodwin et al., 2015). Hallucinogens and cannabinoids are difficult to study using these methods and do not generally maintain robust behavior in most nonhuman species (John et al., 2017; Justinova et al., 2005). A myriad of factors influence behavior in drug self-administration studies, including but not limited to the reinforcement schedule, dose, route of administration, and species. Comprehensive reviews are provided in the literature, and even a cursory summary of these studies is beyond the scope of this article (Müller, 2018; Panlilio and Goldberg, 2007). For the purpose of this review, pain manipulations as modulating stimuli for drug self-administration have been largely limited to the study of IV opioid self-administration in rodents, and this will serve as the focus for this section.
The primary outcome measure for drug self-administration studies is generally the rate of behavior maintained by contingent drug presentation, typically lever presses or nose-poke responses. It is important to understand how drug dose affects response rates under different schedules of reinforcement to make accurate and logical conclusions from these experiments (Sizemore and Martin, 2000). For simple fixed-ratio (FR) schedules, in which a fixed number of responses is required for each injection, an inverted U-shaped dose-effect curve is typical for opioids, but can be difficult to achieve for other drugs, psychostimulants in particular (Figure 1) (Sizemore et al., 1997; Sizemore and Martin, 2000). On the ascending limb of the dose-response curve low doses of drug maintain low rates of behavior, and the response rate increases with increasing drug dose until the maximum rate is achieved. On the descending limb, further increases in drug dose result in decreases in response rate. The descending limb is hypothesized to result from increases in rate-decreasing drug effects (e.g. sedation) with increasing dose, as well as satiety of the reinforcing stimulus in concert with pharmacokinetics and increased duration of effect as the dose is increased. To properly evaluate changes in reinforcement under FR schedules, it is important to know where the dose or doses under study fall on the inverted U-shaped dose-effect curve (Figure 2) (Calabrese, 2008).
Figure 1. Dose-effect curves from FR (fixed ratio), PR (progressive ratio), and FDC (food-drug choice) drug self-administration procedures.
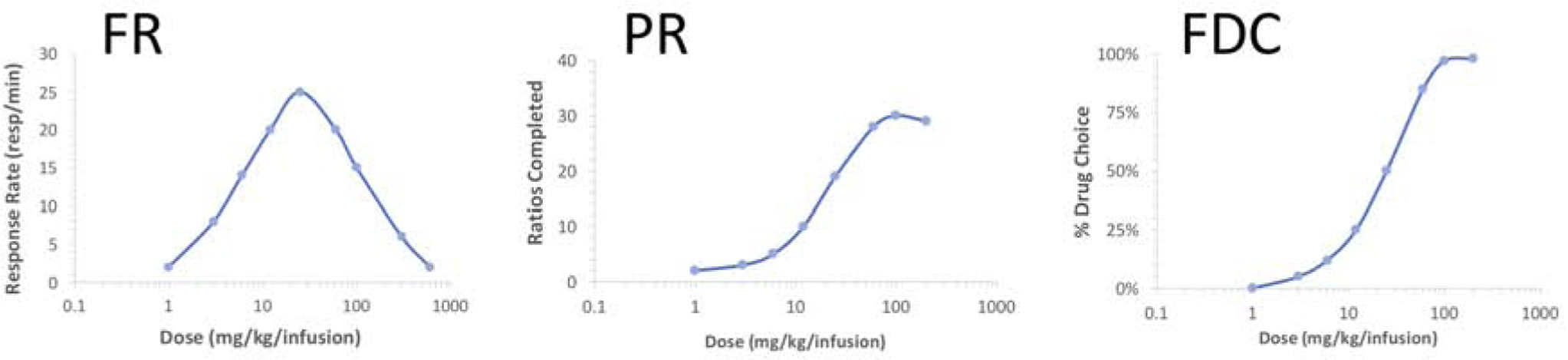
Examples of typical dose-effect curves obtained from drug self-administration procedures using the indicated schedules of reinforcement are shown. Bitonic, inverted U-shaped dose-effect curves are typical of FR schedules, with response rates increasing with increasing dose to a maximal rate at lower doses. Increasing dose further typically leads to decreases in response rate, thought to occur as a result of both rate decreasing effects of higher doses as well as pharmacokinetics. Under the PR schedule, the number of ratios completed (and consequently number of drug infusions) is typically a monotonic function of dose, with decreases in ratios completed after reaching the maximum occurring only at very high doses. Under the FDC schedule, dose-effect curves are also typically a monotonic function of dose, with percentage of trials resulting in drug choice over food increasing with drug dose.
Figure 2. Evaluation of changes in drug reinforcement using FR schedules and effects on dose-effect curves. Figure 2. Overlapping response rates with shifts in dose-effect curves for FR schedules.
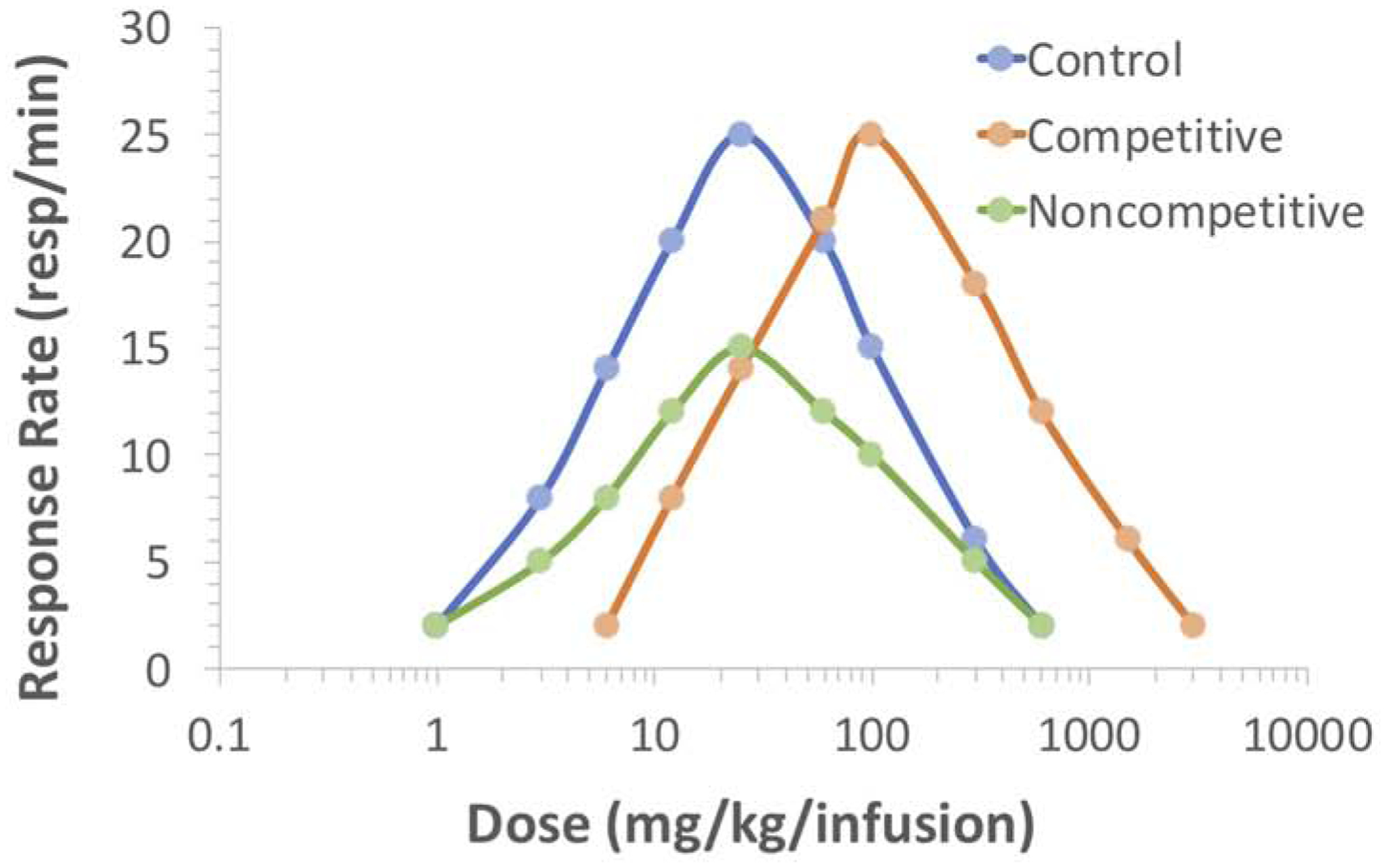
Examples of parallel or downward shifts in dose-effect curves are shown for FR schedules of reinforcement. Parallel shifts typically occur in the presence of competitive antagonists or manipulations that mimic competitive antagonism (orange curve). Downward shifts typically occur with non-competitive antagonists or manipulations that mimic such compounds (green curve). As can be seen, response rates at given doses of the self-administered drug can overlap depending on the nature of the shift. Note response rates at select doses on the control curve (blue) that are not different than that with competitive antagonism (orange) as well as with non-competitive antagonism (green). Likewise, response rates can be similar at select doses with competitive and non-competitive antagonism (orange, green).
Other schedules of reinforcement that have been commonly used include progressive-ratio (PR) and food-drug choice (FDC). For PR schedules, the number of responses required for delivery of the contingent drug infusion increases with subsequent infusions according to a predetermined array, which is generally logarithmic (Richardson and Roberts, 1996). The primary outcome measure is the highest ratio completed, typically defined by limiting the maximal time interval allowed between responses within a given ratio (Figure 1). Psychostimulants maintain robust responding under PR schedules, whereas opioids are often less effective, and both drug classes usually generate a monotonically increasing dose-response curve (Rowlett, 2000; Stafford et al., 1998). For FDC schedules, responses on two separate manipulanda are reinforced by either delivery of drug infusions or food (Banks and Negus, 2017; Negus and Banks, 2018). The subject is free to choose between drug and food, and the available drug dose can be manipulated within daily sessions or across days (Figure 1). The primary outcome measure is the percentage of trials for each component in which the subject chooses drug infusions, and the dose-effect curve is typically monotonically increasing with respect to drug dose. These different schedules of reinforcement add value to the evaluation of reward mechanisms for drugs, including opioids; however, effects of pain manipulations on self-administration of drugs including opioids have been largely documented with FR schedules.
3.1.2. Opioid self-administration
Opioid self-administration typically has been studied using IV delivery and relatively simple schedules of reinforcement, such as FR. More recently others have used PR schedules with the ultrafast acting opioid remifentanil (Panlilio and Schindler, 2000), and FDC studies have been documented in both rats and nonhuman primates (Townsend et al., 2019). Initial studies with opioids in self-administration models primarily examined lever pressing maintained by morphine infusions in rats and nonhuman primates made physically dependent through a schedule of increasing doses of noncontingent infusions or injections (Thompson and Schuster, 1964). Later studies demonstrated that morphine and heroin would maintain robust behavior in non-dependent subjects. The dose-effect curves for numerous MOR agonists demonstrate that the rate of behavior coincides with drug potency and efficacy to activate MORs (Martin et al., 2007). Escalation of intake over several orders of magnitude occurs with continuous access to heroin self-administration and is consistent with the development of tolerance and physical dependence (Sim-Selley et al., 2000). Even relatively modest escalation of heroin intake (3–4 fold) through self-administration is also associated with the development of mechanical hypersensitivity, suggesting that some nociception may be present under these conditions (Edwards et al., 2012). Numerous other reviews summarize the findings of this large literature (Gerak et al., 2020).
3.1.3. Effects of pain manipulations on opioid self-administration
While numerous factors determine the behavioral effects and the nature of the dose-response curve of MOR agonists in self-administration models, most of these variables have not been manipulated or assessed in studies that specifically examine the effects of pain manipulations on reinforcement. Some of the earliest studies that examined how pain models influence opioid self-administration focused on inflammatory pain in arthritic rats induced by the administration of Complete Freund’s Adjuvant (CFA). One study found that arthritic rats self-administered IV morphine at a lower rate than control animals, an effect that was reversed by indomethacin, suggesting that inflammatory pain reduces the reinforcing effects of opioids (Lyness et al., 1989). Conversely, oral intake of fentanyl was shown to increase with the development of CFA-induced arthritis in rats, and intake mirrored the arthritic process (Colpaert et al., 2001, 1982). Additionally, CFA-induced arthritis attenuated low dose heroin self-administration under both FR and PR schedules of reinforcement in rats, whereas high-dose heroin self-administration approximately doubled under the FR schedule but did not change under the PR schedule (Hipólito et al., 2015). In rats with L5/L6 peripheral injury, the effect of neuropathic pain on opioid intake was found to be dependent on the opioid studied and dose, with the dose-effect curves being shifted downward and to the right to varying degrees (Martin et al., 2007). The dose-effect curves for morphine, fentanyl, and hydromorphone were shifted substantially downward, with self-administration being maintained only at the highest doses that maintain the behavior in uninjured rats. Heroin and methadone maintained self-administration in nerve-injured rats over a broader range of doses on the descending limb of the dose-effect curve, but not at doses on the ascending limb. The rate of drug intake of all opioids and at all doses was consistent with modeled titration of reduction in mechanical allodynia in rats with nerve injury, suggesting that drug intake was motivated to some extent by alleviation of hypersensitivity. Moreover, intrathecal clonidine reduced heroin intake through self-administration selectively in nerve-injured rats, while adenosine did not. It is noteworthy that in humans with neuropathic pain, intrathecal clonidine and adenosine both reduce allodynia but only clonidine reduces spontaneous pain (Eisenach et al., 2003, 1995). These data collectively suggest that neuropathy and putative neuropathic pain diminish opioid reinforcement, such that higher doses will be required to maintain self-administration and the effect is dependent upon the relative intrinsic efficacy of the agonist, as the major metabolite of heroin, 6-monoacetylmorphine, and methadone have greater intrinsic efficacy at the MOR than morphine, fentanyl or hydromorphone (Selley et al., 2001, 1998). The sparing effect of intrathecal clonidine but not adenosine suggests that spontaneous pain rather than mechanical allodynia has a major role in the titration of opioid effect through self-administration. It is also noteworthy that intrathecal clonidine will maintain self-administration in rats with L5/L6 nerve ligation, but not in uninjured animals (Martin et al., 2006). These data predict that the presence of pain would result in a preference for higher doses of opioids, and opioids of greater efficacy including heroin. Further, it suggests that successful treatment of chronic pain, such as with intrathecal clonidine, will decrease the propensity to self-administer high doses of heroin or other opioids.
Several studies have addressed the neurobiological changes that occur in the brain following pain manipulations, including changes in classical reward circuitry and in cortical regions including the cingulate cortex and amygdala. These changes are thought to influence opioid reinforcement mechanisms in the presence of chronic pain. MORs are particularly dense in the amygdala, a brain region that integrates noxious peripheral input with the limbic system (Herkenham and Pert, 1982; Sim et al., 1995). Irreversible inhibition of MORs in the central amygdala with the alkylating antagonist beta-funaltrexamine increases the rate of intake for self-administered heroin in nerve-injured but not normal rats, an effect that persists for up to 2 weeks after beta-funaltrexamine treatment (Martin et al., 2011). This suggests that integration of peripheral nociceptive input with the limbic system through the amygdala is important for self-regulation of opioid intake, likely by reducing the influence on downstream limbic targets by direct action on MORs in this region. Other studies have indicated that MOR coupling to G-proteins in the ventral tegmental area (VTA) is reduced in the presence of pain in mice, suggesting another possible mechanism by which pain may shift the dose-response curve for opioid reinforcement (Ozaki et al., 2002). Elevation of dopamine in the NAc during heroin self-administration is attenuated in rats with CFA-induced arthritis, further implicating altered limbic system function in the presence of pain as a mechanism for diminished opioid reinforcement (Hipólito et al., 2015). Increased dynorphin release and an upregulation in kappa opioid receptor activation within the NAc has also been shown with CFA-induced arthritis in rats, and is responsible at least in part for induction of negative affect (Massaly et al., 2019). It has been suggested that the fundamental neurobiology of opioid reinforcement differs depending on the presence or absence of pain, with some potential overlap in negative reinforcing mechanisms between pain and physical dependence/withdrawal (Shurman et al., 2010). The studies cited above suggest several potential sites and mechanisms by which pain may modify opioid reinforcement in animal models. There is a need to examine the interactions between pain and opioid reinforcement and underlying mechanisms in other pain and self-administration models, including more sophisticated models such as FDC procedures.
3.1.4. Summary
The effects of pain manipulations on opioid self-administration suggest that inflammatory or neuropathic pain models decrease the reinforcing effects of opioids. This is evidenced by shifts in the dose-effect curve downward and/or to the right, or by decreased self-administration of lower but not higher doses when the full dose-effect curve was not determined. Potential sites of altered reinforcement mechanisms include the classical dopaminergic pathways from the VTA to the NAc as well as the amygdala. The growing body of literature for neurobiological effects of acute and chronic pain as well as novel behavioral assessments of pain behavior, including novel drug self-administration paradigms, should provide a future framework for evaluating the effects of pain manipulations on opioid self-administration and potential mechanisms.
3.2. Intracranial Self-Stimulation
Like drug self-administration procedures, intracranial self-stimulation (ICSS) is another family of operant behavioral procedures that has proven useful for examining the abuse potential of drugs (Carlezon Jr. and Chartoff, 2007; Kornetsky and Esposito, 1979; Negus and Miller, 2014; Wise, 1996). In ICSS procedures, subjects are equipped with a chronic indwelling microelectrode targeting a brain-reward area and trained to emit an operant response such as pressing a lever to receive pulses of electrical brain stimulation (Figure 3A). In one common variant of ICSS, the frequency of electrical stimulation can then be systematically manipulated during daily sessions from low to high levels that maintain low to high rates of responding (Figure 3B) (Negus and Miller, 2014). This type of “frequency-rate” curve can then serve as a behavioral baseline to examine the effects of drugs, pain states, or their combination. As one example of effects produced by an abused drug on ICSS frequency-rate curves, Figure 3B shows the effects of increasing doses of amphetamine (Bauer et al., 2013). Amphetamine, which promotes the release of dopamine from dopaminergic neurons such as those projecting from the VTA to the NAc, is a known drug of abuse, and it produces effects in the frequency-rate procedure that are typical of drugs with abuse liability: a dose-dependent leftward shift in the frequency-rate curve and increase in low ICSS rates maintained by low brain-stimulation frequencies. This pattern of drug effects is often referred to as “facilitation,” and ICSS facilitation is characteristic of abused drugs (Negus and Miller, 2014). Conversely, drugs with low abuse potential [e.g. the serotonin-selective releaser fenfluramine;(Bauer et al., 2013)] fail to facilitate ICSS up to doses that produce motor impairment.
Figure 3. Overview of intracranial self-stimulation (ICSS).

ICSS procedures are one class of preclinical procedures for assessment of rewarding drug effects. Panel A shows a rat in an operant conditioning chamber pressing a lever for electrical brain stimulation. Stimulation is delivered via a microelectrode that is implanted in a brain-reward area such as the medial forebrain bundle and attached by a cable to an ICSS stimulator located above the chamber. Panel B shows representative data collected using a “frequency-rate” ICSS procedure, in which brain-stimulation frequency is varied during daily behavioral sessions, and rates of responding are monitored during the availability of each frequency. Under baseline conditions, increasing brain-stimulation frequencies maintain increasing response rates, and drugs with abuse liability (e.g. amphetamine, in this case, N=6) typically produce leftward shifts in ICSS frequency-rate curves across some range of doses. Panel C shows that morphine failed to facilitate ICSS in 20 drug-naïve male Sprague-Dawley rats, and instead produced only dose-dependent ICSS depression. These rats were subsequently divided into three groups that received repeated daily treatment with saline (N=6), 1.0 mg/kg/day morphine (N=7), or 3.2 mg/kg/day morphine (N=7). Panel D shows that repeated treatment with 3.2 mg/kg/day morphine produced tolerance to ICSS depression and the emergence of abuse-related ICSS facilitation produced by a test dose of 3.2 mg/kg morphine. This was not observed in the other two groups. These results provide one source of evidence to suggest that pain states do not protect against this emergence of abuse-related ICSS facilitation during repeated morphine (see text). For each panel, the abscissa shows brain-stimulation frequency in log Hz, and the ordinate shows ICSS rate expressed as a percent of the Maximum Control Rate (%MCR), a normalized measure of reinforcement rate. All data show mean ± SEM, doses are in mg/kg, and filled points indicate significantly different from Vehicle (Veh) or Baseline (p<0.05). Data adapted from Bauer et al. 2013 and Miller et al. 2015.
3.2.1. Effects of opioids on ICSS
Under some conditions [e.g. in Fisher-344 rats;(Ewan and Martin, 2012, 2011a, 2011b)], morphine and other MOR agonists produce ICSS facilitation in opioid-naïve subjects. However, MOR agonists often produce a different profile of effects that is strongly influenced by the history of opioid exposure (Negus and Moerke, 2019; Reid, 1987). Specifically, morphine and other MOR agonists often fail to produce ICSS facilitation in opioid-naïve subjects and instead produce primarily only ICSS depression. However, repeated daily treatment with morphine can produce tolerance to ICSS depression and the emergence of ICSS facilitation that becomes more pronounced and occurs earlier in the time course of drug effects as opioid exposure increases (Altarifi et al., 2013; Legakis and Negus, 2018; Miller et al., 2015). For example, Figures 3C and D show data from male Sprague-Dawley rats with electrodes implanted in the medial forebrain bundle and compares the effects of morphine before and after treatment with 3.2 mg/kg/day subcutaneous (SC) morphine for seven days. Before repeated daily treatment, morphine primarily depressed ICSS (Figure 3C); however, following daily treatment, a dose of morphine that depressed ICSS in opioid-naïve animals (3.2 mg/kg, SC) produced facilitation and the leftward shift in the frequency-rate curve typical for drugs of abuse (Figure 3D). These data provide evidence that increases in opioid exposure can increase opioid reward.
3.2.2. Effects of pain manipulations on ICSS
ICSS can also be used to examine the behavioral effects of various pain-related manipulations. Clinically relevant pain states often involve depression of behavior and mood, and preclinical assays have been developed in rodents to assess the expression and treatment of pain-related depression of unconditioned behaviors, such as feeding (Kwilasz and Negus, 2012), wheel running (Kandasamy et al., 2016; Stevenson et al., 2011), and nesting (Negus et al., 2015). Positively reinforced operant behaviors such as ICSS, are also sensitive to depression by some pain manipulations. For example, intraperitoneal (IP) injection of dilute lactic acid can serve as an acute visceral pain stimulus to produce significant, transient (≤1 hr) rightward shifts in ICSS frequency-rate curves (Altarifi et al., 2015; Altarifi and Negus, 2015; Brust et al., 2016; Negus et al., 2010; Pereira Do Carmo et al., 2009). This type of rightward and downward shift in ICSS frequency-rate curves may reflect a combination of pain-related anhedonia (i.e., decreased sensitivity to normally reinforcing stimuli) and/or motor impairment. Moreover, this pain-related depression of ICSS is associated with depression of mesolimbic dopamine release, a neurochemical correlate of anhedonia, and motor impairment (Leitl et al., 2014). Pain-related depression of both ICSS and mesolimbic dopamine release can be blocked by clinically effective analgesics (Leitl et al., 2014).
Relative to the transient ICSS depression produced by IP acid injection, inflammatory pain manipulations such as intraplantar (IPL) injection of CFA and surgical paw incision can produce more sustained ICSS depression for periods of hours (for CFA) to days (for paw incision) (Ewan and Martin, 2014; Leitl et al., 2014; Leitl and Negus, 2016). However, neuropathic pain manipulations generally have little effect on ICSS. For example, L5/L6 spinal nerve ligation (SNL) is a model of neuropathic pain that can produce sustained hypersensitivity of hind paw-withdrawal responses to tactile stimuli for weeks to months, but SNL did not depress ICSS in rats (Ewan and Martin, 2014, 2012, 2011a). Similarly, IPL formalin injection and repeated IP paclitaxel treatment are widely-used models of chemically induced neuropathic pain sufficient to produce mechanical hypersensitivity in rats for weeks to months, but these treatments also failed to reliably depress ICSS (Legakis et al., 2018; Legakis and Negus, 2018; Selley et al., 2020).
3.2.3. Interactions Between Pain Manipulations and Opioids on ICSS
Several studies have examined interactions between putative pain states and opioid effects on ICSS, and in general, these studies have found that pain states either do not alter or decrease opioid-induced ICSS facilitation. No studies have found that pain states increase opioid reward in ICSS procedures. As a result, these ICSS studies suggest that pain states do not increase opioid reward, and may decrease it.
An important variable in studies of pain-opioid interactions is the temporal relationship between exposure to pain and opioid stimuli. Opioid reward can be examined during and/or after a pain manipulation and compared either to opioid reward in separate subjects not exposed to the pain manipulation (a between-subjects comparison) or to opioid reward in the same subjects determined before the pain manipulation (a within-subjects comparison). Clinical exposure to opioids often occurs in the context of treatment for an acute pain episode (e.g. post-surgical pain) in patients who are initially opioid naïve, and as a result, one clinical concern is the degree to which initial exposure to opioids for pain relief might alter later vulnerability to opioid reward when the pain state has resolved. With this in mind, one advantage of ICSS as compared to drug self-administration procedures for examining abuse-related effects of opioids is that the expression of opioid reward can be monitored during the earliest stages of opioid exposure in opioid-naïve subjects with or without pain states. For example, one study evaluated effects produced by daily treatment of male Sprague-Dawley rats with morphine during and after repeated treatment with either IP saline or IP lactic acid as an acute visceral noxious stimulus (Miller et al., 2015). Repeated morphine administration resulted in the emergence of abuse-related ICSS facilitation regardless of whether it was given during and after repeated daily injection of IP acid or acid vehicle; thus, pairing daily morphine with a daily acute pain stimulus failed to protect against the emergence of morphine-induced ICSS facilitation. A related study compared the effects of repeated daily administration of morphine on ICSS in rats treated previously with either saline or paclitaxel in a model of chemotherapy-induced neuropathic pain (Legakis and Negus, 2018). Once again, repeated morphine injections in the vehicle-treated rats resulted in the emergence of abuse-related ICSS facilitation. The paclitaxel treatment regimen (4 total injections of 2.0 mg/kg IP administered every other day for 7 days) was sufficient to produce mechanical hypersensitivity for several weeks (assessed using von Frey filaments to determine threshold mechanical force to elicit paw withdrawal), and this mechanical hypersensitivity was alleviated by daily morphine injections; however, the paclitaxel-induced pain state did not alter baseline ICSS and did not protect against the emergence of morphine-induced ICSS facilitation during repeated morphine treatment. Thus, once again, a putative pain state failed to prevent the emergence of morphine-induced ICSS facilitation.
In contrast to the results described above, which used models of acute pain and chemotherapy-induced neuropathic pain in Sprague-Dawley rats, a different pattern of results has been described in studies using the SNL model of neuropathic pain in male Fischer 344 rats (Ewan and Martin, 2011a, 2011b). In these studies, morphine and other MOR agonists produced dose-dependent ICSS facilitation in opioid-naïve subjects. As with the paclitaxel model of neuropathy described above, subsequent nerve ligation produced sustained mechanical hypersensitivity without altering baseline ICSS responding. However, this SNL model of neuropathy blocked morphine-induced ICSS facilitation, and it attenuated ICSS facilitation produced by the other MOR agonists heroin, methadone, fentanyl, and hydromorphone. Given the acute administration regimen of the MOR agonists, this study did not evaluate the degree to which the SNL model of neuropathic pain might attenuate the trajectory of increased MOR agonist reward with repeated MOR agonist treatment. Nonetheless, these findings suggest a pain-related attenuation of morphine reward.
3.2.4. Summary
MOR agonists produce rewarding effects in ICSS procedures, and these rewarding effects can increase in magnitude with repeated opioid exposure. Pain models either do not alter or decrease the rewarding effects of opioids in ICSS procedures. To date, no ICSS studies have suggested that pain states increase opioid reward. Additionally, ICSS studies suggest that repeated opioid exposure can increase the expression of opioid reward regardless of whether that exposure occurs in the context of a pain state or not.
3.3. Place Conditioning
Place conditioning is a classical conditioning procedure that involves the pairing of an unconditioned stimulus with a conditioned stimulus to produce a learned conditioned response (Bardo and Bevins, 2000; Tzschentke, 2007, 1998). These procedures typically rely on experimental chambers that consist of at least two separate but equally sized compartments distinguished by different environmental stimuli (e.g. different wall colors and floor textures). The experimental design consists of three phases. During the first phase (pre-conditioning baseline), a door separating the compartments is open, subjects are allowed to explore the entire chamber for a specified period of time (e.g. 15 min), and baseline preference for the two chambers is determined. During the second phase (conditioning), the door separating the compartment is closed, and subjects are confined on some occasions to one compartment after treatment with a specified dose of test drug and on other occasions to the other compartment after treatment with vehicle. During this phase, the drug serves as the unconditioned stimulus, and the environmental stimuli of the drug-paired compartment serve as a constellation of stimuli that may become associated with drug effects and come to function as a conditioned stimulus. During the third phase (post-conditioning test), the door separating the compartments is again opened, and subjects can again explore the entire chamber as during Phase 1. If preference increases for the drug-paired compartment, then the drug is considered to produce a conditioned place preference (CPP) as a conditioned response, and this CPP is interpreted as a rewarding effect predictive of abuse potential. Alternatively, low abuse potential is predicted if a drug does not alter preference or produces a conditioned place aversion (CPA, a decrease in preference for the drug-paired compartment).
3.3.1. Effects of Opioids on Place Conditioning
MOR agonists generally produce dose-dependent CPP in the absence of a pain state. As one example, Shippenberg et al. (1988) evaluated the place-conditioning effects of morphine (0.3–5.0 mg/kg, SC) in male Sprague Dawley rats. Each dose was tested in a different group of rats, and morphine doses of 3.0 and 5.0 mg/kg produced CPP, whereas lower doses of 0.3 and 1.0 mg/kg did not. One important variable in place-conditioning procedures is the number of conditioning sessions, and opioid potency tends to decrease as the number of conditioning sessions decreases. For example, in the Shippenberg et al. 1988 study cited above, morphine- and vehicle-conditioning sessions alternated daily for six days such that there were three conditioning sessions with morphine and three with saline before testing on the seventh day. For comparison, Nazarian and colleagues (Armendariz and Nazarian, 2018; Harton et al., 2017) established morphine CPP in male (and female) Sprague Dawley rats using only one morphine and one vehicle conditioning session on sequential days; however, morphine CPP was observed only at 8 mg/kg SC and not at lower doses of 1 or 4 mg/kg.
3.3.2. Effects of pain manipulations on place conditioning
Pain manipulations have been evaluated in two ways using place conditioning procedures. First, relatively rapidly acting and transient pain states lasting on the order of minutes to hours can be used as unconditioned stimuli that are paired with one compartment of the place-conditioning chamber to produce a CPA. For example, Bagdas et al. (Bagdas et al., 2016) tested a range of IP lactic acid concentrations as a transient noxious stimulus to produce a CPA in mice. For these studies, mice received a specified concentration of IP acid for a single conditioning session in the acid-paired compartment and IP vehicle for a single conditioning session in the other compartment. Testing revealed an acid concentration-dependent CPA.
More sustained pain states produced by many inflammatory and neuropathic pain models are not amenable to CPA experimental designs because the slow onset and long duration of the pain state (days to weeks) exceeds the length of a typical conditioning session (minutes to hours). As a result, it is challenging to effectively pair a chronic pain state as an unconditioned stimulus with the environmental stimuli of a single compartment during a conditioning session. Instead, place conditioning studies with chronic pain models use a different experimental design that will be described in more detail below.
3.3.3. Interactions between pain manipulations and opioids on place conditioning
The key issue for this review is whether pain states modify opioid-induced CPP. Relatively transient pain models either do not alter or decrease morphine potency to produce CPP. For example, 10 mg/kg SC morphine was the minimum dose to produce CPP in adult male ICR mice using a single-conditioning session, and co-administration of IP acid as an acute noxious stimulus did not alter this morphine-induced CPP (Bagdas et al., 2016). Similarly, 10 mg/kg morphine IP produced a CPP in male Sprague-Dawley rats, and IPL carrageenan as a transient inflammatory stimulus did not affect this CPP, although it may have attenuated CPP produced by lower morphine doses (van der Kam et al., 2008). In neither study did the pain model increase morphine CPP.
In studies with more chronic pain models, a pain manipulation or its control is implemented before any conditioning, and the putative pain state is presumed to be present throughout conditioning and testing. Under these conditions, the pain state does not become associated with either chamber; rather, the goal is to pair drug as the unconditioned stimulus with one of the two chambers and evaluate the degree to which a putative pain state modifies drug-induced place conditioning (Negus, 2019). Drug-induced CPP under these conditions is interpreted as a summation of both (1) any positive rewarding effects the drug may produce independent of the pain state (as indicated by CPP in a separate group of controls without the pain manipulation) and (2) negative rewarding effects associated with relief of the underlying pain state. Opioid effects under these conditions have been inconsistent. Most studies have found either no change or a decrease in MOR agonist CPP in the context of various chronic pain models (Armendariz and Nazarian, 2018; Betourne et al., 2008; Neelakantan et al., 2016; Niikura et al., 2008; Ozaki et al., 2002; Petraschka et al., 2007; Shippenberg et al., 1988; Sufka, 1994; Suzuki et al., 1996). However, some studies have reported enhancement in either the potency or magnitude of opioid CPP (Armendariz and Nazarian, 2018; Cahill et al., 2013; Navratilova et al., 2015; Nwaneshiudu et al., 2020; Woller et al., 2012; Zhang et al., 2014). As one example of the varied effects of chronic pain models on opioid CPP, Armendariz and Nazarian (2018) determined morphine CPP dose-effect curves in male and female Sprague Dawley rats beginning 1 day or 7 days after IPL CFA or IPL vehicle. In studies beginning one day after IPL CFA, morphine potency to produce CPP was not changed in males and was decreased in females relative to effects in rats treated with IPL vehicle; however, in studies beginning 7 days after IPL CFA, morphine potency to produce CPP was increased in both sexes. This contrasts with results from an earlier study that found no change in the morphine CPP dose-effect curve in male Sprague Dawley rats when conditioning began seven days after IPL CFA (Shippenberg et al., 1988). The critical factors that influence pain-model effects on opioid-induced CPP remain to be determined.
3.3.4. Summary
MOR agonists produce rewarding effects in place-conditioning procedures, consistent with their abuse potential. Relatively transient pain models either do not alter or decrease opioid reward in these procedures. The effects of more chronic pain states have been inconsistent; no changes, decreases, and increases in morphine CPP have been observed in the context of different chronic pain models, and no clear factors have been identified that determine which type of effect is observed.
4. Opioid availability, substance abuse, and pain
Given the weak evidence for pain-related increases in opioid reinforcement and reward, other factors warrant consideration as mediators of the relatively high rates of OUD in pain patients. One obvious potential mediator of higher opioid use by pain patients is higher access to and use of opioids due to medical prescriptions. The distribution of opioid analgesics is regulated by agencies such as the Drug Enforcement Administration in the United States, and licit use requires a prescription. Opioid prescription rates rose steadily in the United States during the early 2000s and peaked in 2012 at approximately 255 million total prescriptions (81.3 prescriptions per 100 persons) before declining by 2018 to approximately 168 million prescriptions (51.4 per 100 persons) (Centers for Disease Control and Prevention; https://www.cdc.gov/drugoverdose/maps/rxrate-maps.html). From the perspective of the pain patient, opioid prescriptions increase opioid availability, provide explicit guidance on opioid use, and specify the occasions for opioid consumption. Of course, pain treatment is the legitimate clinical rationale for this opioid use; however, given that opioid analgesics produce reinforcing effects regardless of clinical intent, any opioid use initially reinforced by adherence to clinical instructions also provides opportunities for the user to experience reinforcement by opioid effects on brain reward systems. Ultimately, drug-taking behavior may come to be maintained by opioid reinforcement independent of clinical instructions or therapeutic rationale.
An additional complication is that, while opioid reinforcement does not appear to be increased by pain states, it can be increased by even modest periods of opioid exposure. In opioid-naïve animals or humans, the reinforcing effects of opioids that increase the probability of drug-taking behavior may be countered and constrained by other opioid effects (e.g. sedation or negative subjective effects) that decrease the probability of drug-taking behavior. However, tolerance can develop relatively quickly to these opposing effects to allow increased impact of opioid reinforcement and reward. One example of preclinical evidence for this phenomenon was described above in the section on ICSS (Legakis and Negus, 2018; Miller et al., 2015). In opioid naïve rats, MOR agonists like morphine often fail to produce abuse-related ICSS facilitation interpreted as reward, and instead produce dose-dependent ICSS depression suggestive of sedation and/or aversive effects. However, repeated daily treatment for as few as six days can produce tolerance to the ICSS rate-decreasing effects of opioids and increase expression of ICSS facilitation. Moreover, this trajectory of increased ICSS facilitation over time with repeated daily opioid exposure is not altered by the presence of a pain state. Repeated opioid exposure can also promote escalation of opioid self-administration (Deneau et al., 1969; Walker et al., 2003; Zernig et al., 2007), enhance expression of opioid-induced CPP (Lett, 1989), and increase reward-related opioid effects on mesolimbic dopamine signaling (Lefevre et al., 2020). Moreover, high levels of opioid consumption can produce physical dependence and introduce opioid withdrawal as a negative reinforcer that further supports continuing opioid use (Negus and Banks, 2018; Shurman et al., 2010). Taken together, these findings suggest the prescription of opioid analgesics to treat pain may promote opioid use not only providing opportunities for opioid reinforcement, but also by producing a pattern of opioid exposure that enhances subsequent opioid reinforcement.
5. Reduced access to non-opioid reinforcers/rewards
Human behavior occurs in environments that contain not only opioids, but also other types of reinforcers such as food, social interaction, and money. Individuals allocate their behavior between the acquisition of opioid and non-opioid reinforcers, and OUD is defined in part by excessive behavioral allocation to opioid use at the expense of more adaptive behaviors maintained by non-drug reinforcers (Banks and Negus, 2017). Both laboratory-animal and human studies have shown convincingly that drug-taking behaviors can be reduced by the introduction of alternative non-opioid reinforcers, or reciprocally, be enhanced by the removal of alternative reinforcers (Banks and Negus, 2012; Carroll, 1993; Higgins, 1997). These findings suggest another plausible mechanism by which pain states could enhance opioid use and increase vulnerability to OUD. Specifically, a common sign of clinically relevant pain is functional impairment, such that pain patients may have difficulty walking, working inside or outside the home, or engaging in other normal activities maintained by non-opioid reinforcers (Dworkin et al., 2005; Jensen et al., 2007). Consistent with this clinical observation, preclinical studies have also reported that some experimental pain states can decrease operant responding maintained by a range of nondrug reinforcers including food (Cone et al., 2018; Martin et al., 2004), electrical brain stimulation (as described for ICSS studies in Section 3.2.2 above), and escape from aversive light (Pahng et al., 2017). Preclinical pain models can also decrease many types of unconditioned behaviors including wheel running, exploration of an open field, nesting/burrowing, and feeding (Negus, 2019; Tappe-Theodor et al., 2019). This pain-related functional impairment can reduce access to alternative reinforcers and thereby reduce the degree to which behaviors maintained by these alternatives can compete with opioid-taking behaviors. Moreover, if functional impairment occurs in tandem with an opioid prescription that increases opioid availability, then behavioral allocation may be further biased toward opioid consumption. One example of this phenomenon could be the relatively high risk of opioid use and misuse by young men engaged in competitive sports, for whom injury may simultaneously decrease their ability to participate in a favored activity while also increasing opioid availability by triggering an opioid prescription (Cottler et al., 2011; Veliz et al., 2014).
Choice procedures in either the animal or human laboratory offer one strategy to examine the impact of pain states on behavioral allocation between opioid vs. non-opioid reinforcers. In these procedures, subjects are trained to choose between an opioid dose or a non-opioid reinforcer such as food (in laboratory animals) or money (in humans), and the effect of experimental manipulations on drug-vs.-nondrug choice can be examined (Banks and Negus, 2012; Jones and Comer, 2013). It is well-established that choice of opioids or other drugs increases when either (a) access to the drug is enhanced (e.g. when large doses are available or the cost of the drug is low) or (b) when access to the alternative non-drug reinforcer is reduced (e.g. by reducing magnitude of the alternative or increasing the cost to obtain it). Opioid choice also increases when opioid-dependent subjects are in a state of withdrawal (Negus and Banks, 2018; Townsend et al., 2019). The impact of a pain state on opioid choice has been examined in one human-laboratory study, which compared the effects of an experimental pain manipulation and a control manipulation (hand immersion in ice water or body-temperature water, respectively) on oxycodone vs. money choice in two groups of subjects: one group that abused prescription opioids, and a second group that had used prescription opioids medically but did not abuse them (Comer et al., 2010). Relative to the control manipulation, the pain manipulation increased opioid choice and reduced money choice in the non-abusers, whereas the abusers choose the opioid under both conditions. These findings support the potential of pain to reduce behavior maintained by a non-opioid reinforcer and produce a reciprocal increase in opioid choice. Studies to examine effects of pain manipulations on opioid-vs.-food choice in rats are underway (unpublished observations, Reiner DJ, Townsend EA, Menendez JO, Applebey SV, Banks ML, Shaham Y, and Negus SS).
6. Cognitive deficits at the intersection of pain and opioid abuse
Impairments in cognitive function represent a cluster of features that are common among individuals suffering from chronic pain conditions, as well as those with OUD. Although a direct causal link in cognitive issues between pain and OUD is difficult to draw, numerous studies have examined impaired cognition in chronic pain patients or people with OUD. Examining cognitive deficits as an intersection between pain and substance abuse is critical due to the role of cognitive dysfunction in compulsive drug-taking and the negative impact of cognitive deficits on substance abuse treatment outcomes and dropout rates (Aharonovich et al., 2006, 2003; Severtson et al., 2010; Walvoort et al., 2012; Yücel and Lubman, 2007). The section below presents a brief examination of clinical and preclinical findings regarding the impact of pain and opioids on cognitive function and the potential cross-section between these two related conditions. This section will also consider preclinical models that can help bridge the gap in understanding the effects and consequences of impaired cognition on pain and opioid abuse. It is noteworthy that findings in the literature demonstrate evidence for deficits in many aspects of cognitive function in people with chronic pain, and those with OUD, which are considered in other reviews (Huysmans et al., 2020; Moriarty et al., 2011; Moriarty and Finn, 2014; Pask et al., 2020). This section will provide an overview of the impact of chronic pain and OUDs on deficits in attention, cognitive flexibility, and decision making, three cognitive functions that are closely linked to substance use and abuse.
6.1. Cognitive deficits in chronic pain patients
People with chronic pain conditions such as musculoskeletal pain, neuropathic pain, or fibromyalgia often complain of cognitive impairments, including impairments in attention, cognitive flexibility, and decision making (Moriarty et al., 2011; Moriarty and Finn, 2014). Chronic pain patients seem to be particularly deficient in complex cognitive tasks such as attentional switching, attentional interference, dual-task performance (i.e., multi-tasking), and decision-making tasks (Dick et al., 2002; Moore et al., 2017, 2012; Moriarty et al., 2011). Attention is an essential part of cognitive functioning that can impact other cognitive processes, including executive function (Antshel et al., 2010; Genova et al., 2013; Strauss et al., 2006). Thus, examining attentional deficits and their association with painful conditions can play a critical role in better understanding the impact of chronic pain conditions on cognitive function. In a hallmark study, Eccleston (1995) showed that patients experiencing high-intensity musculoskeletal pain (primarily low back and limb pain) had notable deficits in attentionally demanding tasks as compared to those with low intensity or no pain (Eccleston, 1995). High-intensity pain patients also had deficits in switching attention from one task to another, indicative of deficiencies in attentional demands and attentional switching. Similar findings have since been reported in patients with neuropathic pain or fibromyalgia (Landrø et al., 2013; Lee et al., 2010). Experimentally induced pain studies in healthy volunteers have also demonstrated that application of a thermal pain stimulus lowered performance on attention span and attentional switching tasks (Keogh et al., 2013; Moore et al., 2017). Optimal performance on many attention tasks requires high cognitive load, which is limited in patients with chronic pain conditions. Indeed, cognitive deficits are, at least in part, due to the redirection of cognitive processes to attend to pain at the expense of other cognitive demands (Eccleston and Crombez, 1999; Grisart and Van der Linden, 2001; Keogh et al., 2013). Aside from attentional tasks, pain-associated cognitive deficits have been reported in executive processing, such as cognitive flexibility and decision making. People with fibromyalgia, lower back, or osteoarthritis pain perform poorly in tasks of cognitive flexibly such as the Wisconsin Card Sorting Task and the Trail Making Test (Karp et al., 2006; Verdejo-García et al., 2009; Weiner et al., 2006). Patients with chronic back pain or complex regional pain syndrome also exhibit deficits in executive function by showing an increase in emotional decision making in the Iowa Gambling Task (Apkarian et al., 2004; Verdejo-García et al., 2009; Walteros et al., 2011). Impaired decision making is also reflected in measures of impulsivity, where patients with chronic pain exhibit an increase in impulsive choice in a Delay Discounting Task (DDT). In this model, chronic pain patients chose to receive an immediate reward of lesser value rather than receiving a larger reward after a period of delay (Tompkins et al., 2016).
6.2. Cognitive deficits in rodent pain models
Rodents with inflammatory or neuropathic pain manipulations exhibit cognitive deficits that are similar to those observed in humans. Impairments in attention have been found in rats experiencing various types of pain manipulations. More recent studies have expanded on these findings by demonstrating that rats with incisional, inflammatory, or neuropathic pain manipulations have deficits in visuospatial attention as measured in the 5-Choice Serial Reaction Time Task (5-CSRTT) (Boada et al., 2020; Higgins et al., 2015; Moazen et al., 2020; Pais-Vieira et al., 2009; Ririe et al., 2018). The 5-CSRTT is an operant conditioning model that requires rats to correctly select one of five apertures to nose-poke upon the brief presentation of a light cue to receive a food reward. The animal is required to be attentive to the light cue presentation in order to select the correct aperture. Incorrect selection of the aperture is interpreted as a measure of inattention. Martin and colleagues (2017) used the 5-CSRTT to demonstrate increasingly impaired attention as a function of increasing pain intensity (Martin et al., 2017). Rats administered IP lactic acid, an acute noxious stimulus of high intensity, required increasingly higher light cue duration to optimally perform as acid concentrations increased. These attentional deficits were reversed by morphine treatment. Similar findings were reported in the visual-signal detection task, another measure of attention, where rats given IP lactic acid exhibited a decrease in response accuracy, indicative of inattention (Freitas et al., 2015). Interestingly, in the same study, rats receiving IPL formalin or CFA, models of inflammatory pain with lower spontaneous pain intensities at the time points tested, had higher response accuracies. These findings are consistent with human experimental pain studies and chronic pain patients that pain stimuli of high intensity produce more cognitive deficits than those of lower intensity, suggesting that higher intensity stimuli may require greater cognitive load at the expense of attentional task performance. Other executive processing tasks related to decision making are also negatively impacted by painful manipulations. For example, in an operant gambling task adapted from the Iowa Gambling Task, rats with kaolin-carrageenan or CFA inflammatory pain manipulations often made decisions considered as more risky, resulting in either large but infrequent rewards or punishment, interpreted as an enhancement in emotional decision making (Ji et al., 2010; Pais-Vieira et al., 2009).
Cognitive flexibility and impulsivity are constructs that have also received recent attention in preclinical studies. Rodents with inflammatory or neuropathic pain manipulations display a decrease in cognitive flexibility and an increase in impulsivity. Findings suggest that rats with SNL exhibit a less profitable behavioral strategy in an operant variable-ratio probabilistic task and were resistant to improving their strategy to a more advantageous method, indicative of impaired cognitive flexibility (Cowen et al., 2018). A study in mice with spared nerve injury to model neuropathic pain showed reduced cognitive flexibility using a rule-shifting task, in which rules and cues for determining which arm of a maze contains a food reward are switched. Mice with a neuropathic pain manipulation required nearly twice as many trials to acquire the new rules to reach the food reward (Shiers et al., 2020). Lastly, changes in impulsivity have recently been reported in rats using a DDT. In the preclinical model of the DDT, rats must choose to press one of two levers, one that will deliver a small but immediate food reward, while the other lever will deliver a larger food reward after a delay. The selection of an immediate but small reward over a larger reward after a delay is interpreted as an impulsive choice. Rats with IPL CFA treatment exhibited an increase in impulsivity, which was gradually worsened over two weeks after CFA treatment (Saputra et al., 2019). The enhanced impulsivity was blocked by morphine administration.
6.3. Cognitive deficits in opioid abuse
Changes in cognitive function can promote compulsive drug taking and difficulty in discontinuing drug use, leading to relapse that is often trigged by drug-associated cues. Demonstration of preexisting cognitive deficits and their direct impact on the development of substance use disorders is primarily found when studying impulsivity. The present consensus is based on preclinical studies showing that trait impulsivity is predictive of increased acquisition of self-administration of drugs such as alcohol, cocaine, or methylphenidate (Cervantes et al., 2013; Dalley et al., 2007; Marusich and Bardo, 2009; Poulos et al., 1995). Clinical studies have demonstrated strong associations between impulsivity and substance abuse disorders, a topic that is thoroughly discussed in this special issue by Verdejo-Garcia and Albein-Urios (Verdejo-Garcia and Albein-Urios, 2021). There is currently a knowledge gap in the predictive effects of impulsivity or other cognitive deficits on the development of OUD. There is greater evidence demonstrating the impact of opioids on cognitive deficits; in fact, cognitive deficits have been extensively reported in people with OUD.
The reported decisions, behaviors, and consequences in people with OUD are a result of aberrant learning and lack of inhibitory control, especially in vulnerable individuals (Everitt et al., 1999; Hyman, 2005; Jentsch and Taylor, 1999; Torregrossa et al., 2011). People with OUD have difficulty switching attentional demands, as demonstrated by having an attentional bias towards cues associated with or predictive of an opioid drug, using the Stroop task (Franken et al., 2000; Marissen et al., 2006; Nejati et al., 2011; Wang et al., 2014). Moreover, people with OUD have deficits in behavioral inhibition, problem-solving, and planning, all of which are essential aspects of cognitive flexibility and decision making. For example, heroin addicts perform poorly on the Tower of London test of planning (Davydov and Polunina, 2004; Ornstein et al., 2000). Similar deficits in planning and problem solving have been reported in opioid-dependent individuals on methadone or buprenorphine therapy, as well as individuals that are opioid abstinent (Ersche et al., 2006). Other studies have demonstrated deficits in cognitive flexibility in people with OUD. Both opioid dependent individuals on methadone maintenance and former opioid dependent individuals that are presently opioid abstinent performed poorly on the Wisconsin Card Sorting and the Trail Making Tasks (Faustino et al., 2019; Lyvers and Yakimoff, 2003; Saroj et al., 2020). Likewise, numerous studies have demonstrated deficits in decision making in individuals with OUD. For example, short- and long-term abstinent heroin addicts performed poorly on the Iowa Gambling Task and a DDT (Bickel and Marsch, 2001; Kirby et al., 1999; Kirby and Petry, 2004; Li et al., 2013). Heroin addicts and opioid-dependent individuals on methadone maintenance therapy exhibited higher motor impulsivity in a Go/NoGo task and made more risky decisions in the Cambridge Gambling Task (Baldacchino et al., 2015; Lee and Pau, 2002).
6.4. Cognitive deficits in pain patients using opioids
Chronic pain and opioids are both known to impair cognitive function. Therefore, it becomes imperative to determine whether opioid analgesics exacerbate or relieve cognitive deficits in chronic pain patients. Findings are mixed with some studies showing that cognitive deficits are improved upon opioid treatment in chronic pain patients, while others show no change or worsening of cognitive deficits. In a clinical study, chronic pain patients were treated with long- or short-acting opioids, and their cognitive flexibility and attention were assessed (Haythornthwaite et al., 1998). Opioid treatment overall did not affect cognitive flexibility, while it improved attention, suggesting the opioid effects on cognition are likely due to the relief of pain. In another study, lower back pain patients were treated with oxycodone or transdermal fentanyl and showed an improvement in their cognitive flexibility as measured by the Trail Making Test (Jamison et al., 2003). By using a DDT, Morasco and colleagues (2019) examined decision making and impulsivity in musculoskeletal pain patients treated with low or high doses of morphine or control. The authors found that morphine dose was inversely related to impulsivity, with the high-dose morphine group showing improvement in impulsivity and decision making (Morasco et al., 2019).
Contrary to the studies cited above, other studies have found no improvement in cognitive flexibility in chronic pain patients given long-term morphine therapy (Tassain et al., 2003). Furthermore, detrimental effects of opioids on cognitive function in chronic pain patients have also been reported. Opioid therapy worsened cognitive flexibility in a group of pain patients with mixed pain etiologies (Kurita et al., 2012). The differences in findings are likely due to study parameters, study design, specific population examined, cognitive assessment methods, and the lack of healthy control group to determine baseline cognitive function. To address these issues, Kurita and colleagues (2015) performed a controlled study to examine cognitive deficits in healthy volunteers that underwent three experiments (Kurita et al., 2015). In the first experiment, healthy volunteers were exposed to an experimental ischemic-pain manipulation (pneumatic tourniquet cuff). In the second experiment, the volunteers were given remifentanil treatment, and in the third experiment, they were exposed to the cuff and remifentanil. The study found that cuffing or remifentanil alone reduced cognitive function, such as attention and cognitive flexibility. The combination of pain and remifentanil further magnified the cognitive impairment, suggesting that pain and opioid treatment seemed to have additive effects on cognitive impairment. These findings further complicate the understanding of the degree to which chronic pain might influence the contribution of cognitive impairment to opioid abuse. To shed light, a recent study utilized a DDT to demonstrate that chronic pain patients considered at high risk of opioid misuse discount future vs. immediate rewards and favor immediate short-term pain relief vs. delayed long-term pain relief (Tompkins et al., 2016). Greater desire to avoid immediate pain can lead a person to use opioids more frequently. This view is further supported by findings showing that negative emotional states enhance impulsivity, which can be a predictor of substance use and dependence (Verdejo-García et al., 2007).
6.5. Summary
At present, the impact of chronic pain and opioids on cognitive function is not fully understood. Clinical and human experimental studies suggest that pain may produce numerous cognitive deficits. Likewise, people with OUD at different stages (active use, methadone or buprenorphine maintenance therapy, or opioid abstinence) have deficits in cognitive function. These findings are further corroborated by preclinical studies in rodents showing that pain manipulations modeling inflammatory or neuropathic pain result in cognitive deficits. When considering the combination of chronic pain and chronic opioid use together in human studies, the findings are mixed and inconclusive. Such inconsistencies are in part due to the limited number of studies and methodological differences that make it difficult to reach a consensus, although, more consistent findings are found in preclinical models. Thus, a greater understanding of the interaction of pain and opioids on cognitive function may be achieved using preclinical rodent models of cognitive function, pain models, and opioid system modulations to generate testable hypotheses for later translational testing in humans.
7. Conclusions and future directions
Pain is a risk factor for OUD, and this review has considered four possible mediators of pain-related increases in OUD prevalence: (1) increased reinforcing effects of opioids, (2) increased access and exposure to opioids, (3) decreased access to alternative non-opioid reinforcers, and (4) cognitive impairment. The results are summarized in Figure 4. Prevailing evidence argues against a role for increased opioid reinforcement. The potential impact of pain states on opioid reward and reinforcement has been extensively evaluated in preclinical studies using procedures of drug self-administration, ICSS, and place conditioning, and this body of literature indicates that experimental pain states in laboratory animals usually either do not alter or decrease metrics of opioid reinforcement and reward. However, pain states can produce both an increase in access and exposure to opioids and a decrease in access to non-opioid alternative reinforcers, and together, these two effects can promote a maladaptive transition in behavioral allocation toward opioid use and away from behaviors reinforced by healthy alternatives. Moreover, pain states can produce cognitive impairments, such as inattention and impulsivity, that further enhance the risk of poor decisions, including the decision to use drugs of abuse. Overall, our review suggests that pain-related increases in OUD prevalence result primarily from increased access and exposure to opioids, decreased access to non-opioid alternative reinforcers, and cognitive impairments that hinder adaptive decision-making.
Figure 4. Pain-related risk factors and their contribution to opioid use and abuse.

Pain is a risk factor for OUD, and this review has considered four possible mediators of pain-related increases in OUD prevalence: (1) increased reinforcing effects of opioids, (2) increased access and exposure to opioids, (3) decreased access to alternative non-opioid reinforcers, and (4) cognitive impairment. The size of the arrows in the figure indicate the apparent contribution of the associated risk factor for OUD. (1) Pain does not appear to increase opioid reinforcement or reward, suggesting little role for this as a mediator. (2) Pain can trigger opioid prescriptions that increase opioid availability and exposure and do increase risk of OUD. (3) Pain also leads to reduced access to non-opioid reinforcers and rewarding experiences, including fewer social interactions and limited mobility. Loss of non-opioid reinforcers can lead to allocation of behavior away from the lost reinforcers and toward opioid use. (4) Lastly, pain can impair cognition and executive processing functions such as attention and decision making. Pain-induced cognitive deficits can lead pain patients to have poor judgement and further promote choice to use and abuse opioids.
Additional preclinical and clinical research would be helpful to further clarify the root causes of pain-related risk for OUD, but evidence summarized in this review is consistent with at least three steps that could be taken to mitigate OUD risk in pain patients. First and most obviously, OUD risk in pain patients can be reduced by using non-opioid treatments when possible and minimizing opioid use for pain treatment when opioids are considered necessary. Opioid use can be minimized not only by limiting the doses and duration of treatment (Dowell et al., 2016), but also by using safer low-efficacy opioids like buprenorphine (Cowan et al., 1977; Khanna and Pillarisetti, 2015), abuse-deterrent opioid formulations (Litman et al., 2018), or intrathecal methods of opioid delivery that minimize drug distribution to brain reward areas (Bolash and Mekhail, 2014; Deer et al., 2017). Second, these steps to reduce access and exposure to opioids in pain patients can be complemented by steps to mitigate the impact of lost access to alternative non-opioid reinforcers. For example, the introduction of virtual reality and video games can provide non-opioid reinforcement for children in pain (Das et al., 2005; Gates et al., 2020; Scapin et al., 2018; Trost et al., 2015), and one recent study found that access to video games decreased both subjective pain scores and opioid use by children with chemotherapy-induced pain (Alonso Puig et al., 2020). Lastly, OUD risk in pain patients may be mitigated by strategies to protect against pain-related cognitive and functional impairment. Methods to improve functioning in chronic pain patients include both cognitive-training approaches such as cognitive behavioral therapy, cognitive functional therapy, and episodic future thinking therapy (Craft et al., 2020; Urits et al., 2019), as well as pharmacotherapeutic options such as psychostimulants.
Highlights.
Pain-related risk factors can help explain increased vulnerability to opioid abuse ·
Pain does not appear to directly increase opioid reinforcement or reward
Pain increases access to opioids and decreases access to non-opioid reinforcers
Pain-induced cognitive deficits may also increase risk of opioid abuse
Understanding these factors can guide development of risk-mitigation strategies
Acknowledgements:
This work was made possible with funds provided by National Institutes of Health grants R01DA030404 (SSN), P30DA033934 (SSN), P01GM113852 (TJM), R01DA048490 (TJM), and funds provided by Western University of Health Sciences. All three authors, AN, SSN and TJM significantly contributed to the writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None
References
- Adan A, Antúnez JM, Navarro JF, 2017. Coping strategies related to treatment in substance use disorder patients with and without comorbid depression. Psychiatry Res 251, 325–332. 10.1016/j.psychres.2017.02.035 [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV, 2006. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 81, 313–322. 10.1016/j.drugalcdep.2005.08.003 [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D, 2003. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 71, 207–11. 10.1016/s0376-8716(03)00092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso Puig M, Alonso-Prieto M, Miró J, Torres-Luna R, Plaza López de Sabando D, Reinoso-Barbero F, 2020. The Association Between Pain Relief Using Video Games and an Increase in Vagal Tone in Children With Cancer: Analytic Observational Study With a Quasi-Experimental Pre/Posttest Methodology. J. Med. Internet Res 22, e16013. 10.2196/16013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Negus SS, 2015. Differential tolerance to morphine antinociception in assays of pain-stimulated vs. pain-depressed behavior in rats. Eur. J. Pharmacol 748, 76–82. 10.1016/j.ejphar.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS, 2015. Effects of mu-opioid receptor agonists in assays of acute pain-stimulated and pain-depressed behavior in male rats: role of mu-agonist efficacy and noxious stimulus intensity. J. Pharmacol. Exp. Ther 352, 208–217. 10.1124/jpet.114.219873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS, 2013. Abuse-related effects of micro-opioid analgesics in an assay of intracranial self-stimulation in rats: modulation by chronic morphine exposure. Behav Pharmacol 24, 459–470. 10.1097/FBP.0b013e328364c0bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antshel KM, Faraone SV, Maglione K, Doyle AE, Fried R, Seidman LJ, Biederman J, 2010. Executive functioning in high-IQ adults with ADHD. Psychol. Med 40, 1909–1918. 10.1017/S0033291709992273 [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR, 2004. Chronic pain patients are impaired on an emotional decision-making task. Pain 108, 129–136. 10.1016/j.pain.2003.12.015 [DOI] [PubMed] [Google Scholar]
- Armendariz A, Nazarian A, 2018. Morphine antinociception on thermal sensitivity and place conditioning in male and female rats treated with intraplantar complete freund’s adjuvant. Behav Brain Res 343, 21–27. 10.1016/j.bbr.2018.01.031 [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR, 2003. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend 70, S55–72. [DOI] [PubMed] [Google Scholar]
- Bagdas D, Muldoon PP, AlSharari S, Carroll FI, Negus SS, Damaj MI, 2016. Expression and pharmacological modulation of visceral pain-induced conditioned place aversion in mice. Neuropharmacology 102, 236–243. 10.1016/j.neuropharm.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldacchino A, Balfour DJK, Matthews K, 2015. Impulsivity and opioid drugs: Differential effects of heroin, methadone and prescribed analgesic medication. Psychol. Med 45, 1167–1179. 10.1017/S0033291714002189 [DOI] [PubMed] [Google Scholar]
- Banks ML, Negus SS, 2017. Insights from Preclinical Choice Models on Treating Drug Addiction. Trends Pharmacol Sci 38, 181–194. 10.1016/j.tips.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS, 2012. Preclinical Determinants of Drug Choice under Concurrent Schedules of Drug Self-Administration. Adv Pharmacol Sci 2012, 281768. 10.1155/2012/281768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbierato M, Zusso M, Skaper SD, Giusti P, 2015. MicroRNAs: emerging role in the endogenous μ opioid system. CNS Neurol Disord Drug Targets 14, 239–250. 10.2174/1871527314666150116123932 [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins R. a., 2000. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl). 153, 31–43. 10.1007/s002130000569 [DOI] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS, 2013. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol 168, 850–862. 10.1111/j.1476-5381.2012.02214.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betourne a, Familiades J, Lacassagne L, Halley H, Cazales M, Ducommun B, Lassalle J-M, Zajac J-M, Frances B, 2008. Decreased motivational properties of morphine in mouse models of cancerous- or inflammatory-chronic pain: implication of supraspinal neuropeptide FF(2) receptors. Neuroscience 157, 12–21. 10.1016/j.neuroscience.2008.08.045 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, 2001. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction 96, 73–86. 10.1046/j.1360-0443.2001.961736.x [DOI] [PubMed] [Google Scholar]
- Boada MD, Ririe DG, Martin CW, Martin SJ, Kim SA, Eisenach JC, Martin TJ, 2020. Nociceptive input after peripheral nerve injury results in cognitive impairment and alterations in primary afferent physiology in rats. Pain 161, 960–969. 10.1097/j.pain.0000000000001782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolash R, Mekhail N, 2014. Intrathecal pain pumps: indications, patient selection, techniques, and outcomes. Neurosurg. Clin. N. Am 25, 735–42. 10.1016/j.nec.2014.06.006 [DOI] [PubMed] [Google Scholar]
- Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I, 2016. Reward deficiency and anti-reward in pain chronification. Neurosci. Biobehav. Rev 68, 282–297. 10.1016/j.neubiorev.2016.05.033 [DOI] [PubMed] [Google Scholar]
- Brooner RK, King VL, Kidorf M, Schmidt CW Jr., Bigelow GE, 1997. Psychiatric and Substance Use Comorbidity Among Treatment-Seeking Opioid Abusers. Arch. Gen. Psychiatry 54, 71–80. 10.1001/archpsyc.1997.01830130077015 [DOI] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, Aube J, Jones SR, Martin TJ, Bohn LM, 2016. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal 9, ra117. 10.1126/scisignal.aai8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Xue L, Grenier P, Magnussen C, Lecour S, Olmstead MC, 2013. Changes in morphine reward in a model of neuropathic pain. Behav. Pharmacol 24, 207–13. 10.1097/FBP.0b013e3283618ac8 [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, 2008. U-shaped dose response in behavioral pharmacology: historical foundations. Crit Rev Toxicol 38, 591–598. 10.1080/10408440802026307 [DOI] [PubMed] [Google Scholar]
- Caputi FF, Rullo L, Stamatakos S, Candeletti S, Romualdi P, 2019. Modulation of the Negative Affective Dimension of Pain: Focus on Selected Neuropeptidergic System Contributions. Int J Mol Sci 20. 10.3390/ijms20164010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA Jr., Chartoff EH, 2007. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2, 2987–2995. 10.1038/nprot.2007.441 [DOI] [PubMed] [Google Scholar]
- Carroll ME, 1993. The economic context of drug and non-drug reinforcers affects acquisition and maintenance of drug-reinforced behavior and withdrawal effects. Drug Alcohol Depend 33, 201–210. 10.1016/0376-8716(93)90061-t [DOI] [PubMed] [Google Scholar]
- Cervantes MC, Laughlin RE, Jentsch JD, 2013. Cocaine self-administration behavior in inbred mouse lines segregating different capacities for inhibitory control. Psychopharmacology (Berl). 229, 515–25. 10.1007/s00213-013-3135-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm-Burns MA, Spivey CA, Sherwin E, Wheeler J, Hohmeier K, 2019. The opioid crisis: Origins, trends, policies, and the roles of pharmacists. Am J Heal. Syst Pharm 76, 424–435. 10.1093/ajhp/zxy089 [DOI] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C, 2009. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J. Pain 10, 113–130. 10.1016/j.jpain.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Harney J, 2015. Shifting Patterns of Prescription Opioid and Heroin Abuse in the United States. N Engl J Med 373, 1789–1790. 10.1056/NEJMc1505541 [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP, 2014. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry 71, 821–826. 10.1001/jamapsychiatry.2014.366 [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Muñoz A, 2005. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002–2004. J Pain 6, 662–672. 10.1016/j.jpain.2005.05.004 [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Lynskey M, Todorov A, Inciardi JA, Surratt HL, 2008. Co-morbid pain and psychopathology in males and females admitted to treatment for opioid analgesic abuse. Pain 139, 127–135. 10.1016/j.pain.2008.03.021 [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Meert T, De Witte P, Schmitt P, 1982. Further evidence validating adjuvant arthritis as an experimental model of chronic pain in the rat. Life Sci. 31, 67–75. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Tarayre JP, Alliaga M, Bruins Slot LA, Attal N, Koek W, 2001. Opiate self-administration as a measure of chronic nociceptive pain in arthritic rats. Pain 91, 33–45. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Kowalczyk WJ, Houser J, 2010. Abuse liability of oxycodone as a function of pain and drug use history. Drug Alcohol Depend. 109, 130–8. 10.1016/j.drugalcdep.2009.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone K, Lanpher J, Kinens A, Richard P, Couture S, Brackin R, Payne E, Harrington K, Rice KC, Stevenson GW, 2018. Delta/mu opioid receptor interactions in operant conditioning assays of pain-depressed responding and drug-induced rate suppression: assessment of therapeutic index in male Sprague Dawley rats. Psychopharmacology (Berl). 235, 1609–1618. 10.1007/s00213-018-4876-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler LB, Ben Abdallah A, Cummings SM, Barr J, Banks R, Forchheimer R, 2011. Injury, pain, and prescription opioid use among former National Football League (NFL) players. Drug Alcohol Depend 116, 188–194. 10.1016/j.drugalcdep.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A, Doxey JC, Harry EJ, 1977. The animal pharmacology of buprenorphine, an oripavine analgesic agent. Br. J. Pharmacol 60, 547–54. 10.1111/j.1476-5381.1977.tb07533.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen SL, Phelps CE, Navratilova E, McKinzie DL, Okun A, Husain O, Gleason SD, Witkin JM, Porreca F, 2018. Chronic pain impairs cognitive flexibility and engages novel learning strategies in rats. Pain 159, 1403–1412. 10.1097/j.pain.0000000000001226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft WH, Tegge AN, Bickel WK, 2020. Episodic Future Thinking Reduces Chronic Pain Severity: A Proof of Concept Study. Drug Alcohol Depend. In Press. 10.1016/j.jns.2019.116544 [DOI] [PubMed] [Google Scholar]
- Crist RC, Clarke TK, Berrettini WH, 2018. Pharmacogenetics of Opioid Use Disorder Treatment. CNS Drugs 32, 305–320. 10.1007/s40263-018-0513-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C, 2018. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep 67, 1001–1006. 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW, 2007. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science (80-.) 315, 1267–1270. 10.1126/science.1137073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das DA, Grimmer KA, Sparnon AL, McRae SE, Thomas BH, 2005. The efficacy of playing a virtual reality game in modulating pain for children with acute burn injuries: a randomized controlled trial [ISRCTN87413556]. BMC Pediatr. 5, 1. 10.1186/1471-2431-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L, Uezato A, Newell JM, Frazier E, 2008. Major depression and comorbid substance use disorders. Curr Opin Psychiatry 21, 14–18. 10.1097/YCO.0b013e3282f32408 [DOI] [PubMed] [Google Scholar]
- Davydov DM, Polunina AG, 2004. Heroin abusers’ performance on the Tower of London Test relates to the baseline EEG alpha2 mean frequency shifts. Prog. Neuro-Psychopharmacology Biol. Psychiatry 28, 1143–1152. 10.1016/j.pnpbp.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Deer TR, Pope JE, Hayek SM, Lamer TJ, Veizi IE, Erdek M, Wallace MS, Grider JS, Levy RM, Prager J, Rosen SM, Saulino M, Yaksh TL, De Andrés JA, Abejon Gonzalez D, Vesper J, Schu S, Simpson B, Mekhail N, 2017. The Polyanalgesic Consensus Conference (PACC): Recommendations for Intrathecal Drug Delivery: Guidance for Improving Safety and Mitigating Risks. Neuromodulation 20, 155–176. 10.1111/ner.12579 [DOI] [PubMed] [Google Scholar]
- Deneau G, Yanagita T, Seevers MH, 1969. Self-administration of psychoactive substances by the monkey. Psychopharmacologia 16, 30–48. [DOI] [PubMed] [Google Scholar]
- Dick B, Eccleston C, Crombez G, 2002. Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Rheum. 47, 639–44. 10.1002/art.10800 [DOI] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH, Zale EL, Meagher MM, 2011. Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol Bull 137, 1065–1093. 10.1037/a0025544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. CDC guideline for prescribing opioids for chronic pain-United States, 2016. JAMA - J. Am. Med. Assoc 315, 1624–1645. 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J, Immpact, 2005. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113, 9–19. 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Eccleston C, 1995. Chronic pain and distraction: An experimental investigation into the role of sustained and shifting attention in the processing of chronic persistent pain. Behav. Res. Ther 33, 391–405. 10.1016/0005-7967(94)00057-Q [DOI] [PubMed] [Google Scholar]
- Eccleston C, Crombez G, 1999. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol. Bull 125, 356–66. 10.1037/0033-2909.125.3.356 [DOI] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF, 2012. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF1 receptor antagonism. Neuropharmacology 62, 1142–51. 10.1016/j.neuropharm.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach JC, DuPen S, Dubois M, Miguel R, Allin D, 1995. Epidural clonidine analgesia for intractable cancer pain. The Epidural Clonidine Study Group. Pain 61, 391–399. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, Rauck RL, Curry R, 2003. Intrathecal, but not intravenous adenosine reduces allodynia in patients with neuropathic pain. Pain 105, 65–70. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, 2016. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron 89, 11–36. 10.1016/j.neuron.2015.11.027 [DOI] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ, 2006. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology 31, 1036–1047. 10.1038/sj.npp.1300889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW, 1999. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann. N. Y. Acad. Sci 877, 412–38. 10.1111/j.1749-6632.1999.tb09280.x [DOI] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ, 2014. Differential suppression of intracranial self-stimulation, food-maintained operant responding, and open field activity by paw incision and spinal nerve ligation in rats. Anesth Analg 118, 854–862. 10.1213/ANE.0000000000000119 [DOI] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ, 2012. Intracranial self-stimulation of the paraventricular nucleus of the hypothalamus: increased faciliation by morphine compared to cocaine. Anesthesiology 116, 1116–1123. 10.1097/ALN.0b013e3182518be3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ, 2011a. Rewarding electrical brain stimulation in rats after peripheral nerve injury: decreased facilitation by commonly abused prescription opioids. Anesthesiology 115, 1271–1280. 10.1097/ALN.0b013e3182330448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ, 2011b. Opioid facilitation of rewarding electrical brain stimulation is suppressed in rats with neuropathic pain. Anesthesiology 114, 624–632. 10.1097/ALN.0b013e31820a4edb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino B, Oliveira J, Lopes P, 2019. Diagnostic precision of the Wisconsin Card Sorting Test in assessing cognitive deficits in substance use disorders. Appl. Neuropsychol 0, 1–8. 10.1080/23279095.2019.1607737 [DOI] [PubMed] [Google Scholar]
- Franken IHA, Kroon LY, Weirs R, Jansen A, 2000. Selective cognitive processing of drug cues in heroin dependence. J. Psychopharmacol 14, 395–400. 10.1177/026988110001400408 [DOI] [PubMed] [Google Scholar]
- Freitas KC, Hillhouse TM, Leitl MD, Negus SS, 2015. Effects of acute and sustained pain manipulations on performance in a visual-signal detection task of attention in rats. Drug Dev. Res 76, 194–203. 10.1002/ddr.21255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofoli M, 2020. Adolescent Substance Abuse. Prim. Care - Clin. Off. Pract 47, 383–394. 10.1016/j.pop.2020.02.013 [DOI] [PubMed] [Google Scholar]
- Gates M, Hartling L, Shulhan-Kilroy J, MacGregor T, Guitard S, Wingert A, Featherstone R, Vandermeer B, Poonai N, Kircher J, Perry S, Graham TAD, Scott SD, Ali S, 2020. Digital Technology Distraction for Acute Pain in Children: A Meta-analysis. Pediatrics 145. 10.1542/peds.2019-1139 [DOI] [PubMed] [Google Scholar]
- Geisser ME, Roth RS, Bachman JE, Eckert TA, 1996. The relationship between symptoms of post-traumatic stress disorder and pain, affective disturbance and disability among patients with accident and non-accident related pain. Pain 66, 207–214. [DOI] [PubMed] [Google Scholar]
- Genova HM, Deluca J, Chiaravalloti N, Wylie G, 2013. The relationship between executive functioning, processing speed, and white matter integrity in multiple sclerosis. J. Clin. Exp. Neuropsychol 35, 631–641. 10.1080/13803395.2013.806649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Maguire DR, France CP, 2020. Behavioral Pharmacology of Drugs Acting at Mu Opioid Receptors, in: Nader MA, Hurd YL (Eds.), Substance Use Disorders: From Etiology to Treatment. Springer International Publishing, Cham, pp. 127–145. 10.1007/164_2019_265 [DOI] [PubMed] [Google Scholar]
- Gibson CA, 2012. Review of posttraumatic stress disorder and chronic pain: the path to integrated care. J Rehabil Res Dev 49, 753–776. [DOI] [PubMed] [Google Scholar]
- Goodwin A, Hiranita T, Paule M, 2015. The Reinforcing Effects of Nicotine in Humans and Nonhuman Primates: A Review of Intravenous Self-Administration Evidence and Future Directions for Research. Nicotine Tob. Res in press. [DOI] [PubMed] [Google Scholar]
- Grisart JM, Van der Linden M, 2001. Conscious and automatic uses of memory in chronic pain patients. Pain 94, 305–13. 10.1016/s0304-3959(01)00366-9 [DOI] [PubMed] [Google Scholar]
- Gupta MA, 2013. Review of somatic symptoms in post-traumatic stress disorder. Int Rev Psychiatry 25, 86–99. 10.3109/09540261.2012.736367 [DOI] [PubMed] [Google Scholar]
- Harton LR, Richardson JR, Armendariz A, Nazarian A, 2017. Dissociation of morphine analgesic effects in the sensory and affective components of formalin-induced spontaneous pain in male and female rats. Brain Res. 1658. 10.1016/j.brainres.2017.01.011 [DOI] [PubMed] [Google Scholar]
- Haythornthwaite JA, Menefee LA, Quatrano-Piacentini AL, Pappagallo M, 1998. Outcome of chronic opioid therapy for non-cancer pain. J. Pain Symptom Manage 15, 185–194. 10.1016/S0885-3924(97)00352-7 [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Cohen C, Heishman SJ, 1991. Drug self-administration methods in abuse liability evaluation. Br. J. Addict 86, 1571–7. 10.1111/j.1360-0443.1991.tb01750.x [DOI] [PubMed] [Google Scholar]
- Herkenham M, Pert CB, 1982. Light microscopic localization of brain opiate receptors: a general autoradiographic method which preserves tissue quality. J Neurosci 2, 1129–1149. 10.1523/jneurosci.02-08-01129.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Van Niekerk A, Desnoyer J, Patrick A, Lau W, Thevarkunnel S, 2015. Enduring attentional deficits in rats treated with a peripheral nerve injury. Behav. Brain Res 286, 347–355. 10.1016/j.bbr.2015.02.050 [DOI] [PubMed] [Google Scholar]
- Higgins ST, 1997. The influence of alternative reinforcers on cocaine use and abuse: a brief review. Pharmacol Biochem Behav 57, 419–427. 10.1016/s0091-3057(96)00446-7 [DOI] [PubMed] [Google Scholar]
- Hipólito L, Wilson-Poe A, Campos-Jurado Y, Zhong E, Gonzalez-Romero J, Virag L, Whittington R, Comer SD, Carlton SM, Walker BM, Bruchas MR, Morón JA, 2015. Inflammatory Pain Promotes Increased Opioid Self-Administration: Role of Dysregulated Ventral Tegmental Area μ Opioid Receptors. J. Neurosci 35, 12217–31. 10.1523/JNEUROSCI.1053-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Fantegrossi WE, 2009. Intravenous Drug Self-Administration in Nonhuman Primates, Methods of Behavior Analysis in Neuroscience. [PubMed] [Google Scholar]
- Huysmans E, Leemans L, Beckwée D, Nijs J, Ickmans K, Moens M, Goudman L, Buyl R, Putman K, Coppieters I, 2020. The Relationship between Cognitive and Emotional Factors and Healthcare and Medication Use in People Experiencing Pain: A Systematic Review. J. Clin. Med 9. 10.3390/jcm9082486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, 2005. Addiction: A disease of learning and memory. Am. J. Psychiatry 162, 1414–1422. 10.1176/appi.ajp.162.8.1414 [DOI] [PubMed] [Google Scholar]
- Jamison RN, Schein JR, Vallow S, Ascher S, Vorsanger GJ, Katz NP, 2003. Neuropsychological effects of long-term opioid use in chronic pain patients. J. Pain Symptom Manage 26, 913–921. 10.1016/S0885-3924(03)00310-5 [DOI] [PubMed] [Google Scholar]
- Jensen MP, Chodroff MJ, Dworkin RH, 2007. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology 68, 1178–1182. 10.1212/01.wnl.0000259085.61898.9e [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, 1999. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl). 146, 373–90. 10.1007/pl00005483 [DOI] [PubMed] [Google Scholar]
- Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V, 2010. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J. Neurosci 30, 5451–5464. 10.1523/JNEUROSCI.0225-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, Martin TJ, Nader MA, 2017. Behavioral Determinants of Cannabinoid Self-Administration in Old World Monkeys. Neuropsychopharmacology 42, 1522–1530. 10.1038/npp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Comer SD, 2013. A review of human drug self-administration procedures. Behav. Pharmacol 24, 384–95. 10.1097/FBP.0b013e3283641c3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Goldberg SR, Heishman SJ, Tanda G, 2005. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav 81, 285–299. 10.1016/j.pbb.2005.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juurlink DN, Dhalla IA, 2012. Dependence and addiction during chronic opioid therapy. J Med Toxicol 8, 393–399. 10.1007/s13181-012-0269-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy R, Calsbeek JJ, Morgan MM, 2016. Home cage wheel running is an objective and clinically relevant method to assess inflammatory pain in male and female rats. J Neurosci Methods 263, 115–122. 10.1016/j.jneumeth.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp JF, Reynolds CF, Butters MA, Dew MA, Mazumdar S, Begley AE, Lenze E, Weiner DK, 2006. The relationship between pain and mental flexibility in older adult pain clinic patients. Pain Med. 7, 444–452. 10.1111/j.1526-4637.2006.00212.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye AD, Jones MR, Kaye AM, Ripoll JG, Galan V, Beakley BD, Calixto F, Bolden JL, Urman RD, Manchikanti L, 2017a. Prescription Opioid Abuse in Chronic Pain: An Updated Review of Opioid Abuse Predictors and Strategies to Curb Opioid Abuse: Part 1. Pain Physician 20, S93–s109. [PubMed] [Google Scholar]
- Kaye AD, Jones MR, Kaye AM, Ripoll JG, Jones DE, Galan V, Beakley BD, Calixto F, Bolden JL, Urman RD, Manchikanti L, 2017b. Prescription Opioid Abuse in Chronic Pain: An Updated Review of Opioid Abuse Predictors and Strategies to Curb Opioid Abuse (Part 2). Pain Physician 20, S111–s133. [PubMed] [Google Scholar]
- Keogh E, Moore DJ, Duggan GB, Payne SJ, Eccleston C, 2013. The disruptive effects of pain on complex cognitive performance and executive control. PLoS One 8. 10.1371/journal.pone.0083272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna IK, Pillarisetti S, 2015. Buprenorphine - an attractive opioid with underutilized potential in treatment of chronic pain. J. Pain Res 8, 859–70. 10.2147/JPR.S85951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, 2004. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction 99, 461–471. 10.1111/j.1360-0443.2003.00669.x [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK, 1999. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen 128, 78–87. 10.1037//0096-3445.128.1.78 [DOI] [PubMed] [Google Scholar]
- Kleiman V, Clarke H, Katz J, 2011. Sensitivity to pain traumatization: a higher-order factor underlying pain-related anxiety, pain catastrophizing and anxiety sensitivity among patients scheduled for major surgery. Pain Res Manag 16, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, Alexander GC, 2015. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Heal. 36, 559–574. 10.1146/annurev-publhealth-031914-122957 [DOI] [PubMed] [Google Scholar]
- Könner a C., Hess S, Tovar S, Mesaros A, Sánchez-Lasheras C, Evers N, Verhagen L. a W., Brönneke HS, Kleinridders A, Hampel B, Kloppenburg P, Brüning JC, 2011. Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell Metab. 13, 720–8. 10.1016/j.cmet.2011.03.021 [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, 1979. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc 38, 2473–2476. [PubMed] [Google Scholar]
- Kurita GP, De Mattos Pimenta CA, Braga PE, Frich L, Jørgensen MM, Nielsen PR, Hojsted J, Sjøgren P, 2012. Cognitive function in patients with chronic pain treated with opioids: Characteristics and associated factors. Acta Anaesthesiol. Scand 56, 1257–1266. 10.1111/j.1399-6576.2012.02760.x [DOI] [PubMed] [Google Scholar]
- Kurita GP, Malver LP, Andresen T, Polianskis R, Drewes AM, Christrup L, Højsted J, Sjøgren P, 2015. Does mutual compensation of the cognitive effects induced by pain and opioids exist? An experimental study. Psychopharmacology (Berl). 232, 1373–1381. 10.1007/s00213-014-3768-y [DOI] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS, 2012. Dissociable effects of the cannabinoid receptor agonists Delta9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther 343, 389–400. 10.1124/jpet.112.197780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrø NI, Fors EA, Våpenstad LL, Holthe Ø, Stiles TC, Borchgrevink PC, 2013. The extent of neurocognitive dysfunction in a multidisciplinary pain centre population. Is there a relation between reported and tested neuropsychological functioning? Pain 154, 972–977. 10.1016/j.pain.2013.01.013 [DOI] [PubMed] [Google Scholar]
- Lee DM, Pendleton N, Tajar A, O’Neill TW, O’Connor DB, Bartfai G, Boonen S, Casanueva FF, Finn JD, Forti G, Giwercman A, Han TS, Huhtaniemi IT, Kula K, Lean MEJ, Punab M, Silman AJ, Vanderschueren D, Moseley CM, Wu FCW, McBeth J, 2010. Chronic widespread pain is associated with slower cognitive processing speed in middle-aged and older European men. Pain 151, 30–36. 10.1016/j.pain.2010.04.024 [DOI] [PubMed] [Google Scholar]
- Lee TMC, Pau CWH, 2002. Impulse control differences between abstinent heroin users and matched controls. Brain Inj. 16, 885–889. 10.1080/02699050210128915 [DOI] [PubMed] [Google Scholar]
- Lees B, Meredith LR, Kirkland AE, Bryant BE, Squeglia LM, 2020. Effect of alcohol use on the adolescent brain and behavior. Pharmacol. Biochem. Behav 192, 172906. 10.1016/j.pbb.2020.172906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre EM, Pisansky MT, Toddes C, Baruffaldi F, Pravetoni M, Tian L, Kono TJY, Rothwell PE, 2020. Interruption of continuous opioid exposure exacerbates drug-evoked adaptations in the mesolimbic dopamine system. Neuropsychopharmacology 1–12. 10.1038/s41386-020-0643-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis LP, Bigbee JW, Negus SS, 2018. Lack of paclitaxel effects on intracranial self-stimulation in male and female rats: comparison to mechanical sensitivity. Behav Pharmacol 29, 290–298. 10.1097/FBP.0000000000000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis LP, Negus SS, 2018. Repeated Morphine Produces Sensitization to Reward and Tolerance to Antiallodynia in Male and Female Rats with Chemotherapy-Induced Neuropathy. J Pharmacol Exp Ther 365, 9–19. 10.1124/jpet.117.246215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Negus SS, 2016. Pharmacological modulation of neuropathic pain-related depression of behavior: effects of morphine, ketoprofen, bupropion and [INCREMENT]9-tetrahydrocannabinol on formalin-induced depression of intracranial self-stimulation in rats. Behav. Pharmacol 27, 364–76. 10.1097/FBP.0000000000000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon W. a, Negus S, 2014. Sustained pain-related depression of behavior: effects of intraplantar formalin and complete freund’s adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol. Pain 10, 62. 10.1186/1744-8069-10-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett BT, 1989. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacol. 98, 357–362. 10.1007/BF00451687 [DOI] [PubMed] [Google Scholar]
- Li X, Zhang F, Zhou Y, Zhang M, Wang X, Shen M, 2013. Decision-making deficits are still present in heroin abusers after short- to long-term abstinence. Drug Alcohol Depend. 130, 61–67. 10.1016/j.drugalcdep.2012.10.012 [DOI] [PubMed] [Google Scholar]
- Litman RS, Pagán OH, Cicero TJ, 2018. Abuse-deterrent Opioid Formulations. Anesthesiology 128, 1015–1026. 10.1097/ALN.0000000000002031 [DOI] [PubMed] [Google Scholar]
- Lyness WH, Smith FL, Heavner JE, Iacono CU, Garvin RD, 1989. Morphine self-administration in the rat during adjuvant-induced arthritis. Life Sci. 45, 2217–2224. [DOI] [PubMed] [Google Scholar]
- Lyvers M, Yakimoff M, 2003. Neuropsychological correlates of opioid dependence and withdrawal. Addict. Behav 28, 605–611. 10.1016/S0306-4603(01)00253-2 [DOI] [PubMed] [Google Scholar]
- Marissen MAE, Franken IHA, Waters AJ, Blanken P, Van Den Brink W, Hendriks VM, 2006. Attentional bias predicts heroin relapse following treatment. Addiction 101, 1306–1312. 10.1111/j.1360-0443.2006.01498.x [DOI] [PubMed] [Google Scholar]
- Martel MO, Shir Y, Ware MA, 2018. Substance-related disorders: A review of prevalence and correlates among patients with chronic pain. Prog Neuropsychopharmacol Biol Psychiatry 87, 245–254. 10.1016/j.pnpbp.2017.06.032 [DOI] [PubMed] [Google Scholar]
- Martin AL, Halket E, Asmundson GJ, Flora DB, Katz J, 2010. Posttraumatic stress symptoms and the diathesis-stress model of chronic pain and disability in patients undergoing major surgery. Clin J Pain 26, 518–527. 10.1097/AJP.0b013e3181e15b98 [DOI] [PubMed] [Google Scholar]
- Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC, 2004. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology 101, 191–203. 10.1097/00000542-200407000-00030 [DOI] [PubMed] [Google Scholar]
- Martin TJ, Buechler NL, Kim SA, Ewan EE, Xiao R, Childers SR, 2011. Involvement of the lateral amygdala in the antiallodynic and reinforcing effects of heroin in rats after peripheral nerve injury. Anesthesiology 114, 633–642. 10.1097/ALN.0b013e318209aba7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC, 2007. Opioid self-administration in the nerve-injured rat: relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology 106, 312–322. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Eisenach JC, 2006. Clonidine maintains intrathecal self-administration in rats following spinal nerve ligation. Pain 125, 257–263. 10.1016/j.pain.2006.05.027 [DOI] [PubMed] [Google Scholar]
- Martin TJ, Strassburg TJ, Grigg AL, Kim SA, Ririe DG, Eisenach JC, 2017. Assessment of behavioral disruption in rats with abdominal inflammation using visual cue titration and the five-choice serial-reaction time task. Anesthesiology 127, 372–381. 10.1097/ALN.0000000000001702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Bardo MT, 2009. Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav. Pharmacol 20, 447–54. 10.1097/FBP.0b013e328330ad6d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaly N, Copits BA, Wilson-Poe AR, Hipólito L, Markovic T, Yoon HJ, Liu S, Walicki MC, Bhatti DL, Sirohi S, Klaas A, Walker BM, Neve R, Cahill CM, Shoghi KI, Gereau R.W. th, McCall JG, Al-Hasani R, Bruchas MR, Morón JA, 2019. Pain-Induced Negative Affect Is Mediated via Recruitment of The Nucleus Accumbens Kappa Opioid System. Neuron 102, 564–573.e6. 10.1016/j.neuron.2019.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell PA, Garland EL, Zubieta JK, Newman-Norlund R, Powers S, Froeliger B, 2020. Impaired frontostriatal functional connectivity among chronic opioid using pain patients is associated with dysregulated affect. Addict Biol 25, e12743. 10.1111/adb.12743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Janes AC, Griffin ML, Taghian N, Greenfield SF, Weiss RD, 2020. Clinical Correlates of Smoking Status in Men and Women with Opioid Use Disorder. Subst Use Misuse 55, 1054–1058. 10.1080/10826084.2020.1725056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicine, A.A. of P., Society, the A.P., 1997. The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain 13, 6–8. [PubMed] [Google Scholar]
- Meisch RA, 2001. Oral drug self-administration: an overview of laboratory animal studies. Alcohol 24, 117–128. 10.1016/s0741-8329(01)00149-5 [DOI] [PubMed] [Google Scholar]
- Miller LL, Altarifi AA, Negus SS, 2015. Effects of repeated morphine on intracranial self-stimulation in male rats in the absence or presence of a noxious pain stimulus. Exp Clin Psychopharmacol 23, 405–414. 10.1037/pha0000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LR, Cano A, 2009. Comorbid chronic pain and depression: who is at risk? J Pain 10, 619–627. 10.1016/j.jpain.2008.12.007 [DOI] [PubMed] [Google Scholar]
- Moazen P, Torabi M, Azizi H, Fathollahi Y, Mirnajafi-Zadeh J, Semnanian S, 2020. The locus coeruleus noradrenergic system gates deficits in visual attention induced by chronic pain. Behav. Brain Res 387. 10.1016/j.bbr.2020.112600 [DOI] [PubMed] [Google Scholar]
- Moore DJ, Eccleston C, Keogh E, 2017. Cognitive load selectively influences the interruptive effect of pain on attention. Pain 158, 2035–2041. 10.1097/j.pain.0000000000001011 [DOI] [PubMed] [Google Scholar]
- Moore DJ, Keogh E, Eccleston C, 2012. The interruptive effect of pain on attention. Q. J. Exp. Psychol 65, 565–586. 10.1080/17470218.2011.626865 [DOI] [PubMed] [Google Scholar]
- Morasco BJ, Dobscha SK, Hyde S, Mitchell SH, 2019. Exploratory study examining associations between prescription opioid dose and delay discounting in patients with chronic pain. J. Opioid Manag 15, 19–25. 10.5055/jom.2019.0482 [DOI] [PubMed] [Google Scholar]
- Moriarty O, Finn DP, 2014. Cognition and pain. Curr. Opin. Support. Palliat. Care 8, 130–6. 10.1097/SPC.0000000000000054 [DOI] [PubMed] [Google Scholar]
- Moriarty O, McGuire BE, Finn DP, 2011. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog. Neurobiol 93, 385–404. 10.1016/j.pneurobio.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Müller CP, 2018. Animal models of psychoactive drug use and addiction - Present problems and future needs for translational approaches. Behav Brain Res 352, 109–115. 10.1016/j.bbr.2017.06.028 [DOI] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, Porreca F, 2015. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J Neurosci 35, 7264–7271. 10.1523/JNEUROSCI.3862-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan H, Ward SJ, Walker EA, 2016. Effects of paclitaxel on mechanical sensitivity and morphine reward in male and female C57Bl6 mice. Exp Clin Psychopharmacol 24, 485–495. 10.1037/pha0000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, 2019. Core Outcome Measures in Preclinical Assessment of Candidate Analgesics. Pharmacol. Rev 71, 225–266. 10.1124/pr.118.017210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Banks ML, 2018. Modulation of drug choice by extended drug access and withdrawal in rhesus monkeys: Implications for negative reinforcement as a driver of addiction and target for medications development. Pharmacol Biochem Behav 164, 32–39. 10.1016/j.pbb.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL, 2014. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev 66, 869–917. 10.1124/pr.112.007419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Moerke MJ, 2019. Determinants of opioid abuse potential: Insights using intracranial self-stimulation. Peptides 112, 23–31. 10.1016/j.peptides.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC, 2010. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacol. 210, 149–159. 10.1007/s00213-009-1770-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL, 2015. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain 156, 1153–1160. 10.1097/j.pain.0000000000000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejati M, Nejati V, Mohammadi MR, 2011. Selective attention and drug related attention bias in methadone maintenance patients. Acta Med. Iran 49, 814–817. [PubMed] [Google Scholar]
- Niikura K, Narita M, Narita M, Nakamura A, Okutsu D, Ozeki A, Kurahashi K, Kobayashi Y, Suzuki M, Suzuki T, 2008. Direct evidence for the involvement of endogenous beta-endorphin in the suppression of the morphine-induced rewarding effect under a neuropathic pain-like state. Neurosci Lett 435, 257–262. 10.1016/j.neulet.2008.02.059 [DOI] [PubMed] [Google Scholar]
- Nwaneshiudu CA, Shi XY, Clark JD, 2020. Incisional Injury Modulates Morphine Reward and Morphine-Primed Reinstatement: A Role of Kappa Opioid Receptor Activation. Anesth Analg 130, 248–257. 10.1213/ANE.0000000000004142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, 2011. NICO-TEEN: neural substrates that mediate adolescent tobacco abuse. Neuropsychopharmacology 36, 356–7. 10.1038/npp.2010.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Nazarian A, 2016. Enhanced vulnerability to tobacco use in persons with diabetes: A behavioral and neurobiological framework. Prog. Neuro-Psychopharmacology Biol. Psychiatry 65, 288–296. 10.1016/j.pnpbp.2015.06.005 [DOI] [PubMed] [Google Scholar]
- O’Donnell ML, Holmes AC, Creamer MC, Ellen S, Judson R, McFarlane AC, Silove DM, Bryant RA, 2009. The role of post-traumatic stress disorder and depression in predicting disability after injury. Med J Aust 190, S71–4. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW, 2000. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology 23, 113–126. 10.1016/S0893-133X(00)00097-X [DOI] [PubMed] [Google Scholar]
- Owens WA, Sevak RJ, Galici R, Chang X, Javors MA, Galli A, France CP, Daws LC, 2005. Deficits in dopamine clearance and locomotion in hypoinsulinemic rats unmask novel modulation of dopamine transporters by amphetamine. J Neurochem 94, 1402–1410. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita Minoru, Narita Michiko, Iino M, Sugita J, Matsumura Y, Suzuki T, 2002. Suppression of the morphine-induced rewarding effect in the rat with neuropathic pain: Implication of the reduction in mu-opioid receptor functions in the ventral tegmental area. J. Neurochem 82, 1192–1198. 10.1046/j.1471-4159.2002.01071.x [DOI] [PubMed] [Google Scholar]
- Pagé MG, Kudrina I, Zomahoun HTV, Ziegler D, Beaulieu P, Charbonneau C, Cogan J, Daoust R, Martel MO, Néron A, Richebé P, Clarke H, 2018. Relative frequency and risk factors for long-term opioid therapy following surgery and trauma among adults: a systematic review protocol. Syst Rev 7, 97. 10.1186/s13643-018-0760-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahng AR, Paulsen RI, McGinn MA, Edwards KN, Edwards S, 2017. Neurobiological Correlates of Pain Avoidance-Like Behavior in Morphine-Dependent and Non-Dependent Rats. Neuroscience 366, 1–14. 10.1016/j.neuroscience.2017.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais-Vieira M, Lima D, Galhardo V, 2009. Sustained attention deficits in rats with chronic inflammatory pain. Neurosci. Lett 463, 98–102. 10.1016/j.neulet.2009.07.050 [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR, 2007. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction 102, 1863–1870. 10.1111/j.1360-0443.2007.02011.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Schindler CW, 2000. Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacol. 150, 61–66. 10.1007/s002130000415 [DOI] [PubMed] [Google Scholar]
- Pask S, Dell’Olio M, Murtagh FEM, Boland JW, 2020. The Effects of Opioids on Cognition in Older Adults With Cancer and Chronic Noncancer Pain: A Systematic Review. J. Pain Symptom Manage 59, 871–893.e1. 10.1016/j.jpainsymman.2019.10.022 [DOI] [PubMed] [Google Scholar]
- Peciña M, Love T, Stohler CS, Goldman D, Zubieta JK, 2015. Effects of the Mu opioid receptor polymorphism (OPRM1 A118G) on pain regulation, placebo effects and associated personality trait measures. Neuropsychopharmacology 40, 957–965. 10.1038/npp.2014.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS, 2009. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain 144, 170–177. 10.1016/j.pain.2009.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraschka M, Li S, Gilbert TL, Westenbroek RE, Bruchas MR, Schreiber S, Lowe J, Low MJ, Pintar JE, Chavkin C, 2007. The absence of endogenous β-endorphin selectively blocks phosphorylation and desensitization of mu opioid receptors following partial sciatic nerve ligation. Neuroscience 146, 1795–1807. 10.1016/j.neuroscience.2007.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenoy RK, 1996. Opioid therapy for chronic nonmalignant pain: a review of the critical issues. J Pain Symptom Manag. 11, 203–217. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL, 1995. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav. Pharmacol 6, 810–814. [PubMed] [Google Scholar]
- Reid LD, 1987. Tests involving pressing for intracranial stimulation as an early procedure for screening the likelihood of addiction of opioids and other drugs, in: Bozarth MJ (Ed.), Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer-Verlag, Berlin, pp. 391–420. [Google Scholar]
- Richardson NR, Roberts DC, 1996. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66, 1–11. 10.1016/0165-0270(95)00153-0 [DOI] [PubMed] [Google Scholar]
- Ririe DG, Danilo Boada M, MacGregor MK, Martin SJ, Strassburg TJ, Kim SA, Eisenach JC, Martin TJ, 2018. Incisional Nociceptive Input Impairs Attention-related Behavior and Is Associated with Reduced Neuronal Activity in the Prefrontal Cortex in Rats. Anesthesiology 129, 778–790. 10.1097/ALN.0000000000002325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins MT, Heinricher MM, Ryabinin AE, 2019. From Pleasure to Pain, and Back Again: The Intricate Relationship Between Alcohol and Nociception. Alcohol Alcohol 54, 625–638. 10.1093/alcalc/agz067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Marsch LA, Joseph H, Portenoy RK, 2008. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol 16, 405–416. 10.1037/a0013628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RS, Geisser ME, Bates R, 2008. The relation of post-traumatic stress symptoms to depression and pain in patients with accident-related chronic pain. J. Pain 9, 588–596. 10.1016/j.jpain.2008.01.333 [DOI] [PubMed] [Google Scholar]
- Rowlett JK, 2000. A labor-supply analysis of cocaine self-administration under progressive-ratio schedules: antecedents, methodologies, and perspectives. Psychopharmacol. 153, 1–16. 10.1007/s002130000610 [DOI] [PubMed] [Google Scholar]
- Saputra S, Espinoza N, Nazarian A, 2019. Pain-induced impulsivity in rats and its reversal by various agents, in: Society for Neuroscience Abstract. [Google Scholar]
- Saroj R, Ghosh A, Subodh BN, Nehra R, Mahintamani T, Rana DK, Basu D, 2020. Neurocognitive functions in patients on buprenorphine maintenance for opioid dependence: A comparative study with three matched control groups. Asian J. Psychiatr 53, 102181. 10.1016/j.ajp.2020.102181 [DOI] [PubMed] [Google Scholar]
- Scapin S, Echevarría-Guanilo ME, Boeira Fuculo Junior PR, Gonçalves N, Rocha PK, Coimbra R, 2018. Virtual Reality in the treatment of burn patients: A systematic review. Burns 44, 1403–1416. 10.1016/j.burns.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Selley DE, Cao CC, Sexton T, Schwegel JA, Martin TJ, Childers SR, 2001. mu Opioid receptor-mediated G-protein activation by heroin metabolites: evidence for greater efficacy of 6-monoacetylmorphine compared with morphine. Biochem. Pharmacol 62, 447–455. [DOI] [PubMed] [Google Scholar]
- Selley DE, Lazenka MF, Sim-Selley LJ, Secor McVoy JR, Potter DN, Chartoff EH, Carlezon WA, Negus SS, 2020. Attenuated dopamine receptor signaling in nucleus accumbens core in a rat model of chemically-induced neuropathy. Neuropharmacology 166, 107935. 10.1016/j.neuropharm.2020.107935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Liu Q, Childers SR, 1998. Signal transduction correlates of mu opioid agonist intrinsic efficacy: receptor-stimulated [35S]GTP gamma S binding in mMOR-CHO cells and rat thalamus. J. Pharmacol. Exp. Ther 285, 496–505. [PubMed] [Google Scholar]
- Severtson SG, von Thomsen S, Hedden SL, Latimer W, 2010. The association between executive functioning and motivation to enter treatment among regular users of heroin and/or cocaine in Baltimore, MD. Addict. Behav 35, 717–20. 10.1016/j.addbeh.2010.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiers S, Mwirigi J, Pradhan G, Kume M, Black B, Barragan-Iglesias P, Moy JK, Dussor G, Pancrazio JJ, Kroener S, Price TJ, 2020. Reversal of peripheral nerve injury-induced neuropathic pain and cognitive dysfunction via genetic and tomivosertib targeting of MNK. Neuropsychopharmacology 45, 524–533. 10.1038/s41386-019-0537-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Stein C, Huber A, Millan MJ, Herz A, 1988. Motivational effects of opioids in an animal model of prolonged inflammatory pain: alteration in the effects of kappa- but not of mu-receptor agonists. Pain 35, 179–186. [DOI] [PubMed] [Google Scholar]
- Shurman J, Koob GF, Gutstein HB, 2010. Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain Med 11, 1092–8. 10.1111/j.1526-4637.2010.00881.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ, 2000. Chronic heroin self-administration desensitizes mu opioid receptor-activated G-proteins in specific regions of rat brain. J. Neurosci 20, 4555–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Childers SR, 1995. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5’-[gamma-[35S]thio]-triphosphate binding. Proc Natl Acad Sci U S A 92, 7242–7246. 10.1073/pnas.92.16.7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore GM, Gaspard TM, Kim SA, Walker LE, Vrana SL, Dworkin SI, 1997. Dose-effect functions for cocaine self-administration: effects of schedule and dosing procedure. Pharmacol Biochem Behav 57, 523–531. 10.1016/s0091-3057(96)00437-6 [DOI] [PubMed] [Google Scholar]
- Sizemore GM, Martin TJ, 2000. Toward a mathematical description of dose-effect functions for self-administered drugs in laboratory animal models. Psychopharmacol. 153, 57–66. 10.1007/s002130000611 [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR, 1998. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacol. 139, 169–184. 10.1007/s002130050702 [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, Jezierski J, Luginbuhl A, Bilsky EJ, 2011. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: implications for preclinical behavioral assessment of chronic pain. Pharmacol Biochem Behav 98, 35–42. 10.1016/j.pbb.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EM, Spreen O, 2006. A compendium of neuropsychological tests: administration, norms, and commentary, 3rd ed. Oxford University Press, New York. [Google Scholar]
- Sufka KJ, 1994. Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain 58, 355–66. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kishimoto Y, Misawa M, 1996. Formalin- and carrageenan-induced inflammation attenuates place preferences produced by morphine, methamphetamine and cocaine. Life Sci. 59, 1667–74. [DOI] [PubMed] [Google Scholar]
- Tappe-Theodor A, King T, Morgan MM, 2019. Pros and Cons of Clinically Relevant Methods to Assess Pain in Rodents. Neurosci. Biobehav. Rev 100, 335–343. 10.1016/j.neubiorev.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassain V, Attal N, Fletcher D, Brasseur L, Dégieux P, Chauvin M, Bouhassira D, 2003. Long term effects of oral sustained release morphine on neuropsychological performance in patients with chronic non-cancer pain. Pain 104, 389–400. 10.1016/S0304-3959(03)00047-2 [DOI] [PubMed] [Google Scholar]
- Thompson T, Schuster CR, 1964. MORPHINE SELF-ADMINISTRATION, FOOD-REINFORCED, AND AVOIDANCE BEHAVIORS IN RHESUS MONKEYS. Psychopharmacologia 5, 87–94. 10.1007/bf00413045 [DOI] [PubMed] [Google Scholar]
- Tompkins DA, Johnson PS, Smith MT, Strain EC, Edwards RR, Johnson MW, 2016. Temporal preference in individuals reporting chronic pain: discounting of delayed pain-related and monetary outcomes. Pain 157, 1724–32. 10.1097/j.pain.0000000000000576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Corlett PR, Taylor JR, 2011. Aberrant learning and memory in addiction. Neurobiol. Learn. Mem 96, 609–623. 10.1016/j.nlm.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Caine SB, Thomsen M, Banks ML, 2019. Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology 44, 2022–2029. 10.1038/s41386-019-0356-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost Z, Zielke M, Guck A, Nowlin L, Zakhidov D, France CR, Keefe F, 2015. The promise and challenge of virtual gaming technologies for chronic pain: the case of graded exposure for low back pain. Pain Manag. 5, 197–206. 10.2217/pmt.15.6 [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, 2007. Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addict. Biol 12, 227–462. 10.1111/j.1369-1600.2007.00070.x [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, 1998. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56, 613–672. 10.1016/s0301-0082(98)00060-4 [DOI] [PubMed] [Google Scholar]
- Urits I, Hubble A, Peterson E, Orhurhu V, Ernst CA, Kaye AD, Viswanath O, 2019. An Update on Cognitive Therapy for the Management of Chronic Pain: a Comprehensive Review. Curr. Pain Headache Rep 23, 57. 10.1007/s11916-019-0794-9 [DOI] [PubMed] [Google Scholar]
- van der Kam EL, Vry JD, Schiene K, Tzschentke TM, 2008. Differential effects of morphine on the affective and the sensory component of carrageenan-induced nociception in the rat. Pain 136, 373–379. 10.1016/j.pain.2007.07.027 [DOI] [PubMed] [Google Scholar]
- Veliz P, Epstein-Ngo QM, Meier E, Ross-Durow PL, McCabe SE, Boyd CJ, 2014. Painfully obvious: a longitudinal examination of medical use and misuse of opioid medication among adolescent sports participants. J Adolesc Heal. 54, 333–340. 10.1016/j.jadohealth.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Albein-Urios N, 2021. Impulsivity traits and neurocognitive mechanisms conferring vulnerability to substance use disorders. Neuropharmacology 183, 108402. 10.1016/j.neuropharm.2020.108402 [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Bechara A, Recknor EC, Pérez-García M, 2007. Negative emotion-driven impulsivity predicts substance dependence problems. Drug Alcohol Depend. 91, 213–219. 10.1016/j.drugalcdep.2007.05.025 [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, López-Torrecillas F, Calandre EP, Delgado-Rodríguez A, Bechara A, 2009. Executive function and decision-making in women with fibromyalgia. Arch. Clin. Neuropsychol 24, 113–122. 10.1093/arclin/acp014 [DOI] [PubMed] [Google Scholar]
- Volkow N, Benveniste H, McLellan AT, 2018. Use and Misuse of Opioids in Chronic Pain. Annu Rev Med 69, 451–465. 10.1146/annurev-med-011817-044739 [DOI] [PubMed] [Google Scholar]
- Walker JR, Chen SA, Moffitt H, Inturrisi CE, Koob GF, 2003. Chronic opioid exposure produces increased heroin self-administration in rats. Pharmacol Biochem Behav 75, 349–354. 10.1016/s0091-3057(03)00094-7 [DOI] [PubMed] [Google Scholar]
- Walteros C, Sánchez-Navarro JP, Muñoz MA, Martínez-Selva JM, Chialvo D, Montoya P, 2011. Altered associative learning and emotional decision making in fibromyalgia. J. Psychosom. Res 70, 294–301. 10.1016/j.jpsychores.2010.07.013 [DOI] [PubMed] [Google Scholar]
- Walvoort SJW, Wester AJ, Egger JIM, 2012. Neurocognitive parameters should be incorporated in the Minnesota Multiphasic Personality Inventory-2 assessment of patients with alcohol use disorders. Drug Alcohol Rev. 31, 550–7. 10.1111/j.1465-3362.2011.00407.x [DOI] [PubMed] [Google Scholar]
- Wang GY, Wouldes TA, Kydd R, Jensen M, Russell BR, 2014. Neuropsychological performance of methadone-maintained opiate users. J. Psychopharmacol 28, 789–799. 10.1177/0269881114538541 [DOI] [PubMed] [Google Scholar]
- Weeks JR, 1962. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science (80-.) 138, 143–144. 10.1126/science.138.3537.143 [DOI] [PubMed] [Google Scholar]
- Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S, 2006. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. 7, 60–70. 10.1111/j.1526-4637.2006.00091.x [DOI] [PubMed] [Google Scholar]
- Wiech K, Tracey I, 2009. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage 47, 987–994. 10.1016/j.neuroimage.2009.05.059 [DOI] [PubMed] [Google Scholar]
- Williams LS, Jones WJ, Shen J, Robinson RL, Kroenke K, 2004. Outcomes of newly referred neurology outpatients with depression and pain. Neurology 63, 674–677. 10.1212/01.wnl.0000134669.05005.95 [DOI] [PubMed] [Google Scholar]
- Wise RA, 1996. Addictive drugs and brain stimulation reward. Annu Rev Neurosci 19, 319–340. 10.1146/annurev.ne.19.030196.001535 [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Vowles KE, 2018. Alcohol and Opioid Use, Co-Use, and Chronic Pain in the Context of the Opioid Epidemic: A Critical Review. Alcohol Clin Exp Res 42, 478–488. 10.1111/acer.13594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woller SA, Moreno GL, Hart N, Wellman PJ, Grau JW, Hook MA, 2012. Analgesia or addiction?: implications for morphine use after spinal cord injury. J Neurotrauma 29, 1650–1662. 10.1089/neu.2011.2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman SC, Alhassoon OM, Hall MG, Stern MJ, Connors EJ, Kimmel CL, Allen KE, Stephan RA, Radua J, 2017. Gray matter abnormalities in opioid-dependent patients: A neuroimaging meta-analysis. Am J Drug Alcohol Abus. 43, 505–517. 10.1080/00952990.2016.1245312 [DOI] [PubMed] [Google Scholar]
- Yeung EW, Craggs JG, Gizer IR, 2017. Comorbidity of Alcohol Use Disorder and Chronic Pain: Genetic Influences on Brain Reward and Stress Systems. Alcohol Clin Exp Res 41, 1831–1848. 10.1111/acer.13491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Lubman DI, 2007. Neurocognitive and neuroimaging evidence of behavioural dysregulation in human drug addiction: implications for diagnosis, treatment and prevention. Drug Alcohol Rev. 26, 33–9. 10.1080/09595230601036978 [DOI] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stockl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A, 2007. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology 80, 65–119. 10.1159/000103923 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tao W, Hou Y-Y, Wang W, Lu Y-G, Pan ZZ, 2014. Persistent pain facilitates response to morphine reward by downregulation of central amygdala GABAergic function. Neuropsychopharmacology 39, 2263–71. 10.1038/npp.2014.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Liu S, Cabeza de Vaca S, Carr KD, 2013. Effects of time of feeding on psychostimulant reward, conditioned place preference, metabolic hormone levels, and nucleus accumbens biochemical measures in food-restricted rats. Psychopharmacology (Berl). 227, 307–20. 10.1007/s00213-013-2981-4 [DOI] [PMC free article] [PubMed] [Google Scholar]


