Abstract
Mammalian cells, with the exception of erythrocytes, harbor mitochondria, which are organelles that provide energy, intermediate metabolites, and additional activities to sustain cell viability, replication, and function. Mitochondria contain multiple copies of a circular genome, or mtDNA, whose individual sequences are rarely identical (homoplasmy) because of inherited or sporadic mutations that result in multiple mtDNA genotypes (heteroplasmy). Here, we examine potential mechanisms for maintenance or shifts in heteroplasmy that occur in induced pluripotent stem cells (iPSCs) generated by cellular reprogramming, and further discuss manipulations that can alter heteroplasmy to impact stem and differentiated cell performance. This additional insight will assist in developing more robust iPSC-based models of disease and differentiated cell therapies.
Keywords: mitochondrial DNA, pluripotency, heteroplasmy, iPSC, reprogramming
iPSCs: Today and the Future
Induced pluripotent stem cells
(iPSCs, see Glossary) and embryonic stem cells (ESCs) are promising cell types for ex vivo disease modeling, drug screening, and upcoming applications in regenerative medicine. iPSCs and ESCs self-renew without limit in tissue culture and can form any cell type in our bodies. Since their introduction in 2006, iPSCs have become a major focus for both basic and applied research in part because of their unique growth characteristics and cellular properties, and their high potential in personalized medicine without the ethical implications carried by the derivation of ESCs [1]. Tremendous effort has been focused on mechanisms and manipulations that regulate and optimize stem cell pluripotency and differentiation. For example, autologous therapies from differentiated iPSCs can be made from any individual with the promise of reduced immunogenicity compared to allogeneic, non-self ESC-derived therapeutics [2]. As of August 2020, there were over 600 active clinical trials involving stem cells of any type, which is an indication of strong and growing interest in applying these cells in future therapies. However, only seven of these trials utilized iPSCs in any manner, typically ex vivo (ClinicalTrials.gov). Furthermore, there currently are no FDA approved treatments involving iPSCs or their derivatives, with a deeper understanding of their derivation, maintenance, and differentiation critical for improving their efficacy and assuring safety in clinical and laboratory applications [3, 4].
Amongst an array of potential impediments to overcome for bringing iPSCs into the clinic, the mitochondria, and its genome, mtDNA, may play key roles. Recent findings indicate that iPSCs and ESCs depend on cellular metabolism, and especially upon mitochondria, to maintain pluripotency and develop functional, differentiated cell types. Beyond optimizing stem cell functions through improved basic understanding, recent studies of iPSCs show that mutations in mtDNA that develop during cellular reprogramming can facilitate transplanted cell immune rejection [5, 6]. Therefore, we now evaluate and discuss what is known about mtDNA changes through somatic cell reprogramming to iPSCs, followed by differentiation into functional cell types. Understanding the dynamics and biology of mtDNA in reprogramming, pluripotency, and differentiation will enable improved disease modeling and drug screening ex vivo, and help to develop safer cell-based regenerative therapies of the future.
Mitochondria and mtDNA Genetics
Mitochondria exist within the cytoplasm of all nucleated mammalian cells as double membrane-bound organelles that contain the circular, maternally inherited double-stranded mtDNA. Structurally, mitochondria exist on a spectrum that spans separate, punctate, ovoid organelles at one extreme to fused, elongated, and branching networks that appear to fill the cell cytoplasm at the other extreme. Each mitochondrion contains dozens to thousands of copies of mtDNA per cell. mtDNA replication occurs independently of nuclear genome (nDNA) replication throughout the cell cycle; however, counter evidence also exists that suggests that the rate of mtDNA replication may vary with cell cycle stage [7, 8]. The ~16.5 Kbp mtDNA encodes for 13 electron transport chain (ETC) proteins in addition to 22 tRNAs and 2 rRNAs for protein translation, all of which are essential for generating ATP by oxidative phosphorylation (OXPHOS). Reports estimating the de novo mtDNA mutation rate range in magnitude; however, this mutation rate is consistently 10x to 100x greater than similar reports for the nDNA [9–11]. mtDNA mutations typically present as deletions or single nucleotide polymorphisms (SNPs) that are either synonymous or nonsynonymous in protein coding regions [12]. In general, cells may exclusively contain mitochondria with only single, identical mtDNA sequences, a condition termed homoplasmy, or they may contain a mixture of different, co-existing mtDNA genotypes, a condition termed heteroplasmy (Box 1) [13–17]. Heteroplasmy is quantified as the copy number ratio of a specific mtDNA sequence to the total mtDNA in a cell, notated as a percentage.
Text Box.
Box 1. Origins and quantification of mtDNA heteroplasmy
Unlike the diploid nuclear genome (nDNA), mtDNA is polyploid and exists as dozens to thousands of copies per cell [107]. mtDNA are dispersed throughout each mitochondrion in tightly compacted nucleoprotein structures, known as nucleoids, that are associated with the matrix side of the mitochondrial inner membrane [108]. Because of the high rate of mtDNA mutation, estimated up to 100x higher than nDNA [10], a cell carrying a single mtDNA sequence (homoplasmy) is comparatively rare. Instead, a mixture of different mtDNA sequences and genotypes are typically present in each cell, creating a condition termed heteroplasmy. Most cells within healthy humans often have low levels of mtDNA mutations, which can remain low or expand and increase over time. These mutations can become significant for human health because mtDNA, which contains no intron sequences unlike nDNA, has coding and non-coding genes and regulatory regions that are essential for the function of OXPHOS.
mtDNA mutations and heteroplasmy are either maternally inherited or may accumulate during aging (Figure 2). The mechanism(s) that cause sporadic mtDNA mutations are heavily debated. Because mtDNA are juxtaposed to the ETC and its oxidative respiratory complexes, one potential mechanism is that ROS introduce base modifications and DNA breaks. However, some studies conclude that ROS does not cause mtDNA mutations [49, 109, 110], but instead that mtDNA lesions are introduced by replication errors [111]. mtDNA replication is continuous even in senescent cells and requires the only nucleus-encoded, mitochondria-localized DNA polymerase, DNA polymerase subunit gamma (Pol γ). Replication errors may occur either by nucleotide imbalances, mutations to Pol γ, or low expression of mitochondrial transcription factor A (TFAM) [76, 112, 113].
To study the role of mtDNA mutations in human health requires robust tools to quantify heteroplasmy, which is defined as the percentage of total copies of a specific mtDNA sequence compared to all mtDNA copies within a cell. Common approaches to quantifying heteroplasmy due to point mutations and deletions include Southern blot, restriction fragment length polymorphism (RFLP), and allele refractory mutation system (ARMS)-qPCR analyses. Advances in digital PCR and next generation sequencing potentially enable even more sensitive methods to quantify mtDNA heteroplasmy [114].
The heteroplasmy percentage of a cell can be dynamic and shift through several different mechanisms. Certain cell types experience reductions in mtDNA copy number during development leading to a genetic bottleneck, in which the proportion of specific mtDNA sequences can be reduced or enriched in the remaining mtDNA population [18]. Moreover, heteroplasmy can shift based on a replicative advantage of certain mtDNAs caused by the biochemical consequences of different mtDNA sequences [19–21]. Some mtDNA mutations cause respiratory dysfunction in cells and confer pathology in organisms, and such mutations were previously considered detrimental only at high mutant heteroplasmy ratios. However, it is now recognized that even low mutant heteroplasmy ratios may result in an increased propensity for certain diseases, with different levels of mutant mtDNA causing threshold effects that lead to different outcomes in cell and organ function. As an example, the m.3243A>G mtDNA mutation is commonly associated with the metabolic disease Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like episodes (MELAS). At 20–30% mutant heteroplasmy, there is an association with type 1 or type 2 diabetes, whereas at 50–80% mutant heteroplasmy the mitochondrial ETC complex I may become dysfunctional and cause cardiomyopathies. Even higher mutant heteroplasmy ratios up to 90–100% are often perinatal lethal or may cause other diseases such as Leigh Syndrome [22–24]. Combined observations over many years indicate that specific cell types, mtDNA mutations, and mutant burden yield cellular and organismal phenotypes that range from unaffected to lethal pathophysiology. Neurological disorders, cardiomyopathies, and muscle dysfunction may occur because specific mtDNA mutations and/or an elevated mutant burden impairs mitochondrial gene expression and/or ETC function, secondarily affecting energy production, the tricarboxylic acid (TCA) cycle, and a range of other essential mitochondrial activities [24, 25]. Because of the importance of heteroplasmy in cellular fitness and disease penetrance, a deep understanding of mitochondria and mtDNA in pluripotent stem cells (PSCs) is essential for effective utilization as research tools and potential therapeutic products.
Pluripotent Stem Cell Mitochondria
The promise of PSC-derivative therapies is inextricably linked to mitochondrial function and intermediate cellular metabolism. Mitochondria in PSCs exist with lower density, perinuclear localization, and punctate morphology compared to mitochondrial features of typical somatic cells. These fragmented PSCs fuse into elongated, branching, filamentous networks with differentiation into cells of the three embryonic germ lineages, ectoderm, endoderm, and mesoderm (Figure 1). Conversely, the mitochondria of somatic cells revert to a lower density, perinuclear localization, and fragmented morphology with disordered mitochondrial cristae and a reduction in mtDNA copy number during cellular reprogramming to pluripotency [26–28]. This shift in mitochondrial morphology from fused networks to punctae upon reprogramming parallels a shift in the stoichiometry of glycolytic and mitochondrial proteins as well. Levels of enzymes and structural proteins that support glycolysis increase, along with ETC complex II, III, and V proteins, whereas the expression of ETC complex I and IV proteins decrease [29, 30]. ETC complex I provides a large multi-subunit protein structure that is essential for regulating OXPHOS. A reduction in the expression of ETC complex I proteins during somatic cell reprogramming shifts energy production and nucleotide biosynthesis for cell replication to an enhanced glycolytic nutrient flux [29] (Figure 1). Accordingly, iPSCs show a relative shift in nutrient utilization towards glycolysis and away from OXPHOS compared to differentiated cells [31]. However, iPSCs also remain dependent on mitochondrial metabolism and the TCA cycle for intermediate metabolites that modify the epigenome, which in turn regulates patterns of gene expression to control stem cell pluripotency and differentiation potential [32–41]. In addition, reactive oxygen species (ROS), often described as a cell damaging and unwanted byproduct of OXPHOS, actually have concentration and species-specific roles in maintaining pluripotency, initiating somatic cell reprogramming, and facilitating iPSC differentiation, all of which depend upon metabolically active mitochondria [42–44]. Yet, despite the reduction in OXPHOS that occurs for iPSCs, mutant heteroplasmy can also reduce somatic cell reprogramming efficiency and affect iPSC performance [22] (Figure 1). Reversible shifts in mitochondrial structure and function associate with pluripotent and terminally differentiated cell states, but to what extent remodeling is a cellular response to or an active driver of cell state conversion and maintenance remains to be fully understood. Quantifying the changes in mitochondrial structure and function during cellular reprogramming helps to elucidate mechanisms of metabolic plasticity and rewiring that occurs during transitions between somatic cells and iPSCs.
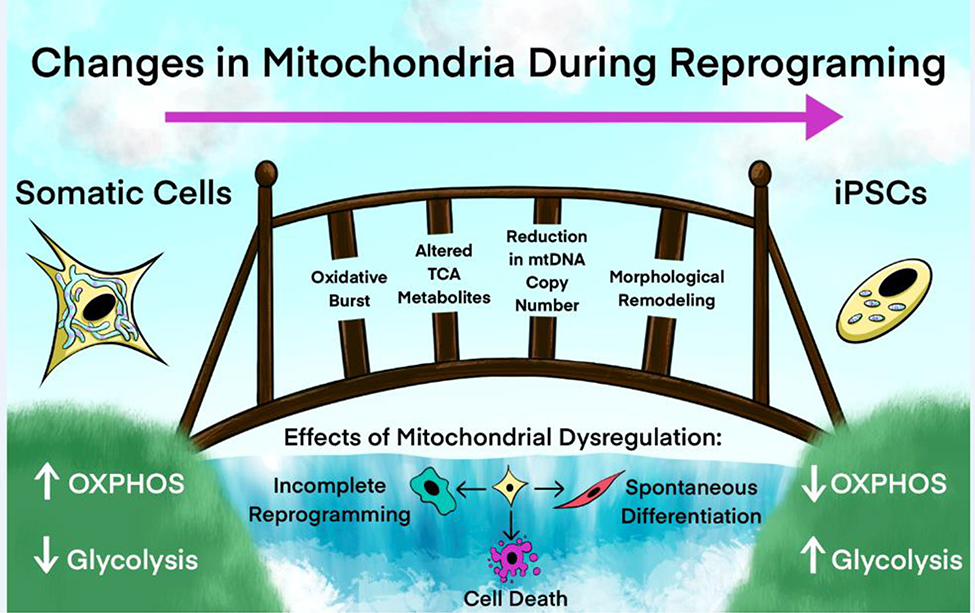
Figure 1.
Remodeling of Mitochondrial Metabolism is Required for Cellular Reprogramming to Pluripotency. Somatic cell reprogramming to iPSCs includes a transition in mitochondrial morphology from an elongated, filamentous and branching network structure to a collection of small, punctate, separate organelles. Concurrent with this morphology shift, metabolism skews from mainly OXPHOS in somatic cells used in this illustration towards mainly glycolytic metabolism in reprogrammed iPSCs. Additional changes that occur during reprogramming include a reduction in mtDNA copy number, alterations in TCA cycle metabolite levels, changes in Ca2+ handling and the production of Fe-S clusters, and a required, time-coordinated oxidative burst. Mitochondrial dysfunction can disrupt these key metabolic transitions and may result in incomplete reprogramming, spontaneous differentiation [19], or cell death.
Somatic Cell Reprogramming and mtDNA Heteroplasmy
Cellular reprogramming does not always generate iPSCs with heteroplasmy identical to the somatic source cells. Mutant mtDNA copy number can be enriched or reduced during somatic cell reprogramming, prolonged iPSC culture, and with iPSC differentiation [5, 45–48]. Monitoring and controlling iPSC mutant heteroplasmy is important because mtDNA mutations can impact iPSC survival, proliferation, and differentiation potential, despite a higher reliance on glycolytic metabolism in pluripotency [49]. For example, iPSCs harboring the m.3243A>G mutation are viable despite decreased OXPHOS and elevated ROS levels; however, reprogramming efficiency was reduced and differentiation potential was impaired, particularly for high energy-demanding cell types such as neurons and cardiomyocytes [22, 50]. In addition, somatic cell reprogramming can cause increased levels and activity of telomerase, which supports indefinite mitotic division [51]. Mitochondrial dysfunction by mutation in somatic cells also reduces telomerase activity and shortens telomere regions of nDNA [52]. It remains unclear whether increased mutant mtDNA heteroplasmy can affect iPSC telomeres; however, differentiated cells from iPSCs with an increased mutant mtDNA burden may show reduced longevity compared to low mutant heteroplasmy counterparts.
Few studies have investigated heteroplasmy changes with somatic cell reprogramming to pluripotency, but the results suggest outcomes with a high degree of variability (Table 1; Figure 2). One study reported that fibroblasts with m.3243A>G mutant heteroplasmy of 77.7% yielded iPSCs with mutant heteroplasmy that ranged from undetectable to 99.4% after reprogramming, with most iPSC clones showing >80% mutant heteroplasmy [53]. A separate study also showed that fibroblasts with elevated mtDNA mutant heteroplasmy also yielded iPSCs with either extremely high or low mutant heteroplasmy [54]. In addition, fibroblasts from different individuals harboring the same mtDNA mutation with similar mutant heteroplasmy ratios may show dramatically different heteroplasmy shifts with reprogramming. In one case, dermal fibroblasts from two different individuals containing the m.3243A>G mutation at similar heteroplasmy levels (69.67% and 66.3%, respectively) showed either an even (1.11% to 85.05%) or a skewed mutant heteroplasmy distribution that contained almost all wild-type (WT) mtDNA in the iPSCs [55]. Reports of elevated mutant heteroplasmy for some iPSC clones do not guarantee that all somatic cells or specific mtDNA mutations with elevated heteroplasmy ratios survive reprogramming. In some cases, iPSCs with >80% mutant heteroplasmy become inviable, enabling iPSCs with more WT mtDNA sequences to overtake a reprogrammed population of cells to skew mutant heteroplasmy lower in the population [48]. Moreover, passaging iPSCs over time may decrease mutant heteroplasmy levels [48, 56, 57].
Table 1.
Heteroplasmy Shifts of mtDNA Mutations Following Cellular Reprogramming to Human iPSCs
| Mutation | Initial heteroplasmy in fibroblasts | Heteroplasmy in iPSCs after reprogramming | Citation |
|---|---|---|---|
| m.3243A>G | 77.70% | 3.6 – 99.4%b | [53] |
| 69.67% | 1.11% – 85.05%b | [55] | |
| 66.30% | 0.67% – 2.00%b | [55] | |
| 29% | 33 – 100%b | [54] | |
| 50% | 47.5 – 97.5%a | [22] | |
| 80% | 62.5% – 92.5%a | [22] | |
| 80% | 67.5% – 97.5%b | [22] | |
| 50% | 47.5% – 92.5%a | [22] | |
| 80% | 72.5% – 97.5%a | [22] | |
| 80% | 67.5% – 97.5%b | [22] | |
| m.13513G>A | 84% | 0 – 100%b | [54] |
| 50% | 0 – 56%b | [48] | |
| m.8993T>C | 52% | 0 – 87%a | [54] |
| 85% | 88% | [104] | |
| 55% | 32% | [105] | |
| m.11778G>C | 100% | 100%* | [106] |
| 100% | 100%* | [106] | |
| m.14484T>C + | 100% | 100%* | [106] |
| m.4160T>C | |||
| m.8344A>G | 90% | 70% | [56] |
| 60% | 60% | [56] |
From a homoplasmic cell line
even distribution
skewed distribution
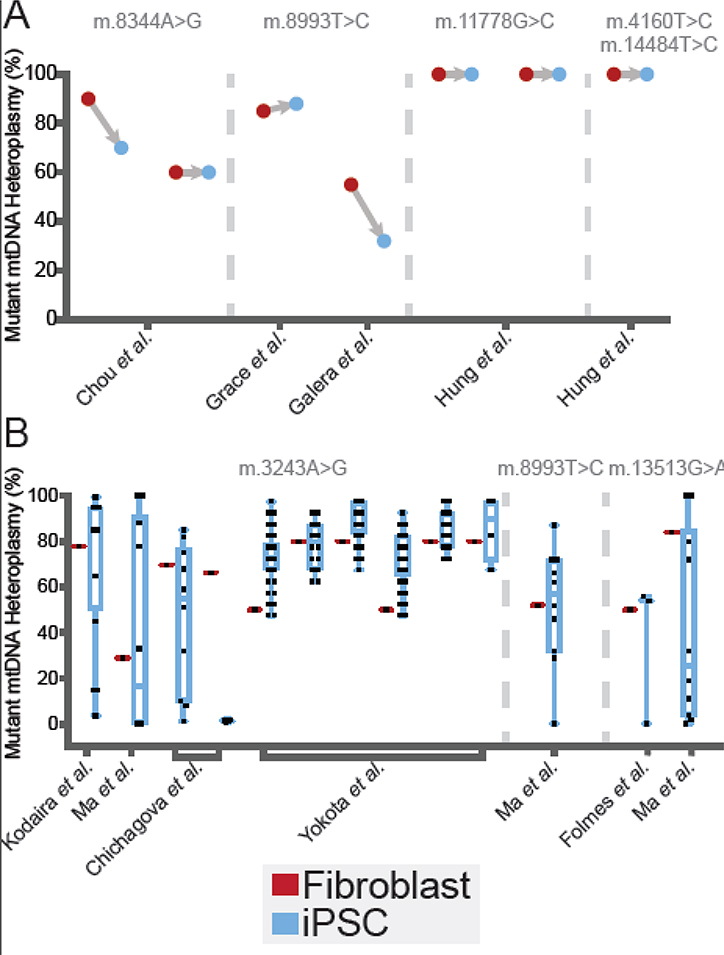
Figure 2.
Meta-Analysis of Heteroplasmy Shifts During Reprogramming. Distributions of measured iPSC clone mutant heteroplasmy from studies listed in Table 1 are plotted alongside the heteroplasmy of the reprogrammed somatic source cells. Reprogrammed somatic cell heteroplasmy is indicated in red and resulting iPSC clone heteroplasmy is indicated in blue. (A) Heteroplasmy shifts measured in a single resulting iPSC clone derived from heteroplasmic starting cell materials. The single iPSC clones analyzed in each experiment are as follows: m.8344A>G from Chou et al. [56], m.8993T>C from Grace et al. [104] and Galera et al. [105], m.11778G>C from Hung et al. [106], and m.4160T>C and m.14484T>C from Hung et al. [106]. (B) Heteroplasmy shifts measured in multiple iPSC clones derived from heteroplasmic starting cell materials. The number of iPSC clones analyzed in each experiment are as follows: m.3243A>G from Kodaira et al. [53] (n= 20, results sourced as averages taken from a binned bar chart and the text), Ma et al. [54] (n=10), Chichagova et al. [55] (listed from left to right: n=10, results sourced from a bar chart and the text, n=4, results sourced from a bar chart and the text), Yokota et al. [22] (results sourced from a bar chart, listed from left to right: n=46, n=18, n=40, n=37, n=20, n=4); m.8993T>C from Ma et al. [54] (n=10); and m.13513G>A from Folmes et al. [48] (n=3), and Ma et al. [54] (n=10).
Altogether, three distinct distributions of mutant heteroplasmy appear to arise in populations of iPSCs generated from heteroplasmic fibroblasts (Figure 3). In one type, iPSCs retain the mutant heteroplasmy ratios of the source somatic cells. In the second mode, we call even distribution, iPSCs show an unbiased range of heteroplasmy from low to high. In the third scenario, we call skewed distribution, mutant heteroplasmy copy number ratios exist as either low, high, or at both extremes relative to WT mtDNA sequences (Table 1). These three distributions suggest potentially different mechanisms of shifting mutant heteroplasmy with reprogramming, which requires further studies to define molecular underpinnings. These observations motivate consideration of heteroplasmy when deriving iPSCs from patient cells and tissues with unknown mutant heteroplasmy levels, or when developing a disease model with iPSCs [46]. Despite the large number of known human mtDNA SNPs (~2,000) and deletions [19, 58], only seven have been studied in iPSCs. This small sample size makes generalizing these phenomena difficult; however, ignoring these shifts can potentially lead to poor reproducibility and differences in functional capabilities for iPSC clones used for developing personalized cell models and regenerative therapeutics.
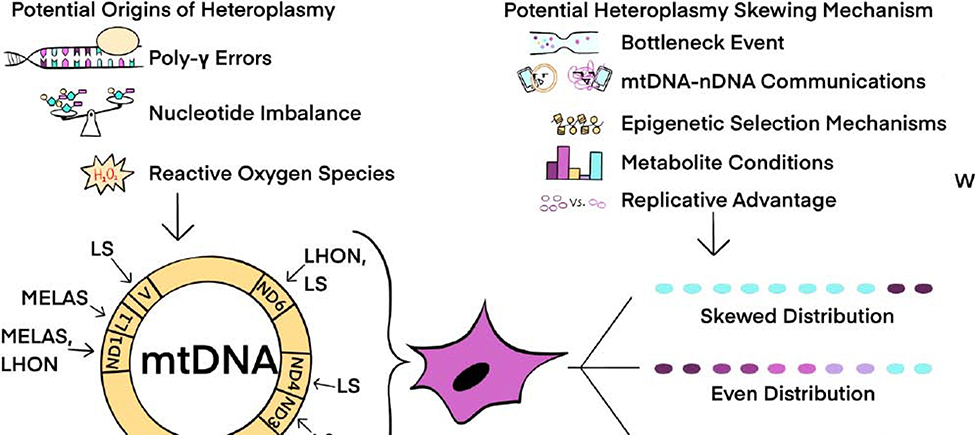
Figure 3.
Potential Origins and Mechanisms of mtDNA Heteroplasmy Shifts During Reprogramming. Every nucleated somatic cell typically contains more than one mtDNA sequence. This situation, called heteroplasmy, can result from mtDNA point mutations and deletions due to several potential mechanisms, including Pol γ replication errors, nucleotide imbalances, and reactive oxygen species-induced mtDNA damage. Fibroblast cell lines with heteroplasmic mtDNA mutations that result in MELAS (Mitochondrial Encephalopathy and Lactic Acidosis Syndrome), LS (Leigh Syndrome), LHON (Leber’s Hereditary Optic Neuropathy), and MERRF (Myoclonic Epilepsy with Ragged Red Fibers) human mtDNA diseases or syndromes have been reprogrammed to iPSCs. Resulting iPSCs can exhibit any of three different potential heteroplasmy patterns between WT and mutant mtDNA, including skewed, even, and retained mtDNA distributions. The mechanism(s) for generating these three distinct mtDNA distributions are not understood, although described mechanisms for somatic cell and germ cell shifts in heteroplasmy may operate during reprogramming and can include a genetic bottleneck, mtDNA-nDNA communication, epigenetic memory, metabolite conditions, and replicative advantages for certain mtDNA sequences. These factors, or their combinations, could lead to the heteroplasmy variations reported for individual iPSC clones.
Potential Mechanisms of Shifting Heteroplasmy with Reprogramming
The mechanisms regulating heteroplasmy shifts during somatic cell reprogramming are currently unknown and understanding them may facilitate the generation of iPSC lines that are functionally consistent. One study measured heteroplasmy in iPSC clones and clonally derived fibroblasts from the same clonal patient-derived fibroblast lines and reported similar heteroplasmic variance in both cell types [22]. This result suggests that heteroplasmy shifts are minimal during reprogramming and that any changes are due to heteroplasmic heterogeneity in the starting cell population. Additional, similar studies will help to confirm whether heteroplasmic drift is from stochastic fluctuations in reprogrammed somatic cells, or whether there are biological pressures that influence heteroplasmy shifts during iPSC derivations. In the absence of additional studies, an inference from work on somatic cells may provide some insight for potentially conserved mechanisms of heteroplasmy selection (Figure 2).
Heteroplasmy selection mechanisms seem to operate at different levels of complexity. mtDNA heteroplasmy is known to shift during reproduction and early development at the cellular level, which has been reviewed extensively elsewhere [59–61]. In these studies, an mtDNA bottleneck, or ‘purifying selection’, occurs during oogenesis with the mtDNA copy number and heteroplasmy ratio dramatically reduced, followed by subsequent re-expansion upon fertilization and implantation. This process can enrich for certain mtDNA sequences and skew heteroplasmy ratios [18]. mtDNA copy number reduction also occurs during cellular reprogramming and may give rise to a similar genetic bottleneck in iPSC generation [27, 28, 62–65]; however, the precise timing of this reduction and segregation during reprogramming remains unclear and warrants further investigations [22, 50, 54].
Recently reported observations show that ‘selective pressure’ for specific heteroplasmy ratios is generated among tissue types with different metabolic requirements. Individual patients can demonstrate tissue-specific mutant heteroplasmy ratios potentially influenced by the unique metabolic demands of different tissues [66]. Specific mtDNA mutations and heteroplasmy ratios may provide a selection advantage through cellular fitness at the mitochondrial level by altering the epigenetic, metabolic, and/or energetic state of a cell. For example, the m.3243A>G mutation can promote tumorigenicity through altered ROS and TCA cycle metabolite concentrations that enhance the proliferation rate of transformed cells [67, 68], and other tumor types show changes in mutant heteroplasmy levels with respect to normal tissue [69–71].
Specifically, work in human prostate cancer shows strong heteroplasmy shifts in malignant cells relative to benign tissue, with higher heteroplasmy ratios correlating with greater metabolic rewiring and reduced patient survival [72]. Similarly, iPSCs require certain metabolic conditions to sustain pluripotency (Figure 1). There may be mtDNA variants that enhance or impair the induction or maintenance of these metabolic states to provide a selective advantage or disadvantage for iPSC proliferation and survival. Reprogramming initiates metabolic rewiring in somatic cells and the energetic and metabolic demands particular to the iPSC fate may generate selective pressure for specific heteroplasmy ratios. Advances in single cell sequencing approaches also help to enable the analysis of cell-to-cell genomic variation [73]. A recent study performed single cell sequencing on patient blood and showed that T cells harbor a reduced level of m.3243A>G heteroplasmy compared to other hematopoietic lineage cells [74]. Moreover, our literature meta-analysis suggests that the analysis of multiple clonal iPSC lines derived from clonal somatic cells are required to unravel heteroplasmy shifts due to reprogramming, which could provide insight into the mechanisms controlling these shifts (Figure 2). Altogether, cellular heterogeneity and tissue-type specific selective pressures may influence heteroplasmy shifts during cellular reprogramming and suggest that single cell approaches to quantify these shifts will provide greater insight into the mechanisms and consequences thereof.
Heteroplasmic selection can occur at the level of mtDNA in individual cells. Mechanisms related to mtDNA replication may influence heteroplasmy, including expression levels and activity of the mitochondrial DNA polymerase, Pol γ, nucleotide imbalances, and selective replication of specific mtDNA sequences, such as those containing deletions [19, 75, 76]. mtDNA deletions and specific point mutations are known to generate a replicative advantage in mitochondrial biogenesis and mtDNA replication [20, 77]. These effects may compound with potential heteroplasmy shifts that occur with cellular reprogramming, followed by mtDNA copy number expansion in iPSCs stimulated to differentiate. The cooperative regulation of mtDNA transcription from elements encoded within the nDNA and mtDNA may represent another genetic level of selective pressure that controls heteroplasmy. In studies of mother-offspring pairs, selection of the mtDNA variants present in the offspring were often influenced by the mother’s nuclear genetic background [16], and the heteroplasmy of iPSCs may be affected by the parental cell source [47]. Additionally, patient fibroblasts with similar heteroplasmy for the same mutation can show unique ranges of iPSC heteroplasmy, perhaps from the influence of nDNA on mtDNA populations [55]. This phenomenon has also been observed in ESCs derived from somatic cell nuclear transfer (SCNT) embryos. ESCs from mtDNA corrected embryos were initially found to contain the donor mtDNA, suggesting that the disease would not arise in future generations [46]. However, these ESCs eventually reverted to the maternal haplotype, indicating likely residual native mtDNA and implying that the compatibility between the mtDNA and the germ cell nDNA could affect mtDNA replication efficiency and the desired heteroplasmy shift. Mitochondrial metabolism may also affect heteroplasmy shifts by influencing the nuclear epigenome through alterations in the levels of alpha-ketoglutarate, succinate, s-adenosylmethionine, and other epigenetic-modifier levels in the cell [24, 78, 79]. Specific heteroplasmy states may poise cells to maintain pluripotency and provide a selective advantage during reprogramming.
Manipulating Heteroplasmy in vitro
With our current lack of detailed mechanistic insight into the regulation of heteroplasmy during cellular reprogramming, methods that manipulate mtDNA sequences and heteroplasmy in somatic tissues may provide a path forward for specifying heteroplasmy ratios in iPSCs. Engineered endonucleases targeted to the mitochondria within cells have been used to degrade mutant mtDNA to enrich for WT heteroplasmy. Mitochondria-targeted Transcription Activator-Like Effector Nucleases (mitoTALENs) degraded mutant mtDNA in fibroblasts prior to reprogramming to pluripotency and differentiation to progeny cells to reduce heteroplasmic levels of m.3243A>G, m.5024 C>T, and m.13513 G>A mutations [57, 80, 81]. To date, only mitoTALENs have shifted the heteroplasmy of iPSCs; however, other endonucleases have the potential to manipulate levels of specific mtDNA sequences in iPSCs including mitochondrial zinc finger nucleases (mitoZFNs) and possibly the CRISPR/Cas gene editing system. mitoZFNs have successfully eliminated mutant mtDNA from human osteosarcoma cells [82], and two studies targeted mitoZFNs to the m.5024C>T mutation in mice, which showed a reduction of mutant mtDNA copy number in heart and skeletal muscle cells. However, neither study fully eliminated mutant heteroplasmy or measured the long-term effects of mitoZFN treatment on mutant heteroplasmy [83, 84]. The CRISPR/Cas gene editing system has also been shown to degrade specific mtDNAs [85, 86]. However, all endonuclease-enabled mtDNA manipulation tools are limited to eliminating specific mtDNA sequences and are unable to yield genetic knock-ins, which require the function of specific DNA repair mechanisms that are not as extensive or robust in the mitochondria as those in the nucleus. Some mtDNA repair mechanisms are well understood and characterized, such as base excision repair, whereas other mechanisms, such as double-stranded DNA and nucleotide excision repair, are poorly elucidated, as reviewed elsewhere [87, 88]. In addition to our current limited knowledge on the effects of endonucleases in stem cells, endonucleases have potential off-target effects, are time consuming to design for specific sequences, and must be engineered for import into the mitochondrial matrix [81, 83]. Investigators have also looked beyond endonucleases to manipulate cellular pools of mtDNA. An exciting new study of a modified bacterial cytidine deaminase toxin showed direct editing of the mtDNA in transformed human cells, which shifted heteroplasmy and induced functional metabolic changes with minimal off-target activity [89]. This technology currently has a ~30% targeting efficiency and has promise for in vitro and in vivo mtDNA editing but has yet to be used in iPSCs or other stem cell types.
The transfer of mitochondria containing WT mtDNA sequences into somatic cells followed by reprogramming to iPSCs provides a potential route towards eliminating deleterious mtDNA mutations. Cytoplasmic hybrids, or ‘cybrids’, provide one type of widely used mitochondrial transfer approach almost exclusively applied to generating transformed, immortalized cells containing an mtDNA genotype of interest. In one study, cybridization corrected cells with homoplasmy for m.14484T>C and m.4160T>C mutations in a patient sample, producing one viable, non-immortal cybrid fibroblast line out of 12 total lines that contained only non-mutant mtDNA [90]. Cells from this one successful line were later reprogrammed to iPSCs and differentiated into retinal ganglion cells that appeared to contain no mutant mtDNA. Currently this is the only study to successfully reprogram cybrids with a corrected mtDNA mutation, and further work is needed to assess the reproducibility, efficiency, or generality of this approach in iPSCs.
Additional approaches to stably introduce exogenous mtDNA sequences involve transferring isolated mitochondria into cells. A growing literature shows mitochondrial coincubation with mammalian cells yields efficient uptake of mitochondria from the culture medium with a range of changes to cell energetics and activity, but the majority of these studies report on effects seen within a limited time frame following transfer [91, 92]. Several reports show stable integration of exogenous mtDNA from isolated mitochondrial coincubation but require high levels of isolated mitochondria or antibiotic selection schemes that limit the mtDNA genotypes that can be transferred [93, 94]. While coincubation facilitates mitochondrial uptake, long-term maintenance of stably integrated exogenous mtDNA is difficult to achieve.
Microinjection has been used to directly transfer mitochondria into the cytoplasm of human cells to yield stable mitochondrial transfer [95], however, the method is laborious and prone to damaging cells by disrupting the plasma membrane when inserting the glass microneedle. One recent technology that reduces the damage caused to recipient cells, called a ‘photothermal nanoblade’, was used to transfer isolated mitochondria into transformed cells that lacked mtDNA to engineer cellular metabolism [96]. However, this approach and its high throughput variant, the Biophotonic Laser Assisted Surgery Tool, have limited accessibility and have not been used directly with PSCs because of their requirement for expertise in lasers and advanced optics [97]. A variation of this technology, called MitoPunch, uses a solenoid driven piston to deliver isolated mitochondria into adherent mtDNA-depleted mammalian cells [98, 99]. This method has been used to generate fibroblasts that stably express homoplasmic exogenous mtDNA and yield functional iPSCs after reprogramming [100]. These methods have not been used to alter heteroplasmy directly in iPSCs, but they represent the potential of altering the mtDNA content of somatic cells that are then reprogrammed for downstream applications. Isolated mitochondrial transfer into somatic cells using these and a range of other methods, including MitoCeption and Magnetomitotransfer, to promote mitochondrial uptake by recipient cells [91, 101], is an exciting new area of research that holds promise for generating future cellular therapeutics for individuals living with mutant mtDNA-caused disorders (Figure 4).
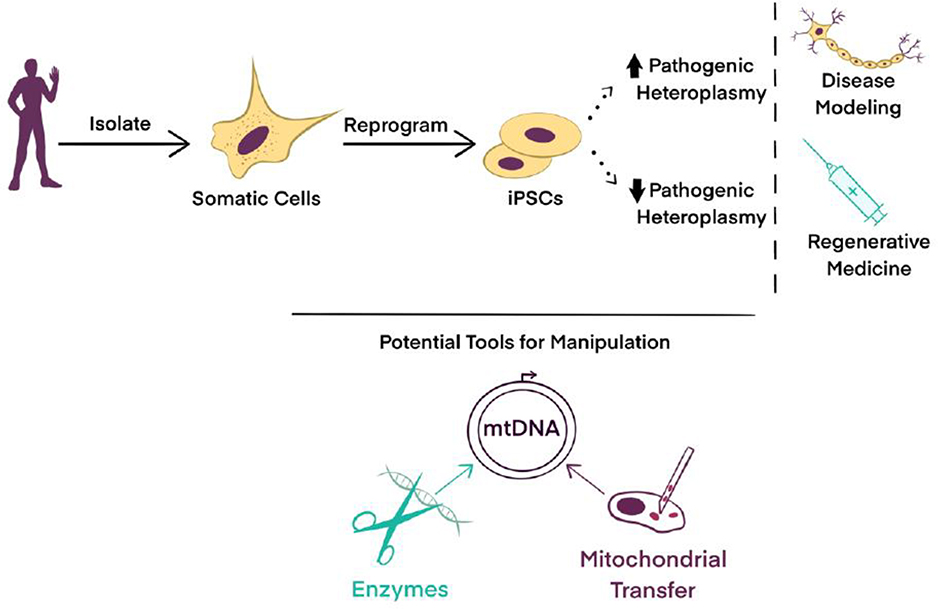
Figure 4.
Controlling Heteroplasmy in iPSCs for Clinical Applications. The utility of reprogramming patient-derived somatic cells into iPSCs may become limited for many reasons including the uncontrolled generation of suboptimal mtDNA heteroplasmy ratios. Tools and approaches have been developed to controllably manipulate the mtDNA content in somatic cells that can then be converted to iPSCs, or in iPSCs themselves, including targeted endonucleases, somatic cell nuclear transfer, and engineered mitochondrial transfer modalities. Methodologies to specify heteroplasmy levels in iPSCs could improve applications in disease modeling, drug screening, and regenerative medicine by tailoring mtDNA populations for specific applications.
Concluding Remarks
iPSCs hold great promise for studies in development, physiology, pathophysiology, and for future treatments of a wide array of diseases. Despite their therapeutic potential, several roadblocks have slowed the progress of iPSC products into clinical applications. Here, we evaluate and discuss one potential barrier that has been largely under the radar concerning mtDNA heteroplasmy shifts during somatic cell reprogramming and the importance of heteroplasmy ratios for iPSC functions and utility. We discuss the metabolic and mitochondrial demands of somatic cell reprogramming and put forth a conceptual framework based on the currently limited experimental data available to define three distinct heteroplasmy distributions identified within iPSC populations. We postulate mechanisms for these shifts based on evidence from studies of somatic and germ cells and encourage the community to expand on these experiments to enhance our understanding of mtDNA genetics in iPSCs. Finally, we explore current and future technologies and techniques to manipulate mtDNA sequences in order to model and possibly correct diseases of the mitochondria. Understanding the mechanisms that foster heteroplasmy shifts in somatic cell reprogramming should lead to the production of reproducible, consistent, and better-defined iPSC populations (see Outstanding Questions). An increased focus in this area of research is necessary to determine whether there are predictable shifts of heteroplasmy during cellular reprogramming, and careful time course experimentation using single cell -omics approaches will aid in understanding which selective pressures may be driving such changes. In addition, implementing recent advances in mitochondrial transfer and mtDNA genome editing techniques in tractable in vitro cell systems will enable studies of how heteroplasmy effects reprogramming, iPSC function, and iPSC differentiation into other cell types. This increased understanding will provide tools to manipulate mutant mtDNA levels in cells for disease modeling or therapeutic applications. Even simpler methods, such as partial mtDNA depletion or single cell expansions, may provide paths forward to remove deleterious mtDNA sequences from established or novel patient-derived iPSC lines [102].
Outstanding Questions.
Will mtDNA heteroplasmy in iPSCs adversely affect differentiated cell therapies in regenerative medicine?
What are the metabolic and genetic regulators of mtDNA heteroplasmy?
What is the timing of mtDNA heteroplasmy shift(s) during reprogramming to pluripotency?
Does the reduction in mtDNA copy number during cellular reprogramming resemble the mtDNA bottleneck that occurs in oocyte development?
Can cytidine deaminase toxin enable efficient, on-target mtDNA editing in stem cells, including iPSCs and ESCs?
There exist hundreds of documented disease-causing mutations to the mtDNA [103], and yet we do not know the rates at which specific mutations expand within cells of different fates, or how other sequences may become eliminated. Further advances in methods to control mtDNA sequences and the ratios of these sequences within cells are needed to minimize the risk of detrimental outcomes for patients treated in the future with stem cell-based products. The role of mitochondria as simple cellular “power plants” is an oversimplification, and the broad range of mitochondrial functions, all of which are affected by mtDNA heteroplasmy, touch on most if not all aspects of cell and organismal biology, directly or indirectly. Perhaps the future of cell based therapeutics depends on our ability to understand and manipulate this second, often overlooked, cellular genome.
Highlights.
Induced pluripotent stem cells (iPSCs) can differentiate into clinically relevant cell types, but understanding how mtDNA changes with reprogramming and how these changes affect iPSC and progeny metabolism will help to maximize their disease modeling, drug screening, and therapeutic potential.
Reprogramming of somatic cells with mtDNA heteroplasmy can yield retained, evenly distributed, or skewed iPSC heteroplasmy ratios that will affect mitochondrial metabolism and potentially cell performance.
mtDNA manipulation techniques have potential for controlling the range of mtDNA genotypes within iPSCs and their differentiated progeny cells for desired applications.
Acknowledgements
A.J.S. has support from two NIH National Research Service Award fellowships (T32GM007185 and T32CA009120). N.M.C was supported by the California Institute for Regenerative Medicine Grant EDUC2-08411. A.N.P. has support from the NIH (T32CA009120) and American Heart Association (18POST34080342). M.A.T. is supported by the Air Force Office of Scientific Research (FA9550-15-1-0406), the NIH (R01GM114188, R01GM073981, R01CA185189, R21CA227480, R01GM127985, and P30CA016042), and by CIRM (RT3-07678).
Glossary
- Biophotonic Laser Assisted Surgery Tool
High-throughput transmembrane delivery device that uses a 532 nm wavelength non-damaging laser pulse to enable the almost simultaneous transfer of up to micron-sized cargo directly into the cytoplasm of ~2 × 105 adherent mammalian cells.
- Genetic bottleneck
Phenomenon that occurs when the mtDNA copy number falls dramatically, leaving a smaller subset of the original mtDNA genotypes that were initially present remaining in a cell. After such an event, the proportions of different mtDNA genotypes can shift and may not recapitulate the same proportions found before the bottleneck.
- Heteroplasmy
The state of more than one mitochondrial genotype existing within a cell. The greater the heteroplasmy of an mtDNA variant, the higher its copy number relative to other mtDNA genotypes within that cell.
- Homoplasmy
The state of only having one mitochondrial genotype within a cell.
- Induced pluripotent stem cell (iPSC)
A pluripotent stem cell derived from somatic cells that have undergone cellular reprogramming and reversion to pluripotency typically by viral transduction or mRNA transfection. iPSCs can be generated from the cells of different tissues of healthy or diseased individuals.
- MitoPunch
High-throughput mitochondrial transfer device that uses pressure to deliver isolated mitochondria directly into the cytoplasm of ~ 2 × 105 cells simultaneously.
- OXPHOS
Abbreviation for mitochondrial oxidative phosphorylation. This biochemical process utilizes the transport of elections sourced from biomolecule catabolism between the protein complexes of the mitochondrial electron transport chain to generate a hydrogen ion gradient and electrochemical potential across the mitochondrial inner membrane, which enables the activity of complex V ATP synthase.
- Photothermal Nanoblade
Single-cell intracellular delivery device that uses a 532 nm wavelength non-damaging laser pulse to enable delivery of up to micron-sized cargo into individual adherent cells.
- Pluripotent
This state describes a cell that has the potential to differentiate into cells of all three embryonic germ layers and, thus, any cell within the body.
- Replicative advantage
Increase in replication rate of specific mtDNA genotypes relative to other mtDNA species in a cell. This may include mtDNA deletions that reduce the number of nucleotides that must be polymerized to increase the speed of mtDNA replication, and some mtDNA point mutations that can enhance replication by other biochemical means.
- Threshold effect
The phenomenon of mutant mtDNA causing different phenotypic effects based on the proportion of mutant to wild type mtDNA present. Many copies of mtDNA exists within cells, allowing wild type mtDNA to offset the deleterious effects of mutant mtDNA with progressively greater penetrance of the mutant phenotype in situations of greater representation within a cell.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takahashi K and Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 [DOI] [PubMed] [Google Scholar]
- 2.Guha P, et al. (2013) Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell 12, 407–412 [DOI] [PubMed] [Google Scholar]
- 3.Liu X, et al. (2017) The Immunogenicity and Immune Tolerance of Pluripotent Stem Cell Derivatives. Frontiers in Immunology 8, 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garreta E, et al. (2018) Roadblocks in the Path of iPSC to the Clinic. Current Transplantation Reports 5, 14–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deuse T, et al. (2019) De novo mutations in mitochondrial DNA of iPSCs produce immunogenic neoepitopes in mice and humans. Nat Biotechnol 37, 1137–1144 [DOI] [PubMed] [Google Scholar]
- 6.Deuse T, et al. (2015) SCNT-derived ESCs with mismatched mitochondria trigger an immune response in allogeneic hosts. Cell Stem Cell 16, 33–38 [DOI] [PubMed] [Google Scholar]
- 7.Magnusson J (2003) Replication of mitochondrial DNA occurs throughout the mitochondria of cultured human cells. 289, 133–142 [DOI] [PubMed] [Google Scholar]
- 8.Sasaki T, et al. (2017) Live imaging reveals the dynamics and regulation of mitochondrial nucleoids during the cell cycle in Fucci2-HeLa cells. Scientific Reports DOI: 10.1038/s41598-017-10843-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludwig LS, et al. (2019) Lineage Tracing in Humans Enabled by Mitochondrial Mutations and Single-Cell Genomics. Cell 176, 1325–1339 e1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart JB and Chinnery PF (2015) The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nature Reviews Genetics 16, 530–542 [DOI] [PubMed] [Google Scholar]
- 11.Lawless C, et al. (2020) The rise and rise of mitochondrial DNA mutations. Open Biology 10, 200061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omasanggar R, et al. (2020) Mitochondrial DNA mutations in Malaysian female breast cancer patients. PLOS ONE DOI: 10.1371/journal.pone.0233461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Temperley RJ, et al. (2010) Human mitochondrial mRNAs--like members of all families, similar but different. Biochim Biophys Acta 1797, 1081–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgstaller JP, et al. (2014) MtDNA segregation in heteroplasmic tissues is common in vivo and modulated by haplotype differences and developmental stage. Cell Rep 7, 2031–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace DC and Chalkia D (2013) Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb Perspect Biol 5, a021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei W, et al. (2019) Germline selection shapes human mitochondrial DNA diversity. Science 364, eaau6520. [DOI] [PubMed] [Google Scholar]
- 17.Soares P, et al. (2009) Correcting for purifying selection: an improved human mitochondrial molecular clock. Am J Hum Genet 84, 740–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaidi AA, et al. (2019) Bottleneck and selection in the germline and maternal age influence transmission of mitochondrial DNA in human pedigrees. Proceedings of the National Academy of Sciences 116, 25172–25178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell OM, et al. (2018) Preferential amplification of a human mitochondrial DNA deletion in vitro and in vivo. Sci Rep DOI: 10.1038/s41598-018-20064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoneda M, et al. (1992) Marked replicative advantage of human mtDNA carrying a point mutation that causes the MELAS encephalomyopathy. 89, 11164–11168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner JT, et al. (2020) Mitochondrial DNA Variation and Selfish Propagation Following Experimental Bottlenecking in Two Distantly Related Caenorhabditis briggsae Isolates. Genes 11, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokota M, et al. (2015) Mitochondrial respiratory dysfunction caused by a heteroplasmic mitochondrial DNA mutation blocks cellular reprogramming. Hum Mol Genet 24, 4698–4709 [DOI] [PubMed] [Google Scholar]
- 23.Matsubara M, et al. (2018) Analysis of mitochondrial function in human induced pluripotent stem cells from patients with mitochondrial diabetes due to the A3243G mutation. Sci Rep DOI: 10.1038/s41598-018-19264-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopinski PK, et al. (2019) Regulation of nuclear epigenome by mitochondrial DNA heteroplasmy. Proc Natl Acad Sci U S A 116, 16028–16035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirose M, et al. (2018) Low-level mitochondrial heteroplasmy modulates DNA replication, glucose metabolism and lifespan in mice. Sci Rep DOI: 10.1038/s41598-018-24290-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo B, et al. (2018) Mitochondrial Dynamics in Stem Cells and Differentiation. International Journal of Molecular Sciences 19, 3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonora M, et al. (2012) ATP synthesis and storage. Purinergic Signal 8, 343–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krebs HA (1940) The citric acid cycle and the Szent-Gyorgyi cycle in pigeon breast muscle. Biochem J 34, 775–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansson J, et al. (2012) Highly coordinated proteome dynamics during reprogramming of somatic cells to pluripotency. Cell Rep 2, 1579–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prigione A, et al. (2014) HIF1alpha modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1–3 and PKM2. Stem Cells 32, 364–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho YM, et al. (2006) Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun 348, 1472–1478 [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, et al. (2018) Metabolism in Pluripotent Stem Cells and Early Mammalian Development. Cell Metab 27, 332–338 [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, et al. (2016) Distinct Metabolic States Can Support Self-Renewal and Lipogenesis in Human Pluripotent Stem Cells under Different Culture Conditions. Cell Rep 16, 1536–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z, et al. (2018) Mitochondrial Metabolism in Major Neurological Diseases. Cells 7, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Appling DR (1991) Compartmentation of folate-mediated one-carbon metabolism in eukaryotes. FASEB J 5, 2645–2651 [DOI] [PubMed] [Google Scholar]
- 36.Watson JA and Lowenstein JM (1970) Citrate and the conversion of carbohydrate into fat. Fatty acid synthesis by a combination of cytoplasm and mitochondria. J Biol Chem 245, 5993–6002 [PubMed] [Google Scholar]
- 37.Carey BW, et al. (2015) Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518, 413–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guitart AV, et al. (2017) Fumarate hydratase is a critical metabolic regulator of hematopoietic stem cell functions. J Exp Med 214, 719–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang Y, et al. (2019) Sirtuins in Metabolic and Epigenetic Regulation of Stem Cells. Trends Endocrinol Metab 30, 177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moussaieff A, et al. (2015) Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab 21, 392–402 [DOI] [PubMed] [Google Scholar]
- 41.Mews P, et al. (2017) Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature 546, 381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou G, et al. (2016) Optimal ROS Signaling Is Critical for Nuclear Reprogramming. Cell Rep 15, 919–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boland MJ, et al. (2014) Epigenetic regulation of pluripotency and differentiation. Circ Res 115, 311–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramakrishna S, et al. (2014) Posttranslational modifications of defined embryonic reprogramming transcription factors. Cell Reprogram 16, 108–120 [DOI] [PubMed] [Google Scholar]
- 45.Hamalainen RH (2016) Mitochondrial DNA mutations in iPS cells: mtDNA integrity as standard iPSC selection criteria? EMBO J 35, 1960–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang E, et al. (2016) Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature 540, 270–275 [DOI] [PubMed] [Google Scholar]
- 47.Perales-Clemente E, et al. (2016) Natural underlying mtDNA heteroplasmy as a potential source of intra-person hiPSC variability. EMBO J 35, 1979–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folmes CD, et al. (2013) Disease-causing mitochondrial heteroplasmy segregated within induced pluripotent stem cell clones derived from a patient with MELAS. Stem Cells 31, 1298–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dahan P, et al. (2019) Metabolism in pluripotency: Both driver and passenger? J Biol Chem 294, 5420–5429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pickrell AM and Youle RJ (2013) Mitochondrial disease: mtDNA and protein segregation mysteries in iPSCs. Curr Biol 23, R1052–R1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allsopp R (2012) Telomere length and iPSC re-programming: survival of the longest. Cell Research 22, 614–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou M-C, et al. (2015) Analysis of association among clinical features and shorter leukocyte telomere length in mitochondrial diabetes with m.3243A>G mitochondrial DNA mutation. BMC Medical Genetics DOI: 10.1186/s12881-015-0238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kodaira M, et al. (2015) Impaired respiratory function in MELAS-induced pluripotent stem cells with high heteroplasmy levels. FEBS Open Bio 5, 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma H, et al. (2015) Metabolic rescue in pluripotent cells from patients with mtDNA disease. Nature 524, 234–238 [DOI] [PubMed] [Google Scholar]
- 55.Chichagova V, et al. (2017) Human iPSC disease modelling reveals functional and structural defects in retinal pigment epithelial cells harbouring the m.3243A > G mitochondrial DNA mutation. Sci Rep DOI: 10.1038/s41598-017-12396-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chou SJ, et al. (2016) Impaired ROS Scavenging System in Human Induced Pluripotent Stem Cells Generated from Patients with MERRF Syndrome. Sci Rep DOI: 10.1038/srep23661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yahata N, et al. (2017) TALEN-mediated shift of mitochondrial DNA heteroplasmy in MELAS-iPSCs with m.13513G>A mutation. Sci Rep DOI: 10.1038/s41598-017-15871-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ju YS, et al. (2014) Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. Elife DOI: 10.7554/eLife.02935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jackson CB, et al. (2020) Therapeutic Manipulation of mtDNA Heteroplasmy: A Shifting Perspective. Trends in Molecular Medicine 26, 698–709 [DOI] [PubMed] [Google Scholar]
- 60.Van Den Ameele J, et al. (2020) Mitochondrial heteroplasmy beyond the oocyte bottleneck. Seminars in Cell & Developmental Biology 97, 156–166 [DOI] [PubMed] [Google Scholar]
- 61.Hahn A and Zuryn S (2019) The Cellular Mitochondrial Genome Landscape in Disease. Trends Cell Biol 29, 227–240 [DOI] [PubMed] [Google Scholar]
- 62.Hamalainen RH, et al. (2013) Tissue- and cell-type-specific manifestations of heteroplasmic mtDNA 3243A>G mutation in human induced pluripotent stem cell-derived disease model. Proc Natl Acad Sci U S A 110, E3622–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pessoa LV, et al. (2015) Mitochondrial DNA dynamics during in vitro culture and pluripotency induction of a bovine Rho0 cell line. Genet Mol Res 14, 14093–14104 [DOI] [PubMed] [Google Scholar]
- 64.Latorre-Pellicer A, et al. (2019) Regulation of Mother-to-Offspring Transmission of mtDNA Heteroplasmy. Cell Metab 30, 1120–1130 e1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Floros VI, et al. (2018) Segregation of mitochondrial DNA heteroplasmy through a developmental genetic bottleneck in human embryos. Nat Cell Biol 20, 144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He Y, et al. (2010) Heteroplasmic mitochondrial DNA mutations in normal and tumour cells. Nature 464, 610–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gammage PA and Frezza C (2019) Mitochondrial DNA: the overlooked oncogenome? BMC Biology DOI: 10.1186/s12915-019-0668-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ishikawa K, et al. (2008) ROS-Generating Mitochondrial DNA Mutations Can Regulate Tumor Cell Metastasis. Science 320, 661–664 [DOI] [PubMed] [Google Scholar]
- 69.Park JS, et al. (2009) A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Human Molecular Genetics 18, 1578–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qi Y, et al. (2016) Heteroplasmy of mutant mitochondrial DNA A10398G and analysis of its prognostic value in non-small cell lung cancer. Oncology Letters 12, 3081–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan Y, et al. (2020) Comprehensive molecular characterization of mitochondrial genomes in human cancers. Nature Genetics 52, 342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schöpf B, et al. (2020) OXPHOS remodeling in high-grade prostate cancer involves mtDNA mutations and increased succinate oxidation. Nature Communications DOI: 10.1038/s41467-020-15237-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gawad C, et al. (2016) Single-cell genome sequencing: current state of the science. Nature Reviews Genetics 17, 175–188 [DOI] [PubMed] [Google Scholar]
- 74.Walker MA, et al. (2020) Purifying Selection against Pathogenic Mitochondrial DNA in Human T Cells. New England Journal of Medicine 383, 1556–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiang ACY, et al. (2019) A Genome-wide Screen Reveals that Reducing Mitochondrial DNA Polymerase Can Promote Elimination of Deleterious Mitochondrial Mutations. Current Biology 29, 4330–4336.e4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song S, et al. (2005) DNA precursor asymmetries in mammalian tissue mitochondria and possible contribution to mutagenesis through reduced replication fidelity. Proceedings of the National Academy of Sciences of the United States of America 102, 4990–4995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fukui H and Moraes CT (2009) Mechanisms of formation and accumulation of mitochondrial DNA deletions in aging neurons. Human Molecular Genetics 18, 1028–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Picard M, et al. (2014) Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proceedings of the National Academy of Sciences 111, E4033–E4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McManus MJ, et al. (2019) Mitochondrial DNA Variation Dictates Expressivity and Progression of Nuclear DNA Mutations Causing Cardiomyopathy. Cell Metabolism 29, 78–90.e75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang Y, et al. (2018) Targeted elimination of mutant mitochondrial DNA in MELAS-iPSCs by mitoTALENs. Protein Cell 9, 283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bacman SR, et al. (2018) Author Correction: MitoTALEN reduces mutant mtDNA load and restores tRNA(Ala) levels in a mouse model of heteroplasmic mtDNA mutation. Nat Med 24, 1940. [DOI] [PubMed] [Google Scholar]
- 82.Gammage PA, et al. (2016) Near-complete elimination of mutant mtDNA by iterative or dynamic dose-controlled treatment with mtZFNs. Nucleic Acids Research 44, 7804–7816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gammage PA, et al. (2018) Genome editing in mitochondria corrects a pathogenic mtDNA mutation in vivo. Nat Med 24, 1691–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bacman SR, et al. (2018) MitoTALEN reduces mutant mtDNA load and restores tRNA(Ala) levels in a mouse model of heteroplasmic mtDNA mutation. Nat Med 24, 1696–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jo A, et al. (2015) Efficient Mitochondrial Genome Editing by CRISPR/Cas9. BioMed Research International 2015, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hussain S-RA, et al. (2020) Adapting CRISPR/Cas9 System for Targeting Mitochondrial Genome. BioRxiv DOI: 10.1101/2020.02.11.944819 [DOI] [Google Scholar]
- 87.Zinovkina LA (2018) Mechanisms of Mitochondrial DNA Repair in Mammals. Biochemistry (Moscow) 83, 233–249 [DOI] [PubMed] [Google Scholar]
- 88.Alexeyev M, et al. (2013) The Maintenance of Mitochondrial DNA Integrity--Critical Analysis and Update. Cold Spring Harbor Perspectives in Biology 5, a012641–a012641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mok BY, et al. (2020) A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature 583, 631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong RCB, et al. (2017) Mitochondrial replacement in an iPSC model of Leber’s hereditary optic neuropathy. Aging (Albany NY) 9, 1341–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caicedo A, et al. (2015) MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci Rep DOI: 10.1038/srep09073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kesner EE, et al. (2016) Characteristics of Mitochondrial Transformation into Human Cells. Sci Rep DOI: 10.1038/srep26057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clark MA and Shay JW (1982) Mitochondrial transformation of mammalian cells. Nature 295, 605–607 [DOI] [PubMed] [Google Scholar]
- 94.Patel D, et al. (2017) Macropinocytic entry of isolated mitochondria in epidermal growth factor-activated human osteosarcoma cells. Sci Rep DOI: 10.1038/s41598-017-13227-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.King MP and Attardi G (1988) Injection of mitochondria into human cells leads to a rapid replacement of the endogenous mitochondrial DNA. Cell 52, 811–819 [DOI] [PubMed] [Google Scholar]
- 96.Wu TH, et al. (2016) Mitochondrial Transfer by Photothermal Nanoblade Restores Metabolite Profile in Mammalian Cells. Cell Metab 23, 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu Y-C, et al. (2015) Massively parallel delivery of large cargo into mammalian cells with light pulses. Nat Methods 12, 439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dawson ER, et al. (2020) Stable retention of chloramphenicol-resistant mtDNA to rescue metabolically impaired cells. Sci Rep DOI: 10.1038/s41598-020-71199-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sercel AJ, et al. (2020) Stable transplantation of human mitochondrial DNA by high-throughput, pressurized mitochondrial delivery. BioRxiv DOI: 10.1101/2020.09.15.298174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patananan AN, et al. Pressure-Driven Mitochondrial Transfer Pipeline Generates Mammalian Cells of Desired Genetic Combinations and Fates. Cell Reports (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Macheiner T, et al. (2016) Magnetomitotransfer: An efficient way for direct mitochondria transfer into cultured human cells. Sci Rep DOI: 10.1038/srep35571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kosanke M, et al. (2020) iPSC culture expansion selects against putatively actionable mutations in the mitochondrial genome. BioRxiv DOI: 10.1101/2020.11.05.369694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tuppen HAL, et al. (2010) Mitochondrial DNA mutations and human disease. Biochimica et Biophysica Acta (BBA) - Bioenergetics 1797, 113–128 [DOI] [PubMed] [Google Scholar]
- 104.Grace HE, et al. (2019) mRNA Reprogramming of T8993G Leigh’s Syndrome Fibroblast Cells to Create Induced Pluripotent Stem Cell Models for Mitochondrial Disorders. Stem Cells Dev 28, 846–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Galera T, et al. (2016) Generation of a human iPSC line from a patient with Leigh syndrome. Stem Cell Res 16, 63–66 [DOI] [PubMed] [Google Scholar]
- 106.Hung SS, et al. (2016) Study of mitochondrial respiratory defects on reprogramming to human induced pluripotent stem cells. Aging (Albany NY) 8, 945–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Robin ED and Wong R (1988) Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. Journal of Cellular Physiology 136, 507–513 [DOI] [PubMed] [Google Scholar]
- 108.Gilkerson R, et al. (2013) The Mitochondrial Nucleoid: Integrating Mitochondrial DNA into Cellular Homeostasis. Cold Spring Harbor Perspectives in Biology 5, a011080–a011080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Greaves LC, et al. (2014) Clonal Expansion of Early to Mid-Life Mitochondrial DNA Point Mutations Drives Mitochondrial Dysfunction during Human Ageing. PLoS Genetics DOI: 10.1371/journal.pgen.1004620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kennedy SR, et al. (2013) Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage. PLoS Genetics DOI: 10.1371/journal.pgen.1003794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zheng W, et al. (2006) Origins of human mitochondrial point mutations as DNA polymerase γ-mediated errors. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 599, 11–20 [DOI] [PubMed] [Google Scholar]
- 112.Nissanka N, et al. (2018) The mitochondrial DNA polymerase gamma degrades linear DNA fragments precluding the formation of deletions. Nature Communications DOI: 10.1038/s41467-018-04895-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Szczepanowska K and Trifunovic A (2015) Different faces of mitochondrial DNA mutators. Biochimica et Biophysica Acta (BBA) - Bioenergetics 1847, 1362–1372 [DOI] [PubMed] [Google Scholar]
- 114.González MDM, et al. (2020) Sensitivity of mitochondrial DNA heteroplasmy detection using Next Generation Sequencing. Mitochondrion 50, 88–93 [DOI] [PubMed] [Google Scholar]