Abstract
Molecular hydrogen (H2) is a physiologically inert gas. However, during the last 10 years, increasing evidence has revealed its biological functions under pathological conditions. More specifically, H2 has protective effects against a variety of diseases, particularly nervous system disorders, which include ischemia/reperfusion injury, traumatic injury, subarachnoid hemorrhage, neuropathic pain, neurodegenerative diseases, cognitive dysfunction induced by surgery and anesthesia, anxiety, and depression. In addition, H2 plays protective roles mainly through anti-oxidation, anti-inflammation, anti-apoptosis, the regulation of autophagy, and preservation of mitochondrial function and the blood-brain barrier. Further, H2 is easy to use and has neuroprotective effects with no major side-effects, indicating that H2 administration is a potential therapeutic strategy in clinical settings. Here we summarize the H2 donors and their pharmacokinetics. Meanwhile, we review the effectiveness and safety of H2 in the treatment of various nervous system diseases based on preclinical and clinical studies, leading to the conclusion that H2 can be a simple and effective clinical therapy for CNS diseases such as ischemia-reperfusion brain injury, Parkinson’s disease, and diseases characterized by cognitive dysfunction. The potential mechanisms involved in the neuroprotective effect of H2 are also analyzed.
Keywords: Molecular hydrogen (H2), Neurological disease, Neuroprotection, Anti-oxidation, Anti-inflammation, Anti-apoptosis
Introduction
Molecular hydrogen (H2) was first discovered by the chemist Henry Cavendish in 1766. It is a colorless, odorless, and physiologically inert gas. The biological functions of H2 were gradually confirmed by scientists in the late 20th century. In 1975, Dole [1] discovered that hyperbaric H2 (2.5% O2 and 97.5% H2) causes regression of squamous cell carcinoma in hairless albino mice. He hypothesized that the effect of H2 might be attributed to its ability to scavenge the most damaging oxidant hydroxyl radical (·OH). In 2001, Gharib [2] found that 0.7 MPa H2 has an anti-inflammatory effect on the chronic liver inflammation associated with schistosomiasis. Several years later, in 2007, Shigeo Ohta demonstrated that H2 exhibits protective effects as an antioxidant on brain tissues under oxidative stress by selectively scavenging the cytotoxic free radical ·OH and peroxynitrite (ONOO−) [3]. Since then, these findings have led to a number of studies that have explored the potential protective effects of H2 against a variety of diseases and the related molecular mechanisms.
Diseases caused by abnormalities in the structure and function of the nervous system are usually most devastating disorders a with high incidence of disability and mortality worldwide. The recovery of neural structure and function from either acute injury or chronic neurodegeneration remains challenging, as most neurological diseases do not have effective approaches to cure and are poorly responsive to traditional interventions such as medications, physical therapies, neuro-rehabilitation, and preventative measures. In contrast, accumulating evidence has shown protective effects of H2 against various neurological diseases, including ischemia/reperfusion injury [4–6], traumatic damage [7, 8], subarachnoid hemorrhage (SAH) [9, 10], neuropathic pain [11, 12], Alzheimer’s disease [13, 14], Parkinson’s disease [15, 16], mood disorders [17, 18], glioblastoma [19], and cerebral infarction [20]. To date, more than 60 clinical trials on the use of H2 in many diseases involving multiple systems have been conducted; at least ten of the trials were on diseases in the nervous system, including acute cerebral ischemia [4], acute cerebral infarction [20], post-cardiac arrest syndrome [21, 22], Parkinson’s disease [23, 24], and mood disorders [18].
As a neuroprotective gas, H2 has a variety of advantages. First, it can cross the blood-brain barrier (BBB), penetrate biomembranes, and diffuse into the cytosol and organelles [3]. In addition, it has no documented major side-effects [4]. Importantly, repeated administration of H2 does not cause tolerance [25]. Further, various easy and convenient approaches to its administration are available [26, 27]. Finally, H2 has protective effects against multiple diseases, including peripheral and central nervous system (CNS) diseases [26, 28]. For instance, in Japan, 2% H2 inhalation has been approved for clinical emergency treatment for cardiac arrest. Thus, H2 is a novel and potential therapeutic strategy for the prevention and treatment of a variety of diseases, including neurological diseases. The advantages of H2 have further promoted the development of the H2 healthcare industry.
In this review, we aim to summarize the current knowledge about the neuroprotective effects of H2 against various diseases in the nervous system (Fig. 1) and the possible mechanisms involved (Table 1).
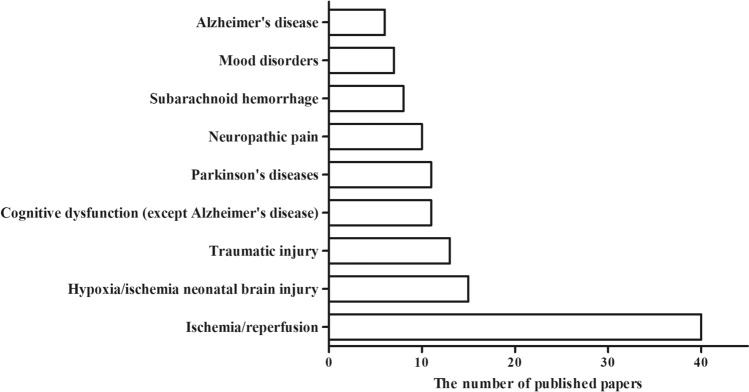
Fig. 1.
The numbers of published papers (clinical trials and animal experiments) in the PubMed database on the use of H2 in various neurological diseases from 2007 to 2020.
Table 1.
Neuroprotective effects of H2 against neurological diseases and related mechanisms.
| Disease type | Object | Hydrogen administration | Mechanism | References |
|---|---|---|---|---|
| Ischemia/reperfusion injury in the CNS | Rat, mouse, rabbit, pig, human | Inhalation, intraperitoneal injection, oral, intravenous injection | Preservation of BBB; preservation of mitochondrial function; inhibition of endoplasmic reticulum stress; anti-oxidation; anti-inflammatory; anti-apoptosis (PI3K/Akt/GSK3β signaling pathway, Cyt c/caspase-3 pathway); microRNA regulation | [5, 6, 20, 29–36] |
| Hypoxia/ischemia neonatal brain injury | Rat, mouse, pig | Inhalation, intraperitoneal injection | Alleviation of oxidative stress (MAPK/HO-1/PGC-1a pathway); inhibition of endoplasmic reticulum stress; promoting autophagy; regulating microglial polarization; improving neurovascular dysfunction | [37–41] |
| Traumatic injury in the CNS | Rat, mouse | Inhalation, intraperitoneal injection, subarachnoid perfusion | Preservation of BBB; attenuation of neuronal apoptosis; preservation of mitochondrial function; anti-oxidation; regulation of oxidative stress-related genes; suppression of astrocyte activation | [7, 8, 42–45] |
| Subarachnoid hemorrhage | Rat, rabbit, | Inhalation, intraperitoneal injection | Preservation of BBB; amelioration of cerebral vasospasm; anti-apoptosis (Akt/GSK3β signaling pathway); inhibition of oxidative stress; inhibition of NF-κB activation and NLRP3 inflammasome formation | [9, 10, 46–49] |
| Neuropathic pain | ||||
| Hyperalgesia | Rat | Intraperitoneal injection | Anti-inflammatory; inhibition of GSK-3β activity and NMDA receptor membrane trafficking; inhibition of oxidative stress (removing ONOO-and blocking MnSOD nitration) | [50–52] |
| Nerve injury | Rat, mouse | Intrathecal infusion, oral, intraperitoneal injection | Suppression of oxidative stress; inhibition of spinal astrocytes and microglia activation; inhibition of inflammation by activating HO-1/CO signaling; down-regulation of p38MAPK and BDNF expression; activation of autophagy via HIF-1α pathways | [11, 12, 25, 53, 54] |
| Parkinson’s disease | Rat, mouse, human | Inhalation, oral | Inhibition of oxidative stress; prevention of the dopaminergic cell loss; activation gastric ghrelin system | [15, 16, 23, 55–58] |
| Alzheimer’s disease | Rat, mouse | Oral, intraperitoneal injection, intracerebral injection | Recovering mitochondrial dysfunction; enhancing the anti-oxidative system by stimulating AMPK and up-regulating Sirt1-FoxO3a axis; suppression of inflammatory response by inhibiting JNK, NF-κB, and NLRP3 activation; activation of E2-ERβ-BDNF signaling pathways | [13, 14, 31, 59–61] |
| Mood disorders | Mouse, human | Inhalation, oral, intraperitoneal injection | Suppression of oxidative stress, anti-apoptosis and anti-inflammation; regulation of hypothalamus-pituitary-adrenal axis activity | [17, 18, 62–64] |
H2 Donors
Currently, there are multiple routes of H2 administration, including inhalation of H2 gas, drinking H2-rich water (HRW), injection of H2-rich saline (HRS), H2-water bathing, intake of a solid H2 carrier (coral calcium hydride), and H2-producing precursors (such as lactulose and L-arabinose) [27, 65–67], as well as functional micro/nanomaterials for targeted H2 delivery [68].
In clinical applications, the common routes of H2 administration mainly include inhalation of H2 gas, drinking HRW, injection of HRS, and H2-water bathing. Inhalation H2 is the simplest and most direct route, and the most commonly used concentrations are 1%–4% (safe concentration). Compared with inhaling H2 gas, drinking HRW is safer and more convenient. HRS is usually administered by intravenous or intraperitoneal injection. Hydrogen bathing is often used for the treatment of skin diseases [69]. When H2 is given by drinking HRW, 59% of the ingested H2 is released in the breath, ~ 0.1% is released from the body surface, and ~ 40% is consumed in the body [70]. After consumption of 500 mL of HRW within 1 min in volunteers, the concentration of breath H2 reaches a peak level of ~ 36 ppm at 10 min and then gradually drops to the baseline level of ~ 7.0 ppm after 60 min [70]. Ono et al. found that the H2 concentration in both the arterial and venous blood rapidly increases and reaches a plateau level (10 μmol/L to 20 μmol/L) in 20 min after the initiation of 3% and 4% H2. When H2 inhalation is discontinued, the H2 concentration in arterial blood decreases to < 10% of the plateau level in ~ 6 min, but in ~ 18 min in venous blood [4]. During 30 min of intravenous HRS (0.8 mmol/L) infusion, the concentration of H2 in both the arterial and venous blood rapidly increases to a maximum (< 1.8 ppm) at ~ 15 min and rapidly decreases with the cessation of HRS infusion [71]. Generally speaking, inhalation leads to a higher blood H2 concentration than intravenous infusion [71]. Animal experiments have also shown that inhalation induces higher H2 concentrations in the brain than the other routes of H2 administration (oral, intraperitoneal, intravenous) [72]. These results suggest that inhalation is the preferred route in H2 therapy for CNS disease.
Although inhalation, oral ingestion, or injection of H2 effectively alleviates diseases in the nervous system, as demonstrated by different research groups [6, 20, 73], there is a lack of comparisons in terms of biological effects between different hydrogen intervention methods in a specific disease. As the H2 concentrations in tissues and organs significantly differ according to which intervention is selected [72, 74], its administration through different routes may have varied effects in the same damaged tissues. Moreover, only a few studies have focused on dose- and time-dependent effects and tolerance to H2 in basic and clinical studies. To choose the most effective H2 therapy method for each disease, it is therefore important to further understand the pharmacokinetics and therapeutic effects of different types of H2 donors. In mouse models of Alzheimer’s disease, intracerebral injection of Pd hydride (PdH) nanoparticles reduces the over-generation of amyloid beta (Aβ) in the brain [14], whereas the intake of HRW does not show a similar effect [13]. H2 has low solubility, and its concentrations in the brain of mice are significantly lower (< 30 ppb/g) via traditional administration (intake of HRW, injection of HRS, or inhalation of 4% H2) [72]. The PdH nanoparticle, which is a high-payload H2 storage material, sustainably releases ~ 6 μmol/L H2 within 60 h [14, 75]. This phenomenon has indicated that Aβ clearance may be correlated with the concentration and duration of H2 in the brain. Namely, a high H2 concentration in the target tissue has a better performance than a low concentration. To maximize the therapeutic action of H2, the developing effective storage and targeted delivery by H2 donors may be one of the future research directions.
Protective Effects of H2 Against Nervous System Diseases
Ischemia/Reperfusion Injury
Ischemia/reperfusion injury is a condition characterized by tissue damage caused by an ischemic or anoxic period, followed by the re-establishment of blood supply to the tissue. Ischemia/reperfusion injury in the CNS is associated with disorders such as stroke, brain trauma, cerebral infarction, and cardiac arrest.
The restoration of circulation after deprivation of an adequate supply of O2 in the blood induces a burst of reactive oxygen species (ROS), which then triggers inflammatory responses and oxidative damage. The production of ROS may directly destroy cell membranes by inducing lipid peroxidation, and thus antioxidant agents are considered as a therapeutic option. First, H2 has been considered as an antioxidant that can buffer the destructive effect of oxidative stress in the brain after focal ischemia/reperfusion by selectively reducing cytotoxic oxygen radicals [3]. This is the basis on which in vivo studies have been conducted to validate the protective role of H2 in ischemia/reperfusion injury in the CNS. In animal models of cerebral ischemia/reperfusion, inhalation of 66.7% H2 significantly increases the activity of SOD and GSH-Px, reduces the level of malondialdehyde (MDA), reduces infarct volume, alleviates brain edema and hemorrhage, and improves neurobehavioral deficits [5, 76, 77]. Brain ischemia/reperfusion injury is a common secondary effect of cardiac arrest which is responsible for mortality and morbidity after cardiopulmonary resuscitation. In experimental cardiac arrest/resuscitation, H2 intervention also significantly diminishes neurologic injury and improves the survival rate and neurological outcome in animals [78, 79]. H2 inhalation or injection after cardiac arrest effectively controls neuronal death and microglial activation in the hippocampus and decreases the serum S100β protein level [80–82]. In addition, H2 inhalation alone or in combination with therapeutic hypothermia has been demonstrated to be superior to hypothermia alone [38, 80, 81, 83].
Clinical ischemia/reperfusion does not often occur in stroke and cerebral infarction, whereas patients who receive thrombolysis treatment or interventional thrombectomy are more likely to develop the most feared brain reperfusion injury. Importantly, the safety and efficacy of H2 has been confirmed in clinical trials for cerebral ischemia, cerebral infarction, and cardiac arrest. In patients with acute cerebral ischemia, inhalation of 3% H2 for 30 min or intravenous H2 administration can deliver sufficient H2 in the blood without compromising safety [4, 84]. A randomized controlled clinical study of patients with acute cerebral infarction showed that inhalation of 3% H2 gas for 1 h twice a day for 7 days improved O2 saturation without causing adverse effects. Patients who receive H2 inhalation therapy have a significantly decreased infarction site, and experience better improvement in the neurological status and the ability to execute activities of daily living relative to controls [20]. Furthermore, intravenous H2 administration in combination with edaravone has more evident and significant favorable effects than edaravone administration alone [85]. In a human study of patients with post-cardiac arrest syndrome, inhalation of a low concentration of H2 for 18 h had a favorable cerebral performance category score after 90 days, with no adverse events reported [21]. To further evaluate the efficacy and safety of H2 inhalation, a larger, phase II clinical trial is being conducted in patients with post-cardiac arrest syndrome in Japan [22]. Although no side-effects of H2 have been found in animal studies, the potential adverse effects should be investigated further, as diarrhea has been reported in a small number of patients after receiving H2 therapy [84].
Hypoxic/Ischemic Neonatal Brain Injury
Hypoxic/ischemic brain injury is a leading cause of death and disability during the perinatal period, and an effective treatment is not available. However, recent studies using rodent models of neonatal hypoxia/ischemia have shown that H2 can protect neonatal brains from injury by hypoxia/reoxygenation. Several studies have demonstrated that intraperitoneal injection of HRS significantly suppresses autophagy and neuro-inflammation, promotes M2 microglia polarization, rescues synaptic loss, and then restores behavioral deficits in a neonatal mouse model of hypoxia/ischemia [39, 40]. Similar results have been obtained from hypoxia/ischemia models in neonatal rats and piglets [37, 38]. In a rat model of neonatal hypoxic-ischemic encephalopathy, H2 inhalation has been shown to reduce the infarct size, neuronal loss, and astrocyte activation in the cortex and hippocampal CA3 region [37]. Meanwhile, the early behavioral reflexes of neonatal rats significantly improve after inhalation of H2 [86]. A study by Htun et al. shows that H2 ventilation combined with mild hypothermia improves the neurological score and walking function in a 5-day neonatal hypoxia/ischemia piglet model [38]. H2 has not only short-term neuroprotective effects but also long-term neurological and neurobehavioral effects. Ten weeks after the hypoxic/ischemic insult in a neonatal rat model, early administration of H2 also improves learning and memory [37]. The neurovascular unit is necessary for maintaining the fragile homeostasis of the brain. ROS produced in the early reoxygenation period severely decreases cerebrovascular reactivity and induces dysfunction of the neurovascular unit. H2 preserves cerebrovascular reactivity and alleviates the development and persistence of delayed neurovascular dysfunction caused by hypoxic stress in newborn piglets [41, 87]. These findings taken together indicate that the use of H2 may be considered a therapeutic approach to neonatal brain injury after asphyxia.
Traumatic Injury
The incidence of traumatic injury in the brain and spinal cord is constantly increasing in modern society. Although progress has been made in prophylactic and therapeutic treatment of traumatic injury, recovery of neural function remains a huge challenge. In animals, trauma in the brain and spinal cord causes hemorrhage, edema, cell death, inflammatory cell infiltration, and increases BBB permeability and neurological deficits; however, H2 treatment significantly improves injuries and promotes the recovery of nerve function [7, 8, 42, 88, 89].
H2 or HRS treatment decreases the expression of caspases-3, caspase-9, and Bax, increases the expression of Bcl-2, and significantly attenuates neuronal apoptosis after mechanical injury [8, 42]. H2 treatment also decreases oxidative products, such as MDA, 8-iso-prostaglandin F2α, 8-hydroxydeoxyguanosine (8-OHdG), and carbonyl protein, increases endogenous antioxidant enzymatic activity (SOD, CAT, and GPx), suppresses the levels of MPO, NOX2, and NOX4, and elevates Sir2, PrxIII, Trx2, and CGRP in contused brain and spinal cord [42, 45, 90, 91]. Moreover, Dohi et al. have demonstrated that treatment with HRW reverses the expression of genes involved in oxidative stress, carbohydrate metabolism, and neuroinflammation after traumatic brain injury [43]. Furthermore, HRS treatment controls the inflammatory process in the brain tissues of traumatic brain injury-challenged rats by decreasing the levels of pro-inflammatory cytokines (TNF-α, IL-1β, and HMGB-1) and the number of inflammatory cells (Iba1) and inflammatory metabolites (Cho) [88, 89]. In addition, H2 molecules may even suppress reactive astrogliosis, which is correlated with oxidative injury in the spinal cord [44]. Notably, in the contused spinal cord of rats, HRS attenuates the local release of pro-inflammatory cytokines and the production of specific markers (STAT3, p-STAT3, and GFAP) expressed by astrocytes, as well as suppressing astrogliosis [44].
Survivors of traumatic brain injury often present with cognitive impairment, including impaired learning and memory. These deficits can be reversed by HRS treatment, as indicated by improved cognitive performance in the Morris water maze after mild traumatic brain injury in the presence of HRS [91]. HRS may ameliorate cognitive deficits after trauma by maintaining synaptic plasticity. In rats with traumatic brain injury, HRS significantly elevates the levels of brain-derived neurotropic factor (BDNF), calcium/calmodulin-dependent protein kinase II, synapsin I, and cyclic AMP-response element binding (CREB) protein in the hippocampus [91]. These molecules are involved in the mediation of synaptic plasticity and cognition. Generally, H2 not only alleviates traumatic brain injury via its common anti-oxidative, anti-inflammatory, and anti-apoptotic effects, but also attenuates traumatic brain injury-induced cognition disorders by improving neuronal synaptic plasticity.
Subarachnoid Hemorrhage
Subarachnoid hemorrhage is a devastating cerebrovascular event with high morbidity and mortality, and a poor prognosis. Oxidative stress is a key factor involved in the pathogenesis of early brain injury after SAH. Therefore, an antioxidant therapy that involves scavenging free radicals is effective in its treatment. H2 is a promising therapeutic method for patients with early-stage SAH. Several studies in animals have shown that HRS treatment remarkably attenuates the early brain injury 24 h after SAH [9, 10]. Similarly, inhalation of 1.3%–2.9% H2 for 2 h after intracerebral hemorrhage (ICH) in rats attenuates brain edema, maintains the integrity of the BBB, reduces apoptosis and neuroinflammation, and improves neurological function, with decreased production of MDA, nitrotyrosine, and 8-OHG in the brain [46, 92]. However, H2 has a neuroprotective effect 24 h after SAH (acute phase), but not after 72 h (delayed phase) [46]. Interestingly, Manaenko et al. have found that 2% H2 inhalation for 1 h significantly decreases brain water content and improves neurological outcomes, whereas H2 inhalation for 2 h does not have any effects 24 h (acute phase) after ICH [93]. Moreover, 72 h after ICH, H2 inhalation is more likely, but not significantly, to improve neurological deficits. In contrast, HRS treatment attenuates the increased levels of MDA, caspase-12, and caspase-3 and substantially alleviates the brain injury and brain edema 72 h after SAH in rabbits [49]. Based on these reports, H2 may have a dose- and time-dependent effect on SAH. In addition, H2 can ameliorate cerebral vasospasm, a common complication in patients with SAH. In rats, HRS attenuates neurological functional deficits and morphological vasospasm of the basilar artery after SAH [47].
Neuropathic Pain
Neuropathic pain is a type of pain attributed to damage or disease affecting the somatosensory nervous system; it significantly affects quality of life. Neuropathic pain is troublesome and extremely challenging to treat. Importantly, H2 treatment may be a therapeutic approach to alleviating neuropathic pain under various pathological conditions, including opioid-induced hyperalgesia, spinal cord injury, and post-herpetic neuralgia.
Postoperative hyperalgesia Remifentanil, a potent, short-acting, synthetic opioid analgesic, is used as an adjunct to an anesthetic during surgery to relieve pain. Hyperalgesia is a side-effect after the administration of intraoperative analgesia with remifentanil. During the development of opioid-induced hyperalgesia, membrane trafficking of the NMDA receptor NR1 and NR2B subunits is increased in the spinal cord, and this trafficking is mediated by the activation of glycogen synthase kinase-3β (GSK-3β) [51]. Zhang et al. have found that remifentanil infusions induce rapid and prolonged mechanical and thermal hyperalgesia and facilitate NR1 membrane trafficking and GSK-3β activation in the dorsal root ganglion (DRG) [51]. More importantly, HRS treatment partially attenuates remifentanil-induced hyperalgesia without affecting the baseline nociceptive threshold, decreases the expression of inflammatory mediators (TNF-α, IL-1β, and IL-6), and suppresses NR1 membrane trafficking through the inhibition of GSK-3β activity in the DRG in a dose-dependent manner [51]. In a rat model of incisional postoperative pain, the production of ONOO- in the spinal cord increases after administration of remifentanil. ONOO- activates divalent metal transporter 1 without iron-responsive element [DMT1(-)IRE] and induces abnormal iron accumulation, leading to the development of hyperalgesia [50]. Meanwhile, intraperitoneal delivery of HRS can remove ONOO- from the spinal cord, protect against remifentanil-induced postoperative hyperalgesia, and attenuate DMT1(-)IRE activation and iron accumulation [50]. In addition, pretreatment with HRS successfully attenuates the postoperative mechanical and thermal hyperalgesia induced by remifentanil in incisional pain in rats, interdicts NR2B expression and membrane trafficking from the intracellular pool to the surface pool, as well as blocking MnSOD nitration in the dorsal horn [52]. These findings indicate that HRS exhibits anti-hyperalgesic effects possibly via the inhibition of oxidative stress and the GSK-3β-NMDA-MnSOD pathway (Fig. 2).
Fig. 2.
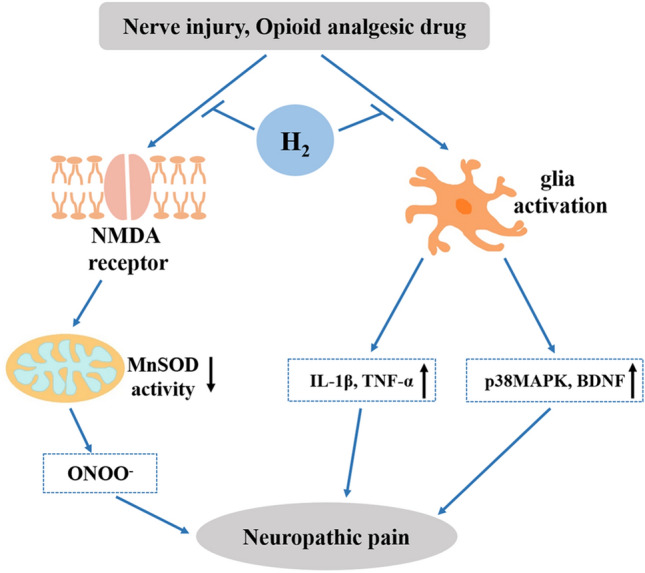
Protective effects of H2 against neuropathic pain and its related molecular mechanisms.
Neuropathic pain after nerve injury Results of a study on neuropathic pain induced by L5 spinal nerve ligation (L5 SNL) in a rat model show that intrathecal infusion of HRS relieves L5 SNL-induced mechanical allodynia and thermal hyperalgesia and provides a relatively long-lasting pre-emptive effect [25]. In chronic constriction-induced injury in another neuropathic pain rat model, intrathecal or intraperitoneal injection of HRS also significantly elevates the mechanical withdrawal threshold and thermal withdrawal latency of neuropathic pain [11, 53]. In a partial sciatic nerve ligation mouse model, the intake of H2 water also significantly alleviates mechanical allodynia and thermal hyperalgesia [12]. In addition to oxidative stress, pain is also triggered by inflammation and the activation of immune cells and glial cells in the DRG [94]. The analgesic effect of H2 might also be associated with the suppression of inflammation and oxidative stress. The administration of H2 inhibits the activation of spinal astrocytes and microglia, reverses the overexpression of pro-inflammatory cytokines (IL-1β, TNF-α, and HMGB), and decreases the levels of tyrosine-nitrated MnSOD, MDA, protein carbonyl, 8-hydroxyguanosine (8-OHG), 8-OHdG, HNE, and MPO (Fig. 2) [12, 25, 53, 54]. In a rat model with sciatic nerve trunk ligation, HRS treatment alleviates pain, decreases pro-inflammatory cytokine levels, and increases HO-1 protein expression and activity in the DRG and spinal cord. Meanwhile, the effects of H2 are reversed by the HO-1 inhibitor SnPP-IX and further enhanced by hemin and CORM-2. Therefore, Chen et al. have indicated that HO-1/CO signaling is involved in the analgesic and anti-inflammatory effects of H2 in neuropathic pain [54].
Parkinson’s Disease
Parkinson’s disease is the second most common neurodegenerative disorder, mainly affecting the motor system. It is characterized by progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta. Thus far, numerous animal experiments and a few human trials have shown that H2 has an attenuating effect on the development of Parkinson’s disease.
A randomized double-blind placebo-controlled trial on 18 patients has found that patients drinking HRW for 48 weeks showed improvement in their total Unified Parkinson’s Disease Rating Scale (UPDRS) scores, whereas patients drinking placebo water had worse UPDRS scores. The preliminarily results indicate that drinking HRW is safe and well-tolerated. Moreover, it has significant effects on Parkinson’s disease [23]. However, in another clinical trial with 20 Parkinson’s disease patients, inhalation of 1.2%–1.4% H2 for 10 min twice a day for 4 weeks had no beneficial effect [95]. Disappointingly, drinking HRW for 72 weeks also failed to improve the total UPDRS scores in 178 patients with Parkinson’s disease in a randomized, double-blind, multicenter trial [96]. Although there were no significant differences in the changes of the score between the HRW drinking group and the placebo group in this trial, it demonstrated the safety of drinking HRW once again [96]. These negative results may be related to the stage of Parkinson’s disease, the phase of the UPDRS score, the H2 concentration in HRW, and the duration of H2 inhalation.
In 6-hydroxydopamine or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) rodent models of Parkinson’s disease, the administration of H2 is effective in inhibiting the development and progression of the disease [16, 55–57]. Further analysis has shown that the therapeutic effects of H2 in Parkinson’s disease models may be correlated with the prevention of dopaminergic neuron loss in the substantia nigra, as well as a decrease in the production of 4-HNE in the SN dopaminergic neurons and accumulation of 8-oxoguanine in the striatum (Fig. 3) [16, 55, 57].
Fig. 3.
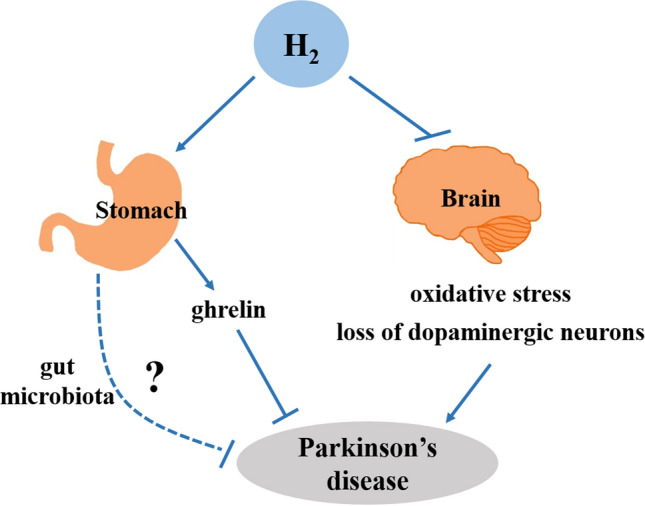
Neuroprotective effects of H2 against Parkinson’s disease.
H2 is one of the main intestinal gases, produced and utilized by gut microbiota. Gut microbiota are closely related to Parkinson’s disease [97]. Thus, the relationship between brain and gut during H2 treatment has become a topic of interest. Matsumoto et al. reported that the oral intake of HRW significantly enhances ghrelin gene expression in the stomach and the ghrelin level in the plasma of mice. More importantly, activation of the β1-adrenergic receptor is required for H2 to induce an increase in ghrelin in plasma [58]. Thus, studies have shown that drinking HRW may ameliorate the pathological process of Parkinson’s disease via activation of the gastric ghrelin system (Fig. 3) [58]. To further validate the contribution of ghrelin in the mouse model of H2-treated Parkinson’s disease, ghrelin-knockout (KO) mice have been used. Yoshii et al. have found that the administration of HRW significantly decreases the loss of dopaminergic cells in ghrelin-KO mice with MPTP insult [15]. Furthermore, the administration of D-Lys3GHRP-6 is not effective in inhibiting the neuroprotective effect of H2 in ghrelin-KO mice with Parkinson’s disease, unlike in wild-type mice [15]. Therefore, they believed that ghrelin is not the only factor associated with the H2-induced neuroprotective effect in Parkinson’s disease (Fig. 3) [15], and more studies are needed to assess the relationship between the brain and stomach during H2 treatment. Mikako et al. have found that consumption of the H2-producing precursor lactulose (substrate for microbial fermentation) increases the H2 concentration in breath and marginally ameliorates the motor deficits in a rat model of Parkinson’s disease [56]. It seems that gut microbiota mediate gut-brain communication by microbial metabolites, including H2 [98], or H2 may modulate the gut microbiota dysbiosis in Parkinson’s disease (Fig. 3).
Cognitive Dysfunction (Alzheimer’s Disease)
Alzheimer’s disease is the most common neurodegenerative disorder worldwide and is the most prevalent cause of dementia in elderly individuals. Women are more commonly affected than men. H2 treatment may be a feasible option for patients with this disease, as it has been reported to improve the cognitive impairment in animal models of the disease. The accumulation of Aβ deposits is widely believed to be the fundamental cause of Alzheimer’s disease. Intracerebral injection of Aβ1-42 in male rats increases the level of oxidative stress in brain tissue, indicating enhanced levels of MDA and 8-OHdG [59, 60]. In a transgenic mouse model of Alzheimer’s disease, the administration of H2 has protective effects [13, 14]. The intake of HRW significantly reduces the level of MDA and improves the activity of T-SOD and GSH in APP/PS1 mice [13]. In mice with 3 × Tg Alzheimer’s disease, the intracerebral injection of PdH nanoparticles (a high-payload H2 carrier) effectively scavenges ·OH, reduces Aβ generation and aggregation, ameliorates mitochondrial dysfunction, reverses the synaptic deficits, and inhibits neuronal death in the brain [14]. In vitro, H2 treatment enhances the anti-oxidative system in human neuroblastoma SK-N-MC cells under Aβ-stimulated oxidative stress by stimulating AMPK and up-regulating the downstream Sirt1-FoxO3a axis, which prevents mitochondrial dysfunction and the production of ROS, thereby ultimately maintaining cell survival (Fig. 4) [61].
Fig. 4.
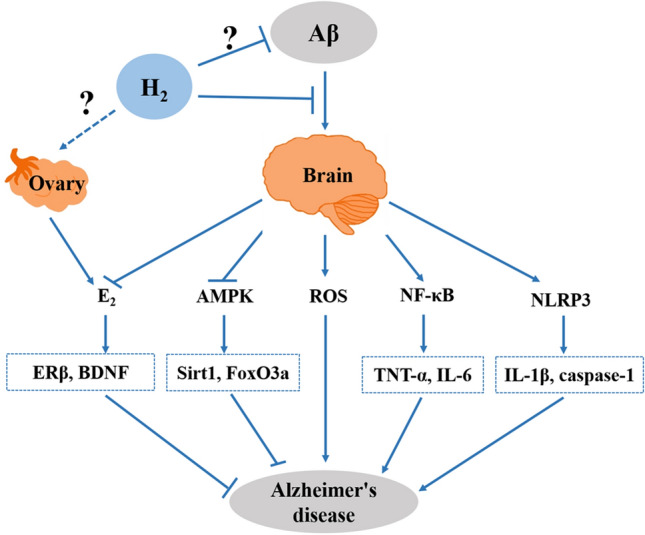
Molecular mechanisms of H2 in ameliorating Aβ-induced Alzheimer’s disease.
H2 improves cognitive impairment in rodent models of Alzheimer’s disease not only by the reduction of oxidative stress but also by the suppression of the inflammatory response in the brain. The administration of H2 effectively prevents excessive neuroinflammation in Aβ1-42-challenged mice, with the suppression of astrocyte activation and inhibition of pro-inflammatory factors (IL-1β, IL-6, and TNF-α) in the brain [59, 60]. In APP/PS1 mice, the intake of H2 water significantly reduces the mRNA levels of IL-6 and TNF-α in the brain (Fig. 4) [13], indicating an anti-inflammatory effect of H2.
Interestingly, H2 has a sex-specific cognitive benefit in APP/PS1 mice without altering Aβ clearance or APP processing. Oral HRW is effective in improving spatial the learning deficits and memory impairment and estrogen (E2) levels in both the brain and serum and in increasing ERβ, BDNF, and TrkB expression in the brains of female, but not male, APP/PS1 mice. This is consistent with the recent studies showing that female 3 × Tg-AD mice display more prominent amyloid plaques, neurofibrillary tangles, neuroinflammation, and spatial cognitive deficits than male 3 × Tg-AD mice [99]. These data indicate that the mechanism underlying the beneficial effect of H2 is most likely through the E2-ERβ-BDNF signaling pathway (Fig. 4) [13]. Using a mouse model of premature ovarian failure induced by zona pellucida 3, He et al. found that HRW improves the serum anti-Müllerian hormone levels and reduces ovarian granulosa cell apoptosis, thereby exerting a protective effect on ovarian function [100]. In addition, the ovaries primarily produce E2. Thus, we speculated that H2 may alleviate cognitive impairment by improving ovarian function in female mice with Alzheimer’s disease (Fig. 4). However, the precise mechanism by which H2 prevents the decline of E2 is unknown and needs further investigation. The overexpression of BDNF, TrkB, and synaptic proteins (postsynaptic density 95, synapsin I, and synaptophysin) [13, 14] has been reported in mouse models of Alzheimer’s disease after H2 treatment, which indicates that H2 may have a neuroprotective effect by restoring neuronal plasticity. In APP/PS1 mice, the researchers also found that oral HRW did not decrease the concentration of Aβ42 or APP nor increase the protein expression of NEP and IDE in the cerebral cortex [13]. These phenomena indicate that H2 does not have an effect on Aβ clearance and APP processing in APP/PS1 mice; in other words, H2 can improve the cognitive deficiency but may not inhibit or reverse the progression of the disease.
In addition to ameliorating the cognitive dysfunction in Alzheimer’s disease, H2 also attenuates the cognitive impairment induced by operation, isoflurane anesthesia, vascular dementia, hypoxia, radiation, stress, status epilepticus, and aging [39, 78, 101–108], which are not discussed here.
Mood Disorders
Mood disorders (such as anxiety and depression) are common but serious mental health diseases worldwide, which have negative effects on a person’s mood, daily life, work, and social communication. Most types of available anti-anxiety drugs and antidepressants are generally safe and effective. However, > 50% of patients do not achieve complete remission after drug therapy. In addition, these drugs have side-effects and warnings regarding their use.
In recent years, animal experiments have revealed that H2 has an anti-anxiety and anti-depression effect. The inhalation of 67% H2 or 4 mL oral HRW per day significantly prevents depressive and anxiety-like behaviors in mice undergoing chronic mild stress (CMS) [17, 62], a rodent model of depression [109]. On the other hand, H2 treatment successfully suppresses the increase in IL-1β, caspase-1, and ROS in the hippocampus and prefrontal cortex of depressed mice [62]. By contrast, the administration of H2 also decreases the serum levels of corticosterone (CORT), ACTH, IL-6, and TNF-α in CMS-challenged mice [17]. Moreover, H2 may have a long-lasting effect on stress resilience in mice, since H2 inhalation in adolescents significantly increases resilience to acute stress in early adulthood [17]. Anxiety is a negative psychological consequence of opioid withdrawal, which can be attenuated by the administration of H2. In a morphine-dependent mouse model of naloxone-precipitated withdrawal, Wen et al. found that the administration of HRS not only significantly reduces body weight loss, jumping behavior, and wet-dog shakes but also suppresses anxiety-like behaviors in mice after naloxone-precipitated withdrawal or a spontaneous withdrawal period [63]. HRS treatment even reverses the hyperactivity of the hypothalamus-pituitary-adrenal axis induced by morphine withdrawal and inhibits the increase of CORT and cortisol levels in the plasma. In an mouse model of autism, the pre- and post-administration of HRW significantly improves the valproic acid-induced anxiety-like behaviors and reduces the serum IL-6 and TNF-α levels in mouse offspring [64].
To further validate the unique role of H2 in emotional regulation, a double-blinded, placebo-controlled study of 31 adult volunteers aged between 20 and 49 years was carried out. Mizuno et al. found that the consumption of HRW for 4 weeks significantly decreases (post versus pretreatment) scores for K6 (mood and anxiety), the Chalder Fatigue Scale (severity of fatigue), and the Pittsburgh Sleep Quality Index (general sleepiness and daytime sleepiness scores) [18]. In addition, the changes in the post/pretreatment ratios for K6 score and low-frequency component power (sympathetic nerve activity test) were significantly lower in the drinking HRW group than in the placebo water group. This finding indicates that the administration of HRW may offer an effective method to reinforce the quality of life and maintain good health by improving mood, anxiety, and autonomic nerve function in daily life.
Mechanisms of the H2-Induced Neuroprotective Effect
Several potential mechanisms may be involved in the neuroprotective effect of H2 against neurological diseases: anti-oxidation, anti-inflammation, anti-apoptosis, regulation of autophagy, preservation of mitochondrial function, and preservation of BBB integrity.
Anti-oxidation Effect of H2
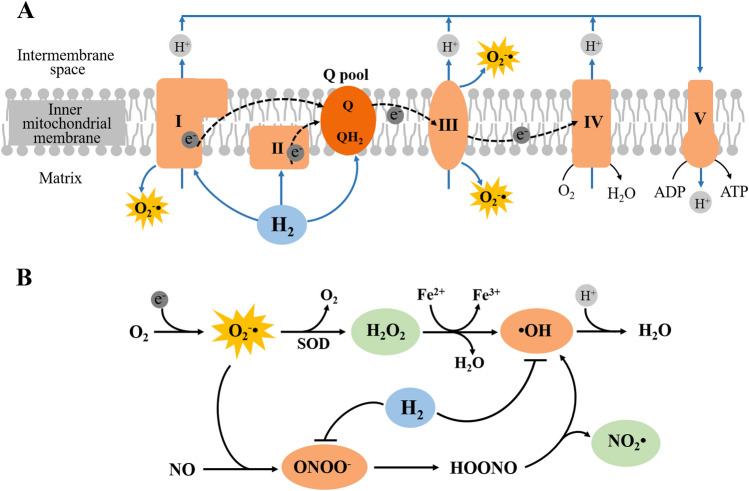
Many diseases are linked to oxidative stress damage which comes from an imbalance between ROS production and scavenging. The excessive production of peroxides and free radicals can damage cellular components, including proteins, lipids, and DNA. H2 is widely recognized as an antioxidant by directly neutralizing cytotoxic ·OH and ONOO− (Fig. 5B), but does not affect other ROS, such as superoxide anion (O2−·), nitric oxide (NO·), and hydrogen peroxide (H2O2), which play physiological roles as signaling molecules [3]. Currently, some researchers propose that H2 may have an antioxidant effect by working upstream of the generation of ROS. ROS mainly originate at the electron transport chain during the process of oxidative phosphorylation (Fig. 5). Meanwhile, mitochondrial complex I has close homology and an evolutionary relationship with [NiFe]-hydrogenases. Therefore Toru Ishibashi proposed that H2 may function as the rectifier of the mitochondrial electron flow to convert ubiquinone to ubiquinol, thereby suppressing excessive electron leakage and ROS generation [110]. A new study by Ishibashi found that H2 suppresses superoxide generation in complex I and reduces the mitochondrial membrane potential (ΔΨm) [111]. Meanwhile, Gvozdjáková et al. also reported that HRW consumption increases the levels of mitochondrial ATP production by complex I and complex II substrates and increases the levels of mitochondrial oxidized coenzyme Q (ubiquinone) in heart tissue of rats [112]. The latest results from Professor Ma’s research team demonstrated that the evolving activity of H2 in eukaryotic mitochondria is closely related to complex I and the activity occurs around the fully oxidized ubiquinone binding site [113]. Moreover, they have also found that H2 significantly enhances the activity of mitochondrial complex I under hypoxic conditions [114]. These results thus reinforce the hypothesis that H2 may control ROS generation at the source by functioning as a rectifier of mitochondrial electron flow in the quinone chamber (Fig. 5A).
Fig. 5.
Hypotheses of the anti-oxidative mechanisms of H2. A The novel hypothesis that H2 suppresses ROS generation by functioning as a rectifier for mitochondrial electron flow in the ubiquinone (Q) pool. B The conventional “scavenger theory” that H2 directly neutralizes ·OH and ONOO- produced by the mitochondrial respiratory chain. Mitochondrial complexes I to V are marked as I, II, III, IV, and V; QH2, ubiquinol; HOONO, peroxynitrous acid; NO2·, nitrogen dioxide.
Apart from its properties as a free radical scavenger and rectifier of the mitochondrial respiratory chain, H2 can also enhance the body’s resistance to excessive oxidative stress by improving the levels of antioxidant enzymes and modulating the expression of redox-related genes. H2 treatment increases the activity of endogenous antioxidant enzymes in the brain and spinal cord, such as SOD, CAT, and GPx [29, 47, 115–117]. Moreover, H2 can regulate the expression of antioxidant genes. Via RNA sequencing and RT-PCR analysis, Chen et al. have found that the expression of oxidative stress-related genes, including Cox8b, Cox6a2, Cox7a1, Hspb7, and Atp2a1, are significantly downregulated following H2 treatment in mice with spinal cord injury [42]. In a model of hypoxia/reoxygenation injury, administration of H2 significantly promotes the expression of HO-1 and nNOS [36, 115]. Strong expression of HO-1 has also been reported after HRS treatment in a rat model of sciatic nerve trunk ligation [54]. Nrf2 is a critical factor for the endogenous antioxidant system, which plays important neuroprotective roles in various neurological diseases by regulating the production of numerous cytoprotective proteins [118]. Yuan et al. have reported that the administration of HRS increases Nrf2 expression and promotes the translocation of Nrf2 from the cytoplasm to the nucleus and increases the expression of downstream factors, such as HO-1 and NQO1 [7]. Meanwhile, enhanced expression of p-p38 MAPK, HO-1, and Nrf2 by H2 treatment have been found in more experiments in vitro and in vivo [37, 119]. Therefore, H2 treatment might induce adaptive responses against oxidative stress in the nervous system by evoking the Nrf2 antioxidant defense system.
Anti-inflammatory Effect of H2
Extensive studies have provided strong evidence for the anti-inflammatory effect of H2 in various diseases in the nervous system. H2 treatment significantly suppresses microglia activation and the secretion of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, and HMGB-1) in animal models of various neurological diseases [5, 26, 120, 121]. In vitro, pretreatment with H2-rich medium significantly inhibits the hypertrophy and proliferation of astrocytes, mitigates the expression of GFAP, and weakens the increased secretion of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-a) in primary astrocytes after H2O2-induced injury [44]. H2 not only effectively inhibits the expression of pro-inflammatory factors, but also enhances the immunosuppressive cytokines IL-10, TGF- β, and YM-1 in the brain [39, 88]. The M1/M2 polarization of microglia is an important participant in neuroinflammation [122]. Ning et al. have reported that H2 treatment markedly inhibits the proportion of M1 microglia, but has no influence on M2 microglia in vitro [123]. Chu et al. have noted the phenomenon that the number of M1 microglia is significantly reduced and the number of M2 microglia is significantly increased in the cortex after HRS treatment of mice with hypoxic/ischemic insult [39]. In addition, fewer amoeboid/round microglia and more intermediate microglia are obtained in mice given a high concentration of H2 [5]. It seems that H2 may suppress neuroinflammation by inhibiting microglial activation and regulating microglial polarization.
The incorrect regulation of NF-κB has been correlated with neuroinflammation. Some studies have indicated that H2 may play an anti-inflammatory role by inhibiting NF-κB activation. In rodents with hypoxia/ischemia, HRS treatment significantly promotes AMPK activation and inhibits NF-κB activation accompanied by miR-21 and miR-210 downregulation in the brain [6, 39]. In a rat model of subarachnoid hemorrhage, HRS treatment decreases the protein levels of p-IκBα and nuclear p65, and increases the level of cytosolic p65 [10, 48]. In a rat model of Alzheimer’s disease, the intraventricular injection of HRS inhibits JNK and NF-κB activation in the hippocampus [60]. Moreover, the intake of HRW significantly attenuates activation of the NLRP3 inflammasome and decreases the expression of its downstream signaling molecules (cleaved caspase-1 and IL-1β) in the brain of female APP/PS1 mice [13]. HRS treatment after subarachnoid hemorrhage also decreases the protein expression of NLRP3, ASC, caspase-1, and IL-1β, and cleaves caspase-3 in the cerebral cortex of rats [10]. These results indicate that, in addition to modulating NF-κB pathways, H2 may also attenuate the inflammatory response via suppressing formation of the NLRP3 inflammasome.
Oxidative stress can induce cell damage and promote inflammation in CNS diseases. Excessive ROS have been shown to stimulate the expression of transcription factors such as NF-κB, and promote the secretion of IL-1β by activating the NLRP3 inflammasome [124, 125]. In fact, the anti-inflammatory effect of H2 is usually paralleled by the antioxidant effect [26, 126]. Therefore, the anti-inflammatory action of H2 may also be due to the mechanism of gene expression changes caused by ROS.
Anti-apoptotic Effect of H2
In addition to the anti-oxidative and anti-inflammatory effects of H2, it also plays an anti-apoptotic role against nerve damage. H2 suppresses the expression of Bax, caspase-3, and caspase-12, promotes the expression of Bcl-2 and Bcl-xL, and increases the Bcl-2/Bax ratio in the brain [9, 37, 42, 48, 101, 103, 107]. Moreover, HRS treatment significantly attenuates the loss of motor neurons, inhibits the release of mitochondrial cytochrome c (Cyt c), and inhibits the activation of downstream caspase-9 and caspase-3 [127]. Further research has shown that H2 treatment significantly decreases the expression levels of Akt, GSK3β, p-Akt, and p-GSK3β in brain tissue and cerebral microvascular endothelial cells (CMECs) after hypoxia/reoxygenation [33]. Other studies have obtained similar results showing that HRS treatment enhances the phosphorylation of Akt and GSK3β after subarachnoid hemorrhage. Furthermore, these beneficial effects of H2 are abolished by Ly294002, a selective inhibitor of the PI3K pathway [9, 33]. These data suggest that H2 may protect the brain against apoptosis by the Akt/GSK3β signaling pathway.
Regulation of Autophagy
Recently, some studies have revealed that H2 may have a neuroprotective effect by regulating the signaling pathways of autophagy. In a rat model of neuropathic pain, Wang et al. found that HRS treatment attenuates hyperalgesia and activates autophagy [11]. Moreover, the intraperitoneal injection of HRS significantly upregulates Beclin-I, HIF-1α, and BNIP3 mRNA and protein expression, downregulates p62 mRNA and protein expression, and increases the number of autophagosomes and autolysosomes in the spinal cord [11]. In a rat model of post-herpetic neuralgia, the intraperitoneal injection of HRS relieves neuralgia and activates autophagy by upregulating the expression of LC3, Beclin-I, and p62 [128]. In addition, in neonatal hypoxic/ischemic brain injury, Bai et al. reported that the administration of HRS elevates the LC3II/LC3I ratio, increases Beclin-1 protein expression, and decreases the levels of phosphorylated mTOR, Stat3, and ERK in the lesioned cortex [40]. Studies have shown that H2 can not only promote autophagy but also inhibit it. In a rat model of vascular dementia, the intake of HRW decreases the number of autophagosomes, which is accompanied by the downregulation of FoxO1 and Atg7 expression levels, attenuation of the LC3-II/I ratio, and upregulation of p62 level [101].
Preservation of Mitochondrial Function
The production of ROS primarily occurs in the mitochondria and it is also commonly affected. Researchers have indicated that HRS can attenuate neuronal injury, possibly by preserving mitochondrial function. Cui et al. found that HRS decreases the degree of mitochondrial swelling and ultrastructural disruption, maintains the integrality of the mitochondrial membrane, and preserves the loss of ΔΨm and Cyt c release in the hippocampus of rats with ischemia/reperfusion injury [35]. In mouse models of amyotrophic lateral sclerosis, HRS attenuates the release of mitochondrial Cyt c, restores the activity of complexes I and IV, suppresses the formation of ROS in mitochondria, and enhances the production of mitochondrial ATP [127]. Mechanical injury damages the structure and function of the mitochondria, leading to mitochondrial permeability transition pore (mPTP) opening and ATP loss in neurons; however, the effects of the damage are reversed by H2 treatment [42, 43]. In human neuroblastoma SH-SY5Y cells, H2 pretreatment enhances mitochondrial activity, indicating an increase of ΔΨm, cellular ATP concentration, and O2 consumption rate [119]. The mPTP is one of the direct sites of ROS [129]; thus, in this study, the neuroprotection by H2 may be associated with the inhibition of mPTP opening. Previous research indicates that the activation of mitochondrial ATP-sensitive K+ (mitoKATP) channels protect neurons against the injury and death caused by ischemia/reperfusion [130]. Zhou et al. speculated that HRS also activates mitoKATP channels, as the beneficial effects of HRS on ischemia/reperfusion in the spinal cord are partially reversed by 5-hydroxydecanoate, a selective mitoKATP channel antagonist [116]. Meanwhile, the relationship between H2 and the mPTP or mitoKATP channels need further validation.
Preservation of the BBB
The BBB, a highly selective and organized structure, is composed of endothelial cells, pericytes, astrocytes, neurons, and extracellular matrix. It is also known as the neurovascular unit and is considered the gatekeeper of the CNS [131]. Disruption of the BBB primarily results from ischemia/reperfusion, spontaneous hypertensive stroke, and mechanical trauma-induced nervous system injury, and H2 has been found to attenuate BBB dysfunction [73, 82, 88, 90]. Takeuchi et al. found that oral intake of HRW reduces the number of 8-OHdG-positive cells and vessels with extravasated albumin in the hippocampus of spontaneously hypertensive stroke-prone rats [73]. Matrix metalloproteinases (MMPs) are important factors in BBB disruption. In animals with traumatic brain injury, the intake of HRW might have protective effects against edema and BBB disruption by suppressing the decrease in AQP-4 and MMP-2 levels and by inhibiting the increase in HIF-1 and MMP-9 levels after cortical impact [43]. In addition, brain-derived CMECs play an important role in the BBB, and H2 treatment inhibits CMEC apoptosis after hypoxia/reoxygenation [33].
Conclusions and Perspectives
H2 has been reported to have a variety of biological properties including anti-oxidative, anti-inflammatory and anti-apoptotic effects, as well as its protection of mitochondria and the BBB. Oxidative stress modulates the expression of a wide range of genes that influence many biological responses, such as apoptosis, autophagy, and the inflammatory response; thus, the anti-oxidative action of H2 may be its most basic property. At present, there are two hypotheses for the mechanisms behind the anti-oxidative action of H2. The generally accepted hypothesis is that H2 directly reacts with ·OH and ONOO−, namely the conventional “scavenger theory”. The other considers that H2 suppresses ROS generation by functioning as a rectifier for mitochondrial electron flow. Although several recently-published papers provide some preliminary evidence for the new hypothesis, more compelling evidence is needed.
The neuroprotective effects of H2 have been demonstrated by growing evidence from animal experiments, meanwhile its efficacy has also been reported in many clinical trials, especially in cerebral ischemia, post-cardiac arrest syndrome, and Parkinson’s disease. Although the protective effect of H2 has rapidly developed from many fundamental studies to preliminary clinical application studies in recent years, more work on the clinical applications is needed. Numerous studies have shown that H2 has no toxic effects, but adverse events including diarrhea, heartburn, and headache have been reported in individual cases [84, 132]. In addition, Wang et al. found that H2 supplementation substantially increases the activity of hydrogenase in Helicobacter pylori. More importantly, higher hydrogenase activity and hydrogen metabolism in H. pylori may induce gastric cancer by promoting the translocation of the carcinogenic factor CagA into host cells [133]. This indicates that H2 may enhance the pathogenicity of pathogens and facilitate the development of diseases in some special cases. Thus, the indications and potential side-effects of H2 cannot be ignored in clinical use.
In some clinic trials with Parkinson’s disease, H2 administration did not have any beneficial effect, which means that the protective effect of H2 is somehow limited in certain pathological conditions. As previously noted, the distribution of H2 in tissue and organs varies with different administration methods and thus influences its biomedical effects. It appears that the limited effects of H2 may be related to the concentration, duration, and route of administration of H2, apart from the stage of disease. Therefore, further studies are required to investigate the pharmacokinetics and the dose-effect relationship of H2 and then develop more effective H2 delivery methods.
Overall, despite limited side-effects and negative results reported in individual studies, H2 may be a promising new treatment modality for numerous nervous system diseases.
Acknowledgements
This review was supported by the National Natural Science Foundation of China (81770855 and 81773717), the Taishan Scholarship from the Shandong Province Government (ts201511057), and the High-Level Talent Training Program of Taishan Medical University (2018GCC08). We are grateful to Nikoli Peacher of West Virginia University for assistance in editing and proofreading.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Han-Ting Zhang, Email: hzhang@hsc.wvu.edu.
Shu-Cun Qin, Email: 13583815481@163.com.
References
- 1.Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science. 1975;190:152–154. doi: 10.1126/science.1166304. [DOI] [PubMed] [Google Scholar]
- 2.Gharib B, Hanna S, Abdallahi OMS, Lepidi H, Gardette B, Reggi MD. Anti-inflammatory properties of molecular hydrogen: investigation on parasite-induced liver inflammation. C R Acad Sci III. 2001;324:719–724. doi: 10.1016/S0764-4469(01)01350-6. [DOI] [PubMed] [Google Scholar]
- 3.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 4.Ono H, Nishijima Y, Adachi N, Sakamoto M, Kudo Y, Kaneko K, et al. A basic study on molecular hydrogen (H2) inhalation in acute cerebral ischemia patients for safety check with physiological parameters and measurement of blood H2 level. Med Gas Res. 2012;2:21. doi: 10.1186/2045-9912-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Liu W, Manaenko A, Sun X, Mei Q, Hu Q. Hydrogen inhibits microglial activation and regulates microglial phenotype in a mouse middle cerebral artery occlusion model. Med Gas Res. 2019;9:127–132. doi: 10.4103/2045-9912.266986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian L, Pan Y, Zeng Q, Bing L, Cai S, Hui K, et al. Neuroprotective effect of hydrogen-rich saline in global cerebral ischemia/reperfusion rats: up-regulated tregs and down-regulated miR-21, miR-210 and NF-κB expression. Neurochem Res. 2016;41:2655–2665. doi: 10.1007/s11064-016-1978-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan J, Wang D, Liu Y, Chen X, Zhang H, Shen F, et al. Hydrogen-rich water attenuates oxidative stress in rats with traumatic brain injury via Nrf2 pathway. J Surg Res. 2018;228:238–246. doi: 10.1016/j.jss.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Zhang Q, Zhu K, Sun J, Zhang Z, Sun J, et al. Hydrogen-rich saline injection into the subarachnoid cavity within 2 weeks promotes recovery after acute spinal cord injury. Neural Regen Res. 2015;10:958–964. doi: 10.4103/1673-5374.158361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong Y, Shao A, Wang J, Chen S, Wu H, Mcbride DW, et al. Neuroprotective effect of hydrogen-rich saline against neurologic damage and apoptosis in early brain injury following subarachnoid hemorrhage: possible role of the Akt/GSK3β signaling pathway. PloS One. 2014;9:e96212. doi: 10.1371/journal.pone.0096212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao A, Wu H, Hong Y, Tu S, Sun X, Wu Q, et al. Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: possible involvement of NF-κB pathway and NLRP3 Inflammasome. Mol Neurobiol. 2016;53:3462–3476. doi: 10.1007/s12035-015-9242-y. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Huo X, Chen H, Li B, Liu J, Ma W, et al. Hydrogen-rich saline activated autophagy via HIF-1α pathways in neuropathic pain model. Biomed Res Int. 2018;2018:4670834. doi: 10.1155/2018/4670834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawaguchi M, Satoh Y, Otsubo Y, Kazama T. Molecular hydrogen attenuates neuropathic pain in mice. PloS One. 2014;9:e100352. doi: 10.1371/journal.pone.0100352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou C, Peng Y, Qin C, Fan F, Liu J, Long J. Hydrogen-rich water improves cognitive impairment gender-dependently in APP/PS1 mice without affecting Aβ clearance. Free Radical Res. 2018;52:1311–1322. doi: 10.1080/10715762.2018.1460749. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Zhao P, Yue C, Jin Z, Liu Q, Du X, et al. Sustained release of bioactive hydrogen by Pd hydride nanoparticles overcomes Alzheimer’s disease. Biomaterials. 2019;197:393–404. doi: 10.1016/j.biomaterials.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 15.Yoshii Y, Inoue T, Uemura Y, Iwasaki Y, Yada T, Nakabeppu Y, et al. Complexity of stomach-brain interaction induced by molecular hydrogen in Parkinson’s disease model Mice. Neurochem Res. 2017;42:2658–2665. doi: 10.1007/s11064-017-2281-1. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi Y, Imamura R, Koyama Y, Kondo M, Kobayashi H, Nonomura N, et al. Renoprotective and neuroprotective effects of enteric hydrogen generation from Si-based agent. Sci Rep. 2020;10:5859. doi: 10.1038/s41598-020-62755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Q, Song H, Wang X, Liang Y, Xi Y, Gao Y, et al. Molecular hydrogen increases resilience to stress in mice. Sci Rep. 2017;7:9625. doi: 10.1038/s41598-017-10362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizuno K, Sasaki AT, Ebisu K, Tajima K, Kajimoto O, Nojima J, et al. Hydrogen-rich water for improvements of mood, anxiety, and autonomic nerve function in daily life. Med Gas Res. 2017;7:247–255. doi: 10.4103/2045-9912.222448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, Xie F, Zhang Y, Wang T, Ma S, Zhao P, et al. Molecular hydrogen suppresses glioblastoma growth via inducing the glioma stem-like cell differentiation. Stem Cell Res Ther. 2019;10:145. doi: 10.1186/s13287-019-1241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ono H, Nishijima Y, Ohta S, Sakamoto M, Kinone K, Horikosi T, et al. Hydrogen gas inhalation treatment in acute cerebral infarction: A randomized controlled clinical study on safety and neuroprotection. J Stroke Cerebrovasc Dis. 2017;26:2587–2594. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Tamura T, Hayashida K, Sano M, Suzuki M, Shibusawa T, Yoshizawa J, et al. Feasibility and safety of hydrogen gas inhalation for post-cardiac arrest syndrome-first-in-human pilot study. Circ J. 2016;80:1870–1873. doi: 10.1253/circj.CJ-16-0127. [DOI] [PubMed] [Google Scholar]
- 22.Tamura T, Hayashida K, Sano M, Onuki S, Suzuki M. Efficacy of inhaled hydrogen on neurological outcome following brain ischemia during post-cardiac arrest care (HYBRID II trial): Study protocol for a randomized controlled trial. Trials. 2017;18:488. doi: 10.1186/s13063-017-2246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoritaka A, Takanashi M, Hirayama M, Nakahara T, Ohta S, Hattori N. Pilot study of H2 therapy in Parkinson’s disease: A randomized double-blind placebo-controlled trial. Mov Disord. 2013;28:836–839. doi: 10.1002/mds.25375. [DOI] [PubMed] [Google Scholar]
- 24.Yoritaka A, Abe T, Ohtsuka C, Maeda T, Hirayama M, Watanabe H, et al. A randomized double-blind multi-center trial of hydrogen water for Parkinson’s disease: protocol and baseline characteristics. BMC Neurol. 2016;16:66. doi: 10.1186/s12883-016-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge Y, Wu F, Sun X, Xiang Z, Yang L, Huang S, et al. Intrathecal infusion of hydrogen-rich normal saline attenuates neuropathic pain via inhibition of activation of spinal astrocytes and microglia in rats. PloS One. 2014;9:e97436. doi: 10.1371/journal.pone.0097436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iketani M, Ohsawa I. Molecular hydrogen as a neuroprotective agent. Curr Neuropharmacol. 2017;15:324–331. doi: 10.2174/1570159X14666160607205417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B, Qin S. Different types of molecular hydrogen donors and their pharmacokinetics in vivo. Sheng Li Xue Bao. 2019;71:371–377. [PubMed] [Google Scholar]
- 28.Wang Y, Li T, Cao H, Yang W. Recent advances in the neuroprotective effects of medical gases. Med Gas Res. 2019;9:80. doi: 10.4103/2045-9912.260649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Xie K, Li J, Xu N, Gong G, Wang G, et al. Beneficial effects of hydrogen gas against spinal cord ischemia-reperfusion injury in rabbits. Brain Res. 2011;1378:125–136. doi: 10.1016/j.brainres.2010.12.071. [DOI] [PubMed] [Google Scholar]
- 30.Gao Y, Gui Q, Jin L, Yu P, Wu L, Cao L, et al. Hydrogen-rich saline attenuates hippocampus endoplasmic reticulum stress after cardiac arrest in rats. Neurosci Lett. 2017;640:29–36. doi: 10.1016/j.neulet.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Wei R, Zhang R, Xie Y, Shen L, Chen F. Hydrogen suppresses hypoxia/reoxygenation-induced cell death in hippocampal neurons through reducing oxidative stress. Cell Physiol Biochem. 2015;36:585–598. doi: 10.1159/000430122. [DOI] [PubMed] [Google Scholar]
- 32.Cole AR, Perry DA, Raza A, Nedder AP, Pollack E, Regan WL, et al. Perioperatively inhaled hydrogen gas diminishes neurologic injury following experimental circulatory arrest in swine. JACC Basic Transl Sci. 2019;4:176–187. doi: 10.1016/j.jacbts.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen K, Wang N, Diao Y, Dong W, Sun Y, Liu L, et al. Hydrogen-rich saline attenuates brain injury induced by cardiopulmonary bypass and inhibits microvascular endothelial cell apoptosis via the PI3K/Akt/GSK3β signaling pathway in rats. Cell Physiol Biochem. 2017;43:1634–1647. doi: 10.1159/000484024. [DOI] [PubMed] [Google Scholar]
- 34.Mo X, Li X, She C, Lu X, Xiao C, Wang S, et al. Hydrogen-rich saline protects rat from oxygen glucose deprivation and reperusion-induced apoptosis through VDAC1 via Bcl-2. Brain Res. 2019;1706:110–115. doi: 10.1016/j.brainres.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 35.Cui Y, Zhang H, Ji M, Jia M, Chen H, Yang J, et al. Hydrogen-rich saline attenuates neuronal ischemia-reperfusion injury by protecting mitochondrial function in rats. J Surg Res. 2014;192:564–572. doi: 10.1016/j.jss.2014.05.060. [DOI] [PubMed] [Google Scholar]
- 36.Hugyecz M, Mracskó E, Hertelendy P, Farkas E, Domoki F, Bari F. Hydrogen supplemented air inhalation reduces changes of prooxidant enzyme and gap junction protein levels after transient global cerebral ischemia in the rat hippocampus. Brain Res. 2011;1404:31–38. doi: 10.1016/j.brainres.2011.05.068. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Zhao M, Chen Z, Wu G, Fujino M, Zhang C, et al. Hydrogen gas attenuates hypoxic-ischemic brain injury via regulation of the MAPK/HO-1/PGC-1a pathway in neonatal rats. Oxid Med Cell Longev. 2020;2020:6978784. doi: 10.1155/2020/6978784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Htun Y, Nakamura S, Nakao Y, Mitsuie T, Nakamura M, Yamato S, et al. Hydrogen ventilation combined with mild hypothermia improves short-term neurological outcomes in a 5-day neonatal hypoxia-ischaemia piglet model. Sci Rep. 2019;9:4088. doi: 10.1038/s41598-019-40674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu X, Cao L, Yu Z, Xin D, Li T, Ma W, et al. Hydrogen-rich saline promotes microglia M2 polarization and complement-mediated synapse loss to restore behavioral deficits following hypoxia-ischemic in neonatal mice via AMPK activation. J Neuroinflammation. 2019;16:104. doi: 10.1186/s12974-019-1488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai X, Song L, Lin Y, Xie Y, Tong L, Wang L, et al. Hydrogen-rich saline mediates neuroprotection through the regulation of endoplasmic reticulum stress and autophagy under hypoxia-ischemia neonatal brain injury in mice. Brain Res. 2016;1646:410–417. doi: 10.1016/j.brainres.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Oláh O, Tóthszűki V, Temesvári P, Bari F, Domoki F. Delayed neurovascular dysfunction is alleviated by hydrogen in asphyxiated newborn pigs. Neonatology. 2013;104:79–86. doi: 10.1159/000348445. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Cui J, Zhai X, Zhang J, Gu Z, Zhi X, et al. Inhalation of hydrogen of different concentrations ameliorates spinal cord injury in mice by protecting spinal cord neurons from apoptosis, oxidative injury and mitochondrial structure damages. Cell Physiol Biochem. 2018;47:176–190. doi: 10.1159/000489764. [DOI] [PubMed] [Google Scholar]
- 43.Dohi K, Kraemer BC, Erickson MA, Mcmillan PJ, Kovac A, Flachbartova Z, et al. Molecular hydrogen in drinking water protects against neurodegenerative changes induced by traumatic brain injury. PloS One. 2016;9:e108034. doi: 10.1371/journal.pone.0108034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu F, Xu S, Xiang Z, Li X, Li J, Yuan H, et al. Molecular hydrogen suppresses reactive astrogliosis related to oxidative injury during spinal cord injury in rats. CNS Neurosci Ther. 2014;20:778–786. doi: 10.1111/cns.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji X, Tian Y, Xie K, Liu W, Qu Y, Fei Z. Protective effects of hydrogen-rich saline in a rat model of traumatic brain injury via reducing oxidative stress. J Surg Res. 2012;178:e9–e16. doi: 10.1016/j.jss.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 46.Zhan Y, Chen C, Suzuki H, Hu Q, Zhi X, Zhang JH. Hydrogen gas ameliorates oxidative stress in early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2012;40:1291–1296. doi: 10.1097/CCM.0b013e31823da96d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan H, Guo S, Sheng C, Sun C, Zhang J, Sun X. Beneficial effect of hydrogen-rich saline on cerebral vasospasm after experimental subarachnoid hemorrhage in rats. J Neurosci Res. 2012;90:1670–1680. doi: 10.1002/jnr.22739. [DOI] [PubMed] [Google Scholar]
- 48.Zhuang Z, Sun X, Zhang X, Liu H, You W, Ma C, et al. Nuclear factor-κB/Bcl-XL pathway is involved in the protective effect of hydrogen-rich saline on the brain following experimental subarachnoid hemorrhage in rabbits. J Neurosci Res. 2013;91:1599–1608. doi: 10.1002/jnr.23281. [DOI] [PubMed] [Google Scholar]
- 49.Zhuang Z, Zhou M, You W, Zhu L, Ma C, Sun X, et al. Hydrogen-rich saline alleviates early brain injury via reducing oxidative stress and brain edema following experimental subarachnoid hemorrhage in rabbits. BMC Neurosci. 2012;13:47. doi: 10.1186/1471-2202-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shu R, Zhang L, Wang C, Li N, Wang H, Xie K, et al. Spinal peroxynitrite contributes to remifentanil-induced postoperative hyperalgesia via enhancement of divalent metal transporter 1 without iron-responsive element-mediated iron accumulation in rats. Anesthesiology. 2015;122:908–920. doi: 10.1097/ALN.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Shu R, Wang C, Wang H, Li N, Wang G. Hydrogen-rich saline controls remifentanil-induced hypernociception and NMDA receptor NR1 subunit membrane trafficking through GSK-3β in the DRG in rats. Brain Resh Bull. 2014;106:47–55. doi: 10.1016/j.brainresbull.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Shu R, Wang H, Yu Y, Wang C, Yang M, et al. Hydrogen-rich saline prevents remifentanil-induced hyperalgesia and inhibits MnSOD nitration via regulation of NR2B-containing NMDA receptor in rats. Neuroscience. 2014;280:171–180. doi: 10.1016/j.neuroscience.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 53.Chen Q, Chen P, Zhou S, Yan X, Zhang J, Sun X, et al. Hydrogen-rich saline attenuated neuropathic pain by reducing oxidative stress. Can J Neurol Sci. 2013;40:857–863. doi: 10.1017/S0317167100016024. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, Chen H, Xie K, Liu L, Li Y, Yu Y, et al. H2 treatment attenuated pain behavior and cytokine release through the HO-1/CO pathway in a rat model of neuropathic pain. Inflammation. 2015;38:1835–1846. doi: 10.1007/s10753-015-0161-x. [DOI] [PubMed] [Google Scholar]
- 55.Fu Y, Ito M, Fujita Y, Ito M, Ichihara M, Masuda A, et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci Lett. 2009;453:81–85. doi: 10.1016/j.neulet.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 56.Mikako I, Masaaki H, Kazuaki Y, Sae G, Masafumi I, Masatoshi I, et al. Drinking hydrogen water and intermittent hydrogen gas exposure, but not lactulose or continuous hydrogen gas exposure, prevent 6-hydorxydopamine-induced Parkinson’s disease in rats. Med Gas Res. 2012;2:15. doi: 10.1186/2045-9912-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujita K, Seike T, Yutsudo N, Ohno M, Yamada H, Yamaguchi H, et al. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. PloS One. 2009;4:e7247. doi: 10.1371/journal.pone.0007247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsumoto A, Yamafuji M, Tachibana T, Nakabeppu Y, Noda M, Nakaya H. Oral ‘hydrogen water’ induces neuroprotective ghrelin secretion in mice. Sci Rep. 2013;3:3273. doi: 10.1038/srep03273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Wang C, Zhang JH, Cai J, Cao Y, Sun X. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer’s disease by reduction of oxidative stress. Brain Res. 2010;1328:152–161. doi: 10.1016/j.brainres.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 60.Wang C, Li J, Liu Q, Yang R, Zhang JH, Cao Y, et al. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-κB activation in a rat model of amyloid-beta-induced Alzheimer’s disease. Neurosci Lett. 2011;491:127–132. doi: 10.1016/j.neulet.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 61.Lin CL, Huang WN, Li HH, Huang CN, Hsieh S, Lai C, et al. Hydrogen-rich water attenuates amyloid β-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem Biol Interact. 2015;240:12–21. doi: 10.1016/j.cbi.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 62.Yi Z, Su W, Ying C, Wu T, Hong G, Shen X, et al. Effects of hydrogen-rich water on depressive-like behavior in mice. Sci Rep. 2016;6:23742. doi: 10.1038/srep23742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wen D, Zhao P, Hui R, Wang J, Shen Q, Gong M, et al. Hydrogen-rich saline attenuates anxiety-like behaviors in morphine-withdrawn mice. Neuropharmacology. 2017;118:199–208. doi: 10.1016/j.neuropharm.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 64.Guo Q, Yin X, Qiao M, Jia Y, Chen D, Shao J, et al. Hydrogen-rich water ameliorates autistic-like behavioral abnormalities in valproic acid-treated adolescent mice offspring. Front Behav Neurosci. 2018;12:170. doi: 10.3389/fnbeh.2018.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ji X, Zhang Q, Zheng W, Yao W. Morphological and molecular response of small intestine to lactulose and hydrogen-rich water in female piglets fed Fusarium mycotoxins contaminated diet. J Anim Sci Biotechnol. 2019;10:9. doi: 10.1186/s40104-019-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao L, Wang Y, Zhang G, Zhang T, Lou J, Liu J. L-arabinose elicits gut-derived hydrogen production and ameliorates metabolic syndrome in C57BL/6J mice on high-fat-diet. Nutrients. 2019;11:3054. doi: 10.3390/nu11123054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hou C, Wang Y, Zhu E, Yan C, Zhao L, Wang X, et al. Coral calcium hydride prevents hepatic steatosis in high fat diet-induced obese rats: A potent mitochondrial nutrient and phase II enzyme inducer. Biochem Pharmacol. 2016;103:85–97. doi: 10.1016/j.bcp.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 68.Zhou G, Goshi E, He Q. Micro/nanomaterials-augmented hydrogen therapy. Adv Healthc Mater. 2019;8:e1900463. doi: 10.1002/adhm.201900463. [DOI] [PubMed] [Google Scholar]
- 69.Zhu Q, Wu Y, Li Y, Chen Z, Wang L, Xiong H, et al. Positive effects of hydrogen-water bathing in patients of psoriasis and parapsoriasis en plaques. Sci Rep. 2018;8:8051. doi: 10.1038/s41598-018-26388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shimouchi A, Nose K, Shirai M, Kondo T. Estimation of molecular hydrogen consumption in the human whole body after the ingestion of hydrogen-rich water. Adv Exp Med Biol. 2012;737:245–250. doi: 10.1007/978-1-4614-1566-4_36. [DOI] [PubMed] [Google Scholar]
- 71.Ono H, Nishijima Y, Adachi N, Sakamoto M, Kudo Y, Nakazawa J, et al. Hydrogen (H2) treatment for acute erythymatous skin diseases. A report of 4 patients with safety data and a non-controlled feasibility study with H2 concentration measurement on two volunteers. Med Gas Res. 2012;2:14. doi: 10.1186/2045-9912-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu C, Kurokawa R, Fujino M, Hirano S, Sato B, Li X. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci Rep. 2014;4:5485. doi: 10.1038/srep05485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takeuchi S, Nagatani K, Otani N, Nawashiro H, Sugawara T, Wada K, et al. Hydrogen improves neurological function through attenuation of blood-brain barrier disruption in spontaneously hypertensive stroke-prone rats. BMC Neurosci. 2015;16:22. doi: 10.1186/s12868-015-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamamoto R, Homma K, Suzuki S, Sano M, Sasaki J. Hydrogen gas distribution in organs after inhalation: Real-time monitoring of tissue hydrogen concentration in rat. Sci Rep. 2019;9:1255. doi: 10.1038/s41598-018-38180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao P, Jin Z, Chen Q, Yang T, Chen D, Meng J, et al. Local generation of hydrogen for enhanced photothermal therapy. Nat Commun. 2018;9:1–12. doi: 10.1038/s41467-017-02088-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen L, Chao Y, Cheng P, Li N, Zheng H, Yang Y. UPLC-QTOF/MS-based metabolomics reveals the protective mechanism of hydrogen on mice with ischemic stroke. Neurochem Res. 2019;44:1950–1963. doi: 10.1007/s11064-019-02829-x. [DOI] [PubMed] [Google Scholar]
- 77.Huang J, Liu W, Sun X. Hydrogen inhalation improves mouse neurological outcomes after cerebral ischemia/reperfusion independent of anti-necroptosis. Med Gas Res. 2018;8:1–5. doi: 10.4103/2045-9912.229596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang L, Applegate RL, II, Applegate PM, Gong L, Ocak U, Boling W, et al. Inhalation of high-concentration hydrogen gas attenuates cognitive deficits in a rat model of asphyxia induced-cardiac arrest. Med Gas Res. 2019;9:122–126. doi: 10.4103/2045-9912.266986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang L, Applegate RL, II, Applegate PM, Boling W, Zhang JH. Inhalation of high concentration hydrogen gas improves short-term outcomes in a rat model of asphyxia induced-cardiac arrest. Med Gas Res. 2018;8:73–78. doi: 10.4103/2045-9912.241063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hayashida K, Sano M, Kamimura N, Yokota T, Suzuki M, Ohta S, et al. Hydrogen inhalation during normoxic resuscitation improves neurological outcome in a rat model of cardiac arrest independently of targeted temperature management. Circulation. 2014;130:2173–2180. doi: 10.1161/CIRCULATIONAHA.114.011848. [DOI] [PubMed] [Google Scholar]
- 81.Chen G, Chen B, Dai C, Wang J, Wang J, Huang Y, et al. Hydrogen inhalation is superior to mild hypothermia for improving neurological outcome and survival in a cardiac arrest model of spontaneously hypertensive rat. Shock. 2018;50:689–695. doi: 10.1097/SHK.0000000000001092. [DOI] [PubMed] [Google Scholar]
- 82.Huo T, Zeng Y, Liu X, Sun L, Han H, Chen H, et al. Hydrogen-rich saline improves survival and neurological outcome after cardiac arrest and cardiopulmonary resuscitation in rats. Anesth Analg. 2014;119:368–380. doi: 10.1213/ANE.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 83.Wang P, Jia L, Chen B, Zhang L, Liu J, Long J, et al. Hydrogen inhalation is superior to mild hypothermia in improving cardiac function and neurological outcome in an asphyxial cardiac arrest model of rats. Shock. 2016;46:312–318. doi: 10.1097/SHK.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 84.Nagatani K, Nawashiro H, Takeuchi S, Tomura S, Otani N, Osada H, et al. Safety of intravenous administration of hydrogen-enriched fluid in patients with acute cerebral ischemia: initial clinical studies. Med Gas Res. 2013;3:13. doi: 10.1186/2045-9912-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ono H, Nishijima Y, Adachi N, Tachibana S, Chitoku S, Mukaihara S, et al. Improved brain MRI indices in the acute brain stem infarct sites treated with hydroxyl radical scavengers, Edaravone and hydrogen, as compared to Edaravone alone. A non-controlled study. Med Gas Res. 2011;1:12. doi: 10.1186/2045-9912-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palanisamy A, Baxter MG, Keel PK, Xie Z, Crosby G, Culley DJ. Rats exposed to isoflurane in utero during early gestation are behaviorally abnormal as adults. Anesthesiology. 2011;114:521–528. doi: 10.1097/ALN.0b013e318209aa71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Domoki F, Oláh O, Zimmermann A, Németh I, Tóthszuki V, Hugyecz M, et al. Hydrogen is neuroprotective and preserves cerebrovascular reactivity in asphyxiated newborn pigs. Pediatr Res. 2010;68:387–392. doi: 10.1203/PDR.0b013e3181f2e81c. [DOI] [PubMed] [Google Scholar]
- 88.Tian R, Hou Z, Hao S, Wu W, Mao X, Tao X, et al. Hydrogen-rich water attenuates brain damage and inflammation after traumatic brain injury in rats. Brain Res. 2016;1637:1–13. doi: 10.1016/j.brainres.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 89.Fu J, Lan Q, Wang D, Wang Y, Liu Y. Effect of hydrogen-rich water on the chondriosome damage and cytokines in brain tissue of rats with traumatic brain injury. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30:317–321. doi: 10.3760/cma.j.issn.2095-4352.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 90.Ji X, Liu W, Xie K, Liu W, Qu Y, Chao X, et al. Beneficial effects of hydrogen gas in a rat model of traumatic brain injury via reducing oxidative stress. Brain Res. 2012;178:e9–e16. doi: 10.1016/j.brainres.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 91.Hou Z, Luo W, Sun X, Hao S, Zhang Y, Xu F, et al. Hydrogen-rich saline protects against oxidative damage and cognitive deficits after mild traumatic brain injury. Brain Res Bull. 2012;88:560–565. doi: 10.1016/j.brainresbull.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 92.Choi K, Kim H, Do S, Hwang S, Yi HJ. Neuroprotective effects of hydrogen inhalation in an experimental rat intracerebral hemorrhage model. Brain Res Bull. 2018;142:122–128. doi: 10.1016/j.brainresbull.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 93.Manaenko A, Lekic T, Ma Q, Ostrowski RP, Zhang JH, Tang J. Hydrogen inhalation is neuroprotective and improves functional outcomes in mice after intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:179–183. doi: 10.1007/978-3-7091-0693-8_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 95.Hirayama M, Ito M, Minato T, Yoritaka A, LeBaron TW, Ohno K. Inhalation of hydrogen gas elevates urinary 8-hydroxy-2′-deoxyguanine in Parkinson’s disease. Med Gas Res. 2018;8:144–149. doi: 10.4103/2045-9912.248264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoritaka A, Ohtsuka C, Maeda T, Hirayama M, Abe T, Watanabe H, et al. Randomized, double-blind, multicenter trial of hydrogen water for Parkinson’s disease. Mov Disord. 2018;33:1505–1507. doi: 10.1002/mds.27472. [DOI] [PubMed] [Google Scholar]
- 97.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ostojic SM. Inadequate production of H2 by gut microbiota and Parkinson disease. Trends Endocrinol Metab. 2018;29:286–288. doi: 10.1016/j.tem.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 99.Yang J, Wang Z, Cai H, Yuan L, Hu M, Wu M, et al. Sex differences in neuropathology and cognitive behavior in APP/PS1/tau triple-transgenic mouse model of Alzheimer’s disease. Neurosci Bull. 2018;34:736–746. doi: 10.1007/s12264-018-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He X, Wang S, Yin C, Wang T, Jia C, Ma Y. Hydrogen-rich water exerting a protective effect on ovarian reserve function in a mouse model of immune premature ovarian failure induced by zona pellucida 3. Chin Med J (Engl) 2016;129:2331–2337. doi: 10.4103/0366-6999.190668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang X, Niu X, Guo Q, Dong Y, Xu J, Yin N, et al. FoxO1-mediated autophagy plays an important role in the neuroprotective effects of hydrogen in a rat model of vascular dementia. Behav Brain Res. 2019;356:98–106. doi: 10.1016/j.bbr.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 102.Liu M, Yuan H, Yin J, Wang R, Song J, Hu B, et al. Effect of hydrogen-rich water on radiation-induced cognitive dysfunction in rats. Radiat Res. 2020;193:16–23. doi: 10.1667/RR15464.1. [DOI] [PubMed] [Google Scholar]
- 103.Li W, Yang S, Yu F, Zhao Y, Sun Z, An J, et al. Hydrogen ameliorates chronic intermittent hypoxia-induced neurocognitive impairment via inhibiting oxidative stress. Brain Res Bull. 2018;143:225–233. doi: 10.1016/j.brainresbull.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 104.Nagata K, Nakashimakamimura N, Mikami T, Ohsawa I, Ohta S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacol. 2009;34:501–508. doi: 10.1038/npp.2008.95. [DOI] [PubMed] [Google Scholar]
- 105.Jia R, Jia N, Yang F, Liu Z, Li R, Jiang Y, et al. Hydrogen alleviates necroptosis and cognitive deficits in lithium-pilocarpine model of status epilepticus. Cell Mol Neurobiol. 2019;39:857–869. doi: 10.1007/s10571-019-00685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gu Y, Huang CS, Inoue T, Yamashita T, Ishida T, Kang KM, et al. Drinking hydrogen water ameliorated cognitive impairment in senescence-accelerated mice. J Clin Biochem Nutr. 2010;46:269–276. doi: 10.3164/jcbn.10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li C, Hou L, Chen D, Lin F, Chang T, Li M, et al. Hydrogen-rich saline attenuates isoflurane-induced caspase-3 activation and cognitive impairment via inhibition of isoflurane-induced oxidative stress, mitochondrial dysfunction, and reduction in ATP levels. Am J Transl Res. 2017;9:1162–1172. [PMC free article] [PubMed] [Google Scholar]
- 108.Tian Y, Guo S, Zhang Y, Xu Y, Zhao P, Zhao X. Effects of hydrogen-rich saline on hepatectomy-induced postoperative cognitive dysfunction in old mice. Mol Neurobiol. 2017;54:2579–2584. doi: 10.1007/s12035-016-9825-2. [DOI] [PubMed] [Google Scholar]
- 109.Luo H, Liu Z, Liu B, Li H, Yang Y, Xu ZQD. Virus-mediated overexpression of ETS-1 in the ventral hippocampus counteracts depression-like behaviors in rats. Neurosci Bull. 2019;35:1035–1044. doi: 10.1007/s12264-019-00412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ishibashi T. Therapeutic efficacy of molecular hydrogen: A new mechanistic insight. Curr Pharm Des. 2019;25:946–955. doi: 10.2174/1381612825666190506123038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ishihara G, Kawamoto K, Komori N, Ishibashi T. Molecular hydrogen suppresses superoxide generation in the mitochondrial complex I and reduced mitochondrial membrane potential. Biochem Biophys Res Commun. 2020;522:965–970. doi: 10.1016/j.bbrc.2019.11.135. [DOI] [PubMed] [Google Scholar]
- 112.Gvozdjáková A, Kucharská J, Kura B, Vančová O, Rausová Z, Sumbalová Z, et al. A new insight into the molecular hydrogen effect on coenzyme Q and mitochondrial function of rats. Can J Physiol Pharmacol. 2020;98:29–34. doi: 10.1139/cjpp-2019-0281. [DOI] [PubMed] [Google Scholar]
- 113.Zhang X, Zhang Z, Wei Y, Li M, Zhao P, Adzavon YM, et al. Mitochondria in higher plants possess H2 evolving activity which is closely related to complex I. arXiv 2020, 2001.02132. https://arxiv.org/abs/2001.02132.
- 114.Ma X, Zhang X, Xie F, Zhao P, Zhang Z, Yi Y, et al. Bio-enzyme basis of hydrogen in biological system. Current Biotechnology. 2020;10:15–22. [Google Scholar]
- 115.Wang X, Zhang L, Zhao W, Liu T. The protective effects of hydrogen on HO-1 expression in the brainafter focal cerebral ischemia reperfusion in rats. Turk J Med Sci. 2016;46:1534–1539. doi: 10.3906/sag-1502-3. [DOI] [PubMed] [Google Scholar]
- 116.Zhou L, Wang X, Xue W, Xie K, Huang Y, Chen H, et al. Beneficial effects of hydrogen-rich saline against spinal cord ischemia-reperfusion injury in rabbits. Brain Res. 2013;1517:150–160. doi: 10.1016/j.brainres.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 117.Wang T, Zhao L, Liu M, Xie F, Ma X, Zhao P, et al. Oral intake of hydrogen-rich water ameliorated chlorpyrifos-induced neurotoxicity in rats. Toxicol Appl Pharmacol. 2014;280:169–176. doi: 10.1016/j.taap.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 118.Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murakami Y, Ito M, Ohsawa I. Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PloS One. 2017;12:e0176992. doi: 10.1371/journal.pone.0176992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shi Y, Wang G, Li J, Yu W. Hydrogen gas attenuates sevoflurane neurotoxicity through inhibiting nuclear factor κ-light-chain-enhancer of activated B cells signaling and proinflammatory cytokine release in neonatal rats. Neuroreport. 2017;28:1170–1175. doi: 10.1097/WNR.0000000000000899. [DOI] [PubMed] [Google Scholar]
- 121.Liu Y, Dong F, Guo R, Zhang Y, Qu X, Wu X, et al. Hydrogen-rich saline ameliorates experimental autoimmune encephalomyelitis in C57BL/6 mice via the Nrf2-ARE signaling pathway. Inflammation. 2019;42:586–597. doi: 10.1007/s10753-018-0915-3. [DOI] [PubMed] [Google Scholar]
- 122.Qin C, Zhou L, Ma X, Hu Z, Yang S, Chen M, et al. Dual functions of microglia in ischemic stroke. Neurosci Bull. 2019;35:921–933. doi: 10.1007/s12264-019-00388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ning K, Liu W, Huang J, Lu H, Sun X. Effects of hydrogen on polarization of macrophages and microglia in a stroke model. Med Gas Res. 2018;8:154–159. doi: 10.4103/2045-9912.248266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev. 2015;2015:610813. doi: 10.1155/2015/610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Minutoli L, Puzzolo D, Rinaldi M, Irrera N, Marini H, Arcoraci V, et al. ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxid Med Cell Longev. 2016;2016:2183026. doi: 10.1155/2016/2183026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tao G, Song G, Qin S. Molecular hydrogen: current knowledge on mechanism in alleviating free radical damage and diseases. Acta Biochim Biophys Sin (Shanghai) 2019;51:1189–1197. doi: 10.1093/abbs/gmz121. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Y, Li H, Yang C, Fan D, Guo D, Hu H, et al. Treatment with hydrogen-rich saline delays disease progression in a mouse model of amyotrophic lateral sclerosis. Neurochem Res. 2016;41:770–778. doi: 10.1007/s11064-015-1750-7. [DOI] [PubMed] [Google Scholar]
- 128.Hongtao MA, Chen H, Dong A, Wang Y, Bian Y, Xie K. Hydrogen-rich saline attenuates hyperalgesia and reduces cytokines in rats with post-herpetic neuralgia via activating autophagy. Chinese Journal of Cellular & Molecular Immunology. 2017;33:155–158. [PubMed] [Google Scholar]
- 129.Pérez MJ, Ponce DP, Aranguiz A, Behrens MI, Quintanilla RA. Mitochondrial permeability transition pore contributes to mitochondrial dysfunction in fibroblasts of patients with sporadic Alzheimer’s disease. Redox Biol. 2018;19:290–300. doi: 10.1016/j.redox.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arabian M, Aboutaleb N, Soleimani M, Ajami M, Habibey R, Pazoki-Toroudi H. Activation of mitochondrial KATP channels mediates neuroprotection induced by chronic morphine preconditioning in hippocampal CA-1 neurons following cerebral ischemia. Adv Med Sci. 2018;63:213–219. doi: 10.1016/j.advms.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 131.Khatri R, Mckinney AM, Swenson B, Janardhan V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012;79:S52–S57. doi: 10.1212/WNL.0b013e3182697e70. [DOI] [PubMed] [Google Scholar]
- 132.Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J Clin Biochem Nutr. 2010;46:140–149. doi: 10.3164/jcbn.09-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang G, Romero-Gallo J, Benoit SL, Piazuelo MB, Dominguez RL, Morgan DR, et al. Hydrogen metabolism in Helicobacter pylori plays a role in gastric carcinogenesis through facilitating CagA translocation. MBio. 2016;7:e01016–e01022. doi: 10.1128/mBio.01022-16. [DOI] [PMC free article] [PubMed] [Google Scholar]