Abstract
Regulation of glucocorticoids (GCs), important mediators of physiology and behavior at rest and during stress, is multi-faceted and dynamic. The 11ß hydroxysteroid dehydrogenases 11ß-HSD1 and 11ß-HSD2 catalyze the regeneration and inactivation of GCs, respectively, and provide peripheral and central control over GC actions in mammals. While these enzymes have only recently been investigated in just two songbird species, central expression patterns suggest that they may function differently in birds and mammals, and little is known about how peripheral expression regulates circulating GCs. In this study, we utilized the 11ß-HSD inhibitor carbenoxolone (CBX) to probe the functional effects of 11ß-HSD activity on circulating GCs and central GC-dependent gene expression in the adult zebra finch (Taeniopygia guttata). Peripheral CBX injection produced a marked increase in baseline GCs 60min after injection, suggestive of a dominant role for 11ß-HSD2 in regulating circulating GCs. In the adult zebra finch brain, where 11ß-HSD2 but not 11ß-HSD1 is expressed, co-incubation of micro-dissected brain regions with CBX and stress-level GCs had no impact on expression of several GC-dependent genes. These results suggest that peripheral 11ß-HSD2 attenuates circulating GCs, whereas central 11ß-HSD2 has little impact on gene expression. Instead, rapid 11ß-HSD2-based regulation of local GC levels might fine-tune membrane GC actions in brain. These results provide new insights into the dynamics of GC secretion and action in this important model organism.
Keywords: 11ß-hydroxysteroid dehydrogenase, glucocorticoids, stress response, carbenoxolone, zebra finch
1. Introduction:
Allostasis, the process of responding and adapting to physical and psychological challenges while maintaining bodily systems, is crucial for short-term survival as well as long-term health and well-being (McEwen, BS, 1998; McEwen and Wingfield, 2003; Sterling, 1988). The vertebrate hypothalamic-pituitary-adrenal (HPA) axis, in conjunction with the sympatho-adrenomedullary system, mediates allostasis through the production and regulation of glucocorticoids (GCs) by the adrenal glands. GCs such as corticosterone (CORT; the primary avian GC) act on nearly every tissue in the body to induce genomic and non-genomic effects that regulate the immune, cardiovascular, metabolic, reproductive, and cognitive systems (Sapolsky et al., 2000).
Precise control over GC levels and action is necessary to ensure optimal function in the face of challenging conditions. GCs are regulated by negative feedback loops, most notably at the level of the hippocampus (HP), hypothalamus (HYP), and pituitary, where excess GCs induce down-regulation of corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), respectively, through binding of the glucocorticoid receptor (GR) and mineralocorticoid receptor (MR). MR primarily mediates basal GC effects, particularly in the HP, which exerts inhibitory neuronal control over the HYP under basal conditions. In contrast, lower-affinity GR are bound when GC levels are elevated during stress (de Kloet et al., 1998; de Kloet et al., 2005a; de Kloet et al., 2005b). While classical GR and MR are nuclear receptors mediating genomic effects, CORT also binds to neural membrane receptors to rapidly modulate intra- and extra-cellular signaling (Breuner and Orchinik, 2009; Orchinik et al., 1997). Up- or down-regulation of receptor expression or activity in the HPA axis therefore alters sensitivity to negative feedback. In addition, selective alterations to GR or MR expression can modify target tissue sensitivity to basal and stress-induced GCs (Lattin and Romero, 2014; Oitzl et al., 2010; Sapolsky et al., 1984).
While modifying receptor expression can regulate GC effects, other mechanisms may provide more flexible and dynamic control over GC actions. For example, local GC synthesis from steroid precursors has been established or inferred in rodent brain, immune tissues, and intestine, as well as in songbird immune tissues (Schmidt and Soma, 2008; Taves et al., 2015, 2011). In addition, reversible GC metabolism via the expression of the 11ß hydroxysteroid dehydrogenase enzymes may enable tissues to selectively regenerate (11ß-HSD1) or inactivate (11ß-HSD2) GCs in the absence of alterations in HPA axis activity, circulating GCs, or receptor expression (Holmes and Seckl, 2006; Seckl et al., 2000). Recent reports of 11ß-HSD1 and 11ß-HSD2 expression in a variety of peripheral tissues (e.g., muscle, liver, kidney) suggest the potential for such widespread local peripheral GC control in songbirds (Pérez et al., 2020; Pradhan et al., 2019; Rensel et al., 2018).
The 11ß-HSDs play diverse roles in the mammalian periphery and central nervous system. In the kidney, 11ß-HSD2 selectively inactivates GCs to preserve MR access for aldosterone, while its expression in the placenta and fetal central nervous system prevents excess GC action at GR (Benediktsson et al., 1997; Cottrell et al., 2014; Edwards et al., 1988; Holmes et al., 2006; Räikkönen et al., 2017; Welberg et al., 2000). In contrast, 11ß-HSD1 is expressed in the mammalian liver, where it regulates glucose metabolism (Jamieson et al., 2000, 1999; Kotelevtsev et al., 1997). This enzyme is relatively absent from the developing fetal brain, but is expressed in the adult rodent HP and HYP, where it regulates diurnal and stress-induced changes in HP GCs, affects negative feedback and fear-based memory, and potentiates the impacts of age-related cognitive decline and kainate-induced neurotoxicity (Ajilore and Sapolsky, 1999; Holmes et al., 2010; Holmes and Seckl, 2006; Rajan et al., 1996; Sarabdjitsingh et al., 2014; Yau et al., 2015, 2001).
While these molecular mechanisms of GC regulation in the brain and periphery are well-established in mammals, relatively little is known about whether and how they operate in other vertebrate taxa. Songbirds are important model organisms for addressing impacts of environmental perturbations, including climate change, on wild species and modeling the impacts of stress on neurodegeneration and cognition (Clayton and Emery, 2015; Thompson and Brenowitz, 2010; Wingfield, 2008). Many features of the songbird stress axis appear to be conserved, including most aspects of GR and MR-based action and expression and GC-dependent signaling (Breuner and Orchinik, 2009; Rensel et al., 2018; Rensel and Schlinger, 2020). In addition, 11ß-HSD2 and 11ß-HSD1 are highly expressed in the songbird kidney and liver, respectively, suggesting conservation of function in these tissues (Pérez et al., 2020; Rensel et al., 2018).
Some elements of GC regulation differ between songbirds and mammals, however. MR expression is more widespread in the songbird than mammalian brain, and corticosteroid-binding-globulins, which circulate in the bloodstream and regulate free GC access to target tissues, differentially bind GCs and other steroids in birds and mammals (Breuner and Orchinik, 2009; Deviche et al., 2001; Rensel et al., 2018). Moreover, while 11ß-HSD2 is relatively absent and 11ß-HSD1 is widely expressed in the adult rodent brain, our recent work suggests that the converse is true in zebra finches (Rensel and Schlinger, 2016). Indeed, we and others have documented widespread, if low, expression and activity of 11ß-HSD2 in the adult zebra finch brain, but no measurable 11ß-HSD1 (Katz et al., 2010; Rensel et al., 2018, 2014; but see Pérez et al. (2020)). Thus, while the basic mechanics of the stress response are conserved across vertebrate taxa, the molecular mechanisms governing local GC actions are dynamic and diverse.
While we have an emerging picture of 11ß-HSD1 and 11ß-HSD2 expression in the songbird periphery and brain, the functional significance of this expression remains relatively unknown (but see Pérez et al., 2020). For example, renal 11ß-HSD2 not only preserves aldosterone access to MR, but expression in other peripheral tissues, particularly the adrenals, may regulate circulating GC levels in mammals (Coulter et al., 1999; Mazzocchi et al., 1998; Pérez et al., 2020; Roland and Funder, 1996; Shimojo et al., 1996; Stewart et al., 1994; Yang and Matthews, 1995). To assess the functional significance of 11ß-HSD2 expression in a songbird, we conducted two experiments in adult zebra finches. First, we investigated the relationship between peripheral 11ß-HSD2 expression and circulating CORT levels. Birds were injected with carbenoxolone (CBX), a synthetic licorice derivative that inhibits the 11ß-HSDs in mammals (Ajilore and Sapolsky, 1999; Jellinck et al., 1993; Monder et al., 1989; Rajan et al., 1996; van Haarst et al., 1996), and we measured CORT under baseline followed by stressed conditions. CBX, a compound with well-established dose and kinetic parameters, inhibits the activity of both 11ß-HSD1 and 11ß-HSD2, but only 11ß-HSD2 is expressed in the adult zebra finch brain (Rensel et al., 2018). Because 11ß-HSD2 activity appears to be unaffected by circulating CORT levels (Zallocchi et al., 2004) and because 11ß-HSD2 activity predominates in the site of mammalian GC synthesis, the adrenals (Mazzocchi et al., 1998), we predicted that inhibiting both enzymes would cause an increase in both baseline and stress-induced CORT levels.
In the second experiment, we tested the hypothesis that 11ß-HSD2 activity in specific brain regions attenuates GC-dependent gene expression. In addition to demonstrating widespread expression of 11ß-HSD2 across the adult zebra finch brain (Rensel et al., 2018), we recently showed that stress levels of CORT up-regulate expression of two CORT-dependent genes, FKBP5 and SGK1, in brain slice culture (Rensel and Schlinger, 2020). We hypothesized that incubating similar brain slices with CBX would enhance GC-dependent downstream gene expression, particularly in regions that express relatively more 11ß-HSD2 in the zebra finch brain, the cerebellum (CER) and caudal telencephalon (cTEL; Rensel et al., 2018).
2. Materials and Methods:
2.1. Animal Care
Adult, non-breeding zebra finches were obtained from our colony at UCLA, where they were maintained on a 14h light: 10h dark cycle and fed a diet of millet supplemented with vitamins, egg mix, grit, and cuttle bone. Prior to experiments, individuals were housed in groups of ~35 single-sex individuals per cage, and all birds in the colony were in visual and vocal contact with each other. All housing and experimental procedures were approved and monitored by the UCLA Chancellor’s Animal Research Committee in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.2. Experiment 1: Effect of Carbenoxolone on Circulating CORT
2.2.1. Chemicals:
Carbenoxolone disodium salt (Sigma C4790) was dissolved in 0.1M sterile phosphate buffered saline. Experimental birds received a ~100mg/kg I.M. injection (see below), which inhibits 11ß-HSD1 and 11ß-HSD2-based activity and effects in rodents (Heine and Rowitch, 2009; Jellinck et al., 1993).
2.2.2. Injection Protocol:
Adult zebra finches were caught in the main colony room and moved to individual soundproof chambers (n = 4 males and 4 females) over the course of two capture dates (2 males and 2 females were caught on each date). Animals were removed from the aviaries in the dark to reduce the stress of capture and handling and to ensure random selection. Upon delivery to the soundproof chambers, one animal of each sex was randomly allocated to chambers designated for either the CBX or vehicle injection group (animals were taken from the small carrying cage in the dark to prevent stress and bias in treatment allocation). Because isolation can induce a stress response in songbirds (Apfelbeck and Raess, 2008; Banerjee and Adkins-Regan, 2011; Remage-Healey et al., 2003), birds were acclimated in the chambers for 5 full days, where the chamber door was opened once per day to change food and water. On day 6, the chamber door was opened between the hours of 8:30 and 10:30AM (to minimize variation due to diel CORT rhythms; Rensel et al., 2014) and a baseline blood sample (~15ul) was obtained from the brachial vein within 3min of capture. Each bird then received a 50ul I.M. injection of CBX or sterile saline (vehicle used to dissolve CBX) into the pectoral muscle, followed by return to the home cage for 60min to enable sufficient time for the CBX to inhibit enzyme activity (van Haarst et al., 1996) and to allow physiological recovery from the stress of handling, blood sampling, and injection. At the 60min time point, each bird was caught and bled once again within 3min of capture, followed by 30min in an opaque cloth bag and a subsequent blood sample to measure the CORT handling and restraint response. To determine whether peripheral CBX treatment had lasting effects on the HPA axis, these same individuals underwent an additional restraint stressor 24 hours later with the same protocol minus the injection (baseline blood sampling within 3min of disturbance followed by stress-induced blood sampling after 30min of restraint in a cloth bag).
Blood samples were kept on ice until centrifugation at 10,000g for 10min, separation of the plasma portion, and freezing at −80°C.
2.2.3. Solid Phase Extraction & ELISA
Prior to ELISA, plasma CORT samples were extracted using a solid phase extraction protocol based on (Rensel and Schlinger, 2020). Plasma samples were thawed and briefly vortexed, then 6.5ul plasma was added to 10ml ultrapure water. Samples were extracted with C18 extraction cartridges (6ml; Thermo Scientific Hypersep; 500mg bed weight) placed in an extraction manifold. Columns were initially primed with HPLC grade methanol, followed by two rounds of water wash, two rounds of sample loading, an additional two rounds of water wash, and a final elution in HPLC grade methanol followed by storage at −20°C. In preparation for the ELISA, all samples were dried under a gentle stream of nitrogen in a 37°C water bath, then resuspended in assay buffer to represent a 1:20 dilution. Water blanks and cold CORT standards (417pg/ml) were extracted in parallel.
Extracted plasma CORT samples were quantified with the Cayman Chemical Corticosterone ELISA Kit (catalog no. 501320). The cross-reactivity of the Cayman kit is as follows: corticosterone: 100%; 11-deoxycorticosterone: 15.8%; prednisolone: 3.4%; 11-dehydrocorticosterone: 2.9%; cortisol: 2.5%. The assay standard curve ranges from 8-5000pg/ml, with an assay sensitivity limit of 30pg/ml. All samples were run across two plates, where samples from a given individual were always run on the same plate but randomly distributed spatially within the plate. The average recovery based on extracted and un-extracted standards was 59%. While this recovery is lower than anticipated, all samples but one was above the 30pg/ml level of sensitivity (mean sample values before accounting for dilution factor = 467.7pg/ml; standard deviation = 485.3pg/ml). The intra-assay CV, based on the average CV for sample duplicates, was 9.4%. The inter-assay CV was 9.6% when calculated based on standard curve values (determined based on the curve fitting equation for each assay) and 24% when calculated based on 3 un-extracted assay standards run on each plate. Because of this elevated inter-assay CV, we included assay ID as a random factor in our statistical model to account for any sample variability due to plate. We previously validated and utilized these solid phase extraction and ELISA protocols for zebra finch plasma (Rensel and Schlinger, 2020).
2.3. Experiment 2: Effect of Carbenoxolone on CORT-Dependent Gene Expression in Brain
2.3.1. Chemicals:
Crystalline CORT (Sigma) was dissolved in absolute ethanol and then diluted to a working solution of 30nM in aCSF to represent typical zebra finch stress-induced plasma CORT levels (aCSF: NaCl (199mM), NaHCO3 (26.2mM), Glucose (11mM), KCl (2.5mM), NaH2PO4 (1mM), MgSO4 (1.3mM), and CaCl (2.5mM) (pH 7.4; Katz et al., 2010; Rensel et al., 2014; Tam and Schlinger, 2007; Taves et al., 2010; Wada et al., 2008). The final ethanol concentration was < 0.001%.
To obtain a 1μM working carbenoxolone (CBX) solution, CBX powder was dissolved in aCSF to generate a 10mM stock that was then further diluted in aCSF. This concentration inhibited 11ß-HSD1 activity (Rajan et al., 1996; van Haarst et al., 1996), prevented 11ß-HSD1-mediated neurotoxicity in the presence of kainic acid and 11-DHC in rat hippocampal cell cultures (Ajilore and Sapolsky, 1999) and inhibited 11ß-HSD2-mediated preservation of external granule cell layer proliferation in the presence of prednisone in neonatal mouse cerebellum (Heine and Rowitch, 2009).
2.3.2. Slice Culture
Microsections of zebra finch brain were incubated with either CORT or CORT + CBX in a paired design following the methods in (Rensel and Schlinger, 2020). This design enabled us to investigate the impact of CBX on GC-dependent gene expression by pairing bilateral sections taken from the same coronal slice (1 section received the CORT treatment and the other CORT + CBX). Briefly, 6 zebra finches (3 males and 3 females) were euthanized by rapid decapitation within 40sec of turning off the light in the zebra finch colony room. Individuals were caught in the dark from large single-sex aviaries in the colony room to avoid investigator bias in subject selection. Two birds (1 male and 1 female) were caught per day, for a total of 3 days of capturing and conducting the slice culture experiment. The same aviary cage was never entered more than once over the course of 48+ hours to ensure a lack of stress in experimental subjects.
After sacrifice, the head was immediately buried in wet ice and transported to the laboratory, where removal of the brain was accomplished within 12min of sacrifice. The cerebellum was separated from the rest of the brain and both were maintained before and after sectioning and microdissection in ice-cold aCSF with bubbling carbogen (95% O2, 5% CO2). 500μm coronal sections of the brain and CER were taken using an NSVL Vibroslice (World Precision Instruments). Each coronal section was then visualized on an ice-cold wet petri dish situated under a dissecting microscope and the following regions were dissected bilaterally with a carbon steel micro knife: HP (6–7 slices per bird), HYP (4–5 slices per bird), and cTEL incorporating the caudomedial nidopallium and caudal nidopallium (Rensel and Schlinger, 2020) (4–5 slices each per bird). The remaining cTEL was also saved for incubation and later production of qPCR standard curves. Bilaterally dissected regions were randomly allocated to Netwells situated in bubbling ice-cold aCSF to enable a paired design. While we did not track the side that each section came from when allocating to treatment groups, the fact that sections were free-floating in aCSF prior to microdissection and that samples for each region were taken from multiple coronal sections makes it unlikely that treatment groups were composed of only one side. Therefore, we did not expect any effects of lateralization on gene expression in this experiment. The CER was sectioned last and alternating slices were allocated to each of the two treatment groups.
Once microdissection was complete, Netwells containing the tissue from each designated region and treatment group were incubated for 1h at 40°C in aCSF bubbled with carbogen with gentle shaking (“recovery”). Next, Netwells were transferred to 12-well plates and incubated for 1h at 40°C in either aCSF or aCSF + 1μM CBX to allow the CBX to inhibit enzyme activity before CORT application (“pre-treatment”). Finally, Netwells were transferred to plates containing either 30nM CORT or 30nM CORT + 1μM CBX and incubated for 6h at 40°C, followed by rapid freezing and storage at −80°C until further processing. We chose 6h as the incubation time because treatment with 30nM CORT induces up-regulation of SGK1 and FKBP5 within this time frame (dependent on region) but does not affect GR or MR expression (Rensel and Schlinger, 2020).
2.3.3. RNA Extraction, cDNA Preparation, and Quantitative RT-PCR
RNA was extracted using the RNeasy mini system (Qiagen) coupled with the Qiagen on-column DNAse treatment. Sample concentration and purity were determined with an ND-1000 nanodrop, and samples with concentrations below 12.5ng/ul were concentrated with the RNeasy MinElute cleanup kit (Qiagen). Sample A260/280 values were inspected and accepted if they were ≥ 1.80. Following RNA extraction, 100ng RNA was reverse transcribed into cDNA with the Superscript III cDNA synthesis system (Invitrogen), and cDNA samples were further checked for sample integrity with PCR to amplify a highly expressed gene (GR, MR, or GAPdH). Blanks and no-RT controls were included in each round of cDNA synthesis. Inspection of sample bands on a gel confirmed the presence of a single amplification product at the correct molecular weight.
The relative expression of 5 GC target genes, FKBP5, SGK1, GILZ, MR, and GR, was determined in samples via quantitative RT-PCR using the Sybr Green method, following our previously published methods (Rensel and Schlinger, 2020). FKBP5, SGK1, and GILZ are known GC targets in mammals (Ayroldi and Riccardi, 2009; Juszczak and Stankiewicz, 2018), and both GR and MR expression can be regulated by CORT (Paskitti et al., 2000). Briefly, primer specificity and optimal reaction conditions were established prior to sample assays (Rensel et al., 2018; Rensel and Schlinger, 2020; Table 1). Samples were run in duplicate at a 1:10 dilution, and all paired samples from a given brain region were run on the same plate for each gene. Sample values were normalized to GAPdH expression, as GAPdH is relatively stable in the songbird brain (Zinzow-Kramer et al., 2014). Duplicate sample values differing by more than 0.5CT were either re-run (in conjunction with the paired sample) or excluded from analysis (Hellemans and Vandesompele, 2011). A single calibrator sample was run on every plate.
Table 1.
Quantitative RT-PCR Primers
| Gene | Primer Sequence | [Primer] (μM)† | Mean Efficiency (%) |
|---|---|---|---|
| SGK1 | F: AGGCGTCTGGTCCTACCTTA R: TGAACTTCAGGGTGCTTGCAT |
0.6 | 97.4 |
| GILZ | F: CTGCAACAGGAACATCGACC R: TTTTTCACAAGATCCATCGCCT |
0.3 | 99.8 |
| FKBP5 | F: GGCAAGGGCCAGGTAATCAA R: CCTCAAACAAGTCCTCGCCT |
0.3 | 103.8 |
| GR | F: TGCAGTACTCCTGGATGTTCC R: GAGCATGTGTTTGCATTGTTC |
0.6 | 99.8 |
| MR | F: AAGAGTCGGCCAAACATCCTTGTTCT R: AAGAAACGGGTGGTCCTAAAATCCCAG |
0.3 | 96.3 |
| GAPdH | F: TGACCTGCCGTCTGGAAAA R: CCATCAGCAGCAGCCT |
0.3 | 97.8 |
per reaction
Quality control measures were employed to confirm the reliability and accuracy of each assay, including: 1) Verification that standard curves included in each plate (generated from pooled cTEL tissue) had efficiencies of 90 -110% and linearity ≥ 0.98); 2) Inclusion of water blanks and no-RT controls to confirm the lack of DNA contamination in samples and reaction mixtures; 3) Visual inspection of dissociation curves to confirm the presence of a single peak indicative of one amplification product.
Expression of each of the target genes was calculated using the 2^-ΔΔCT method for every gene except for FKBP5. Because efficiencies for this gene regularly exceeded the ~5% difference cutoff with respect to GAPdH, we used the Pfaffl method, which accounts for variable efficiencies of target and reference genes, to quantify FKBP5 expression (Pfaffl, 2001). Patterns of statistical significance for FKBP5 were unaffected by whether we used the Pfaffl or 2^-ΔΔCT methods, so results from the Pfaffl method are presented below.
Finally, to confirm the presence of 11ß-HSD2 expression in our slice culture preparations, we measured 11ß-HSD2 expression in a subset of slices prepared from each of the brain regions and treatment groups used in this study, employing previously published primers: F: AAAACAGGGACAACATGCGA, R: CCCCTCTGTGATGCTGTTCA (Rensel et al., 2018). Raw CT values in 5-9 samples taken from each region and across both treatment groups ranged from CT 27-31 (mean = 29.5; SD = 1.23), with expression values averaging ~0.2-0.6 (mean = 0.37; SD = 0.24). These values are remarkably similar to our previously published data on 11ß-HSD2 expression in fresh frozen zebra finch brain tissue (Rensel et al., 2018).
2.4. Statistics
We used a generalized linear mixed model to assess the impact of CBX injection on circulating CORT before and during a standard restraint stressor protocol. Treatment (CBX or vehicle), sample number, and the interaction between the two were included as fixed effects. Sex was not included as a fixed effect because there were only two individuals per treatment group per sex. Degrees of freedom were assessed using a Kenworth-Roger approximation because of relatively small sample sizes, and assay ID was included as a random effect. Because we took 5 samples from each bird, we accounted for non-independence of samples by comparing multiple models that used bird identity in either the “repeated” or “random” functions. We selected the best fit model by comparing the Akaike and Bayesian information criteria and the graph of predicted vs. observed data points generated from each model. The best fit model incorporated bird ID as a random variable alongside assay ID. Post-hoc comparisons of pairwise differences between sampling time points were made using a sequential Sidak test. Finally, we visually inspected the histogram and Q-Q plot for model residuals to verify normal distribution of the data.
On day 1 of sampling, one bird in the CBX injection group escaped prior to the first baseline CORT sample and again at the 60min post-injection time point. While the bird was recaught and sampled within an appropriate time frame for baseline CORT estimation (under 3min), the impact of this additional “stressor” could affect circulating CORT dynamics, and therefore these day 1 samples were removed before analysis. Day 2 CORT samples from this individual were retained in the final analysis; running the model with and without these samples produced the same patterns of significance.
To assess the effects of CBX in conjunction with CORT incubation in slice cultures, we conducted paired t-tests for each gene in each brain region. Sex was not considered in the analyses because of small sample sizes (3 individuals per sex maximum). We used 1-tailed tests for analyses of FKBP5, GILZ, and SGK1 expression because we predicted a priori that CBX would reduce 11ß-HSD2 activity, thereby enhancing the effects of CORT exposure on CORT-regulated gene expression. We used 2-tailed tests to examine MR and GR expression, as CORT exposure may both up- and down-regulate expression of these receptors (e.g., Ahmed et al., 2006; Paskitti et al., 2000; Patchev et al., 1994; Sapolsky et al., 1984). Because of small sample sizes, we also analyzed the data with non-parametric Wilcoxon’s Signed Rank Tests, which yielded the same significance patterns (data not shown). Finally, we confirmed that GAPdH expression was unaffected by CBX treatment by conducting 2-tailed, paired t-tests for GAPdH CT values in each assay. GAPdH CT values were unaffected by CBX in all tests (all Ps > 0.29).
Analyses were run in SPSS v26 and graphs were created in GraphPad Prism. Error bars in figures represent means ± 1 standard error.
3. Results:
3.1. Experiment 1: Effect of Carbenoxolone on Circulating CORT
Overall, plasma CORT varied significantly across the 5 sampling timepoints (F4,20 = 9.2; P < 0.001) but not between CBX and vehicle-injected birds (F1,4 = 3.4; P = 0.133). However, there was a significant interaction between treatment group and sampling timepoint (F4,20 = 7.7; P = 0.001), such that CORT levels were significantly higher in CBX than saline-injected birds 60min after injection (F1,8 = 17.9; P = 0.003) but not at any other time point (all Ps > 0.12; Fig. 1).
Figure 1. Plasma CORT levels before and after a single CBX or vehicle injection and in response to a handling and restraint stressor.
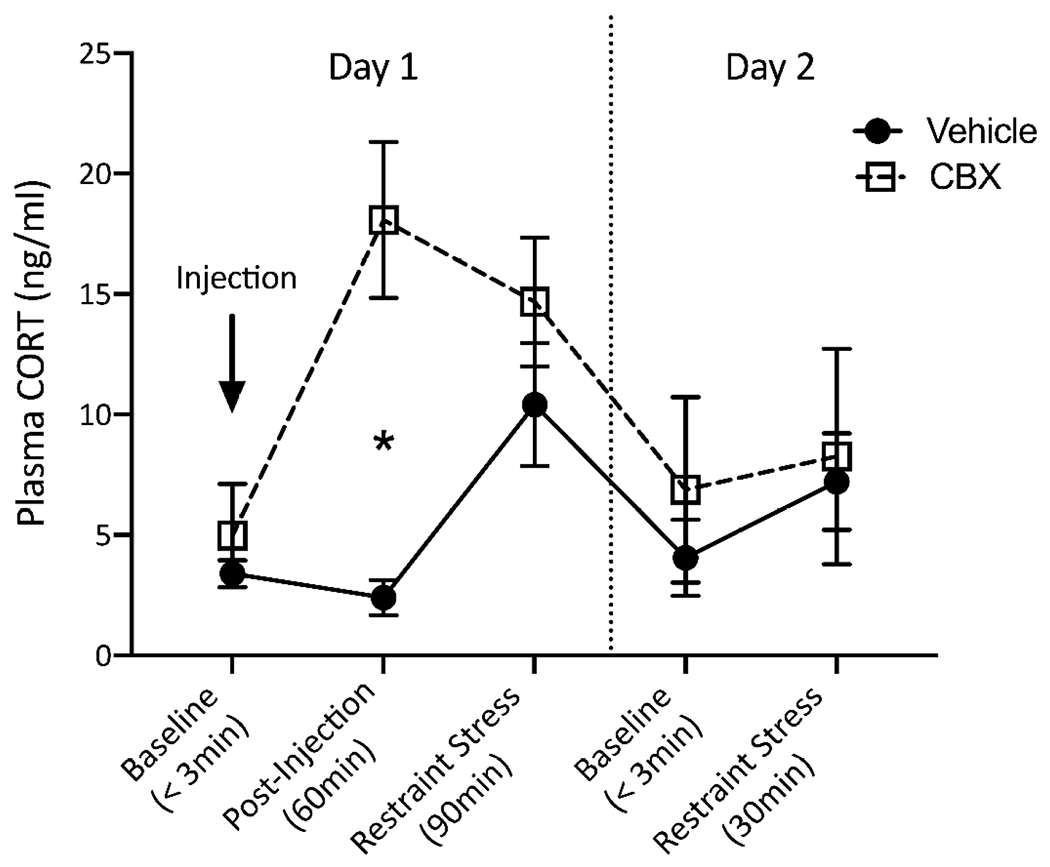
The stressor was repeated on day 2 to examine lasting effects of CBX on circulating CORT. * indicates a significant (P < 0.05) difference between CBX and vehicle-injected animals at a given time point. For pairwise comparisons between sampling timepoints within each treatment group, see Table 2. (CBX = carbenoxolone)
CORT also differed significantly across sampling timepoints in both CBX (F4,20 = 12.0; P < 0.0001) and vehicle-injected birds (F4,20 = 3.8; P = 0.018), but with different patterns of significance in each group. As expected, vehicle injection had no impact on CORT levels 60min post-injection (P = 0.89), and CORT levels increased significantly in response to restraint stress after this timepoint (P = 0.029; Fig. 1; Table 2). Interestingly, stress-induced CORT levels were not significantly different from initial, pre-injection CORT levels in this group, although this trended towards significance (P = 0.067). Of the 4 vehicle-injected birds, all but one exhibited a marked increase in plasma CORT with handling and restraint stress (Fig. 2). In CBX-injected birds, CORT levels were significantly elevated 60min after injection and again 30min later (after restraint stress) relative to the pre-injection baseline sample (P = 0.001 and 0.01, respectively; Fig. 1; Table 2), while there was no evidence of a CORT increase in response to the restraint stressor (P = 0.64). Examination of each individual’s CORT response in this group shows a robust increase in CORT for all after CBX injection, then a very slight increase in 2 of 3 of birds and a large decrease in plasma CORT for the 3rd after handling and restraint (Fig. 2).
Table 2.
Post-hoc comparisons of plasma CORT in vehicle and CBX-injected animals obtained from generalized linear mixed model (significant comparisons in bold).
| t | P^ | |
|---|---|---|
| Vehicle | ||
| Day 1 Baseline*Day 1 Post-Injection | 0.42 | 0.898 |
| Day 1 Baseline*Day 1 Restraint Stress | −2.96 | 0.067 |
| Day 1 Baseline*Day 2 Baseline | −0.28 | 0.898 |
| Day 1 Baseline*Day 2 Restraint Stress | −1.61 | 0.543 |
| Day 1 Post-Injection*Day 1 Restraint Stress | −3.38 | 0.029 |
| Day 1 Post-Injection*Day 2 Baseline | −0.70 | 0.870 |
| Day 1 Post-Injection*Day 2 Restraint Stress | −2.03 | 0.331 |
| Day 1 Restraint Stress*Day 2 Baseline | 2.68 | 0.109 |
| Day 1 Restraint Stress*Day 2 Restraint Stress | 1.35 | 0.657 |
| Day 2 Baseline*Day 2 Restraint Stress | −1.34 | 0.657 |
| CBX | ||
| Day 1 Baseline*Day 1 Post-Injection | −4.79 | 0.001 |
| Day 1 Baseline*Day 1 Restraint Stress | −3.54 | 0.01 |
| Day 1 Baseline*Day 2 Baseline | 0.56 | 0.918 |
| Day 1 Baseline*Day 2 Restraint Stress | 0.03 | 0.974 |
| Day 1 Post-Injection*Day 1 Restraint Stress | 1.25 | 0.644 |
| Day 1 Post-Injection*Day 2 Baseline | 5.53 | <0.001 |
| Day 1 Post-Injection*Day 2 Restraint Stress | 5.01 | 0.001 |
| Day 1 Restraint Stress*Day 2 Baseline | 4.24 | 0.003 |
| Day 1 Restraint Stress*Day 2 Restraint Stress | 3.71 | 0.008 |
| Day 2 Baseline*Day 2 Restraint Stress | −0.58 | 0.918 |
Sequential Sidak adjustment for multiple comparisons
Figure 2. Plasma CORT levels of individual birds injected with vehicle (left panel circles) or CBX (right panel squares) on day 1 (open symbols) or day 2 (closed symbols).
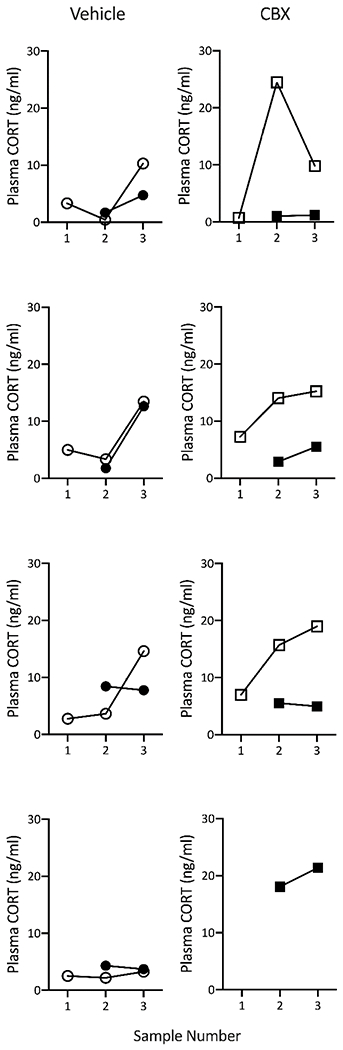
Day 1 datapoints for one CBX-injected bird were not included because of multiple escapes during day 1 sampling. (Sample Number: 1 = pre-injection baseline sample on day 1; 2 = baseline sample collected 60min after injection day 1 and again on day 2 without prior injection; 3 = restraint stress sample collected 30min after baseline sample on days 1 and 2.)
On day 2, CORT in CBX-treated birds was no longer elevated with respect to levels on day 1 after CBX injection (all Ps < 0.008), and baseline CORT levels for both groups were no different from baseline CORT levels on day 1 (both Ps > 0.89; Fig. 1; Table 2). Interestingly, CORT did not increase in response to handling and restraint in either treatment group (both Ps > 0.6). Indeed, a robust CORT response was only observed in 1 bird from the vehicle injection group, while all other birds either exhibited very small increases, no increase, or a slight decrease in plasma CORT with handling and restraint. A 4th CBX-injected bird (who was eliminated from day 1 analysis because of multiple escapes) had elevated CORT levels on day 2 (Fig. 2).
3.2. Experiment 2: Effect of Carbenoxolone on CORT-Dependent Gene Expression in Brain
All genes were expressed in all slices from all treatment groups. There was a trend towards elevated GILZ expression in HYP slices exposed to CORT + CBX (P = 0.07), with a large effect size (d = 0.877), and a trend towards elevated GR expression in CER slices treated with CORT + CBX (P = 0.084; d = 0.712). Otherwise, CBX treatment in conjunction with CORT had no statistically significant impact on expression of FKBP5, SGK1, GILZ, MR, or GR in any region, although some comparisons yielded moderate effect sizes, potentially indicative of the presence of a significant effect with a larger sample size (all Ps > 0.1; Fig. 3; Table 3). Finally, as mentioned above, we used qPCR to confirm the presence of 11β-HSD2 transcripts in a subset of samples from the slice culture preparation, using established primers (Rensel et al., 2018).
Figure 3. Relative expression of CORT-regulated genes in micro-dissected slices of zebra finch brain incubated with 30nM CORT or 30nM CORT + 1μM CBX.
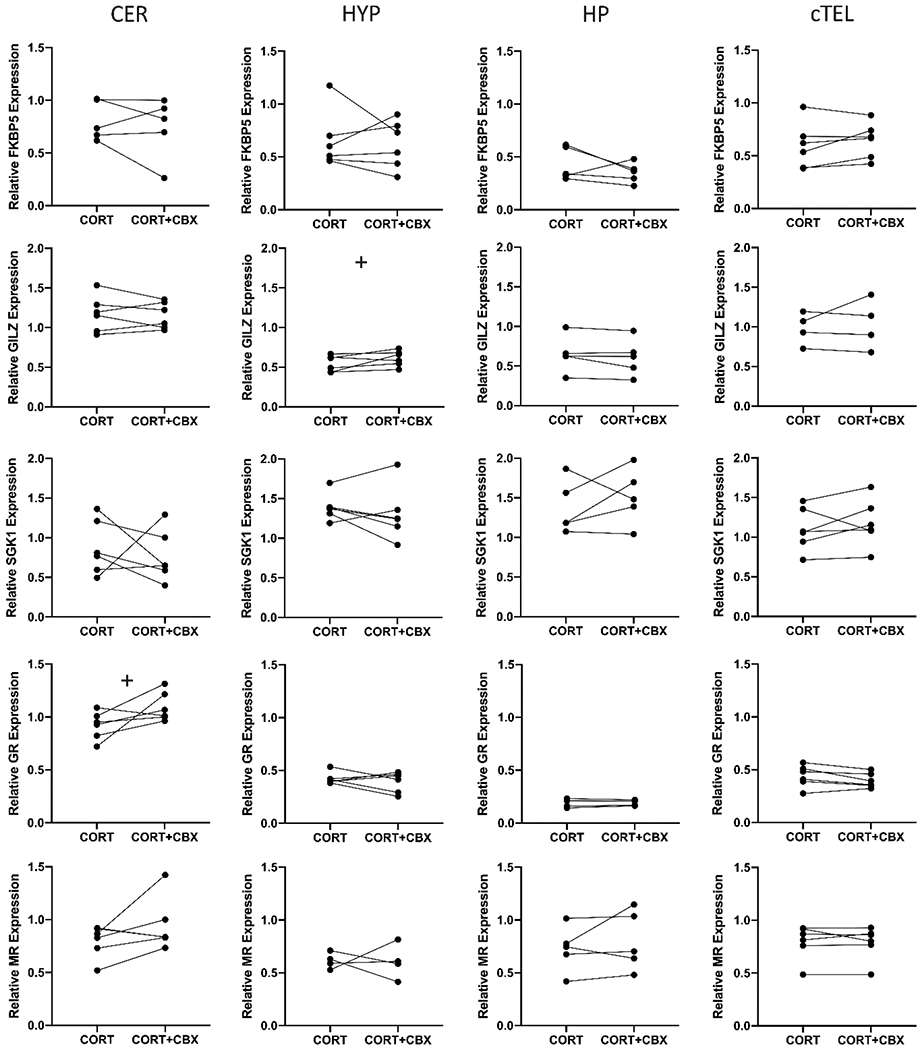
Symbols and lines indicate individual animals and paired samples. + indicates P < 0.1. (CER = cerebellum; HYP = hypothalamus; HP = hippocampus; cTEL = caudal telencephalon.)
Table 3.
Results of paired t-tests examining effect of CBX treatment on GC-dependent gene expression in slice culture of hypothalamus (HYP), hippocampus (HP), caudal telencephalon (cTEL), and cerebellum (CER).
| t(df) | 95% CI [LL, UL] | Cohen’s d | P | |
|---|---|---|---|---|
| HYP | ||||
| SGK1 | 0.86(5) | [−0.168, 0.337] | 0.350 | 0.215 |
| FKBP5 | 0.35(5) | [−0.228, 0.299] | 0.142 | 0.371 |
| GILZ | −1.74(5) | [−0.166, 0.032] | 0.712 | 0.071 |
| MR | 0.07 (5) | [−0.341, 0.356] | 0.036 | 0.948 |
| GR | 0.72(5) | [−0.078, 0.139] | 0.296 | 0.502 |
| HP | ||||
| SGK1 | −0.89(4) | [−0.593, 0.306] | 0.397 | 0.213 |
| FKBP5 | 1.15(4) | [−0.118, 0.284] | 0.513 | 0.158 |
| GILZ | 1.46(4) | [−0.037, 0.118] | 0.652 | 0.109 |
| MR | −0.94(4) | [−0.296, 0.146] | 0.420 | 0.401 |
| GR | −0.54(3) | [−0.026, 0.018] | 0.271 | 0.625 |
| cTEL | ||||
| SGK1 | −0.95(5) | [−0.294, 0.136] | 0.386 | 0.194 |
| FKBP5 | −1.31(5) | [−0.153, 0.050] | 0.536 | 0.124 |
| GILZ | −0.53(3) | [−0.354, 0.252] | 0.267 | 0.315 |
| MR | 0.35(5) | [−0.053, 0.070] | 0.144 | 0.738 |
| GR | 1.84(5) | [−0.016, 0.098] | 0.751 | 0.125 |
| CER | ||||
| SGK1 | 0.54(5) | [−0.425, 0.648] | 0.218 | 0.308 |
| FKBP5 | 0.72(4) | [−0.192, 0.327] | 0.322 | 0.256 |
| GILZ | 0.37(5) | [−0.117, 0.157] | 0.152 | 0.363 |
| MR | −1.51(5) | [−0.394, 0.102] | 0.617 | 0.191 |
| GR | −2.15(5) | [−0.385, 0.034] | 0.877 | 0.084 |
4. Discussion:
Results presented here support the view that a primary function of 11ß-HSD2 in songbirds is to regulate circulating CORT levels. Moreover, whereas 11ß-HSD2 is expressed in the songbird brain and both central CORT levels and CORT-dependent gene expression are subject to regional regulation, our data do not support the view that neural 11ß-HSD2 is the chief mechanism responsible for this regulation.
Blockage of peripheral 11ß-HSD activity with CBX injection had a powerful effect on circulating CORT levels, elevating those levels more than 4-fold 60min after injection. We believe this effect of CBX reflected a change in baseline CORT levels, primarily because all of these birds were unrestrained and undisturbed for those 60min, a condition that allowed circulating CORT levels to return to baseline in vehicle-injected birds. These results suggest that peripheral 11ß-HSD2 is responsible for helping to modulate baseline levels of circulating CORT.
It is difficult to make any solid conclusions about the potential effect of CBX on stress-induced CORT levels in this study. In that CORT levels at 90min were indistinguishable between control and CBX-injected birds (i.e., 30 mins after application of a stressor and 60min after CBX injection) it is likely that inhibition of 11ß-HSD had no effect on stress-induced levels of CORT. However, it is possible that CBX was cleared from the circulation by 90 mins post-injection, as has been demonstrated for rodents (Leshchenko et al., 2006). If so, then CORT levels in CBX-injected birds may have declined to baseline after 60min (as CBX exposure also declined), only to be restored by stress at the 90min timepoint. It is also possible that the biosynthetic capacity of the adrenals to secrete CORT was exhausted by the elevated baseline levels due to CBX-treatment, preventing further synthesis and secretion upon exposure to stress. In another study of a songbird, treatment with the 11ß-HSD2 antagonist sodium diethyldithiocarbamate trihydrate (DETC) coincident with a stressor did elevate stress-induced levels of CORT (Pérez et al., 2020). Thus, 11ß-HSD may function to regulate both baseline and stress-induced CORT levels, detectable only under specific experimental conditions.
Our finding of elevated plasma CORT after CBX injection suggests that these enzymes, particularly 11ß-HSD2, play a key role in modulating circulating GCs. The manner in which this occurs is difficult to determine, as 11ß-HSD1 and 2 are expressed across numerous peripheral tissues and may work synergistically or antagonistically to regulate peripheral levels. Most likely it is activity of 11ß-HSD2 in the adrenals that is most important for regulation of circulating CORT. In mammals, 11ß-HSD2 expression and activity is abundant in the adrenals, particularly in the GC-producing zona fasciculata (Coulter et al., 1999; Mazzocchi et al., 1998; Roland and Funder, 1996; Shimojo et al., 1996; Stewart et al., 1994; Yang and Matthews, 1995) and 11ß-HSD2 activity predominates over expression and activity of 11ß-HSD1 (Mazzocchi et al., 1998). Moreover, in white-crowned sparrows (Zonotrichia leucophrys), adrenal 11ß-HSD2 expression exceeds kidney levels, with much lower levels of expression in gonads and liver and a virtual absence in other peripheral tissues (Pérez et al., 2020). As noted by some of these authors, adrenal 11ß-HSD2 expression may be an autocrine mechanism preventing excess local GC exposure and regulating GC effects on aldosterone and sex steroid production in the zona glomerulosa and zona reticularis. In addition, local 11ß-HSD2 expression provides a mechanism to regulate circulating GCs at their source (Pérez et al., 2020; Yang and Matthews, 1995).
While the effects of CBX on plasma CORT, indicative of peripheral 11ß-HSD2 inhibition, were short-lived, our results suggest that experiencing as little as a single acute stressor may alter the sensitivity of the HPA axis to future stressors. When we applied a 30min handling and restraint stressor 24 hours after the initial injection and stress protocol, we saw no significant stress response, regardless of whether individuals received CBX or saline injections the previous day. These results resemble previous observations. In rodents, repeated stressors produced habituation (Chen and Herbert, 1995; Cockrem and Silverin, 2002; Gadek-Michalska and Bugajski, 2003; Love et al., 2003) while a single capture and restraint experience during brooding diminished CORT responses in female Eastern bluebirds (Sialia sialis) during their next brood (Lynn et al., 2010). Lastly, a single handling and restraint procedure increased baseline CORT levels 24h later in Japanese quail (Coturnix japonica; Malisch et al., 2010). Together, these results suggest that, at least within our population of zebra finches, stressor habituation may occur rapidly, leading to the conclusion that repeated versus single stressors must be accounted for in the design and interpretation of both field and lab-based behavioral research.
While 11ß-HSDs appear important peripherally, with respect to central gene expression, our results indicate a limited or non-existent role for 11ß-HSD2. We predicted that application of CBX would amplify CORT upregulation of at least two genes, FKBP5 and SGK1, short feedback regulators of GC action (Juszczak and Stankiewicz, 2018), but we observed no additional amplification by CORT in the presence of CBX, and we saw no impact of CBX and CORT on other genes that were examined across any of the brain regions studied. There was a trend towards increased hypothalamic GILZ and cerebellar GR expression in the presence of CBX, but these effects were not significant, suggesting, at best, a limited effect. Studies with larger sample numbers, varying incubation conditions, or alternative pharmacological treatments might better reveal a role for neural 11ß-HSD2 in songbirds. In addition, while we verified 11ß-HSD2 expression in our slice culture preparation, we did not measure enzyme activity; determination of 11ß-HSD2 protein and activity in these cultures will be a key future step. Whereas we found no evidence of a role for 11ß-HSD2 in central gene expression, Pérez et al. (2020) observed that systemic stress-induced CORT levels were elevated after central 11ß-HSD2 inhibition, an unexpected response since 11ß-HSD2 inhibition should theoretically increase CORT in the HYP and thereby down-regulate the stress response (Pérez et al., 2020). Thus, a role for neural 11ß-HSD2 in songbirds cannot be eliminated, but it may function in unexpected ways. One possibility is that while 11ß-HSD2 may not regulate CORT-dependent gene expression (an effect that would be predicted if 11ß-HSD2 was co-localized with GR and/or MR-expressing cells) this enzyme might regulate rapid membrane actions of CORT in brain. Membrane receptors that respond to CORT binding rapidly influence neuronal excitability and function (Orchinik et al., 1997; Rose et al., 1993), including in the songbird brain (Breuner and Orchinik, 2009; Schmidt et al., 2010). In addition, in mammals, both 11ß-HSD1 and 11ß-HSD2 expression have been documented in glia (Chalbot and Morfin, 2012; Gottfried-Blackmore et al., 2010; Groyer et al., 2006). If this cellular location is similar in songbirds, glial 11ß-HSD2 might be positioned to locally regulate CORT levels that act on nearby neuronal membranes. Neuroanatomical studies to assess the cellular location of the 11ß-HSDs in the songbird brain would prove useful, as would neurophysiological studies to assess rapid actions of CORT when 11ß-HSD activities are modified.
4.1. Conclusions
In conclusion, our results and those of Pérez et al. (2020) support the hypothesis that peripheral 11ß-HSD2 activity, most likely in the adrenals, regulates circulating CORT in songbirds. While adrenal 11ß-HSD2 expression has been well-documented in mammals, only Mazzocchi et al. (1998) documented the potential impact of this expression on circulating CORT. Future work across taxa should evaluate this rarely considered mechanism for directly regulating CORT at the source. In addition, while we previously documented 11ß-HSD2 expression and activity in the zebra finch brain (Katz et al., 2010; Rensel et al., 2018, 2014), it appears that neural 11ß-HSD2 plays little role in regulating GC-dependent gene expression, suggesting other possible mechanisms of action.
Highlights.
11ß-HSD expression is widespread in the songbird, but its function is unclear
Peripheral 11ß-HSD inhibition induced a 4-fold increase in baseline corticosterone
Central 11ß-HSD2 inhibition did not alter corticosterone-dependent gene expression
5. Acknowledgements:
We are grateful for the excellent animal care provided by the Department of Laboratory Animal Management at UCLA. Many thanks to Xuqi Chen and Devaleena Pradhan for assistance with qRT-PCR and slice culture logistics, respectively, as well as to Mohak Kumar for assistance with laboratory work. We also thank the two anonymous reviewers for their constructive feedback. This work was supported by NIMH grant 5R03MH108921 awarded to MAR and BAS.
7. Funding:
This work was supported by the National Institutes of Mental Health (5R03MH108921 awarded to M.A.R and B.A.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. Competing Interests:
The authors have no conflicts of interest to declare.
8. References:
- Ahmed T, Frey JU, Korz V, 2006. Long-Term Effects of Brief Acute Stress on Cellular Signaling and Hippocampal LTP. J. Neurosci 26, 3951–3958. 10.1523/JNEUROSCI.4901-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajilore OA, Sapolsky RM, 1999. In vivo Characterization of 11β-Hydroxysteroid Dehydrogenase in Rat Hippocampus Using Glucocorticoid Neuroendangerment as an Endpoint. Neuroendocrinology 69, 138–144. 10.1159/000054412 [DOI] [PubMed] [Google Scholar]
- Apfelbeck B, Raess M, 2008. Behavioural and hormonal effects of social isolation and neophobia in a gregarious bird species, the European starling (Sturnus vulgaris). Horm. Behav 54, 435–441. 10.1016/j.yhbeh.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Ayroldi E, Riccardi C, 2009. Glucocorticoid-induced leucine zipper (GILZ): a new important mediator of glucocorticoid action. FASEB J. 23, 3649–3658. 10.1096/fj.09-134684 [DOI] [PubMed] [Google Scholar]
- Banerjee SB, Adkins-Regan E, 2011. Effect of isolation and conspecific presence in a novel environment on corticosterone concentrations in a social avian species, the zebra finch (Taeniopygia guttata). Horm. Behav 60, 233–238. 10.1016/j.yhbeh.2011.05.011 [DOI] [PubMed] [Google Scholar]
- Benediktsson R, Calder AA, Edwards CRW, Seckl JR, 1997. Placental 11β-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin. Endocrinol. (Oxf.) 46, 161–166. 10.1046/j.1365-2265.1997.1230939.x [DOI] [PubMed] [Google Scholar]
- Breuner CW, Orchinik M, 2009. Pharmacological characterization of intracellular, membrane, and plasma binding sites for corticosterone in house sparrows. Gen. Comp. Endocrinol 163, 214–224. 10.1016/j.ygcen.2009.01.027 [DOI] [PubMed] [Google Scholar]
- Chalbot S, Morfin R, 2012. Cytochrome P450-7B1 and 11β-hydroxysteroid dehydrogenase type 1 distribution in human tissues. Horm. Mol. Biol. Clin. Investig 9, 179–189. 10.1515/hmbci-2011-0138 [DOI] [PubMed] [Google Scholar]
- Chen X, Herbert J, 1995. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience 64, 675–685. 10.1016/0306-4522(94)00532-a [DOI] [PubMed] [Google Scholar]
- Clayton NS, Emery NJ, 2015. Avian Models for Human Cognitive Neuroscience: A Proposal. Neuron 86, 1330–1342. 10.1016/j.neuron.2015.04.024 [DOI] [PubMed] [Google Scholar]
- Cockrem JF, Silverin B, 2002. Variation within and between Birds in Corticosterone Responses of Great Tits (Parus major). Gen. Comp. Endocrinol 125, 197–206. 10.1006/gcen.2001.7750 [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR, Holmes MC, Wyrwoll CS, 2014. Foetal and placental 11β-HSD2: a hub for developmental programming. Acta Physiol. Oxf. Engl 210, 288–295. 10.1111/apha.12187 [DOI] [PubMed] [Google Scholar]
- Coulter CL, Smith RE, Stowasser M, Sasano H, Krozowski ZS, Gordon RD, 1999. Expression of 11β-hydroxysteroid dehydrogenase type 2 (11βHSD-2) in the developing human adrenal gland and human adrenal cortical carcinoma and adenoma. Mol. Cell. Endocrinol 154, 71–77. 10.1016/S0303-7207(99)00077-5 [DOI] [PubMed] [Google Scholar]
- de Kloet E. Ron, Joëls M, Holsboer F, 2005. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci 6, 463–475. 10.1038/nrn1683 [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Schmidt M, Meijer OC, 2005. Chapter 3.1 - Corticosteroid receptors and HPA-axis regulation, in: Steckler T, Kalin NH, Reul JMHM (Eds.), Techniques in the Behavioral and Neural Sciences, Handbook of Stress and the Brain. Elsevier, pp. 265–294. 10.1016/S0921-0709(05)80016-1 [DOI] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M, 1998. Brain Corticosteroid Receptor Balance in Health and Disease. Endocr. Rev 19, 269–301. 10.1210/edrv.19.3.0331 [DOI] [PubMed] [Google Scholar]
- Deviche P, Breuner C, Orchinik M, 2001. Testosterone, Corticosterone, and Photoperiod Interact to Regulate Plasma Levels of Binding Globulin and Free Steroid Hormone in Dark-Eyed Juncos, Junco hyemalis. Gen. Comp. Endocrinol 122, 67–77. 10.1006/gcen.2001.7613 [DOI] [PubMed] [Google Scholar]
- Edwards CRW, Burt D, Mcintyre MA, De Kloet ER, Stewart PM, Brett L, Sutanto WS, Monder C, 1988. Localisation of 11β-hydroxy steroid dehydrogenase - tissue specific protector of the mineralocorticoid receptor. The Lancet, Originally published as Volume 2, Issue 8618 332, 986–989. 10.1016/S0140-6736(88)90742-8 [DOI] [PubMed] [Google Scholar]
- Gadek-Michalska A, Bugajski J, 2003. Repeated handling, restraint, or chronic crowding impair the hypothalamic-pituitary-adrenocortical response to acute restraint stress. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc 54, 449–459. [PubMed] [Google Scholar]
- Gottfried-Blackmore A, Sierra A, Mcewen BS, Ge R, Bulloch K, 2010. Microglia express functional 11β-hydroxy steroid dehydrogenase type 1. Glia 58, 1257–1266. 10.1002/glia.21007 [DOI] [PubMed] [Google Scholar]
- Groyer G, Eychenne B, Girard C, Rajkowski K, Schumacher M, Cadepond F, 2006. Expression and Functional State of the Corticosteroid Receptors and 11β-Hydroxysteroid Dehydrogenase Type 2 in Schwann Cells. Endocrinology 147, 4339–4350. 10.1210/en.2005-1625 [DOI] [PubMed] [Google Scholar]
- Heine VM, Rowitch DH, 2009. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11βHSD2-dependent mechanism. J. Clin. Invest JCI36376. 10.1172/JCI36376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans J, Vandesompele J, 2011. qPCR data analysis – unlocking the secret to successful results. PCR Troubl. Optim. Essent. Guide 13. [Google Scholar]
- Holmes MC, Carter RN, Noble J, Chitnis S, Dutia A, Paterson JM, Mullins JJ, Seckl JR, Yau JLW, 2010. 11β-Hydroxysteroid Dehydrogenase Type 1 Expression Is Increased in the Aged Mouse Hippocampus and Parietal Cortex and Causes Memory Impairments. J. Neurosci 30, 6916–6920. 10.1523/JNEUROSCI.0731-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MC, Sangra M, French KL, Whittle IR, Paterson J, Mullins JJ, Seckl JR, 2006. 11β-Hydroxy steroid dehydrogenase type 2 protects the neonatal cerebellum from deleterious effects of glucocorticoids. Neuroscience 137, 865–873. 10.1016/j.neuroscience.2005.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MC, Seckl JR, 2006. The role of 11β-hydroxysteroid dehydrogenases in the brain. Mol. Cell. Endocrinol, The International Workshop on llbeta and 17beta-Hydroxysteroid Dehydrogenases 248, 9–14. 10.1016/j.mce.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Jamieson PM, Nyirenda MJ, Walker BR, Chapman KE, Seckl JR, 1999. Interactions between oestradiol and glucocorticoid regulatory effects on liver-specific glucocorticoid-inducible genes: possible evidence for a role of hepatic 11 -hydroxysteroid dehydrogenase type. J. Endocrinol 7. [DOI] [PubMed] [Google Scholar]
- Jamieson PM, Walker BR, Chapman KE, Andrew R, Rossiter S, Seckl, 2000. 11 beta-hydroxysteroid dehydrogenase type lisa predominant 11 beta-reductase in the intact perfused rat liver. J. Endocrinol 165, 685–692. 10.1677/joe.0.1650685 [DOI] [PubMed] [Google Scholar]
- Jellinck PH, Monder C, McEwen BS, Sakai RR, 1993. Differential inhibition of 11β-hydroxysteroid dehydrogenase by carbenoxolone in rat brain regions and peripheral tissues. J. Steroid Biochem. Mol. Biol 46, 209–213. 10.1016/0960-0760(93)90296-9 [DOI] [PubMed] [Google Scholar]
- Juszczak GR, Stankiewicz AM, 2018. Glucocorticoids, genes and brain function. Prog. Neuropsychopharmacol. Biol. Psychiatry 82, 136–168. 10.1016/j.pnpbp.2017.11.020 [DOI] [PubMed] [Google Scholar]
- Katz A, Oyama RK, Feng N, Chen X, Schlinger BA, 2010. 11beta-hydroxysteroid dehydrogenase type 2 in zebra finch brain and peripheral tissues. Gen. Comp. Endocrinol 166, 600–605. 10.1016/j.ygcen.2010.01.016 [DOI] [PubMed] [Google Scholar]
- Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CRW, Seckl JR, Mullins JJ, 1997. 11β-Hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc. Natl. Acad. Sci. U. S. A 94, 14924–14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattin CR, Romero LM, 2014. Chronic stress alters concentrations of corticosterone receptors in a tissue-specific manner in wild house sparrows (Passer domesticus). J. Exp. Biol 217, 2601–2608. 10.1242/jeb.103788 [DOI] [PubMed] [Google Scholar]
- Leshchenko Y, Likhodii S, Yue W, Burnham WM, Perez Velazquez JL, 2006. Carbenoxolone does not cross the blood brain barrier: an HPLC study. BMC Neurosci. 7, 3. 10.1186/1471-2202-7-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love OP, Shutt LJ, Silfies JS, Bird DM, 2003. Repeated restraint and sampling results in reduced corticosterone levels in developing and adult captive American kestrels (Falco sparverius). Physiol. Biochem. Zool. PBZ 76, 753–761. 10.1086/376431 [DOI] [PubMed] [Google Scholar]
- Lynn SE, Prince LE, Phillips MM, 2010. A single exposure to an acute stressor has lasting consequences for the hypothalamo–pituitary–adrenal response to stress in free-living birds. Gen. Comp. Endocrinol 165, 337–344. 10.1016/j.ygcen.2009.07.018 [DOI] [PubMed] [Google Scholar]
- Malisch JL, Satterlee DG, Cockrem JF, Wada EL, Breuner CW, 2010. How acute is the acute stress response? Baseline corticosterone and corticosteroid-binding globulin levels change 24h after an acute stressor in Japanese quail. Gen. Comp. Endocrinol 165, 345–350. 10.1016/j.ygcen.2009.08.003 [DOI] [PubMed] [Google Scholar]
- Mazzocchi G, Rossi GP, Neri G, Malendowicz LK, Albertin G, Nussdorfer GG, 1998. 11β-Hydroxysteroid dehydrogenase expression and activity in the human adrenal cortex. FASEB J. 12, 1533–1539. 10.1096/fasebj.12.14.1533 [DOI] [PubMed] [Google Scholar]
- McEwen BS, S. B, 1998. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci 840, 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC, 2003. The concept of allostasis in biology and biomedicine. Horm. Behav 43, 2–15. [DOI] [PubMed] [Google Scholar]
- Monder C, Stewart PM, Lakshmi V, Valentino R, Burt D, Edwards CRW, 1989. Licorice Inhibits Corticosteroid 1lβ-Dehydrogenase of Rat Kidney and Liver: In Vivo and in Vitro Studies. Endocrinology 125, 1046–1053. 10.1210/endo-125-2-1046 [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Champagne DL, van der Veen R, de Kloet ER, 2010. Brain development under stress: Hypotheses of glucocorticoid actions revisited. Neurosci. Biobehav. Rev., Special Section: Developmental determinants of sensitivity and resistance to stress: A tribute to Seymour “Gig” Levine 34, 853–866. 10.1016/j.neubiorev.2009.07.006 [DOI] [PubMed] [Google Scholar]
- Orchinik M, Hastings N, Witt D, McEwen BS, 1997. High-affinity binding of corticosterone to mammalian neuronal membranes: possible role of corticosteroid binding globulin. J. Steroid Biochem. Mol. Biol 60, 229–236. [DOI] [PubMed] [Google Scholar]
- Paskitti ME, McCreary BJ, Herman JP, 2000. Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: time-course analysis. Mol. Brain Res 80, 142–152. 10.1016/S0169-328X(00)00121-2 [DOI] [PubMed] [Google Scholar]
- Patchev VK, Brady LS, Karl M, Chrousos GP, 1994. Regulation of HSP90 and corticosteroid receptor mRNA by corticosterone levels in vivo. Mol. Cell. Endocrinol. 103, 57–64. 10.1016/0303-7207(94)90069-8 [DOI] [PubMed] [Google Scholar]
- Pérez JH, Swanson RE, Lau HJ, Cheah J, Bishop VR, Snell KRS, Reid AMA, Meddle SL, Wingfield JC, Krause JS, 2020. Tissue-specific expression of 11β-HSD and its effects on plasma corticosterone during the stress response. J. Exp. Biol 223. 10.1242/jeb.209346 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan DS, Van Ness R, Jalabert C, Hamden JE, Austin SH, Soma KK, Ramenofsky M, Schlinger BA, 2019. Phenotypic flexibility of glucocorticoid signaling in skeletal muscles of a songbird preparing to migrate. Hormones and Behavior 116, 104586. 10.1016/j.yhbeh.2019.104586 [DOI] [PubMed] [Google Scholar]
- Räikkönen K, Martikainen S, Pesonen A-K, Lahti J, Heinonen K, Pyhälä R, Lahti M, Tuovinen S, Wehkalampi K, Sammallahti S, Kuula L, Andersson S, Eriksson JG, Ortega-Alonso A, Reynolds RM, Strandberg TE, Seckl JR, Kajantie E, 2017. Maternal Licorice Consumption During Pregnancy and Pubertal, Cognitive, and Psychiatric Outcomes in Children. Am. J. Epidemiol 185, 317–328. 10.1093/aje/kww172 [DOI] [PubMed] [Google Scholar]
- Rajan V, Edwards RW, Seckl JR, 1996. 11P-Hydroxysteroid Dehydrogenase in Cultured Hippocampal Cells Reactivates Inert 11-Dehydrocorticosterone, Potentiating Neurotoxicity. J Neurosci 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Adkins-Regan E, Romero LM, 2003. Behavioral and adrenocortical responses to mate separation and reunion in the zebra finch. Horm. Behav 43, 108–114. 10.1016/S0018-506X(02)00012-0 [DOI] [PubMed] [Google Scholar]
- Rensel MA, Comito D, Kosarussavadi S, Schlinger BA, 2014. Region-Specific Neural Corticosterone Patterns Differ From Plasma in a Male Songbird. Endocrinology 155, 3572–3581. 10.1210/en.2014-1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensel MA, Ding JA, Pradhan DS, Schlinger BA, 2018. 11β-HSD Types 1 and 2 in the Songbird Brain. Front. Endocrinol 9, 86. 10.3389/fendo.2018.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensel MA, Schlinger BA, 2020. The stressed brain: regional and stress-related corticosterone and stress-regulated gene expression in the adult zebra finch (Taeniopygia guttata). J. Neuroendocrinol 32, e12852. 10.1111/jne.12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensel MA, Schlinger BA, 2016. Determinants and Significance of Corticosterone Regulation in the Songbird Brain. Gen. Comp. Endocrinol 227, 136–142. 10.1016/j.ygcen.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland BL, Funder JW, 1996. Localization of 11beta-hydroxysteroid dehydrogenase type 2 in rat tissues: in situ studies. Endocrinology 137, 1123–1128. 10.1210/en.137.3.1123 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEWEN BS, 1984. Stress Down-Regulates Corticosterone Receptors in a Site-Specific Manner in the Brain. Endocrinology 114, 287–292. 10.1210/endo-114-1-287 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU, 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev 21, 55–89. 10.1210/edrv.21.1.0389 [DOI] [PubMed] [Google Scholar]
- Sarabdjitsingh RA, Zhou M, Yau JLW, Webster SP, Walker BR, Seckl JR, Joëls M, Krugers HJ, 2014. Inhibiting 11β-hydroxysteroid dehydrogenase type 1 prevents stress effects on hippocampal synaptic plasticity and impairs contextual fear conditioning. Neuropharmacology 81, 231–236. 10.1016/j.neuropharm.2014.01.042 [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Malisch JL, Breuner CW, Soma KK, 2010. Corticosterone and cortisol binding sites in plasma, immune organs and brain of developing zebra finches: intracellular and membrane-associated receptors. Brain. Behav. Immun 24, 908–918. 10.1016/j.bbi.2010.02.008 [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Soma KK, 2008. Cortisol and corticosterone in the songbird immune and nervous systems: local vs. systemic levels during development. Am. J. Physiol. Regul. Integr. Comp. Physiol 295, R103–110. 10.1152/ajpregu.00002.2008 [DOI] [PubMed] [Google Scholar]
- Seckl JR, Cleasby M, Nyirenda MJ, 2000. Glucocorticoids, 11β-hydroxy steroid dehydrogenase, and fetal programming. Kidney Int. 57, 1412–1417. 10.1046/j.1523-1755.2000.00984.x [DOI] [PubMed] [Google Scholar]
- Shimojo M, Condon J, Whorwood CB, Stewart PM, 1996. Adrenal 11β-hydroxysteroid dehydrogenase. Endocr. Res 22, 771–780. 10.1080/07435809609043775 [DOI] [PubMed] [Google Scholar]
- Sterling P, 1988. Allostasis : A New Paradigm to Explain Arousal Pathology. Handb. Life Stress Cogn. Health [Google Scholar]
- Stewart PM, Murry BA, Mason JI, 1994. Type 2 11 beta-hydroxysteroid dehydrogenase in human fetal tissues. J. Clin. Endocrinol. Metab 78, 1529–1532. 10.1210/jcem.78.6.8200959 [DOI] [PubMed] [Google Scholar]
- Tam H, Schlinger BA, 2007. Activities of 3beta-HSD and aromatase in slices of developing and adult zebra finch brain. Gen. Comp. Endocrinol 150, 26–33. 10.1016/j.ygcen.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves MD, Gomez-Sanchez CE, Soma KK, 2011. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am. J. Physiol. Endocrinol. Metab 301, E11–24, 10.1152/ajpendo.00100.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves MD, Plumb AW, Sandkam BA, Ma C, Van Der Gugten JG, Holmes DT, Close DA, Abraham N, Soma KK, 2015. Steroid profiling reveals widespread local regulation of glucocorticoid levels during mouse development. Endocrinology 156, 511–522. 10.1210/en.2013-1606 [DOI] [PubMed] [Google Scholar]
- Taves MD, Schmidt KL, Ruhr IM, Kapusta K, Prior NH, Soma KK, 2010. Steroid concentrations in plasma, whole blood and brain: effects of saline perfusion to remove blood contamination from brain. PloS One 5, e15727. 10.1371/journal.pone.0015727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Brenowitz EA, 2010. Neuroprotective effects of testosterone in a naturally occurring model of neurodegeneration in the adult avian song control system. J. Comp. Neurol 518, 4760–4770. 10.1002/cne.22486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haarst AD, Welberg LAM, Sutanto W, Oitzl MS, Kloet E.R. de, 1996. 11β-Hydroxysteroid Dehydrogenase Activity in the Hippocampus: Implications for in vivo Corticosterone Receptor Binding and Cell Nuclear Retention. J. Neuroendocrinol 8, 595–600. 10.1111/j.1365-2826.1996.tb00693.x [DOI] [PubMed] [Google Scholar]
- Wada H, Salvante KG, Stables C, Wagner E, Williams TD, Breuner CW, 2008. Adrenocortical responses in zebra finches (Taeniopygia guttata): individual variation, repeatability, and relationship to phenotypic quality. Horm. Behav 53, 472–480. 10.1016/j.yhbeh.2007.11.018 [DOI] [PubMed] [Google Scholar]
- Welberg LAM, Seckl JR, Holmes MC, 2000. Inhibition of 11β-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur. J. Neurosci 12, 1047–1054. 10.1046/j.1460-9568.2000.00958.x [DOI] [PubMed] [Google Scholar]
- Wingfield JC, 2008. Comparative endocrinology, environment and global change. Gen. Comp. Endocrinol 157, 207–216. 10.1016/j.ygcen.2008.04.017 [DOI] [PubMed] [Google Scholar]
- Yanga K, Matthew SG, n.d. Cellular localization of 11/3-hydroxysteroid dehydrogenase 2 gene expression in the ovine adrenal gland 5. [DOI] [PubMed] [Google Scholar]
- Yau JLW, Noble J, Kenyon CJ, Hibberd C, Kotelevtsev Y, Mullins JJ, Seckl JR, 2001. Lack of tissue glucocorticoid reactivation in 11β-hydroxy steroid dehydrogenase type 1 knockout mice ameliorates age-related learning impairments. Proc. Natl. Acad. Sci. U. S. A 98, 4716–4721. 10.1073/pnas.071562698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JLW, Noble J, Kenyon CJ, Ludwig M, Seckl JR, 2015. Diurnal and stress-induced intra-hippocampal corticosterone rise attenuated in 11β-HSD1-deficient mice: a microdialysis study in young and aged mice. Eur. J. Neurosci 41, 787–792. 10.1111/ejn.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallocchi M, Matkovic L, Damasco MC, 2004. Adrenal 11-beta hydroxy steroid dehydrogenase activity in response to stress. Can. J. Physiol. Pharmacol 82, 422–425. 10.1139/y04-035 [DOI] [PubMed] [Google Scholar]
- Zinzow-Kramer WM, Horton BM, Maney DL, 2014. Evaluation of reference genes for quantitative real-time PCR in the brain, pituitary, and gonads of songbirds. Horm. Behav 66, 267–275. 10.1016/j.yhbeh.2014.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]


