Abstract
Based on the adverse consequences and inadequate evidence of effectiveness for long-term opioid therapy (LOT), the CDC developed recommendations to decrease the use of LOT and morphine equivalent dose (MED) for patients receiving LOT. However, the majority of these patients report that opioid medication is significantly beneficial for pain management and are hesitant to reduce/decrease its use. Compounding the problem is poor access to nonpharmacologic therapies for many patients due to insurance reimbursement structures and limited pain-service availability. EMPOWER is an intent-to-treat, two-arm, open-label, randomized controlled trial evaluating a web-based self-management chronic pain program (E-health) that has been found to reduce self-reported MED, while also decreasing pain, in two randomized controlled trials. Approximately 400 chronic pain patients receiving LOT at a daily average prescribed MED ≥ 20 mg at one of two U.S. healthcare systems, located in North Carolina and Ohio, will be randomized in a 1:1 ratio to treatment as usual (TAU) or TAU plus E-health (E-health+). TAU consists of LOT from a prescribing clinician. E-health+ participants are provided with a 4-month E-health subscription (active treatment phase). All participants will complete web-based self-report measures at baseline, the end of the active treatment phase, and 6-months post-active treatment. Opioid prescription information will be collected from the participants’ electronic health record (EHR) from baseline through 6 months post-active treatment. This paper describes design considerations for this unique trial which is conducted completely remotely, with no in-person visits, and utilizes the EHR for participant identification and primary outcome collection.
Keywords: chronic pain, web-based intervention, primary care, EHR, self-management, remote randomized trial
1.0. Introduction
More than 50 million U.S. adults suffer from chronic pain and 19.6 million experience high-impact chronic pain severe enough to interfere with daily living or work activities [1]. The treatment of chronic non-cancer pain in recent decades has, due to healthcare system constraints, often relied on long-term opioid therapy (LOT) [2, 3]. The present U.S. opioid crisis has been attributed, in part, to increased opioid prescribing during the first decade of the 21st century [4]. Thus, the Centers for Disease Control and Prevention (CDC) created the Guideline for Prescribing Opioids for Chronic Pain, which was designed to decrease the use of LOT and the morphine equivalent dose (MED) for patients receiving LOT [5]. Concerns have been raised, however, by professional pain treatment leaders that reducing access for the 18 million Americans receiving LOT will cause needless suffering [6]. A majority of people receiving LOT report that opioids are significantly beneficial and even critical to managing their pain [7–9] and patients have reported pessimism about non-opioid pain strategies and fear of opioid withdrawal [10]. Providers have expressed frustration with their ability to: 1) maintain patient satisfaction while changing opioid therapy [11] and 2) taper opioid medications due to patients’ inadequate access to alternative pain treatments [11].
The importance of testing adjunctive therapies to reduce MED was noted in the National Institute on Drug Abuse strategic plan for 2016–2020 [12]. This aligns with the U.S. National Pain Strategy’s recommendation for development of effective, individualized, pain self-management strategies, which would provide consistent education and skills training to prevent, cope with, and reduce pain [13]. These programs may be an effective means to improve quality of life and health functioning while reducing health care resource utilization [14, 15] and would likely be more affordable than other pain management alternatives. Web-based interventions are more accessible than face-to-face interventions and they have been found to have equivalent efficacy [16, 17]. While studies of web-based, self-management chronic-pain interventions have been undertaken, the majority have not evaluated their impact on reducing LOT reliance [18].
An exception is research on the Goalistics Chronic Pain Management Program (referred to here as E-Health). Two prior randomized controlled trials found that the E-Health program had efficacy for decreasing both self-reported pain and medication use [19, 20]. While promising, the results need to be replicated in a larger trial with an objective measure of MED. This paper describes the design of the EMPOWER trial (NCT03308188), which will evaluate the effects of E-Health, relative to treatment as usual (TAU), for patients receiving LOT for chronic pain. In addition to being the first larger-scale, multi-site trial of an accessible web-based intervention for this patient population, EMPOWER is unique in several respects, including: the study is completed entirely remotely, with no in-person study visits; potential participants are identified using electronic health records (EHR); and the primary outcome is collected via EHR. This paper describes the key design considerations associated with the trial.
2. Research design and study organization
2.1. Research questions
The primary objective of EMPOWER is to evaluate the impact of treatment as usual (TAU) relative to TAU plus E-health (E-Health+). It is hypothesized that the E-Health+, relative to TAU, group will have a significantly greater proportion of participants with: 1) ≥15% reduction in daily MED (primary outcome); and 2) clinically significant decrease in pain intensity (key secondary outcome) at 6 months post-treatment. The secondary objective is to test a conceptual model of E-Health’s mechanisms of change, including hypothesized mediators and moderators of E-Health’s impact on decreasing MED and pain intensity. This paper is focused on the design considerations associated with the primary objective.
2.2. Research design
EMPOWER is a two-site intent-to-treat, two-arm, open-label, remote randomized controlled trial in which 400 chronic pain patients being treated with LOT will be randomized in a 1:1 ratio to E-Health+ or TAU. TAU consists of LOT from a prescribing clinician. E-health+ participants are provided with a 4-month E-health subscription (active treatment phase). All participants complete self-report measures at baseline, the end of the active treatment phase, and 6-months post-active treatment in REDCap, a web-based software toolset and workflow methodology for collection and management of clinical research data [21]. Opioid prescription information will be collected from the participants’ EHR from baseline through 6 months post-active treatment. The study schema is provided in Figure 1. The rationale for key design decisions is provided below.
Figure 1.
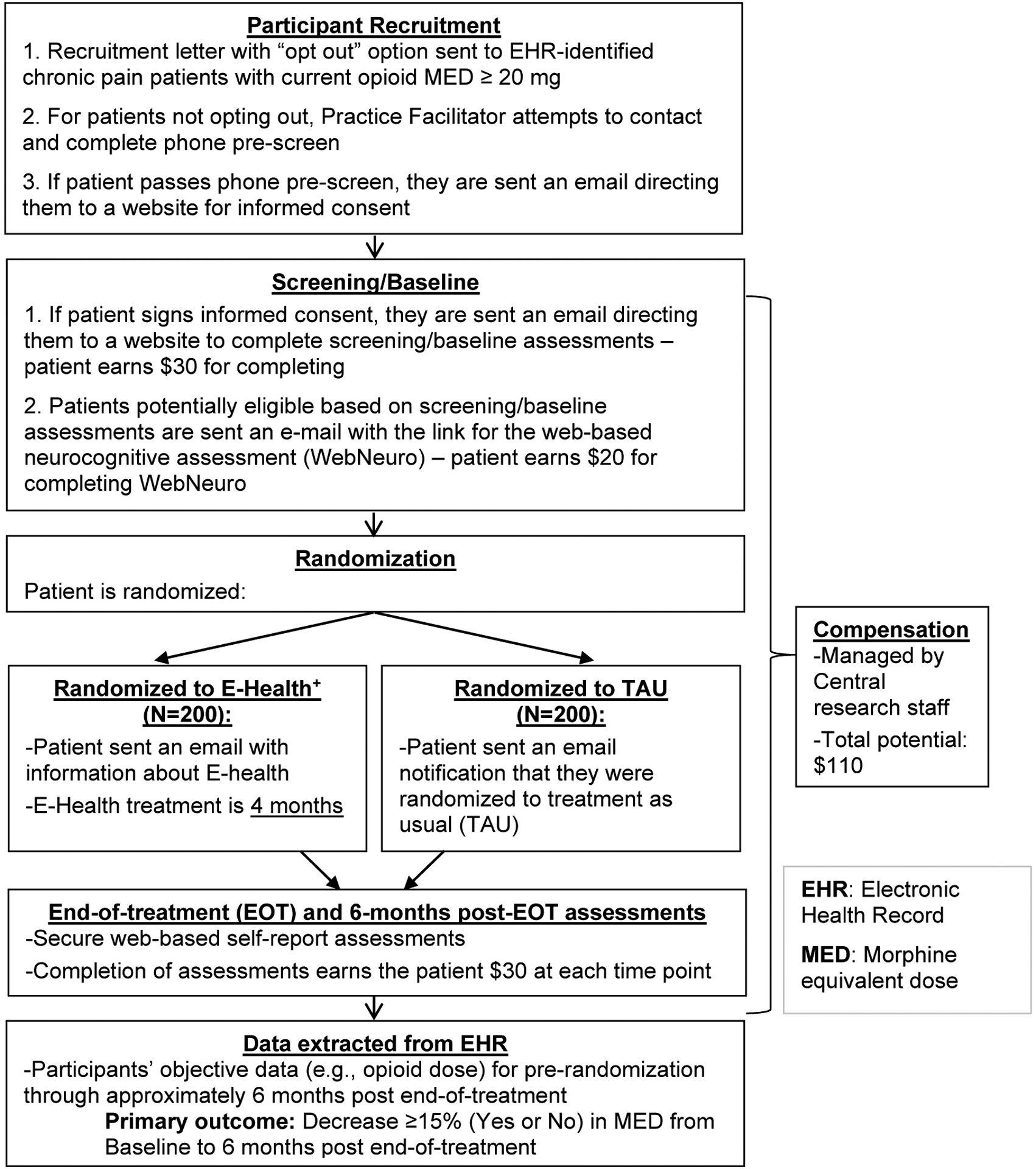
Study schema.
2.2.1. Comparator: Treatment as usual (TAU)
Due, in part, to the mounting adverse consequences associated with using LOT as the principal treatment for chronic pain (i.e., opioid overdoses, deaths, addiction, etc.), the question of whether there are viable alternative treatments has been asked frequently including by the Institute of Medicine [2] and National Institutes of Health [3]. In order to be viable, the alternative treatment needs to be accessible – both in terms of availability and affordability – and effective. Unfortunately, many potential treatment alternatives, including effective multidisciplinary or interprofessional treatments, are associated with significant barriers, including limited availability in many areas, lack of coverage by many insurance plans, and limited patient acceptance [22–24]. It was decided that the comparator should be TAU at the practices from which the participants are recruited given the lack of compelling alternative treatments; in addition, utilizing the standard care as the comparator increases the external validity of the trial.
2.2.2. Randomization: Participant level vs. cluster randomized
Randomization will be at the level of participants and stratified by site. An alternative study design would be a cluster-randomized trial in which clinical sites would be randomized to E-Health+ or to TAU. This design was not selected because differential changes in TAU over time at the study sites could reduce the internal validity of the trial, resulting in E-health appearing to be more, or less, effective than it is in actuality. This is a particularly important consideration for this trial in that there has been a significant increase in efforts to improve LOT prescribing practices the past few years [25–27].
2.2.3. Remote clinical trial
The EMPOWER trial was designed and initiated in 2017, several years before the COVID-19 crisis that began in 2020. The decision to design the trial to be conducted remotely was based on several considerations. First, the intervention being evaluated is accessed via the internet and, thus, in-person visits were not required for intervention administration. Second, the primary outcome is a ≥15% reduction in daily MED as assessed by the EHR; obtaining this objective measure does not require an in-person assessment. Third, participants were being recruited from two healthcare systems that included a total of 47 different practices; the staffing required for in-person recruitment of patients as they attended appointments would have added substantial cost to the trial and would have burdened the practices, which would be required to host research staff. Finally, the population of patients with chronic pain are likely to struggle with mobility and with transportation issues related to disability, which increases the difficulty of attending in-person visits.
There are a number of potential barriers to utilizing EHRs to streamline clinical trials, including the need for extensive programming to support identification, assessment, and follow-up of patients [28]. For EMPOWER, our team developed an innovative solution. The two participating healthcare systems were guided in developing two EHR query types: one to identify potential participants (Type I) and one to extract randomized participants’ study outcome data during the baseline period through 6-months post-active treatment (Type II). The study leadership decided on the data to include in the query results, and the requirements were provided to a University of Cincinnati (UC) Health informatics analyst to implement the query for UC Health’s EHR database (Epic Clarity). After initial programming, the queries went through several iterations of testing, feedback, and revision until they were finalized. The code for running the queries at UC Health was sent to Duke Health informatics staff, who adapted it for use on Duke’s Epic Clarity database. The query data are stored in password-protected, restricted-access computers and are transferred using secure methods.
The Type I query is auto-generated by each healthcare system on a weekly basis and imported into a Microsoft Access recruitment database at each research site. The database, developed by one of the authors (DL), analyzes the dataset to identify eligible patients and, using Microsoft Word’s mail merge function, generates personalized recruitment letters. The results from the Type II query are imported into a central Microsoft Access database, developed by one of the authors (DL), to extract the data needed for analysis of the primary outcome (MED). This approach offers efficiency gains over traditional data collection because the data are a by-product of routine clinical care and the outcome measures are missing only in rare circumstances. Research staff are also automatically provided updated contact information, which can assist in participant follow-up efforts. This approach removes common barriers to the use of EHRs for research by: 1) not requiring healthcare systems to modify their EHR; and 2) not requiring significant programming by healthcare systems because the programming is completed centrally.
2.3. Study Sites
The two healthcare systems participating in this trial, UC Health and Duke Health, have successfully participated in primary care and chronic pain research and have a sufficient pool of potential participants to support the target recruitment rate (approximately 5.1/month). Due to the need to characterize TAU at the clinical practices, only primary care/pain practices completing the required assessments (see section 3.1.1) were eligible to participate. The total number of practices from which patients will be recruited were 42 primary care (16 from UC Health and 26 from Duke), and 5 pain clinics (3 from UC Health and 2 from Duke).
2.4. Study Population
The participant eligibility criteria are listed in Table 1. To identify chronic pain patients prescribed LOT, a weekly query is run on the EHR data of the participating health systems (Type I query, described above in Section 2.2.3). This query identifies patients who are age 25–80, have at least one chronic pain diagnosis, have recently attended visits at one of the eligible primary care or pain medicine clinics, and have recent opioid medication prescriptions. These patients’ opioid prescription data are then processed by a database application at the health system to identify patients with an average daily MED of at least 20 over the prior 90 days. Those patients are flagged as being eligible to receive an Institutional Review Board (IRB)-approved personalized recruitment letter and brochure describing the study, which is sent via the US Postal Service. The recruitment letter explains that the recipient may be eligible for the EMPOWER study, and that they should expect to be called by a research staff member to discuss the study. The recipient is also given instructions on how to opt out from receiving any further contact about the study. The site research staff calls patients who have not opted out to inform them about the study, including key details from the informed consent document, and offer to complete a pre-screen interview. If the patient is interested in participating and passes the pre-screen interview, an automated email is sent by the EMPOWER REDCap system, inviting the patient to a secure form for informed consent. Patients are encouraged to discuss any questions or concerns with study staff prior to electronically signing the consent form. Patients who decide to participate sign the electronic consent form (e.g., using a mouse or touch screen). When a participant has signed the electronic consent, an email notification is automatically sent to the centralized research staff. These research staff review the signed consent form before sending an email with a link to the REDCap-based screening assessments, and participants are mailed a hard copy of their signed consent form.
Table 1.
Eligibility criteria for the EMPOWER trial
| Inclusion Criteria |
|---|
Potential participants must:
|
| Exclusion Criteria |
Potential participants must not:
|
In accordance with the CDC table of MED conversion factors [36], buprenorphine does not contribute to MED; thus a patient prescribed only buprenorphine would not have met this eligibility criterion.
WebNeuro is a standalone web-based neuropsychological battery [51] that was included for the objective of testing moderators/mediators; individuals who report being unable/unwilling to complete it during the pre-screen are deemed ineligible for study participation
2.5. Randomization
Eligible participants are randomized in a 1:1 ratio to TAU or to TAU plus E-Health (E-Health+). Randomization is stratified by site. The number in each treatment group will never differ by more than a factor of b/2 where b is the block size; this helps ensure treatment balance. In order to ensure unpredictability, block size randomly alternated between b = 2 and b = 4. The randomization process is performed within REDCap and the randomization sequence is unknown to the research staff.
3.0. Treatments
3.1. Treatment as Usual (TAU)
TAU primarily consists of LOT. Opioid dose, physician visits, and referrals to nonpharmacological treatments (e.g., physical therapy, behavioral therapy, etc.) are tracked through the EHR. Participant self-report is also used to assess whether the patient received referrals for nonpharmacologic treatment and the type of nonpharmacologic treatment(s) received. In addition, TAU at the practice level will be characterized both prior to the start of participant recruitment and following the end of data collection; this pre-post assessment was included since there have been significant efforts to improve LOT prescribing practices the past few years [25–27] and, hence, TAU at the practice might change during the course of the trial.
3.1.1. Clinical practice measures to characterize TAU
TAU at the participating practices will be characterized using three assessments, completed by an administrative person from each practice. An attempt will be made to have the same person complete the assessments at the pre-recruitment and post-data collection assessment time points; individuals will be compensated for completing these assessments between $50 and $100 based on the number of assessments completed and the staffing required to complete them (e.g., a staff member could complete the assessments for multiple practices, etc.).
Clinical Practice Characteristics. A questionnaire will be used to obtain information about the general characteristics of each practice, including size (e.g., number of full-time equivalent providers, staff, patients), geographic location, and provider types (e.g., family medicine, internal medicine, nurse practitioners, etc.).
Practice-level Payer Data. A questionnaire will be used to obtain information about the payer types for each practice.
Assessment of Chronic Illness Care (ACIC). A modified version of the Assessment of Chronic Illness Care (ACIC, version 3.5) [29] will be used to assess the practice’s approach to treating chronic pain. The ACIC assesses six elements that encourage high-quality care as outlined in the Chronic Care Model [30]. The ACIC version 3.5 assesses: 1) health care organization; 2) community linkages; 3) self-management support; 4) decision support; 5) delivery system design; and 6) clinical information systems. In addition, the degree to which there is integration of these elements is assessed. The ACIC has been utilized in a number of quality improvement projects and has been shown to have adequate reliability and validity [31, 32].
3.2. E-health
Participants randomized to the E-Health+ condition receive a free, 4-month subscription to the online Goalistics Chronic Pain Management Program. The Goalistics Chronic Pain Management Program, referred to as the E-Health program in EMPOWER, was developed from cognitive, behavioral, interpersonal, and self-management interventions with demonstrated efficacy in traditional face-to-face or group settings. It is patient-centered, having been developed based on substantial input from people with chronic pain and chronic pain professionals. Specifically, in the initial project funded by the National Institute of Neurological Disorders and Stroke (NINDS), a prototype for the pain program was developed, reviewed, and twice revised. A second NINDS-funded project included the final development of the program and a randomized controlled trial to test its efficacy [33].
The E-Health program includes five learning centers - the suggested order of completion is customized for each user based on priorities identified from completing the Profile of Chronic Pain [34]. Table 2 provides an overview of the content and therapeutic approach for each learning center. Developing new skills and integrating them into a patient’s life requires practice and persistence on the part of the patient and the E-Health intervention was designed to promote both. A set of self-management tools are integrated into the learning centers to enhance tracking, troubleshooting, and attainment of program activities and goals; descriptions of these E-Health tools are provided in Table 3. The length of time needed to complete the learning centers depends on the activities offered and the participant’s level of engagement and speed. In general, all activities can be completed within 8 weeks by spending approximately 1–2 hours per week. Additional time may be devoted to implementing and monitoring goals, tracking symptoms and practicing new skills.
Table 2.
E-Health learning center content and therapeutic approach
| 1. Thinking Better - Derived from cognitive models of pain management, this learning center teaches the person with chronic pain how to recognize, interrupt, challenge, and replace dysfunctional thinking. Users create a custom plan to decrease self-defeating thoughts while increasing effective thinking. |
| 2. Feeling Better - Provides training in fundamental methods of emotion regulation, including identifying negative and positive emotional triggers, the role of relaxation training in emotion regulation, and incorporating positive emotional triggers into daily life. A set of relaxation sessions offer practice in using breathing as a trigger for relaxation, progressive muscle relaxation, guided imagery, and mindfulness meditation. |
| 3. Doing More - The primary goals are to increase activity and exercise and promote goal-based activities. This learning center is based on behavioral and motivational models of pain self-management. |
| 4. Relating Better - This is derived from interpersonal/transactional models of pain management. The user learns about types of social support and how to differentiate effective versus ineffective support. A personalized support plan is created. |
| 5. Understanding Pain - This learning center teaches basic concepts underlying the biopsychosocial perspective on chronic pain and its management. It consists of a series of videos covering such topics as medication management, the safe use of pain medication, reducing reliance on pain medication, relaxation, cognitive behavior therapy, biomedical treatment options, and hypnosis. |
Table 3.
E-Health tools to promote and track lifestyle changes
| Navigator Calendar: Allows the user to schedule program activities and goals and send email and text-message reminders, with the ultimate goal that they will become automatic and self-sustaining. |
| Self-Monitoring Tool: Allows the user to select a self-monitoring exercise (e.g., monitoring positive emotions), schedule it on the calendar, and send automated email or text alerts throughout the day. |
| Pacing Tool: Used to create a custom plan to safely and slowly increase an activity over time. The user selects an activity to be paced (e.g., walking, swimming, meditating, etc.), identifies the amount of time it can be comfortably completed, and sets a target goal. The system creates a plan that increases the duration of the activity by 10% each week until the target time is reached. |
| Daily Check-In: Assists the user in tracking progress over time. This is also linked to a separate set of program features: Mood Boosters, Pain Soothers, and Activity Boosters. These tools allow the user to create a set of activities or experiences to boost mood, decrease pain, and increase activity. These are brief and easy-to-implement. Low mood, pain management, or activity ratings over several days triggers a reminder to schedule a Mood Booster, Pain Soother, or Activity Booster. |
| Self-Reward tool: Allows the patient to create a personalized list of inexpensive, easy-to-implement rewards to self-administer for progress and completing activities. |
| My Progress: Daily entries about what helps and interferes with activity completion are displayed, and compared to those of other program users to help the user understand his/her progress and to troubleshoot lack of progress. The system also displays progress in terms of Activity Completion Rate (the percentage of times that the user completed a scheduled activity). Each week, if the overall completion rate is reasonably high (e.g., ≥75%), a congratulatory message will be sent along with the suggestion that the user select a reward from his/her Self-Reward Menu. |
3.2.1. E-health Adherence
Adherence to the E-Health program is assessed by the presence of “star ratings” selected by the participant after completing each activity; these ratings indicate that the participant has engaged with the activity. Consistent with the adherence scoring method used in a randomized controlled trial of E-Health [19], these star ratings are used to derive an adherence score for each E-Health+ participant. In EMPOWER, E-Health Adherence Scores range from 0 to 6, with higher scores representing a greater number of learning centers in which activities were completed.
To encourage utilization, E-Health+ participants will receive weekly e-mails with instructions on how to use the program and goals for weekly activity completion. Participants’ progress will be monitored by research staff. Research staff will monitor the participants’ progress in the program and will attempt to make phone contact with any participant who does not significantly engage with the program. A number of strategies will be used to encourage participants to engage with the E-Health program, including providing specific feedback and encouragement about activities that the participant has completed. Research staff will also be prepared to address any technological issues that may interfere with successful program engagement (e.g., problems with the participant’s web browser, forgotten login information).
4.0. Assessments
The primary outcome is derived from EHR data and all other assessments are REDCap-based surveys completed by the participants at screening/baseline, the end of the active treatment phase, and 6-months post-active treatment.
4.1. Primary and key secondary outcome measures
The primary outcome measure is whether there was a ≥15% decrease (yes/no) in MED, based on the opioid prescribing information in the participant’s EHR, between baseline and 6-month post-treatment follow-up. There is no universally agreed upon method of calculating MED, which can result in inconsistent conversions among clinicians [35]. For EMPOWER, the calculation of MED will use the Opioid Morphine Equivalent Conversion Factors table created by the CDC [36]. There is also no standard definition for what constitutes a significant decrease in MED but a ≥15% decrease has been defined as a significant change in past research [37]. The key secondary outcome is whether there is a clinically meaningful decrease (yes/no) in pain intensity (≥2 points) as measured by the Brief Pain Inventory (BPI) Pain Intensity 11-point Scale [38] at 6-month post-treatment follow-up. The definition of ≥2 points as clinically significant is based on a study by Farrar and colleagues finding that a reduction of approximately two points on an 11-point Pain Intensity scale represented a clinically important decrease [39]. The BPI is a well-validated, reliable instrument that consists of a 4-item pain intensity subscale and a 7-item pain interference subscale. The BPI has been used in more than 400 studies worldwide [40], including a trial testing the efficacy of E-Health for patients receiving opioid therapy [19].
4.2. Other secondary outcome measures
Other secondary outcomes include: pain-related sleep problems, global health, current opioid misuse, and healthcare utilization. The 3-item Sleep Problems Index will be used to assess sleep interference resulting from pain; this measure has been found to have good construct validity and reliability (Cronbach’s α > 0.90) [41]. Global Health, which is a quality of life measure, will be assessed with the Patient-Reported Outcomes Measurement Information System (PROMIS) 10-item measure for Global Health, which briefly but comprehensively assesses physical, mental, and social health; this is a reliable (Cronbach’s α > 0.80) measure with demonstrated construct validity [42]. The Current Opioid Misuse Measure (COMM) [43, 44] will be used to assess opioid misuse. The COMM is a 17-item self-assessment used to monitor patients on opioid therapy and to assess whether patients are currently exhibiting behaviors indicative of misuse. The COMM has good predictive validity and reliability (Cronbach’s α > 0.82) [43, 44]. Test-retest reliability has been established and construct validity demonstrated with positive correlations with urine toxicology results [45]. The Stanford University Patient Education Research Center Health Care Utilization survey will be used to track health care utilization; the instrument has good test-retest reliability, and validity has been established through chart audits [46].
4.3. Safety Measures
Adverse events (AE) for this trial testing a low-risk, internet-based, intervention will be assessed using the BPI [38] and the Depression, Anxiety, and Stress Scales (DASS-21) [47]. The DASS-21 has established validity and reliability and assesses three constructs: 1) depression; 2) anxiety; and 3) stress [47–49]. A potential AE is defined as >30% symptom deterioration from baseline as indicated by any of the following: 1) Pain Intensity score or Pain Interference score as measured by the BPI; 2) Depression, Anxiety, or Stress score from the DASS-21. Additionally, to meet criteria for an AE, these follow-up scores must fall within at least the “moderate” range of severity for the respective measures. A report identifying participants with AEs (i.e., scores meeting the above criteria) is generated on a weekly basis. A research staff member attempts to contact any participants experiencing an AE to obtain additional information. During the process of obtaining additional information, the research staff assesses for potential Serious AEs.
5. Sample Size Estimation
The sample size calculation assumed an α level of 0.05 (two-tail). Study completion rates in the two prior E-Health randomized controlled trials were 92.4% [33] and 79% [19]. Data completeness for our primary outcome measure, obtained from the EHR, is expected to be higher than the completeness of participant-completed assessments. The pilot randomized controlled trial, which required participants to have an opioid prescription, found that 21% of E-Health, compared to 7% of wait-list control, participants reported decreasing their opioid medication[19]; this difference equates to an odds ratio (OR) of 3.6. While EMPOWER uses a different MED reduction outcome (≥15%) than the pilot (any reduction), the data from the pilot was the most pertinent available for estimating power. The EMPOWER trial has a target sample size of 400 (200/arm) participants – using a conservative estimated completion rate of 75% yields 300 total completers (150/arm). Having 300 completers would provide 80% power to detect an E-Health+ treatment effect if ≥18% participants have a meaningful MED reduction (i.e., of at least 15%) against 7% of TAU participants having a meaningful MED reduction; this equates to an OR of ≥2.77.
6. Analytic Plan
Each primary and secondary efficacy outcome measure will be analyzed for the intent-to-treat (ITT) population. No interim efficacy analyses are planned. All statistical tests will be conducted at the 5% Type I error rate (two-sided).
6.1. Primary Outcome
The primary hypothesis is that a significantly greater proportion of E-Health+, relative to TAU, participants will have a ≥15% decrease in MED between baseline and 6-months post-active treatment. The MED outcome may be prone to site effects in that the strategies used by the healthcare system to treat chronic pain may influence prescribing behavior; thus, the primary analysis will test for potential site effects. Specifically, a logistic regression will test for treatment (E-Health+ vs. TAU), site, and treatment-by-site interaction effects, with treatment being the effect of interest.
6.2. Secondary Outcomes
The key secondary hypothesis, that a significantly greater proportion of E-Health+, relative to TAU, participants will have a clinically significant decrease (i.e. ≥2 points [50]) in Pain Intensity score at 6-months post-active treatment, will be tested using logistic regression with treatment (E-Health+ vs. TAU) as the effect of interest. For consistency with the primary analysis, models including site effects will be evaluated. Most of the secondary outcomes consist of longitudinal variables that can reasonably be treated as continuous numeric values. These will be analyzed using random-intercept mixed model regressions including treatment, time, and treatment-by-time interaction as predictor variables. For consistency with the primary analysis, models including effects of site will be evaluated. The impact of utilizing non-study chronic pain treatments (i.e., as self-reported on the pain-related treatment assessment or documented in the EHR), other than LOT, on decrease in MED and pain intensity (yes/no) will be tested with logistic regressions including treatment (E-Health+ vs. TAU), use of other chronic pain treatment (yes/no), and their interaction. In the E-Health+ group, the relationship between treatment adherence and outcome (e.g., MED, pain) will be evaluated with logistic regression with treatment adherence score as the covariate.
7. Oversight of Data and Safety
Oversight is provided by multiple boards including the single IRB of record (University of Cincinnati). A Data and Safety Monitoring Board (DSMB) will examine accumulating data to assure protection of participants’ safety while the study’s scientific goals are being met. The DSMB will determine whether there is support for continuation of the trial, or evidence that study procedures should be changed, or if the trial should be halted, for reasons relating to the safety of the study participants, the efficacy of the treatment under study, or inadequate trial performance (e.g., poor recruitment). A Data and Safety Monitoring Plan for the trial has been approved by the study sponsor.
8. Current status of the trial
Study recruitment began in February of 2018 and was completed in November 2020. Participant data collection will be completed in September of 2021.
9. Summary
Our study will determine whether an accessible E-Health intervention can assist with reduced opioid reliance in chronic pain patients while also decreasing pain, which can, ultimately, reduce risks of unintended opioid overdose and death. This trial was designed to be completed remotely, which has proven to be advantageous during the COVID-19 pandemic. Our study design, which utilizes an innovative approach to using EHRs to streamline the conduct of a clinical trial, can be used as a template for trials seeking to use EHR databases to assist with efficient recruitment and collection of clinically-relevant outcomes.
Role of the funding source
This work was supported by the National Institute on Drug Abuse, National Institutes of Health, by grant R01DA044248 (Winhusen). The funding source had no further involvement in the study design, the writing of the report, or the decision to submit this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
Dr. Wilson reported receiving consulting fees in 2018 for work on a grant to Goalistics, LLC. All other authors have no potential conflicts of interest to report.
REFERENCES
- [1].Dahlhamer J, Lucas J, Z elaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C, Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016, MMWR. Morbidity and mortality weekly report 67(36) (2018) 1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Institute of Medicine Committee on Advancing Pain, Research, Care, and Education, Relieving pain in America a blueprint for transforming prevention, care, education, and research, National Academies Press, Washington, D.C., 2011. [PubMed] [Google Scholar]
- [3].Reuben DB, Alvanzo AA, Ashikaga T, Bogat GA, Callahan CM, Ruffing V, Steffens DC, National Institutes of Health Pathways to Prevention Workshop: the role of opioids in the treatment of chronic pain, Annals of internal medicine 162(4) (2015) 295–300. [DOI] [PubMed] [Google Scholar]
- [4].Centers for Disease Control and Prevention, Vital Signs: Overdoses of Prescription Opioid Pain Relievers - United States, 1999–2008, Morbidity and Mortality Weekly Report 60(43) (2011) 1487–1492. [PubMed] [Google Scholar]
- [5].Dowell D, Haegerich TM, Chou R, CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016, Jama-Journal of the American Medical Association 315(15) (2016) 1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Darnall BD, Juurlink D, Kerns RD, Mackey S, Van Dorsten B, Humphreys K, Gonzalez-Sotomayor JA, Furlan A, Gordon AJ, Gordon DB, Hoffman DE, Katz J, Kertesz SG, Satel S, Lawhern RA, Nicholson KM, Polomano RC, Williamson OD, McAnally H, Kao MC, Schug S, Twillman R, Lewis TA, Stieg RL, Lorig K, Mallick-Searle T, West RW, Gray S, Ariens SR, Sharpe Potter J, Cowan P, Kollas CD, Laird D, Ingle B, Julian Grove J, Wilson M, Lockman K, Hodson F, Palackdharry CS, Fillingim RB, Fudin J, Barnhouse J, Manhapra A, Henson SR, Singer B, Ljosenvoor M, Griffith M, Doctor JN, Hardin K, London C, Mankowski J, Anderson A, Ellsworth L, Davis Budzinski L, Brandt B, Hartley G, Nickels Heck D, Zobrosky MJ, Cheek C, Wilson M, Laux CE, Datz G, Dunaway J, Schonfeld E, Cady M, LeDantec-Boswell T, Craigie M, Sturgeon J, Flood P, Giummarra M, Whelan J, Thorn BE, Martin RL, Schatman ME, Gregory MD, Kirz J, Robinson P, Marx JG, Stewart JR, Keck PS, Hadland SE, Murphy JL, Lumley MA, Brown KS, Leong MS, Fillman M, Broatch JW, Perez A, Watford K, Kruska K, Sophia You D, Ogbeide S, Kukucka A, Lawson S, Ray JB, Wade Martin T, Lakehomer JB, Burke A, Cohen RI, Grinspoon P, Rubenstein MS, Sutherland S, Walters K, Lovejoy T, International Stakeholder Community of Pain Experts and Leaders Call for an Urgent Action on Forced Opioid Tapering, Pain medicine 20(3) (2019) 429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Robinson JP, Dansie EJ, Wilson HD, Rapp S, Turk DC, Attitudes and Beliefs of Working and Work-Disabled People with Chronic Pain Prescribed Long-Term Opioids, Pain medicine 16(7) (2015) 1311–24. [DOI] [PubMed] [Google Scholar]
- [8].Jamison RN, Anderson KO, Peeters-Asdourian C, Ferrante FM, Survey of opioid use in chronic nonmalignant pain patients, Regional anesthesia 19(4) (1994) 225–30. [PubMed] [Google Scholar]
- [9].Watson CP, Watt-Watson J, Chipman M, The long-term safety and efficacy of opioids: a survey of 84 selected patients with intractable chronic noncancer pain, Pain research & management : the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur 15(4) (2010) 213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kennedy LC, Binswanger IA, Mueller SR, Levy C, Matlock DD, Calcaterra SL, Koester S, Frank JW, “Those Conversations in My Experience Don’t Go Well”: A Qualitative Study of Primary Care Provider Experiences Tapering Long-term Opioid Medications, Pain medicine 19(11) (2018) 2201–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Frank JW, Levy C, Matlock DD, Calcaterra SL, Mueller SR, Koester S, Binswanger IA, Patients’ Perspectives on Tapering of Chronic Opioid Therapy: A Qualitative Study, Pain medicine 17(10) (2016) 1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].National Institute on Drug Abuse, 2016–2020 NIDA Strategic Plan: Advancing Addiction Science, National Institutes of Health, U.S. Department of Health and Human Services, 2015. [Google Scholar]
- [13].National Institutes of Health Office of Pain Policy, Interagency Pain Research Coordinating Committee, Federal Pain Research Strategy, 2018. https://www.iprcc.nih.gov/sites/default/files/FPRS_Research_Recommendations_Final_508C.pdf. (Accessed August 18 2020).
- [14].Boyers D, McNamee P, Clarke A, Jones D, Martin D, Schofield P, Smith BH, Cost-effectiveness of self-management methods for the treatment of chronic pain in an aging adult population: a systematic review of the literature, The Clinical journal of pain 29(4) (2013) 366–75. [DOI] [PubMed] [Google Scholar]
- [15].Lorig KR, Ritter PL, Laurent DD, Plant K, The internet-based arthritis self-management program: a one-year randomized trial for patients with arthritis or fibromyalgia, Arthritis and rheumatism 59(7) (2008) 1009–17. [DOI] [PubMed] [Google Scholar]
- [16].Andersson G, Cuijpers P, Carlbring P, Riper H, Hedman E, Guided Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: a systematic review and meta-analysis, World psychiatry : official journal of the World Psychiatric Association 13(3) (2014) 288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Carlbring P, Andersson G, Cuijpers P, Riper H, Hedman-Lagerlof E, Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis, Cognitive behaviour therapy 47(1) (2018) 1–18. [DOI] [PubMed] [Google Scholar]
- [18].Bender JL, Radhakrishnan A, Diorio C, Englesakis M, Jadad AR, Can pain be managed through the Internet? A systematic review of randomized controlled trials, Pain 152(8) (2011) 1740–50. [DOI] [PubMed] [Google Scholar]
- [19].Wilson M, Roll JM, Corbett C, Barbosa-Leiker C, Empowering Patients with Persistent Pain Using an Internet-based Self-Management Program, Pain management nursing : official journal of the American Society of Pain Management Nurses 16(4) (2015) 503–14. [DOI] [PubMed] [Google Scholar]
- [20].Mun C, Karoly P, Okun M, Ruehlman L, Does fear of pain mediate the effect of an online chronic pain management program on medication use?, 123rd Annual Convention of the American Psychological Association, Toronto, Ontario, Canada, 2015. [Google Scholar]
- [21].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support, Journal of biomedical informatics 42(2) (2009) 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Leverence RR, Williams RL, Potter M, Fernald D, Unverzagt M, Pace W, Parnes B, Daniels E, Skipper B, Volk RJ, Brown AE, Rhyne RL, Clinicians PN, Chronic non-cancer pain: a siren for primary care--a report from the PRImary Care MultiEthnic Network (PRIME Net), Journal of the American Board of Family Medicine : JABFM 24(5) (2011) 551–61. [DOI] [PubMed] [Google Scholar]
- [23].Elder NC, Simmons T, Regan S, Gerrety E, Care for patients with chronic nonmalignant pain with and without chronic opioid prescriptions: a report from the Cincinnati Area Research Group (CARinG) network, Journal of the American Board of Family Medicine : JABFM 25(5) (2012) 652–60. [DOI] [PubMed] [Google Scholar]
- [24].Lynch ME, Campbell F, Clark AJ, Dunbar MJ, Goldstein D, Peng P, Stinson J, Tupper H, A systematic review of the effect of waiting for treatment for chronic pain, Pain 136(1–2) (2008) 97–116. [DOI] [PubMed] [Google Scholar]
- [25].Bohnert ASB, Guy GP, Losby JL, Opioid Prescribing in the United States Before and After the Centers for Disease Control and Prevention’s 2016 Opioid Guideline, Annals of internal medicine 169(6) (2018) 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Strickler GK, Zhang K, Halpin JF, Bohnert ASB, Baldwin GT, Kreiner PW, Effects of mandatory prescription drug monitoring program (PDMP) use laws on prescriber registration and use and on risky prescribing, Drug and alcohol dependence 199 (2019) 1–9. [DOI] [PubMed] [Google Scholar]
- [27].Volkow ND, Baler R, A prescription for better opioid prescribing?, Nature Medicine 24(10) (2018) 1496–1498. [DOI] [PubMed] [Google Scholar]
- [28].Mc Cord KA, Al-Shahi Salman R, Treweek S, Gardner H, Strech D, Whiteley W, Ioannidis JPA, Hemkens LG, Routinely collected data for randomized trials: promises, barriers, and implications, Trials 19(1) (2018) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].MacColl Institute for Healthcare Innovation, Assessment of Chronic Illness Care Version 3.5, 2000. http://www.improvingchroniccare.org/downloads/acic_v3.5a.pdf. (Accessed September 12 2016). [Google Scholar]
- [30].Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A, Improving chronic illness care: translating evidence into action, Health affairs 20(6) (2001) 64–78. [DOI] [PubMed] [Google Scholar]
- [31].Bonomi AE, Wagner EH, Glasgow RE, VonKorff M, Assessment of chronic illness care (ACIC): a practical tool to measure quality improvement, Health services research 37(3) (2002) 791–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cramm JM, Strating MM, Tsiachristas A, Nieboer AP, Development and validation of a short version of the Assessment of Chronic Illness Care (ACIC) in Dutch disease management programs, Health and quality of life outcomes 9 (2011) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ruehlman LS, Karoly P, Enders C, A randomized controlled evaluation of an online chronic pain self management program, Pain 153(2) (2012) 319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ruehlman LS, Karoly P, Newton C, Aiken LS, The development and preliminary validation of the Profile of Chronic Pain: Extended Assessment Battery, Pain 118(3) (2005) 380–9. [DOI] [PubMed] [Google Scholar]
- [35].Rennick A, Atkinson T, Cimino NM, Strassels SA, McPherson ML, Fudin J, Variability in Opioid Equivalence Calculations, Pain medicine (2015). [DOI] [PubMed] [Google Scholar]
- [36].National Center for Injury Prevention and Control, CDC compilation of benzodiazepines, muscle relaxants, stimulants, zolpidem, and opioid analgesics with oral morphine milligram equivalent conversion factors, 2017. https://www.cdc.gov/drugoverdose/data-files/CDC_Oral_Morphine_Milligram_Equivalents_Sept_2017.xlsx. (Accessed November 2 2020). [Google Scholar]
- [37].Chen L, Vo T, Seefeld L, Malarick C, Houghton M, Ahmed S, Zhang Y, Cohen A, Retamozo C, St Hilaire K, Zhang V, Mao J, Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients, The journal of pain : official journal of the American Pain Society 14(4) (2013) 384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cleeland CS, Ryan KM, Pain assessment: global use of the Brief Pain Inventory, Annals of the Academy of Medicine, Singapore 23(2) (1994) 129–38. [PubMed] [Google Scholar]
- [39].Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, Poole RM, Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale, Pain 94(2) (2001) 149–58. [DOI] [PubMed] [Google Scholar]
- [40].Cleeland C, The Brief Pain Inventory user guide, 2009. http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/BPI_UserGuide.pdf. (Accessed February 2014).
- [41].Kosinski M, Janagap CC, Gajria K, Schein J, Psychometric testing and validation of the Chronic Pain Sleep Inventory, Clinical therapeutics 29 Suppl (2007) 2562–77. [DOI] [PubMed] [Google Scholar]
- [42].Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D, Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items, Quality of Life Research 18(7) (2009) 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Butler SF, Budman SH, Fanciullo GJ, Jamison RN, Cross validation of the current opioid misuse measure to monitor chronic pain patients on opioid therapy, The Clinical journal of pain 26(9) (2010) 770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, Jamison RN, Development and validation of the Current Opioid Misuse Measure, Pain 130(1–2) (2007) 144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wasan AD, Butler SF, Budman SH, Benoit C, Fernandez K, Jamison RN, Psychiatric history and psychologic adjustment as risk factors for aberrant drug-related behavior among patients with chronic pain, The Clinical journal of pain 23(4) (2007) 307–15. [DOI] [PubMed] [Google Scholar]
- [46].Lorig K, Stewart A, Ritter P, Gonzalez V, Laurent D, Lynch J, Outcome measures for health education and other health care interventions, Sage Publications, Thousand Oaks, 1996. [Google Scholar]
- [47].Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP, Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample, Psychological Assessment 10(2) (1998) 176–181. [Google Scholar]
- [48].DasMahapatra P, Chiauzzi E, Pujol LM, Los C, Trudeau KJ, Mediators and moderators of chronic pain outcomes in an online self-management program, The Clinical journal of pain 31(5) (2015) 404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Smitherman TA, Davis RE, Walters AB, Young J, Houle TT, Anxiety sensitivity and headache: diagnostic differences, impact, and relations with perceived headache triggers, Cephalalgia : an international journal of headache 35(8) (2015) 710–21. [DOI] [PubMed] [Google Scholar]
- [50].Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, Farrar JT, Hertz S, Raja SN, Rappaport BA, Rauschkolb C, Sampaio C, Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations, Pain 146(3) (2009) 238–44. [DOI] [PubMed] [Google Scholar]
- [51].Silverstein SM, Berten S, Olson P, Paul R, Willams LM, Cooper N, Gordon E, Development and validation of a World-Wide-Web-based neurocognitive assessment battery: WebNeuro, Behavior research methods 39(4) (2007) 940–9. [DOI] [PubMed] [Google Scholar]


