Abstract
Introduction
Sepsis is a complication in acute-on-chronic liver failure (ACLF) patients associated with high rates of mortality and morbidity. Early diagnosis of sepsis in ACLF patients can improve prognosis. This study aimed to explore potential effective biomarkers for the early diagnosis of sepsis in ACLF patients.
Methods
Ninety-four ACLF patients with sepsis were enrolled from 10 hospitals across China from January 2015 to June 2016 as well as 49 ACLF patients without infection from Xiangya Hospital. The first-day admission data and SOFA score and CLIF-SOFA score were collected. The differences of indicators between groups were compared with Kruskal-Wallis test. The receiver-operating characteristic (ROC) curve was analyzed to evaluate the diagnostic efficiency of the selected factors.
Results
Soluble triggering receptor expressed on myeloid cell-1 (sTREM-1) and presepsin were significantly higher in ACLF-sepsis patients compared with ACLF patients with no infection (P < 0.001). sTREM-1 and presepsin presented higher diagnostic value in sepsis for ACLF patients compared with other biomarkers [white blood cells (WBC), procalcitonin (PCT) and C-reactive protein (CRP)]. Combining sTREM-1 or presepsin with the CLIF-SOFA score increased the diagnostic efficiency (AUC = 0.876 or AUC = 0.913, respectively).
Conclusions
sTREM-1 and presepsin are potential biomarkers for the early diagnosis of sepsis in ACLF patients. The combination of presepsin and the CLIF-SOFA score is a promising method for diagnosing sepsis in ACLF patients.
Trial Registration
ClinicalTrials.gov identifier, NCT02457637.
Keywords: Acute-on-chronic liver failure, Early diagnosis, Multicenter study, Presepsin, Sepsis, sTREM-1
Key Summary Points
| Why carry out this study? |
| Sepsis can lead to high mortality in ACLF patients. |
| Early diagnosis of sepsis in ACLF patients can improve prognosis. |
| sTREM-1 and/or presepsin may be early diagnostic biomarkers of sepsis in ACLF patients. |
| What was learned from the study? |
| Combining presepsin with the CLIF-SOFA score is a potential way to diagnose sepsis in ACLF patients. |
| Presepsin and sTREM-1 are potential biomarkers for diagnosing sepsis in ACLF patients. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13096340.
Introduction
Acute-on-chronic liver failure (ACLF) is an acute deterioration of pre-existing chronic liver disease with high short-term mortality [1–4]. ACLF patients are more susceptible to infection and developing sepsis since they usually also have intestinal barrier destruction, intestinal microecologic disorder, immune activation and ascites [5]. Sepsis is not only one of the leading causes of death in critically ill patients in the intensive care unit (ICU) [6, 7], but also one of the pathologic features of ACLF [8, 9]. Sepsis can activate the immune cells and promote the release of proinflammatory factors to mediate liver inflammation, necrosis and disorder of liver cell regeneration, eventually leading to multiple organ dysfunction or death [10–12]. One hour delay of antimicrobial treatment decreases the survival rate of sepsis patients by 7.6% [13]. Therefore, early diagnosis of sepsis is vital for proper patient management to improve prognosis. Unfortunately, current methods for the diagnosis of sepsis, such as blood culture, are often time consuming and have low sensitivity. Xue et al. reported that the ratio of PLT/WBC indicated the occurrence of sepsis in HBV-ACLF patients [14]. However, more effective biomarkers are still needed for the early diagnosis of sepsis for ACLF patients.
Triggering receptor expressed on myeloid cell-1(TREM-1) is a member of the immunoglobulin superfamily and is expressed on the surface of immune cells, such as neutrophils, monocytes and macrophages. Soluble TREM-1 (sTREM-1) is a new inflammatory biomarker. It is a soluble TREM-1 and can be tested in plasma, urine and pleural effusion. The level of sTREM-1 is increased in various infections and sepsis [15–19]. sTREM-1 could be a biomarker for early diagnosis of sepsis, but few studies have focused on sTREM-1 in ACLF-sepsis.
Presepsin, also called soluble CD14 subtype (sCD14-st), is another new inflammatory biomarker. The secretion of presepsin stimulates monocyte phagocytosis [20, 21]. Previous studies showed that serum presepsin levels were elevated in different infectious diseases [22–25]. Early increasing levels of presepsin in sepsis make it an attractive indicator in diagnosing sepsis, and it has been associated with the severity and prognosis of sepsis [26–29]. However, the effect of presepsin on the diagnosis and prognosis of ACFL-sepsis is not known.
In this multicenter study, we analyzed sTREM-1, presepsin and other inflammatory biomarkers, such as white blood cells (WBC), procalcitonin (PCT) and C-reactive protein (CRP), between ACLF-sepsis patients and ACLF patients with no infection. Our aim was to explore potential biomarkers for the early diagnosis of sepsis in ACLF patients.
Methods
Patients and Groups
Ninety-four ACLF patients with sepsis were enrolled from ten hospitals across China during January 2015 to June 2016 as the ACLF-sepsis group. Forty-nine ACLF patients without infection (no fever, normal percentage of white blood cells and neutrophils, no infectious ascites with WBC < 250/µl, normal pulmonary imaging examination and without anti-infection treatment) were enrolled from Xiangya Hospital as the ACLF group.
Patients who met one of the following criteria were excluded: (1) age < 15 years old or ≥ 80 years old; (2) pregnancy; (3) malignant tumor; (4) serious chronic history of extrahepatic organs, such as COPD with respiratory failure, coronary heart disease with ≥ NYHA III, diabetes with serious complications, or chronic kidney disease with renal failure; (5) receiving immunosuppressive therapy; (6) patients or family members refused participation in the clinical trial. All patients gave written informed consent according to the Declaration of Helsinki. The study was approved by Xiangya Hospital Ethics Committee, Central South University, and the committees of each center. All documents approved by Clinical Research Committee of Ren Ji Hospital Affiliated to Shanghai Jiao Tong University were sent to each institution and approved by each institution under the same IRB name. The National Clinical Trial number (NCT no.) is NCT02457637.
Diagnostic Criteria
ACLF
The diagnostic criteria of ACLF were based on the 2019 consensus recommendations for chronic and acute liver failure developed by the Asian-Pacific Association for the Study of the Liver (APASL) [3], which was defined as acute liver damage with clinical manifestations of jaundice and/or clotting dysfunction on the basis of known or unrecognized chronic liver disease, complicated within 4 weeks by ascites and/or hepatic encephalopathy.
Sepsis
The definition of sepsis referred to the criteria proposed by European Society of Intensive Care Medicine (ESICM) and Society of Critical Care Medicine (SCCM) [30]. Patients with infection and SOFA score ≥ 2 were diagnosed with sepsis. Meeting any of the following criteria was considered as indicating infection:
Systemic inflammation: systemic inflammatory response syndrome (SIRS) and increase of inflammatory indicators (WBC, PCT, CRP and so on).
Clinical manifestations of infection: cough, yellow pus sputum, frequent urination, odynuria, abdominal tenderness and rebound pain, and so on.
Infection confirmed by radiograph.
Infection confirmed by specimens: ascites WBC ≥ 250/µl, middle segment urine leukocyte ≥ ++ and positive results in culture.
Collection of Data
The general condition, vital signs and consciousness state of the patient after admission.
WBC, PCT, CRP, liver function, renal function, electrolyte, coagulation function and other indicators were tested on the first day of admission.
SOFA score and CLIF-SOFA score were assessed within 24 h of admission.
Detection of sTREM-1 and Presepsin in Plasma
The plasma used for detection of sTREM-1 and presepsin was collected from the patient on the first day of admission. The ELISA-based sTREM-1 quantitative assay kit was purchased from Hycult Biotech, The Netherlands (HK348). The assay was carried out according to the kit instructions. Presepsin quantitative reagent strips were purchased from Mitsubishi of Japan (PATHFASTTM), and the chemiluminescence immunoanalyzer was also from Mitsubishi Corp. To test presepsin, we added 100 ul of plasma into the sample hole of each reagent bar and then put the reagent rack into the instrument to react for 15 min.
Statistical Analysis
Counting data were analyzed with chi-square test. Measurement data with normal distribution data were expressed as mean ± standard deviation (SD). The measurement data with non-normal distribution were represented by the median [25th, 75th] (M [P25, P75]). Kruskal-Wallis test was used for comparison of index level differences between groups. The receiver-operating characteristic (ROC) curve was drawn for diagnosis efficiency. All data were analyzed by SPSS20.0 software. P < 0.05 was statistically significant.
Results
The Clinical Data and General Information
We enrolled 94 patients (77 males and 17 females) in the ACLF-sepsis group and 49 patients (42 males and 7 females) in the ACLF group, respectively. In the ACLF-sepsis group, patients had infections in different organs or sites: 58 patients with pulmonary infection, 26 patients with abdominal infection and 10 patients with other infections. The age and gender between the two groups were comparable. Liver injury was more severe in ACLF-sepsis patients compared with ACLF patients (Table 1).
Table 1.
Clinical characteristics of patients
| Variable | ACLF-sepsis group (N = 94) | ACLF group (N = 49) | P value |
|---|---|---|---|
| Age (years) | 47 (40, 53) | 44 (38, 50) | 0.339 |
| Gender (male, female) | 77, 17 | 42, 7 | 0.733 |
| Fever (yes, no) | 41, 53 | 12, 37 | 0.029 |
| TBIL (µmol/l) | 353.2 ± 183.3 | 282.2 ± 149.4 | 0.025 |
| ALT (U/l) | 171.7 (62, 459) | 550.8 (206.2,1077.8) | 0.000 |
| INR | 2.15 (1.76, 2.70) | 1.67 (1.50, 2.07) | 0.000 |
| SOFA score | 6 (5, 7) | 5 (4, 6) | 0.000 |
| CLIF-SOFA score | 7 (7, 8.25) | 6 (5, 7) | 0.000 |
| 28-Day mortality rate | 21.3%(20/94) | 4.1%(2/49) | |
| 90-Day mortality rate | 38.2%(36/94) | 81.6%(4/49) |
TBIL total bilirubin, ALT alanine transaminase, INR international normalized ratio
Comparison of Biomarkers Between the ACLF-Sepsis Group and ACLF Group
We compared inflammatory biomarkers (WBC, PCT, CRP, sTREM-1 and presepsin) between the ACLF-sepsis group and ACLF group. The results showed all the inflammatory biomarkers in the ACLF-sepsis group were significantly higher than those in the ACLF group (all P < 0.001) (Table 2).
Table 2.
Comparison of biomarkers between the ACLF-sepsis and ACLF group
| Biomarkers M[P25, P75] | ACLF-sepsis group | ACLF group | P value |
|---|---|---|---|
| WBC (^109/l) | 10.06 [5.75, 12.56] | 5.5 [4.45, 6.1] | 0.000 |
| PCT (ng/ml) | 1.16 [0.48, 2.4] | 0.44 [0.30, 0.74] | 0.000 |
| CRP (mg/l) | 15.65 [10.48,23.38] | 7.76 [5.80, 12.3] | 0.000 |
| sTREM-1 (pg/ml) | 723.48 [387.45, 1060.48] | 302.32 [123.13, 562.92] | 0.000 |
| Presepsin (pg/ml) | 508.5 [393, 847] | 284 [216, 522] | 0.000 |
WBC white blood cell, PCT procalcitonin, CRP C-reactive protein, sTREM-1 soluble triggering receptor expressed on myeloid cell-1
ROC Analysis of Biomarkers for Diagnosing Sepsis in ACLF Patients
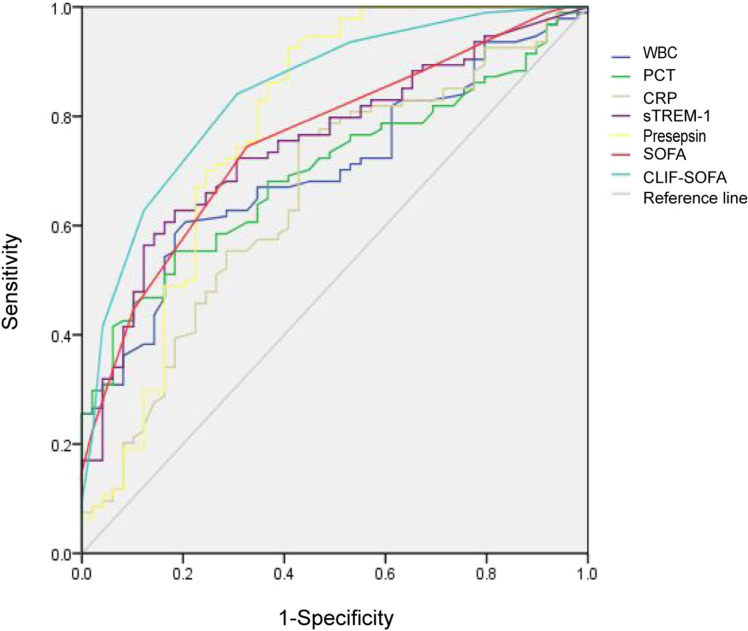
ROC analysis showed that sTREM-1 and presepsin had higher efficiency in diagnosing sepsis for ACLF patients compared with the other biomarkers (WBC, PCT and CRP). The CLIF-SOFA score was better than the SOFA score in diagnosing sepsis among ACLF patients (Fig. 1). The area under the curve (AUC) of presepsin was 0.790. The sensitivity and specificity were 96.8% and 59.2%, respectively, when taking presepsin 404.5 pg/ml as the cutoff value. The single indicator sTREM-1 or presepsin only had a moderate diagnostic efficiency (AUC = 0.752 or AUC = 0.790, respectively). However, combining sTREM-1 or presepsin with CLIF-SOFA increased the diagnostic efficiency (AUC = 0.876 or AUC = 0.913, respectively) (Table 3).
Fig. 1.
ROC curve of indicators in sepsis diagnosis
Table 3.
Efficiency of indicators in the diagnosis of sepsis in ACLF patients
| Indicators | AUC | Critical point | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|---|
| WBC | 0.701 | 6.15^109/l | 60.6 | 79.6 | 73.4 | 78 |
| PCT | 0.690 | 0.765 ng/ml | 55.3 | 81.6 | 62.7 | 80 |
| CRP | 0.654 | 13.5 mg/l | 68.5 | 57.7 | 62.7 | 70 |
| sTREM-1 | 0.752 | 607.94 pg/ml | 62.8 | 81.6 | 64.8 | 78 |
| Presepsin | 0.790 | 404.5 pg/ml | 96.8 | 59.2 | 71.2 | 66 |
| SOFA score | 0.758 | 5.5 | 76.5 | 67.3 | 63.9 | 66 |
| CLIF-SOFA score | 0.878 | 7 | 72.3 | 85.7 | 77.7 | 68 |
| Presepsin + CLIF-SOFA score | 0.913 | – | – | – | – | – |
| sTREM-1 + CLIF-SOFA score | 0.876 | – | – | – | – | – |
WBC white blood cell, PCT procalcitonin, CRP C-reactive protein, sTREM-1 soluble triggering receptor expressed on myeloid cell-1, AUC area under the curve
Discussion
ACLF patients were susceptible to bacterial infection because of dramatically decreasing immunity [31]. Infection was an important risk factor leading to aggravation and even death of ACLF patients [32, 33]. Due to various pathogens of sepsis, severe conditions, complicated clinical symptoms and individual differences of ACLF patients, clinicians usually have difficulty determining sepsis in ACLF patients. In addition, both pathogen-associated molecular patterns (PAMPs, expressed by pathogens) and damage-associated molecular patterns (DAMPs, released by damaged body tissues and cells) can activate pattern recognition receptors (PRRs) and trigger inflammatory responses [34–36], so it is difficult to recognize sepsis in ACLF patients.
In this study, we showed that WBC, PCT and CRP were not suitable indicators for diagnosing sepsis in ACLF patients, although they increased significantly in the ACLF-sepsis group compared with the ACLF group with no infection. WBC is a common clinical indicator for the diagnosis of infection. However, most ACLF patients are in the decompensated stage of cirrhosis and their immune system is in a state of continuous activation, so WBC lacks sensitivity and specificity in the diagnosis of sepsis in ACLF patients. PCT was not effective in distinguishing sepsis from non-infectious systemic inflammatory response syndrome (SRIS) with low sensitivity and low specificity [37] because PCT could be increased in non-infectious inflammation. In our study, PCT in the ACLF-sepsis group was higher than in the ACLF group, but the area under the ROC curve was only 0.690. CRP can be increased in infection, inflammation and stress. The poor specificity and sensitivity were the main disadvantages of CRP as a biomarker [38].
sTREM-1 and presepsin are new inflammatory indicators. Both sTREM-1 and presepsin play important roles in the development of sepsis. sTREM-1 was significantly increased in the serum of the animal with sepsis shock induced by lipopolysaccharides (LPS), and the antagonist of TREM-1 could reduce the occurrence of shock and death in LPS-mediated sepsis mice [39]. The plasma presepsin in sepsis patients was significantly higher than that in non-infectious SRIS patients and was correlated with organ dysfunction and prognosis [40]. Our study found that sTREM-1 and presepsin were superior to WBC, PCT and CRP, but the diagnostic efficiency of a single indicator was at a medium level. However, when sTREM-1 or presepsin was combined with the CLIF-SOFA score, the AUC could be increased to 0.876 or 0.913 respectively.
Sequential organ failure assessment (SOFA) comprehensively assesses organ function from six aspects including the respiratory system, circulatory system, nervous system, liver function, coagulation function and kidney function. The SOFA score is a criterion for evaluating organ dysfunction in the diagnosis of sepsis. Although a high SOFA score was associated with mortality in ACLF-sepsis patients [41], it had limitations in evaluating organ dysfunction for diagnosing sepsis in ACLF patients. The SOFA score could not distinguish between organ dysfunction caused by the infection itself and that caused by sepsis, and the diagnostic criteria also had limitations for patients who had existing organ dysfunction before sepsis such as ACLF [30]. Due to severe organ dysfunction with hypoimmunity, ACLF patients develop infections easily and are susceptible to sepsis, and in turn sepsis accelerates organ failure. Therefore, the SOFA score cannot distinguish between liver failure caused by sepsis and that from ACLF when ACLF patients have sepsis. The Sequential Organ Failure Assessment of Chronic Liver Failure (CLIF-SOFA) is the organ function assessment system for patients with chronic liver disease. In this study, we analyzed the effectiveness of CLIF-SOFA in diagnosing sepsis of ACLF. The results showed that the CLIF-SOFA score was more effective than the SOFA score in diagnosing sepsis of ACLF, and the combination with sTREM-1 or presepsin could increase diagnostic efficiency.
In summary, we investigated the potential biomarkers for early diagnosis of sepsis in ACLF patients through a multicenter study. We found that the diagnostic efficiency of sTREM-1 and presepsin was higher than that of WBC, PCT, and CRP in sepsis diagnosis among ACLF patients. In addition, combining sTREM-1 or presepsin with the CLIF-SOFA score could increase diagnostic efficiency. The combination of presepsin and CLIF-SOFA score had high efficacy in diagnosing sepsis in ACLF patients. Our results provide a reference for early diagnosis of sepsis in ACLF patients. However, further study should be carried out to verify the results.
There are some shortcomings in this study. First, the sample is not large enough in this multicenter study, and the patients in the ACLF group were from one center. Second, the effect of biomarkers (sTREM-1, presepsin) on the prognosis of ACLF-sepsis is not analyzed in this study. Third, the patient population is limited in this study, and whether sTREM-1 or presepsin applies to early diagnosis of sepsis in ACLF patients with HCC or other chronic diseases is not kown. Besides, we did not analyze the etiology of the liver disease related to ACLF in this study. Therefore, more sample data and well-designed studies are needed to further verify the results.
Conclusions
sTREM-1 and presepsin are potential biomarkers for the early diagnosis of sepsis for ACLF patients. In clinical practice, combining presepsin with the CLIF-SOFA score could be a promising method to diagnose sepsis in ACLF patients.
Acknowledgements
We thank the participants in the study: Ren Ji Hospital, Shanghai Jiao Tong University: Shan Yin, Wen-Yi Gu, Yan Zhang and Liang Qiao. Union Hospital, Huazhong University of Science and Technology: Cong-Cong Zou and Jing Liu. Nanfang Hospital, Southern Medical University: Bei-Ling Li and Xiu Hua Jiang. Taihe Hospital, Hubei University of Medicine: Yuan-Yuan Chen. The First Affiliated Hospital of Xinjiang Medical University: Yan-Fang Zhu. Southwest Hospital, Third Military Medical University: Xiao-Mei Xiang. Beijing Ditan Hospital, Capital Medical University: Qun Zhang.
Funding
This work was supported by the National Key R&D Program of China (no. 2017YFC0908104); the National Science and Technology Major Project of China (no. 2018ZX10732-202–001-007); the National Natural Science Foundation of China (no. 81970550); the Natural Science Foundation of Hunan Province, China (2019JJ40496); the National Natural Science Foundation of China (81700561). The Rapid Service Fees were funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Jun Chen, Ze-Bing Huang, Hai Li, Xin Zheng, Jin-Jun Chen, Xian-Bo Wang, Zhi-Ping Qian, Xiao-Xiao Liu, Xue-Gong Fan, Xing-Wang Hu, Cheng-Jin Liao, Li-Yuan Long and Yan Huang declare that they have no conflict of interest in this research.
Compliance with Ethics Guidelines
All patients gave written informed consent according to the Declaration of Helsinki. The study was approved by Xiangya Hospital Ethics Committee, Central South University, and the committees of each center. All documents approved by the Clinical Research Committee of Ren Ji Hospital Affiliated to Shanghai Jiao Tong University were sent to each institution and approved by each institution under the same IRB name. The National Clinical Trial number (NCT no.) is NCT02457637.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Jun Chen and Ze-Bing Huang contributed equally.
References
- 1.Asrani SK, Simonetto DA, Kamath PS. Acute-on-chronic liver failure. Clin Gastroenterol Hepatol. 2015;13:2128–2139. doi: 10.1016/j.cgh.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernaez R, Sola E, Moreau R, Gines P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541–553. doi: 10.1136/gutjnl-2016-312670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353–390. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gawande A, Gupta GK, Gupta A, Wanjari SJ, Goel V, Rathore V, Bhardwaj H, et al. Acute-on-chronic liver failure: etiology of chronic and acute precipitating factors and their effect on mortality. J Clin Exp Hepatol. 2019;9:699–703. doi: 10.1016/j.jceh.2019.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Premkumar M, Saxena P, Rangegowda D, Baweja S, Mirza R, Jain P, Bhatia P, et al. Coagulation failure is associated with bleeding events and clinical outcome during systemic inflammatory response and sepsis in acute-on-chronic liver failure: an observational cohort study. Liver Int. 2019;39:694–704. doi: 10.1111/liv.14034. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, Jaton K, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 7.Gustot T, Felleiter P, Pickkers P, Sakr Y, Rello J, Velissaris D, Pierrakos C, et al. Impact of infection on the prognosis of critically ill cirrhotic patients: results from a large worldwide study. Liver Int. 2014;34:1496–1503. doi: 10.1111/liv.12520. [DOI] [PubMed] [Google Scholar]
- 8.Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453–471. doi: 10.1007/s12072-014-9580-2. [DOI] [PubMed] [Google Scholar]
- 9.Kim TY, Kim DJ. Acute-on-chronic liver failure. Clin Mol Hepatol. 2013;19:349–359. doi: 10.3350/cmh.2013.19.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nesseler N, Launey Y, Aninat C, Morel F, Malledant Y, Seguin P. Clinical review: the liver in sepsis. Crit Care. 2012;16:235. doi: 10.1186/cc11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernsmeier C, Singanayagam A, Patel VC, Wendon J, Antoniades CG. Immunotherapy in the treatment and prevention of infection in acute-on-chronic liver failure. Immunotherapy. 2015;7:641–654. doi: 10.2217/imt.15.27. [DOI] [PubMed] [Google Scholar]
- 12.Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghoner A, Vidacek D, Siewert E, Bach J, et al. Patients with acute on chronic liver failure display "sepsis-like" immune paralysis. J Hepatol. 2005;42:195–201. doi: 10.1016/j.jhep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 14.Xue R, Zhu Y, Liu H, Meng Q. The clinical parameters for the diagnosis of hepatitis B virus related acute-on-chronic liver failure with sepsis. Sci Rep. 2019;9:2558. doi: 10.1038/s41598-019-38866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibot S, Kolopp-Sarda MN, Bene MC, Cravoisy A, Levy B, Faure GC, Bollaert PE. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med. 2004;141:9–15. doi: 10.7326/0003-4819-141-1-200407060-00009. [DOI] [PubMed] [Google Scholar]
- 16.Siranovic M, Kovac J, Gopcevic S, Kelecic M, Kovac N, Rode B, Vucic M. Human soluble TREM-1: lung and serum levels in patients with bacterial ventilator associated pneumonia. Acta Clin Croat. 2011;50:345–349. [PubMed] [Google Scholar]
- 17.Phua J, Koay ES, Zhang D, Tai LK, Boo XL, Lim KC, Lim TK. Soluble triggering receptor expressed on myeloid cells-1 in acute respiratory infections. Eur Respir J. 2006;28:695–702. doi: 10.1183/09031936.06.00005606. [DOI] [PubMed] [Google Scholar]
- 18.Adly AAM, Ismail EA, Andrawes NG, El-Saadany MA. Circulating soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) as diagnostic and prognostic marker in neonatal sepsis. Cytokine. 2014;65:184–191. doi: 10.1016/j.cyto.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Altay FA, Elaldi N, Şentürk GÇ, Altin N, Gözel MG, Albayrak Y, Şencan İ. Serum sTREM-1 level is quite higher in Crimean Congo hemorrhagic fever, a viral infection. J Med Virol. 2016;88:1473–1478. doi: 10.1002/jmv.24496. [DOI] [PubMed] [Google Scholar]
- 20.Chenevier-Gobeaux C, Borderie D, Weiss N, Mallet-Coste T, Claessens Y-E. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin Chim Acta. 2015;450:97–103. doi: 10.1016/j.cca.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Arai Y, Mizugishi K, Nonomura K, Naitoh K, Takaori-Kondo A, Yamashita K. Phagocytosis by human monocytes is required for the secretion of presepsin. J Infect Chemother. 2015;21:564–569. doi: 10.1016/j.jiac.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Memar MY, Alizadeh N, Varshochi M, Kafil HS. Immunologic biomarkers for diagnostic of EarlyOnset Neonatal Sepsis. J Matern Fetal Neonatal Med. 2017;32:143–153. doi: 10.1080/14767058.2017.1366984. [DOI] [PubMed] [Google Scholar]
- 23.Qi Z-J, Yu H, Zhang J, Li C-S. Presepsin as a novel diagnostic biomarker for differentiating active pulmonary tuberculosis from bacterial community acquired pneumonia. Clin Chim Acta. 2018;478:152–156. doi: 10.1016/j.cca.2017.12.045. [DOI] [PubMed] [Google Scholar]
- 24.Tsuji S, Kitatoube A, Kikuchi-Taura A, Oguro E, Shigesaka M, Okita Y, Shimizu T, et al. Elevated soluble CD14-subtype (PRESEPSIN; P-SEP) levels in rheumatoid arthritis (RA) patients with bacterial infection. Mod Rheumatol. 2017;27:718–720. doi: 10.1080/14397595.2016.1246119. [DOI] [PubMed] [Google Scholar]
- 25.Koakutsu T, Sato T, Aizawa T, Itoi E, Kushimoto S. Postoperative changes in presepsin level and values predictive of surgical site infection after spinal surgery: a single-center, prospective observational study. Spine (Phila Pa 1976) 2018;43:578–584. doi: 10.1097/BRS.0000000000002376. [DOI] [PubMed] [Google Scholar]
- 26.Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother. 2011;17:764–769. doi: 10.1007/s10156-011-0254-x. [DOI] [PubMed] [Google Scholar]
- 27.Behnes M, Bertsch T, Lepiorz D, Lang S, Trinkmann F, Brueckmann M, Borggrefe M, et al. Diagnostic and prognostic utility of soluble CD 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Crit Care. 2014;18:507. doi: 10.1186/s13054-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulla M, Pizzolato E, Lucchiari M, Loiacono M, Soardo F, Forno D, Morello F, et al. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: a multicenter prospective study. Crit Care. 2013;17(4):R168. doi: 10.1186/cc12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpio R, Zapata J, Spanuth E, Hess G. Utility of presepsin (sCD14-ST) as a diagnostic and prognostic marker of sepsis in the emergency department. Clin Chim Acta. 2015;450:169–175. doi: 10.1016/j.cca.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, et al. The third international consensus definition for sepsis and septic shock(sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruns T, Zimmermann HW, Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014;20:2542–2554. doi: 10.3748/wjg.v20.i10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arvaniti V, Gennaro D, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 34.Saïd-Sadier N, Ojcius DM. Alarmins, inflammasomes and immunity. Biomed J. 2012;35:437–449. doi: 10.4103/2319-4170.104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui J, Chen Y, Wang HY, Wang RF. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum Vaccine Immunother. 2014;10:3270–3285. doi: 10.4161/21645515.2014.979640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Tang BMP, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis. 2007;7:210–217. doi: 10.1016/S1473-3099(07)70052-X. [DOI] [PubMed] [Google Scholar]
- 38.Jaye DL, Waites KB. Clinical applications of C-reactive protein in pediatrics. Pediatr Infect Dis J. 1997;16:735–746. doi: 10.1097/00006454-199708000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi G, Shibata S, Ishikura H, Miura M, Fukui Y, Inoue Y, et al. Presepsin in the prognosis of infectious diseases and diagnosis of infectious disseminated intravascular coagulation: a prospective, multicenter, observational study. Eur J Anaesthesiol. 2015;32:199–206. doi: 10.1097/EJA.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 41.Cold F, Schiødt FV, Pott FC, Strandkjær N, Christensen E. Sepsis-related Organ Failure Assessment Score is a strong predictor of survival in acute-on-chronic liver failure. Danish Med J. 2019;66:5557. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.