Dear Editor,
Small, short-chain fatty acids (SCFAs) (acetic acid, propionic acid, and butyric acid: conjugate bases, acetate, propionate, and butyrate) as well as the alpha-hydroxy acid, L-lactic acid (conjugate base, L-lactate) are important energy substrates and signaling molecules in the central nervous system (CNS) [1, 2]. L-lactic acid is produced by glycolysis [3] and gut microbes [4] and is released in large quantities during exercise [5]. Within the CNS, L-lactate has been shown to be a rapid, excitatory signaling molecule [6–8], displaying both neuroprotective [9] and neurotoxic [10, 11] properties depending on concentration and time. The dysregulation of L-lactic acid/L-lactate metabolism in age-related cognitive decline [12] as well as certain neurodegenerative diseases [11–14] has been documented, however the contribution of L-lactate to the induced cellular pathologies in such diseases has yet to be explored. While studies have examined the role of L-lactate-induced cytotoxicity in ischemia/reperfusion injury, stroke, and traumatic brain injury, the mechanisms remain unclear. Thus, the bolstering of mechanistic insight into L-lactate signaling may advance targeted intervention. This may be especially beneficial in several neurodegenerative diseases, as the connections between elevated L-lactate and disease progression mechanisms have yet to be made. Given the evidence suggesting that L-lactate is an excitatory signaling molecule [6, 7], coupled with its documented elevation and/or dysregulation in several neurodegenerative diseases (multiple sclerosis [13], amyotrophic lateral sclerosis [14], and Alzheimer’s disease [15]) and age-related cognitive decline [12], we investigated the effects, if any, of pathophysiological concentrations of L-lactate on the development of neurodegeneration-like cellular pathologies. Specifically, we investigated L-lactate-induced excitotoxicity, the generation of reactive oxygen species (ROS), and changes in cytosolic Ca2+, and screened for alterations in gene expression known to be affected by neurodegenerative diseases.
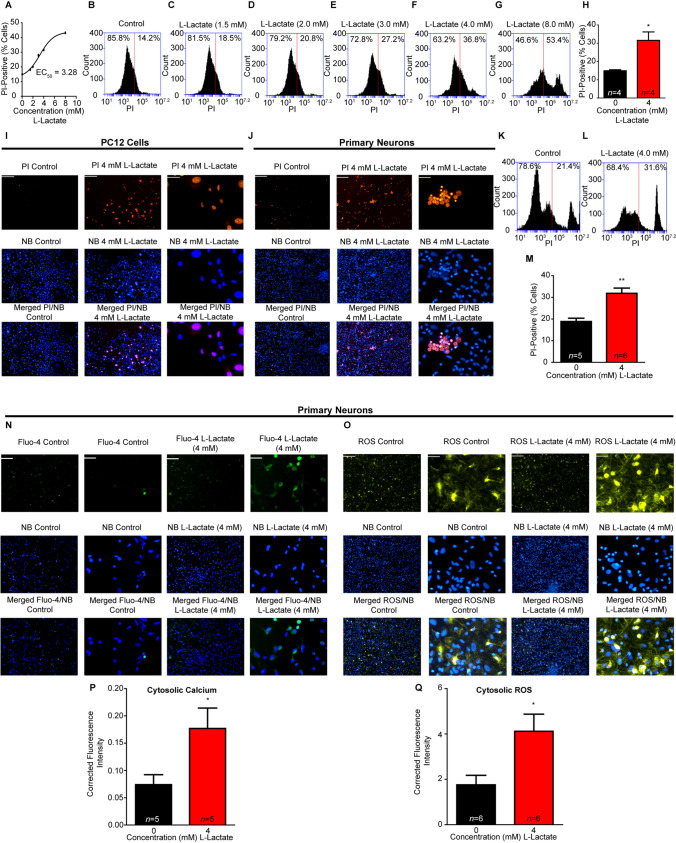
To construct a toxicity dose-response curve to L-lactate (Fig. 1A), nerve growth factor-induced PC12 cells were incubated with different concentrations of L-lactate (0–8 mM) for 4 h (Fig. 1B–G). Toxicity was determined using a propidium iodide (PI) exclusion assay [16] analyzed via flow-cytometry. From the dose-response curve, we calculated an EC50 value for L-lactate of 3.28 mM (Fig. 1A) and used 4 mM (Fig. 1H) for all subsequent experiments. Having an EC50 value, we then recapitulated the flow cytometry toxicity using real-time imaging in PC12 cells (Fig. 1I) and in primary neurons (Fig. 1J), as well as flow cytometric analysis in primary neurons (Fig. 1K–M).
Fig. 1.
Effects of L-lactate on excitotoxicity, cytosolic Ca2+, and ROS. A Dose-dependent response for the propidium iodide (PI) exclusion assay measured by flow cytometry for L-lactate treatment (4 h), EC50 = 3.28 mM. B–G Representative flow cytometry charts for increasing concentrations of L-lactate (0, 1.5, 2, 3, 4, and 8 mM). H L-lactate treatment (4 mM, 4 h) significantly (*P < 0.05, unpaired t-test) increases PC12 cytotoxicity compared to control. I PI and NucBlue (NB, Hoechst 33342 DNA stain) fluorescence imaging in PC12 cells for control treatment (4 h) (left, scale bars, 200 μm), and PC12 cells after L-lactate treatment (4 mM, 4 h) (middle, scale bars, 200 μm) and magnified images in PC12 cells after L-lactate treatment (right, scale bars, 50 μm). J PI and NB staining in primary neuronal cultures for control (left, scale bars, 200 μm), L-lactate-treated (4 mM) (middle, scale bars, 200 μm), magnified L-lactate-treated (right, scale bars, 50 μm). K, L Representative flow cytometry charts for primary neuronal toxicity PI assay, for baseline control (K) and L-lactate (4 mM) (L). M Summary data for L-lactate-induced excitotoxicity in primary neuronal cultures from flow cytometry. N L-lactate increases cytosolic Ca2+ in primary neuronal cultures; control (left, scale bars, 200 μm), magnified control (middle left, scale bars, 50 μm) for L-lactate-treated (4 mM) (middle right, scale bars, 200 μm) and magnified L-lactate-treated primary neuronal cultures (right, scale bars, 50 μm). O L-lactate increases cytosolic ROS in primary neuronal cultures; control (left, scale bars, 200 μm), magnified control (middle left, scale bars, 50 μm), L-lactate-treated (4 mM) (middle right, scale bars, 200 μm) and magnified L-lactate-treated primary neuronal cultures (right, scale bars, 50 μm). P Quantified cytosolic Ca2+ fluorescence intensity is significantly increased (*P < 0.05, unpaired t-test) in L-lactate-treated (4 mM) neurons compared to control (0 mM). Q Quantified ROS fluorescence intensity is significantly increased (*P < 0.05, unpaired t-test) in L-lactate-treated (4 mM) neurons compared to control (0 mM).
We then investigated what other major cellular pathologies are also present in neurodegenerative diseases besides excitotoxicity. Calcium dysregulation from excitotoxicity as well as increases in reactive oxygen species/oxidative stress (ROS) were among the most prominent cellular pathologies noted in neurodegenerative diseases. We then screened primary neuronal cultures for L-lactate-induced alterations in cytosolic Ca2+ and ROS using real-time imaging (Fig. 1N–O) and quantified the relative fluorescence intensity with ImageJ (NIH) (Fig. 1P–Q). L-lactate (4 mM) significantly increased both cytosolic Ca2+ and ROS compared to vehicle control.
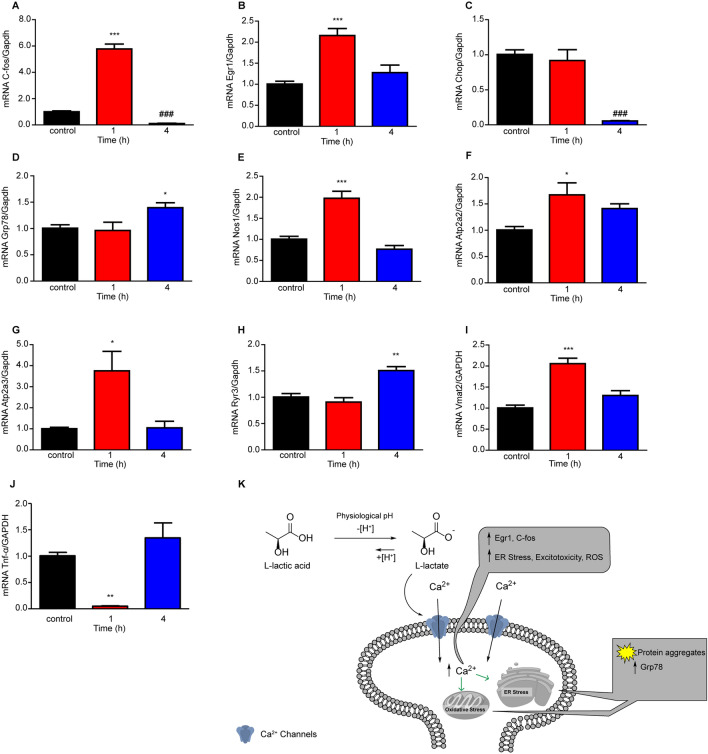
Given the finding that L-lactate increased neuronal cytosolic Ca2+ and ROS, we next determined whether there were any L-lactate induced alterations in gene expression related to Ca2+ or ROS signaling. We found significant changes in C-fos (Fig. 2A), a marker for neuronal activity, as well as the early growth response 1 (Egr1) gene (Fig. 2B), a Ca2+ -dependent marker for neuronal activity and synapse-dependent processes. Furthermore, the endoplasmic reticulum (ER) stress biomarkers, DNA damage inducible transcript 3 (Chop) and binding immunoglobulin protein (Grp78) as well as ER-Ca2+-dependent ATP pumps (Atp2a2 and Atp2a3) and the primarily brain ER-Ca2+ release ryanodine 3 (Ryr3) ion channel were significantly altered in response to L-lactate (Fig. 2). Regarding L-lactate increases in neuronal cytosolic ROS, neuronal NOS (Nos1) was also significantly upregulated (Fig. 2E). Our group had previously reported the SCFA acetate (6 mM) significantly increases tumor necrosis factor alpha (Tnf-α) in dopaminergic-like PC12 cells [16] and anticipated a similar effect with L-lactate (4 mM). Contrary to this hypothesis, L-lactate downregulated Tnf-α, suggesting a possible mechanism in the reduction of neuroinflammation, the extent of which is yet to be determined. Interestingly, we found significant upregulation of the vesicular monoamine transporter (Vmat2) in response to L-lactate, suggesting possible modulation of monoamines (dopamine, norepinephrine, epinephrine, serotonin, and histamine) which may be relevant to certain neurodegenerative diseases.
Fig. 2.
L-lactate (4 mM) increases expression of early response genes and activates the unfolded protein response in primary neuronal cultures (n = 4 wells per treatment). A C-fos gene expression is significantly upregulated (***P < 0.001) after L-lactate treatment (4 mM, 1 h) and significantly downregulated (###P < 0.001) after L-lactate treatment (4 mM, 4 h) compared to control [one-way ANOVA with Bonferroni post hoc analysis (F(2,9) = 191)]. B Early growth response 1 (Egr1) gene expression is significantly upregulated (***P < 0.001) after L-lactate treatment (4 mM, 1 h) compared to control [one-way ANOVA with Bonferroni post hoc analysis (F(2,9) = 16.86)]. C DNA damage-inducible transcript 3 (Chop) gene expression is significantly downregulated (###P < 0.001) after L-lactate treatment (4 mM, 4 h) compared to control [one-way ANOVA with Bonferroni post hoc analysis (F(2,9) = 29.5)]. D Binding immunoglobulin protein (Grp78) gene expression is significantly upregulated (*P < 0.05) after L-lactate treatment (4 mM, 4 h) compared to control [one-way ANOVA with Bonferroni post hoc analysis (F(2,9) = 4.56)]. E Nitric oxide synthase 1 (Nos1) is significantly upregulated (***P < 0.001) 1 h post L-lactate treatment (4 mM) compared to control [one-way ANOVA with Bonferroni post hoc analysis (F(2,9) = 31.22)]. F The SERCA-ATP pump, Atp2a2 is significantly upregulated (*P < 0.05) 1 h post L-lactate treatment (4 mM) compared to control [one-way ANOVA with Bonferroni post hoc analysis (F(2,9) = 5.25)]. G SERCA-ATP, Atp2a3 is significantly upregulated (*P < 0.05) at 1 h post L-lactate treatment (4 mM) compared to control [one-way ANOVA with Bonferroni post hoc analysis (F(2,9) = 7.73)]. H Ryanodine receptor 3 (Ryr3) is significantly upregulated (**P < 0.01) at 4 h post L-lactate treatment (4 mM) compared to control [one-way ANOVA with Bonferroni post hoc analysis (F(2,9) = 18.36)]. I L-lactate significantly increased Vmat2 mRNA expression (***P < 0.001) 1 h post lactate treatment compared to control [one-way ANOVA with Bonferroni post hoc analysis (F(2,9) = 25.89)]. J L-lactate significantly downregulated mRNA expression of the pro-inflammatory cytokine Tnf-α (**P < 0.01) at 1 h post L-lactate treatment compared to control [one-way ANOVA with Bonferroni post hoc analysis (F(2,9) = 15.94)]. K Hypothetical model for L-lactate-induced neurodegeneration-like cellular pathology.
In perspective, we investigated the effects of elevated and sustained L-lactate on many aspects of the cellular pathologies (Fig. 2K) associated with several neurodegenerative diseases. Like any signaling molecule (e.g., glutamate, GABA, dopamine, and glucose) too much or too little can result in severe cognitive impairment and health effects. Excess glutamate results in excitotoxicity, while uncontrolled diabetes and fluctuations in glucose levels contribute to severe cognitive impairment. We believe time and concentration are important aspects to keep in mind when investigating the impact of signaling molecules on neuronal and brain function. In this case and preparation, we linked what is seen clinically in several neurodegenerative disorders regarding elevated L-lactate concentrations with neurotoxicity and associated cellular pathologies.
We cannot however ignore the beneficial aspects of L-lactate as a signaling molecule and an energy source. Exercise increases circulating L-lactate levels and is prescribed for reducing the progression of many neurodegenerative diseases and age-related cognitive decline. Exercise induction of brain-derived neurotropic factor is thought to play a critical role in this rescue [17]. Furthermore, exercise-induced increases in monocarboxylate transporters (transporters of SCFAs and L-lactate) may play a critical role in the sequestration and utilization of L-lactate and thus maintain homeostatic circulating levels. This in turn may mitigate or reverse neurodegenerative disease progression. Conversely, impaired monocarboxylate transporters in ALS [18] has been reported whereby oligodendroglia are starved for energy (L-lactate and pyruvate), but we also speculate that this impaired transport of L-lactate may contribute to a vicious cycle of excitotoxic buildup of L-lactate. Similarly, mitochondrial dysfunction in neuropathological disease progression may precipitate excess L-lactate, further exacerbating neurodegenerative processes through excitatory signaling mechanisms.
In summary, we have demonstrated that primary neuronal cell cultures exposed to pathophysiological concentrations of L-lactate display many of the cellular pathologies associated with neurodegenerative disease. While portions of this information have been reported to a certain degree, the direct connection of L-lactate-specific pathologies to neurodegenerative disease had yet to be made. Here we showed that elevated L-lactate increased the subsequent: neuronal toxicity, cytosolic Ca2+, cytosolic ROS, and the mRNA expression of Egr1, C-fos, Grp78, Vmat2, Atp2a2, Atp2a3, and Ryr3, as well as downregulation of Chop and Tnf-α. Future studies will aim to translate the cellular mechanisms to in vivo mechanisms, coupled with behavioral models. This would include either intracerebral ventricular infusion of L-lactate to discern global brain effects or site-specific brain delivery (e.g., hippocampus infusion coupled with learning-memory tasks; and primary motor cortex, brainstem, and spinal cord infusion coupled with rotarod tasks). These studies would examine any L-lactate induced behavioral pathology associated with a certain neurodegenerative disease. Translatability studies to humans would include interventions aimed at normalizing L-lactate, among which exercise is one. Increasing L-lactate metabolism/sequestration or buffering pH may also prove beneficial. As some have suggested, reducing and/or regulating brain lactate levels in ischemia reperfusion injury and traumatic brain injury may promote neuronal survival and favorable outcomes [10, 11] and the same may be true regarding neurodegenerative diseases.
Footnotes
Andrew D. Chapp and Jessica E. Behnke have contributed equally to this work.
Contributor Information
Andrew D. Chapp, Email: achapp@umn.edu
Qing-Hui Chen, Email: qinghuic@mtu.edu.
References
- 1.Magistretti PJ, Allaman I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci. 2018;19:235–249. doi: 10.1038/nrn.2018.19. [DOI] [PubMed] [Google Scholar]
- 2.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogatzki MJ, Ferguson BS, Goodwin ML, Gladden LB. Lactate is always the end product of glycolysis. Front Neurosci. 2015;9:22. doi: 10.3389/fnins.2015.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruning J, Chapp A, Kaurala GA, Wang R, Techtmann S, Chen QH. Gut microbiota and short chain fatty acids: Influence on the autonomic nervous system. Neurosci Bull. 2020;36:91–95. doi: 10.1007/s12264-019-00410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodwin ML, Harris JE, Hernández A, Gladden LB. Blood lactate measurements and analysis during exercise: a guide for clinicians. J Diabetes Sci Technol. 2007;1:558–569. doi: 10.1177/193229680700100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang F, Lane S, Korsak A, Paton JF, Gourine AV, Kasparov S, et al. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat Commun. 2014;5:3284. doi: 10.1038/ncomms4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I, et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A. 2014;111:12228–12233. doi: 10.1073/pnas.1322912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin YN, Hu J, Wei YL, Li ZL, Luo ZC, Wang RQ, et al. Astrocyte-derived lactate modulates the passive coping response to behavioral challenge in male mice. Neurosci Bull. 2020 doi: 10.1007/s12264-020-00553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berthet C, Lei H, Thevenet J, Gruetter R, Magistretti PJ, Hirt L. Neuroprotective role of lactate after cerebral ischemia. J Cereb Blood Flow Metab. 2009;29:1780–1789. doi: 10.1038/jcbfm.2009.97. [DOI] [PubMed] [Google Scholar]
- 10.Kawamata T, Katayama Y, Hovda DA, Yoshino A, Becker DP. Lactate accumulation following concussive brain injury: the role of ionic fluxes induced by excitatory amino acids. Brain Res. 1995;674:196–204. doi: 10.1016/0006-8993(94)01444-M. [DOI] [PubMed] [Google Scholar]
- 11.Lama S, Auer RN, Tyson R, Gallagher CN, Tomanek B, Sutherland GR. Lactate storm marks cerebral metabolism following brain trauma. J Biol Chem. 2014;289:20200–20208. doi: 10.1074/jbc.M114.570978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross JM, Öberg J, Brené S, Coppotelli G, Terzioglu M, Pernold K, et al. High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc Natl Acad Sci U S A. 2010;107:20087–20092. doi: 10.1073/pnas.1008189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albanese M, Zagaglia S, Landi D, Boffa L, Nicoletti CG, Marciani MG, et al. Cerebrospinal fluid lactate is associated with multiple sclerosis disease progression. J Neuroinflammation. 2016;13:36. doi: 10.1186/s12974-016-0502-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang YJ, Fan DS. Elimination Rate of Serum Lactate is Correlated with Amyotrophic Lateral Sclerosis Progression. Chin Med J (Engl) 2016;129:28–32. doi: 10.4103/0366-6999.172561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liguori C, Stefani A, Sancesario G, Sancesario GM, Marciani MG, Pierantozzi M. CSF lactate levels, τ proteins, cognitive decline: a dynamic relationship in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2015;86:655–659. doi: 10.1136/jnnp-2014-308577. [DOI] [PubMed] [Google Scholar]
- 16.Chapp AD, Behnke JE, Driscoll KM, Fan Y, Hoban E, Shan Z, et al. Acetate Mediates Alcohol Excitotoxicity in Dopaminergic-like PC12 Cells. ACS Chem Neurosci. 2019;10:235–245. doi: 10.1021/acschemneuro.8b00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller P, Duderstadt Y, Lessmann V, Müller NG. Lactate and BDNF: Key mediators of exercise induced neuroplasticity? J Clin Med 2020, 9. [DOI] [PMC free article] [PubMed]
- 18.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]