Abstract
P2X3 monomeric receptors (P2X3Rs) and P2X2/3 heteromeric receptors (P2X2/3Rs) in primary sensory neurons and microglial P2X4 monomeric receptors (P2X4Rs) in the spinal dorsal horn (SDH) play important roles in neuropathic pain. In particular, P2X4R in the spinal microglia during peripheral nerve injury (PNI), experimental autoimmune neuritis, and herpes models are useful to explore the potential strategies for developing new drugs to treat neuropathic pain. Recently, novel P2X4 antagonists, NP-1815-PX and NC-2600, were developed, which demonstrated potent and specific inhibition against rodent and human P2X4Rs. The phase I study of NC-2600 has been completed, and no serious side effects were reported. The roles played by purinergic receptors in evoking neuropathic pain provide crucial insights into the pathogenesis of neuropathic pain.
Keywords: Neuropathic pain, P2X3, P2X2/3, P2X4, Primary sensory neurons, Microglia
Introduction
Acute nociceptive pain has physiological significance as a warning system under normal conditions. The primary sensory neurons in the dorsal root ganglion (DRG) are pseudo-monopolar cells having one short axonal process that is divided into two directions. One of them is distributed peripherally as a peripheral branch receiving inputs through receptors. The other branch becomes the dorsal root of the spinal cord and transmits impulses from the peripheral receptors to the spinal cord. The primary sensory neurons of DRG are composed of several types of neurons with or without myelin sheaths. Among them, Aδ-fibers, with myelin sheaths, and C-fibers, without sheath, conduct pain-related spikes to spinal dorsal horn (SDH) neurons. Painful stimuli evoke action potentials in the distal ends of the C-fibers or Aδ-fibers of DRG neurons, and these signals are conducted to the central ends of these DRG neurons and transmitted to the secondary sensory neurons in the SDH. When these signals finally reach the sensory cortex, pain sensation occurs. Evidence suggest that ionotropic P2XRs play important roles in pain signaling under normal conditions [1].
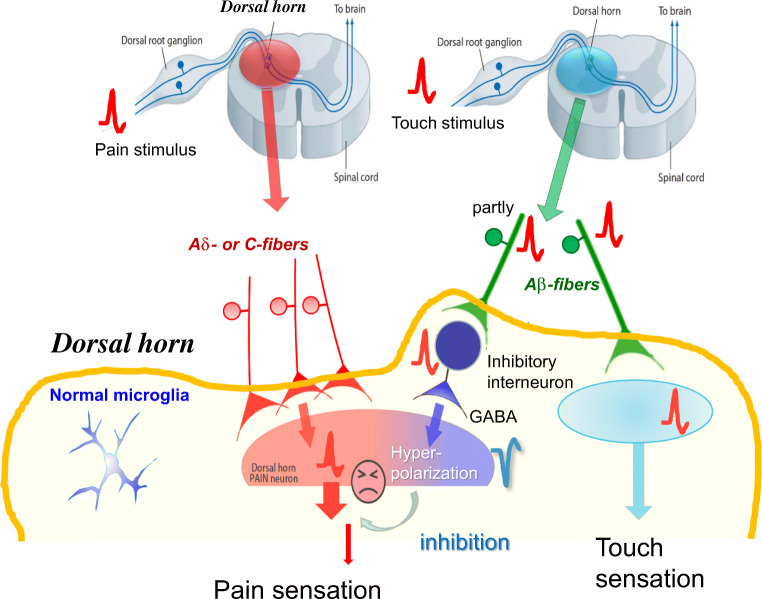
Touch stimuli evoke action potentials in the Aβ-fibers of DRG neurons, and these signals are transmitted to the sensory cortex, resulting in touch sensation. These action potentials can also be partially transmitted to the inhibitory interneurons in the SDH, resulting in the release of the inhibitory neurotransmitters, γ-aminobutyric acid (GABA), and glycine. GABA evokes the hyperpolarization of secondary neurons, which inhibits pain signaling. Thus, light touch stimuli do not cause pain sensations but, instead, inhibit pain signaling under normal conditions (Fig.1). However, under pathological condition light touch stimuli can cause painful sensations. Among pathological pain types, neuropathic pain is one of the most important because no fundamental drugs have been identified that can inhibit this pain. Neuropathic pain often develops when the nerves become damaged by trauma, due to accidents, surgical operations, diabetes, or infections, even after the tissue damage has healed [2–4]. The most characteristic symptom of neuropathic pain is tactile allodynia (an abnormal hypersensitivity to innocuous stimuli). Evidence has indicated that functional alterations in primary sensory neurons and in the SDH play very important roles in the pathogenesis of neuropathic pain after peripheral nerve injury (PNI) [2–4]. An increasing body of evidence has suggested that P2X3Rs and P2X2/3Rs in primary sensory neurons [5–9] and P2X4Rs in the SDH [10] have important roles in neuropathic pain, as described below.
Fig. 1.
Pain signaling under normal conditions
Painful stimuli evoke action potentials in the distal ends of C-fibers or Aδ-fibers of DRG neurons, and these signals are conducted to the central ends of these DRG neurons and transmitted to the secondary sensory neurons in the SDH. These signals finally reach to the sensory cortex resulting in pain sensation. Touch stimuli evoke action potentials in Aβ-fibers of DRG neurons, and these signals are transmitted to the sensory cortex, resulting in touch sensation. These action potentials can also be partially transmitted to the inhibitory interneurons in the SDH, resulting in the release of the inhibitory neurotransmitters, γ-aminobutyric acid (GABA), and glycine. GABA evokes the hyperpolarization of secondary neurons, which inhibits pain signaling.
The role of P2X3 or P2X2/3Rs in primary sensory neurons for evoking neuropathic pain
Although the messenger RNA (mRNA) and protein for all P2XRs, except for P2X7R, have reportedly been detected in primary sensory neurons [11], electrophysiological studies showed that homomeric P2X3R and heteromeric P2X2R and P2X3R (P2X2/3R) are the predominant functional P2XRs in these neurons [12, 13].
Cytosolic phospholipase A2 (cPLA2) is a key enzyme for the generation of arachidonic acid and subsequent lipid mediators [14, 15]. Spinal nerve injuries increase the level of phosphorylated cPLA2 (phospho-cPLA2) levels, which represents the active form [16] of cPLA2. In DRG neurons, phospho-cPLA2 localizes in the vicinity of plasma membrane [17], which is not a major target of cPLA2 [18, 19]. The number of DRG neurons displaying redistributed phospho-cPLA2 progressively increases after PNI, and the time-course of this increase is correlated well with the strength of tactile allodynia [17]. A selective inhibitor for cPLA2 can suppress both the number of DRG neurons with redistributed phospho-cPLA2 and allodynia after PNI.
Recent evidence has indicated that P2XRs in DRG neurons are involved in the development of neuropathic pain [20–22] and that ATP causes an increase in intracellular Ca2+ levels in DRG neurons [23]. Extracellular ATP has also been shown to cause increase the level of phospho-cPLA2 in cultured DRG neurons [17]. Treatment with αβ-methylene ATP (αβmeATP), a P2X1R and P2X3R agonist [24], increases the level of phospho-cPLA2. ATP-induced cPLA2 phosphorylation can be prevented by A-317491, a potent and selective P2X3R and P2X2/3R antagonist [25], and the ATP- and αβmeATP-mediated increases in cPLA2 can be inhibited by a cPLA2α inhibitor [17]. In an in vivo experiment, A-317491 significantly decreased the number of DRG neurons displaying the redistribution of phospho-cPLA2 and inhibited tactile allodynia after PNI [17]. These data indicated that tactile allodynia after PNI depends on the unique activation of cPLA2, through the stimulation of P2X3Rs and P2X2/3Rs, in damaged DRG neurons.
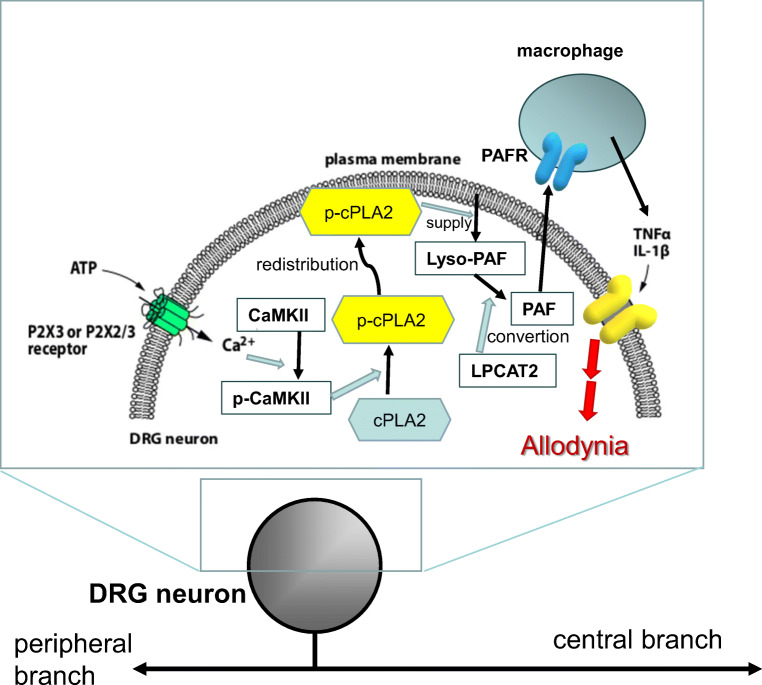
Determining which lipid mediators are associated with the development of tactile allodynia through the downstream activation of signal cascades following cPLA2 activation is the next step. One potential candidate is the platelet-activating factor (PAF) because the pharmacological blockade of PAF receptors (PAFRs) has been shown to reduce tactile allodynia after PNI [26]. PAFR mRNA expression is increased in DRG macrophages ipsilateral to PNI. Mice lacking PAFRs show a reduction of tactile allodynia after PNI, and a marked suppression of upregulation of tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β) in the injured DRG [26]. TNFα and IL-1β are well-known pro-inflammatory cytokines associated with pain hypersensitivity [27, 28]. Moreover, a single injection of PAF causes tactile allodynia, in a dose-dependent manner, and an increase in the expression of mRNAs for TNFα and IL-1β [26]. These results indicate that the PAF/PAFR system may play an important role in the production of TNFα and IL-1β in the DRG and tactile allodynia following PNI (Fig.2).
Fig. 2.
Schema of the proposed mechanism for the P2X3R- and P2X2/3Rs-involved PAF/PAFR system-mediated tactile allodynia after PNI
In DRG neurons, ATP stimulates P2X3Rs and P2X2/3Rs to increase internal concentration of Ca2+ after PNI, leading activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) which phosphorylates cPLA2. Phosphorylated cPLA2 (phospho-cPLA2) localizes in the vicinity of plasma membrane to cut out lyso-PAF from the cell membrane, which in turn converts into PAF by the lyso-PAF-acetyltransferase (LPCAT2). In macrophages, stimulation of PAFR by PAF may lead to produce and release of pro-inflammatory cytokines, TNFα and IL-1β. These cytokines may increase the excitability of DRG neurons that link to PNI-induced tactile allodynia
The role of P2X4Rs in activated DH microglia in neuropathic pain
PNI causes tactile allodynia in rats and mice in which the DH microglia have been activated. Activated microglia over-express P2X4Rs exclusively in the DH after PNI, and P2X4R knockout animals do not show tactile allodynia after PNI [29, 30]. Tactile allodynia after PNI can also be inhibited by a P2X4R blocker [29].These results indicated that tactile allodynia after PNI depends on microglial P2X4R activity.
ATP stimulates microglial P2X4Rs resulting in the synthesis and release of brain-derived neurotrophic factor (BDNF) from activated microglia [30, 31]. BDNF binds to transmembrane tyrosine kinase B (TrkB) in secondary sensory neurons, triggering the downregulation of the KCC2 potassium-chloride transporter, resulting in the increased concentration of intracellular chloride ions [Cl−]i, and causing a depolarizing shift in the anion reversal potential (Eanion) [32]. PNI or the injection of ATP stimulated microglia into the DH of animal models causes a similar shift of the Eanion and tactile allodynia [32].
Extracellular ATP in the SDH stimulates microglia, which play essential roles in neuropathic pain after PNI. However, which cells release ATP within the spinal cord remains unknown. Recently, the vesicular nucleotide transporter (VNUT) in DH neurons has been identified as a key molecule associated with ATP release and neuropathic pain [33]. The extracellular ATP contents ([ATP]e) of the DH, VNUT expression, and pain hypersensitivity all increase after PNI in wild-type mice, while the [ATP]e increase and tactile allodynia are prevented in VNUT-deficient mice [33]. The increase of spinal [ATP]e and tactile allodynia are inhibited only in mice with the specific deletion of VNUT in DH neurons, not in mice with the specific deletion of VNUT in primary sensory neurons, microglia, or astrocytes after PNI [33]. DH neurons can be classified as inhibitory and excitatory neurons. Recent findings have shown that inhibitory interneurons may be crucial for the release of ATP because the increase of spinal [ATP]e could be suppressed in mice lacking VNUT in inhibitory neurons, which corresponds with the suppression of tactile allodynia after PNI [10]. Thus, the VNUT-dependent release of ATP from DH inhibitory neurons may be important for evoking tactile allodynia in neuropathic pain.
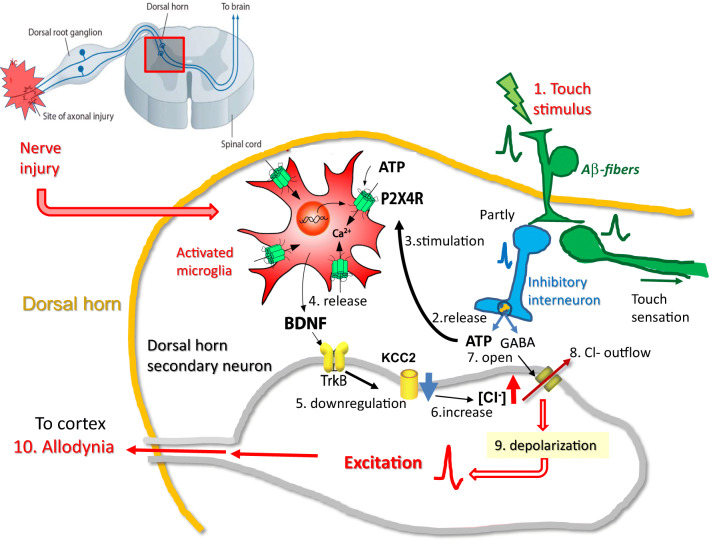
By combining the above data, the following hypothesis has been developed. Under pathological condition, touch stimulation causes ATP and GABA release from the inhibitory interneurons of the DH. Released ATP stimulates microglial P2X4Rs, resulting in BDNF secretion, which acts on secondary neurons to increase [Cl−]i.. Furthermore, released GABA affects the Cl− channels of secondary neurons, leading to increased Cl− outflow, which depolarize these neurons to evoke action potentials that reach the cortex, causing innocuous touch stimuli to be mistakenly recognized as pain; this hypothesis describes a potential mechanism for allodynia (Fig.3).
Fig.3.
A hypothesis of the mechanism of allodynia involving microglial P2X4Rs PNI
activates spinal microglia to overexpress P2X4Rs. In this pathological condition, touch stimuli (1) cause ATP and GABA release (2) from inhibitory interneurons of the DH. Released ATP stimulates (3) microglial P2X4Rs to secrete BDNF (4) which acts on secondary neurons to increase [Cl−]i(6). Furthermore, released GABA affects the Cl− channels of secondary neurons (7), leading to increase Cl− outflow (8), resulting in depolarizing these neurons to evoke action potentials (9). These spikes reach the cortex and evoke pain (10). In this way, innocuous touch stimuli are mistakenly recognized as pain
Clinical significance of P2XRs in pain
These results have indicated that microglial P2X4Rs play central roles in the pathogenesis of tactile allodynia associated with neuropathic pain [10]. P2X4R antagonists may serve as therapeutic agents against neuropathic pain in humans. Herpes zoster, which is characterized by clustered blisters, strong pain, and tactile allodynia, is caused by the reactivation of the varicella-zoster virus in the sensory ganglia [34]. Recent studies have shown that herpes simplex virus type 1 (HSV-1) inoculated into mouse skin caused DRG neuron damage, allodynia, and the activation of spinal microglia [35–37]. The expression of P2X4R mRNA in HSV-1 inoculated mice increased progressively in microglia [38]. A novel and selective P2X4R antagonist, NP-1815-PX, was able to inhibit tactile allodynia in a model of herpetic pain [38]. These results indicated that P2X4Rs in the spinal microglia play important roles in the allodynia associated with herpetic pain and that P2X4R antagonists may represent good candidates for therapeutic agents against herpetic neuropathic pain.
Guillain–Barré Syndrome (GBS) is an acute inflammatory demyelinating disorder, in which the immune system attacks portions of the peripheral nervous system. Neuropathic pain develops in many GBS patients. The autoimmune experimental neuritis method of immunization using a peptide derived from peripheral nerve myelin has been used to generate an animal model of GBS. In this model, the number of microglial cells and P2X4R expression levels continuously increase, in parallel with the clinical score [39, 40]. The time-course of tactile allodynia in this model is similar to those observed for microglial activation and P2X4R expression. Paroxetine, an antidepressant with P2X4R antagonistic effects [41], inhibits this allodynia. These results indicated that P2X4Rs in spinal microglia play a primary role in evoking allodynia in a rat model of GBS and that a P2X4R antagonist might represent a therapeutic agent against neuropathic pain associated with GBS [39].
Recently, gefapixant, a selective P2X3 and P2X2/3 receptor antagonist, was reported to inhibit the pain of rat models of hypersensitivity and neuropathic sensation [42], and reduce cough reflex sensitivity of patients with chronic cough in a phase II study [43]. In the phase II study, dysgeusia was the most frequent adverse event that can be easily inferred from the report that P2X3 is involved in the transmission of taste signaling [44]. Since homomeric P2X4R is thought not to be involved in the taste signaling, it is speculated that P2X4R antagonists may not evoke dysgeusia if they are used as anti-pain drugs in human.
Conclusions
An increasing body of evidence has suggested that P2X3Rs and P2X2/3Rs in primary sensory neurons and microglial P2X4Rs in the SDH play important roles in neuropathic pain. In particular, P2X4Rs in the spinal microglia are active after PNI in autoimmune experimental neuritis and herpes animal models, which may indicate potential strategies for developing new drugs against neuropathic pain. Spinal microglia also express other purinergic receptors, including P2X7R, P2Y12R, and P2Y6R, which show interesting functions related to neuropathic pain. The roles played by purinergic receptors in microglia and DRG neurons for the evocation of neuropathic pain provide crucial insights into the pathogenesis of allodynia and suggest potential strategies for the development of novel neuropathic pain treatments.
In memory of Geoffrey Burnstock (Fig.4)
Fig. 4.
Photos in memories with Geoffrey Burnstock
In 2003, when the Japan Purine Club was officially launched, at the 8th Japan Purine Meeting, Geoff was very pleased and participated in the Tokyo meeting to support us. While I was preparing the 2012 International Purine Meeting which was to be held in Fukuoka Japan, a very strong earthquake hit our country, in 2011, and a nuclear power plant suffered catastrophic damage. Although many researchers around the world wondered whether the meeting could be held in Fukuoka, Geoff strongly encouraged me to keep the meeting in Fukuoka. Geoff had visited Fukuoka many times and he was familiar with the charming character of Fukuoka. I wrote a welcome message indicating that Fukuoka is located 1075 km from the Fukushima Power Plant, a distance similar to that between the Chernobyl Nuclear Power Plant and Prague or Vienna, and further than the distance between London and Milan (940 km). This description seemed to succeed in calming the anxieties of many people, and the meeting was attended by approximately 200 participants from overseas. At a social gathering, we rented a tour boat in Fukuoka Bay, and Prof. Fusao Kato’s piano performance on the boat was enjoyed by everyone. The meeting was success due to Geoff’s powerful support.
In purinergic signaling field, I feel as though we are sailing a ship that has lost its compass. That should not be the case, so we have to fill the large hole left in Geoff’s absence and continue to develop research in this area.
Geoff, thank you very much for your continued support. Please rest peacefully in heaven while watching and protecting us.
Funding
This work was supported partly by JSPS KAKENHI Grant Numbers 25117013 and the Core Research for Evolutional Science and Technology (AMED-CREST) program from Japan Agency for Medical Research and Development. I also thank Drs. Makoto Tsuda, Hidetoshi Tozaki-Saitoh, Takahiro Masuda and many students for the experiments. I thank Lisa Giles, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for suggesting to edit a draft of this manuscript.
Compliance with ethical standards
Conflict of interest
Kazuhide Inoue declares that he/she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article is part of the Topical Collection on A Tribute to Professor Geoff Burnstock.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110:433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Aldskogius H, Kozlova EN. Central neuron–glial and glial–glial interactions following axon injury. Prog Neurobiol. 1998;55:1–26. doi: 10.1016/s0301-0082(97)00093-2. [DOI] [PubMed] [Google Scholar]
- 3.Carson MJ. Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia. 2002;40:218–231. doi: 10.1002/glia.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eikelenboom P, Bate C, Van Gool WA, Hoozemans JJ, Rozemuller JM, Veerhuis R, Williams A. Neuroinflammation in Alzheimer’s disease and prion disease. Glia. 2002;40:232–239. doi: 10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- 5.Colburn RW, Rickman AJ, Deleo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- 6.Sweitzer S, Martin D, Deleo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103:529–539. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- 7.Garden GA. Microglia in human immunodeficiency virusassociated neurodegeneration. Glia. 2002;40:240–251. doi: 10.1002/glia.10155. [DOI] [PubMed] [Google Scholar]
- 8.Inoue K. P2 receptors and chronic pain. Purinergic Signal. 2007;3:135–144. doi: 10.1007/s11302-006-9045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho T, Chaban VV. Expression of P2X3 and TRPV1 receptors in primary sensory neurons from estrogen receptors-α and estrogen receptor-β knockout mice. Neuroreport. 2012;23:530–534. doi: 10.1097/WNR.0b013e328353fabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci. 2018;19:138–152. doi: 10.1038/nrn.2018.2. [DOI] [PubMed] [Google Scholar]
- 11.Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rae MG, Rowan EG, Kennedy C. Pharmacological properties of P2X3-receptors present in neurones of the rat dorsal root ganglia. Br J Pharmacol. 1998;124:176–180. doi: 10.1038/sj.bjp.0701803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueno S, Tsuda M, Iwanaga T, Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. Br J Pharmacol. 1999;126:429–436. doi: 10.1038/sj.bjp.0702319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 15.Hirabayashi T, Shimizu T. Localization and regulation of cytosolic phospholipase a(2) Biochim Biophys Acta. 2000;1488:124–138. doi: 10.1016/s1388-1981(00)00115-3. [DOI] [PubMed] [Google Scholar]
- 16.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa S, Kohro Y, Tsuda M, Inoue K. Activation of cytosolic phospholipase A2 in dorsal root ganglion neurons by Ca2+/calmodulin-dependent protein kinase II after peripheral nerve injury. Mol Pain. 2009;5:22. doi: 10.1186/1744-8069-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schievella AR, Regier MK, Smith WL, Lin LL. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J Biol Chem. 1995;270:30749–30754. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- 19.Stahelin RV, Rafter JD, Das S, Cho W. The molecular basis of differential subcellular localization of C2 domains of protein kinase C-alpha and group IVa cytosolic phospholipase A2. J Biol Chem. 2003;278:12452–12460. doi: 10.1074/jbc.M212864200. [DOI] [PubMed] [Google Scholar]
- 20.Barclay J, Patel S, Dorn G, Wotherspoon G, Moffatt S, Eunson L, Abdel'al S, Natt F, Hall J, Winter J, Bevan S, Wishart W, Fox A, Ganju P. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J Neurosci. 2002;22:8139–8147. doi: 10.1523/JNEUROSCI.22-18-08139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honore P, Mikusa J, Bianchi B, McDonald H, Cartmell J, Faltynek C, Jarvis MF. TNP-ATP, a potent P2X(3) receptor antagonist, blocks acetic acid- induced abdominal constriction in mice: comparison with reference analgesics. Pain. 2002;96:99–105. doi: 10.1016/s0304-3959(01)00434-1. [DOI] [PubMed] [Google Scholar]
- 22.Tsuda M, Koizumi S, Kita A, Shigemoto Y, Ueno S, Inoue K. Mechanical allodynia caused by intraplantar injection of P2X receptor agonist in rats: involvement of heteromeric P2X2/3 receptor signaling in capsaicin-insensitive primary afferent neurons. J Neurosci. 2000;20:RC90. doi: 10.1523/JNEUROSCI.20-15-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouvier MM, Evans ML, Benham CD. Calcium influx induced by stimulation of ATP receptors on neurons cultured from rat dorsal root ganglia. Eur J Neurosci. 1991;3:285–291. doi: 10.1111/j.1460-9568.1991.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 24.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 25.Jarvis MF, Khakh BS. ATP-gated P2X cation-channels. Neuropharmacol. 2009;56:208–215. doi: 10.1016/j.neuropharm.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa S, Kohro Y, Shiratori M, Ishii S, Shimizu T, Tsuda M, Inoue K. Role of PAF receptor in proinflammatory cytokine expression in the dorsal root ganglion and tactile allodynia in a rodent model of neuropathic pain. PLoS One. 2010;5:e10467. doi: 10.1371/journal.pone.0010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 30.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29:3518–3528. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 33.Masuda T, Ozono Y, Mikuriya S, Kohro Y, Tozaki-Saitoh H, Iwatsuki K, Uneyama H, Ichikawa R, Salter MW, Tsuda M, Inoue K. Dorsal horn neurons release extracellular ATP in neuropathic pain in a VNUT-dependent manner. Nat Commun. 2016;7:12529. doi: 10.1038/ncomms12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loeser JD. Herpes zoster and postherpetic neuralgia. Pain. 1986;25:149–164. doi: 10.1016/0304-3959(86)90089-8. [DOI] [PubMed] [Google Scholar]
- 35.Takasaki I, Andoh T, Shiraki K, Kuraishi Y. Allodynia and hyperalgesia induced by herpes simplex virus type-1 infection in mice. Pain. 2000;86:95–101. doi: 10.1016/s0304-3959(00)00240-2. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki A, Inomata Y, Serizawa K, Andoh T, Kuraishi Y. Contribution of sensory C-fiber neuron injury to mechanical dynamic allodynia in a murine model of postherpetic neuralgia. Neuroreport. 2013;24:137–141. doi: 10.1097/WNR.0b013e32835df4d9. [DOI] [PubMed] [Google Scholar]
- 37.Takasaki I, Taniguchi K, Komatsu F, Sasaki A, Andoh T, Nojima H, Shiraki K, Hsu DK, Liu FT. Kato I et al.:contribution of spinal galectin-3 to acute herpetic allodynia in mice. Pain. 2012;153:585–592. doi: 10.1016/j.pain.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Matsumura Y, Yamashita T, Sasaki A, Nakata E, Kohno K, Masuda T, Tozaki-Saitoh H, Imai T, Kuraishi Y, Tsuda M, Inoue K. A novel P2X4 receptor-selective antagonist produces anti-allodynic effect in a mouse model of herpetic pain. Sci Rep. 2016;6:32461. doi: 10.1038/srep32461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imai T, Nakata E, Inoue K. Inhibition of P2X4 receptor on spinal microglia attenuates mechanical allodynia in experimental autoimmune neuritis rats. Pain Res. 2012;27:27–36. [Google Scholar]
- 40.Zhang Z, Zhang ZY, Fauser U, Schluesener HJ. Mechanical allodynia and spinal up-regulation of P2X4 receptor in experimental autoimmune neuritis rats. Neuroscience. 2008;152:495–501. doi: 10.1016/j.neuroscience.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 41.Nagata K, Imai T, Yamashita T, Tozaki-Saitoh H, Tsuda M, Inoue K. Antidepressants inhibit P2X4 receptor function: a possible involvement in neuropathic pain relief. Mol Pain. 2009;5:20. doi: 10.1186/1744-8069-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards D, Gever JR, Ford AP, Fountain SJ. Action of MK-7264 (Gefapixant) at human P2X3 and P2X2/3 receptors and in vivo efficacy in models of sensitization. Br J Pharmacol. 2019;176:2279–2291. doi: 10.1111/bph.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morice AH, Kitt MM, Ford AP, Tershakovec AM, Wu WC, Brindle K, Thompson R, Thackray-Nocera S, Wright C. The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J. 2019;54:1900439. doi: 10.1183/13993003.00439-2019. [DOI] [PubMed] [Google Scholar]
- 44.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]