Abstract
Experimental experience suggests that microbial agents including probiotics and prebiotics (representative microbial agents) play a critical role in defending against respiratory virus infection. We aim to systematically examine these agents’ effect on respiratory viral infection and encourage research into clinical applications. An electronic literature search was conducted from published data with a combination of a microbial agents search component containing synonyms for microbial agents-related terms and a customized search component for respiratory virus infection. Hazard ratio (HR), risk ratio (RR) and standard deviation (SD) were employed as effect estimates. In 45 preclinical studies, the mortality rates decreased in the respiratory viral infection models that included prebiotics or prebiotics as interventions (HR: 0.70; 95% confidence interval (CI): 0.56–0.87; P=0.002). There was a significant decrease in viral load due to improved gut microbiota (SD: −1.22; 95% CI: −1.50 to −0.94; P<0.001). Concentrations of interferon (IFN)-α (SD: 1.05; 95% CI: 0.33–1.77; P=0.004), IFN-γ (SD: 0.83; 95% CI: 0.01–1.65; P=0.05) and interleukin (IL)-12 (SD: 2.42; 95% CI: 0.32–4.52; P=0.02), IL-1β (SD: 0.01; 95% CI: −0.37 to 0.40; P=0.94) increased, whereas those of TNF-α (SD: −0.58; 95% CI: −1.59 to 0.43; P=0.26) and IL-6 (SD: −0.59; 95% CI: −1.24 to 0.07; P=0.08) decreased. Six clinical studies had lower symptom scores (SD: −0.09; 95% CI: −0.44 to 0.26; P=0.61) and less incidence of infection (RR: 0.80; 95% CI: 0.64–1.01; P=0.06). Our research indicates that probiotics and prebiotics pose a defensive possibility on respiratory viral infection and may encourage the clinical application.
Keywords: Antivirus, Gut microbiota, Gut-lung axis, Prebiotics, Probiotics, Viral pneumonia
Background
Annually, approximately 200 million people experience viral community-acquired pneumonia (CAP) worldwide [1], 24.5% of which were infected with respiratory syncytial virus (RSV), influenza virus and rhinovirus among the most prevalent types [2]. According a review in 2015, an estimated 300–500 million severe cases of the CAP are caused by infection with influenza virus, whereas nearly 14000 in-hospital deaths are related to RSV infection [3]. The emergence of the infectious diseases especially severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) caused by coronavirus, has led to the life-threatening nature of viral pneumonia.
More recently, novel coronavirus pneumonia (COVID-19) has become a pandemic catastrophe, with a fatality rate rising to 49% in critical cases [4]. At the time of this analysis, updated by the World Health Organization (WHO) for the COVID-19 pandemic, the pandemic caused by the SARS-CoV-2 coronavirus infection had brought over 1.9 million deaths globally out of 88 million [5]. Depending on the host’s immunocompetence, the severity of this respiratory disease can vary from mild symptoms to fatal complications, such as acute respiratory distress and multiple organ dysfunction [6]. However, current strategies to combat SARS-CoV-2 are far from satisfactory. Other than the two neuraminidase inhibitors: acyclovir and ganciclovir, there are few efficacious and efficient antiviral agents.
Preclinical research on the pathology of the viral pneumonia has shown that microbial agents including probiotics (exogenous salutary bacteria like Bifidobacterium) and prebiotics (indigestible substance promoting the growth of salutary bacteria, such as oligosaccharides) may modulate the composition of gastrointestinal flora and provide beneficial effects for patients with respiratory diseases, attributing to the gut–lung axis theory. When mice were manually exposed to a germ-free state through antibiotic treatment, both virus-specific CD4 and CD8 T cells and the influenza-specific antibody levels were markedly decreased, leading to a delay of the invasive virus elimination [7]. It was observed that the population of intestinal Bacteroidetes was significantly increased while the content of Firmicutes was decreased in murine models of RSV. By administering probiotic supplements, the incidence of upper respiratory tract infections was reduced and the disease duration was shortened. According to the microbiota–gut–lung axis theory, the gut microbiota plays the critical role in response to viral lung infections [8].
So far, the studies on probiotics and prebiotics treatments for respiratory viral infections failed to yield the expected results. Thus, in the present study, we proposed and evaluated the existing scientific evidence and credible results from the published preclinical and clinicalstudies, aiming to provide some useful information and suggestions for the future studies of stopping the rampant respiratory virus infection.
Methods
Search strategy
We conducted a meta-analysis following the recommended Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. We searched for the published studies up to December 2020 in PubMed, the Cochrane Library, Embase, the China National Knowledge Infrastructure, the Chinese Biomedical Literature Database and VIP databases, using combinations of a microbial agents search component containing synonyms for microbial agents-related terms and a customized search component for viral pneumonia. Retrieved articles were exported to an EndNote file. After removing duplicates, we screened the titles, abstracts and full texts according to the study selection criteria. There was no restriction for language, and a consensus was reached by mutual discussion.
Study selection
We included only the randomized controlled studies that investigated directly the effects of microbial agents on the respiratory viral infections. We excluded the articles that reported only the preventive effects on respiratory infection events which also contained bacteria-related events but did not specifically refer to the viral infection. We also excluded the comments, letters and other similar articles from which no relevant data could be extracted. There were no restrictions for specific variables such as different virus species and types of microbial agents.
Quality assessment and data extraction
To assess the quality for the studies included, we applied the Cochrane Risk Assessment Scale to the clinical studies, and used SYRCLE’s risk of bias tool (RoB) for animal studies. The following general information from the studies were extracted: first author, publication year, age, sex, animal species, sample size, condition at baseline, respiratory virus pathogens, intervention (type, dose, duration), control, samples collected for outcome evaluation and effect estimates. Disagreements between the authors during the data abstraction were resolved by referring to the original article.
Statistical analysis
We evaluated the heterogeneity between study-specific estimates using the I2 statistic and Cochran’s Q test, for which an I2 value >50% or a P-value <0.10 was considered as significant heterogeneity. Considering the significant differences in study design among the selected studies, we pooled data using a random-effects model. In cases with significant heterogeneity, we carried out subgroup and sensitivity analyses if permitted. We also estimated the publication bias using Egger’s test. The analyses for preclinical and clinical trials were conducted separately. We extracted data separately for evaluation from various sampling locations, microbial agents types and viral species in the individual studies. We employed the software applications GetData 2.20, RevMan 5.3 and Stata 12.0 for the data extraction and synthesis. For all statistical procedures (except for heterogeneity), we defined a P-value of <0.05 as statistically significant.
Results
Literature search
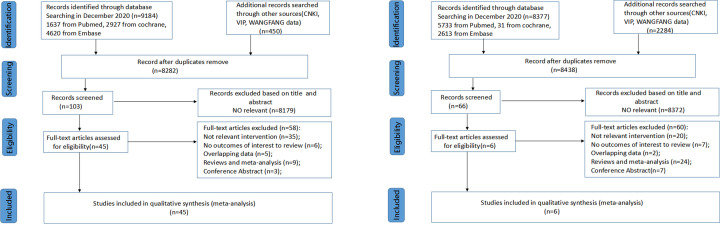
As shown in Figure 1A,B, the broad database searches resulted in 9634 preclinical and 10661 clinical hits, which we reduced to 8282 and 8438, respectively, after removing duplicates. After screening separately the titles and abstracts meeting with the protocol eligibility criteria, we included preclinical 104 and clinical 65 articles, respectively. After examining the full text, only 45 preclinical and 6 clinical studies were eligible for the data extraction and final evaluation.
Figure 1. Flow diagram according to the PRISMA protocol.
We retrieved related articles from databases and included preclinical studies (A) and clinical studies (B).
Study characteristics
Preclinical studies
Among the 45 selected studies (Table 1), most of them (42) were conducted using mice model; only one using chickens, one using preweaned dairy calves [9] and one using pigs [10–12]. Among the challenge viruses, influenza A had the highest frequency, followed by RSV. In terms of microbial agents, the included animal studies mainly determined the effects of administering prebiotics and probiotics on respiratory viral infection, in which different soluble oligosaccharides were regarded as prebiotics, and live or heat-inactivated lactic acid bacteria were selected as probiotics. The datasets from two studies that used ribavirin (a broad-spectrum antiviral drug) were combined as an additional control group [13,14], distinguishing them from the other studies just employing phosphate saline buffer or saline.
Table 1. Study characteristics of included preclinical studies investigating the effect of probiotics and prebiotics on respiratory viral infection.
| Author, year | Animal model | Total sample size | Virus infection | Intervention of experimental group | Duration | Comparison | Outcome |
|---|---|---|---|---|---|---|---|
| Iwabuchi, 2011 | SPF female BALB/c mice, 4 weeks old | 20 | Intranasally with 5 × 106 pfu of IFV A/PR/8/34 (H1N1) | Lyophilized BB536 (Bifidobacterium longum) at a dose of 2109 colony forming unit/0.2 ml/mouse | 2 weeks | Saline | Symptom score↓, body weight loss↓, virus titer↓, IFN-γ↓, IL-6↓, IL-10↑ |
| Kim, 2018 | Male BALB/c mice, 8 weeks old | 24 | A/Puerto Rico/8/34 H1N1 (PR8) strain of IAV | Heat-treated Lactobacillus plantarum nF1-fortified yogurt 200 μl each day | 21 days | PBS solution | Spleen index↑, thymus index↑, IFN-α↓, IL-2↑, IL-12↑, Klrb1, CD69, NK cell cytotoxicity↑, IL-1β mRNA↑, IL-6 mRNA↑, survival rate |
| Kiso, 2013 | Female BALB/c mice, 6 weeks old | 145 | Influenza A virus (A/California/04/2009 (CA04) virus) | Heat-killed Lactobacillus pentosus b240 a dose of 10 mg/mouse | 5 weeks | Saline | Survival rate↑, body weight↑, IL-10↑, GM-CSF, IL-5↑, G-CSF |
| Kobayashi, 2011 | Female BALB/c/Cr Slc (SPF) mice, 5 weeks old | 143 | IFV A/PR8/34 H1N1 at a dose of 50 μl of 107.5 TCID50/ml | Heat-killed Lactobacillus pentosus strain b240 (ONRIC b0240: b240) at doses of 0.4, 2 or 10 mg/mouse | 3 weeks | Saline | Survival rate↑, virus titer↓, anti-IFV IgA↑, anti-IFV IgG↑, CTLL-2 |
| Chiba, 2013 | Female BALB/c mice, 3 weeks old | NM | 106 PFU of human RSV strain A2 | Lactobacillus rhamnosus CRL1505 at a dose of 108 cells/mouse/day | 5 days | No treatment | Body weight↑, virus titer↓, lung wet:dry weight ratio↑, BAL albumin↓, BAL LDH↓, IFN-β↑, TNF-α, IFN-γ↑, IL-6 ↑, IL-10↑ |
| Maede, 2009 | Female C57BL/6 mice, 7 weeks old | 45 | Intranasally with influenza virus A/FM/1/47(H1N1) 100 pfu/mouse | Heat-killed Lactobacillus plantarum L-137 (HK-LP) at a dose of 75 mg/kg/day | 2 weeks | PBS solution | Survival rate↑, virus titer↓, IFN-β |
| Mahooti, 2019 | Female BALB/c mice, 6–8 weeks old | 20 | 1 LD90 of human influenza virus A/PR/8/34 (H1N1) | Bifidobacterium bifidum | 21 days | PBS | Stimulation index↑, IgG↑, IgG1↑, IgG2a↑, IFN-γ↑, IL-4↑, IL-6↓, survival rate↑ |
| Park, 2018 | Female BALB/c mice, 5 weeks old | 42 | Intranasally with influenza A (H1N1 and H3N2 subtypes), influenza B (Yamagata lineage) viruses | Heat-killed Lactobacillus plantarum (nF1) 0.05 or 10 mg | 4 weeks | PBS | Body weight↑, survival rate↑, virus titer↓ |
| Yasui, 2004 | BALB/c mice at 2-day-old (male and female) and 2-, 3-, 5-, 7- and 13-week-old (female) | 88 | Influenza A/PR/8/34 (PR8, H1N1) virus | Lactobacillus casei strain Shirota of 10⁁8.6 CFU/mouse | 3 weeks | Saline | Survival rate↑, virus titer↓, pulmonary NK cells↑, IL-12↑ |
| Youn, 2012 | SPF female BALB/c mice, 6 weeks old | 30 | Influenza A/NWS/33 (H1N1) virus | Live Lactobacillus rhamnosus or dead Lactobacillus rhamnosus | 21 days | Skim milk | Survival rate↑, virus titer↓, IgA ↓, TNF-α↓, IL-6↓, IL-1β↓ |
| Muramatsu, 2012 | Male C57BL/6N mice, 8-week-old | 21 | A/Puerto Rico/8/34 (PR8; H1N1) strain of influenza A virus | β-Glucan derived from Aureobasidium pullulans, 2 mg/ml, 0.2 ml/mouse | 24 days | PBS | Survival rate↑, body weight loss↓, virus titer↓, IL-1β↓, IFN-γ, TNF-α↓, IL-6 mRNA expression, lung immune cells, CSF2, CSF3, RIG-1, MDA5 mRNA expression |
| Chen, 2019 | Male BALB/c mice, 6 weeks | 24 | High pathogenicity influenza virus H1N1 (A/FM/1/47) | Houttuynia cordata polysaccharide (HCP), 40 mg/kg/day | 4 days | Ribavirin (positive control) No treatment (negative control) | Mucosubstances in goblet cells↓, HIH-1α↓, zo-1↑, TLR2 and TLR4 levels↓, IL-1β↓, IL-10↑ |
| Zhu, 2018 | Male BALB/c mice, 4–6 weeks old | 60 | Intranasally with high pathogenicity influenza virus H1N1 A/FM/1/47 at a dose of LD100 | 20 or 40 mg/kg/day of Houttuynia cordata (HCP) once daily | 7 days | Ribavirin (positive control) 5‰ CMC (negative control) | Survival rate↑, lung index↓, IgA↑ TLR4, NF-κB, TNF-α↓, IL-6↓, IFN-α↓, IL-1↓, MIP-1↓, RANTES↓, MCP-1↓, IP-10↓ |
| Ohta, 2007 | Female BALB/c mice, 5 weeks old | 32 | Intranasally with Influenza A virus (NWS strain,H1N1) of 2 × 105 PFU/mouse | Cordyceps militaris dissolved in H2O (2.5 mg/0.1 ml in PBS) or APS at a dose of 0.1 mg/15 µl per mouse | 10 days | H2O | Survival rate↑, body weight↑, virus titer↓, TNF-α↑, IFN-γ↑, NO production↑, iNOS expression↑, IL-1β↑, IL-6↑, IL-10↑ |
| Kawashima, 2011 | Female BALB/c mice | 50 | Intranasal with influenza A virus (A/NWS/33,H1N1) (IFV) of 2 × 104 PFU/mouse | Lactobacillus plantarum strain YU: LpYU (0.011, 0.21 or 2.1 mg/day) | 14 days | Saline | Virus titer↓, IL-12↑, IgE levels↓, IFN-γ↑, IL-4, IL-10, fecal IgA↑, NK cell activity↑, neutralization antibody titer↑ |
| Kondoh, 2012 | Male C57BL/6N mice, 6 weeks old | 38 | Intranasally with A/Puerto Rico/8/34 (H1N1; PR8), 103 PFU of PR8 at 0.05 ml | Heat-treated FK-23(SLFK), 15 mg per mouse | 7 days | Saline | Survival rate↑, body weight↑, IL-10↑, IL-1β, TNF-α, IFN-β, IFN-γ mRNA expression |
| Maruo, 2012 | Female BALB/c mice, 8 weeks old | 56 | Influenza virus A New Caledonia 2099 (H1N1), 25 μl (200 FFU per mouse) intranasally | 100 μl of milk fermented with Lactococcus lactis subsp. Cremoris FC | 12 days | Sterile physiologic saline | Survival rate↑, body weight loss↓, virus titer↓ |
| Hori, 2002 | Female BALB/c mice, 15 months old | 49 | Intranasally with influenza A/PR/8/34 (H1N1) (PR8) virus | Lactobacillus casei strain Shirota | 4 months | MM-3 diet | NK cell activity↑, viral titer↓, IFN-γ↑, TNF-α↑, IL-4 |
| Jounai, 2015 | Female wildtype DBA/2JJcl mice, 6-10 weeks old | 37 | Intranasal administration of a 25 μl drop containing 64 hemagglutination units (HAU) of mPIV1 | Fed AIN93G containing of 1 mg heat-killed Lactococcus lactis subsp. Lactis JCM5805/day/mouse and water ad libitum | 29 days | AIN93G (Oriental Yeast, Tokyo, Japan) | Survival rate↑, body weight loss↓, lung tissue damage↓, expression of MHC class II on pDCs, IFN-α, IFN-β mRNA expression, lung lymphocytes↑ |
| Jung, 2004 | Colostrum-deprived 5-day-old pigs | 40 | Intranasally with 3 ml of tissue culture fluid containing 2 × 106 tissue culture infective doses 50% (TCID50)/ml of Swine influenza virus H1N1, SNUVR030925 strain | Saccharomyces cerevisiae β-glucan (50 mg/day/pig) | 3 days | Culture medium | The severity of clinical sings↓, rectal temperature↓, microscopics lesions↓, nucleic acid↓, IFN-γ↑, NO↑ |
| Takeda, 2011 | Female SPF BALB/c mice, 6 weeks old | 94 | IFV A/PR/8/34 (H1N1), intranasally infected or mock-infected with 500 PFU in 20 μl of PBS | Lactic acid bacteria, L. plantarum 06CC2 strain, 0.2 ml per mouse twice daily | 9 days | Distilled water | Survival rate↑, body weight loss↓, viral titer↓, IFN-γ, IL-12, IFN-γ↑, TNF-α, IL-6↓, infiltrated cells in BALF↓, NK cell activity↑ |
| Bae, 2018 | Female BALB/c mice, 5 weeks old | 228 | A/Korea/01/2009 (2009 pandemic influenza A H1N1 virus, rK09); A/Puerto Rico/8/1934 expressing green fluorescent protein (rPR8-GFP); A/Anhui/01/2013 (avian influenza A H7N9 subtype 6:2 vaccine virus, rAH01) | Lactobacillus plantarum (Lp) or Leuconostoc mesenteroides (Lm) strain (109 CFU in 200 µl PBS) once daily | 14 days | PBS | Survival rate↑, body weight↑, viral plaques↓ |
| Antunes, 2019 | Female BALB/c mice, 6–8 weeks old | NM | Intranasally with 107 PFU/ml of RSV A2 strain | A high-fiber diet (HF) containing cellulose and pectin from citrus | 4 weeks | Cellulose | Body weight↑, viral load↓, total cells, lung histological score↓, CD11c, CD86 cells in axillary lymph nodes, TNF-α, IL-10↑, IL-4, CD4 T cell↓, IFN-γ, IL-17a, FoxP3-CD11CD86 |
| Belkacem, 2017 | Female BALB/c mice, 6 weeks old | NM | Influenza A virus, A/Scotland/ 20/74 (H3N2); IAV 50 μl | Lactobacillus paracasei CNCM I-1518 strain, 200 μl with 2 × 108 CFU | 17 days | PBS | Viral load↓, body weight↑, temperature loss↓, clinical score↑, total cells in lung, gut microbiota, IL-1α, IL-1β↑, MIP-1α, MIP-1β, IFN-γ, MCP-1 IL-33↑, IL-10↑ |
| Chen, 2016 | C57BL/6 male mice | NM | Influenza A virus strain (A/WSN/33) | Heat-killed Enterococcus faecalis, 17 mg/kg/day | 12 days | PBS | Survival rate↑, body weight loss↓, viral titer↓, leukocytes↓, IL-6, TNF-α, IL-1β, IFN-γ, IL-10, IL-17, MCP-1↑, TGFβ |
| Eguchi, 2019 | Female BALB/c mice, 5–7 weeks old | 25 | RSV A2 strain, 5 × 106 TCID50/head | Lactobacillus gasseri SBT2055, 2 × 109 cfu/head | 25 days | 25% trehalose solution | Body weight loss↓, viral titer↓, TNF-α↓, CCL2↓, IL-1β, IL-6↓ |
| Goto, 2013 | Female BALB/c mice, 4 weeks old | 104 | Intranasally with Influenza A/PR/8/34 (H1N1), 5 × 105 plaque-forming units (PFUs)/mouse | Expt I: doses of 300 ml of non-live L-92 suspension/day, Expt II: doses of 300 ml of live L-92 suspension/day | 21 days | Saline | Symptom score↓, viral titer↓, NK cell activity↑, consolidation score↓, neutrophils↓, Eotaxin↑, M-CSF↑, IL-1β↑, RANTES↑,IFN-α↑, IL-4, IL-6, IL-17 |
| Jiang, 2017 | SPF BALB/c mice, 6–7 weeks old | 44 | Intranaslly with H9N2 subtype Avian influenza virus at the dose of 0.05 ml 10× LD50 of H9N2 | Recombinant NC8 strains at the dose of 1.0 × 109 CFU/0.1 mL phosphate buffered saline (PBS) | 3 days | PBS | The activation and polarization of T cells, IgA↑, IFN-γ, IL-4, IL-17. survival rate↑, body weight↑ |
| Kawahara, 2015 | Female BALB/c mice, 6 weeks old | 26 | Intranaslly with influenza virus A/Puerto Rico/8/1934 (PR8, H1N1) virus | 200 μl of phosphate buffered saline (PBS) containing 2.0 × 109 CFU of Bifidobacterium longum MM-2(MM-2) | 17 days | PBS | Survival rate↑, symptom score↑, body weight↑, viral titer↓, NK cell activity↑, IFN-α↑, IL-6↓, TNF-α↓ |
| Nakayama, 2014 | Male C57BL/6N mice, 5–7 weeks old | 34 | Intranasally with A/Puerto Rico/8/34 (PR8; H1N1) at a titer of 1000 pfu | Lactobacillus gasseri SBT2055 | 41 days | 25% trehalose solution | Survival rate↑, body weight, virus titers, IL-6↓, BALF cells, LDH activity↓, antiviral genes |
| Nogusa, 2009 | Male C57BL/6– mice, 6–8 weeks old | 48 | Intranasally with influenza A/Puerto Rico/8/34 | Active hexose correlated compound at daily doses of 0.05, 0.1, 0.5 and 1 g/kg | 17 days | No treatment | Survival rate↑, body weight↑,NK cell cytotoxicity and lytic efficiency↑ |
| Kawase, 2010 | Female BALB/c mice, 4 weeks old | 39 | Intranasally with Flu A/PR/8/34 (H1N1), 5 × 106 PFU per mouse | 10 mg of Lyophilized Lactobacillus rhamnosus GG and Lactobacillus gasseri TMC0356 in 200 ul of sterile physiologic saline | 19 days | sterile physiologic saline | Clinical symptom score↓, virus titers↓, histopathology |
| Kechaou, 2013 | SPF BALB/c mice, 6 weeks old | 49 | Intranasally with influenza virus H1N1 strain A Puerto Rico/8/1934 (A/PR8/34; a mouse-adapted strain) | 1 × 109 CFU of Lactobacillus casei DN114-001, Lactobacillus rhamnosus GG or Lactobacillus plantarum CNRZ1997 | 24 days | PBS | Weight loss↓, clinical symptoms↓, virus proliferation↓ |
| Nagai, 2011 | Female BALB/c mice, 7 weeks old | 27 | Intranaslly with influenza virus A/PR/8/34 (A/PR8, H1N1) | Expt I: yogurt at a dose of 0.4 ml (20 μg as polysaccharide)/mouse/day Expt II: EPS at a dose of 20 μg/mouse(∼1 mg/kg body weight) | 27 days | Water | Survival rate↑, virus titers↓,virus antibodies titers↑, NK cell activity↑ |
| Kawase, 2011 | Female BALB/c mice, 4 weeks old | 48 | Intranasally with Flu A/PR/8/34 (H1N1), 5 × 106 PFU per mouse | Heat-killed Lactobacillus gasseri TMC0356 10 mg of lyophilized lactobacilli (TMC0356-70, group 1; TMC0356-90, group 2) | 19 days | Sterile physiologic saline | Body weight↑, clinical symptom score↑, virus titer↓, histopathology,NK cell activity, (IL)-12↑, IL-15↑, IL-21↑, IFN-c↑, TNF↑, IL-12a↑, IL-12rbl↑, IL-2rb↑, perforin 1↑ |
| Song, | SPF female BALB/c mice, 4 weeks old | 20 | Intranasally with influenza virus A/NWS/3 3 (H1N1) | 0.3 ml of 1 × 109 colony forming units/ml Lactobacillus rhamnosus M21 (KCTC 10965BP) | 34 days | Skim milk | Survival rate↑, pneumonia↓, IL1β, IL-6, IL-4, IFN-γ↑, IL-2↑, IgA levels↑ |
| Park, 2013 | Female BALB/c mice, 6–8 weeks old | 27 | Influenza virus strains A/Puerto Rico/8/1934 (H1N1; A/PR8) and A/Philippines/82 (H3N2 subtype) | Intragastric 200 ml of suspension containing 109 or 108 CFU of live L. plantarum DK119 once daily | 24 days | No treatment | Body weight↑, viral titers↓,IL-4↑, IL-6↑, TNF-a↑, IL-12↑, IFN-γ↑, lung histopathology |
| Pugh, 2015 | Female BALB/c mice, 6–8 weeks old | 60 | Intranasally with human influenza A/PR/8/34 (H1N1) virus of 5 μl of viral inoculum per nostril | Fed rodent diet AIN-93M supplemented with 0.35% Immulina (the average daily feed intake was 3.0–3.5 g/mouse) | 51 days | Rodent diet AIN-93M | Feed intake↑, weight loss↓, clinical signs↓, lung histopathology scores↓, |
| Seo, 2012 | SPF chickens, 5 weeks old | 100 | Orally challenged with low-pathogenic avian influenza (LPAI) (H9N2) | Live Leuconostoc mesenteroides YML003 or heat-killed Leuconostoc mesenteroides YML003 | 2 weeks | Normal diet | Antiviral activity↑, viral gene titers↓, IFN-γ levels↑ |
| Waki, 2013 | Female SPF BALB/c mice, 7–8 weeks old | 60 | Intranasally with 2× MLD50 (50% mouse lethal dose) IFV A/PR/8/34 (H1N1) | Lactobacillus brevis KB290 (KB290) | 14 days | Potato starch | Body weight↑, physical conditions↑, IgA level↑, IFN-α↑, |
| Zheng, 2006 | ICR mice of either sex | NM | Intranasally with influenza virus (H1N1) | Exopolysaccharide from Aphanothece halophytica (Chroococcales) at doses of 10, 20, 40, 60 and 80 mg/kg/day | 4 days | Ribavirin (Sigma) at a dose of 40 mg/kg (positive control), Distilled water (negative control) | Endotoxin level, pulmonary edema↓, NK cells↑, lymphocyte proliferation↑, IL-2↑, IL-1β↓, phagocytic capacity↓ |
| Yin, 2013 | Female BALB/c mice, 5 weeks old | 28 | Intranasal with 50 μl of Influenza A/PR/8/34 virus (H1N1 subtype) | Korean red ginseng (RG) total extract, RG polysaccharide and RG saponin were directly given into digestive tracts of mice by oral feedings | 28 days | PBS (negative control), oseltamivir (positive control) | Survival rate↑,body weight↑, TNF-α/inducible nitric oxide synthase (iNOS)-producing dendritic cells (tipDCs)↓ |
| Yitbarek, 2018 | 1-day-old SPF layer chickens | 100 | via the oral-nasal route with 400 μl of 107 tissue culture infectious dose 50 (TCID50/ml) of A/turkey/Wisconsin/1/ (H9N2) infuenza virus | Administration of probiotic combination (1 ml/day by gavaging to the crop using a 1 ml syringe) or FMT | 4 days | Normal diet | Gut microbiota dicersity↓,virus shedding, INF I, IL-22↑, villus, VH:CD↓, Pearson’s correlations |
| Mahmoud 2020 | Holstein×Angus mixed sex | 24 | NutriTek was fed at a rate of 5 g/day, top-dressed on to the calf starter | SCFP supplemented | 31 days | Base milk replacer and calf starter | Body weight↑, clinical score, cortisal, lung pathology, Virus isolation, NS2 copy number, CD4 T↑, CD8 T, δT, IL-6↑, TNFα↑, IL-1β |
| Trompette, 2018 | Female BALB/c or C57BL/6 mice, 8–12 week-old | 36 | Influenza virus strain PR8 (A/Puerto Rico8/34, H1N1) 1100 PFU for high-dose infection of BALB/c, 4500 PFU for high-dose infection in C57BL/6, and 100 PFU for low-dose infections | Inulin (high-fiber diet) | Since born | cellulose | Survival rate↑,clinical score↓, compliance in response to methacholine↑, RBC accumulation↓, albumin levels↓, MPO activity↓,gut microbial composition, SCFA↑, CXCL1 Production↓, macrophage population↑, CD8+ T Cell |
Abbreviations: CFU, colony-forming unit; FMT, fecal microbiota transplantation; IAV, influenza A virus; IFN, interferon; IFV, influenza virus; IL, interleukin; NM, not mentioned; PBS, phosphate buffer saline; PFU, plaque forming unit; SCFA, short-chain fatty acid; SPF, specific-pathogen-free.
↑, the effect in intervention group was greater than control group; ↓, the effect in intervention group was smaller than control group.
Clinical studies
Only six clinical studies were eligible under the predetermined criteria (Table 2), and all were the randomized, double-blind, placebo-controlled trials. Rhinovirus was chosen as the final infectious target in the studies, except for two studies choosing human influenza virus [15] and SARS-CoV-2 [16]. Various probiotics or prebiotics were used. Turner et al. selected Bifidobacterium animalis subspecies lactis Bl-04 (Bl-04) as the intervention, while Tapiovaara et al. employed heat-inactivated Lactobacillus rhamnosus GG [17,18]. Similarly, Kumpu et al. investigated whether live or heat-inactivated L. rhamnosus GG had equivalent effects against rhinovirus, while Tetsu et al. examined the protective effect of Lactococcus lactis ssp. lactis JCM5805 [15,19]. Luoto et al. used a mixture of two prebiotics (galacto-oligosaccharide and polydextrose at a 1:1 ratio) and also a probiotic (L. rhamnosus GG) as the intervention factors in their study. They wanted to determine the prophylactic effect in 94 preterm infants with the gestational ages greater than 32 + 0 or less than 36 + 6 weeks and birth weight >1500 g [20]. In study from Ettorre et al., participants were 70 hospitalized patients positive for COVID-19. Oral bacteriotherapy using a multistrain formulation, which contained various probiotics such as Streptococcus thermophilus and L. acidophilus, were selected as intervention.
Table 2. Study characteristics of included clinical studies.
| Author, year | Study design | Sample size | Characteristics of participants | Virus | Intervention | Comparator | Quality assessment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Turner, 2017 | Randomized, double-blind, placebo controlled, parallel trial | 115 | Healthy adult volunteers serum neutralizing antibody titer of ≤1:4 | Rhinovirus 100 tissue culture infectious dose 50 (TCID50) of virus by intranasal drops | Bifidobacterium animalis subsp. lactis Bl-04 (Bl-04) a minimum of 2 × 109 cfu of Bl-04 mixed with 1 g of sucrose for 33 days | 1 g of sucrose | 6 | CXCL8↓, CXCL10, G-CSF concentration, virus titer↓, NO, total symptom score |
| Tapiovaara, 2016 | Randomized, double-blind, placebo-controlled, pilot study | 50 | Healthy subjects aged 18–65 years | Human rhinovirus (HRV)39 100–300 tissue culture infectious dose (TCID)50 | Juice enriched with live or heat-inactivated L. rhamnosus GG for 6 weeks | Control juice | 4 | Virus titer↓, NO, total symptom score↓ |
| Luoto, 2014 | Randomized, double-blind, placebo-controlled trial | 68 | Preterm infants gestational age between 32 + 0 and 36 + 6 weeks birth weight greater than 1500 g | Rhinovirus | Prebiotics (galacto_x0002_oligosaccharide and polydextrose mixture, 1:1) probiotic (L. rhamnosus GG, ATCC 53103), between days 3 and 60 of life | Microcrystalline cellulose | 6 | The incidence of RTIs↓, rhinovirus infections↓, virus load↓, |
| Ettorre, 2020 | Randomized, double blind, placebo-controlled, pilot trial | 59 | Healthy volunteers aged 18–65 years | Rhinovirus immunotype 39 100–300 tissue culture infectious dose (TCID)50 | Live or heat-inactivated L. rhamnosus GG for 6 weeks | Carrier juice | 4 | Diarrhea and other symptoms↓, estimated risk of developing respiratory failure↓, prevalence of patients transferred to ICU and mortality↓ |
| Kumpu, 2015 | Randomized, placebo-controlled, double-blind, experiment | 213 | Healthy adults (30–59 years old) from Japan female:male = 121:92 | Human influenza virus A/H1N1 (A/PR/8/34) | Lactococcus lactis ssp. lactis JCM5805 for 10 weeks | Placebo beverage | 5 | Occurrence and severity of cold symptoms↓, number of subjects with rhinovirus infection↓ |
| Tetsu, 2015 | Randomized, placebo-controlled, double-blind, experiment | 213 | Healthy adults (30–59 years old) from Japan female:male = 121:92 | Human influenza virus A/H1N1 (A/PR/8/34) | Lactococcus lactis ssp. lactis JCM5805 for 10 weeks | Placebo beverage | 5 | Major symptoms of an influenza-like illness↓, IFN-α↑, IGSF15↑ |
Quality assessment according to the Cochrane Risk Assessment Scale.
Abbreviations: IFN, interferon; TCID, tissue culture infective dose.
↑, the effect in intervention group was greater than control group; ↓, the effect in intervention group was smaller than control group.
Primary outcomes in preclinical studies
Survival analysis
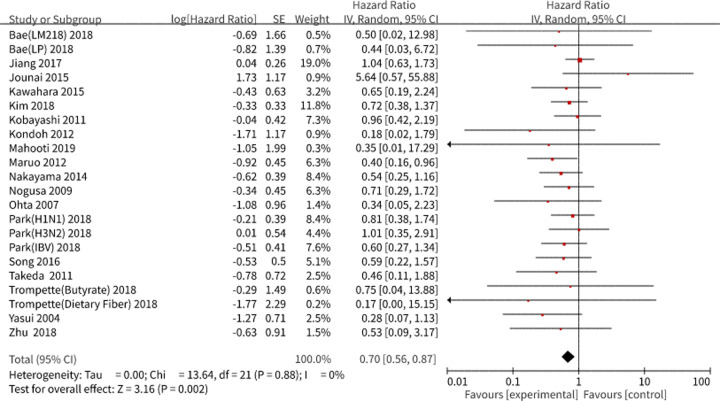
Twenty studies comprising 24 subgroups were included in the meta-analysis using the random effects model. As a result, a pooled hazard ratio (HR) of 0.70, with a 95% confidence interval (CI) of 0.56–0.87 was obtained, which corresponded to a higher survival rate for the microbial intervention group than that for the control group after the virus challenge, without heterogeneity (I2 = 0.0%, P=0.88) (Figure 2). Notably, none of the pooled effect estimate in each selected study had statistical significance, probably due to the small sample size except for the data from one study by Maruo et al. [21]. We also evaluated the publication bias using Stata software 12.0, with no quantitative publication bias exists in Egger’s test (P=0.10).
Figure 2. Survival analysis in preclinical studies.
We performed a forest plot of the survival analysis in preclinical studies using RevMan 5.3. The pooled results are expressed as HRs with their 95% CI. I2 and P-values represent the heterogeneity among the studies, while an I2 value >50% and a P-value <0.1 indicate considerable heterogeneity. The 95% CI of the result intersecting with the solid vertical line represents no statistical significance. Abbreviations: H1N1, infected with influenza A (H1N1 subtypes); H3N2, infected with influenza A (H3N2 subtypes); IBV, infected with influenza B (Yamagata lineage) viruses; LM218, treated with Leuconostoc mesenteroides strain; LP, treated with Lactobacillus plantarum 920 strain.
Viral load
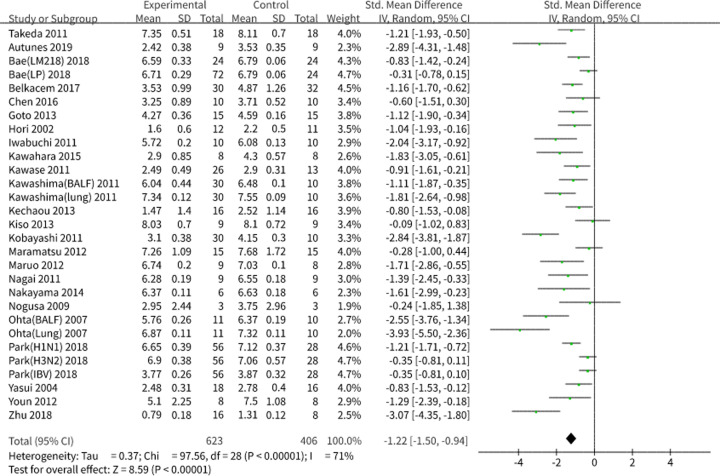
Results showed a pooled standard deviation (SD) of −1.22 and 95% CI of −1.50 to −0.94 (P<0.001), revealing that the consumption of probiotics or prebiotics alleviated the viral load after a respiratory viral infection (Figure 3). Due to the significant heterogeneity (I2 = 71.3%, P<0.001), we performed a subgroup analysis and sensitivity analysis (Table 3). We observed that the heterogeneity would be significantly affected if the eligible studies were grouped into ‘SD’ (I2 = 79%, P<0.001) and ‘standard error of the mean (SEM)’ (I2 = 22.3%, P=0.24) based on the effect estimates adopted by the individual authors. We sequentially analyzed the studies in which ‘SD’ was employed as the effect estimate. Heterogeneity was significantly affected when the studies were grouped into ‘H1N1’ (I2 = 73%, P<0.001) and ‘others’ (I2 = 0.0%, P=0.53) according to the specific virus challenge but was not affected when the studies were divided into ‘probiotics’ (I2 = 84%, P<0.001) and ‘prebiotics’ (I2 = 90%, P<0.001) according to the types of microbial agents, indicating that the differences between the specific virus species might cause the final heterogeneity. Considering that there was no clear decrease in heterogeneity in the subgroup analyses by microbial agents’ types, we conducted a sensitivity analysis and discovered that the study performed by Maramatsu et al. was the main source of heterogeneity. We also evaluated the publication bias using Stata data analysis and determined a distinct publication bias based on Egger’s test (P<0.001).
Figure 3. Analysis of viral load in preclinical studies.
We performed a forest plot of viral load in preclinical studies using RevMan 5.3. We included studies using SD and SEM, transforming SEM to SD for better construction. The data were pooled using a random effects model and expressed as SD with 95% CIs. I2 and P-values represent the heterogeneity among the studies, while an I2 value >50% and a P-value <0.1 indicate considerable heterogeneity. Abbreviations: LM218, treated with Leuconostoc mesenteroides 218 strain; LP, treated with L. plantarum 920 strain; IBV, infected with influenza B (Yamagata lineage) viruses; H3N2, infected with influenza A (H3N2 subtypes); H1N1, infected with influenza A (H1N1 subtypes); Heat-killed, treated with heat-killed Enterococcus faecalis; Lung, virus titers detected in the lung; BALF, virus titers detected in bronchoalveolar lavage fluids.
Table 3. Subgroup and sensitivity analysis of viral load in preclinical studies.
| Total number of studies | Total sample size | Subgroup analysis | Sensitivity analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exp. | Ctr. | SMD (95% CI) | I2 | P | SMD (95% CI) | I2 | P | ||
| Effect estimates | |||||||||
| SEM | 10 | 132 | 121 | −1.14 (−1.47, −0.82) | 22% | 0.24 | −1.05 (−1.33, −1.77) | 0% | 0.73 |
| SD | 19 | 461 | 275 | −1.26 (−1.63, −0.88) | 79% | <0.001 | −1.16 (−1.51, −0.80) | 75% | <0.001 |
| Viral type | |||||||||
| H1N1 | 15 | 283 | 181 | −1.54 (−1.98, −1.10) | 73% | <0.001 | −1.44 (−1.86, −1.01) | 69% | <0.001 |
| Others | 4 | 208 | 104 | −0.42 (−0.66, −0.18) | 0% | 0.53 | - | - | - |
| Microbial agents | |||||||||
| Prebiotics | 4 | 53 | 43 | −2.39 (−4.16, −0.61) | 90% | <0.001 | −3.07 (−3.83, −2.30) | 0% | 0.4 |
| Probiotics | 15 | 438 | 242 | −1.00 (−1.46, −0.54) | 84% | <0.001 | −0.86 (−1.25, −0.46) | 79% | <0.001 |
Data were analyzed using a random-effects model. We analyzed the Effect estimates group for the included preclinical studies regarding viral load. We analyzed the viral type and microbial agent groups in the preclinical studies using SD as the effect estimate. Abbreviations: Ctr, control group; Exp, experimental group.
Cytokines
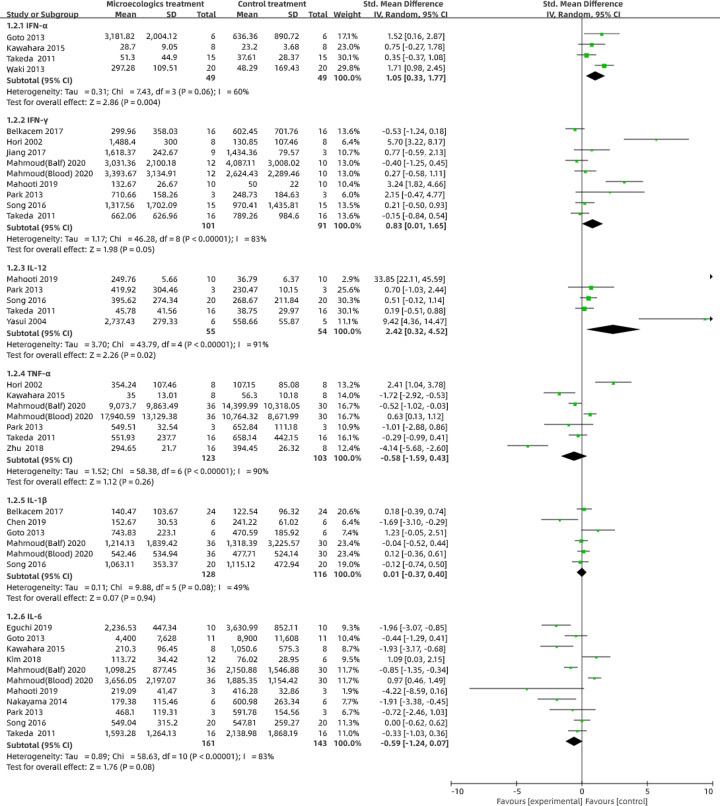
We conducted forest plots to assess the changes in cytokines including interferon (IFN)-α, IFN-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-12 and IL-6 levels in the studies (Figure 4). Based on the established readings, the consumption of probiotics or prebiotics increased the concentrations of IFN-α (SD: 1.05; 95% CI: 0.33–1.77; P=0.004), IFN-γ (SD: 0.83; 95% CI: 0.01–1.65; P=0.05), IL-12 (SD: 2.42; 95% CI: 0.32–4.52; P=0.02) and IL-1β (SD: 0.01; 95% CI: −0.37 to 0.40; P=0.94), with significant heterogeneity (I2 = 60%, P=0.06; I2 = 83%, P<0.001; I2 = 91%, P<0.001 and I2 = 49%, P=0.08, respectively) while it decreased the concentrations of TNF-α (SD: −0.58; 95% CI: −1.59 to 0.43; P=0.26) and IL-6 (SD: −0.59; 95% CI: −1.24 to 0.07; P=0.08), with significant heterogeneity (I2 = 90%, P<0.001 and I2 = 83%, P<0.001, respectively).
Figure 4. Evaluation of cytokines in preclinical studies.
We evaluated the IFN-α, IFN-γ, IL-12, TNF-α, IL-1β and IL-6 concentrations compared with the control group through a random-effects models using RevMan 5.3. The pooled data are expressed as SD with 95% CIs. I2 and P-values represent the heterogeneity among the studies, while an I2 value >50% and a P-value <0.1 indicate considerable heterogeneity. The 95% CI of the result intersecting with the solid vertical line represents no statistical significance.
Adverse events
In most animal studies, the adverse events of probiotics and prebiotics are not recorded for lack of observable consequences. The reported aspiration pneumonia could be induced by either intranasally administrating high doses of live L. rhamnosus or the same dose of dead L. rhamnosus [22].
Primary outcomes in clinical studies
Clinical symptoms
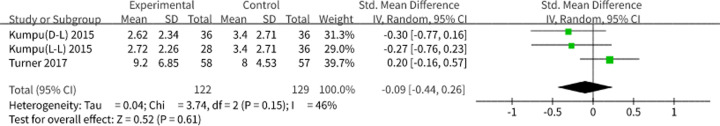
Results from the pooled random-effects model analysis, showed a decrease in clinical symptom scores (SMD: −0.09; 95% CI: −0.44 to 0.26, P=0.61), with moderate heterogeneity (I2 = 46%, P= 0.15) (Figure 5), implying a reduction in disease severity, although the result did not have a statistical significance because of small samples.
Figure 5. Symptom scores in the clinical studies.
The total symptom scores reported in the studies represent the symptom severity evaluations, and higher scores represent more severe symptoms. The pooled results are expressed as SD with 95% CI. I2 and P-values represent the heterogeneity among the studies, while an I2 value >50% and a P-value <0.1 indicate considerable heterogeneity. When the result intersects the invalid line, the result has no statistical significance. Abbreviations: D-L, dead Lactobacillus; L-L, live Lactobacillus.
Infection rate
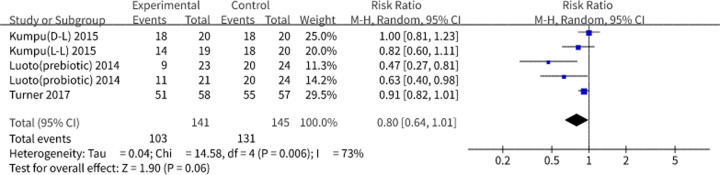
Three studies that consisted of five subgroups including a highly heterogenetic subgroup focused on prebiotics were pooled for analysis using the random-effects model, As a consequence, an overall risk ratio (RR) and 95% CI of 0.80 and 0.64–1.01 (P=0.06) were shown in Figure 6. The results suggested that a decreased viral morbidity was due to the treatment of probiotics and prebiotics. If larger sample size were used for the analysis, the observed treatment effects could be statistically significant [20].
Figure 6. Viral infection rates in the clinical studies.
The pooled results are expressed as RR with 95% CI. I2 and P-values represent the heterogeneity among the studies, while an I2value >50% and a P-value <0.1 indicate considerable heterogeneity. When the result intersects the invalid line, the result has no statistical significance. Abbreviations: D-L, dead Lactobacillus; L-L, live Lactobacillus.
Adverse events
Among the studies were pooled for analysis, only Turner et al. reported gastrointestinal adverse events after treatment with probiotics, but no details were described [18].
Quality assessment
For clinical studies, all targets included for evaluation had low risk of selection bias and performance bias because they were randomized, double-blind, placebo-controlled trials. Most of the studies had low risk of reporting bias and other bias except for one study that had high risk of attrition bias [18] (Table 2). Quality evaluating in preclinical studies was detailed in Supplementary Table S1.
Discussion
With the exploration of microbial agents, their properties that favor successful defense against respiratory virus infection have gaining a mass of interests. Olaimat et al. presented clinical fruits of the use of probiotic supplementation to prevent or treat respiratory tract infections [23] while Shinde et al. determined to identify evidence relating to potential mechanisms [24]. However, they just elaborated this subject qualitatively. Our study aimed to evaluate the effectiveness of on probiotics and prebiotics for viral pneumonia quantitatively, through the published data from 45 preclinical and 6 clinical studies. The probiotics included the live probiotics such as L. rhamnosus [25–28], Bifidobacterium [29,30] and a heat-inactivated one Enterococcus faecalis [13,31]. The prebiotics were commonly used such as inulin [32], polysaccharide [13,33–35] and oligosaccharide [36]. As a result, we found that both probiotics and prebiotics played a crucial role in treating viral pneumonia.
In viral pneumonia, inflammatory cascades could be commonly observed. Our results showed that probiotics or prebiotics helped to reduce the viral load leading to increase the overall survival through the up-regulation of the antiviral cytokines, IFN-α, IFN-γ, IL-1β and IL-12 and the down-regulation of the other cytokines IL-6 and TNF-α. The results from the cohort clinical studies on the influenza virus are generally consistent with those from preclinical studies. Patients treated with probiotics or prebiotics had lower disease severity and fewer infections. However, these findings warrant further studies to understand the effectiveness of probiotics and prebiotics in preventing viral pneumonia becuase of currently insufficient clinical studies and coherent indicators.
Interferon is a crucial antiviral factor that has a vital role in assessing host immunity. Unfortunately, there was no measure valid for the benefit of interferon in clinic yet. Our analysis revealed that all the probiotics or prebiotics tested could notably increase the interferon levels, consistent with a previous study that healthy athletes increased interferon secretion after a 1-month course of L. acidophilus. through a mechanism of engaging Toll-like receptors on the surface of antigen-presenting cells, which would, in turn, affect the subsequent cytokine secretion pattern [37,38], thereby indicating that probiotics and prebiotics work energetically by enhancing the host’s antiviral capabilities. Toll-like receptors (TLRs) play crucial roles in the innate immune system by recognizing pathogen-associated molecular patterns derived from various microbes. It has been reported that both neutrophil granulocytes and regulatory T cells put a halt to the reaction by releasing quantities of anti-inflammatory chemicals when an inflammatory response occurs within a capable immune system [14,39]. However, when the host is immunocompromised, effective responses break down. Accordingly, viral duplication that exacerbates tissue damages is attributed to a burst of inflammatory cytokines induced. Typically, the critical COVID-19 patients reported with higher plasma concentrations of IL-7, IL-8, IL-9, basic fibroblast growth factor, granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor were in high fatality [6].
A well-documented postbiotic is called short-chain fatty acids (SCFAs) is that are produced by bacterial fermentation of indigestible fibers. The most abundant SCFAs refer to propionate, acetate and butyrate. SCFAs act on G protein-coupled receptors (GPRs) 41 and GPR43, [40,41] or as histone deacetylase inhibitors [42] to down-regulate proinflammatory chemokine and combat cytokine cascades. Our findings demonstrated that immune cells took advantage of bacterial metabolites to enhance antiviral response. The mucosal immune system, specifically the lymphoid tissues on the mucosal surface, was also involved in modulating anti-virus immunity [43,44]. Microfold cells could absorb bacterial metabolites into the circulation, with a bond of mucosal immune system where they would stimulate immune cells and rapidly recruit them [45].
IL-12 and IL-6 are representative activators to Th1 and Th2, respectively. In severe influenza infections, there was a marked Th polarization shift from Th2 to Th1 [46]. However, during probiotics or prebiotics treatment, this inflammation-oriented polarization could be reversed through up-regulation of IL-12 and down-regulation of IL-6. Furthermore, Chen et al. and other researchers discovered that production of IL-10 from Treg cells was increased [13,25]. IL-10 functions to limit the host immune response to pathogens, thereby preventing damage to the host and maintaining normal tissue homeostasis. Thus, we can speculate that probiotics and prebiotics could recruit Treg cells and up-regulate IL-10 concentrations to achieve an antiviral effect by preventing immoderate inflammatory responses through inhibiting production of the inflammatory cytokines, TNF-α and IL-6.
Concerning secondary infections incurred by enterogenous endotoxemia during severe viral pneumonia, the gut microbiota could notably defend it. The gut mucosal barrier is composed of mucus, symbiotic flora, tight junctions between intestinal epithelial cells and mucosal immune cells, making it difficult for opportunistic infections to take root. Once the virus fiercely strikes at the respiratory tissues, it would possibly bring about systematic hypoxia, where the intestinal epithelial cells would dysfunction and act to weaken the mucosal barrier [14]. In addition, the misuse or overuse of broad-spectrum antibiotics could invariably result in dysbiosis, blemishing intestinal permeability and endamaging gut mucosal barrier during antiviral treatment [47].
Probiotic supplements were considered to be an optimal approach to restore the gut mucosal barrier function in viral pneumonia. Probiotics can bind to Toll-like receptor-4, whose population could increase with the help of inactivated L. salivarius and fructo-oligosaccharides [48], thereby competing against harmful bacteria. In addition, probiotics and its metabolic profiles including bacteriocin, hydrogen peroxide, antimicrobial peptides and defensin, help to modulate the local immunity and drive enterocyte and goblet cells to secret mucus as a consequence to strengthen the mucosal barrier at length [49,50].
For COVID-19 patients, SARS-CoV-2 binds its spike proteins to angiotensin‐converting enzyme 2 (ACE2). ACE2 is highly expressed in the bronchi and gastrointestinal tract to facilitate to viral invading and replication [51,52]. Since the invasive bindings to ACE2, ACE2 located in the gut might not function effectively, potentially altering the symbiotic flora and undermining the intestinal barrier, leading to patients prone to secondary infections. A published study reported that treatment of an irritant, compared with wildtype littermates, caused gut microbiota alteration to promote profoundly inflammatory reaction in ACE2 mutation mice, which could be directly regulated by microbial agents [53]. Encouragingly, probiotics and prebiotics treatment has been incorporated as adjuvant therapy for critical patients to prevent secondary infections in the fourth Trial Edition of COVID-19 Diagnosis and Treatment Plan by the National Health Commission of China, [54].
In summary, our study suggested that probiotics and prebiotics could be an inspiration for healthcare givers when treating viral pneumonia. This therapy could limit inflammatory responses, stimulate both innate and adaptive immune cells to defend against the viral attacks and preventing secondary infections. Our findings implied a promising target and encourage probiotics or prebiotics to be incorporated into regular treatments for patients infected with respiratory virus, particularly for the patients with severe viral pneumonia.
Limitations
The present study has several limitations. Firstly, most of the clinical studies related to the topic were not included because they focused generally on the respiratory tract infections but not particularly on respiratory viral infections. Thus, only a small number of clinical studies was eligible for our analysis, thereby influencing the extrapolation of outcomes. Secondly, considering the differences in experimental designs, we did not conduct a direct comparison of the merits of individual microecological agents tested. We also did not confirm optimal dosage, dosage form and duration, which need further investigation. Thirdly, despite remarkable functions showed in applying probiotics and prebiotics to treat viral pneumonia, the effects only limit to a certain amount of bacteria species and their products. Therefore, it is appropriate to specify the individual probiotics or prebiotics with more explorations.
Supplementary Material
Acknowledgements
We acknowledge the help of Chaoyi Wang in data extraction for this meta-analysis.
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- CAP
community-acquired pneumonia
- CI
confidence interval
- COVID-19
novel coronavirus pneumonia
- GPR
G protein-coupled receptor
- HR
hazard ratio
- IFN
interferon
- IL
interleukin
- RR
risk ratio
- RSV
respiratory syncytial virus
- SARS
severe acute respiratory syndrome
- SCFA
short-chain fatty acid
- SD
standard deviation
- TNF
tumor necrosis factor
Contributor Information
Changlong Xu, Email: xchlong@163.com.
Xiangyang Xue, Email: wzxxy001@163.com.
Data Availability
All data generated or analyzed during the present study are included in this published article.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Zhejiang Provincial Natural Science Foundation of China [grant number LY20H180010]; the Wenzhou Science and Technology Bureau [grant number Y20180142]; and the Science and Technology Department of Zhejiang [grant number 2017C33068].
Author Contribution
C.l.X. and F.y.W. conceived the study idea. S.X. and Z.h.X. retrieved and collected the papers. J.m.Z. and Y.w.W. filtered the literature. B.h.P. drafted the manuscript and T.t.Z. conducted data analysis. S.X. and Z.h.X. extracted the data. F.y.W., X.y.X. and Q.h.Z. revised the manuscript. Z.h.X. and Y.f.B. designed and made pictures and charts. All co-authors had full access to and approved the final version of the manuscript. All authors had no conflicts of interest with regard to this manuscript.
References
- 1.Ruuskanen O., Lahti E., Jennings L.C. and Murdoch D.R. (2011) Viral pneumonia. Lancet 377, 1264–1275 10.1016/S0140-6736(10)61459-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burk M., El-Kersh K., Saad M., Wiemken T., Ramirez J. and Cavallazzi R. (2016) Viral infection in community-acquired pneumonia: a systematic review and meta-analysis. Eur. Respir. Rev. 25, 178–188 10.1183/16000617.0076-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi T., Denouel A., Tietjen A.K.et al. (2019) Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: a systematic review and meta-analysis. J. Infect. Dis. 10.1093/infdis/jiz059 [DOI] [PubMed] [Google Scholar]
- 4.Wu Z. and McGoogan J.M. (2020) Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 323, 1239–1242 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (2021) . Coronavirus disease (COVID-19) pandemic. WHO https://www.who.int/publications/m/item/weekly [Google Scholar]
- 6.Huang C., Wang Y., Li X.et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichinohe T., Pang I.K., Kumamoto Y.et al. (2011) Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. U.S.A. 108, 5354–5359 10.1073/pnas.1019378108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groves H.T., Cuthbertson L., James P., Moffatt M.F., Cox M.J. and Tregoning J.S. (2018) Respiratory disease following viral lung infection alters the murine gut microbiota. Front. Immunol. 9, 182 10.3389/fimmu.2018.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmoud A.H.A., Slate J.R., Hong S., Yoon I. and McGill J.L. (2020) Supplementing a Saccharomyces cerevisiae fermentation product modulates innate immune function and ameliorates bovine respiratory syncytial virus infection in neonatal calves. J. Anim. Sci. 98, 10.1093/jas/skaa252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung K., Ha Y., Ha S.K.et al. (2004) Antiviral effect of Saccharomyces cerevisiae β-glucan to swine influenza virus by increased production of interferon-γ and nitric oxide. J. Vet. Med. Ser. B 51, 72–76 10.1111/j.1439-0450.2004.00732.x [DOI] [PubMed] [Google Scholar]
- 11.Seo B.J., Rather I.A., Kumar V.J.et al. (2012) Evaluation of Leuconostoc mesenteroides YML003 as a probiotic against low-pathogenic avian influenza (H9N2) virus in chickens. J. Appl. Microbiol. 113, 163–171 10.1111/j.1365-2672.2012.05326.x [DOI] [PubMed] [Google Scholar]
- 12.Yitbarek A., Taha-Abdelaziz K., Hodgins D.C.et al. (2018) Gut microbiota-mediated protection against influenza virus subtype H9N2 in chickens is associated with modulation of the innate responses. Sci. Rep. 8, 13189 10.1038/s41598-018-31613-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen M.Y., Li H., Lu X.X.et al. (2019) Houttuynia cordata polysaccharide alleviated intestinal injury and modulated intestinal microbiota in H1N1 virus infected mice. Chin J. Nat. Med. 17, 187–197 10.1016/S1875-5364(19)30021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu H., Lu X., Ling L.et al. (2018) Houttuynia cordata polysaccharides ameliorate pneumonia severity and intestinal injury in mice with influenza virus infection. J. Ethnopharmacol. 218, 90–99 10.1016/j.jep.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 15.Sugimura T., Takahashi H., Jounai K.et al. (2015) Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br. J. Nutr. 114, 727–733 10.1017/S0007114515002408 [DOI] [PubMed] [Google Scholar]
- 16.d’Ettorre G., Ceccarelli G., Marazzato M.et al. (2020) Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front. Med. (Lausanne) 7, 389 10.3389/fmed.2020.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tapiovaara L., Kumpu M., Makivuokko H.et al. (2016) Human rhinovirus in experimental infection after peroral Lactobacillus rhamnosus GG consumption, a pilot study. Int. Forum Allergy Rhinol. 6, 848–853 10.1002/alr.21748 [DOI] [PubMed] [Google Scholar]
- 18.Turner R.B., Woodfolk J.A., Borish L.et al. (2017) Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection - a randomised controlled trial. Benef. Microbes 8, 207–215 10.3920/BM2016.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumpu M., Kekkonen R.A., Korpela R.et al. (2015) Effect of live and inactivated Lactobacillus rhamnosus GG on experimentally induced rhinovirus colds: randomised, double blind, placebo-controlled pilot trial. Benef. Microbes 6, 631–639 10.3920/BM2014.0164 [DOI] [PubMed] [Google Scholar]
- 20.Luoto R., Ruuskanen O., Waris M., Kalliomaki M., Salminen S. and Isolauri E. (2014) Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 133, 405–413 10.1016/j.jaci.2013.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maruo T., Gotoh Y., Nishimura H., Ohashi S., Toda T. and Takahashi K. (2012) Oral administration of milk fermented with Lactococcus lactis subsp. cremoris FC protects mice against influenza virus infection. Lett. Appl. Microbiol. 55, 135–140 10.1111/j.1472-765X.2012.03270.x [DOI] [PubMed] [Google Scholar]
- 22.Youn H.N., Lee D.H., Lee Y.N.et al. (2012) Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antiviral Res. 93, 138–143 10.1016/j.antiviral.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 23.Olaimat A.N., Aolymat I., Al-Holy M.et al. (2020) The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Sci. Food 4, 17 10.1038/s41538-020-00078-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinde T., Hansbro P.M., Sohal S.S., Dingle P., Eri R. and Stanley R. (2020) Microbiota modulating nutritional approaches to countering the effects of viral respiratory infections including SARS-CoV-2 through promoting metabolic and immune fitness with probiotics and plant bioactives. Microorganisms 8, 10.3390/microorganisms8060921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiba E., Tomosada Y., Vizoso-Pinto M.G.et al. (2013) Immunobiotic Lactobacillus rhamnosus improves resistance of infant mice against respiratory syncytial virus infection. Int. Immunopharmacol. 17, 373–382 10.1016/j.intimp.2013.06.024 [DOI] [PubMed] [Google Scholar]
- 26.Kim D.H., Chung W.C., Chun S.H., Han J.H., Song M.J. and Lee K.W. (2018) Enhancing the natural killer cell activity and anti-influenza effect of heat-treated Lactobacillus plantarum nF1-fortified yogurt in mice. J. Dairy Sci. 101, 10675–10684 10.3168/jds.2018-15137 [DOI] [PubMed] [Google Scholar]
- 27.Kiso M., Takano R., Sakabe S.et al. (2013) Protective efficacy of orally administered, heat-killed Lactobacillus pentosus b240 against influenza A virus. Sci. Rep. 3, 1563 10.1038/srep01563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi N., Saito T., Uematsu T.et al. (2011) Oral administration of heat-killed Lactobacillus pentosus strain b240 augments protection against influenza virus infection in mice. Int. Immunopharmacol. 11, 199–203 10.1016/j.intimp.2010.11.019 [DOI] [PubMed] [Google Scholar]
- 29.Iwabuchi N., Xiao J.Z., Yaeshima T. and Iwatsuki K. (2011) Oral administration of Bifidobacterium longum ameliorates influenza virus infection in mice. Biol. Pharm. Bull. 34, 1352–1355 10.1248/bpb.34.1352 [DOI] [PubMed] [Google Scholar]
- 30.Mahooti M., Abdolalipour E., Salehzadeh A., Mohebbi S.R., Gorji A. and Ghaemi A. (2019) Immunomodulatory and prophylactic effects of Bifidobacterium bifidum probiotic strain on influenza infection in mice. World J. Microbiol. Biotechnol. 35, 91 10.1007/s11274-019-2667-0 [DOI] [PubMed] [Google Scholar]
- 31.Kondoh M., Fukada K., Fujikura D.et al. (2012) Effect of water-soluble fraction from lysozyme-treated Enterococcus faecalis FK-23 on mortality caused by influenza A virus in mice. Viral Immunol. 25, 86–90 10.1089/vim.2011.0056 [DOI] [PubMed] [Google Scholar]
- 32.Trompette A., Gollwitzer E.S., Pattaroni C.et al. (2018) Dietary fiber confers protection against Flu by shaping Ly6c(-) patrolling monocyte hematopoiesis and CD8(+) T cell metabolism. Immunity 48, 992.e1008–1005.e1008 10.1016/j.immuni.2018.04.022 [DOI] [PubMed] [Google Scholar]
- 33.Muramatsu D., Iwai A., Aoki S.et al. (2012) beta-Glucan derived from Aureobasidium pullulans is effective for the prevention of influenza in mice. PLoS ONE 7, e41399 10.1371/journal.pone.0041399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin S.Y., Kim H.J. and Kim H.J. (2013) A comparative study of the effects of whole red ginseng extract and polysaccharide and saponin fractions on influenza A (H1N1) virus infection. Biol. Pharm. Bull. 36, 1002–1007 10.1248/bpb.b13-00123 [DOI] [PubMed] [Google Scholar]
- 35.Zheng W., Chen C., Cheng Q., Wang Y. and Chu C. (2006) Oral administration of exopolysaccharide from Aphanothece halophytica (Chroococcales) significantly inhibits influenza virus (H1N1)-induced pneumonia in mice. Int. Immunopharmacol. 6, 1093–1099 10.1016/j.intimp.2006.01.020 [DOI] [PubMed] [Google Scholar]
- 36.Nogusa S., Gerbino J. and Ritz B.W. (2009) Low-dose supplementation with active hexose correlated compound improves the immune response to acute influenza infection in C57BL/6 mice. Nutr. Res. 29, 139–143 10.1016/j.nutres.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 37.Clancy R.L., Gleeson M., Cox A.et al. (2006) Reversal in fatigued athletes of a defect in interferon gamma secretion after administration of Lactobacillus acidophilus. Br. J. Sports Med. 40, 351–354 10.1136/bjsm.2005.024364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen H.R., Frokiaer H. and Pestka J.J. (2002) Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 168, 171–178 10.4049/jimmunol.168.1.171 [DOI] [PubMed] [Google Scholar]
- 39.Hartshorn K.L., White M.R., Tecle T., Holmskov U. and Crouch E.C. (2006) Innate defense against influenza A virus: activity of human neutrophil defensins and interactions of defensins with surfactant protein D. J. Immunol. 176, 6962–6972 10.4049/jimmunol.176.11.6962 [DOI] [PubMed] [Google Scholar]
- 40.Le Poul E., Loison C., Struyf S.et al. (2003) Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 10.1074/jbc.M301403200 [DOI] [PubMed] [Google Scholar]
- 41.Maslowski K.M., Vieira A.T., Ng A.et al. (2009) Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 10.1038/nature08530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aoyama M., Kotani J. and Usami M. (2010) Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 26, 653–661 10.1016/j.nut.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 43.Newton A.H., Cardani A. and Braciale T.J. (2016) The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin. Immunopathol. 38, 471–482 10.1007/s00281-016-0558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tulic M.K., Piche T. and Verhasselt V. (2016) Lung-gut cross-talk: evidence, mechanisms and implications for the mucosal inflammatory diseases. Clin. Exp. Allergy 46, 519–528 10.1111/cea.12723 [DOI] [PubMed] [Google Scholar]
- 45.Maeda N., Nakamura R., Hirose Y.et al. (2009) Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int. Immunopharmacol. 9, 1122–1125 10.1016/j.intimp.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 46.Graham B.S., Johnson T.R. and Peebles R.S. (2000) Immune-mediated disease pathogenesis in respiratory syncytial virus infection. Immunopharmacology 48, 237–247 10.1016/S0162-3109(00)00233-2 [DOI] [PubMed] [Google Scholar]
- 47.Wu Y.Y., Hsu C.M., Chen P.H., Fung C.P. and Chen L.W. (2014) Toll-like receptor stimulation induces nondefensin protein expression and reverses antibiotic-induced gut defense impairment. Infect. Immun. 82, 1994–2005 10.1128/IAI.01578-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsay T.B., Yang M.C., Chang W.H., Chen P.H. and Chen L.W. (2018) Lactobacillus salivarius reverse antibiotic-induced lung defense impairment in a ventilator model. J. Transl. Med. 16, 225 10.1186/s12967-018-1597-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abreu M.T. (2010) Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 10, 131–144 [DOI] [PubMed] [Google Scholar]
- 50.Kreth J., Merritt J. and Qi F. (2009) Bacterial and host interactions of oral streptococci. DNA Cell Biol. 28, 397–403 10.1089/dna.2009.0868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gurwitz D. (2020) Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 10.1002/ddr.21656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harmer D., Gilbert M., Borman R. and Clark K.L. (2002) Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 532, 107–110 10.1016/S0014-5793(02)03640-2 [DOI] [PubMed] [Google Scholar]
- 53.Hashimoto T., Perlot T., Rehman A.et al. (2012) ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487, 477–481 10.1038/nature11228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.General Office of the National Health Commission (2020) Notice on the issuance of COVID-19 diagnosis and treatment plan (for trial version 4) [EB/OL]. State Administration of Hospital Administration http://www.nhc.gov.cn/yzygj/s7653p/202001/4294563ed35b43209b31739bd0785e67.shtml [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.