Abstract
OBJECTIVES:
Geriatric nutritional risk index (GNRI) might predict the all-cause mortality in patients with heart failure (HF). We performed a meta-analysis to evaluate the correlation between GNRI and all-cause mortality in patients with HF.
METHODS:
We searched the PubMed, Medline, Cochrane Library, and Embase databases for clinical trials investigating the association between GNRI and all-cause mortality in patients with HF, having the primary endpoint as all-cause mortality.
RESULTS:
In total, nine studies involving 7,659 subjects were included in the systematic review and meta-analysis. The results indicated that major risk and moderate risk GNRI (GNRI<92) was associated with an increased risk of all-cause mortality in elderly patients with HF (hazard ratios [HR] 1.59, 95% confidence intervals [CI] 1.37-1.85). Low risk GNRI (GNRI<98) group predicted all-cause mortality in elderly HF patients (HR 1.56, 95%CI 1.12-2.18) when compared with the high GNRI value group. A subgroup analysis indicated that the relationship between GNRI and HF might differ based on the subtype of heart failure.
CONCLUSIONS:
GNRI is a simple and well-established nutritional assessment tool to predict all-cause mortality in patients with HF.
Keywords: Geriatric Nutritional Risk Index, Heart Failure, All-Cause Mortality
INTRODUCTION
Heart failure (HF) is associated with high morbidity and mortality. Approximately 1-2% of adults have HF, and the incidence increases to 10% among people aged above 70 years (1-4). The all-cause mortality rate has been reported to be 5.0-30.0% in 12 months, and the one-year hospitalization rates are 18.9-65.0% (5-7). Some studies indicated that malnutrition was common in patients with HF, and it might be useful in predicting the all-cause mortality, risk of cardiovascular (CV) events, and hospitalization for HF (11-16). Among malnutrition scores, the prognostic nutrition status (PNI), controlling nutrition status (CONUT), and geriatric nutritional risk index (GNRI) have been studied, and the GNRI is reported to be very important in elderly patients. The GNRI is calculated based on the serum albumin and body mass index (BMI). It could be a simple tool in predicting the prognosis for various cancers (8,9), such as large B-cell lymphoma, esophageal squamous cell carcinoma, and others. Some studies indicated that it could also be a simple and well-established nutritional assessment tool and a significant prognostic factor for HF. However, there have been no systematic reviews and meta-analyses on the association between GNRI and HF.
The objective of our meta-analysis was to investigate the relationship between GNRI and all-cause mortality among patients with HF.
MATERIALS AND METHODS
Search strategy
We searched for articles in PubMed, Medline, Embase, and the Cochrane Library published up to November 15, 2019, without language restrictions. The following MESH terms or text words were used for our search: HF, GNRI, malnutrition, and mortality. Completed trials among human beings whose abstracts or full texts were available were selected.
Geriatric nutritional risk index
The GNRI was calculated from the serum albumin and BMI:
GNRI=14.89* serum albumin (g/dl) +41.7*BMI/22 kg/m2
The cut-off values of GNRI were based on the report by Bouillanne et al. (10). They defined four grades of nutrition-related risk of morbidity and mortality for elderly patients: major risk (GNRI<82), moderate risk (82≤GNRI<92), low risk (92≤GNRI<98), and no risk (GNRI>98). We defined two groups of nutrition-related risk in our meta-analysis as follows: a low GNRI group (GNRI<92) with a risk of malnutrition-related morbidity and mortality and a high GNRI (GNRI≥92) group with low or no risk of malnutrition- related morbidity and mortality.
Inclusion and exclusion criteria
Two investigators independently reviewed the title and abstracts of all potentially eligible studies reporting the effect of GNRI on all-cause mortality in patients. Two researchers independently extracted the data using standardized data extraction forms and the defined eligibility criteria. Disagreements were solved by consensus. To ensure the reliability of the studies, the following criteria were used for the selection: a) Adults patients with HF, b) GNRI available and associated with all-cause mortality, c) cut-off GNRI value of 92, The following studies were excluded: a) overlapping or duplicate reports, b) Non-human experiments, c) The cut-off value not meeting our defined criteria.
Data extraction and quality assessment
After removing all duplicate citations, two researchers extracted all the outcome data independently. The following data were extracted: publication characteristics, study regions, follow-up duration, BMI, GNRI, serum albumin, and all-cause mortality/CV events. The quality of the studies selected was scored by two reviewers using Newcastle-Ottawa Scale (NOS). We considered studies as high quality if they had a score of more than six.
Statistical analysis
All data were analyzed using STATA statistical software (version 14.0). Results are presented as mean±x when normally distributed and as median and interquartile range otherwise. Heterogeneity between the studies was evaluating the Cochrane’s Q and I2 tests. A fixed-effect model was used in the absence of significant heterogeneity (I2<50%), else the random effect model was used. Publication bias in studies with different samples size was assessed by Egger’s funnel plots. We considered two-sided probability values of <0.05 as statistically significant.
RESULTS
Literature search and included studies
Initially, a total of 47 articles were selected from the primary search in the major databases. After screening the title and abstracts, 31 articles were excluded. Finally, nine studies published from 2013 to 2019 were selected (11-19). The flow diagram of the study selection is shown in Figure 1. A total of 7,659 patients were enrolled, and the characteristics of the included studies are listed in Table 1. All of them were observational studies, and six studies were conducted in Japan. All the studies reported all-cause mortality, while four studies also reported CV events. The mean follow-up duration was 2.8±1.0 years (range 189 days to 1,573 days). The mean age of the enrolled patients was 74.7±5.1 years, and 37.9% of them were female. Three studies included HF patients with preserved ejection fraction (11-12,19), while two studies included patients with acute HF (17-18), four studies had a mixed cohort (13-16). The hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were selected from each study. According to the Newcastle-OttawaScale scores (NOS), all cohort studies were of high quality and had scores of six or more.
Figure 1. Flowchart of the selection of published studies selected for the meta-analysis.
Table 1. Main characteristics of the included studies.
| Study | Heart failure type | Number of patients | Age (year, mean±s) | Female (n,%) | Follow-up | Country | All-cause mortality/CV events | BMI kg/m2 | Serum albumin (g/l) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Minamisawa et al. (11) | HFpEF | 1677 | 72.4 (64.1, 79.5) | 516 (48.5) | 2.9 years | USA, Canada, Brazil, Argentina | All-cause mortality, CV events | 32.4 (27.8,37.8) | 4.1 (3.9,4.3) | 8 |
| Nishi et al. (12) | HFpEF | 110 | 78.5±7.2 | 51 (46.4) | 503 days | Japan | All-cause mortality | 23.1±4.1 | 3.6 (3.2,3.9) | 7 |
| Nakamura et al. (13) | HF | 213 | 87.2±4.9 | 120 (56.3) | 540 days | Japan | All-cause mortality | low 18.2±2.3 high 23.0±3.0 | low 3.2±0.4 high 3.7±0.3 | 7 |
| Yoshihisa et al. (14) | HF | 1274 | 66.5 | 482 (37.8) | 1146 days | Japan | All-cause mortality | NA | NA | 7 |
| Sze et al. (15) | HF | 3386 | 75.0 (67.0, 81) | 1320 (39.0) | 1573 days | UK | All-cause mortality | NA | NA | 7 |
| Sargento et al. (16) | HFrEF | 143 | 75.4±6.5 | 43 (30.1) | 3 years | Portugal | All-cause mortality | 28.8±7.9 | 4.1±0.5 | 7 |
| Ouchi et al. (17) | Acute HF | 214 | 73.0 (64.0, 82.0) | 99 (46.3) | 22.6±14.2 months | Japan | All-cause mortality | 22.9 (20.4-25.7) | 3.4 (3.0,3.6) | 7 |
| Honda et al. (18) | Acute HF | 490 | 79.0±7.0 | 203 (41.0) | 189 days | Japan | All-cause mortality, CV events | 22.6±3.7 | 3.8 (3.4,4.0) | 7 |
| Kinugasa et al. (19) | HFpEF | 152 | 77.0±11.0 | 70 (46.1) | 2.1 years | Japan | All-cause mortality, CV events | 21.7±4.0 | 3.5±0.5 | 7 |
HFpEF heart failure with preserved ejection fraction; CV cardiovascular; BMI body mass index; NOS Newcastle-Ottawa Scale.
GNRI and all-cause mortality in HF
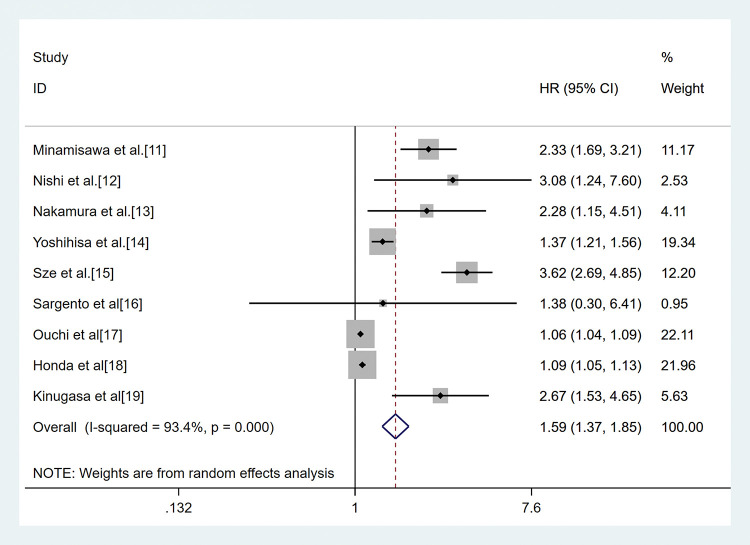
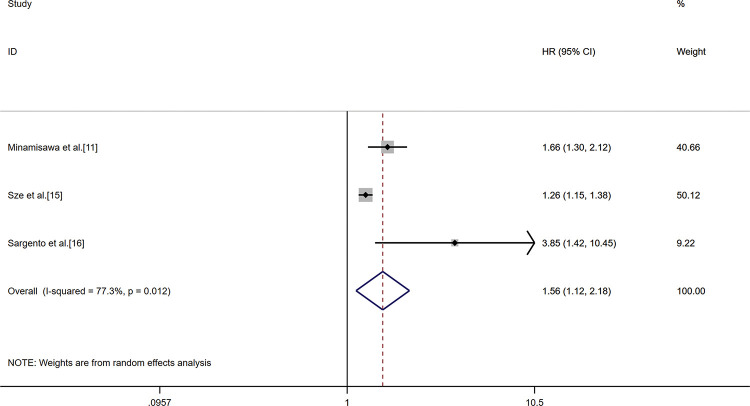
The combined analysis of nine cohorts, including 7,659 patients, showed the association between GNRI and all-cause mortality. Patients were divided into two malnutrition-related risk groups: moderate to serve risk (GNRI<92), low or no risk (GNRI≥92). The pooled outcome for the low GNRI group showed a 1.59 (95%CI 1.37-1.85) times higher risk compared with the high GNRI group (p<0.01, random effect, Figure 2) for all-cause mortality. Three studies had a GNRI cut-off value of 98 (11,15-16). The analysis indicated that the low GNRI group had higher all-cause mortality in HF (HR 1.56, 95%CI 1.12-2.18, (p<0.01, random effect) compared to the high GNRI group (Figure 3).
Figure 2. Result of the meta-analysis of HR values for all-cause mortality (the cut-off value of GNRI was 92). Each square denotes the HR for each trial compared with the corresponding 95% confidence intervals. The size of the square is directly proportional to the amount of information contributed by the trial. The diamonds represent the overall outcome of HR and 95%CI.
Figure 3. Result of the meta-analysis of HR values for all-cause mortality (the cut-off value of GNRI was 98). Each square denotes the HR for each trial compared with the corresponding 95% confidence intervals. The size of the square is directly proportional to the amount of information contributed by the trial. The diamonds represent the overall outcome of HR and 95%CI.
Subgroup analysis of GNRI and all-cause mortality
Due to extreme heterogeneity between the studies (I2=93.7%, p<0.01), we conducted four subgroup analyses based on the study region (western countries, Eastern countries), sample size (>500 and ≤500), age (>75 years and ≤75 years), follow-up duration (>2 years and ≤2 years), and type of heart failure [heart failure with preserved ejection fraction (HFpEF), Acute heart failure, Mixed]. When grouped by study region, GNRI predicted the all-cause mortality in Western countries (HR 2.78, 95%CI 1.86-4.17, random effect) (11,15-16) as well as Eastern countries (HR 1.21, 95%CI 1.09-1.34, random effect) (12-14,17-19). On comparison by sample size, similar results were seen in groups with large sample (HR 2.24, 95%CI 1.20-2.18, random effect) (11,14-15) and small sample size (HR 1.13, 95%CI 1.03-1.24, random effect) (12-13,16,18-19). The prognostic role of GNRI in predicting all-cause mortality was seen in subjects >75 years old (HR 1.91, 95%CI 1.07-3.38, random effect) (12-13,15,17-18) and ≤75 years old (HR 1.82, 95%CI 1.21-2.73, random effect) (11,14-15,17-18). GNRI predicted all-cause mortality in the groups with short duration (HR 1.09, 95%CI 1.02-1.17, random effect) (12-13,15,17-18) as well as long duration of follow-up (HR 2.23, 95%CI 1.35-3.68, random effect) (11,14-16,19). Three studies enrolled patients with HFpEF (11-12,19), while two studies included patients of acute heart failure (17-18), four studies included a mixed cohort. GNRI predicted all-cause mortality in the HFpEF group (HR 2.46, 95%CI 1.88-3.21, random effect), acute heart failure group (HR 1.07, 95%CI 1.05-1.09, random effect), and the mixed group (HR 2.10, 95%CI 1.07-4.12, random effect). The results are listed in Table 2.
Table 2. Results of the subgroup analysis on prognostic significance of GRNI in HF.
| Analysis | Categories | No of Studies (n) | Patients (n) | Model | HR (95%CI) | Z | p | I2 (%) | ph |
|---|---|---|---|---|---|---|---|---|---|
| Overall | 9 | 7659 | Random | 1.59 (1.37, 1.85) | 5.96 | 0.00 | 93.40 | 0.00 | |
| Study region | Western country | 3 | 5206 | Random | 2.78 (1.86, 4.17) | 4.95 | 0.00 | 58.70 | 0.08 |
| Eastern country | 6 | 2453 | Random | 1.21 (1.09, 1.34) | 3.58 | 0.00 | 85.80 | 0.00 | |
| Sample size | >500 | 3 | 6337 | Random | 2.24 (1.20, 4.18) | 2.52 | 0.01 | 95.00 | 0.00 |
| ≤500 | 6 | 1332 | Random | 1.13 (1.03, 1.24) | 2.57 | 0.01 | 76.30 | 0.00 | |
| Age | >75 years | 4 | 1108 | Random | 1.91 (1.07, 3.38) | 2.21 | 0.02 | 79.50 | 0.00 |
| ≤75 years | 5 | 6551 | Random | 1.82 (1.21, 2.73) | 2.89 | 0.00 | 97.00 | 0.00 | |
| Follow-up duration | >2 years | 5 | 6632 | Random | 2.23 (1.35, 3.68) | 3.14 | 0.00 | 90.60 | 0.00 |
| ≤2 years | 4 | 1027 | Random | 1.09 (1.02, 1.17) | 2.48 | 0.01 | 72.10 | 0.01 | |
| HF type | Acute HF | 2 | 704 | Random | 1.07 (1.05, 1.09) | 6.24 | 0.00 | 0.00 | 0.36 |
| HFpEF | 3 | 1939 | Random | 2.46 (1.88, 3.21) | 6.61 | 0.00 | 0.00 | 0.81 | |
| Mixed | 4 | 5106 | Random | 2.10 (1.07, 4.12) | 2.15 | 0.03 | 91.70 | 0.00 |
GRNI geriatric nutritional risk index; HF heart failure; HFpEF heart failure with preserved ejection fraction; HR hazard ratios; CI confidence interval; ph p value of heterogeneity.
Sensitivity analysis and publication bias
Sensitivity analysis indicated that some included studies led to heterogeneity in the meta-analysis (Figure 4). The subgroup analysis indicated that the type of HF might be the main cause of heterogeneity, and further studies should focus on the predictive role of GNRI by the subtype of HF. There was some publication bias seen on Egger’s funnel plot, which might have been caused due to the small number of studies included.
Figure 4. Result of the sensitivity analysis. The middle vertical line indicates the combined OR, and the two vertical lines represent the 95% CI values. Every round hollow indicates the pooled OR when the left study was omitted in a meta- analysis with a random model.
DISCUSSION
HF is a growing health problem and is associated with high mortality. In patients with HF, malnutrition is common due to the change in systemic metabolism, the increase of body consumption and the dysfunction of gastrointestinal. A previous study indicated that approximately one-third of the patients with HF had accompanying malnutrition (11) and that malnutrition was associated with an increased risk for CV events, hospitalization due to HF, and all-cause death.
The Geriatric Nutritional Risk Index (GNRI) is an-elderly-specific index that has been proposed to assess the nutrition-related risk of morbidity and mortality for elderly patients.Some studies demonstrated that low GNRI was associated with an increased risk for cancer and other diseases (8,20). Our meta-analysis confirmed that GNRI could be a simple and feasible tool to assess nutrition in patients with HF.
The present study indicated that GNRI was associated with an increased risk for all-cause mortality in patients with HF. Patients in our study were divided into two groups, low GNRI (<92) indicating moderate to serve malnutrition, and high GNRI (≥92) indicating low or no risk of malnutrition. Our meta-analysis indicates that low GNRI predicted all-cause mortality in patients with HF (p<0.05). A further analysis involving 5206 subjects demonstrated that even a low risk of malnutrition (<98) was associated with an increased risk of all-cause mortality in patients with HF, which was in line with previous studies. Mastatoshi et al. also reported that low GNRI was strongly associated with increased CV events and re-hospitalization due to HF.
In our subgroup analysis, we grouped patients by region, age, sample size, follow-up duration, and subtype of HF. The analysis of the correlation between GNRI and clinical features demonstrated the prognostic role of GNRI in predicting mortality was higher in studies in the Western countries, those with large sample size, and having a long-term follow-up. One study enrolled extremely old patients (>80 years old), while patients in the other studies were elderly (≥65 years old). The result demonstrated that an association between low GNRI and poor prognosis in patients with HF was seen at all ages. In the analysis by subtype of HF, the significance of GNRI in predicting all-cause mortality in acute HF was low, while it was high in chronic HF. Thus, low GNRI might be better in predicting all-cause mortality in chronic heart failure than acute heart failure, especially in HFpEF.
There was some heterogeneity in our study; we conducted some subgroup and sensitivity analyses to detect the origin of the heterogeneity. The subgroup analysis indicated the type of HF as the main cause of heterogeneity. The included studies comprised patients with acute heart failure, HFpEF, HFrEF, and mixed type of heart failure, which might lead to some heterogeneity. However, the limited number of studies precluded a more detailed analysis of classification.
GNRI was calculated from serum albumin and BMI, and was a more useful parameter for predicting mortality than BMI and serum albumin in HF. Seven of the included studies reported BMI, of which the mean BMI was above 28 kg/m2 in one study, while it was below 28 kg/m2 in others. GNRI might underestimate malnutrition in overweight patients. Moreover, different nutrition-related risk results might be seen with different assessment methods, such as CONUT or PNI. Hence, future studies should focus on a better method to estimate the nutrition in HF patients.
To the best of our knowledge, this is the first meta-analysis that analyzed the association between GNRI and all-cause mortality in patients with HF. However, there are several limitations of the present systematic review and meta-analysis. First, some of the included studies had a small sample size in the low GNRI group, which could have affected the results. Second, studies that lacked information concerning the GNRI and mortality were excluded from the analyses. Third, the number of women enrolled was lower than men, which might have influenced the results. The population in studies from Eastern countries was entirely from Japan, which might have influenced some results. Besides, all the included studies were observational studies, which can influence the accuracy of the study. Despite these limitations, GNRI could be an effective marker in predicting the risk of mortality in HF.
CONCLUSIONS
In elderly patients with HF, GNRI is a simple and well-established nutritional assessment tool that can help in predicting all-cause mortality. More studies should focus on GNRI and the subtype of HF and suggest methods to improve malnutrition in patients with HF.
AUTHOR CONTRIBUTIONS
Dong CH and Chen SY designed and conceived this study. Dong CH and Chen SY completed data collection and manuscript writing. Pan J participated in data analysis and manuscript preparation. Zeng HL and Yang B provided important suggestions for article revision. Chen SY approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank all authors whose publications were included in our meta-analysis.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 2.Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25(18):1614–9. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 3.Ceia F, Fonseca C, Mota T, Morais H, Matias F, de Sousa A, et al. EPICA Investigators. Prevalence of chronic heart failure in Southwestern Europe: the EPICA study. Eur J Heart Fail. 2002;4(4):531–9. doi: 10.1016/S1388-9842(02)00034-X. [DOI] [PubMed] [Google Scholar]
- 4.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–46. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, et al. Heart Failure Association of the European Society of Cardiology (HFA). EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot) Eur J Heart Fail. 2013;15(7):808–17. doi: 10.1093/eurjhf/hft050. [DOI] [PubMed] [Google Scholar]
- 6.Hendricks V, Schmidt S, Vogt A, Gysan D, Latz V, Schwang I, et al. Case management program for patients with chronic heart failure: effectiveness in terms of mortality, hospital admissions and costs. Dtsch Arztebl Int. 2014;111(15):264–70. doi: 10.3238/arztebl.2014.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly JP, Hammill BG, Doll JA, Felker GM, Heidenreich PA, Bhatt DL, et al. The Potential Impact of Expanding Cardiac Rehabilitation in Heart Failure. J Am Coll Cardiol. 2016;68(9):977–8. doi: 10.1016/j.jacc.2016.05.081. [DOI] [PubMed] [Google Scholar]
- 8.Yamana I, Takeno S, Shimaoka H, Yamashita K, Yamada T, Shiwaku H, et al. Geriatric Nutritional Risk Index as a prognostic factor in patients with esophageal squamous cell carcinoma -retrospective cohort study. Int J Surg. 2018;56:44–8. doi: 10.1016/j.ijsu.2018.03.052. [DOI] [PubMed] [Google Scholar]
- 9.Kanemasa Y, Shimoyama T, Sasaki Y, Hishima T, Omuro Y. Geriatric nutritional risk index as a prognostic factor in patients with diffuse large B cell lymphoma. Ann Hematol. 2018;97(6):999–1007. doi: 10.1007/s00277-018-3273-1. [DOI] [PubMed] [Google Scholar]
- 10.Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–83. doi: 10.1093/ajcn/82.4.777. [DOI] [PubMed] [Google Scholar]
- 11.Minamisawa M, Seidelmann SB, Claggett B, Hegde SM, Shah AM, Desai AS, et al. Impact of Malnutrition Using Geriatric Nutritional Risk Index in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2019;7(8):664–75. doi: 10.1016/j.jchf.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Nishi I, Seo Y, Hamada-Harimura Y, Yamamoto M, Ishizu T, Sugano A, et al. Ibaraki Cardiovascular Assessment Study-Heart Failure Investigators. Geriatric nutritional risk index predicts all-cause deaths in heart failure with preserved ejection fraction. ESC Heart Fail. 2019;6(2):396–405. doi: 10.1002/ehf2.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura T, Matsumoto M, Haraguchi Y, Ishida T, Momomura SI. Prognostic impact of malnutrition assessed using geriatric nutritional risk index in patients aged ⩾80 years with heart failure. Eur J Cardiovasc Nurs. 2020;19(2):172–7. doi: 10.1177/1474515119864970. [DOI] [PubMed] [Google Scholar]
- 14.Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Miyata M, et al. Impact of nutritional indices on mortality in patients with heart failure. Open Heart. 2018;5(1):e000730. doi: 10.1136/openhrt-2017-000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sze S, Pellicori P, Kazmi S, Rigby A, Cleland JGF, Wong K, et al. Prevalence and Prognostic Significance of Malnutrition Using 3 Scoring Systems Among Outpatients With Heart Failure: A Comparison With Body Mass Index. JACC Heart Fail. 2018;6(6):476–86. doi: 10.1016/j.jchf.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Sargento L, Vicente Simões A, Rodrigues J, Longo S, Lousada N, Palma Dos Reis R. Geriatric nutritional risk index as a nutritional and survival risk assessment tool in stable outpatients with systolic heart failure. Nutr Metab Cardiovasc Dis. 2017;27(5):430–7. doi: 10.1016/j.numecd.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Ouchi S, Miyazaki T, Shimada K, Sugita Y, Shimizu M, Murata A, et al. Low Docosahexaenoic Acid, Dihomo-Gamma-Linolenic Acid, and Arachidonic Acid Levels Associated with Long-Term Mortality in Patients with Acute Decompensated Heart Failure in Different Nutritional Statuses. Nutrients. 2017;9(9):956. doi: 10.3390/nu9090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda Y, Nagai T, Iwakami N, Sugano Y, Honda S, Okada A, et al. Usefulness of Geriatric Nutritional Risk Index for Assessing Nutritional Status and Its Prognostic Impact in Patients Aged ≥65 Years With Acute Heart Failure. Am J Cardiol. 2016;118(4):550–5. doi: 10.1016/j.amjcard.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 19.Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, et al. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J. 2013;77(3):705–11. doi: 10.1253/circj.CJ-12-1091. [DOI] [PubMed] [Google Scholar]
- 20.Kuo IC, Huang JC, Wu PY, Chen SC, Chang JM, Chen HC. A Low Geriatric Nutrition Risk Index Is Associated with Progression to Dialysis in Patients with Chronic Kidney Disease. Nutrients. 2017;9(11):1228. doi: 10.3390/nu9111228. [DOI] [PMC free article] [PubMed] [Google Scholar]