Summary
The insect sex determination and the intimately linked dosage compensation pathways represent a challenging evolutionary puzzle that has been solved only in Drosophila melanogaster. Analyses of orthologs of the Drosophila genes identified in non-drosophilid taxa1,2 revealed that evolution of sex determination pathways is consistent with a bottom-up mode,3 where only the terminal genes within the pathway are well conserved. doublesex (dsx), occupying a bottom-most position and encoding sex-specific proteins orchestrating downstream sexual differentiation processes, is an ancient sex-determining gene present in all studied species.2,4,5 With the exception of lepidopterans, its female-specific splicing is known to be regulated by transformer (tra) and its co-factor transformer-2 (tra2).6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Here we show that in the African malaria mosquito Anopheles gambiae, a gene, which likely arose in the Anopheles lineage and which we call femaleless (fle), controls sex determination in females by regulating splicing of dsx and fruitless (fru; another terminal gene within a branch of the sex determination pathway). Moreover, fle represents a novel molecular link between the sex determination and dosage compensation pathways. It is necessary to suppress activation of dosage compensation in females, as demonstrated by the significant upregulation of the female X chromosome genes and a correlated female-specific lethality, but no negative effect on males, in response to fle knockdown. This unexpected property, combined with a high level of conservation in sequence and function in anopheline mosquitoes, makes fle an excellent target for genetic control of all major vectors of human malaria.
Keywords: Anopheles gambiae, transgenesis, sex determination pathway evolution, female-specific lethality, malaria vectors, genetic vector control
Highlights
-
•
fle is a new sex determination pathway element conserved in Anopheles mosquitoes
-
•
fle may have originated in the Anopheles lineage and is highly conserved in Anopheles
-
•
fle suppresses activation of dosage compensation in females
-
•
Depletion of fle transcripts is lethal or otherwise deleterious to females
Krzywinska et al. identify a new element of the sex determination pathway in Anopheles. femaleless (fle), in addition to controlling splicing of dsx and fru, is essential for suppression of dosage compensation and viability of females. Female-deleterious effects upon fle knockdown make fle a promising target for control of malaria mosquitoes.
Results and Discussion
fle Is a Sex Determination Pathway Component in Anopheles
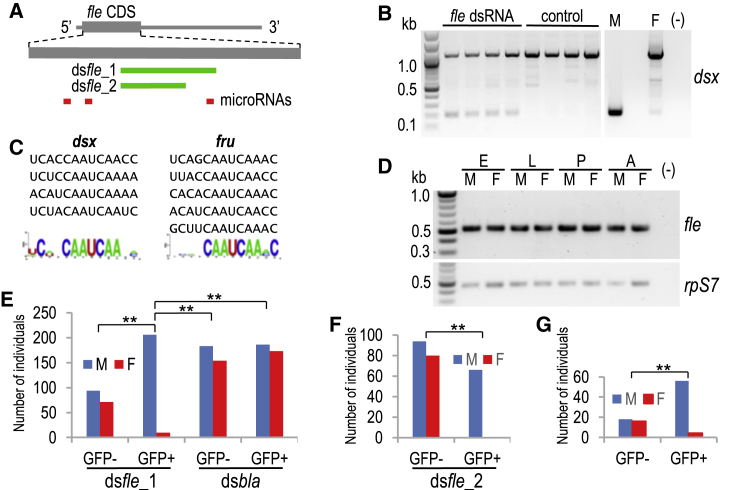
In the mosquitoes from the genus Anopheles, sex is chromosomally determined, with the XX individuals developing as females and the XY individuals developing into males. Three components from the molecular pathway controlling this process have been identified in the African malaria mosquito Anopheles gambiae: a Y-chromosome-linked primary signal gene, Yob, conferring maleness21,22 and, on the opposite end, dsx23 and fru.24 To search for molecules transducing the sex-determining instruction, we queried the A. gambiae genome with the D. melanogaster Tra2 sequence using a BLAST algorithm. Tra2 is an RNA-binding protein with a characteristic structure consisting of an RNA recognition motif (RRM) flanked by two arginine-rich/serine-rich (RS) domains containing multiple serine-arginine dipeptides.25 In all significant BLAST hits, sequence similarity was limited to the RRM region. The top two hits, AGAP029421 (e value = 1e−28) and AGAP006798 (e value = 1e−25), encoding proteins with a structure typical of Tra2, are regarded here as the tra2 homologs, named, respectively, tra2b and tra2a. To assess whether they are involved in sex determination, we investigated the effect of their transient knockdown on dsx splicing in the A. gambiae Sua5.1 female-like cell line. Transfection experiments using the in-vitro-synthesized double-stranded RNA (dsRNA) of either tra2a or tra2b did not yield the expected change in the dsx splicing pattern from the female to the male mode, indicating that neither has the sex-determining role (Figures S1A and S1B), consistent with the findings that tra2 homologs have no role in dsx splicing in a culicine mosquito, Aedes albopictus.26 In contrast, knockdown of the third-best hit, AGAP013051 (e value = 5e−18), caused a clear decrease of the female, and gain of the male, dsx transcript isoforms, as compared with the control non-transfected Sua5.1 cells (Figures 1A, 1B, and S1C). This result, further validated in transgenic A. gambiae lines with a stable AGAP013051 knockdown (see below), demonstrated that AGAP013051 represents a sex determination pathway element regulating dsx splicing in A. gambiae females.
Figure 1.
fle Is a Sex Determination Gene Necessary for dsx Splicing and Survival in Anopheles Females
(A) A schematic of the A. gambiae fle transcript, with the location of RNAi targets within the coding sequence.
(B) RT-PCR analysis of the dsx splicing pattern in A. gambiae Sua5.1 cells transfected with in vitro-synthesized fle_1 dsRNA, as compared with control non-transfected cells.
(C) Sequences of the putative Fle binding sites in the A. gambiae dsx and fru female-specific exons. Presented are 13-nt fragments with similarity to the Drosophila repeat elements [TC(T/A)(T/A)CAATCAACA].
(D) RT-PCR analysis of fle transcription during A. gambiae development. E, embryos; L, larvae; P, pupae; A, adults; (−), negative control; M, male; F, female. Ribosomal protein S7 (rpS7) transcript levels were used as a gel loading control.
(E and F) Knockdown of fle expression causes female-specific lethality in A. gambiae embryos. A summary of three independent microinjection experiments using fle_1 (E) and fle_2 (F) dsRNA fragments, with bla dsRNA used as control. GFP−/+ denote cohorts of individuals scored during the first larval instar as GFP-negative or GFP-positive. ∗∗p < 0.0001; Fisher’s exact test.
(G) Knockdown of fle ortholog expression causes female-specific lethality in A. stephensi embryos. A summary of two independent fle dsRNA microinjection experiments. GFP−/+ as in (E). ∗∗p < 0.0001; Fisher’s exact test.
See also Figures S1–S3.
Apart from similarity within the RRM, AGAP013051 substantially differs from insect tra2 orthologs in the length of the coding region (420 amino acids compared to 232–299 amino acids in the described Tra2 proteins)10,11,14,16,27, 28, 29, 30 and structure of the encoded protein, including lack of typical RS domains and presence of two additional putative RRM regions in the N-terminal half of the protein (Figures S2A and S2B). Combined with the results of a phylogenetic analysis, in which AGAP013051 does not cluster with Tra2 proteins but is grouped with a putative splicing factor, Nix, functioning as the male determiner in Aedes aegypti31 (Figures S2C and S2D), the above indicates that AGAP013051 and tra2 may be only distantly related. We named the gene femaleless (fle), to reflect the associated knockdown phenotypes described below.
The RRM is a highly abundant motif in various eukaryotic proteins. It folds into a β1α1β2β3α2β4 sandwich topology, with the β1 and β3 sheets encompassing, respectively, ribonucleoprotein 2 (RNP2) and RNP1 sequence elements that are required for specific binding to the RNA sequences.25,32,33 In Drosophila, Tra2 molecules bind six 13-nt repeat elements [UC(U/A)(U/A)CAAUCAACA], clustered on the dsx pre-mRNA within the untranslated region (UTR) of the female-specific dsx exon 4, to promote the use of an adjacent upstream weak splice acceptor site, leading to splicing of dsx into the female form.8,34,35 fle may directly target a (U/A)C(U/A)(U/C/A)CAAUCAA(U/C/A)(C/A) sequence, which forms a four-repeat cluster within the UTR of the A. gambiae female-specific dsx exon 5 (Figure 1C). These putative fle targets map to regions of very low nucleotide diversity in natural A. gambiae populations36 and coincide with blocks of increased conserved sequence across Anopheles species (data not shown), which lends support to the notion of their functional significance. The A. gambiae fru gene, whose sex-specific splicing is also fle dependent (this study; see below), contains three putative fle binding sites clustered at the 5' end of the female-specific exon 3 and two binding sites in the sex-specifically processed exon 4. Their consensus sequence shares the same invariant core (CAATCAA) with the A. gambiae dsx repeat elements, as well as with the counterparts from the Drosophila dsx and fru (Figure 1C). Considerable variation within the nucleotide positions flanking the core suggests that only the core may be important for efficient fle binding.
We queried the NCBI whole-genome shotgun contigs database with the fle amino acid sequence using tBLASTn to evaluate the phylogenetic distribution of the fle orthologs. Significant hits were identified only in the genomes of genus Anopheles representatives, with highly conserved 1-to-1 orthologs detected in each case (Figure S3A), indicating that fle has been subject to strong functional constraints throughout approximately 100 million years of Anopheles lineage evolution.37,38 A lack of discernible orthologs beyond anopheline genomes suggests that fle may have originated in a recent ancestor of the Anopheles lineage. To test whether fle could have arisen through a gene duplication, we searched the A. gambiae annotated proteins for sequences similar to Fle. The top hit was to AGAP001643 (the gene restricted to the family Culicidae [mosquitoes]), which at the amino acid level shares a significant similarity (BLASTp, e value = 4e−20) to the N-terminal half of Fle and a lower similarity to the C-terminal RRM (Figures S3B and S3C). The first intron in AGAP001643 and the intron in fle split a codon located at the putative homologous alignment position, further suggesting a common origin of the two genes. Thus, fle may represent an ancient paralog of AGAP001643, which after a gene duplication rapidly diverged to assume essential developmental functions. In that case, the similarity between Tra2 and Fle within the RRM could represent a case of convergent evolution driven by the adaptation of fle to efficiently bind highly conserved target sequences. Alternatively, fle could have originated from a fusion between an AGAP001643 copy and a tra2 copy, followed by subsequent rearrangements. A likely evolutionary scenario cannot be reliably inferred because the sequences in question are highly divergent.
Transient fle Knockdown Kills Anopheles Female Embryos
The fle gene produces a single transcript and is constitutively expressed throughout development in both A. gambiae sexes (Figure 1D). To assess whether a transient fle knockdown has an effect on A. gambiae early development, we injected the fle dsRNA into non-sexed preblastoderm embryos using an established protocol.21 The injection mix contained a control plasmid with a green fluorescent protein (GFP) expression cassette, which allowed for an easy identification of individuals that received sufficient amounts of nucleic acids. Surviving first-instar larvae were sorted into GFP-negative and GFP-positive cohorts, and at the pupal stage mosquitoes were sexed. A very strong male bias was observed among the GFP-positive mosquitoes (Figures 1E and 1F), whereas in the GFP-negative group and in the control group of A. gambiae embryos injected with heterologous bla dsRNA the sex ratio was not significantly biased. A random sample of individuals (n = 20) from the strong GFP cohort were sexed using PCR21 and confirmed to have the XY karyotype (data not shown). Similar results, with a strongly male-biased survival, were obtained after microinjection of Anopheles stephensi fle dsRNA into embryos of A. stephensi (Figure 1G), a species that diverged from the A. gambiae lineage over 40 million years ago (mya).37,39 These results indicated that embryonic knockdown of fle is lethal to genetic females but apparently has no discernible effect on the development of genetic males. The female death was occurring during the embryo stage, because the numbers of hatched GFP-positive larvae were comparable to the numbers of pupating individuals.
fle Links the Sex Determination and Dosage Compensation Pathways
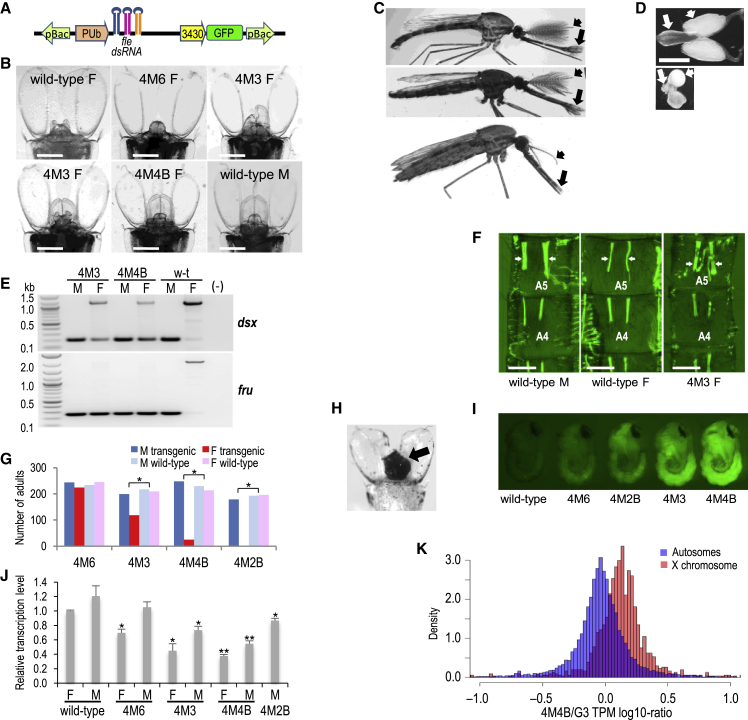
Using piggyBac-based transgenesis, we generated six A. gambiae lines that stably produce a polycistronic transcript encoding three microRNAs designed to silence expression of fle (Figure 2A). In each line, fle knockdown affected the development of females, with phenotypic effects defined by the genomic location of the transgene insertions (none known to interrupt a gene; Table S1). The abnormalities in sexually dimorphic characters ranged from mild, such as asymmetric development or loss of cerci (terminal appendages of the female abdomen) in line 4M6, to an extensive masculinization manifested in the development of a nearly normal male copulatory apparatus and male-like head appendages in line 4M4B (Figures 2B and 2C). The internal reproductive system was similarly affected, including underdeveloped or atrophied ovaries, atrophied spermatheca, partially developed male accessory glands, and a rudimentary ejaculatory pump (Figure 2D). The level of masculinization of transgenic females was correlated with a substantially altered splicing of dsx (similar to the effects of fle knockdown in Sua5.1 cells) and, additionally, fru (Figure 2E), whose sex-specific splicing is conserved between Anopheles and Drosophila.24 In Drosophila, female-specific fru transcripts do not code for a functional protein, whereas the male-specific isoforms encode a protein produced in a small subset of neurons in the central nervous system, where it regulates male sexual behavior40 and specifies the development of a male-specific muscle of Lawrence (MOL) in the fifth abdominal segment.41 The fru ortholog apparently performs the same function in A. gambiae.24 Consistent with that notion, we found a male-like, bilaterally paired muscle reminiscent of MOL in the fifth abdominal segment of the A. gambiae transgenic females (Figure 2F). Moreover, in none of the lines were transgenic females attracted to a blood source, likely because they produce the male form of fru.
Figure 2.
Stable fle Knockdown in A. gambiae Causes Female Masculinization and Death through Abnormal Upregulation of Transcription from the X Chromosome
(A) Schematic representation of the transgenic fle knockdown construct.
(B) Pupal abdominal termini of the wild-type and fle knockdown transgenic mosquito lines. Scale bars, 200 μm.
(C) Adults. From top: wild-type male, 4M4B line female, wild-type female. Note male-like antennae (short arrows) and palps (long arrows), and a slender abdomen with a male-like uninverted copulatory apparatus.
(D) Reproductive system: ejaculatory pump (long white arrows) and accessory glands (short white arrows) in a wild-type male and 4M4B female. Scale bar, 100 μm.
(E) RT-PCR analysis of dsx and fru splicing patterns in females from selected transgenic lines.
(F) A sexually dimorphic pair of dorsal muscles (indicated by white arrows) in the fifth segment of the abdomen (A5), likely homologous to the Drosophila muscle of Lawrence, is of similar thickness in wild-type males and transgenic females, and considerably thicker than in wild-type females. Scale bars, 200 μm.
(G) Proportions of individuals reaching adulthood in selected transgenic lines. The lines have been maintained by backcrossing of transgenic males with the wild-type line females at every generation, which should yield 50% of wild-type progeny and equal proportions of the sexes in the transgenic and wild-type groups. However, apart from line 4M6, a highly significant deficiency of transgenic females was observed (∗p < 0.0001; chi-square test). In line 4M2B, no transgenic females developed beyond the embryonic stage.
(H) Pupal abdominal terminus with a characteristic black tumor (arrow) in a 4M4B female.
(I) Fluorescent marker expression in selected transgenic lines.
(J) Levels of fle transcripts in the pupae of the wild-type line G3 and in selected transgenic lines quantified by qRT-PCR and normalized relative to the fle/rpS7 transcription level in wild-type females. The error bars represent standard deviations. ∗p < 0.01, ∗∗p < 0.001; Student’s t test.
(K) Comparison of transcription levels from the autosomes and the X chromosomes in transgenic line 4M4B and wild-type line G3 females. Shown are transcripts per kilobase million (TPM) ratios. The median expression ratio between the 4M4B and G3 lines for the autosomes (0.92) and for the X chromosomes (1.34) indicates that the X chromosome genes in the transgenic line females are significantly overexpressed; p < 10−15; Mann-Whitney U test. A similar trend for the median shrunken fold change for the autosomal (0.985) and for the X linked (1.22) transcripts confirms that the effect is statistically robust.
See also Table S1.
Beyond masculinization, fle depletion caused partial or complete female lethality in some lines (Figure 2G). In line 4M2B, only one-third of hatched larvae at each generation were transgenic. They developed exclusively as males, which indicated that transgenic females were dying during embryonic stage, consistent with the female-specific embryonic lethality in transient fle knockdown experiments. In line 4M4B, female death was occurring during late developmental stages; a small proportion of individuals that survived to adulthood died shortly (up to 3 days) after eclosion. The majority of females dying as pupae developed a distinctive black tumor at the abdominal terminus (Figure 2H). In contrast to females, males appeared not to be affected in any of the transgenic lines.
The severity of the aberrant female development in transgenic lines was roughly correlated with the level of expression of the GFP transformation marker (Figure 2I) and with the extent of the fle knockdown (Figure 2J) in pupae. Line 4M2B, with an invariable female embryonic-lethal phenotype but with a relatively low GFP expression and a low fle knockdown level in male pupae, represented a notable exception, suggesting an involvement of an embryonic enhancer driving higher microRNA expression and a resulting increased fle knockdown during early development. Overall, the observed levels of knockdown were surprisingly low, even in a line exhibiting strong masculinization (Figure 2J), suggesting that fle is a haploinsufficient gene. Presumably, fle knockdown below a certain critical threshold leads to female lethality.
Sex-specific lethality caused by loss-of-function mutations or knockdown of sex determination genes results from misregulation of dosage compensation in Drosophila and a silk moth, Bombyx mori.19,42, 43, 44 In Drosophila, dosage compensation relies on a two-fold upregulation of transcription from the single male X chromosome to the levels of expression from both X chromosomes in females.45 Dosage compensation machinery is not assembled in Drosophila females, because SXL, a female-specific protein involved in sex determination, prevents translation of MSL-2, a key protein of the dosage compensation complex.46 Mutations in Sxl, or in genes involved in Sxl regulation, lead to overexpression of X linked genes and female death during embryogenesis.42,43,47,48 In A. gambiae, dosage compensation also operates by upregulation of the X chromosome in males and that process is controlled by the primary sex determiner gene Yob.21,49 Similarly, in A. stephensi, the X chromosome dosage in males is regulated by the Y linked maleness gene guy-1.50 Female-specific lethality observed in this study suggests that dosage compensation is activated in Anopheles females in response to depletion of fle transcripts. To evaluate whether misregulation of the X chromosome transcription is indeed involved, we compared transcriptomes of female pupae from a wild-type line and from the 4M4B transgenic line, in which female lethal effects occur during late stages of development. Relative to the autosomes, transcription from the X chromosomes in transgenic females was significantly upregulated (Mann-Whitney U test, p < 10−15), with an overall transcription increase by more than 40% (Figure 2K), which is apparently toxic and leads to preimaginal death. The X-chromosome-wide overtranscription caused by misregulation of dosage compensation mechanisms mimics X chromosome aneuploidy, and is known to lead to tumorigenesis in Drosophila and cancers in mice,51,52 and apparently is the cause of abdominal tumors developing in the 4M4B female pupae (Figure 2G). However, tumors in the genital area were also observed in a moth, Plutella xylostella, female mutants with disrupted dsxF transcripts.53
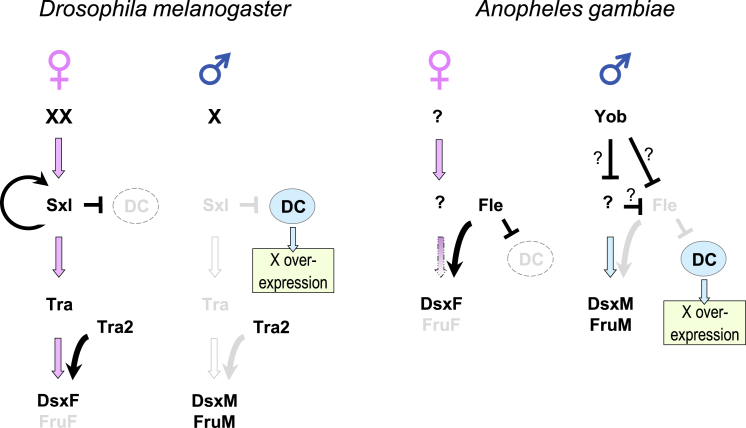
Control of the dsx and fru female-specific splicing by a non-sex-specifically expressed fle provokes a question about the mechanism of that process. In most studied holometabolous insects, the female specificity of dsx splicing relies on Tra, which is produced only in females, and whose interaction with Tra2 via RS domains is necessary to stabilize the spliceosome assembly on the weak acceptor splice site.8,9,11,14,16,25 Considering an apparent absence of tra in the A. gambiae genome,2,54 as well as a larger size and a structural dissimilarity of Fle as compared to Tra2, it is conceivable that Fle does not have an obligatory Tra-like partner and that binding of Fle alone to its putative targets (Figure 1C) is sufficient to promote the spliceosome assembly in females, whereas in males that process is interrupted by Yob (Figure 3).
Figure 3.
Proteins Determining Sex in Somatic Tissues of D. melanogaster and A. gambiae
In fruit fly females, a double dose of X linked elements activates productive transcription of Sxl, leading to establishment of a positive feedback loop of Sxl production. Sxl controls splicing of tra into a productive form, which, together with Tra2, is necessary for splicing of dsx into a productive form and fru into a non-productive form. Sxl also prevents assembly of the dosage compensation (DC) complex by blocking translation of msl2, a critical component of the complex. In fruit fly males, a single dose of X linked proteins is insufficient to initiate a productive transcription of Sxl. As a result, dosage compensation is activated, tra is spliced into a non-productive form, and the default splicing of dsx and fru productive male forms occurs. In Anopheles females, Fle is necessary for the splicing of dsx and fru into productive and non-productive forms, respectively, as well as for repression of dosage compensation. The upstream sex-determining molecules remain to be identified; it is unclear whether Fle requires a cofactor to promote splicing of dsx and fru. In males, the primary sex determiner Yob triggers the sex determination pathway and inactivates Fle by a yet unknown mechanism, allowing activation of dosage compensation. Fle does not take part in the splicing of dsx or fru into male forms.
In summary, in addition to a vital role in the splicing of dsx and fru into a female form, fle represses dosage compensation in A. gambiae females through a yet unknown mechanism. As such, fle is only the second known, after Sxl in Drosophila, molecular link between the sex determination and dosage compensation pathways in insects (Figure 3). Female lethality in response to fle knockdown indicates that dosage compensation is regulated by fle also in A. stephensi.
fle as a Target Molecule for Anopheles Control
Mosquitoes from the genus Anopheles are the exclusive vectors of Plasmodium parasites that cause human malaria, a disease infecting annually nearly 230 million people and causing over 400,000 deaths worldwide.55 Over 90% of malaria cases occur in sub-Saharan Africa, where A. gambiae is the primary vector. Control of the disease relies heavily on the use of insecticides, which are increasingly ineffective because of the emergence and spread of insecticide resistance in natural Anopheles populations.55,56 Various genetic control approaches, proposed to complement the existing insecticidal tools,57,58 rely on mass releases of irradiated or otherwise genetically modified males, which through mating with wild-type females spread desirable traits, such as sterility, female lethality, or inability to transmit pathogens, to cause mosquito population suppression or modification. The requirement for male-only releases is dictated by ethical and safety considerations, because only females feed on blood, and released modified females could contribute to biting and parasite transmission. To date, maleness genes Yob and guy-1 have been found to have female-killing properties,21,59 and thus could be used to conditionally eliminate females of the A. gambiae species complex and A. stephensi, respectively, in genetic control operations.58 This study, in addition to advancing our understanding of sex determination and dosage compensation regulation in insects, identifies fle as a universal molecule, conserved in sequence and function in anopheline mosquitoes, that could be targeted in genetic control to eliminate females of all major malaria vector species. The apparent haploinsufficiency makes fle unsuitable as a target of homing gene drives; however, female-specific deleterious effects could be exploited in conditional fle knockdown transgenic lines to suppress populations of various Anopheles vector species.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| E. coli DH10B ElectroMax | Thermo Fisher Scientific | Cat#18290015 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Lipofectamine 2000 Transfection Reagent | Invitrogen | Cat#11668019 |
| Schneider’s Modified Medium | Lonza | Cat#04-351Q |
| Critical Commercial Assays | ||
| PureLink RNA Micro Kit | Invitrogen | Cat#12183016 |
| ActinGreen 488 ReadyProbes Reagent | Thermofisher Scientific | Cat#R37110 |
| LunaScript RT SuperMix Kit | New England Biolabs | Cat#E3010S |
| Luna Universal qPCR Master Mix | New England Biolabs | Cat#M3003S |
| DNeasy Blood & Tissue Kit | QIAGEN | Cat#69504 |
| MEGAscript RNAi T7 Kit | Life Technologies | Cat#AM1626 |
| One-Step RT-PCR System | Invitrogen | Cat#12574026 |
| Deposited Data | ||
| RNA-Seq data | This study | ENA: PRJEB38605 |
| Experimental Models: Cell Lines | ||
| A. gambiae Sua5.1 | 60 | N/A |
| Experimental Models: Organisms/Strains | ||
| A. gambiae G3 strain | BEI Resources | MRA-112 |
| A. stephensi AF-SDA500 strain | Infravec2 | V.2.3.1.L.FR.1.0 |
| Oligonucleotides | ||
| See Table S2 for detailed information on used oligonucleotides | This study | N/A |
| Recombinant DNA | ||
| p165 | 61 | GenBank: KU189142 |
| pUAST-attB | 62 | GenBank: EF362409 |
| pBac_pattPs_fle_3miR | This study | GenBank: MW147152 |
| pENTR R4-vas2-Transposase-R3 | 63 | N/A |
| pGEM-T Easy Vector System | Promega | Cat#A1360 |
| Software and Algorithms | ||
| BLAST | 64 | https://blast.ncbi.nlm.nih.gov/Blast.cgi |
| Jpred-4 | 65 | http://www.compbio.dundee.ac.uk/jpred/ |
| ClustalX2 | 66 | https://clustalx.software.informer.com/2.1/ |
| MEGA v. 7.0.26 | 67 | https://www.megasoftware.net/ |
| WebLogo | 68 | https://weblogo.berkeley.edu/logo.cgi |
| ImageJ | 69 | https://imagej.nih.gov/ij/ |
| Kallisto v0.46 | 70 | https://pachterlab.github.io/kallisto/ |
| DESeq2 | 71 | http://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| apeGLM | 72 | https://bioconductor.org/packages/release/bioc/html/apeglm.html |
| Other | ||
| QuantStudio 3 Real-Time PCR System | Applied Biosystems | Cat#A28136 |
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jaroslaw Krzywinski (jaroslaw.krzywinski@pirbright.ac.uk).
Materials Availability
All relevant data supporting the key findings of this study are available within the article and its Supplementary Information files or from the corresponding author upon a reasonable request.
Data and Code Availability
The RNA-seq data generated during this study are available at the European Nucleotide Archive with the accession number PRJEB38605.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mosquito strains
A. gambiae G3 strain, transgenic lines generated on the A. gambiae G3 strain background, and A. stephensi AF-SDA500 strain were reared at 28°C and 80% humidity on 12 h: 12 h light:dark cycle, following the standard protocol.73 Larvae were reared in plastic trays filled with 1 L of deionised water and provided with ground TetraMin tropical fish food flakes (Tetra). The adults were kept in BugDorm-1 (30 × 30 × 30 cm) or BugDorm-4 (24.5 × 24.5 × 24.5 cm) cages (BugDorm) and provided with 10% sucrose solution ad libitum. A 1:1 mixture of time-expired human red blood cells and plasma sourced from a blood bank was used for feeding females through the Hemotek membrane feeder.
Mosquito cells
The A. gambiae female-like Sua5.1 cells60 were cultured at 28°C in Schneider’s Modified medium (Lonza) supplemented with 10% fetal bovine serum (PAA), and 100 U/ml Penicillin and 100 μg/ml Streptomycin (Life Technologies).
METHOD DETAILS
Sequence analyses
All similarity searches were conducted using BLAST,64 with the word size = 2. The search for the A. gambiae dsx splicing factors within the A. gambiae PEST strain genome was conducted using a BLASTp and tBLASTn algorithm, with the expect value 1e-7, and the D. melanogaster Tra2 (FlyBase IDs CG10128-PA) or Aedes aegypti Nix (a distant homolog of D. melanogaster tra2; GenBank accession AHW46195.1) protein sequences used as query. The phylogenetic distribution of the fle orthologs was evaluated through a tBLASTn search against the NCBI whole-genome shotgun contigs (wgs) database using as query the amino acid translation of fle. The search for a putative fle paralog was conducted using a local BLASTp search against a database of the A. gambiae AgamP4.12 peptides downloaded from Vectorbase, and for other Anopheles species by implementing BLASTp search against a protein database for a respective species in Vectorbase. Structure of the proteins was derived by searches of the Conserved Domain Database74 and through structure prediction using Jpred-4.65 Sequence alignments were conducted using ClustalX2.66 Maximum likelihood phylogenetic analysis was conducted in MEGA767 using JTT matrix-based model75 with Gamma distributed evolutionary rate differences among sites. The sequence logos were generated using WebLogo.68
dsRNA synthesis
Open reading frame fragments of the A. gambiae or A. stephensi genes were amplified through PCR from a genomic DNA template or in a one-step RT-PCR (Invitrogen) reaction from the pupal total RNA templates using gene-specific primers flanked at their 5′ ends with the T7 promoter sequence (for details on primers see Table S2). The resulting products, cloned into pGEM T-easy vector (Promega), were used directly, or after reamplification with the same primer pairs, as a template to synthesize double-stranded RNA (dsRNA) using the MEGAscript RNAi T7 kit (Life Technologies) according to manufacturer’s recommendation. Similarly, a fragment of β-lactamase (bla) gene was amplified by PCR from the pGEM-T Easy vector using bla-specific primers, each containing the T7 promoter sequence at the 5' end, and dsRNA was synthesized as described above.
Transfection experiments
Prior to transfection, the A. gambiae Sua5.1 cells were split into new culture flasks and transfection experiments were performed when cells’ confluency reached 60%–80%. Approximately 1 × 106 cells per well were seeded onto 24 well plates and transfected in suspension, using 3 μl of Lipofectamine 2000 transfection reagent (Life Technologies) and 1.5 μg of dsRNA of a tested gene per well. In parallel, cells in a separate set of wells were transfected with a plasmid (0.3 μg per well) containing the eGFP open reading frame under the control of A. gambiae polyubiquitin promoter as a control of transfection efficiency. In addition, non-transfected control cells were cultured in a set of wells in each experiment. After approximately 24 hours, the transfection efficiency was evaluated using fluorescence microscopy. If at least 30% of the plasmid control cells per well were GFP-positive on a given plate, experimental and non-transfected control cells from that plate were harvested 48 hours post transfection to isolate total RNA with PureLink Micro kit (Life Technologies). Transfection experiments were repeated 3 times.
Analysis of sex-specific splicing
The effect of knockdown of the analyzed genes on the pattern of the dsx splicing was evaluated through RT-PCR using total RNA templates from the transfected Sua5.1 cells and primers dsxF2 and dsxR5m. Similarly, RT-PCR was used to analyze the effect of a stable fle knockdown on the splicing pattern of dsx and fru (the latter using primers Aga_fruF and Aga_fruR) in pupae of selected transgenic A. gambiae lines.
Transient gene silencing
Transient gene silencing experiments were conducted as described earlier.21 Briefly, early preblastoderm A. gambiae or A. stephensi embryos of unknown sex were microinjected with a solution containing dsRNA (1-1.5 μg/μl) of either fle or bla (as a control) gene and a plasmid (0.2 μg/μl) with the GFP gene downstream of the Drosophila melanogaster actin 5C promoter, or with plasmid alone as a control. Surviving first instar larvae were screened for the presence of GFP marker in the midgut cells using an M165 FC microscope equipped with a GFP filter. The larvae were sorted into GFP-negative and GFP-positive groups (cf. Figure S9 in Krzywinska et al.21). After pupation, sex of individuals was determined based on morphological characters. The experiments in A. gambiae were repeated three times with each of the two dsRNAs (cf. Figure 2A), and in A. stephensi were repeated two times.
Transgenic construct plasmid and transgenesis
To create the transgenic construct we used the p165 plasmid61 backbone by digesting the plasmid with MluI and NotI and ligating a linker containing the SfiI site and compatible MluI/NotI ends. The resulting plasmid was digested with SfiI and NotI, and two in vitro synthesized inserts were incorporated in a single ligation reaction. One insert, with SfiI and NheI ends, encoded the puromycin resistance gene pac under the control of the AGAP004395 promoter; the other insert, with NheI/NotI ends, encoded SV40 terminator, followed by GFP under the control of the AGAP003430 promoter (both promoters drive expression in the Sua5.1 cells, and the AGAP003430 promoter in vivo throughout development in gastric caeca, anterior and posterior stomach, Malpighian tubules and rectum, and at lower level in the brain, thoracic muscles and anal papillae). The resulting plasmid was digested with MluI and SfiI to clone a PCR-generated A. gambiae polyubiquitin promoter76 with MluI/BsaI-FseI ends and a PCR-generated SV40 terminator with FseI/SfiI ends. Finally, that plasmid was digested with BsaI and FseI to clone a fle_3miR fragment encoding a polycistronic transcript designed to silence expression of fle. The fle_3miR fragment contained three microRNAs targeting fle and was constructed by annealing overlapping oligonucleotides (Table S2) and PCR amplification, as previously described.77 After cloning into pUAST-attB vector,62 the fle_3miR fragment was released using BsaI and FseI. Thus engineered construct was excised using MluI and AsiSI and cloned into a MluI and AsiSI-cut p165-based plasmid backbone flanked by piggyBac arms and a φC31 attB site added to each end in opposite orientation, to create a transformation plasmid pBac_attBs_fle-3miR (GenBank: MW147152).
Early preblastoderm A. gambiae embryos were microinjected with a solution of pBac_pattPs_fle_3miR (0.4 μg/μl) and a helper plasmid pENTR R4-vas2-Transposase-R3 (0.2 μg/μl), containing piggy-Bac transposase reading frame under the control of the vas2 regulatory sequences,63 following previously described methods.78 Injection of approximately 1200 embryos yielded 252 G0 larvae, of which 108 individuals, that exhibited a transient fluorescent marker expression, reached the pupal stage. The emerging adults (59 males and 49 females) were placed in 9 same-sex pools for crosses with the wild-type G3 strain mosquitoes. Over 120 transgenic G1 mosquitoes were recovered from four pools of male founders, whereas no transgenic G1 individuals were produced by the female founders. Selected G1 males originating from different founder cages or exhibiting different intensity of fluorescent marker expression (if originating from the same cage; Figure 2I) were crossed to wild-type females to establish 7 independent lines. Progeny from these crosses were screened for inheritance of fluorescent marker to evaluate transgene copy number. In three lines approximately 50% of G2 individuals were transgenic, indicative of single insertions. Four other lines exhibited 60%–89% transgene inheritance, with transgenic G2 individuals representing up to three discernible classes of fluorescence intensity or pattern per line, indicative of multiple insertions. In an effort to isolate single insertion sub-lines, males derived from multiple-insertion lines and representing different fluorescence classes were backcrossed with wild-type females at consecutive generations and the number of fluorescence phenotypes was monitored at each generation. Sub-lines that, after 6 generations, produced more than one fluorescence phenotype, or in which more than 50% of the individuals inherited the transgene, were eliminated. Finally, molecular characterization was used to confirm that each line from the final set possessed a single, unique genomic transgene integration site.
No efforts have been made to generate fle loss-of-function mutants in this study out of feasibility concerns. Tra2 in Drosophila, in addition to female sex determination, is necessary for male germline development – loss of Tra2 function leads to male sterility.79 Fle may perform a similar role during the Anopheles spermatogenesis, which, combined with an apparent fle haploinsufficiency in females, could make recovering fle knockout mutants biologically impossible.
Identification of transgene insertion sites
The integration sites of the piggyBac element within the genome has been identified using the splinkerette PCR protocol80 or by inverse PCR. DNA isolated from individual pupae was used for both approaches. For inverse PCR, the DNA was digested with CviQI, HaeIII, MspI, Sau3AI, or TaqI (NEB), circularized by ligation, and amplified by PCR using primers ITRL1F and ITRL1R for piggyBac left arm, or ITRR1F81 and InpBacR2R for piggyBac right arm (Table S2) to isolate flanking genomic regions. The products containing genomic sequences flanking the piggyBac elements were sequenced directly, or after cloning, and genomic location of the integration sites was identified by BLAST search.
Abdominal musculature
Adult mosquito abdomens were dissected in phosphate-buffered saline (PBS) to release tergites with the associated musculature. The tissues were fixed in PBS containing 4% paraformaldehyde for 15 min, washed three times for 5 min in PBS, and incubated in ActinGreen 488 ReadyProbes Reagent containing AlexaFluor 488-conjugated phalloidin. After three short washes the tissues were mounted on slides and photographed with a Leica DFC365 FX camera mounted on a Leica M165 FC microscope equipped with a GFP filter. Images were processed with ImageJ.69
Real-time PCR
Total RNA was extracted from individual A. gambiae pupae using PureLink RNA Micro Kit (Invitrogen) according to manufacturer’s recommendations. For each sample, 500 ng of total RNA was used to synthesize cDNA with LunaScript RT SuperMix Kit (NEB). Quantitative PCR was conducted using primer pairs JK1051/JK1052 and JK1053/JK1054 to amplify, respectively, a fragment of fle and of the housekeeping gene encoding ribosomal protein S7 (rpS7, AGAP010592) used to normalize the expression. QuantStudio 3 Real-Time PCR System (Applied Biosystems) was employed to run the reactions using Luna Universal qPCR Master Mix (NEB) at annealing temperature of 59°C. Expression levels were calculated using 2−ΔΔCt method,82 with triple technical and three biological replicates for each sample, and all data normalized to the relative fle/rpS7 expression in the samples of the wild-type female pupae.
RNA-seq analysis
Total RNA was extracted using the Trizol method and quality-checked using TapeStation (Agilent). Triplicate samples of female pupae from wild-type G3 line and from transgenic 4M4B line were used for transcriptome sequencing. The TruSeq library preparation protocol (Illumina) was followed by 150 bp paired-end sequencing using NovaSeq 6000 sequencing system (Illumina). The reads were pseudo-aligned to the A. gambiae transcriptome genebuild AgamP4.12 using Kallisto v0.46.70 Transcripts per kilobase million (TPM) value was quantified for each transcript and averaged across multiple replicates of the same sample. As a further check of statistical robustness, differential expression analysis was performed using DESeq2,71 with filtering out transcripts covered by less than 10 reads among all samples, and then shrinking log2-fold changes using apeGLM.72
QUANTIFICATION AND STATISTICAL ANALYSIS
For the transient fle experiments, the probability of the observed microinjection results under the null hypothesis that there is no sex bias difference between the GFP-positive and GFP-negative (or control) groups was calculated using Fisher’s exact test. For the real-time PCR experiments, Student’s t test was used to evaluate statistical differences between the relative fle expression levels in the wild-type and transgenic strains after performing a goodness of fit test.
Acknowledgments
We thank Neil Hobbs and Aqib Ali for their technical support in maintenance of mosquito colonies. This study was supported by funds from UK Biotechnology and Biological Sciences Research Council (BBSRC) grant BB/P019269/1 (to J.K.), and through strategic BBSRC grants BBS/E/I/00007033, BBS/E/I/00007038, and BBS/E/I/00007039 to the Pirbright Institute.
Author Contributions
E.K. and J.K. conceived the study; E.K. performed molecular biology, cell line, and mosquito experiments, with contributions from J.K.; J.L. designed and conducted qRT-PCR experiments; E.K., J.-C.L., and C.-H.C. designed and generated plasmid constructs; L.F. performed RNA-sequencing (RNA-seq) data analysis; E.K. and J.K. wrote the paper with input from the other authors.
Declaration of Interests
The authors declare no competing interests.
Published: January 7, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.cub.2020.12.014.
Supplemental Information
References
- 1.Saccone G., Pane A., Polito L.C. Sex determination in flies, fruitflies and butterflies. Genetica. 2002;116:15–23. doi: 10.1023/a:1020903523907. [DOI] [PubMed] [Google Scholar]
- 2.Geuverink E., Beukeboom L.W. Phylogenetic distribution and evolutionary dynamics of the sex determination genes doublesex and transformer in insects. Sex Dev. 2014;8:38–49. doi: 10.1159/000357056. [DOI] [PubMed] [Google Scholar]
- 3.Wilkins A.S. Moving up the hierarchy: a hypothesis on the evolution of a genetic sex determination pathway. BioEssays. 1995;17:71–77. doi: 10.1002/bies.950170113. [DOI] [PubMed] [Google Scholar]
- 4.Wexler J., Delaney E.K., Belles X., Schal C., Wada-Katsumata A., Amicucci M.J., Kopp A. Hemimetabolous insects elucidate the origin of sexual development via alternative splicing. eLife. 2019;8:e47490. doi: 10.7554/eLife.47490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhulst E.C., van de Zande L. Double nexus—Doublesex is the connecting element in sex determination. Brief. Funct. Genomics. 2015;14:396–406. doi: 10.1093/bfgp/elv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amrein H., Maniatis T., Nöthiger R. Alternatively spliced transcripts of the sex-determining gene tra-2 of Drosophila encode functional proteins of different size. EMBO J. 1990;9:3619–3629. doi: 10.1002/j.1460-2075.1990.tb07573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedley M.L., Maniatis T. Sex-specific splicing and polyadenylation of dsx pre-mRNA requires a sequence that binds specifically to tra-2 protein in vitro. Cell. 1991;65:579–586. doi: 10.1016/0092-8674(91)90090-l. [DOI] [PubMed] [Google Scholar]
- 8.Inoue K., Hoshijima K., Higuchi I., Sakamoto H., Shimura Y. Binding of the Drosophila transformer and transformer-2 proteins to the regulatory elements of doublesex primary transcript for sex-specific RNA processing. Proc. Natl. Acad. Sci. USA. 1992;89:8092–8096. doi: 10.1073/pnas.89.17.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pane A., Salvemini M., Delli Bovi P., Polito C., Saccone G. The transformer gene in Ceratitis capitata provides a genetic basis for selecting and remembering the sexual fate. Development. 2002;129:3715–3725. doi: 10.1242/dev.129.15.3715. [DOI] [PubMed] [Google Scholar]
- 10.Burghardt G., Hediger M., Siegenthaler C., Moser M., Dübendorfer A., Bopp D. The transformer2 gene in Musca domestica is required for selecting and maintaining the female pathway of development. Dev. Genes Evol. 2005;215:165–176. doi: 10.1007/s00427-004-0464-7. [DOI] [PubMed] [Google Scholar]
- 11.Salvemini M., Robertson M., Aronson B., Atkinson P., Polito L.C., Saccone G. Ceratitis capitata transformer-2 gene is required to establish and maintain the autoregulation of Cctra, the master gene for female sex determination. Int. J. Dev. Biol. 2009;53:109–120. doi: 10.1387/ijdb.082681ms. [DOI] [PubMed] [Google Scholar]
- 12.Hediger M., Henggeler C., Meier N., Perez R., Saccone G., Bopp D. Molecular characterization of the key switch F provides a basis for understanding the rapid divergence of the sex-determining pathway in the housefly. Genetics. 2010;184:155–170. doi: 10.1534/genetics.109.109249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verhulst E.C., Beukeboom L.W., van de Zande L. Maternal control of haplodiploid sex determination in the wasp Nasonia. Science. 2010;328:620–623. doi: 10.1126/science.1185805. [DOI] [PubMed] [Google Scholar]
- 14.Geuverink E., Rensink A.H., Rondeel I., Beukeboom L.W., van de Zande L., Verhulst E.C. Maternal provision of transformer-2 is required for female development and embryo viability in the wasp Nasonia vitripennis. Insect Biochem. Mol. Biol. 2017;90:23–33. doi: 10.1016/j.ibmb.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Shukla J.N., Palli S.R. Sex determination in beetles: production of all male progeny by parental RNAi knockdown of transformer. Sci. Rep. 2012;2:602. doi: 10.1038/srep00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shukla J.N., Palli S.R. Tribolium castaneum Transformer-2 regulates sex determination and development in both males and females. Insect Biochem. Mol. Biol. 2013;43:1125–1132. doi: 10.1016/j.ibmb.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J., Chen S., Zeng B., James A.A., Tan A., Huang Y. Bombyx mori P-element somatic inhibitor (BmPSI) is a key auxiliary factor for silkworm male sex determination. PLoS Genet. 2017;13:e1006576. doi: 10.1371/journal.pgen.1006576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki M.G., Imanishi S., Dohmae N., Nishimura T., Shimada T., Matsumoto S. Establishment of a novel in vivo sex-specific splicing assay system to identify a trans-acting factor that negatively regulates splicing of Bombyx mori dsx female exons. Mol. Cell. Biol. 2008;28:333–343. doi: 10.1128/MCB.01528-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiuchi T., Koga H., Kawamoto M., Shoji K., Sakai H., Arai Y., Ishihara G., Kawaoka S., Sugano S., Shimada T. A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature. 2014;509:633–636. doi: 10.1038/nature13315. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Z.Z., Sun X., Zhang B., Pu J., Jiang Z.Y., Li M., Fan Y.J., Xu Y.Z. Alternative splicing regulation of doublesex gene by RNA-binding proteins in the silkworm Bombyx mori. RNA Biol. 2019;16:809–820. doi: 10.1080/15476286.2019.1590177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krzywinska E., Dennison N.J., Lycett G.J., Krzywinski J. A maleness gene in the malaria mosquito Anopheles gambiae. Science. 2016;353:67–69. doi: 10.1126/science.aaf5605. [DOI] [PubMed] [Google Scholar]
- 22.Krzywinska E., Krzywinski J. Effects of stable ectopic expression of the primary sex determination gene Yob in the mosquito Anopheles gambiae. Parasit. Vectors. 2018;11(Suppl 2):648. doi: 10.1186/s13071-018-3211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scali C., Catteruccia F., Li Q., Crisanti A. Identification of sex-specific transcripts of the Anopheles gambiae doublesex gene. J. Exp. Biol. 2005;208:3701–3709. doi: 10.1242/jeb.01819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gailey D.A., Billeter J.C., Liu J.H., Bauzon F., Allendorfer J.B., Goodwin S.F. Functional conservation of the fruitless male sex-determination gene across 250 Myr of insect evolution. Mol. Biol. Evol. 2006;23:633–643. doi: 10.1093/molbev/msj070. [DOI] [PubMed] [Google Scholar]
- 25.Amrein H., Hedley M.L., Maniatis T. The role of specific protein-RNA and protein-protein interactions in positive and negative control of pre-mRNA splicing by Transformer 2. Cell. 1994;76:735–746. doi: 10.1016/0092-8674(94)90512-6. [DOI] [PubMed] [Google Scholar]
- 26.Li X., Jin B., Dong Y., Chen X., Tu Z., Gu J. Two of the three Transformer-2 genes are required for ovarian development in Aedes albopictus. Insect Biochem. Mol. Biol. 2019;109:92–105. doi: 10.1016/j.ibmb.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amrein H., Gorman M., Nöthiger R. The sex-determining gene tra-2 of Drosophila encodes a putative RNA binding protein. Cell. 1988;55:1025–1035. doi: 10.1016/0092-8674(88)90247-4. [DOI] [PubMed] [Google Scholar]
- 28.Martín I., Ruiz M.F., Sánchez L. The gene transformer-2 of Sciara (Diptera, Nematocera) and its effect on Drosophila sexual development. BMC Dev. Biol. 2011;11:19. doi: 10.1186/1471-213X-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nissen I., Müller M., Beye M. The Am-tra2 gene is an essential regulator of female splice regulation at two levels of the sex determination hierarchy of the honeybee. Genetics. 2012;192:1015–1026. doi: 10.1534/genetics.112.143925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Concha C., Scott M.J. Sexual development in Lucilia cuprina (Diptera, Calliphoridae) is controlled by the transformer gene. Genetics. 2009;182:785–798. doi: 10.1534/genetics.109.100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall A.B., Basu S., Jiang X., Qi Y., Timoshevskiy V.A., Biedler J.K., Sharakhova M.V., Elahi R., Anderson M.A., Chen X.G. A male-determining factor in the mosquito Aedes aegypti. Science. 2015;348:1268–1270. doi: 10.1126/science.aaa2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dreyfuss G., Swanson M.S., Piñol-Roma S. Heterogeneous nuclear ribonucleoprotein particles and the pathway of mRNA formation. Trends Biochem. Sci. 1988;13:86–91. doi: 10.1016/0968-0004(88)90046-1. [DOI] [PubMed] [Google Scholar]
- 33.Maris C., Dominguez C., Allain F.H. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 2005;272:2118–2131. doi: 10.1111/j.1742-4658.2005.04653.x. [DOI] [PubMed] [Google Scholar]
- 34.Burtis K.C., Baker B.S. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989;56:997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- 35.Lynch K.W., Maniatis T. Synergistic interactions between two distinct elements of a regulated splicing enhancer. Genes Dev. 1995;9:284–293. doi: 10.1101/gad.9.3.284. [DOI] [PubMed] [Google Scholar]
- 36.Anopheles gambiae 1000 Genomes Consortium Genome variation and population structure among 1142 mosquitoes of the African malaria vector species Anopheles gambiae and Anopheles coluzzii. Genome Res. 2020;30:1533–1546. doi: 10.1101/gr.262790.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neafsey D.E., Waterhouse R.M., Abai M.R., Aganezov S.S., Alekseyev M.A., Allen J.E., Amon J., Arcà B., Arensburger P., Artemov G. Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science. 2015;347:1258522. doi: 10.1126/science.1258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krzywinski J., Grushko O.G., Besansky N.J. Analysis of the complete mitochondrial DNA from Anopheles funestus: an improved dipteran mitochondrial genome annotation and a temporal dimension of mosquito evolution. Mol. Phylogenet. Evol. 2006;39:417–423. doi: 10.1016/j.ympev.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Moreno M., Marinotti O., Krzywinski J., Tadei W.P., James A.A., Achee N.L., Conn J.E. Complete mtDNA genomes of Anopheles darlingi and an approach to anopheline divergence time. Malar. J. 2010;9:127. doi: 10.1186/1475-2875-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryner L.C., Goodwin S.F., Castrillon D.H., Anand A., Villella A., Baker B.S., Hall J.C., Taylor B.J., Wasserman S.A. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/s0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- 41.Gailey D.A., Taylor B.J., Hall J.C. Elements of the fruitless locus regulate development of the muscle of Lawrence, a male-specific structure in the abdomen of Drosophila melanogaster adults. Development. 1991;113:879–890. doi: 10.1242/dev.113.3.879. [DOI] [PubMed] [Google Scholar]
- 42.Lucchesi J.C., Skripsky T. The link between dosage compensation and sex differentiation in Drosophila melanogaster. Chromosoma. 1981;82:217–227. doi: 10.1007/BF00286106. [DOI] [PubMed] [Google Scholar]
- 43.Gergen J.P. Dosage compensation in Drosophila: evidence that daughterless and Sex-lethal control X chromosome activity at the blastoderm stage of embryogenesis. Genetics. 1987;117:477–485. doi: 10.1093/genetics/117.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cline T.W., Meyer B.J. Vive la différence: males vs females in flies vs worms. Annu. Rev. Genet. 1996;30:637–702. doi: 10.1146/annurev.genet.30.1.637. [DOI] [PubMed] [Google Scholar]
- 45.Lucchesi J.C., Kelly W.G., Panning B. Chromatin remodeling in dosage compensation. Annu. Rev. Genet. 2005;39:615–651. doi: 10.1146/annurev.genet.39.073003.094210. [DOI] [PubMed] [Google Scholar]
- 46.Bashaw G.J., Baker B.S. The regulation of the Drosophila msl-2 gene reveals a function for Sex-lethal in translational control. Cell. 1997;89:789–798. doi: 10.1016/s0092-8674(00)80262-7. [DOI] [PubMed] [Google Scholar]
- 47.Cline T.W. Two closely linked mutations in Drosophila melanogaster that are lethal to opposite sexes and interact with daughterless. Genetics. 1978;90:683–698. doi: 10.1093/genetics/90.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hilfiker A., Amrein H., Dübendorfer A., Schneiter R., Nöthiger R. The gene virilizer is required for female-specific splicing controlled by Sxl, the master gene for sexual development in Drosophila. Development. 1995;121:4017–4026. doi: 10.1242/dev.121.12.4017. [DOI] [PubMed] [Google Scholar]
- 49.Rose G., Krzywinska E., Kim J., Revuelta L., Ferretti L., Krzywinski J. Dosage compensation in the African malaria mosquito Anopheles gambiae. Genome Biol. Evol. 2016;8:411–425. doi: 10.1093/gbe/evw004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi Y., Wu Y., Saunders R., Chen X.G., Mao C., Biedler J.K., Tu Z.J. Guy1, a Y-linked embryonic signal, regulates dosage compensation in Anopheles stephensi by increasing X gene expression. eLife. 2019;8:e43570. doi: 10.7554/eLife.43570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clemente-Ruiz M., Murillo-Maldonado J.M., Benhra N., Barrio L., Pérez L., Quiroga G., Nebreda A.R., Milán M. Gene dosage imbalance contributes to chromosomal instability-induced tumorigenesis. Dev. Cell. 2016;36:290–302. doi: 10.1016/j.devcel.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Yildirim E., Kirby J.E., Brown D.E., Mercier F.E., Sadreyev R.I., Scadden D.T., Lee J.T. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell. 2013;152:727–742. doi: 10.1016/j.cell.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y., Chen X., Liu Z., Xu J., Li X., Bi H., Andongma A.A., Niu C., Huang Y. Mutation of doublesex induces sex-specific sterility of the diamondback moth Plutella xylostella. Insect Biochem. Mol. Biol. 2019;112:103180. doi: 10.1016/j.ibmb.2019.103180. [DOI] [PubMed] [Google Scholar]
- 54.Salvemini M., D’Amato R., Petrella V., Aceto S., Nimmo D., Neira M., Alphey L., Polito L.C., Saccone G. The orthologue of the fruitfly sex behaviour gene fruitless in the mosquito Aedes aegypti: evolution of genomic organisation and alternative splicing. PLoS ONE. 2013;8:e48554. doi: 10.1371/journal.pone.0048554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.World Health Organization World malaria report 2019. 2019. https://apps.who.int/iris/handle/10665/330011
- 56.Ranson H., Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Knipling E.F. Sterile-male method of population control. Science. 1959;130:902–904. doi: 10.1126/science.130.3380.902. [DOI] [PubMed] [Google Scholar]
- 58.Alphey L. Genetic control of mosquitoes. Annu. Rev. Entomol. 2014;59:205–224. doi: 10.1146/annurev-ento-011613-162002. [DOI] [PubMed] [Google Scholar]
- 59.Criscione F., Qi Y., Tu Z. GUY1 confers complete female lethality and is a strong candidate for a male-determining factor in Anopheles stephensi. eLife. 2016;5:e19281. doi: 10.7554/eLife.19281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Müller H.M., Dimopoulos G., Blass C., Kafatos F.C. A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J. Biol. Chem. 1999;274:11727–11735. doi: 10.1074/jbc.274.17.11727. [DOI] [PubMed] [Google Scholar]
- 61.Hammond A., Galizi R., Kyrou K., Simoni A., Siniscalchi C., Katsanos D., Gribble M., Baker D., Marois E., Russell S. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 2016;34:78–83. doi: 10.1038/nbt.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bischof J., Maeda R.K., Hediger M., Karch F., Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Volohonsky G., Terenzi O., Soichot J., Naujoks D.A., Nolan T., Windbichler N., Kapps D., Smidler A.L., Vittu A., Costa G. Tools for Anopheles gambiae transgenesis. G3 (Bethesda) 2015;5:1151–1163. doi: 10.1534/g3.115.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 65.Drozdetskiy A., Cole C., Procter J., Barton G.J. JPred4: a protein secondary structure prediction server. Nucleic Acids Res. 2015;43:W389–W394. doi: 10.1093/nar/gkv332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 67.Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crooks G.E., Hon G., Chandonia J.M., Brenner S.E. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bray N.L., Pimentel H., Melsted P., Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 71.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu A., Ibrahim J.G., Love M.I. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics. 2019;35:2084–2092. doi: 10.1093/bioinformatics/bty895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benedict M.Q. Care and maintenance of Anopheline mosquito colonies. In: Crampton J.M., Beard C.B., Louis C., editors. The Molecular Biology of Insect Disease Vectors. Chapman & Hall; 1997. pp. 3–12. [Google Scholar]
- 74.Lu S., Wang J., Chitsaz F., Derbyshire M.K., Geer R.C., Gonzales N.R., Gwadz M., Hurwitz D.I., Marchler G.H., Song J.S. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res. 2020;48(D1):D265–D268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones D.T., Taylor W.R., Thornton J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 76.Adolfi A., Pondeville E., Lynd A., Bourgouin C., Lycett G.J. Multi-tissue GAL4-mediated gene expression in all Anopheles gambiae life stages using an endogenous polyubiquitin promoter. Insect Biochem. Mol. Biol. 2018;96:1–9. doi: 10.1016/j.ibmb.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 77.Chen C.H., Huang H., Ward C.M., Su J.T., Schaeffer L.V., Guo M., Hay B.A. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science. 2007;316:597–600. doi: 10.1126/science.1138595. [DOI] [PubMed] [Google Scholar]
- 78.Lombardo F., Lycett G.J., Lanfrancotti A., Coluzzi M., Arcà B. Analysis of apyrase 5¢ upstream region validates improved Anopheles gambiae transformation technique. BMC Res. Notes. 2009;2:24. doi: 10.1186/1756-0500-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mattox W., McGuffin M.E., Baker B.S. A negative feedback mechanism revealed by functional analysis of the alternative isoforms of the Drosophila splicing regulator transformer-2. Genetics. 1996;143:303–314. doi: 10.1093/genetics/143.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Potter C.J., Luo L. Splinkerette PCR for mapping transposable elements in Drosophila. PLoS ONE. 2010;5:e10168. doi: 10.1371/journal.pone.0010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lycett G.J., Amenya D., Lynd A. The Anopheles gambiae alpha-tubulin-1b promoter directs neuronal, testes and developing imaginal tissue specific expression and is a sensitive enhancer detector. Insect Mol. Biol. 2012;21:79–88. doi: 10.1111/j.1365-2583.2011.01112.x. [DOI] [PubMed] [Google Scholar]
- 82.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data generated during this study are available at the European Nucleotide Archive with the accession number PRJEB38605.