Abstract
Background.
Postoperative complications (POCs) are associated with worse oncologic outcomes in several cancer types. The implications of complications after rectal cancer surgery are not well studied.
Methods.
The United States Rectal Cancer Consortium (2007–2017) was reviewed for primary rectal adenocarcinoma patients who underwent R0/R1 resection. Ninety-day POCs were categorized as major or minor and were grouped into infectious, cardiopulmonary, thromboembolic, renal, or intestinal dysmotility. Primary outcomes were overall survival (OS) and recurrence-free survival (RFS).
Results.
Among 1136 patients, the POC rate was 46% (n = 527), with 63% classified as minor and 32% classified as major. Of all POCs, infectious complications comprised 20%, cardiopulmonary 3%, thromboembolic 5%, renal 9%, and intestinal dysmotility 19%. Compared with minor or no POCs, major POCs were associated with both worse RFS and worse OS (both p < 0.01). Compared with no POCs, a single POC was associated with worse RFS (p < 0.01), while multiple POCs were associated with worse OS (p = 0.02). Regardless of complication grade, infectious POCs were associated with worse RFS (p < 0.01), while cardiopulmonary and thromboembolic POCs were associated with worse OS (both p < 0.01). Renal POCs were associated with both worse RFS (p < 0.001) and worse OS (p = 0.01). After accounting for pathologic stage, neoadjuvant therapy, and final margin status, Multivariable analysis (MVA) demonstrated worse outcomes with cardiopulmonary, thromboembolic, and renal POCs for OS (cardiopulmonary: hazard ratio [HR] 3.6, p = 0.01; thromboembolic: HR 19.4, p < 0.01; renal: HR 2.4, p = 0.01), and renal and infectious POCs for RFS (infectious: HR 2.1, p < 0.01; renal: HR 3.2, p < 0.01).
Conclusions.
Major complications after proctectomy for cancer are associated with decreased RFS and OS. Given the association of infectious complications and postoperative renal dysfunction with earlier recurrence of disease, efforts must be directed towards defining best practices and standardizing care.
In recent decades, advances in surgical techniques and perioperative treatment have improved survival following rectal cancer surgery. Total mesorectal excision alone has been shown to achieve local recurrence rates of only 10% and cancer-specific survival of 70%.1 Furthermore, randomized clinical trials of neoadjuvant radiotherapy or chemoradiotherapy for locally advanced rectal cancer have demonstrated reductions in local recurrence of up to 25%.2,3 Despite these advances in the oncologic landscape, the morbidity of neoadjuvant therapy combined with resection is significant, as evidenced by the high rate of postoperative complications (POCs) after rectal cancer surgery. Recent trials in the field, such as the 2013 Laparoscopic versus Open Surgery for Rectal Cancer (COLOR II) trial, have demonstrated POC rates as high as 40% regardless of operative approach.4
The association between adverse postoperative events and decreased long-term outcomes has been demonstrated repeatedly in the literature for most fields of surgery.5–9 The linkage between major adverse events such as cardiovascular or renal complications and increased all-cause mortality is intuitive given the well understood natural history of these disease processes and their effect on other major organ systems. The consequences of potentially less serious complications, such as infectious complications, on cancer-related outcomes are more subtle but are predominantly driven by the downstream effect of a chronic inflammatory response, a critically important component of tumor progression.
Moreover, the postoperative period is of particular importance in oncology given that tissue injury during resection results in a surge of inflammatory cells that release growth factors, promote angiogenesis, and alter the extracellular matrix to facilitate invasion.10 These processes can be further augmented by POCs, and this synergism may lead to earlier cancer recurrence and decreased survival. Certain series have actually shown that the postoperative period is more important in determining the survival after major surgery than preoperative patient risk factors.11
Although the functional relationship between postoperative inflammation and cancer is not new, establishing a clear association between POCs and worse long-term outcomes is paramount to enable identification of a point of intervention to allow systematic improvements in processes that will subsequently impact the outcomes for these patients. Importantly, analyzing the grade and type of complication will enable more targeted quality improvement efforts. Therefore, the aim of our study was to utilize a large, multi-institutional database to assess the association of POCs and the grade and specific type of complication with overall survival (OS) and recurrence-free survival (RFS) after rectal cancer surgery.
METHODS
Data Source
The United States Rectal Cancer Consortium (USRCC) represents a collaboration of six academic institutions, including Emory University, University of Michigan, University of Pittsburgh Medical Center, The Ohio State University, Vanderbilt University Medical Center, and Washington University School of Medicine in St Louis. Institutional Review Board (IRB) approval was obtained at each institution prior to data collection. Patients who underwent an R0 or R1 low anterior resection (LAR) or abdominoperineal resection (APR) for primary rectal adenocarcinoma from 2007 to 2017 were included. Patients who were preoperatively determined to undergo palliative resection were excluded.
Demographic, intraoperative, histopathologic, and postoperative outcome data were collected by retrospective review of the medical records. Staging was based on the American Joint Committee on Cancer (AJCC) 8th edition guidelines. Data regarding neoadjuvant and adjuvant therapy, disease recurrence, and survival were also recorded. Postoperative 90-day complications were dichotomized into single or multiple complications and also subcategorized, according to the highest Clavien–Dindo grade of complication, into minor complications (Clavien–Dindo I or II) or major complications (Clavien–Dindo III or IV). To determine whether the type of complication influenced outcome, POCs were also categorized into four groups: (1) infectious, including superficial surgical site infection, deep surgical site infection, intra-abdominal infection, pneumonia, urinary tract infection, anastomotic leak, and postoperative systemic sepsis; (2) cardiopulmonary, including cardiac arrest, myocardial infarction, unplanned intubation, and tracheostomy; (3) thromboembolic, including cerebrovascular accident, deep venous thrombosis, or pulmonary embolus; and (4) intestinal dysmotility, including the need for postoperative tube feeds or total parenteral nutrition. The primary aim was to assess the association between the presence of any POC, as well as the grade and type, with OS or RFS.
Statistical Analysis
Statistical analyses were performed using the SPSS statistical package version 25.0 (IBM Corporation, Armonk, NY, USA). Statistical significance was predefined as a two-tailed p value < 0.05. The Chi square or Fisher’s exact tests were used for categorical variables, while continuous variables were analyzed using t tests or the Wilcoxon signed-rank test. Comparative analyses were conducted between patients who experienced a minor complication or a major complication. Survival was estimated using the Kaplan–Meier (KM) method, and the logrank test was used for comparison of survival between no complication and any POC, grade of POC, or type of POC, and pairwise comparisons for all individual strata were performed. Univariate Cox regression was performed to determine the association of any POC, grade of POC, or type of POC with long-term outcomes, including OS and RFS. Multivariable Cox regression was performed by adjusting for patient-related risk factors such as age, body mass index (BMI), and number of comorbidities, if applicable, and by including other clinicopathologic factors that were significantly associated with OS or RFS on univariate analysis.
RESULTS
Demographic and Clinicopathologic Characteristics
Among 1881 patients in the USRCC, 1136 met the inclusion criteria. Demographic and clinicopathologic characteristics of the entire cohort are listed in Table 1. Median age was 59 years (interquartile range [IQR] 51–67), 61% were male (n = 693), median BMI was 28 kg/m2 (IQR 24–32), and 62% of patients had at least one comorbidity (n = 699). An R0 resection was carried out in 95% (n = 1080) of patients. Median follow-up was 31 months (IQR 13–54). A majority of patients (76%) underwent neoadjuvant chemoradiation (n = 867), while 22% underwent neoadjuvant chemotherapy alone (n = 251) and 65% had adjuvant chemotherapy (n = 659). Utilization of enhanced recovery pathways (ERPs) was documented in 29% (n = 326) of patients. POCs were identified in 46% (n = 523) of patients, of which 20% were infectious (n = 104), 3% were cardiopulmonary (n = 14), 5% were thromboembolic (n = 25), and 19% were intestinal dysmotility (n = 100).
TABLE 1.
Demographic and clinicopathologic factors of the entire cohort and univariate comparison of factors by presence of minor versus major postoperative complications
| All patients | Postoperative complications | None vs. minor or major p value | Minor vs. major p value | |||
|---|---|---|---|---|---|---|
| [N = 1136] | None [N = 529] | Minor [N = 330] | Major [N = 170] | |||
| Preoperative factors | ||||||
| Age at diagnosis [median (IQR)] | 59 (51–67) | 58 (50–66) | 61 (53–69) | 60 (52–69) | < 0.01 | 0.99 |
| Sex | ||||||
| Female | 443 (39) | 202 (39) | 134 (41) | 53 (31) | 0.70 | 0.10 |
| Male | 693 (61) | 316 (61) | 196 (59) | 117 (69) | ||
| Race | ||||||
| White | 1012 (89) | 417 (91) | 292 (88) | 145 (85) | 0.02 | 0.03 |
| Black | 95 (8) | 30 (6) | 34 (10) | 22 (13) | ||
| Other | 29 (3) | 71 (14) | 4 (1) | 3 (2) | ||
| Number of comorbidities | ||||||
| 0 | 262 (23) | 113 (22) | 76 (23) | 34 (20) | 0.30 | 0.21 |
| 1 | 373 (33) | 165 (32) | 113 (34) | 64 (38) | ||
| 2 | 203 (18) | 83 (16) | 62 (19) | 41 (24) | ||
| 3 | 79 (7) | 30 (6) | 25 (8) | 17 (10) | ||
| ≥ 4 | 44 (4) | 16(3) | 21 (6) | 5 (3) | ||
| BMI [median (IQR)] | 28 (24–32) | 28 (24–31) | 28 (24–33) | 27 (24–32) | 0.95 | 0.33 |
| Preoperative serum albumin | 4 (3.7–4.3) | 4 (3.7–4.3) | 4 (3.6–4.2) | 4 (3.6–4.2) | 0.03 | 0.95 |
| Intraoperative factors | ||||||
| Type of resection | ||||||
| LAR | 799 (70) | 406 (78) | 213 (64) | 95 (56) | < 0.01 | < 0.01 |
| APR | 337 (30) | 112 (22) | 117 (35) | 75 (44) | ||
| Operative approach | ||||||
| Open | 420 (37) | 150 (29) | 144 (44) | 77 (45) | < 0.01 | < 0.01 |
| Hand-assist | 221 (19) | 114 (22) | 55 (17) | 31 (18) | ||
| Laparoscopic | 153 (13) | 60 (12) | 58 (18) | 28 (16) | ||
| Robotic | 151 (13) | 74 (14) | 37 (11) | 21 (12) | ||
| Hybrid (laparoscopic + robotic) | 160 (14) | 104 (20) | 35 (11) | 12 (7) | ||
| Drain placement | 755 (66) | 350 (68) | 226 (68) | 123 (72) | 0.26 | 0.44 |
| Diverting loop ileostomy | 621 (55) | 316 (61) | 178 (54) | 71 (42) | < 0.01 | < 0.01 |
| Intraoperative complication | 54 (5) | 10 (2) | 24 (7) | 19 (11) | < 0.01 | < 0.01 |
| Estimated blood loss, mL [median (IQR)] | 200 (100–400) | 200 (100–300) | 200 (150–425) | 300 (104–500) | < 0.01 | < 0.01 |
| Intraoperative blood transfusion | 65 (6) | 12 (2) | 31 (9) | 19 (11) | < 0.01 | < 0.01 |
| Histopathology | ||||||
| Tumor grade | ||||||
| Well-differentiated | 35 (3) | 15 (3) | 9 (3) | 8 (5) | 0.65 | 0.83 |
| Moderately differentiated | 664 (58) | 275 (53) | 222 (67) | 115 (67) | ||
| Poorly differentiated | 60 (5) | 23 (4) | 22 (7) | 12 (7) | ||
| Undifferentiated | 8 (1) | 5 (1) | 2 (1) | 1 (1) | ||
| Pathologic stage (AJCC 8th edition) | ||||||
| Stage I | 358 (32) | 168 (32) | 96 (29) | 59 (35) | 0.10 | 0.17 |
| Stage II | 267 (24) | 105 (20) | 95 (29) | 42 (35) | ||
| Stage III | 340 (30) | 147 (28) | 94 (28) | 53 (31) | ||
| Final resection status | ||||||
| R0 | 1080 (95) | 495 (96) | 312 (95) | 162 (95) | 0.84 | 0.88 |
| R1 | 56 (5) | 23 (4) | 18 (5) | 8 (5) | ||
| Multimodality therapy | ||||||
| Neoadjuvant chemotherapy | 251 (22) | 123 (24) | 76 (23) | 37 (22) | 0.90 | 0.93 |
| Neoadjuvant chemoradiation | 867 (76) | 390 (75) | 261 (79) | 139 (82) | 0.06 | 0.11 |
| Adjuvant chemotherapy | 659 (58) | 320 (62) | 195 (59) | 94 (55) | 0.11 | 0.16 |
| Delay in adjuvant chemotherapy | 43 (7) | 9 (2) | 14 (7) | 17 (18) | 0.07 | < 0.01 |
| Postoperative outcomes | ||||||
| ERP utilization | 326 (29) | 199 (38) | 90 (27) | 33 (19) | < 0.01 | < 0.01 |
| Any POC | 523 (46) | – | – | |||
| Infectious POCs | 104 (20) | – | 38 (12) | 60 (35) | – | < 0.01 |
| Cardiopulmonary POCs | 14 (3) | – | 2 (1) | 12 (7) | – | < 0.01 |
| Thromboembolic POCs | 25 (5) | – | 13 (4) | 12 (7) | – | < 0.01 |
| Renal POCs | 46 (9) | – | 28 (8) | 17 (10) | – | < 0.01 |
| Intestinal dysmotility POCs | 100 (19) | – | 56 (17) | 38 (22) | – | < 0.01 |
| Discharge destination | ||||||
| Home | 1023 (9) | 496 (96) | 323 (98) | 159 (93) | < 0.01 | < 0.01 |
| Non-home | 12 (1) | 14 (3) | 3 (1) | 5 (3) | ||
Data are expressed as n (%) unless otherwise specified
Bold data indicates statistical significance
LAR low-anterior resection, APR abdominoperineal resection, ERP enhanced recovery pathway, POCs postoperative complications, BMI body mass index, IQR interquartile range, AJCC American Joint Committee on Cancer
Comparison of None, Minor, and Major Complications
Among all POCs, 63% were classified as minor complications (n = 330) and 32% (n = 170) as major complications. Compared with patients who experienced either a minor or major complication, those who had no POCs were younger (median 58 vs. 61 vs. 60 years, p < 0.01), more likely to have an LAR (78% vs. 64% vs. 56%, p < 0.01) with placement of a diverting loop ileostomy (61% vs. 54% vs. 42%, p < 0.01). Additionally, these patients had fewer intraoperative complications (2% vs. 7% vs. 11%, p < 0.01) and a lower rate of intraoperative blood transfusion (2% vs. 9% vs. 11%, p < 0.01). However, these cohorts were otherwise well-matched for histopathologic factors, including tumor grade, pathologic stage, and final resection status, and receipt of neoadjuvant or adjuvant therapy (Table 1).
A comparison of patients who experienced a minor versus major complication demonstrated that they were well-matched for most preoperative prognostic factors, including age, number of comorbidities, BMI, and preoperative serum albumin (Table 1). With respect to treatment, there were no differences in the rate of neoadjuvant chemoradiation (79% vs. 82%, p = 0.11), neoadjuvant chemotherapy (23% vs. 22%, p = 0.93), or adjuvant chemotherapy (59% vs. 55%, p = 0.16) between the minor or major complication cohorts. However, patients with major complications had a higher rate of delay in adjuvant chemotherapy initiation (7% vs. 18%, p < 0.01), and lower rate of ERP utilization (27% vs. 19%, p < 0.01). Patients with major complications were more likely to have an APR (35% vs. 44%, p < 0.01), had more intraoperative complications (7% vs. 11%, p < 0.01), higher median estimated blood loss (200 ml vs. 300 ml, p < 0.01), and a higher rate of intraoperative transfusion (9% vs. 11%, p < 0.01). Importantly, those with major complications were more likely to have a non-home discharge (1% vs. 3%, p < 0.01).
Survival Analysis: Any Versus No Postoperative Complication
When compared with no POCs, the presence of any complication was associated with worse RFS (76% vs. 61%, p < 0.01) [Fig. 1a]. When evaluating whether the number of complications was prognostic for recurrence, multivariable analysis demonstrated that both a single complication (hazard ratio [HR] 1.68, 95% confidence interval [CI] 1.17–2.42, p < 0.01) [Table 2, multivariable analysis A] or multiple complications (HR 1.76, 95% CI 1.24–2.51, p < 0.01) [Table 2, multivariable analysis A] were associated with worse RFS when adjusting for receipt of neoadjuvant chemotherapy or neoadjuvant chemoradiation, and pathologic stage. Notably, neither receipt nor delay in initiation of adjuvant therapy were associated with RFS (Table 2). Compared with no POCs, only multiple complications however were associated with worse OS (79% vs. 63%, p < 0.01) [Fig. 1b] and this persisted on multivariable analyses when accounting for age, number of comorbidities, receipt of neoadjuvant chemotherapy or neoadjuvant chemoradiation, and pathologic stage (HR 1.57, 95% CI 1.02–2.40, p = 0.03) [Table 3, multivariable analysis A].
FIG. 1.
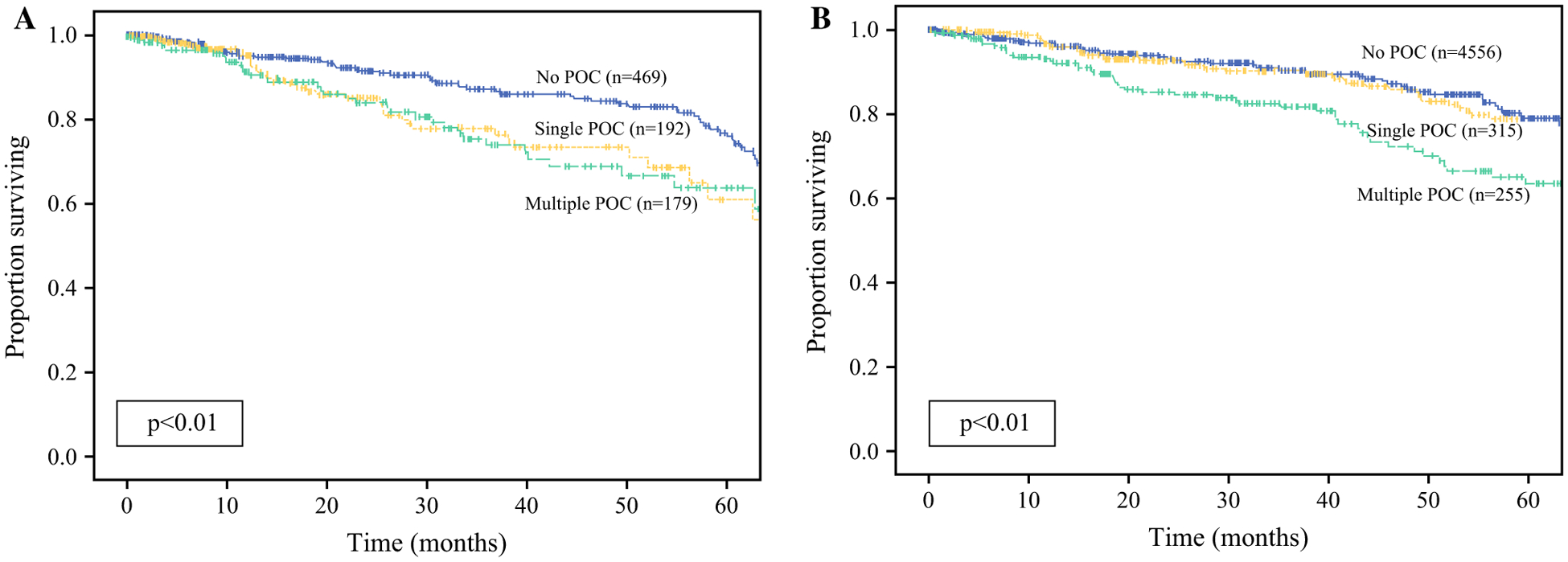
Kaplan-Meier analysis for a recurrence-free survival and b overall survival, comparing none, single, and multiple POCs. POCs postoperative complications
TABLE 2.
Cox regression for recurrence-free survival
| Univariate analysis | Multivariable analysis A: number of POCs | Multivariable analysis B: grade of POCs | Multivariable analysis C: infectious POCs | Multivariable analysis D: renal POCs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | 1.00 (0.99–1.01) | 0.95 | – | – | – | – | – | – | – | – |
| BMI | 1.03 (1.01–1.05) | 0.01 | 1.03 (1.01–1.05) | < 0.01 | 1.03 (1.01–1.05) | < 0.01 | 1.03 (1.01–1.05) | < 0.01 | 1.03 (1.01–1.05) | < 0.01 |
| NAC | 0.51 (0.35–0.77) | < 0.01 | 0.89 (0.64–1.23) | 0.46 | 0.56 (0.35–0.91) | 0.02 | 0.56 (0.35–0.91) | 0.02 | 0.56 (0.35–0.90) | 0.02 |
| Neoadjuvant CRT | 1.5 (0.99–2.21) | 0.06 | – | – | – | – | – | – | – | – |
| Adjuvant chemotherapy | 1.10 (0.78–1.54) | 0.58 | ||||||||
| Delay in adjuvant therapy | 1.28 (0.68–2.43) | 0.44 | ||||||||
| Tumor differentiation | ||||||||||
| Well | Reference | – | – | – | – | – | – | – | – | |
| Moderate | 0.67 (0.27–1.65) | 0.38 | – | – | – | – | – | – | – | – |
| Poor | 0.83 (0.29–2.37) | 0.73 | – | – | – | – | – | – | – | – |
| Undifferentiated | 1.53 (0.29–7.88) | 0.62 | – | – | – | – | – | – | – | – |
| Pathologic stage (AJCC 8th edition) | ||||||||||
| I | Reference | Reference | Reference | Reference | Reference | |||||
| II | 3.01 (1.80–5.03) | < 0.01 | 2.65 (1.69–4.14) | < 0.01 | 2.47 (1.44–4.25) | < 0.01 | 2.47 (1.44–4.25) | < 0.01 | 2.52 (1.47–4.33) | < 0.01 |
| III | 3.84 (2.41–6.14) | < 0.01 | 3.66 (2.44–5.48) | < 0.01 | 2.85 (1.75–4.66) | < 0.01 | 2.85 (1.75–4.66) | < 0.01 | 3.06 (1.87–5.00) | < 0.01 |
| Number of POCs | ||||||||||
| No POCs | Reference | Reference | ||||||||
| Single | 1.89 (1.41–2.54) | < 0.01 | 1.68 (1.17–2.42) | < 0.01 | ||||||
| Multiple | 1.80 (1.36–2.38) | < 0.01 | 1.76 (1.24–2.51) | < 0.01 | ||||||
| Grade of POCs | ||||||||||
| No POCs | Reference | – | – | Reference | – | – | – | – | ||
| Minor POCs | 1.47 (0.95–2.29) | 0.08 | – | – | 1.37 (0.84–2.24) | 0.20 | – | – | – | – |
| Major POCs | 2.75 (1.78–4.26) | < 0.01 | – | – | 2.26 (1.39–3.67) | < 0.01 | – | – | – | – |
| Type of POCs | – | – | ||||||||
| No POCs | Reference | – | – | – | – | Reference | – | – | ||
| Other POCs | 2.63 (1.62–4.27) | < 0.01 | – | – | – | – | 1.48 (0.96–2.29) | 0.08 | – | – |
| Infectious POCs | 3.66 (1.75–7.62) | < 0.01 | – | – | – | – | 2.13 (1.27–3.58) | < 0.01 | – | – |
| Type of POCs | ||||||||||
| No POCs | Reference | – | – | – | – | – | – | Reference | ||
| Other POCs | 1.88 (1.31–2.69) | < 0.01 | – | – | – | – | – | – | 1.61 (1.08–2.39) | 0.02 |
| Renal POCs | 3.65 (1.75–7.62) | < 0.01 | – | – | – | – | – | – | 3.18 (1.49–6.75) | < 0.01 |
Bold data indicates statistical significance
HR hazard ratio, CI confidence interval, NAC neoadjuvant chemotherapy, CRT chemoradiotherapy, POCs postoperative complications, BMI body mass index, AJCC American Joint Committee on Cancer Multivariable analyses were omitted from the table for cardiopulmonary, thromboembolic, and intestinal dysmotility type of POC
TABLE 3.
Cox regression for overall survival
| Univariate analysis | Multivariable analysis A: number of POCs | Multivariable analysis B: grade of POCs | Multivariable analysis C: cardiopulmonary POCs | Multivariable analysis D: thromboembolic POCs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age | 1.02 (1.01–1.04) | < 0.01 | 1.02 (1.01–1.03) | < 0.01 | 1.02 (1.01–1.03) | 0.02 | 1.02 (1.00–1.03) | 0.03 | 1.02 (1.00–1.03) | 0.04 |
| BMI | 0.99 (0.97–1.02) | 0.68 | – | – | – | – | – | – | – | – |
| Number of comorbidities | ||||||||||
| 0 | Reference | Reference | Reference | Reference | Reference | |||||
| 1 | 1.48 (0.91–2.41) | 0.11 | 1.34 (0.81–2.22) | 0.26 | 1.18 (0.69–1.98) | 0.54 | 1.22 (0.73–2.03) | 0.45 | 1.26 (0.75–2.10) | 0.38 |
| 2 | 1.62 (0.93–2.81) | 0.08 | 1.78 (1.03–3.07) | 0.04 | 1.59 (0.91–2.79) | 0.10 | 1.57 (0.91–2.72) | 0.11 | 1.61 (0.93–2.79) | 0.09 |
| 3 | 1.65 (0.78–3.51) | 0.19 | 1.49 (0.72–3.07) | 0.28 | 1.55 (0.73–3.27) | 0.25 | 1.58 (0.75–3.33) | 0.23 | 1.54 (0.73–3.24) | 0.25 |
| ≥ 4 | 2.23 (1.11–4.49) | 0.03 | 2.29 (1.07–4.89) | 0.03 | 2.29 (1.06–4.93) | 0.04 | 2.02 (0.95–4.33) | 0.07 | 2.12 (0.99–4.53) | 0.05 |
| NAC | 2.14 (1.46–3.14) | < 0.01 | 2.50 (1.57–3.98) | < 0.01 | 2.24 (1.37–3.67) | < 0.01 | 2.20 (1.36–3.55) | < 0.01 | 2.31 (1.43–3.72) | < 0.01 |
| Neoadjuvant CRT | 1.56 (1.04–2.33) | 0.03 | 1.24 (0.79–1.94) | 0.35 | 1.48 (0.91–2.41) | 0.11 | 1.56 (0.97–2.51) | 0.07 | 1.48 (0.92–2.39) | 0.11 |
| Tumor differentiation | ||||||||||
| Well | Reference | |||||||||
| Moderate | 3.25 (0.80–13.21) | 0.09 | – | – | – | – | – | – | – | – |
| Poor | 8.12 (1.88–34.97) | 0.01 | – | – | – | – | – | – | – | – |
| Undifferentiation | 5.74 (0.52–65.5) | 0.15 | – | – | – | – | – | – | – | – |
| Pathologic stage (AJCC 8th edition) | ||||||||||
| I | Reference | Reference | Reference | Reference | Reference | |||||
| II | 1.43 (0.89–2.28) | 0.13 | 1.41 (0.85–2.34) | 0.11 | 1.44 (0.85–2.41) | 0.17 | 1.40 (0.84–2.33) | 0.20 | 1.47 (0.88–2.45) | 0.14 |
| III | 2.32 (1.56–3.45) | < 0.01 | 2.41 (1.57–3.71) | < 0.01 | 2.25 (1.46–3.49) | < 0.01 | 2.22 (1.44–3.42) | < 0.01 | 2.35 (1.52–3.64) | < 0.01 |
| Number of POCs | ||||||||||
| No POCs | Reference | Reference | ||||||||
| Single | 1.07 (0.82–1.40) | 0.63 | 0.74 (0.47–1.17) | 0.20 | ||||||
| Multiple | 1.52 (1.18–1.97) | < 0.01 | 1.57 (1.02–2.40) | 0.03 | ||||||
| Grade of POCs | ||||||||||
| No POCs | Reference | – | – | Reference | – | – | – | – | ||
| Minor POCs | 1.01 (0.67–1.49) | 0.95 | – | – | 0.80 (0.51–1.25) | 0.65 | – | – | – | – |
| Major POCs | 2.05 (1.39–3.03) | < 0.01 | – | – | 1.62 (1.04–2.54) | 0.03 | – | – | – | – |
| Type of POCs | – | – | ||||||||
| No POCs | Reference | – | – | – | – | Reference | – | – | ||
| Non-cardiopulmonary POCs | 1.45 (1.02–2.05) | 0.04 | – | – | – | – | 1.02 (0.69–1.49) | 0.93 | – | – |
| Cardiopulmonary POCs | 1.23 (0.73–2.05) | 0.44 | – | – | – | – | 2.78 (1.01–7.88) | 0.05 | – | – |
| Type of POCs | – | – | – | – | ||||||
| No POCs | Reference | – | – | – | – | – | – | Reference | ||
| Non-thromboembolic POCs | 1.24 (0.88–1.73) | 0.22 | – | – | – | – | – | – | 0.97 (0.66–1.43) | 0.87 |
| Thromboembolic POCs | 2.31 (1.24–4.31) | 0.01 | – | – | – | – | – | – | 16.6 (6.37–43.4) | < 0.01 |
| Recurrence | 3.82 (2.79–5.24) | < 0.01 | – | – | – | – | – | – | – | – |
Bold data indicates statistical significance
HR hazard ratio, CI confidence interval, NAC neoadjuvant chemotherapy, CRT chemoradiotherapy, POCs postoperative complications, BMI body mass index, AJCC American Joint Committee on Cancer Multivariable analyses were omitted from the table for infectious, renal, and intestinal dysmotility type of POCs
Survival Analysis: Complication Grade
When compared with no POCs or minor POCs, major POCs were associated with both worse 5-year RFS (76% vs. 63% vs. 48%, p < 0.01) [Fig. 2a] and worse 5-year OS (80% vs. 76% vs. 64%, p < 0.01) [Fig. 2b]. On multivariable analysis, when accounting for prognostic factors, including BMI, receipt of neoadjuvant chemotherapy, and pathologic stage, major POCs resulted in worse RFS when compared with no complications (HR 2.26, 95% CI 1.39–3.67, p < 0.01) [Table 2, multivariable analysis B], while minor POCs were not prognostic for recurrence (HR 1.37, 95% CI 0.84–2.24, p = 0.20) [Table 2, multivariable analysis B]. Multivariable analysis for OS demonstrated similar findings, as minor complications did not result in worse OS (HR 0.80, 95% CI 0.51–1.25, p = 0.65) [Table 3, multivariable analysis B], while major complications did (HR 1.62, 95% CI 1.04–2.54, p = 0.03) [Table 3, multivariable analysis B].
FIG. 2.
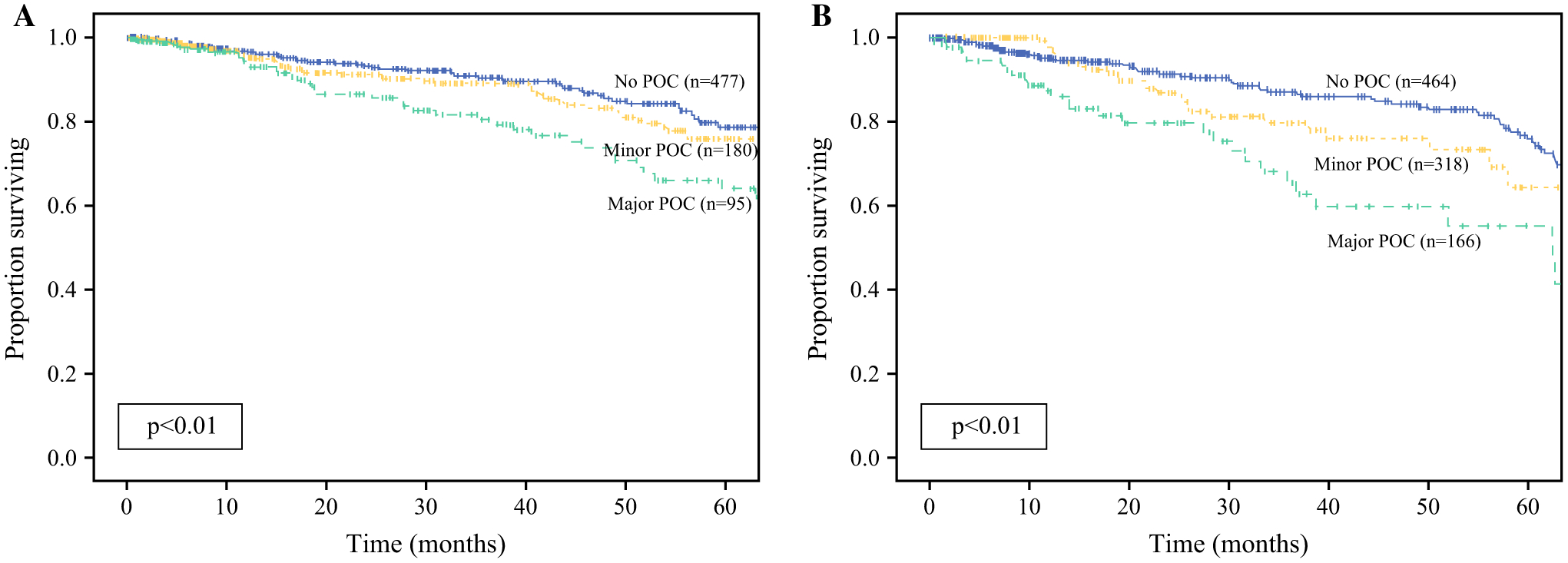
Kaplan-Meier analysis for a recurrence-free survival and b overall survival, comparing none, minor, and major POCs. POCs postoperative complications
Survival Analysis: Type of Postoperative Complication
Compared with no POCs, regardless of complication grade, infectious complications as well as intestinal dysmotility complications were associated with worse RFS (infectious: 56% vs. 76%, p < 0.01; intestinal dysmotility: 43% vs. 77%, p < 0.01), while cardiopulmonary and thromboembolic complications were associated with reduced OS (cardiopulmonary: 40% vs. 78%, p < 0.01; thromboembolic: 63% vs. 78%, p < 0.01). Postoperative renal dysfunction was associated with both worse RFS (26% vs. 76%, p < 0.001) and worse OS (62% vs. 78%, p = 0.01). These results persisted on multivariable analysis for RFS when accounting for BMI, receipt of neoadjuvant therapy (chemotherapy or chemoradiation), and pathologic stage (infectious: HR 2.13, 95% CI 1.27–3.58, p < 0.01) [Table 2, multivariable analysis C]; renal: HR 3.18, 95% CI 1.49–6.75, p < 0.01 [Table 2, multivariable analysis D]; intestinal dysmotility: HR 1.95, 95% CI 1.11–3.43, p = 0.02). For OS, cardiopulmonary and thromboembolic complications remained independently prognostic for worse survival when adjusting for age, number of comorbidities, receipt of neoadjuvant therapy, and pathologic stage (cardiopulmonary: HR 2.78, 95% CI 1.01–7.88, p = 0.05; thromboembolic: HR 16.63, 95% CI 6.37–43.39, p < 0.01; renal: HR 2.39, 95% CI 1.27–4.50, p = 0.01) [Table 3, multivariable analysis C].
DISCUSSION
To the authors’ knowledge, this is the largest study to date to evaluate the influence of POCs on long-term oncologic outcomes in patients with rectal adenocarcinoma. The utilization of a large, multi-institutional database also enabled a robust analysis of the oncologic impact of complication grade and type. The results of the present study demonstrate that while the presence of any POC, whether minor or major, can result in worse RFS (HR 1.68, 95% CI 1.17–2.42, p < 0.01) [Table 2, multivariable analysis A], only multiple (HR 1.81, 95% CI 1.19–2.73, p < 0.01) [Table 3, multivariable analysis A] or major complications (HR 1.81, 95% CI 1.19–2.73, p < 0.01) [Table 3, multivariable analysis B] impact OS. Additionally, when evaluating complication type, infectious, renal, or intestinal dysmotility complications led to earlier recurrence of disease, while cardiopulmonary or thromboembolic complications were associated with decreased survival.
Several mechanisms have been shown to be responsible for the association between POCs, particularly infectious POCs, and tumor recurrence. Anastomotic leaks, in particular, have been well-studied in this regard and contribute to the risk of systemic, peritoneal, or local recurrence from colorectal cancer.12–16 It has been suggested that one of the mechanisms in which POCs alter long-term outcomes is related to the surge of host inflammatory cells, which produce more transforming growth factor (TGF)-β than tumor cells, thus leading to inhibition of host tumor immune surveillance, which may lead to cancer cell escape. Via this mechanism, anastomotic leaks, and likely other infectious and non-infectious complications, also potentiate the prometastatic nature of the innate cellular, cytokine, and neurohormonal surgical response. In fact, a 2014 study by Salvans et al. found that postoperative peritoneal infection in patients with resected colorectal cancer enhanced both cell migration and invasion.17 A second mechanism relates to a shift towards a T-helper (Th)-2-type lymphocyte pattern as a result of the systemic inflammatory response syndrome. Th-2 cytokines, such as interleukin (IL)-10, downregulate tumor-specific immune responses by directly suppressing interferon (IFN)-γ and IL-12 production. This in turn causes a reduction in major histocompatibility complex expression on the surface of tumor cells and inhibits tumor antigen presentation by antigen-presenting cells, thus allowing proliferation of occult or dormant cancer cells.18 This particular mechanism is so important that some have even hypothesized that a reduction in the magnitude of the postoperative systemic inflammatory response with the use of perioperative corticosteroids may improve long-term outcomes following surgery for colorectal cancer.19,20 Lastly, increased expression of proangiogenic factors, such as vascular endothelial growth factor, released in response to surgical trauma and further amplified by POCs, may facilitate survival and growth of residual tumor cells.21
As demonstrated in our study, early cancer recurrence is a mediator to decreased survival (HR 3.82, 95% CI 2.79–5.24, p < 0.01) [Table 3], therefore, despite not being directly associated with worse OS in our results, minor complications may also predict a patient’s earlier cancer-specific death. However, it is not surprising that major complications, particularly those affecting major organ systems, such as cardiopulmonary and thromboembolic complications, are independently associated with worse OS. In a 2005 study of 105,951 patients, Khuri et al. demonstrated that 30-day POCs are more important than preoperative patient risk factors in determining long-term survival after major surgery.11 The reduction in median survival independently attributed to specific complication groups ranged from 42% for wound complications to 99% for cardiac complications.
Although our results regarding POCs and worse long-term outcomes are intuitive based on previously published literature on the subject, the concept that even a single or minor postoperative infectious complication can result in earlier cancer recurrence underscores the important role of prevention through adherence to evidence-based practice guidelines, and provides further evidence for continuing quality improvement efforts in the field of colorectal surgery. Over the past decade, high compliance with systematic approaches, or bundles, have been shown to reduce the risk of postoperative infectious complications in patients who undergo colorectal surgery.22 Although the rate of ERP compliance in our study was only 29%, this is likely secondary to the inclusion of patients prior to widespread implementation of ERP protocols. Further evidence regarding the use of additional perioperative bundles to prevent other complication types is necessary and this presents an area of future study. Our findings also warrant further experimental studies, such as comparison of circulating cancer cells or cytokines in patients who experience POCs and those who do not. Lastly, perhaps POCs should be included in recurrence nomograms with the ultimate goal of individualizing surveillance strategies to carefully monitor patients at higher risk of recurrence due to their postoperative course.
The present study must be interpreted with some limitations. Although its retrospective design invites some selection bias, the use of the USRCC mitigates single-institution bias and enables the generalizability of our results. Although strict definitions for each complication type were used during data extraction, the diagnosis of each complication type was not standardized across institutions. Similarly, it is possible that due to the limitations of the medical records, some minor, yet important, POCs were not well-documented and were therefore not extracted into the dataset.
CONCLUSION
While major complications after proctectomy for cancer are associated with reduced OS, both minor and major complications portend worse RFS. Given the association of infectious complications and postoperative renal dysfunction with earlier recurrence of disease, efforts must be directed towards defining best practices and standardizing care.
FUNDING
This work was supported in part by The Abraham J. & Phyllis Katz Foundation.
Footnotes
Meeting Presentation 2020 Society of Surgical Oncology.
DISCLOSURES Adriana C. Gamboa, Rachel M. Lee, Michael K. Turgeon, Christopher Varlamos, Scott E. Regenbogen, Katherine A. Hrebinko, Jennifer Holder-Murray, Jason T. Wiseman, Aslam Ejaz, Michael P. Feng, Alexander T. Hawkins, Philip Bauer, Matthew Silviera, Shishir K. Maithel, and Glen C. Balch have no disclosures relevant to this study.
REFERENCES
- 1.Enker WE, Merchant N, Cohen AM, et al. Safety and efficacy of low anterior resection for rectal cancer: 681 consecutive cases from a specialty service. Ann Surg. 1999;230(4):544–52; discussion 552–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–82. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40. [DOI] [PubMed] [Google Scholar]
- 4.Bonjer HJ, Deijen CL, Haglind E, Group CIS. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;373(2):194. [DOI] [PubMed] [Google Scholar]
- 5.Laurent C, Sa Cunha A, Couderc P, Rullier E, Saric J. Influence of postoperative morbidity on long-term survival following liver resection for colorectal metastases. Br J Surg. 2003;90(9):1131–6. [DOI] [PubMed] [Google Scholar]
- 6.Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg. 2015;261(3):497–505. [DOI] [PubMed] [Google Scholar]
- 7.Rizk NP, Bach PB, Schrag D, et al. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg. 2004;198(1):42–50. [DOI] [PubMed] [Google Scholar]
- 8.Dorcaratto D, Mazzinari G, Fernandez M, et al. Impact of postoperative complications on survival and recurrence after resection of colorectal liver metastases: systematic review and meta-analysis. Ann Surg. 2019;270(6):1018–27. [DOI] [PubMed] [Google Scholar]
- 9.Lagarde SM, de Boer JD, ten Kate FJ, Busch OR, Obertop H, van Lanschot JJ. Postoperative complications after esophagectomy for adenocarcinoma of the esophagus are related to timing of death due to recurrence. Ann Surg. 2008;247(1):71–6. [DOI] [PubMed] [Google Scholar]
- 10.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242(3):326–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita S, Teramoto T, Watanabe M, Kodaira S, Kitajima M. Anastomotic leakage after colorectal cancer surgery: a risk factor for recurrence and poor prognosis. Jpn J Clin Oncol. 1993;23(5):299–302. [PubMed] [Google Scholar]
- 13.Walker KG, Bell SW, Rickard MJ, et al. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004;240(2):255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katoh H, Yamashita K, Wang G, Sato T, Nakamura T, Watanabe M. Anastomotic leakage contributes to the risk for systemic recurrence in stage II colorectal cancer. J Gastrointest Surg. 2011;15(1):120–9. [DOI] [PubMed] [Google Scholar]
- 15.Law WL, Choi HK, Lee YM, Ho JW, Seto CL. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg. 2007;11(1):8–15. [DOI] [PubMed] [Google Scholar]
- 16.Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253(5):890–9. [DOI] [PubMed] [Google Scholar]
- 17.Salvans S, Mayol X, Alonso S, et al. Postoperative peritoneal infection enhances migration and invasion capacities of tumor cells in vitro. Ann Surg. 2014;260(5):939–44. [DOI] [PubMed] [Google Scholar]
- 18.Xu M, Mizoguchi I, Morishima N, Chiba Y, Mizuguchi J, Yoshimoto T. Regulation of antitumor immune responses by the IL-12 family cytokines, IL-12, IL-23, and IL-27. Clin Dev Immunol. 2010;2010:832454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McSorley ST, Watt DG, Horgan PG, McMillan DC. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol. 2016;23(9):2832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watt DG, McSorley ST, Park JH, Horgan PG, McMillan DC. A postoperative systemic inflammation score predicts short- and long-term outcomes in patients undergoing surgery for colorectal cancer. Ann Surg Oncol. 2017;24(4):1100–9. [DOI] [PubMed] [Google Scholar]
- 21.Alonso S, Pascual M, Salvans S, et al. Postoperative intra-abdominal infection and colorectal cancer recurrence: a prospective matched cohort study of inflammatory and angiogenic responses as mechanisms involved in this association. Eur J Surg Oncol. 2015;41(2):208–14. [DOI] [PubMed] [Google Scholar]
- 22.Zywot A, Lau CSM, Stephen Fletcher H, Paul S. Bundles prevent surgical site infections after colorectal surgery: meta-analysis and systematic review. J Gastrointest Surg. 2017;21(11):1915–30. [DOI] [PubMed] [Google Scholar]


