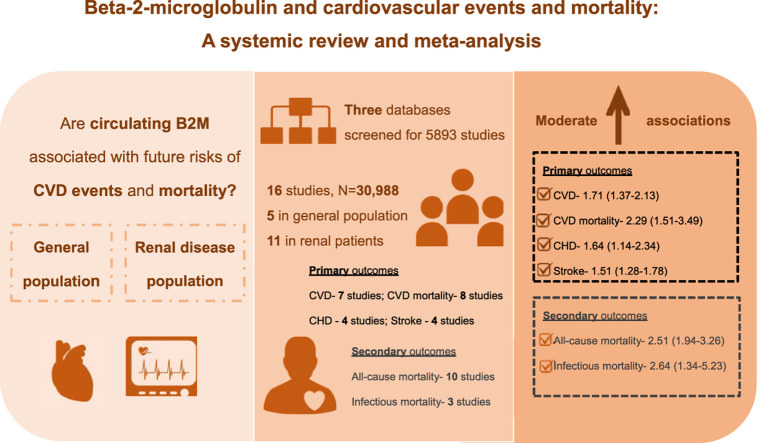
Abstract
Background and aims
Beta-2-microglobulin (B2M) has been suggested as an emerging biomarker for cardiovascular diseases (CVD), including coronary heart disease (CHD) and stroke, and mortality.
Methods
Three databases were searched from inception to January 2, 2020, supplemented by scanning reference lists of identified studies. We identified studies that reported associations of baseline serum or plasma B2M and CVD incidence, CVD mortality, or CHD and stroke separately, in either general populations or patients with renal disease. Relative risks (RR) were extracted and harmonized to a comparison of the highest versus lowest third of the distribution of B2M, and the results were aggregated.
Results
Sixteen studies (5 in general populations, and 11 in renal disease populations) were included, involving 30,988 participants and 5391 CVD events. Based on random-effects meta-analysis, the pooled adjusted RRs comparing the highest versus lowest third of the distribution of B2M were 1.71 (95%CI: 1.37–2.13) for CVD, 2.29 (1.51–3.49) for CVD mortality, 1.64 (1.14–2.34) for CHD, and 1.51 (1.28–1.78) for stroke, with little to high heterogeneity between studies (0.0% ≤ I2 ≤ 80.0%). The positive associations between B2M and risks of CVD outcomes remained broadly significant across subgroup analyses. Moreover, the pooled adjusted RRs were 2.51 (1.94–3.26; I2 = 83.7%) for all-cause mortality and 2.64 (1.34–5.23; I2 = 83.1%) for infectious mortality.
Conclusions
Available observational data show that there are moderate positive associations between B2M levels and CVD events and mortality, although few studies have been conducted in general populations.
Keywords: Beta-2-microglobulin, Cardiovascular diseases, Mortality, Meta-analysis
Graphical abstract
Highlights
-
•
Available epidemiological evidence shows that circulating B2M levels are moderately associated with CVD events and mortality.
-
•
Future large-scale general population-based prospective studies and genetic studies are needed to assess the causality.
1. Introduction
Beta-2-microglobulin (B2M) first discovered in 1964 is a 100-amino acid protein (11.8 kDa) encoded by a gene in chromosome 15 in humans [1]. B2M is an important component of the major histocompatibility complex class I (MHC-I) molecule that is expressed on the surface of almost all nucleated cells [1]. B2M is necessary for the cell surface expression and structural stability of the MHC-I molecule [2], which plays key roles in antigen presentation and processing, inflammation, the complement cascade, and stress response [3,4]. B2M also complexes with many non-classical MHC-I like molecules such as CD1, MR1, HLA-E, –F, -G and neonatal Fc receptor [[5], [6], [7], [8]] that are involved in mucosal immunity, tumour surveillance, immunoglobulin and albumin homeostasis [9]. Moreover, B2M is constantly secreted into circulation from cell surfaces or intracellular release (0–3 mg/L concentration in plasma or serum) and is eliminated from blood predominantly by the kidneys under normal physiological conditions [2,10], and has particularly been studied as a biomarker of renal function [9].
In view of the high morbidity and mortality burden of cardiovascular diseases (CVD), there is great interest in discovering novel biomarkers that distinguish individuals at a higher risk of CVD [11]. Circulating B2M may be a potential biomarker given the associations of elevated B2M with inflammatory responses and declining glomerular filtration rate (GFR) [[12], [13], [14]], together with the involvement of inflammation and impaired GFR in the pathogenesis of vascular disease [[15], [16], [17], [18]]. Recent epidemiological studies have suggested higher B2M levels associated with higher CVD risk both in general populations studies [19], and in individuals with renal conditions [20]. However, the association between B2M and CVD has not yet been systematically assessed.
To address the above uncertainties, we conducted a systemic review and meta-analysis of published studies to primarily quantify the observational association of B2M and CVD outcomes, both in general populations and in renal patients; and investigated the associations of B2M with non-cardiovascular and all-cause mortality in the same cohorts.
2. Materials and methods
2.1. Search strategy and selection criteria
This review followed the guidelines in the MOOSE (Meta-analysis of Observational Studies in Epidemiology) statement [21]. A systematic search was conducted in PubMed, Web of Science and Embase databases from inception to January 2, 2020 for relevant studies reporting associations between B2M and CVD in general populations and people with renal diseases, motivated by the specific use of B2M as a renal biomarker [9]. The combined literature search terms were related to B2M and the outcomes (CVD or CVD mortality or CHD or cerebrovascular disease) with the restriction to English language (Supplementary Table 1). The literature search was complemented by reviewing reference lists of the identified studies.
Studies were eligible for inclusion if they met the following criteria: (1) full-length publication in English language available; (2) were prospective (nested case-control and prospective cohort studies) or retrospective (case-control and retrospective cohort studies) studies; (3) reported associations between baseline B2M (serum or plasma) and outcomes, i.e. CVD or CVD mortality or CHD (defined as non-fatal myocardial infarction, coronary heart disease death, or coronary revascularization) or stroke; (4) participants were primarily sampled from the general population or, secondarily, in renal disease populations. Studies that solely selected participants (in cohort studies) or controls (in nested case-control studies) on the basis of pre-existing CVD or metabolic abnormalities other than renal disease were excluded; (5) participants were adults (aged ≥18); (6) relative risk (RR) measures and corresponding 95% confidence interval (CI) were provided.
2.2. Data extraction and quality assessment
From each retrieved article, the following characteristics were extracted: name of first author, year of publication, study design, geographical location, data source, assay method, population type, proportion of female participants, age of participants, follow-up years, relevant outcome definitions, number of cases, mean and standard deviation of B2M, reported estimates of B2M association with outcome, scale of reported estimates, and degree of statistical adjustment for covariates. The estimate adjusted for conventional cardiovascular risk factors was chosen if more than one estimates were reported. Quality of the studies was assessed by Newcastle-Ottawa scale (NOS) [22] (Supplementary Data 2), by two reviewers (FS and LS) independently, and discussed with the third reviewer (SK). Study scores of 0–3, 4–6, and 7–9 were considered as low, moderate and high quality, respectively.
2.3. Statistics analysis
The overall associations between baseline B2M and CVD outcomes were estimated in cohorts or nested case-control studies. Hazard ratios, risk ratios, and odds ratios were assumed to approximate the same measure of RR on the basis of low incidence of the outcomes studied. When studies reported RRs only in subgroups (e.g. by sex), a single pooled estimate was first obtained for the study using fixed-effect meta-analysis. The study-specific relative risk estimates were transformed to correspond to a comparison of risk in the highest versus lowest third of the distribution of B2M using established methods [23] (further details provided in Supplementary Data 3). Non-cardiovascular and all-cause mortality in the selected studies were secondarily investigated.
A random-effects meta-analysis was conducted using the DerSimonian-Laird method to account for potential heterogeneity between studies. The heterogeneity between studies was assessed by Cochran's Q test and I2 statistics [24]. I2 statistics of <25%, 25–50%, 50–75% and >75% was considered as “no or little heterogeneity”, “low heterogeneity”, “moderate heterogeneity” and “high heterogeneity” respectively [24]. Due to the relatively small number of contributing studies, subgroup analyses were conducted based on random-effects meta-regression with hypothesis tests based on the t-distribution to explore study-level characteristics potentially explaining heterogeneity [25]. Funnel plots were used to assess publication or small study bias. Sensitivity analyses by omitting one study at a time were conducted to assess the influence of individual studies. To further evaluate whether renal function altered the results, analyses were conducted, where available, based on estimates adjusted for markers of renal function (e.g. estimated GFR (eGFR)), or restricted to the participants without chronic renal diseases (i.e. eGFR≥60 mL/min/1.73 m2). All analyses used Stata version 15.1 [26], and two-sided p < 0.05 was interpreted as statistically significant.
3. Results
3.1. Overall characteristics of selected studies
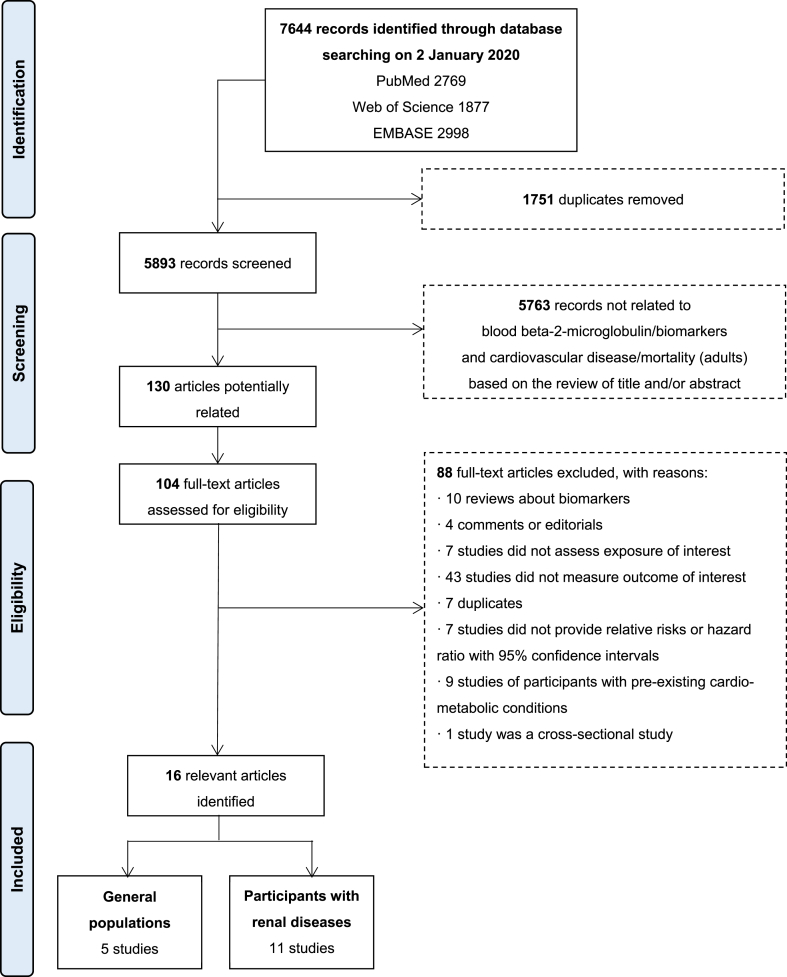
Electronic searching from PubMed, Web of Science, and Embase identified 5893 relevant articles (Fig. 1). After a detailed assessment of 104 full-text available articles, 16 articles were included in this review. Table 1 summarises the characteristics of the studies included. In aggregate, 5391 cardiovascular events, including 1866 CVD mortality cases, 2352 CHD cases and 1257 stroke cases, were reported in fourteen prospective studies (28,486 participants), and two retrospective studies (2502 participants). Five studies were conducted in the general populations (in the United States (US)), while eleven studies were primarily conducted in participants with renal diseases (one in Europe; four in the US; and six in Asia), among which Matsushita et al. study [20] also reported estimates for people without chronic kidney disease (CKD). B2M was measured from plasma samples in four studies, and from serum samples in 12 studies. The overall quality assessed by NOS was relatively high (seven or more stars, with one study [27] having six stars) (Supplementary Table 2).
Fig. 1.
Literature review flow diagram.
Matsushita et al. study [20], which primarily focused on CKD patients and was included into the 11 studies on participants with renal diseases here, also reported estimates for participants without CKD. CKD: chronic kidney disease.
Table 1.
Characteristics of 16 studies included in the review of the association between beta-2-microglobulin and cardiovascular disease.
| Study | Study design | Region | Data source | Baseline survey | Population | B2M assay |
Events for analysis | Sample size (female %) | Age (y) | Median follow-up (y) | B2M (mg/L) (mean ± SD) | No. of events |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ssource | Method | CVD | CVDM | CHD | Sstroke | |||||||||||
| General populations | ||||||||||||||||
| Astor, 2012 [39] | Prospective cohort | US | ARIC study | 1990–1992 | Community-based | Serum | PEINA (Siemens) | CHD | 9988 (43.1) | 62.9 ± 5.2j | 10.2 | 2.1 ± 0.9j | – | – | 1279 | – |
| Foster, 2013 [33] | Prospective cohort | US | NHANES III Cystatin C project | 1988–1994 | Population-based | Serum | LA (Siemens) | CVDM | 6445 (53.6) | ≧20 | 14.4 | 1.8 | – | 1079 | 605d | – |
| CHDM | ||||||||||||||||
| Prentice, 2013 [28] | Nested case-control | US | WHI HT trials | 1993–1998 | Postmenopausal women | Plasma | ELISA (CalBiotech) | CHD | 710 (100.0) | 50–79 | 7c | 110l | – | – | 358 | 362 |
| Strokeh | 708 (100.0) | |||||||||||||||
| Matsushita, 2014 [20] | Prospective cohort | US | ARIC study | 1996–1998 | Community-based (only non-CKDs) | Serum | PEINA (Siemens) | CVD | 7682 (59) | 62 ± 6 | 11.9 | 1.9 ± 0.4j | 1336 | – | – | 277 |
| Strokei | ||||||||||||||||
| Rist, 2017 [40] | Nested case-control | US | NHS | 1989–1990 | Female nurses | Plasma | ITA (Roche) | Ischaemic stroke | 946 (100.0) | 60.8 ± 6.0j | 9.0 | 1.9 ± 0.4m | – | – | – | 473 |
| Ho, 2018 [19] |
Prospective cohort | US | FHS | 1998–2005 | Community-based | Plasma | ELISA (Sigma-Aldrich) | CVD | 3523 (53.3) | 62 ± 8 | 14.3 | NR | 392e | 167 | – | – |
| CVDM | ||||||||||||||||
| Renal disease populations | ||||||||||||||||
| Cheung, 2008 [34] | Prospective cohort | US | HEMO study | 1995–2000 | HD patients (ESRD) | Serum | RIA (Abbott) | CVDM | 1813 (56.0) | 57.6 ± 14.1 | 2.6a | 37.6 ± 11.9 | – | 315f | – | – |
| Okuno, 2009 [35] | Prospective cohort | Japan | Hospital | 1999 | HD patients (ESRD) | Serum | LIA (Mitsubishi) | CVDM | 490 (41.2) | 60.1 ± 11.8 | 3.3a | 32.5 ± 7.2 | – | 36 | – | – |
| Liabeuf, 2012 [29] | Prospective cohort | France | Hospital | 2006–2007 | CKD stage 1–5 patients | Plasma | INA (Siemens) | CVD | 142 (39.4) | 67 ± 12 | 2.9 | 13.5 ± 12.5 | 49 | 24 | – | – |
| CVDM | ||||||||||||||||
| Astor, 2013 [36] | Retrospective cohort | US | Hospital | 1996–2009 | Kidney transplant recipients | Serum | MEIA (Abbott), ITA (Hitachi, Roche), NA (Siemens) | CVDM | 2190 (40.3) | 50.2 ± 13.0j | 4.1 | 3.3 | – | 114 | – | – |
| Matsushita, 2014 [20] | Prospective cohort | US | ARIC study | 1996–1998 | CKD stage 1–5 patients | Serum | PEINA (Siemens) | CVD | 940 (59.5) | 64.5 ± 5.5j | 11.9 | 2.4 ± 0.7j | 336 | – | – | 94 |
| Strokei | ||||||||||||||||
| Matsui, 2016 [27] | Prospective cohort | Japan | Medical university | 2010 | PD patients (ESRD) | Serum | NR | CVD | 40 (37.5) | 62.8 ± 12.3j | 1.5 | 20.8 ± 10.3j | 13 | – | – | – |
| Foster, 2016 [30] | Prospective cohort | US | CRIC study | 2005–2008 | CKD stage 1–3 patients | Serum | NA (Siemens) | CVD | 2405 (47.9) | 56.0 ± 11.6 | 6 | 4.2 ± 2.2 | 292 | – | 110g | 51 |
| MI | ||||||||||||||||
| Strokei | ||||||||||||||||
| Wu, 2017 [31] |
Retrospective cohort | China (Taiwan) | Hospital | 2009–2015 | CKD stage 3–5 patients | Serum | MEIA (Abbott) | CVD | 312 (38.1) | 70.9 ± 18.0j | 3.3a | 53.1 ± 23.2j | 27 | – | – | – |
| Yamashita, 2018 [37] | Prospective cohort | Japan | Hospital | 2012 | HD patients (ESRD) | Serum | NR | CVDM | 307 (38.8) | 68 ± 13 | 2b | 26.9 ± 6.4 | – | 25 | – | – |
| Chang, 2019 [38] | Prospective cohort | Korea | Hospital | 2006–2011 | PD patients (ESRD) | Serum | LIA | CVDM | 725 (44.4) | 59.3 ± 13.9 | 3.2 | 9.6 ± 8.3 | – | 106 | – | – |
| Nishimura, 2019 [32] | Prospective cohort | Japan | Hospital | 2005 | HD patients (ESRD) | Serum | NR | CVD | 244 (48.4) | 64 ± 11 | 4.7a | 41.4 ± 4.7k | 78 | – | – | – |
ARIC Study: Atherosclerosis Risk in Communities Study; B2M: Beta-2-microglobulin; CHD: Coronary Heart Disease; CHDM: CHD Mortality; CKD: Chronic Kidney Disease; CRIC Study: Chronic Renal Insufficiency Cohort Study; CVD: Cardiovascular Disease; CVDM: CVD Mortality; ELISA: Enzyme-linked immunosorbent assay; ESRD: End Stage Renal Disease; FHS: Framingham Heart Study; HD: Hemodialysis; HEMO Study: Hemodialysis Study; INA: Immunonephelometric assay; ITA: Immunoturbidimetric assay; LA: Latex assay; LIA: Latex immunoassay; MEIA: Microparticle enzyme immunoassay; MI: Myocardial Infarction; NA: Nephelometric assay; NHANES III: The Third National Health and Nutrition Examination Survey; NHS: Nurses' Health Study; NR: Not Reported; PD: Peritoneal Dialysis; PEINA: Particle-enhanced immunonephelometric assay; RIA: Radioimmunoassay; US: United States; WHI HT Trials: Women's Health Initiative postmenopausal hormone therapy trials.
Mean.
Maximum.
Minimum.
No. of CHD mortality.
No. of atherosclerotic CVD.
No. of cardiac death.
No. of MI.
Both haemorrhagic and ischaemic stroke were included but cases number of subtypes were not reported.
Whether haemorrhagic or ischaemic stroke was not specified.
Mean ± SD calculated using https://home.ubalt.edu/ntsbarsh/business-stat/otherapplets/Pooled.htm and/or http://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html.
Calculated and the unit of this figure from the original study is ng/mL.
Geometric mean reported in control groups was 0.11 mg/mL and equalled to 110 mg/L;m mean ± SD in the control group.
3.2. Associations of B2M and cardiovascular outcomes
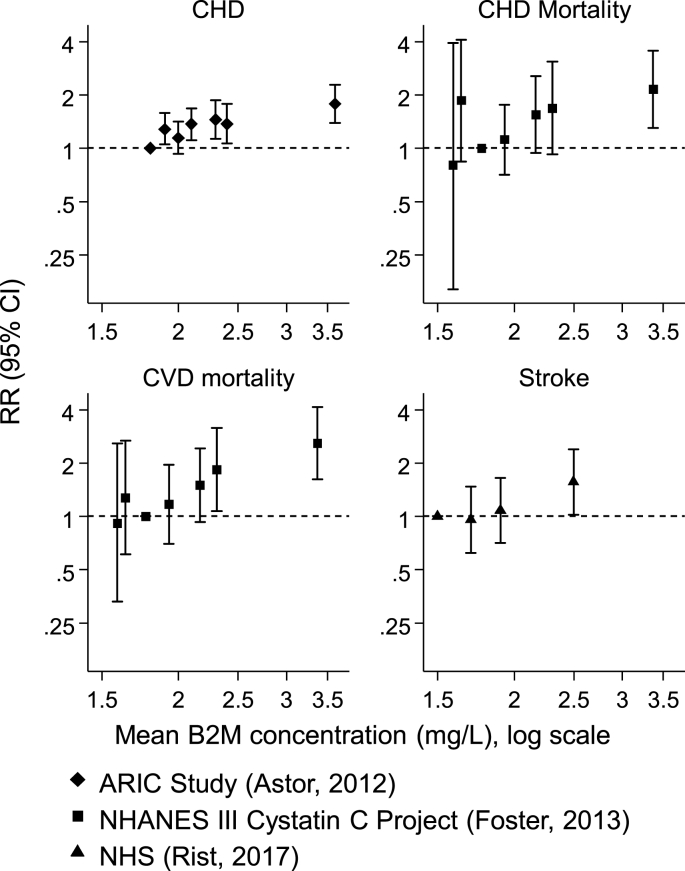
The RR estimates were converted into a comparison of highest versus lowest third of the distribution of B2M, except one study [28] where the standard deviation of B2M was not available (Supplementary Table 3). Fig. 2 shows the dose-response plots constructed for studies that used at least four categories of B2M levels, suggesting that a log-linear association of B2M and risk of CVD outcomes was reasonable.
Fig. 2.
Relative risk of cardiovascular events according to categories of B2M levels for studies that provided results for quartiles or quintiles of B2M levels.
ARIC Study: Atherosclerosis Risk in Communities Study; B2M: Beta-2-microglobulin; CVD: Cardiovascular Disease; CHD: Coronary Heart Disease; NHANES III: The Third National Health and Nutrition Examination Survey; NHS: Nurses' Health Study.
3.2.1. Cardiovascular disease outcomes
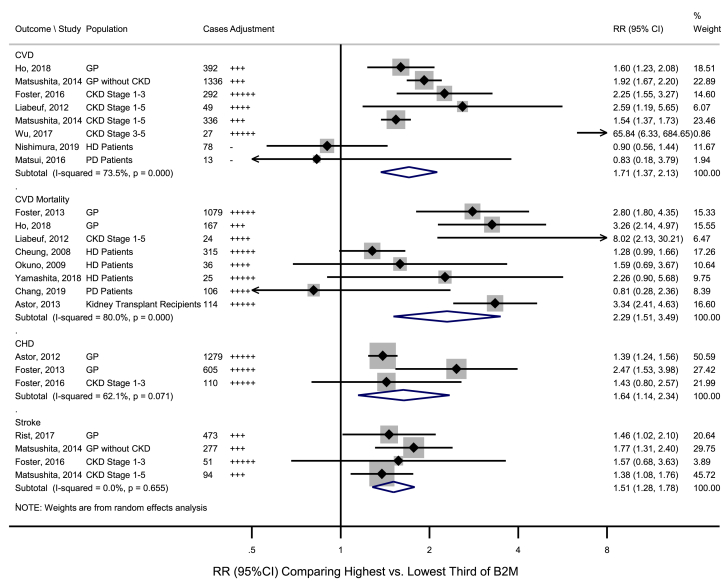
Of the seven studies [19,20,27,[29], [30], [31], [32]] investigating the association between B2M and CVD, five [19,20,[29], [30], [31]] reported significant positive associations (Fig. 3 and Supplementary Table 3). The pooled RR for CVD comparing the highest versus lowest third of B2M was 1.71 (95%CI: 1.37–2.13; I2 = 73.5%, phet < 0.001) (Fig. 3), and the pooled RR was 1.69 (1.33–2.14) for studies with further adjustments for renal function (Supplementary Figure 1). Sensitivity analysis omitting one study iteratively suggested that none of the included studies significantly influenced the pooled estimates, with RRs ranging from 1.63 (1.28–2.07) to 1.84 (1.49–2.26) (Supplementary Figure 2). The retrospective study by Wu et al. [31] reporting an RR of 65.84 (95%CI: 6.33–684.54) was considered as an outlier (meta-regression p = 0.034, by study design) (Table 2). Of the remaining prospective studies, the pooled RR was 1.66 (1.39, 1.99) with a moderate heterogeneity between studies (I2 = 64.7%, phet = 0.009) (Table 2).
Fig. 3.
Association of B2M with risk for cardiovascular outcomes, comparing highest versus lowest third of B2M.
HD/PD patients and those at CKD Stage 5 are normally ESRD patients. B2M: Beta-2-microglobulin; CHD: Coronary Heart Disease; CI: Confidence Interval; CKD: Chronic Kidney Disease; CVD: Cardiovascular Disease; ESRD: End Stage Renal Disease; GP: General Populations; HD: Hemodialysis; PD: Peritoneal Dialysis; RR: Relative Risk. Adjustment: no adjustment, + adjusted for age and/or sex, ++ age, sex, and non-lipid risk factors (e.g. race, medication use), +++ adjusted for age, sex, diabetes, body mass index/blood pressure/smoking and/or lipid markers, ++++adjusted for preceding plus inflammatory markers; +++++adjusted for preceding plus urinary indices.
Table 2.
Association of B2M with risk for CVD, CVD mortality, and all-cause mortality by recorded study level characteristics.
| Subgroup | CVD |
CVD mortality |
All-cause mortality |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studiesa | No of cases | RR (95%CI) | I2 (%) | pmeta-regression | No of studies | No of cases | RR (95%CI) | I2 (%) | pmeta-regression | No of studies | No of cases | RR (95%CI) | I2 (%) | pmeta-regression | |
| All studies | 8 | 2523 | 1.71 (1.37, 2.13) | 73.5 | – | 8 | 1866 | 2.29 (1.51, 3.49) | 80.0 | – | 10 | 6165 | 2.51 (1.94, 3.26) | 83.7 | – |
| Study design | |||||||||||||||
| Retrospective | 1 | 27 | 65.84 (6.33, 684.54) | – | 0.034 | 1 | 114 | 3.34 (2.41, 4.63) | – | 0.457 | 2 | 481 | 5.79 (1.06, 31.65) | 58.5 | 0.315 |
| Prospective | 7 | 2496 | 1.66 (1.39, 1.99) | 64.7 | 7 | 1752 | 2.13 (1.34, 3.37) | 76.4 | 8 | 5684 | 2.36 (1.80, 3.09) | 84.8 | |||
| Population | |||||||||||||||
| General population | 2 | 1728 | 1.82 (1.54, 2.14) | 31.2 | 0.954 | 2 | 1246 | 3.03 (2.23, 4.11) | 0 | 0.407 | 3 | 4572 | 2.00 (1.68, 2.39) | 65.3 | 0.263 |
| Renal disease patients | 6 | 795 | 1.75 (1.13, 2.72) | 76.2 | 6 | 620 | 2.04 (1.16, 3.59) | 81.9 | 7 | 1593 | 2.94 (1.96, 4.39) | 72.0 | |||
| Geographical location | |||||||||||||||
| America | 4 | 2356 | 1.75 (1.49, 2.05) | 63.2 | 0.520 | 4 | 1675 | 2.47 (1.45, 4.20) | 89.1 | 0.227 | 5 | 5677 | 2.56 (1.87, 3.49) | 90.6 | 0.332 |
| Asia | 3 | 118 | 2.70 (0.37, 19.49) | 84.0 | 3 | 167 | 1.51 (0.87.2.60) | 3.3 | 4 | 444 | 2.04 (1.16, 3.61) | 57.8 | |||
| Europe | 1 | 49 | 2.59 (1.19, 5.66) | – | 1 | 24 | 8.02 (2.13, 30.23) | – | 1 | 44 | 6.61 (2.53, 17.28) | – | |||
| Study quality (NOS) | |||||||||||||||
| <8 | 3 | 140 | 1.30 (0.59, 2.83) | 62.8 | 0.359 | 1 | 24 | 8.02 (2.13, 30.23) | – | 0.155 | 1 | 44 | 6.61 (2.53, 17.28) | – | 0.174 |
| ≧ 8 | 5 | 2383 | 1.83 (1.46, 2.28) | 77.1 | 7 | 1842 | 2.11 (1.39, 3.21) | 80.7 | 9 | 6121 | 2.39 (1.84, 3.09) | 83.9 | |||
| Proportion of female participants | |||||||||||||||
| <50% | 5 | 459 | 2.06 (0.96, 4.42) | 80.5 | 0.885 | 5 | 305 | 2.36 (1.30, 4.28) | 62.7 | 0.899 | 8 | 3018 | 2.70 (1.80, 4.03) | 87.0 | 0.660 |
| ≧50% | 3 | 2064 | 1.69 (1.44, 1.98) | 66.2 | 3 | 1561 | 2.23 (1.17, 4.23) | 88.9 | 2 | 3147 | 2.18 (1.86, 2.56) | 3.1 | |||
| Adjust for renal markers | |||||||||||||||
| No | 6 | 2204 | 1.59 (1.31, 1.92) | 65.5 | 0.267 | 4 | 333 | 2.34 (1.09, 5.02) | 69.4 | 0.939 | 4 | 1148 | 2.16 (1.36, 3.43) | 66.1 | 0.498 |
| Yes | 2 | 319 | 9.91 (0.37, 264.28) | 87.2 | 4 | 1533 | 2.26 (1.28, 3.98) | 87.1 | 6 | 5017 | 2.78 (1.90, 4.08) | 89.2 | |||
B2M: Beta-2-microglobulin; CI: Confidence Interval; CVD: Cardiovascular disease; NOS: Newcastle-Ottowa Scale; RR: Relative Risk.
Matsushita et al. study [20] was counted twice for CVD because estimates were provided for two populations, respectively.
3.2.2. Cardiovascular mortality
Eight studies [19,29,[33], [34], [35], [36], [37], [38]] assessed the association between B2M and CVD mortality (Fig. 3 and Supplementary Table 3), with two [19,33] studies conducted in general populations reporting significant positive associations, and six [29,[34], [35], [36], [37], [38]] studies conducted in patients with renal diseases showing inconsistent associations. The pooled RR for CVD mortality comparing the highest versus lowest third of B2M was 2.29 (1.51–3.49; I2 = 80.0%, phet<0.001) (Fig. 3). Further adjustments for estimated renal function did not alter the results (Supplementary Figure 1). Subgroup and meta-regression analyses by study-level characteristics did not identify characteristics explaining heterogeneity (Table 2 and Supplementary Figure 4). Sensitivity analysis omitting one study at a time suggested that none of the individual studies significantly influenced the pooled estimates (Supplementary Figure 2).
3.2.3. Coronary heart disease and stroke
Four studies reported associations with B2M on CHD [28,[30], [33],39] and stroke [20,28,30,40], respectively, which were all significantly positive in general populations. The pooled RR comparing the highest vs lowest thirds of B2M distribution was 1.64 (1.14–2.34; I2 = 62.1%, phet = 0.071) for CHD, and 1.51 (1.28–1.78; I2 = 0.0%, phet = 0.655) for stroke (Fig. 3), which remained significant with further adjustment for renal function or restricted to those with eGFR≥60 mL/min/1.73 m2 (Supplementary Figure 1 and 3). One study [28] was not included in the present meta-analysis due to inability to convert reported RRs (Supplementary Table 3), in which, 30% higher B2M was associated with RRs of 1.21 (1.06–1.37) for CHD, and 1.46 (1.21–1.78) for stroke, respectively.
3.3. Associations of B2M with non-cardiovascular and all-cause mortality
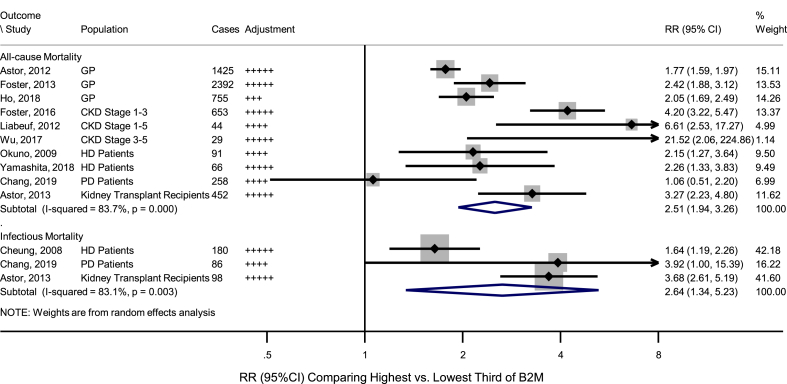
Among the included studies, 6165 all-cause mortality cases were reported by ten studies[19,[29], [30], [31],33,[35], [36], [37], [38], [39]], about 22.1%–54.5% of which were cardiovascular mortality; meanwhile, 364 infectious mortality cases were reported by three [34,36,38] studies (Supplementary Table 4). The pooled RR comparing the highest vs lowest thirds of B2M distribution was 2.51 (1.94–3.26; I2 = 83.7%, phet <0.001) for all-cause mortality and 2.64 (1.34–5.23; I2 = 83.1%, phet = 0.003) for infectious mortality (Fig. 4), which remained significant in all the sensitivity analyses (Supplementary Figure 1-3).
Fig. 4.
Association of B2M with risk for all-cause and infectious mortality, comparing highest versus lowest third of B2M.
HD/PD patients and those at CKD Stage 5 are normally ESRD patients. B2M: Beta-2-microglobulin; CI: Confidence Interval; CKD: Chronic Kidney Disease; ESRD: End Stage Renal Disease; GP: General Populations; HD: Hemodialysis; PD: Peritoneal Dialysis; RR: Relative Risk. Adjustment: no adjustment, + adjusted for age and/or sex, ++ age, sex, and non-lipid risk factors (e.g. race, medication use), +++ adjusted for age, sex, diabetes, body mass index/blood pressure/smoking and/or lipid markers, ++++adjusted for preceding plus inflammatory markers; +++++adjusted for preceding plus urinary indices.
3.4. Publication bias
Since limited numbers of studies were available for each outcome, only CVD, CVD mortality and all-cause mortality studies (8, 8, and 10 estimates reported, respectively) were deemed suitable for the assessment of publication or small study bias using funnel plots. There was little evidence of publication or small study bias (Egger's test p > 0.05 and Begg's test p > 0.05 for all) among the studies of CVD, CVD mortality and all-cause mortality (Supplementary Figure 5).
4. Discussion
By integrating observational evidence from 16 studies, including 30,988 participants and 5391 CVD events, we primarily found positive associations of higher B2M levels and CVD outcomes, independent of conventional CVD risk factors as well as renal function; and secondarily found higher B2M levels were associated with increased risks of infectious and all-cause mortality. The associations between higher B2M levels and increased risk of CVD events and mortality persisted and remained broadly significant across study-level characteristics.
Previous individual studies on the associations of B2M and CVD outcomes have reported inconsistent results, with either positive [19,20,[28], [29], [30], [31],33,36,39,40] or no statistically significant [27,32,34,35,37,38] associations between B2M levels and CVD outcomes. In individual studies comprising models with different degrees of adjustment, the estimates of the association between B2M and cardiovascular outcomes were attenuated towards null [30,40] when further adjusting for eGFR. The present meta-analysis found significant associations between higher B2M levels and increased risks of CVD outcomes even after adjustment for inflammatory markers (e.g. albumin and C-reactive protein) and renal markers (e.g. eGFR), which were broadly consistent across the study-level characteristics assessed. The CVD and CVD mortality associations were somewhat stronger in general populations than in renal patients. In addition, the positive association of B2M and CVD, CHD or stroke appeared slightly stronger in individuals without chronic kidney disease than those with chronic kidney disease in one study [20]. The precise mechanisms linking B2M with CVD has not been fully understood, and it has been suggested that it may be partly due to renal function. B2M has been recognized as a marker of renal function [9], because it can be freely filtered by the glomerulus and reabsorbed and metabolized by the proximal tubule under normal kidney condition [2,10], and its circulating level rises when GFR declines [13]. Inflammation [12,13] has also been suggested as a potential mechanism linking B2M and CVD. Evidence suggested that higher B2M levels were positively associated with inflammatory markers [41].
Our meta-analysis found that B2M was also associated with all-cause mortality [19,[29], [30], [31],33,[35], [36], [37], [38], [39]] and mortality from infectious diseases [34,36,38] in the same cohorts, and the relevance of B2M with all-cause mortality was consistent across the study characteristics assessed in the present analyses. The positive associations with infectious and all-cause mortality were independent of renal function markers in line with previous findings in older age populations [42]. B2M has previously been found to be associated with other non-cardiovascular outcomes, such as various cancers [10], though not reported in many of the studies included in our review. Existing evidence has suggested that B2M is probably a general biomarker that reflects the acute or chronic changes during inflammation, infection, or immune dysregulation [2]. In our meta-analysis, however, compared to those for cardiovascular outcomes, the number of studies reporting B2M with non-cardiovascular outcomes was relatively limited, and the majority of study population patients had renal diseases [[29], [30], [31],[34], [35], [36], [37], [38]]. Hence, interpretations of the findings on B2M with non-cardiovascular mortality warrants cautions. This limitation emphasizes the need for prospective studies in general populations to compare the dose-response and magnitude of associations of B2M levels and incident disease outcomes.
B2M levels were markedly elevated with the progression of CKD and peaked in ESRD [29,36]. Compared to the general populations, the positive associations with CVD, CVD mortality and all-cause mortality seemed to be modest in ESRD patients undergoing dialysis [27,29,32,34,35,37,38]. These patients are special as high-flux dialysis could remove putatively atherogenic middle molecules [43,44] that may contribute to CVD events, such as advanced glycosylation end products [44]. Moreover, associations of B2M with CVD outcomes and all-cause mortality were found to be non-significant or even become significantly negative among patients that had undergone dialysis for over 3.7 years [34,45].
Although the majority of included studies comprehensively adjusted for potential confounders, the associations could still be subject to residual confounding or reserve causation bias, as our meta-analysis was based on observational studies. In the present meta-analysis, the association of B2M with CVD outcomes seemed to be attenuated according to some study level characteristics, such as smaller sample size and greater proportion of females, though no significant differences were found, which may be due to the low statistical power with few studies available. Longitudinal studies on changes of B2M demonstrated that B2M changes conveyed greater disease risk for CVD events [28,38]. While application of Mendelian randomization (MR) approaches may be informative (i.e. utilizing genotypes information fixed at conception to avoid reverse causation or confounding inherent in observational epidemiological studies [46]), we could not identify genetic instruments from currently available studies [47] that strictly fulfill assumptions underlying MR [48], thereby hampering further investigation. Hence, a comprehensive evaluation of associations in future well-powered genetic studies or randomized clinical trials of B2M and CVD are needed, given the evolving literature on B2M as potential drug targets [49,50] and gaps in translating research findings into clinical practice [15].
Our study has strengths. Our meta-analysis, by combining all available evidence so far, has provided improved statistical power than individual studies on the association between B2M levels and CVD outcomes. Our analyses were able to quantify the magnitude of the association between B2M and CVD outcomes, by harmonizing the reporting scales in individual studies, and to explore potential sources of heterogeneity between studies. Further, the linearity assumption underlying the RR conversions were satisfied based on checking studies that provided results by quartiles or quintiles of B2M levels.
Our study also had some limitations. First, the number of eligible studies identified reporting individual CVD outcomes was relatively small, in particular CHD, stroke, and stroke pathological types (e.g. ischaemic stroke and haemorrhagic stroke), reducing statistical power to detect heterogeneity [24]. Second, we only used aggregate data as reported or calculated in the published articles rather than analysis of individual participant data, thereby limiting the explorations of the contributions of individual level characteristics (e.g. observation time) to observed heterogeneity, or conducting a dose-response meta-analysis across all studies. Third, while publication or small study bias could in principle affect the results, it was not detected or possible to assess for all outcomes analysed in this study. Furthermore, the statistical tests concerning Begg's rank correlation and Egger's funnel plot asymmetry were less informative, given the small number of high-quality studies included [51]. Finally, our results only reflect the measurement of B2M at a single time point rather than longitudinal changes in B2M. Few studies [28,38,52] have so far explored the association between the change in B2M and cardiovascular diseases, although time-varying B2M demonstrated stronger associations with risk of CVD than baseline B2M [[28], [38]].
In summary, combined evidence from available observational studies shows positive associations between B2M level and risk of CVD outcomes, independent of conventional CVD risk factors, and estimated renal function. Future studies can help assess the causal nature of associations between B2M and CVD outcomes.
Financial support
FS received funds from the China Scholarship Council, and Cambridge Commonwealth European and International Trust. LS and SK are supported by grant from the British Heart Foundation (BHF) (RG/18/13/33946).
CRediT authorship contribution statement
Fanchao Shi: Study conception and design, literature search, inclusion, and statistical analysis, interpretation, first draft of manuscript, revision, All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy. Luanluan Sun: literature search, inclusion, and statistical analysis, interpretation, revision, All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy. Stephen Kaptoge: Study conception and design, literature search, inclusion, and statistical analysis, interpretation, revision, All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.atherosclerosis.2021.01.018.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Berggård I., Bearn A.G. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J. Biol. Chem. 1968;243(15):4095–4103. doi: 10.1016/s0021-9258(18)93284-9. [DOI] [PubMed] [Google Scholar]
- 2.Li L., Dong M., Wang X.-G. The implication and significance of beta 2 microglobulin. Chin Med J (Engl). 2016;129(4):448–455. doi: 10.4103/0366-6999.176084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horton R., Wilming L., Rand V. Gene map of the extended human MHC. Nat. Rev. Genet. 2004;5(12):889–899. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- 4.Trowsdale J., Knight J.C. Major histocompatibility complex genomics and human disease. Annu. Rev. Genom. Hum. Genet. 2013;14(1):301–323. doi: 10.1146/annurev-genom-091212-153455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi H., Hashimoto K. Association of MR1 protein, an MHC class I-related molecule, with β2-microglobulin. Biochem. Biophys. Res. Commun. 2002;290(2):722–729. doi: 10.1006/bbrc.2001.6277. [DOI] [PubMed] [Google Scholar]
- 6.Gonen-Gross T., Achdout H., Arnon T.I. The CD85J/leukocyte inhibitory receptor-1 distinguishes between conformed and β2-microglobulin-free HLA-G molecules. J. Immunol. 2005;175(8):4866–4874. doi: 10.4049/jimmunol.175.8.4866. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhury C., Mehnaz S., Robinson J.M. The major histocompatibility complex–related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J. Exp. Med. 2003;197(3):315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyzik M., Rath T., Lencer W.I., Baker K., Blumberg R.S. FcRn: the architect behind the immune and nonimmune functions of IgG and albumin. J. Immunol. 2015;194(10):4595–4603. doi: 10.4049/jimmunol.1403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argyropoulos C.P., Chen S.S., Ng Y. Rediscovering beta-2 microglobulin as a biomarker across the spectrum of kidney diseases. Front. Med. 2017;4(June) doi: 10.3389/fmed.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prizment A.E., Linabery A.M., Lutsey P.L. Circulating beta-2 microglobulin and risk of cancer: the atherosclerosis risk in Communities study (ARIC) Cancer Epidemiol. Biomark. Prev. 2016;25(4):657–664. doi: 10.1158/1055-9965.EPI-15-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas H., Diamond J., Vieco A. Global atlas of cardiovascular disease 2000-2016: the path to prevention and control. Glob. Heart. 2018;13(3):143–163. doi: 10.1016/j.gheart.2018.09.511. [DOI] [PubMed] [Google Scholar]
- 12.Vraetz T., Ittel T.H., Mackelenbergh MG Van, Heinrich P.C., Sieberth H.G., Graeve L. Regulation of B2-microglobulin expression in different human cell lines by proinflammatory cytokines. Nephrol. Dial. Transplant. 1999;14(9):2137–2143. doi: 10.1093/ndt/14.9.2137. [DOI] [PubMed] [Google Scholar]
- 13.Cooper E.H., Forbes M.A., Hambling M.H. Serum beta 2- microglobulin and C reactive protein concentrations in viral infections. J. Clin. Pathol. 1984;37(10):1140–1143. doi: 10.1136/jcp.37.10.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schardijn G.H.C., van Eps L.W.S. β2-microglobulin: its significance in the evaluation of renal function. Kidney Int. 1987;32(5):635–641. doi: 10.1038/ki.1987.255. [DOI] [PubMed] [Google Scholar]
- 15.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 16.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 17.Matsushita K., van der Velde M., Astor B.C. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita K., Coresh J., Sang Y. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–525. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho J.E., Lyass A., Courchesne P. Protein biomarkers of cardiovascular disease and mortality in the community. J. Am. Heart Assoc. 2018;7(14):e008108. doi: 10.1161/JAHA.117.008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushita K., Sang Y., Ballew S.H. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease: the Atherosclerosis Risk in Communities study. Arterioscler. Thromb. Vasc. Biol. 2014;34(8):1770–1777. doi: 10.1161/ATVBAHA.114.303465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 22.Wells G.A., Shea B., O'Connell D. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 23.Ceu Stata programs-cardiovascular epidemiology unit [internet] http://www.phpc.cam.ac.uk/ceu/erfc/programs/
- 24.Higgins J.P.T., Thompson S.G, Deeks J.J, Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knapp G., Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003;22(17):2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 26.StataCorp . StataCorp LLC; College Station, TX: 2017. (Stata Statistical Software: Release 15). [Google Scholar]
- 27.Matsui M., Samejima K., Takeda Y. Angiogenic factors and risks of technique failure and cardiovascular events in patients receiving peritoneal dialysis. Cardiorenal Med. 2016;6(3):251–259. doi: 10.1159/000444886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prentice R.L., Zhao S., Johnson M. Proteomic risk markers for coronary heart disease and stroke: validation and mediation of randomized trial hormone therapy effects on these diseases. Genome Med. 2013;5(12):112. doi: 10.1186/gm517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liabeuf S., Lenglet A., Desjardins L. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82(12):1297–1303. doi: 10.1038/ki.2012.301. [DOI] [PubMed] [Google Scholar]
- 30.Foster M.C., Coresh J., Hsu C. Serum β-trace protein and β2-microglobulin as predictors of ESRD, mortality, and cardiovascular disease in adults with CKD in the chronic renal insufficiency cohort (CRIC) study. Am. J. Kidney Dis. 2016;68(1):68–76. doi: 10.1053/j.ajkd.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H.-C., Lee L.-C., Wang W.-J. Associations among serum beta 2 microglobulin, malnutrition, inflammation, and advanced cardiovascular event in patients with chronic kidney disease. J. Clin. Lab. Anal. 2017;31(3) doi: 10.1002/jcla.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura M., Tokoro T., Takatani T. Circulating aminoterminal propeptide of type III procollagen as a biomarker of cardiovascular events in patients undergoing hemodialysis. J. Atherosclerosis Thromb. 2019;26(4):340–350. doi: 10.5551/jat.45138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster M.C., Inker L.A., Levey A.S. Novel filtration markers as predictors of all-cause and cardiovascular mortality in US adults. Am. J. Kidney Dis. 2013;62(1):42–51. doi: 10.1053/j.ajkd.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung A.K., Greene T., Leypoldt J.K. Association between serum β2 -microglobulin level and infectious mortality in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2008;3(1):69–77. doi: 10.2215/CJN.02340607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okuno S., Ishimura E., Kohno K. Serum β2-microglobulin level is a significant predictor of mortality in maintenance haemodialysis patients. Nephrol. Dial. Transplant. 2009;24(2):571–577. doi: 10.1093/ndt/gfn521. [DOI] [PubMed] [Google Scholar]
- 36.Astor B.C., Muth B., Kaufman D.B., Pirsch J.D., Michael Hofmann R., Djamali A. Serum β2 -microglobulin at discharge predicts mortality and graft loss following kidney transplantation. Kidney Int. 2013;84(4):810–817. doi: 10.1038/ki.2013.172. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita K., Mizuiri S., Nishizawa Y., Shigemoto K., Doi S., Masaki T. Addition of novel biomarkers for predicting all-cause and cardiovascular mortality in prevalent hemodialysis patients. Ther. Apher. Dial. 2018;22(1):31–39. doi: 10.1111/1744-9987.12593. [DOI] [PubMed] [Google Scholar]
- 38.Chang T.I., Lim H., Park C.H. Lower serum beta-2 microglobulin levels are associated with worse survival in incident peritoneal dialysis patients. Nephrol. Dial. Transplant. 2019;34(1):138–145. doi: 10.1093/ndt/gfy193. [DOI] [PubMed] [Google Scholar]
- 39.Astor B.C., Shafi T., Hoogeveen R.C. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am. J. Kidney Dis. 2012;59(5):653–662. doi: 10.1053/j.ajkd.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rist P.M., Jiménez M.C., Rexrode K.M. Prospective association between β2 -microglobulin levels and ischemic stroke risk among women. Neurology. 2017;88(23):2176–2182. doi: 10.1212/WNL.0000000000004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juraschek S.P., Coresh J., Inker L.A. Comparison of serum concentrations of β-trace protein, β2-microglobulin, Cystatin C, and Creatinine in the US population. Clin. J. Am. Soc. Nephrol. 2013;8(4):584–592. doi: 10.2215/cjn.08700812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shinkai S., Chaves P.H.M., Fujiwara Y. β2-microglobulin for risk stratification of total mortality in the elderly population: comparison with cystatin C and C-reactive protein. Arch. Intern. Med. 2008;168(2):200–206. doi: 10.1001/archinternmed.2007.64. [DOI] [PubMed] [Google Scholar]
- 43.Weiss M.F., Erhard P., Kader-Attia F.A. Mechanisms for the formation of glycoxidation products in end-stage renal disease. Kidney Int. 2000;57(6):2571–2585. doi: 10.1046/j.1523-1755.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- 44.Makita Z., Radoff S., Rayfield E.J. Advanced glycosylation end products in patients with diabetic nephropathy. N. Engl. J. Med. 1991;325(12):836–842. doi: 10.1056/nejm199109193251202. [DOI] [PubMed] [Google Scholar]
- 45.Delmez J.A., Yan G., Bailey J. Cerebrovascular disease in maintenance hemodialysis patients: results of the HEMO study. Am. J. Kidney Dis. 2006;47(1):131–138. doi: 10.1053/j.ajkd.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 46.Smith G.D., Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003;32(1):1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 47.Yao C., Chen G., Song C. Genome‐wide mapping of plasma protein QTLs identifies putatively causal genes and pathways for cardiovascular disease. Nat. Commun. 2018;9(1):3268. doi: 10.1038/s41467-018-05512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burgess S., Scott R.A., Timpson N.J., Smith G.D., Thompson S.G., EPIC- InterAct Consortium Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 2015;30(7):543–552. doi: 10.1007/s10654-015-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nomura T., Huang W-C., Zhau H., Josson S., Mimata H., Chung L. Beta2-Microglobulin-mediated signaling as a target for cancer therapy. Anticancer Agents Med Chem. 2014;14(3):343–352. doi: 10.2174/18715206113139990092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navya P., Hema K., Munikumar M., Swargam S., Umamaheswari A. Molecular docking of a beta-2-microglobulin drug target. Online J Bioinforma. 2012;13:222–231. [Google Scholar]
- 51.Higgins J.P., Green S. In: Cochrane Handbook for Systematic Reviews of Interventions. Higgins J.P., Green S., editors. John Wiley & Sons, Ltd; Chichester, UK: 2008. [DOI] [Google Scholar]
- 52.Rebholz C.M., Grams M.E., Matsushita K. Change in multiple filtration markers and subsequent risk of cardiovascular disease and mortality. Clin. J. Am. Soc. Nephrol. 2015;10(6):941–948. doi: 10.2215/CJN.10101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.