Abstract
Purpose
Not all patients with stage III non-small cell lung cancer (NSCLC) are suitable for concurrent chemoradiation therapy (CRT). Local failure rate is high for sequential concurrent CRT. As such, there is a rationale for treatment intensification.
Methods and Materials
Isotoxic intensity modulated radiation therapy (IMRT) is a multicenter feasibility study that combines different intensification strategies including hyperfractionation, acceleration, and dose escalation facilitated by IMRT. Patients with unresectable stage III NSCLC, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2, and unsuitable for concurrent CRT were recruited. A minimum of 2 cycles of platinum-based chemotherapy was compulsory before starting radiation therapy (RT). Radiation dose was increased until a maximum dose of 79.2 Gy was reached or 1 or more of the organs at risk met predefined constraints. RT was delivered in 1.8-Gy fractions twice daily, and an RT quality assurance program was implemented. The primary objective was the delivery of isotoxic IMRT to a dose >60 Gy equivalent dose in 2-Gy fractions (EQD2 assuming an α/β ratio of 10 Gy for acute reacting tissues).
Results
Thirty-seven patients were recruited from 7 UK centers. Median age was 69.9 years (range, 46-86 years). The male-to-female ratio was 17:18. ECOG PS was 0 to 5 in 14.2% of patients; PS was 1 to 27 in 77.1% of patients; PS was 2 to 3 in 8.6% of patients. Stage IIIA:IIIB ratio was 22:13 (62.9%:37.1%). Of 37 patients, 2 (5.4%) failed to achieve EQD2 > 60 Gy. Median prescribed tumor dose was 77.4 Gy (range, 61.2-79.2 Gy). A maximum dose of 79.2Gy was achieved in 14 patients (37.8%). Grade 3 esophagitis was reported in 2 patients, and no patients developed grade 3 to 4 pneumonitis. There were 3 grade 5 events: acute radiation pneumonitis, bronchopulmonary hemorrhage, and acute lung infection. Median follow-up at time of analysis was 25.4 months (range, 8.0-44.2) months for 11 of 35 survivors. The median survival was 18.1 months (95% confidence interval [CI], 13.9-30.6), 2-year overall survival was 33.6% (95% CI, 17.9-50.1), and progression-free survival was 23.9% (95% CI, 11.3-39.1).
Conclusions
Isotoxic IMRT is a well-tolerated and feasible approach to treatment intensification.
Introduction
The current 5-year survival of stage III non-small cell lung cancer (NSCLC) with standard treatment is approximately 20% to 30% at best,1, 2, 3 highlighting the urgency to improve outcomes. Concurrent chemoradiotherapy (cCRT), chemotherapy, and radiation therapy (RT) given at the same time is the standard of care in stage III NSCLC followed by durvalumab in patients who are fit and have responded to treatment.4 However, for most patients, this treatment is unsuitable because of comorbidities and poor performance status (PS).5 A national audit of the management and outcomes of stage III NSCLC conducted in England in 2016 showed that only 17% of stage III patients were treated with curative-intent RT. For patients receiving chemotherapy and curative-intent RT, only 34% received cCRT. Sequential CRT was delivered almost twice as often as concurrent.6 Sequential CRT has inferior local control and survival rates.1 Local control with standard 3-dimensional conformal RT (3D-CRT) alone remains poor, with reported 2-year locoregional control rates of 20% to 44%.1,7,8 However, meta-analysis data has shown that improved local control in lung cancer can lead to improvement in survival.1
Personalized medicine is an increasing facet of modern cancer treatment, but its implementation has been less evident in radiation oncology. To date, patients still receive fixed doses of radiation that do not account for the volume or stage of disease (IIIA or IIIB), patient physiology, or location of tumor.9 There is heterogeneity in stage III NSCLC and therefore a need to move away from a broad-brush treatment approach. To improve local control, one strategy is to escalate the dose of radiation delivered to the tumor. A clear dose-response relationship in NSCLC was established by Martel et al,10 with 84 Gy needed to achieve 50% probability of tumor control at 3 years.
However, in the RTOG 0617 phase III study in which patients were randomized between 60 Gy in 30 daily fractions and 74 Gy in 37 daily fractions, the high-dose arm failed to demonstrate a survival advantage, indicating that dose escalation using conventionally fractionated RT is not going to deliver improved patient outcomes. This failure was most likely multifactorial and might have been due to prolonged treatment time combined with poorer treatment delivery (with fewer patients receiving cCRT), reduced compliance to RT, issues with quality assurance, and unreported toxicity, including cardiac toxicity.11 Following the publication of RTOG 0617, 60 Gy delivered in 2 Gy per fraction is now considered standard treatment in patients with stage III NSCLC.12 Accelerated hyperfractionation has been studied in an attempt to reduce the overall treatment time and to counteract repopulation in lung cancer. Modified fractionation (hyperfractionation, acceleration, or both) has been shown to improve overall survival in NSCLC compared with conventional schedules, resulting in an absolute benefit of 2.5% at 5 years.13
The Maastro group developed the concept of “isotoxic RT” using individualized tailored dose escalation.14 They showed that with hyperfractionated, accelerated 3D-CRT, increasing radiation dose to prespecified normal tissue dose constraints can lead to increased tumor control probability with the same normal tissue complication probability.14,15 In the sequential CRT setting, a mean dose of 61.2 Gy could be delivered to patients with stage III disease; however, <10% of patients received the maximum dose of 79.2 Gy in 44 fractions twice daily.14 Importantly, intensity modulated radiation therapy (IMRT), which enables a reduction in dose to organs at risk (OAR), and potential dose escalation were not used in the Maastro trial.16
Given the need to intensify treatment in the sequential CRT setting, a UK study was established to investigate the feasibility of delivering image-guided isotoxic IMRT using a hyperfractionated accelerated schedule in patients with stage III NSCLC.
Methods and Materials
Study design and participants
The isotoxic IMRT study was a prospective, multicenter, nonrandomized feasibility study with early stopping rules; details of the trial design have been published.17 Patients were treated with individualized doses of radiation based on prespecified normal tissue doses (eg, heart, brachial plexus, lung tissue, spinal cord, great vessels/proximal bronchial tree; see Table 1) up to a maximum of dose 79.2 Gy in 44 fractions.
Table 1.
Prespecified normal tissue doses
| Organ at risk | Prespecified normal tissue doses |
|---|---|
| Brachial plexus | Max dose = EQD2 ≤ 66 Gy |
| Heart | Max dose = EQD2 ≤ 76 Gy Mean dose ≤ 46Gy |
| Lung | Mean lung dose (lung-GTV) ≤ 20 Gy |
| Mediastinal envelope∗ | Max dose = EQD2 ≤ 76 Gy |
| Spinal canal PRV | Max dose = EQD2 ≤ 50 Gy |
Abbreviations: GTV = gross tumor volume; PRV = planning organ at risk volume; EQD2 = Equivalent dose in 2 Gy fractions.
Comprising the heart, proximal bronchial tree, trachea, and esophagus and edited manually to include the blood vessels in the upper mediastinum.
Patients were enrolled from 7 UK centers: Addenbrookes Hospital (Cambridge), Beatson Cancer Centre (Glasgow), The Christie NHS Foundation Trust (Manchester), Northern Ireland Cancer Centre (Belfast), The Royal Marsden (London), St James's Hospital (Leeds) and Weston Park Hospital (Sheffield). Eligible patients were age 18 years or older and had inoperable stage III NSCLC (T3 N1-3, any T4, or any N2-3 according to TNM version 7) that was confirmed with histologic or cytologic analysis and on PET scanning, with or without mediastinoscopy or thoracoscopy. Patients with Eastern Cooperative Oncology Group PS of 0 to 2 (PS 2 allowed if due to disease-related symptoms not comorbidities) and who were unsuitable for cCRT were included. Prior treatment with a minimum of 2 cycles of platinum- based induction chemotherapy (per the local standard of care) and the ability to commence RT within 5 weeks of the last cycle of chemotherapy was compulsory.
Before trial registration, mandatory investigations included a contrast-enhanced computed tomographic (CT) scan of the thorax and upper abdomen (within 4 weeks before registration), contrast-enhanced CT (or magnetic resonance imaging) brain scan (within 4 weeks before registration), fluorodeoxyglucose (FDG)-positron emission tomography (PET) CT within 4 weeks before registration and lung function tests. Participants gave written informed consent and the study was conducted according to Good Clinical Practice Guidelines and the Declaration of Helsinki. The trial was reviewed in the United Kingdom by the National Research Ethics Service Committee, which granted ethics approval for the study on 8th August 2013. The protocol was also approved by the institutional review board at each study center.
An RT planning scan using free-breathing 4-dimensional (4D) CT with intravenous contrast injection was mandatory to account for tumor motion (patients with a medical contraindication to contrast were excluded from the study). Patients were planned and treated in the supine position, immobilized using either a chest board and fixed arm position above the head or a 5-point fixation shell.
The gross tumor volume (GTV) was contoured depending on local practice to include (1) the combined GTV exhale (defined on the maximum exhale 4D-CT data set) and GTV inhale (defined on the maximum inhale 4D-CT data set), (2) combined GTV from all phases of the 4D-CT data set, or (3) GTV as defined on the maximum intensity projection data set. The GTV is defined as identifiable tumor and involved lymph nodes from cross-sectional imaging, using CT to define nodal involvement if nodes ≥1cm in short axis or PET positive lymph nodes (SUV > 3 if information on uptake of blood pool is not available). As induction chemotherapy was mandated, the GTV included the post-chemotherapy tumor volume and the prechemotherapy lymph node volume.18
The clinical target volume (CTV) comprised the GTV with a 5 mm margin of radiologically normal tissue in all directions. In case of a complete remission of a lymph node, the whole anatomic area as defined by Chapet et al19 was included. Manual editing of the CTV was permitted to reduce the dose to organs at risk (eg, when disease is adjacent to a structure, such as a vertebra, but is not thought to invade the structure). Elective nodal irradiation was not permitted. The planning target volume (PTV) comprised the CTV with a 0.9-cm margin superiorly and inferiorly and a 0.7-cm margin laterally. Editing of the PTV was not permitted. The RT planning guidelines and quality assurance document were provided as a reference to contour OAR. Treatment planning and optimization of inverse planned IMRT was undertaken by an experienced dosimetrist and physicist in lung planning. The use of volumetric modulated arc therapy–rapid arc–tomotherapy–fixed-beam IMRT was allowed in this study. The OAR tolerance doses were specified to a volume of 1 mL, with the exception of a mean heart dose and mean lung dose (MLD; see Table 1). At least 95% of the PTV should have received 90% (ideally 95%) of the prescribed dose, and the mean dose to the CTV should have been 100%. Hotspots did not exceed 107% of the prescribed dose within a 1-mL volume. The dose of radiation was increased until one or more of the OAR tolerances or the maximum dose of 79.2 Gy was reached. A case example with dose distribution is shown in Figure 1. Using image guidance with cone beam CT (CBCT), RT was delivered twice-daily (a minimum 6-hour interval between fractions) on consecutive weekdays in 1.8 Gy per fraction over a maximum of 44 fractions.
Fig. 1.
Case example of a 65-year-old man with T2 N3 M0 adenocarcinoma of the left lung stage IIIB, radiation therapy dose of 70.2 Gy in 39 fractions delivered. Motion-adapted gross tumor volume is outlined in green, clinical target volume is outlined in purple, planning target volume is outlined in light blue, mediastinal envelope is outlined in red, and brachial plexus is outlined in brown. (A color version of this figure is available at https://doi.org/10.1016/j.ijrobp.2020.11.040.)
The trial was subject to an RT quality assurance (QA) program tailored to the technical requirements of lung IMRT. The QA program for the study was coordinated by the National Cancer Research Institute Radiotherapy Trials Quality Assurance (RTTQA) Group.20 Before recruitment each center delineated a benchmark case. The first patients’ RT plan from each center was reviewed prospectively before a second patient was recruited. All RT plans were reviewed retrospectively by the RTTQA group to ensure adherence to the trial protocol.
Data regarding any serious adverse events (SAEs; as defined by GCP) was collected at each follow-up visit. All SAEs causally related to the RT treatment were reported to the Manchester Academic Health Science Centre Trials Coordination Unit and followed until they resolved or stabilized. Acute and late radiation toxicities continue to be recorded at each follow-up visit (according to the Common Terminology Criteria for Adverse Events V.4.0 grading system).
Patients were followed-up for 5 years after treatment (every 4 months in years 1 and 2, and semiannually during years 2-5). At each visit, a late toxicity assessment was performed. CT scans were performed every 4 months for the first 2 years.
Outcomes and statistics
The primary objective was the delivery of isotoxic IMRT to a dose >60 Gy equivalent dose in 2-Gy fractions (EQD2). The secondary objectives included the suitability of the study population for isotoxic IMRT, acceptability of isotoxic IMRT among patients, estimation of recruitment rates, estimation of patients with acute grade 3 or more nonhematological toxicity, estimation of local control, estimation of overall survival, and the development of a robust QA process for lung IMRT.
This feasibility study was 2-stage (with early stopping rules) using the design of Bryant and Day with 85% power and 15% type-1 error for both completion (an acceptable rate of 90% and unacceptable rate of 70% of patients receiving >60 Gy EQD2) and acute radiation pneumonitis rates (an acceptable rate of grade 3 or more acute radiation pneumonitis of 8.5% and unacceptable rate of 22.5%).21
After the first 11 of 35 patients were enrolled (stage 1 of the study) if fewer than 8 of 11 patients were planned to a dose >60 Gy EQD2 or more than 2 of 11 patients had experienced grade 3 or more acute radiation pneumonitis the trial would be stopped. Further investigation of the intervention would be recommended if more than 27 of 35 patients were planned to a dose >60 Gy EQD2 and less than 6 of 35 patients experienced grade 3 or more acute radiation pneumonitis.
Results
Between June 2014 and March 2016, 37 patients were enrolled from 7 UK centers; the baseline characteristics are shown in Table 2. Initial recruitment target was 35 patients.
Table 2.
Patient characteristics
| Patient characteristics | On trial (n = 35) | Off trial (n = 2) (unable to dose escalate) |
|
|---|---|---|---|
| Patient 1 | Patient 2 | ||
| Sex | |||
| Female | 18 (51%) | 1 | — |
| Male | 17 (49%) | — | 1 |
| Age, years | 52 | 48 | |
| Median | 69.9 | ||
| Range | 46-86 | ||
| ECOG PS | |||
| 0 | 5 (14.2%) | 1 | 1 |
| 1 | 27 (77.1%) | ||
| 2 | 3 (8.6%) | ||
| Stage | |||
| IIIa | 22 (62.9%) | IIIa | IIIb |
| IIIb | 13 (37.1%) | ||
| Histology | |||
| Squamous | 16 (45.7%) | ||
| Adenocarcinoma | 14 (40%) | 1 | 1 |
| Other | 5 (14.3) | ||
| Median lung function (range) | |||
| FEV1 (L) | 1.8 (0.7-3.4) | 2.7 | 3.3 |
| DLCO (% predicted) | 66.8 (26.2-102) | 83 | 88 |
| Gross tumor volume, cm3 | |||
| Median | 57.1 | Not specified | 97.4 |
| Range | 8.2-260.7 | ||
| Planning target volume, cm3 | |||
| Median | 330 | Not specified | 753.4 |
| Range | 146-807 | ||
Abbreviations: DLCO = diffusing capacity for carbon monoxide; ECOG = Eastern Cooperative Oncology Group; FEV = forced expiratory volume; PS = performance status.
Of 37 patients, 2 (5.4%) failed to achieve a planned dose of EQD2 > 60 Gy because of large tumor size and inability to meet OAR constraints. Both patients who failed to achieve a dose of >60 Gy EQD2 also received 55 Gy in 20 fractions once daily over 4 weeks. As a result, 35 of 37 patients achieved an EQD2 > 60 Gy and received treatment per the trial protocol (Table 2). The median prescribed tumor dose for the 35 patients treated with dose >60 Gy EQD2 was 77.4 Gy (range, 61.2-79.2 Gy) with the maximum dose of 79.2 Gy delivered to 14 (37.8%) patients. In addition to the prescribed tumor dose, the doses delivered to the normal tissues are summarized in Table 3. All patients completed RT as scheduled, except 1 patient for whom treatment was stopped because of disease progression after 8 fractions (4 days). One patient underwent replanning because of tumor growth on CBCT.
Table 3.
Prescribed tumor doses and normal tissue dosimetry in 35 patients treated per protocol, with doses > 60 Gy EQD2
| Radiotherapy planning parameters | Median (range) |
|---|---|
| PTV | |
| Prescribed dose (Gy) | 77.4 Gy (61.2-79.2) |
| Lung | |
| V5 (Lung-PTV) | 63.2% (29.2-91.5) |
| V20 (Lung-PTV) | 26.6% (14-41.4) |
| MLD (Lung-GTV) | 18.5 Gy (6.8-20.0) |
| Esophagus | |
| V35 | 28.6% (0-69) |
| V50 | 21.7% (0-62.5) |
| V60 | 17.4% (0-53.5) |
| Mean | 21.5 Gy (8.0-44.4) |
| Max 1 cm3 | 74.1 Gy (21.2-78.6) |
| Heart∗ | |
| V5 | 47.6% (8.2-100) |
| V30 | 23.6% (0.1-93.1) |
| V40 | 18.2% (3.4-63.3) |
| V50 | 11.2% (0.9-34.7) |
| Mean | 17.0 Gy (1.4-45.7) |
| Max 1 cm3 | 77.6 Gy (21.8-79.1) |
| Other OARs | |
| Max 1 cm3 brachial plexus | 4.51 Gy (0.6-72.4) |
| Max 1 cm3 mediastinal envelope | 78.2 Gy (39.2-79.2) |
| Max 1 cm3 proximal tree | 77.2 Gy (20.1-79.0) |
| Max 1 cm3 trachea | 65.6 Gy (1.12-79.2) |
| Max 1 cm3 spinal canal | 43.7 Gy (14.3-57.7) |
| Max 1 cm3 spinal canal + 0.5 cm | 49.4 Gy (14.9-59.1) |
Abbreviations: GTV = gross tumor volume; Gy = Gray; MLD = mean lung dose; OAR = organs at risk; PTV = planning target volume.
V5, V20, V35, V50, and V60 denote the volume receiving more than 5, 20, 35, 50, and 60 Gy, respectively.
Toxicity
Thirteen grade 3 to 4 acute events and 3 grade 5 events occurred in 8 individuals. The most common grade 3 acute adverse events included dypsnoea (n = 2; 5.7%), lung infection (n = 2; 5.7%), and radiation esophagitis (n = 2; 5.7%; Table E1). None of the patients with grade 3 radiation esophagitis required feeding tubes. No patient developed grade ≥3 anemia or neutropenia.
Three grade 5 events resulted from acute radiation pneumonitis, a bronchopulmonary hemorrhage, and acute lung infection. The first 2 grade 5 events were deemed as probable treatment-related deaths, and causality was not determined for the third patient. The 3 patients all received the maximum dose of 79.2 Gy in 44 fractions and had similar volumes of disease (PTV 311, 344, and 281.6 cm3, respectively). The first patient died from acute radiation pneumonitis 4 months after completion of RT. Lung V20 was 23.5%, and MLD was 19.5 Gy. The second patient died of a bronchopulmonary hemorrhage 18 months after completion of RT. Their RT plan demonstrated a V20 of 27.1% and an MLD of 15 Gy. The 1-mL max dose to the proximal tree was 78.25 Gy. The third patient died 16 days after completion of RT; lung V20 was 29.7%, and MLD was 16.7 Gy. Grade 3 late toxicities included fatigue (n = 1; 2.9%) and dyspnoea (n = 3; 8.6%), and 1 (2.9%) case of late grade 4 lung infection (Table E1).
Survival and local control
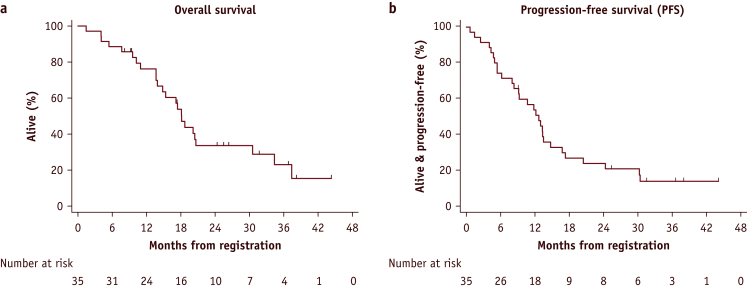
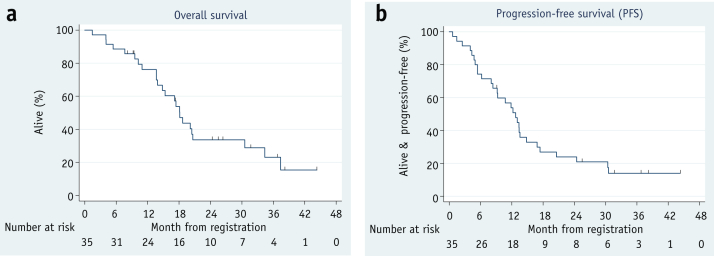
At the time of analysis, the median follow-up was 25.4 months (range, 8.0-44.2 months) for 11 of 35 survivors. The 2-year overall survival (Fig. 2a) was 33.6% (95% confidence interval [CI], 17.9-50.1), and progression-free survival was 23.9% (95% CI, 11.3-39.1; Fig. 2b). The median survival was 18.1 months (95% CI, 13.9-30.6).
Fig. 2.
(a) The 2-year overall survival was 33.6% (95% confidence interval, 17.9-50.1). (b) The progression-free survival was 23.9% (95% confidence interval, 11.3-39.1).
Radiation therapy quality assurance
Treatment plans for each study patient were assessed within 1 week of the start of treatment. For the treatment planning benchmark case, 2 of the 7 centers (28.6%) were able to achieve the maximum dose per number of fractions (79.2 Gy/44). Dose escalation was limited in all cases by the dose to 1 mL of the mediastinal envelope. Thirty-four on-trial treatment plans were reviewed, and QA reports were sent to each center. For all 34 plans, the OAR dose constraints were met. Of the 34 treatment plans, 2 failed to meet one of the PTV criteria (the PTV D [Total Volume –1 mL] ≥ 85%), but were within 2% of the acceptable limit. There were minor deviations from the protocol for OAR outlining, which was highlighted to the appropriate center.
Discussion
Despite technological advances, a conventional RT fractionation of 60 Gy in 30 fractions and chemotherapy devised more than 30 years ago remain the international standard of care in stage III inoperable NSCLC.9,11,12,22 This study combines a number of strategies to intensify RT: personalized dose escalation, acceleration, and hyperfractionation, facilitated by the use of image guided IMRT. The primary endpoint was met, as it was feasible to intensify treatment using isotoxic IMRT, with 35 of 37 patients (95%) receiving dose-escalated RT. The overall survival of 76% at 1 year and 34% at 2 years and PFS of 54% at 1 year and 24% at 2 years compare favorably to historical survival reported in sequential CRT trials.1 However, because of small numbers, it is not appropriate to compare the results of isotoxic IMRT to large contemporary data sets of patients treated with concurrent CRT, such as RTOG0617 and PACIFIC.4,12
It is important that the potential survival benefit of dose intensification is balanced with the risk of treatment-related toxicity. RTOG 0617, which compared 60 Gy in 30 daily fractions to 74 Gy in 37 daily fractions concurrently with chemotherapy, showed a detrimental effect on survival in the high-dose arm. As an alternative to using conventional RT fractionation in NSCLC, isotoxic IMRT along with other trials, including IDEAL (isotoxic dose-escalated concurrent chemoradiotherapy), CHART-ED (continuous hyperfractionated accelerated radiation therapy without chemotherapy), and PLANET (dose escalation to 84 Gy with concurrent chemotherapy) have investigated dose escalation schedules.23, 24, 25
Despite the intensified regime used in our study, the rate of grade 3 to 5 pneumonitis (3%) was comparable to the standard arm of RTOG 0617 and other intensified schedules (Table E2). The incidence of grade ≥3 esophagitis (6%) was the same as in the IDEAL study,23 and less than reported in the high dose arm of RTOG 0617,12 CHART-ED,24 and PLANET25 (Table E2). This observation can be explained in part by the fact that the chemotherapy was delivered sequentially in the isotoxic IMRT study, as opposed to concurrently. It should be noted that all 3 patients with grade 5 events in our study had an MLD ≥ 15 Gy and a V20 > 23%. However, the maximum threshold of 20 Gy for MLD was not exceeded in any of these patients as per protocol. Furthermore V20 was <30% in all 3 cases.
Results thus far suggested that dose escalation in thoracic RT might be limited by pneumonitis and esophagitis,25 but other toxicities such as bronchopulmonary hemorrhage and the development of fistulas may be rarer, but related to severe morbidity and mortality.22,26 A phase 1 trial delivering hypofractionated RT using IMRT up to doses of 85.5 Gy in 25 fractions reported no cases of grade 3 radiation pneumonitis or esophagitis, but it was prematurely terminated after 5 treatment-related deaths that included 3 from fatal hemotypsis.26 It is well documented that centrally located tumors are associated with a high risk of grade 5 hemoptysis.22,26, 27, 28 This phase 1 trial protocol did not include any OAR constraints for the proximal bronchial tree, but it demonstrated a dose-response relationship, with higher doses to the proximal bronchial tree resulting in severe late toxicity.
In our study, the maximum dose permitted to the mediastinal envelope (which includes mediastinal blood vessels, heart, trachea, esophagus, and proximal bronchial tree) was EQD2 ≤ 76 Gy. The mediastinal envelope was the structure limiting dose escalation. For future studies, including the dose limits for individual OAR rather than to the mediastinal envelope might facilitate dose escalation.
We have reported on 2 deaths that were probably related to treatment: one from acute pneumonitis and the other from a late bronchopulmonary hemorrhage. One patient had a nonfatal grade 3 tracheoesophageal fistula. A treatment-related death rate of 5.7% is comparable to other dose-escalation studies (4.8% IDEAL)23 and less than the 74-Gy high-dose arm of RTOG 0617 (7.5%).12 In the PET-Boost trial (dose escalation to the entire primary tumor or redistributed to regions of high pretreatment FDG-uptake in patients with inoperable stage II-III NSCLC), fatal pulmonary hemorrhages and esophageal fistulae were observed in 9 of 107 patients (8.5%). Patients were treated with an isotoxic integrated boost of ≥72 Gy in 24 fractions, with or without chemotherapy and strict dose constraints for organs at risk. Acute and late ≥G3 was reported in 41% and 25% of patients, respectively.
A key strength of our study is the mandatory use of IMRT to allow dose escalation. Although IMRT is now routinely used for radical lung cancer, IMRT was not a standard of care in the United Kingdom when the trial was conducted. The higher median prescribed tumor dose of 77.4 Gy (range, 61.2-79.2 Gy) in isotoxic IMRT study compared with the median dose within the Maastro study (also including patients treated with sequential CRT) of 65 Gy (range, 51-69 Gy) can be explained by the mandatory use of IMRT. In a subsequent study from the Maastro group, isotoxic accelerated RT given concurrently with chemotherapy was delivered using IMRT.29 The mean tumor dose was 66.0 ± 12.8 Gy (range, 36-73 Gy) delivered in a mean of 39.7 fractions. The marginal increase in dose delivered in this study compared with the previous one could be explained by patient selection (median tumor volume of 50.3 mL in the Maastro sequential isotoxic cohort compared with 72.6 mL in the Maastro concurrent isotoxic cohort).
The most robust data supporting the use of IMRT in the treatment of lung cancer come from a secondary analysis of RTOG 0617. It demonstrated that despite the IMRT cohort having larger planning treatment volumes and more stage IIIB disease, survival outcomes were the same as those treated in the 3D-CRT cohort.30 Furthermore, quality of life was better in the IMRT group, despite some unfavorable prognostic factors.31 The incidence of grade 3 radiation-related toxicities of pneumonitis, cardiovascular, and esophagitis were lower in the IMRT group (3.5%, 4.8%, 13.2%) compared with 3DCRT (7.9%, 8.3%, and 15.4%). The lung V20 did not differ between the 2 groups, but the lung V5 was higher in the IMRT group, and heart doses were significantly less in those receiving IMRT compared with 3DCRT.
As reported in the secondary analysis of RTOG 0617, an advantage of IMRT over 3DCRT is that the dose to the heart can be reduced. Over the years, dose constraints to the heart have been poorly defined and RT-related cardiac toxicity is often underreported. In RTOG 0617, the heart dose was higher in the high-dose arm; in addition, on multivariate analysis of the survival data, the higher heart V5Gy and V30Gy was associated with poorer survival.30 Recently, further evidence has shown that heart dose is significantly and independently associated with overall survival12,32, 33, 34 and cardiac events.35
In addition to mandating IMRT, strengths of this study include the multicenter setting and the delivery of state-of-the-art image-guided RT overseen with a robust QA program. Furthermore, the study protocol mandated the use of bidaily CBCT. Recent data have shown the benefit of strict image guidance protocols, with residual setup errors toward the heart being linked with poorer survival in patients with lung cancer.36
Conversely, the authors are mindful of the limitations of this single-arm study incorporating a heterogeneous group of patients with stage III cancer. The proximity of tumor bulk to OAR in addition to histologic subtype and genomic variations can affect outcomes greatly.15 It is this heterogeneity of stage III disease that warrants investigating a move away from a one-size-fits-all approach to improve patient outcomes. Clinical trials incorporating heterogeneity in their design to individualize treatment include boosting subvolumes of the tumor based on FDG-PET, as used in Artforce/PET (ClinicalTrials.gov: NCT01024829) and RTOG 1106/ACRIN 667 (ClinicalTrials.gov: NCT01507428). Other strategies include combining radical RT with targeted drugs, the addition of immunotherapy in the sequential CTRT setting, using hypofractionated stereotactic body RT to the primary site and conventionally fractionated RT to central mediastinal lymph nodes (ClinicalTrials.gov: NCT01933568), adaptive techniques, and targeting hypoxia. Future studies should involve the use of genomic signatures to predict radioresistant tumors and tumors that have a higher risk of relapse who might benefit from dose escalation. Currently, there are limited treatment strategies in the routine setting for stage III NSCLC that take into account molecular or genomic tumor characteristics and circulating tumor cells and DNA to personalize treatment.
Conclusion
There is an unmet need to intensify treatment in patients with stage III NSCLC who are unsuitable for concurrent CRT, as outcomes are poor. We have demonstrated that isotoxic IMRT is a well-tolerated treatment intensification strategy with promising outcomes. This regimen is currently being tested in a UK phase 2 randomized controlled trial (ADSCAN- ISRCTN47674500)37 alongside 3 other dose-escalated and accelerated sequential CRT schedules.
Acknowledgments
The authors acknowledge the support of Damian Mullan and Ben Taylor (radiology input), Carl Rowbottom and Gareth Webster (protocol development), Dave Ardron (patient representative), Tsang Yatman/Elizabeth Miles/Romaana Mir (NCRI Radiation therapy Quality Assurance Group), Glenda Laviste (senior clinical trials nurse), Sally Falk (research project manager).
Footnotes
This research is jointly funded by Cancer Research UK’s (CRUK) Clinical Trials Awards and Advisory Committee (CTAAC) & British Lung Foundation (grant reference number C17052/A15702). C.F-F. is supported by the NIHR Manchester Biomedical Research Center.
Disclosures: F.M. reports grants from MSD during the conduct of this study.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2020.11.040.
Supplementary Materials
References
- 1.Auperin A., Le Pechoux C., Rolland E. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 2.Bayman N., Blackhall F., McCloskey P., Taylor P., Faivre-Finn C. How can we optimise concurrent chemoradiotherapy for inoperable stage III non-small cell lung cancer? Lung Cancer. 2014;83:117–125. doi: 10.1016/j.lungcan.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Bradley J.D., Hu C., Komaki R.R. Long-term results of NRG Oncology RTOG 0617: Standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2020;38:706–714. doi: 10.1200/JCO.19.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonia S.J., Villegas A., Daniel D. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. New Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 5.De Ruysscher D., Botterweck A., Dirx M. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: A prospective, population-based study. Ann Oncol. 2009;20:98–102. doi: 10.1093/annonc/mdn559. [DOI] [PubMed] [Google Scholar]
- 6.Adizie J.B., Khakwani A., Beckett P. Stage III non-small cell lung cancer management in England. Clin Oncol. 2019;31:688–696. doi: 10.1016/j.clon.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Le Chevalier T., Arriagada R., Quoix E. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: First analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991;83:417–423. doi: 10.1093/jnci/83.6.417. [DOI] [PubMed] [Google Scholar]
- 8.Machtay M., Paulus R., Moughan J. Defining local-regional control and its importance in locally advanced non-small cell lung carcinoma. J Thorac Oncol. 2012;7:716–722. doi: 10.1097/JTO.0b013e3182429682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson A., Chan C., Woolf D. Is heterogeneity in stage 3 non-small cell lung cancer obscuring the potential benefits of dose-escalated concurrent chemo-radiotherapy in clinical trials? Lung Cancer. 2018;118:139–147. doi: 10.1016/j.lungcan.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Martel M., Ten Haken R., Hazuka M. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer. 1999;24:31–37. doi: 10.1016/s0169-5002(99)00019-7. [DOI] [PubMed] [Google Scholar]
- 11.Faivre-Finn C. Dose escalation in lung cancer: Have we gone full circle? Lancet Oncol. 2015;16:125–127. doi: 10.1016/S1470-2045(15)70001-X. [DOI] [PubMed] [Google Scholar]
- 12.Bradley J.D., Paulus R., Komaki R. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauguen A., Le Pechoux C., Saunders M.I. Hyperfractionated or accelerated radiotherapy in lung cancer: An individual patient data meta-analysis. J Clinical Oncol. 2012;30:2788–2797. doi: 10.1200/JCO.2012.41.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Baardwijk A., Reymen B., Wanders S. Mature results of a phase II trial on individualised accelerated radiotherapy based on normal tissue constraints in concurrent chemo-radiation for stage III non-small cell lung cancer. Eur J Cancer. 2012;48:2339–2346. doi: 10.1016/j.ejca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 15.van Baardwijk A., Bosmans G., Boersma L. Individualized radical radiotherapy of non-small-cell lung cancer based on normal tissue dose constraints: A feasibility study. Int J Radiat Oncol Bio Phys. 2008;71:1394–1401. doi: 10.1016/j.ijrobp.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 16.Chan C., Lang S., Rowbottom C., Guckenberger M., Faivre-Finn C., IASLC Advanced Radiation Technology Committee Intensity-modulated radiotherapy for lung cancer: Current status and future developments. J Thorac Oncol. 2014;9:1598–1608. doi: 10.1097/JTO.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 17.Haslett K., Franks K., Hanna G.G. Protocol for the isotoxic intensity modulated radiotherapy (IMRT) in stage III non-small cell lung cancer (NSCLC): A feasibility study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nestle U., De Ruysscher D., Ricardi U. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. Radiother Oncol. 2018;127:1–5. doi: 10.1016/j.radonc.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Chapet O., Kong F.M., Quint L.E. CT-based definition of thoracic lymph node stations: An atlas from the University of Michigan. Int J Radiat Oncol Biol Phys. 2005;63:170–178. doi: 10.1016/j.ijrobp.2004.12.060. [DOI] [PubMed] [Google Scholar]
- 20.www.rttrialsqa.org.uk Available from: Accessed November 12, 2020.
- 21.Bryant J., Day R. Incorporating toxicity considerations into the design of two-stage phase II clinical trials. Biometrics. 1995;51:1372–1383. [PubMed] [Google Scholar]
- 22.Barrett S., Hanna G.G., Marignol L. An overview on personalisation of radiotherapy prescriptions in locally advanced non-small cell lung cancer: Are we there yet? Radiother Oncol. 2018;128:520–533. doi: 10.1016/j.radonc.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Landau D.B., Hughes L., Baker A. IDEAL-CRT: A phase 1/2 trial of isotoxic dose-escalated radiation therapy and concurrent chemotherapy in patients with stage II/III non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2016;95:1367–1377. doi: 10.1016/j.ijrobp.2016.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatton M.Q., Hill R., Fenwick J.D. Continuous hyperfractionated accelerated radiotherapy - escalated dose (CHART-ED): A phase I study. Radiother Oncol. 2016;118:471–477. doi: 10.1016/j.radonc.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Hallqvist A., Bergstrom S., Bjorkestrand H. Dose escalation to 84 Gy with concurrent chemotherapy in stage III NSCLC appears excessively toxic: Results from a prematurely terminated randomized phase II trial. Lung Cancer. 2018;122:180–186. doi: 10.1016/j.lungcan.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Cannon D.M., Mehta M.P., Adkison J.B. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol. 2013;31:4343–4348. doi: 10.1200/JCO.2013.51.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wanet M., Delor A., Hanin F.X. An individualized radiation dose escalation trial in non-small cell lung cancer based on FDG-PET imaging. Strahlenther Onkol. 2017;193:812–822. doi: 10.1007/s00066-017-1168-z. [DOI] [PubMed] [Google Scholar]
- 28.van Diessen J., De Ruysscher D., Sonke J.J. The acute and late toxicity results of a randomized phase II dose-escalation trial in non-small cell lung cancer (PET-boost trial) Radiother Oncol. 2019;131:166–173. doi: 10.1016/j.radonc.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 29.De Ruysscher D., van Baardwijk A., Wanders R. Individualized accelerated isotoxic concurrent chemo-radiotherapy for stage III non-small cell lung cancer: 5-Year results of a prospective study. Radiother Oncol. 2019;135:141–146. doi: 10.1016/j.radonc.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Chun S.G., Hu C., Choy H. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: A secondary analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol. 2017;35:56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Movsas B., Hu C., Sloan J. Quality of life analysis of a radiation dose-escalation study of patients with non-small-cell lung cancer: A secondary analysis of the Radiation Therapy Oncology Group 0617 Randomized Clinical Trial. JAMA Oncol. 2016;2:359–367. doi: 10.1001/jamaoncol.2015.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speirs C.K., DeWees T.A., Rehman S. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol. 2017;12:293–301. doi: 10.1016/j.jtho.2016.09.134. [DOI] [PubMed] [Google Scholar]
- 33.McWilliam A., Kennedy J., Hodgson C., Vasquez Osorio E., Faivre-Finn C., van Herk M. Radiation dose to heart base linked with poorer survival in lung cancer patients. Eur J Cancer. 2017;85:106–113. doi: 10.1016/j.ejca.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 34.Stam B., van der Bijl E., van Diessen J. Heart dose associated with overall survival in locally advanced NSCLC patients treated with hypofractionated chemoradiotherapy. Radiother Oncol. 2017;125:62–65. doi: 10.1016/j.radonc.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Wang K., Eblan M.J., Deal A.M. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: Pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35:1387–1394. doi: 10.1200/JCO.2016.70.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson-Hart C.N., Price G.J., Faivre-Finn C., Aznar M.C., van Herk M. Residual setup errors towards the heart after image guidance linked with poorer survival in lung cancer patients: Do we need stricter IGRT protocols? Int J Radiat Oncol Biol Phys. 2018;102:434–442. doi: 10.1016/j.ijrobp.2018.05.052. [DOI] [PubMed] [Google Scholar]
- 37.Hatton M.Q.F., Lawless C.A., Faivre-Finn C. Accelerated, dose escalated, sequential chemoradiotherapy in non-small-cell lung cancer (ADSCaN): A protocol for a randomised phase II study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2017-019903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.