Abstract
Background
The prognosis of left ventricular noncompaction (LVNC) remains elusive despite its recognition as a clinical entity for >30 years. We sought to identify clinical and imaging characteristics and risk factors for mortality in patients with LVNC.
Methods and Results
339 adults with LVNC seen between 2000 and 2016 were identified. LVNC was defined as end‐systolic noncompacted to compacted myocardial ratio >2 (Jenni criteria) and end‐diastolic trough of trabeculation‐to‐epicardium (X):peak of trabeculation‐to‐epicardium (Y) ratio <0.5 (Chin criteria) by echocardiography; and end‐diastolic noncompacted:compacted ratio >2.3 (Petersen criteria) by magnetic resonance imaging. Median age was 47.4 years, and 46% of patients were female. Left ventricular ejection fraction <50% was present in 57% of patients and isolated apical noncompaction in 48%. During a median follow‐up of 6.3 years, 59 patients died. On multivariable Cox regression analysis, age (hazard ratio [HR] 1.04; 95% CI, 1.02–1.06), left ventricular ejection fraction <50% (HR, 2.37; 95% CI, 1.17–4.80), and noncompaction extending from the apex to the mid or basal segments (HR, 2.11; 95% CI, 1.21–3.68) were associated with all‐cause mortality. Compared with the expected survival for age‐ and sex‐matched US population, patients with LVNC had reduced overall survival (P<0.001). However, patients with LVNC with preserved left ventricular ejection fraction and patients with isolated apical noncompaction had similar survival to the general population.
Conclusions
Overall survival is reduced in patients with LVNC compared with the expected survival of age‐ and sex‐matched US population. However, survival rate in those with preserved left ventricular ejection fraction and isolated apical noncompaction was comparable with that of the general population.
Keywords: ejection fraction, mortality, noncompaction, prognosis, survival
Subject Categories: Cardiomyopathy, Risk Factors, Cardiovascular Disease, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- LVNC
left ventricular noncompaction
- NC:C
noncompacted:compacted
- TTE
transthoracic echocardiography
Clinical Perspective
What Is New?
We report the outcomes of 339 patients meeting echocardiographic and/or magnetic resonance imaging diagnostic criteria for left ventricular noncompaction (LVNC).
Increased age, lower left ventricular ejection fraction, and noncompaction extending beyond the left ventricular apex to the midbasal segments was associated with increased mortality among patients with LVNC.
The 5‐year survival of patients meeting LVNC diagnostic criteria but with normal left ventricular ejection fraction and noncompaction limited to the apical segment was similar to the age‐ and sex‐matched US population.
What Are the Clinical Implications?
These data can assist clinicians in risk stratifying patients meeting contemporary morphological diagnostic criteria for LVNC.
Patients with LVNC but with preserved systolic function and noncompaction limited to the apical segment have a benign prognosis.
Regular assessment and clinical follow‐up for LVNC with reduced ejection fraction might be beneficial, as the prognosis appears to worsen substantially with impairment of LV systolic function.
Left ventricular noncompaction (LVNC) is characterized by a bilayered appearance of the myocardium, with excessive trabeculations and deep intertrabecular recesses. 1 , 2 LVNC was initially described as a cardiomyopathy with a malignant course, characterized by heart failure, arrhythmias, stroke, and increased mortality. 3 , 4 Subsequently, LVNC was variably defined as a distinct cardiomyopathy or a morphologic feature common to other cardiomyopathies. 5 , 6 The debate regarding the existence of LVNC as a distinct cardiomyopathy versus a morphologic feature of ventricular remodeling continues in recent times. 7 , 8 , 9
Despite almost 30 years since the initial description of LVNC as a clinical entity, the prognosis of patients meeting the morphologic diagnostic criteria of LVNC remains uncertain. 10 , 11 , 12 Thus, we sought to describe the prognosis of patients meeting the morphologic diagnostic criteria of LVNC in a large tertiary care center. Our objectives were (1) to identify predictors of all‐cause mortality on the basis of clinical and imaging characteristics and (2) to compare the overall survival of patients with LVNC with that of the general population.
Methods
This retrospective cohort included 339 adults meeting echocardiographic and/or magnetic resonance imaging (MRI) criteria seen at Mayo Clinic, Rochester (MN) between 2000 and 2016. The study was approved by the Mayo Clinic Institutional Review Board. The study was considered minimal risk, and informed consent was waived for the study. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
The inclusion criteria were the presence of LVNC based on established echocardiographic and magnetic resonance imaging (MRI) criteria. Imaging studies were individually reviewed and offline measurements performed by 2 coauthors (V.R.V. and M.L.). For the assessment of noncompacted myocardium, the left ventricle was divided into 9 segments (basal anterior, basal lateral, basal inferior, basal septal, midanterior, midlateral, midinferior, midseptal, and apical segments) and end‐diastolic and end‐systolic measurements of noncompacted and compacted myocardial thickness performed. Measurements that included the papillary muscles were intentionally avoided to minimize measurement bias. To further increase specificity and given the lack of a gold standard, patients with transthoracic echocardiography (TTE) were required to fulfill both Chin and Jenni echocardiographic criteria, and patients undergoing MRI studies were required to meet the Petersen criteria. 3 , 4 , 13 Patients with TTE or MRI alone were included, and patients with both investigations available were required to meet both TTE and MRI diagnostic criteria.
Definitions
Chin criteria were defined as any one segment with the ratio of trough of trabeculation‐to‐epicardium (X):peak of trabeculation‐to‐epicardium (Y) <0.5 at end diastole, 3 whereas Jenni criteria as any segment with maximum end‐systolic noncompacted (NC):compacted (C) thickness ≥2. 4 Petersen criteria were defined as end‐diastolic NC:C thickness ≥2.3. 13
The institutional echocardiographic and MRI databases as well as electronic medical records were searched for adult patients (age ≥18) with a possible or definite diagnosis of LVNC between 2000 and 2016 (Figure 1). Among 388 patients, 17 were excluded for presence of complex congenital heart disease (eg, tetralogy of Fallot, transposition of great arteries, and Ebstein anomaly), 8 because of nonavailability of digital images, and 1 because of missing clinical data. Among 362 remaining patients, 339 met the inclusion criteria for diagnosis of LVNC and comprised the study cohort.
Figure 1. Derivation of the cohort.
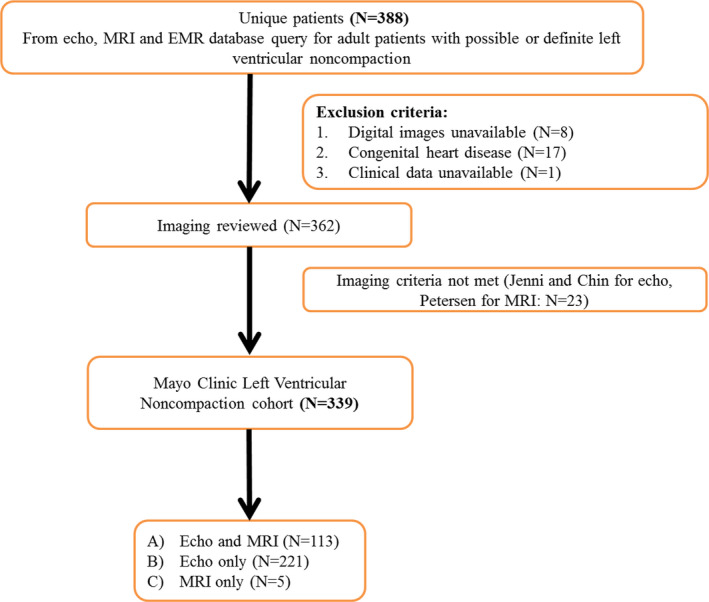
EMR indicates electronic medical record; and MRI, magnetic resonance imaging.
Data Collection
Clinical information was abstracted from the medical records, including demographics, comorbidities, cardiovascular implantable electronic device presence at baseline, medications, laboratory, and electrocardiography data. Echocardiography data including left ventricular ejection fraction (LVEF), end‐diastolic and end‐systolic left ventricular diameters, increased left ventricular wall thickness (defined as LV mass index >95 g/m2 in women and >115 g/m2 in men), left atrial volume index, and right ventricular systolic pressure were also collected. Left ventricular systolic dysfunction was defined as LVEF <50% by echocardiography, because TTE was present in the majority patients included in the cohort. 14 For the remaining patients, the LVEF by MRI was included. Noncompaction extent was categorized into 2 different groups: isolated apical segment involvement versus mid or basal segment involvement. If the Chin and Jenni echocardiographic criteria were met for the apical segment alone, this was defined as isolated apical noncompaction. Similarly, for patients without echocardiography and MRI images only (N=5), if the Petersen criteria were met for the apical segment alone, this was defined as isolated apical noncompaction. In contrast, if the Chin, Jenni, or Petersen criteria were met for any segment in the mid or basal left ventricle, this was defined as mid or basal noncompaction extent. Because the majority of the patients underwent TTE, if there was discordance regarding the presence of isolated apical noncompaction between echocardiography and MRI, we chose to classify them based on echocardiography data. For patients with ≥1 segment meeting echocardiographic (or MRI) diagnostic criteria, the maximum NC:C ratio and the minimum X:Y ratio were selected for purposes of statistical analysis. MRI data, including left and right ventricular end‐systolic and end‐diastolic volumes, and presence of late gadolinium enhancement, were also collected when available.
Outcome Ascertainment
Survival status and date of death were determined by 2 methods. First, the institution medical record was reviewed and date of death for deceased patients abstracted. We then used an institutionally‐approved location service (Accurint, LexisNexis, Philadelphia, PA) for the remaining patients in order to ascertain the most recent vital status. Similar methodology has been used in prior studies. 15 , 16
Statistical Analysis
Continuous variables were expressed as median and interquartile range (IQR) (25th–75th percentile). Comparisons between baseline variables were performed using the chi‐square test for categorical variables and the unpaired t test for interval variables. Overall mortality was estimated using the Kaplan‐Meier method. Cox proportional hazards models were used to calculate hazard ratios (HR) and 95% CI for all‐cause mortality. Because of the limited number of outcome events, variables that were significantly associated with mortality on univariate analysis, and felt to have considerable clinical impact in the opinion of the investigators, were selected for inclusion in the multivariable model. Variable selection for inclusion in the multivariable model was based on statistical significance on univariate analysis and a priori knowledge. Overall expected mortality was calculated for various groups using the age‐ and sex‐specific death rates from the US population. 17 This expected survival was compared with the cohorts using a 1‐sample log‐rank test. A 2‐tailed P<0.05 was considered significant.
Results
The final cohort included 339 patients. TTE was performed in 334 patients (99%) and MRI in 118 patients (35%). TTE and MRI were both performed in 113 patients (33%); when both studies were performed the median time difference between them was 2 days (IQR 0–31 days). For the remaining patients, 221 had only TTE and 5 patients had only MRI.
Baseline Demographics
The median age was 47.4 years (IQR 34–61) and 46% of the patients were female (Table 1). Hypertension was present in 31% of patients, smoking in 31%, and diabetes mellitus in 12%. Atrial fibrillation or flutter was present in 22% and 6% had a prior history of stroke or transient ischemic attack. Cardiovascular implantable electronic devices were present in 17% of patients at baseline. Dyspnea was the commonest symptom and was present in 49% and 29% had received a clinical diagnosis of heart failure, whereas 26% of patients were asymptomatic at presentation. The commonest cardiac medications were beta blockers (64%) followed by angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers (49%); 25% were on anticoagulation at baseline (87% warfarin, 13% novel oral anticoagulants) and 42% were on aspirin. The indication for initial echocardiography referral was most frequently for evaluation of known cardiomyopathy (54%); followed by atrial or ventricular arrhythmias (13%), symptoms suspected to be of cardiac origin (12%), other indications (12%), abnormal physical examination findings (4%), prior history of simple congenital heart defects (4%), and evaluation of hemodynamic abnormalities (1%).
Table 1.
Baseline Demographics of the Entire Cohort
| N (%)/Median (IQR) | |
|---|---|
| Age, y | 47.4 (34–61) |
| Female sex | 157 (46%) |
| Hypertension | 106 (31%) |
| Diabetes mellitus | 40 (12%) |
| Smoking | 105 (31%) |
| Stroke | 20 (6%) |
| Coronary artery disease | 49 (14%) |
| Congestive heart failure | 99 (29%) |
| Left ventricular ejection fraction (%) | 45 (30–58) |
| Left ventricular ejection fraction <50% | 194 (57%) |
| Atrial fibrillation/flutter | 76 (22%) |
| Any cardiovascular implantable device | 57 (17%) |
| Implantable cardioverter defibrillator | 38 (11%) |
| Permanent pacemaker | 6 (2%) |
| Cardiac resynchronization therapy | 13 (4%) |
| Symptoms at presentation | |
| Dyspnea | 165 (49%) |
| Chest pain | 38 (11%) |
| Presyncope/syncope | 76 (22%) |
| Sudden cardiac death | 6 (2%) |
| Medication use | |
| Beta blocker | 217 (64%) |
| Angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker | 167 (49%) |
| Aldosterone antagonist | 50 (15%) |
| Diuretic | 97 (29%) |
| Antiarrhythmic drug | 45 (13%) |
| Anticoagulation* | 85 (25%) |
| Aspirin | 143 (42%) |
| Other antiplatelet agent | 12 (4%) |
| Indication for echocardiography | |
| Evaluation of known cardiomyopathy | 180 (54%) |
| Atrial or ventricular arrhythmias | 42 (13%) |
| Symptoms suspected to be of cardiac origin | 41 (12%) |
| Other indications | 41 (12%) |
| Simple adult congenital heart defects | 13 (4%) |
| Abnormal physical examination | 12 (4%) |
| Evaluation of hemodynamic abnormalities | 5 (1%) |
IQR indicates 25th to 75th percentile interquartile range.
Either warfarin (87%) or novel oral anticoagulant (13%).
Imaging Characteristics
The median LVEF was 45% (IQR 30–58, Table 2); LV dysfunction (EF <50%) was present in 57% of patients. The median left atrial volume index was 35 mL/m2 (IQR 27–47) and right ventricular systolic pressure, 30 mm Hg (IQR 24–39). The median number of segments meeting echocardiographic criteria for LVNC were 2 (IQR 1–3; Figure 2), with the most frequently involved segment being the apex (93%), followed by the midlateral segment (43%). Isolated apical involvement was present in 48% of patients and any mid or basal segment involvement in 52%. The median end‐systolic NC: C ratio by echocardiography was 2.8 (IQR 2.4–3.3), whereas the median X:Y ratio was 0.25 (IQR 0.21–0.28). Similar to TTE, the most frequently involved segment on MRI was the apex (89%), followed by the midlateral segment (38%). The median end‐diastolic NC: C ratio by MRI was 4.0 (3.2–5.0).
Table 2.
Imaging Characteristics of the Entire Cohort
| N (%)/Median (IQR) | |
|---|---|
| Echocardiography (N=334) | |
| Left ventricular end‐diastolic diameter, mm | 55 (50–62) |
| Left ventricular end‐systolic diameter, mm | 41 (34–51) |
| Left atrial volume index, mL/m2 | 35 (27–47) |
| Left atrial enlargement (left atrial volume index >34 mL/m2) | 160 (50%) |
| Right ventricular systolic pressure, mm Hg | 30 (24–39) |
| Any RV enlargement | 85 (26%) |
| Any RV dysfunction | 90 (27%) |
| Moderate or greater mitral regurgitation | 43 (13%) |
| LV septal thickness, mm | 10 (9–11) |
| LV posterior wall thickness, mm | 10 (9–11) |
| LV mass index, g/m2 | 108 (89–138) |
| Increased LV wall thickness (LV mass index >95 g/m2: women; >115 g/m2: men) | 161 (48%) |
| Echocardiographic noncompaction measurements | |
| Minimum systolic compacta thickness, mm | 6 (5–7) |
| Maximum systolic noncompacta thickness, mm | 18 (15–21) |
| Maximum end‐systolic NC:C ratio (Jenni criteria) | 2.8 (2.4–3.3) |
| Minimum end‐diastolic X:Y ratio (Chin criteria) | 0.25 (0.21–0.28) |
| Number of noncompacted segments | 1 (1–3) |
| Extent of noncompaction | |
| Apex only involvement | 161 (48%) |
| Mid or basal involvement | 173 (52%) |
| Magnetic resonance imaging (N=118) | |
| Left ventricular end‐diastolic volume, mL | 161 (131–209) |
| Left ventricular end‐systolic volume, mL | 72 (51–111) |
| Left ventricular stroke volume, mL | 84 (68–98) |
| MRI noncompaction measurements | |
| Minimum diastolic compacta thickness, mm | 5 (5–7) |
| Maximum diastolic noncompacta thickness, mm | 17 (14–21) |
| Maximum end‐diastolic NC:C ratio (Petersen criteria) | 4.0 (3.2–5.0) |
| Number of noncompacted segments | 1 (1–2) |
| Delayed gadolinium enhancement | 17 (15%) |
IQR indicates interquartile range; LV, left ventricle; NC:C, noncompacted to compated; RV, right ventricle; and X:Y, trough of trabeculation‐to‐epicardium (X):peak of trabeculation‐to‐epicardium (Y).
Figure 2. Distribution of noncompaction segments; NC:C (noncompacted:compacted) ratios and X:Y ratios by echocardiography.
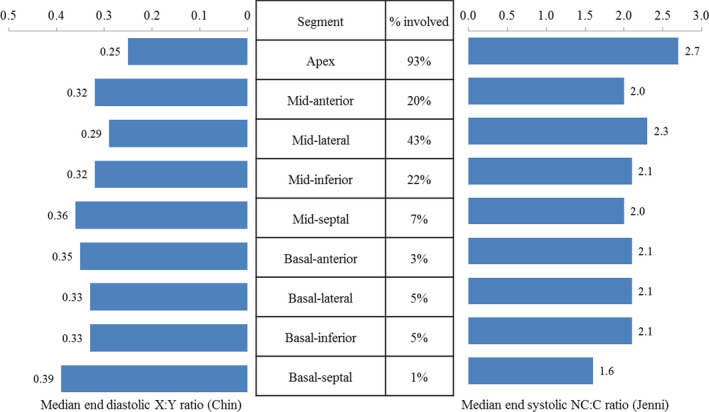
NC:C indicates noncompacted to compacted; and X:Y, trough of trabeculation‐to‐epicardium (X):peak of trabeculation‐to‐epicardium (Y).
Survival Analysis
Over a median follow‐up of 6.3 years (IQR 3.1–10.8), there were 59 deaths. The cause of death was not available for 42 patients. Among 17 patients for whom cause of death was available, cardiovascular causes were responsible for 71% of deaths and noncardiovascular causes for 29%. The cardiovascular causes of death were end‐stage congestive heart failure or cardiogenic shock in 8 and cardiac arrest or ventricular arrhythmia in 4. The noncardiovascular causes of death were sepsis in 2 patients; and chronic kidney disease, metastatic cancer, and idiopathic pulmonary fibrosis in 1 patient each.
Univariate analysis is presented in Table 3. Traditional cardiovascular risk factors, such as age, hypertension, diabetes mellitus, history of stroke or transient ischemic attack, and smoking were associated with all‐cause mortality. Asymptomatic presentation was associated with lower all‐cause mortality (HR, 0.34; 95% CI, 0.15–0.75; P<0.001). Notably, left ventricular EF, left ventricular end‐diastolic and end‐systolic diameter, increased LV wall thickness, right ventricular (RV) enlargement, and RV dysfunction were associated with all‐cause mortality; but measures used to define noncompaction such as the NC:C ratio and X:Y ratio were not. Isolated apical noncompaction was associated with decreased all‐cause mortality, compared with mid or basal noncompaction extent (HR, 0.48; 95% CI, 0.28–0.84; P=0.009). Maximum systolic noncompacta thickness was associated with increased all‐cause mortality (HR, 1.06; 95% CI, 1.02–1.10; P=0.006), whereas minimum systolic compacta thickness was not (HR, 1.16; 95% CI, 0.95–1.42; P=0.15).
Table 3.
Univariate Cox Proportional Hazard Analysis for Variables Associated With Overall Mortality
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Clinical variables | ||
| Age | 1.05 (1.03–1.07) | <0.001 |
| Female sex | 1.19 (0.71–2.00) | 0.52 |
| Hypertension | 1.77 (1.06–2.96) | 0.03 |
| Diabetes mellitus | 3.12 (1.79–5.43) | <0.001 |
| Smoking | 1.86 (1.11–3.10) | 0.019 |
| Stroke/transient ischemic attack | 5.08 (2.02–12.76) | <0.001 |
| Coronary artery disease | 1.32 (0.70–2.50) | 0.39 |
| Congestive heart failure | 2.56 (1.54–4.27) | <0.001 |
| LVEF <50% | 3.96 (2.00–7.82) | <0.001 |
| Atrial fibrillation/flutter | 1.51 (0.87–2.64) | 0.14 |
| Asymptomatic presentation | 0.34 (0.15–0.75) | <0.001 |
| Anticoagulation at baseline | 1.32 (0.74–2.26) | 0.34 |
| Aspirin use at baseline | 1.47 (0.88–3.29) | 0.14 |
| Echocardiographic variables | ||
| LVEF (continuous) | 0.96 (0.94–0.97) | <0.001 |
| Left ventricular end‐diastolic diameter | 1.06 (1.03–1.09) | <0.001 |
| Left ventricular end‐systolic diameter | 1.06 (1.04–1.08) | <0.001 |
| Left atrial volume index (LAVI) | 1.02 (1.01–1.03) | <0.001 |
| Left atrial enlargement (LAVI >34 mL/m2) | 3.20 (1.71–6.00) | <0.001 |
| Right ventricular systolic pressure | 1.06 (1.04–1.08) | <0.001 |
| Any RV enlargement | 2.05 (1.22–3.44) | 0.007 |
| Any RV dysfunction | 2.43 (1.45–4.08) | 0.001 |
| Moderate or greater mitral regurgitation | 3.46 (1.95–6.13) | <0.001 |
| LV mass index, g/m2 | 1.01 (1.01–1.02) | <0.001 |
| Increased LV wall thickness (LV mass index >95 g/m2: women; >115 g/m2: men) | 3.04 (1.73–5.34) | <0.001 |
| Minimum systolic compacta thickness | 1.16 (0.95–1.42) | 0.15 |
| Maximum systolic noncompacta thickness | 1.06 (1.02–1.10) | 0.006 |
| Maximum end‐systolic NC:C ratio (Jenni criteria) | 1.23 (0.94–1.60) | 0.13 |
| Minimum end‐diastolic X:Y ratio (Chin criteria, per 0.1 unit increase in the ratio) | 1.26 (0.76–2.07) | 0.37 |
| Number of segments involved | 1.21 (1.03–1.43) | 0.024 |
| Extent of noncompaction | ||
| Isolated apical involvement | 0.48 (0.28–0.84) | 0.009 |
LV indicates left ventricle; LVEF, left ventricular ejection fraction; NC:C, noncompacted to compacted; RV, right ventricle; TIA, transient ischemic attack; and X:Y, trough of trabeculation‐to‐epicardium (X):peak of trabeculation‐to‐epicardium (Y).
Based on the number of outcome events, 6 variables were included in the initial multivariable model: age, diabetes mellitus, history of stroke/transient ischemic attack, left ventricular EF <50%, asymptomatic presentation (absence of dyspnea, chest pain, presyncope, syncope, or cardiac arrest), and extent of noncompaction (isolated apical versus mid or basal involvement). Age (HR, 1.04; 95% CI, 1.02–1.06; P<0.001), LVEF <50% (HR, 2.37; 95% CI, 1.17–4.80; P=0.01), and noncompaction extending to the mid or basal segments (HR, 2.11; 95% CI, 1.21–3.68; P=0.016) emerged as statistically significant predictors of all‐cause mortality (Table 4). Asymptomatic presentation was associated with a trend toward reduced all‐cause mortality (HR, 0.50; 95% CI, 0.22–0.85; P=0.09).
Table 4.
Multivariable Cox Proportional Hazard Analysis for Variables Associated With Overall Mortality
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age | 1.04 (1.02–1.06) | <0.001 |
| Diabetes mellitus | 1.50 (0.83–2.74) | 0.19 |
| Stroke/transient ischemic attack | 1.62 (0.64–4.13) | 0.31 |
| Asymptomatic presentation | 0.50 (0.22–0.85) | 0.09 |
| Left ventricular ejection fraction <50% | 2.37 (1.17–4.80) | 0.01 |
| Isolated apical noncompaction | 0.47 (0.21–0.85) | 0.016 |
We performed additional multivariable analyses to assess whether age, noncompaction extent, and LVEF <50% were associated with all‐cause mortality independent of right ventricular dysfunction, increased left ventricular wall thickness, left ventricular size, and left atrial enlargement. Age (HR, 1.05; 95% CI, 1.03–1.07; P<0.001) and noncompaction extent (isolated apical versus midbasal extent: HR, 0.41; 95% CI, 0.23–0.76; P=0.004) continued to be significantly associated with all‐cause mortality, while controlling for LVEF <50%, increased LV wall thickness, LA enlargement, and RV dysfunction in the multivariable models (Tables 5 and 6).
Table 5.
Multivariable Cox Proportional Hazard Analysis for Variables Associated With Overall Mortality (Model 2)
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age | 1.05 (1.03–1.07) | <0.001 |
| Left ventricular ejection fraction <50% | 1.81 (0.86–3.82) | 0.11 |
| Isolated apical vs midbasal noncompaction extent | 0.41 (0.23–0.76) | 0.004 |
| Left atrial enlargement (left atrial volume index >34 cc/m2) | 1.22 (0.61–2.48) | 0.57 |
| Increased left ventricle wall thickness | 1.64 (0.84–3.21) | 0.15 |
| Any right ventricle dysfunction | 1.98 (1.10–3.54) | 0.02 |
Table 6.
Multivariable Cox Proportional Hazard Analysis for Variables Associated With Overall Mortality (Model 3)
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age | 1.05 (1.03–1.08) | <0.001 |
| Left ventricular ejection fraction <50% | 1.07 (0.45–2.56) | 0.88 |
| Isolated apical vs midbasal noncompaction extent | 0.39 (0.20–0.75) | 0.003 |
| Left atrial enlargement (left atrial volume index >34 cc/m2) | 1.07 (0.52–2.23) | 0.85 |
| Left ventricle end‐diastolic diameter | 1.05 (1.01–1.09) | 0.009 |
| Maximum systolic noncompacta thickness | 0.98 (0.90–1.05) | 0.54 |
To determine the relative impact of reduced left ventricular EF and extent of LVNC, we stratified the patients into 4 groups: LVEF >50% with isolated apical involvement, LVEF >50 with mid or basal involvement, LVEF <50% with isolated apical involvement, and LVEF <50% with mid or basal involvement. Overall mortality significantly differed among these 4 groups (overall P<0.001, Figure 3); and those with LVEF <50% and mid or basal segment involvement appeared to have the worst prognosis. Comparisons between individual groups are listed in the figure legend.
Figure 3. Overall survival of patients with noncompaction, stratified by left ventricular ejection fraction and extent of left ventricular noncompaction.
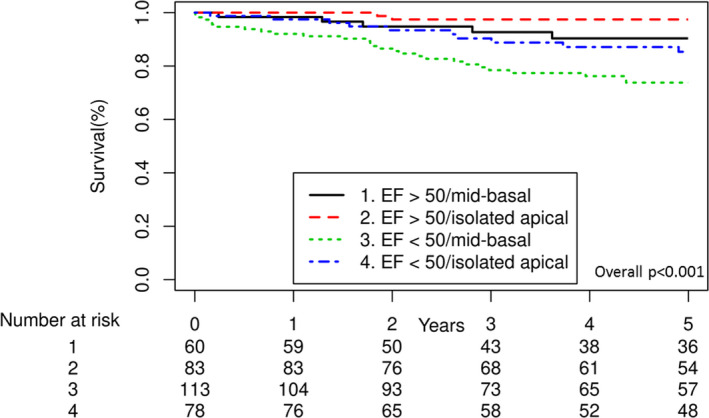
P values for comparison between each group: LVEF >50%, apex only vs LVEF >50%, mid or basal P=0.06; LVEF >50%, apex only vs LVEF <50%, apex only P=0.001; LVEF >50%, apex only vs LVEF <50%, mid or basal P<0.001; LVEF >50%, mid or basal vs LVEF <50%, apex only P=0.20; LVEF >50%, mid or basal vs LVEF <50%, mid or basal P=0.01; LVEF <50%, apex only vs LVEF <50%, mid or basal P=0.18. EF indicates ejection fraction.
Comparison of Clinical Characteristics of Patients With Isolated Apical Noncompaction Versus Midbasal Noncompaction Extent
Because isolated apical noncompaction was associated with lower risk of all‐cause mortality compared with midbasal noncompaction extent, we sought to determine differences in clinical characteristics of these patients. Patients with isolated apical noncompaction were similar to midbasal noncompaction with regard to traditional cardiovascular risk factors such as age, hypertension, diabetes mellitus, stroke/transient ischemic attack, coronary disease, and atrial fibrillation (P=NS, Table 7). However, patients with isolated apical LVNC had higher LVEF (median 50%, IQR 36%–60% versus median 40%, IQR 25%–55%, P<0.001) and were more frequently asymptomatic (32% versus 11%, P=0.012) than those with midbasal noncompaction extent. There were no differences in left atrial volume index, RV enlargement, RV dysfunction, or moderate or severe mitral regurgitation (P=NS). However, patients with isolated apical LVNC had lower LV end‐systolic diameter (39 versus 43 mm, P=0.012), greater right ventricular systolic pressure (28 versus 31 mm Hg, P=0.02), lower noncompacta thickness (17 versus 19 mm, P<0.0001), and lower NC:C ratio (2.6 versus 2.9, P=0.011). Despite these differences between the groups at baseline, noncompaction extent (isolated apical versus midbasal) was associated with all‐cause mortality, after adjusting for age, LVEF, and symptoms status at presentation (Table 4; isolated apical versus midbasal: HR, 0.47; 95% CI, 0.21–0.85; P=0.02).
Table 7.
Comparison of Baseline Characteristics Between Patients With LVNC With Isolated Apical Noncompaction Versus Mid‐Basal Noncompaction
| Variables | Isolated Apical Noncompaction N=163 | Mid‐Basal Noncompaction N=173 | P Value |
|---|---|---|---|
| Age, y | 45 (34–61) | 49 (33–61) | 0.82 |
| Female sex | 67 (41%) | 90 (51%) | 0.06 |
| Hypertension | 49 (30%) | 57 (32%) | 0.64 |
| Diabetes mellitus | 18 (11%) | 22 (13%) | 0.68 |
| Smoking | 43 (26%) | 62 (35%) | 0.08 |
| Stroke/TIA | 11 (7%) | 9 (5%) | 0.52 |
| Coronary artery disease | 22 (14%) | 27 (15%) | 0.63 |
| Congestive heart failure | 38 (23%) | 61 (35%) | 0.02 |
| Left ventricular ejection fraction (%) | 50% (36–60) | 40% (25–55) | <0.001 |
| Left ventricular ejection fraction <50% | 79 (49%) | 114 (65%) | 0.002 |
| Atrial fibrillation/flutter | 40 (25%) | 36 (21%) | 0.37 |
| Any cardiovascular implantable device | 22 (14%) | 35 (20%) | 0.11 |
| Asymptomatic at presentation | 52 (32%) | 36 (11%) | 0.012 |
| Echocardiographic variables | |||
| Left ventricular end‐diastolic diameter | 55 (50–61) | 55 (49–63) | 0.31 |
| Left ventricular end‐systolic diameter | 39 (33–48) | 43 (34–54) | 0.012 |
| Left atrial dilation present (LAVI >34 mL/m2) | 71 (46%) | 89 (54%) | 0.15 |
| Right ventricular systolic pressure, mm Hg | 28 (24–36) | 31 (26–42) | 0.02 |
| Any RV enlargement | 43 (27%) | 42 (25%) | 0.7 |
| Any RV dysfunction | 42 (26%) | 48 (28%) | 0.61 |
| Moderate or greater mitral regurgitation | 20 (13%) | 23 (14%) | 0.7 |
| LV mass index, g/m2 | 104 (85–137) | 111 (93–139) | 0.09 |
| Minimum systolic compacta thickness, mm | 6 (5–7) | 6 (5–6) | <0.001 |
| Maximum systolic noncompacta thickness, mm | 17 (14–20) | 19 (17–22) | <0.001 |
| Maximum end‐systolic NC:C ratio (Jenni criteria) | 2.6 (2.3–3.2) | 2.9 (2.5–3.4) | 0.011 |
| Minimum end‐diastolic X:Y ratio (Chin criteria, per 0.1 unit increase in the ratio) | 0.25 (0.21–0.29) | 0.25 (0.21–0.28) | 0.15 |
| Number of segments involved | 1 (1–1) | 3 (2–4) | <0.001 |
| Delayed gadolinium enhancement on MRI | 7 (14%) | 10 (17%) | 0.64 |
LAVI indicates left atrial volume index; LV, left ventricular; LVNC, left ventricular noncompaction; MRI, magnetic resonance imaging; NC:C, noncompacted:compacted; RV, right ventricular; and TIA, transient ischemic attack.
Survival Compared With Expected US Age‐ and Sex‐Matched Population Rates
In comparison to the expected age‐ and sex‐matched US population death rates, patients with LVNC had reduced overall survival (observed survival at 5 years 86% [95% CI, 82%–90%], expected survival 95%, P<0.001; Figure 4). As expected, patients with LVNC with left ventricular EF <50% had significantly increased mortality compared with the general population (observed survival 79% at 5 years versus expected survival 94%; P<0.001). However, patients with LVNC with left ventricular EF ≥50% had no excess mortality compared with the general population (observed survival 95% at 5 years versus expected survival 97%; P=0.45; Figure 5). Similarly, patients with LVNC with apical only involvement had similar survival compared with the general population (observed survival 92% at 5 years versus expected survival 95%, P=0.20), and patients with mid or basal involvement had reduced survival compared to the general population (observed survival 79% at 5 years, expected survival 95%; P<0.001).
Figure 4. Comparison of overall mortality between left ventricular noncompaction and expected US age‐ and sex‐matched population rates.
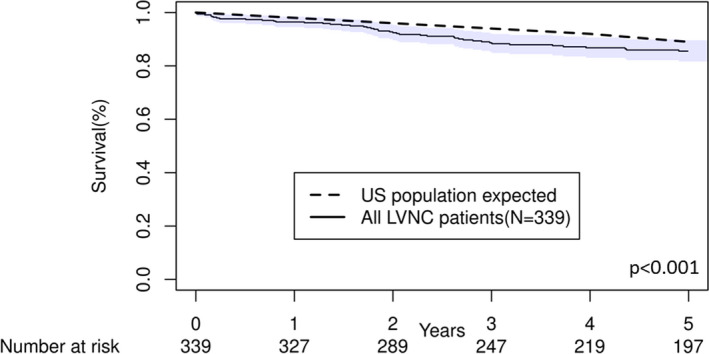
Shaded region indicates 95% CIs. LVNC indicates left ventricular noncompaction.
Figure 5. Comparison of overall mortality between left ventricular noncompaction and expected US age‐ and sex‐matched population rates, stratified by left ventricular ejection fraction (A) and noncompaction extent (B).
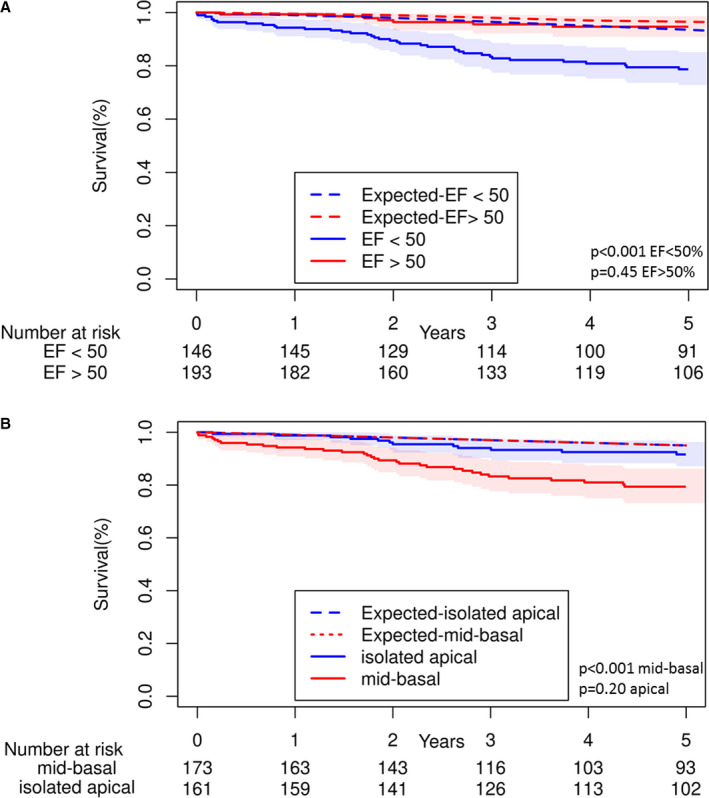
Shaded regions indicate 95% CIs. EF indicates ejection fraction.
Comparison of Patients With and Without LA Dilatation
Left atrial dilation (defined as left atrial volume index >34) was present in 50% of the patients, and the median left atrial volume index was 35 cc/m2. We performed further characterization of patients with left atrial dilation, including a statistical comparison of baseline variables among those with and without LA dilation (Table S1). The clinical features of patients with and without LA dilatation were different; patients with LA dilatation were significantly older, had greater frequency of hypertension, congestive heart failure, and atrial fibrillation. On echocardiographic assessment, patients with LA dilatation were more likely to have lower EF, increased LV wall thickness and more frequently had moderate or greater severity mitral regurgitation. Among patients with LVNC with LA dilatation, 25% died (n=39), compared with 8% of patients without LA dilatation (n=13). On univariate analysis, LA dilatation was significantly associated with all‐cause mortality (HR, 3.20; 95% CI, 1.71–6.00; P<0.001). Among 39 patients with LA dilatation who died, the cause of death was cardiovascular in 9, non‐cardiovascular in 3, and unknown in 27. Among 13 patients without LA dilatation, the cause of death was cardiovascular in 2, and unknown in 11.
Discussion
Main Findings
To our knowledge, this study represents the largest reported cohort of adult patients with LVNC. Our findings showed that (1) reduced left ventricular EF and extent of LVNC (isolated apical versus mid or basal extent) were significantly associated with all‐cause mortality; (2) patients with LVNC have reduced overall survival compared with the general population but that (3) patients with LVNC with preserved systolic function and isolated apical noncompaction have similar survival rates to the general individuals.
LVNC is classified as a distinct cardiomyopathy by the American Heart Association and is characterized by a bilayered appearance of the left ventricular myocardium, comprising a thick layer with prominent trabeculations and deep intertrabecular recesses and a disproportionately thinner compacted layer. 1 , 2 , 6 The embryonal myocardium has a trabeculated appearance after cardiac looping, gradually progressing to the adult compacted appearance by month 4 in human embryos. 18 Initially, it was thought that the trabeculated embryonal myocardium coalesces to contribute to the thickness of the compacted myocardium. 19 However, direct evidence supporting the notion that the embryonal “noncompacted” myocardium undergoes “compaction” to contribute to the myocardial free wall is lacking. Recent immunohistochemical studies instead contend that the proliferation of the compact zone of the embryonal myocardium into the trabeculated zone, forming an intermediate “hybrid zone,” is responsible for the change from a trabeculated, noncompacted embryonal appearance to a compacted appearance of the myocardium. 20 Inhibition of compact layer proliferation results in noncompacted myocardial appearance and may be one of the mechanisms underlying LVNC pathogenesis. Despite the emergence of novel insights that do not consistently support the notion of “compaction” of trabeculated layer, we retained the term “noncompacted” to refer to the trabeculated layer because of widespread usage of this term in the clinical setting.
Currently, the most widely accepted diagnostic criteria for LVNC in adults depend upon a bilayered myocardial appearance and the relative thickness of the noncompacted and compacted layers, as measured by echocardiography or cardiac MRI. 3 , 4 , 13 The trabeculae can have a lace‐like appearance, especially on cardiac MRI. However, there are controversy and equipoise regarding the existence of LVNC as a distinct cardiomyopathy and the prognosis of patients meeting current diagnostic criteria. 11 , 12
Prognostic Variables in LVNC
Apart from measurements of noncompacted and compacted myocardial thickness, cardiac imaging provides parameters of global left ventricular function, such as the ejection fraction and measures of ventricular size and volume. Among larger cohorts of adult patients with LVNC, markers of global left ventricular function including LVEF are consistently associated with increased mortality and adverse clinical outcomes. 21 , 22 , 23 , 24 , 25 , 26 , 27 Our data further highlight the importance of left ventricular systolic function in the prognosis of patients with LVNC, and LVEF <50% at diagnosis was a statistically significant predictor of overall mortality.
Cardiac imaging can also provide measures of the extent and severity of noncompaction. Such measures include the various ratios used to diagnose noncompaction, number of involved segments, and noncompacted and compacted myocardial thickness. With MRI, additional measures such as noncompacted mass and fractal analysis can be assessed using sophisticated measurement techniques. 28 , 29 However, these markers of the extent and severity of noncompaction were not associated with mortality and adverse clinical outcomes in several studies of adult patients with LVNC. 21 , 26 , 30 , 31 In contrast, Stacey et al reported increased risk of congestive heart failure and combined cardiovascular events among patients meeting end‐systolic MRI criteria for LVNC. 32 Similar to most reports, ratios used to define noncompaction did not correlate with overall mortality in our study. Whether these ratios might be used to identify patients at increased risk for cardiovascular morbidity requires further investigation.
The novel observation from our cohort is that the extent of noncompaction (ie, noncompaction extent as limited to the apex or extending to the mid or basal segments) is of prognostic value. In our study, 48% patients had noncompaction isolated to the apical segment. On multivariable analysis, mid or basal noncompaction extent was significantly associated with increased overall mortality compared with isolated apical noncompaction. This finding was independent of the LVEF, structural abnormalities such as increased LV wall thickness, RV dysfunction, and traditional cardiovascular risk factors in multivariable analysis.
The reasons for the association of mid or basal noncompaction with increased all‐cause mortality are not completely elucidated by our study. It is known that the embryonal myocardium has a highly noncompacted appearance, extending to most of the left ventricle. As the embryonal myocardium develops, it has an increasingly compacted appearance. This process appears to start from the base of the ventricles and continues toward the apices. We speculate that patients with midbasal noncompaction might have an earlier arrest in normal myocardial development, a larger burden of noncompacted myocardium, and therefore the risks associated with this defect, such as heart failure and mortality. Noncompaction is associated with regional wall motion abnormalities of the involved segments, and increased number of noncompacted segments has been associated with reduced LVEF. 33 Mid or basal involvement could be a marker of ventricular systolic or diastolic dysfunction later in the course of LVNC, or a marker of increased tendency toward ventricular arrhythmias. Indeed, on comparison of baseline characteristics of patients with isolated apical versus midbasal noncompaction, patients with isolated apical LVNC had greater LVEF and lower rate of symptomatic presentation but had similar distribution of traditional cardiovascular risk factors. However, despite adjustment for LVEF and symptom status, midbasal noncompaction extent was associated with worse all‐cause mortality in patients with LVNC.
LVEF and extent of LVNC are imaging characteristics that are readily available and can be assessed in any patient with suspected LVNC. Clinicians can incorporate these data to counsel patients and further prognosticate patients with morphological diagnosis of LVNC (Figure 6, Videos S1 and S2). Interestingly, asymptomatic status at baseline was associated with a trend toward improved survival but with borderline statistical significance. This observation deserves further investigation.
Figure 6. Summarizing illustration.
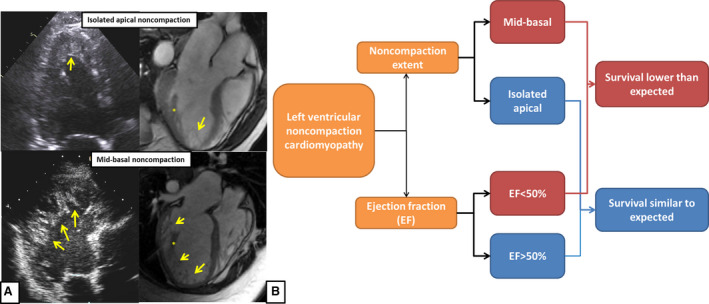
A, Imaging characteristics of patients with isolated apical vs midbasal noncompaction. In patients with isolated apical noncompaction the noncompacted layer (yellow arrows) is limited to the ventricular apex. In patients with midbasal noncompaction, the noncompacted myocardium extends up to or beyond the midventricular level, identified by presence of papillary muscles distinct from the noncompacted myocardium (*). B, Prognostic factors in adult patients with left ventricular noncompaction.
LVNC as a Distinct Cardiomyopathy
Although noncompaction was classified as a distinct genetic cardiomyopathy by the American Heart Association, there are questions about whether it is a unique cardiomyopathy or represents a subtype of ventricular remodeling. 6 In one study, 8% of athletes met LVNC criteria by echocardiography, had either normal or mildly reduced LVEF, and had no adverse events over 4 years of follow‐up. 34 LVNC criteria have also been reported in pregnant patients and those with sickle cell anemia. 35 , 36 Among 2742 Multi‐Ethnic Study of Atherosclerosis participants undergoing MRI at baseline and ≈10 years later, patients with highest quintile of NC:C ratio (2.46–5.41) had no reduction in LVEF or increase in LV size at follow‐up. 37 However, among 5004 patients with initial MRI, only 3016 patients underwent the 9.5‐year follow‐up MRI, bringing up the possibility of survival bias.
Even among patients with known cardiomyopathy, the presence of the noncompaction phenotype has uncertain significance. Among patients with known systolic dysfunction referred for cardiac MRI, Amzulescu et al noted no difference in cardiovascular outcomes based on the presence of the Petersen criteria for LVNC or increased noncompacted mass. 26 In contrast, Sedaghat‐Hamedani et al observed increased cardiovascular events among patients with LVNC and a relatively high proportion of late gadolinium enhancement on MRI, compared with an age‐ and sex‐matched population with dilated cardiomyopathy. 38
Because it is uncertain whether individuals meeting morphological criteria for LVNC have an increased risk of mortality, we compared observed survival of patients with LVNC with the expected survival of age‐ and sex‐matched US population. We found that as a group, patients with LVNC had reduced overall survival compared with the general population. However, the survival of those with preserved LVEF and isolated apical noncompaction was similar to that of the general population. These results add to the data indicating that LVNC limited to the apical segment in patients with preserved EF most likely has a more benign course and is not associated with excess mortality. These data could assist in risk‐stratifying patients with echocardiographic findings suggestive of LVNC in routine clinical practice. However, further studies are required to determine whether any subgroup of patients with LVNC with preserved LVEF and isolated apical involvement demonstrate increased mortality.
Prognosis of LVNC: Comparisons With Prior Data
The prognosis of patients with cardiomyopathy can vary according to the etiology of the cardiomyopathy. For example, peripartum cardiomyopathy appears to have favorable prognosis, compared with idiopathic, ischemic, infiltrative, and chemotherapy‐related cardiomyopathies. 39 Additionally, the prognosis of cardiomyopathies and heart failure changes over time, as evidenced by improvement in overall mortality associated with heart failure and hypertrophic cardiomyopathy. 40 , 41 , 42 Knowledge of the prognosis of LVNC in a contemporary cohort can be helpful in the management and counseling of patients. Initial cohorts of LVNC suggested 35% to 38% mortality over median 5 to 11 years of follow‐up. 3 , 43 Our data suggest that LVNC prognosis is overall favorable; the 5‐year survival in our study was 86%. The differences in LVNC prognosis between earlier studies and ours could be owing to heterogeneity among the studies, selection of patients with various extent of disease, technological advancement in cardiac imaging over time, and improvements in medical and device‐based therapies for heart failure with reduced ejection fraction.
Future Directions
There are several unanswered questions about prognosis in LVNC. For example, the impact of diastolic function on patients with LVNC with preserved or reduced ejection fraction remains unclear and is beyond the scope of the current study. Also, the impact of heart failure therapies such as guideline‐directed medical therapy, cardiac resynchronization, ventricular assist device, and heart transplantation remains unknown and could not be ascertained from our data. Systematic genetic evaluation of patients with LVNC with commercially available genetic panels or whole genome sequencing may provide unique insights into the genetic contributors of this condition and genotype‐phenotype correlation. Lastly, subsequent studies are warranted to, first, replicate our observation that patients with isolated apical noncompaction and preserved LVEF do not have excess mortality compared with the general population and second, to identify patients within this group that might have worse prognosis.
Limitations
Several important limitations must be considered while interpreting our data. First, this is an observational study with limitations inherent to the retrospective design. Second, information on genetic testing was not consistently present and not reported in the current study. The 2011 Heart Rhythm Society/European Heart Rhythm Association expert consensus statement on genetic testing for cardiomyopathies suggests that genetic testing can be useful in patients with an established diagnosis of LVNC, and recent studies have reported the genotype and genotype‐phenotype correlations among patients with LVNC. 25 , 38 , 44 , 45 We agree that further characterization of the genotype in LVNC, including whole genome sequencing and genotype‐phenotype correlation, is paramount in furthering our ability to diagnose and manage patients with LVNC. However, the focus of the current study was to identify clinical and imaging variables that are routinely available in current clinical practice, which can be used to risk‐stratify patients fulfilling the morphologic diagnostic criteria of LVNC. Genetic counseling and detailed 3‐generation of family history were not performed on all patients. Therefore, data on family history of LVNC or cardiomyopathy were likely underestimated in our study.
Third, late gadolinium enhancement on MRI is increasingly recognized as a predictor of adverse cardiovascular outcomes in patients with LVNC. 46 MRI was performed approximately one third of patients included in the cohort. 46 Furthermore, only 6 patients who underwent MRI died during follow‐up, so we could not evaluate the impact of delayed gadolinium enhancement in the assessment of prognosis. Fourth, comparison with the US general population is subject to confounding factors, because other critical baseline variables and comorbidities are not accounted for in this analysis. However, despite the lack of adjustment for comorbidities, patients with preserved LVEF and isolated apical noncompaction appear to have similar prognosis to the general population, indicative of a more benign clinical course in these patients.
Fifth, we were unable to assess the dimensions of individual trabeculations because of inadequate spatial resolution of imaging. We were also unable to classify the trabeculations qualitatively into hypertrophied versus lace‐like trabeculae, which could be an important assessment in future studies. Sixth, we acknowledge that there is no widely held consensus on the most accurate diagnostic criteria for LVNC. We were unable to apply all published diagnostic criteria for the diagnosis of LVNC in our cohort and used the Chin, Jenni, and Petersen criteria. However, we reviewed a random subset of 20 patients from our cohort and found that 90% of the patients did fulfill the Stollberger criteria for LV hypertrabeculation/noncompaction. 47 Seventh, we were unable to compare noncompacta and compacta dimensions to a population of individuals lacking excessive trabeculations in the current study. Finally, data on the cause of death were not available for majority (71%) of the patients. However, among the patients with available cause of death, cardiovascular causes were responsible for almost three fourths of the deaths and we expected similar rates for the rest of cohort.
Conclusions
In this contemporary study, long‐term survival was lower in patients with LVNC compared with the general population. Unlike patients with reduced LVEF and midbasal extent, patients with preserved systolic function and isolated apical noncompaction had similar survival compared with the general population.
Sources of Funding
Dr Melduni is supported by National Institutes of Health (NIH) K01 (HL 135288).
Disclosures
None.
Supporting information
Table S1
Video S1
Video S2
(J Am Heart Assoc.2021;10:e015563. DOI: 10.1161/JAHA.119.015563.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.015563
For Sources of Funding and Disclosures, see page 14.
See Editorial by Jefferies
References
- 1. Towbin JA, Lorts A, Jefferies JL. Left ventricular non‐compaction cardiomyopathy. Lancet. 2015;386:813–825. [DOI] [PubMed] [Google Scholar]
- 2. Arbustini E, Weidemann F, Hall JL. Left ventricular noncompaction: a distinct cardiomyopathy or a trait shared by different cardiac diseases? J Am Coll Cardiol. 2014;64:1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82:507–513. [DOI] [PubMed] [Google Scholar]
- 4. Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non‐compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kuhl U, Maisch B, McKenna WJ, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–276. [DOI] [PubMed] [Google Scholar]
- 6. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB; American Heart A . Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–1816. [DOI] [PubMed] [Google Scholar]
- 7. Oechslin E, Jenni R. Left ventricular non‐compaction revisited: a distinct phenotype with genetic heterogeneity? Eur Heart J. 2011;32:1446–1456. [DOI] [PubMed] [Google Scholar]
- 8. Oechslin E, Jenni R. Left ventricular noncompaction: from physiologic remodeling to noncompaction cardiomyopathy. J Am Coll Cardiol. 2018;71:723–726. [DOI] [PubMed] [Google Scholar]
- 9. Ross SB, Semsarian C. Clinical and genetic complexities of left ventricular noncompaction: preventing overdiagnosis in a disease we do not understand. JAMA Cardiol. 2018;3:1033–1034. [DOI] [PubMed] [Google Scholar]
- 10. Jenni R, Goebel N, Tartini R, Schneider J, Arbenz U, Oelz O. Persisting myocardial sinusoids of both ventricles as an isolated anomaly: echocardiographic, angiographic, and pathologic anatomical findings. Cardiovasc Intervent Radiol. 1986;9:127–131. [DOI] [PubMed] [Google Scholar]
- 11. Oechslin E, Jenni R. Nosology of noncompaction cardiomyopathy: the emperor still wears clothes!. Can J Cardiol. 2017;33:701–704. [DOI] [PubMed] [Google Scholar]
- 12. Anderson RH, Jensen B, Mohun TJ, Petersen SE, Aung N, Zemrak F, Planken RN, MacIver DH. Key questions relating to left ventricular noncompaction cardiomyopathy: is the emperor still wearing any clothes? Can J Cardiol. 2017;33:747–757. [DOI] [PubMed] [Google Scholar]
- 13. Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, Watkins H, Neubauer S. Left ventricular non‐compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101–105. [DOI] [PubMed] [Google Scholar]
- 14. Hsu JJ, Ziaeian B, Fonarow GC. Heart failure with mid‐range (borderline) ejection fraction: clinical implications and future directions. JACC Heart Fail. 2017;5:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 16. DeSimone CV, Friedman PA, Noheria A, Patel NA, DeSimone DC, Bdeir S, Aakre CA, Vaidya VR, Slusser JP, Hodge DO, et al. Stroke or transient ischemic attack in patients with transvenous pacemaker or defibrillator and echocardiographically detected patent foramen ovale. Circulation. 2013;128:1433–1441. [DOI] [PubMed] [Google Scholar]
- 17. Therneau TMSJ, Bergstralh EJ, Offord JR. Expected survival based on hazard rates, Section of Biostatistics, Mayo Clinic, Technical Report Series No. 52. March 1994.
- 18. Risebro CA, Riley PR. Formation of the ventricles. ScientificWorldJournal. 2006;6:1862–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental patterning of the myocardium. Anat Rec. 2000;258:319–337. [DOI] [PubMed] [Google Scholar]
- 20. Tian X, Li Y, He L, Zhang H, Huang X, Liu Q, Pu W, Zhang L, Li Y, Zhao H, et al. Identification of a hybrid myocardial zone in the mammalian heart after birth. Nat Commun. 2017;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aras D, Tufekcioglu O, Ergun K, Ozeke O, Yildiz A, Topaloglu S, Deveci B, Sahin O, Kisacik HL, Korkmaz S. Clinical features of isolated ventricular noncompaction in adults long‐term clinical course, echocardiographic properties, and predictors of left ventricular failure. J Card Fail. 2006;12:726–733. [DOI] [PubMed] [Google Scholar]
- 22. Greutmann M, Mah ML, Silversides CK, Klaassen S, Attenhofer Jost CH, Jenni R, Oechslin EN. Predictors of adverse outcome in adolescents and adults with isolated left ventricular noncompaction. Am J Cardiol. 2012;109:276–281. [DOI] [PubMed] [Google Scholar]
- 23. Andreini D, Pontone G, Bogaert J, Roghi A, Barison A, Schwitter J, Mushtaq S, Vovas G, Sormani P, Aquaro GD, et al. Long‐term prognostic value of cardiac magnetic resonance in left ventricle noncompaction: a prospective multicenter study. J Am Coll Cardiol. 2016;68:2166–2181. [DOI] [PubMed] [Google Scholar]
- 24. Ivanov A, Dabiesingh DS, Bhumireddy GP, Mohamed A, Asfour A, Briggs WM, Ho J, Khan SA, Grossman A, Klem I, et al. Prevalence and prognostic significance of left ventricular noncompaction in patients referred for cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2017;10:e006174. [DOI] [PubMed] [Google Scholar]
- 25. van Waning JI, Caliskan K, Hoedemaekers YM, van Spaendonck‐Zwarts KY, Baas AF, Boekholdt SM, van Melle JP, Teske AJ, Asselbergs FW, Backx A, et al. Genetics, clinical features, and long‐term outcome of noncompaction cardiomyopathy. J Am Coll Cardiol. 2018;71:711–722. [DOI] [PubMed] [Google Scholar]
- 26. Amzulescu MS, Rousseau MF, Ahn SA, Boileau L, de Meester de Ravenstein C, Vancraeynest D, Pasquet A, Vanoverschelde JL, Pouleur AC, Gerber BL. Prognostic impact of hypertrabeculation and noncompaction phenotype in dilated cardiomyopathy: a CMR study. JACC Cardiovasc Imaging. 2015;8:934–946. [DOI] [PubMed] [Google Scholar]
- 27. Aung N, Doimo S, Ricci F, Sanghvi MM, Pedrosa C, Woodbridge SP, Al‐Balah A, Zemrak F, Khanji MY, Munroe PB, et al. Prognostic significance of left ventricular noncompaction: systematic review and meta‐analysis of observational studies. Circ Cardiovasc Imaging. 2020;13:e009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Captur G, Zemrak F, Muthurangu V, Petersen SE, Li C, Bassett P, Kawel‐Boehm N, McKenna WJ, Elliott PM, Lima JA, et al. Fractal analysis of myocardial trabeculations in 2547 study participants: Multi‐Ethnic Study of Atherosclerosis. Radiology. 2015;277:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacquier A, Thuny F, Jop B, Giorgi R, Cohen F, Gaubert JY, Vidal V, Bartoli JM, Habib G, Moulin G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non‐compaction. Eur Heart J. 2010;31:1098–1104. [DOI] [PubMed] [Google Scholar]
- 30. Lofiego C, Biagini E, Pasquale F, Ferlito M, Rocchi G, Perugini E, Bacchi‐Reggiani L, Boriani G, Leone O, Caliskan K, et al. Wide spectrum of presentation and variable outcomes of isolated left ventricular non‐compaction. Heart. 2007;93:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Habib G, Charron P, Eicher JC, Giorgi R, Donal E, Laperche T, Boulmier D, Pascal C, Logeart D, Jondeau G, et al. Isolated left ventricular non‐compaction in adults: clinical and echocardiographic features in 105 patients. results from a French registry. Eur J Heart Fail. 2011;13:177–185. [DOI] [PubMed] [Google Scholar]
- 32. Stacey RB, Andersen MM, St Clair M, Hundley WG, Thohan V. Comparison of systolic and diastolic criteria for isolated LV noncompaction in CMR. JACC Cardiovasc Imaging. 2013;6:931–940. [DOI] [PubMed] [Google Scholar]
- 33. Dellegrottaglie S, Pedrotti P, Roghi A, Pedretti S, Chiariello M, Perrone‐Filardi P. Regional and global ventricular systolic function in isolated ventricular non‐compaction: pathophysiological insights from magnetic resonance imaging. Int J Cardiol. 2012;158:394–399. [DOI] [PubMed] [Google Scholar]
- 34. Gati S, Chandra N, Bennett RL, Reed M, Kervio G, Panoulas VF, Ghani S, Sheikh N, Zaidi A, Wilson M, et al. Increased left ventricular trabeculation in highly trained athletes: do we need more stringent criteria for the diagnosis of left ventricular non‐compaction in athletes? Heart. 2013;99:401–408. [DOI] [PubMed] [Google Scholar]
- 35. Gati S, Papadakis M, Papamichael ND, Zaidi A, Sheikh N, Reed M, Sharma R, Thilaganathan B, Sharma S. Reversible de novo left ventricular trabeculations in pregnant women: implications for the diagnosis of left ventricular noncompaction in low‐risk populations. Circulation. 2014;130:475–483. [DOI] [PubMed] [Google Scholar]
- 36. Gati S, Papadakis M, Van Niekerk N, Reed M, Yeghen T, Sharma S. Increased left ventricular trabeculation in individuals with sickle cell anaemia: physiology or pathology? Int J Cardiol. 2013;168:1658–1660. [DOI] [PubMed] [Google Scholar]
- 37. Zemrak F, Ahlman MA, Captur G, Mohiddin SA, Kawel‐Boehm N, Prince MR, Moon JC, Hundley WG, Lima JA, Bluemke DA, et al. The relationship of left ventricular trabeculation to ventricular function and structure over a 9.5‐year follow‐up: the MESA study. J Am Coll Cardiol. 2014;64:1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sedaghat‐Hamedani F, Haas J, Zhu F, Geier C, Kayvanpour E, Liss M, Lai A, Frese K, Pribe‐Wolferts R, Amr A, et al. Clinical genetics and outcome of left ventricular non‐compaction cardiomyopathy. Eur Heart J. 2017;38:3449–3460. [DOI] [PubMed] [Google Scholar]
- 39. Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long‐term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. [DOI] [PubMed] [Google Scholar]
- 40. Elliott PM, Gimeno JR, Thaman R, Shah J, Ward D, Dickie S, Tome Esteban MT, McKenna WJ. Historical trends in reported survival rates in patients with hypertrophic cardiomyopathy. Heart. 2006;92:785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roger VL, Weston SA, Redfield MM, Hellermann‐Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community‐based population. JAMA. 2004;292:344–350. [DOI] [PubMed] [Google Scholar]
- 42. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Kober L, et al. Declining risk of sudden death in heart failure. N Engl J Med. 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 43. Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long‐term follow‐up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36:493–500. [DOI] [PubMed] [Google Scholar]
- 44. Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8:1308–1339. [DOI] [PubMed] [Google Scholar]
- 45. van Waning JI, Caliskan K, Michels M, Schinkel AFL, Hirsch A, Dalinghaus M, Hoedemaekers YM, Wessels MW, IJpma AS, Hofstra RMW, et al. Cardiac phenotypes, genetics, and risks in familial noncompaction cardiomyopathy. J Am Coll Cardiol. 2019;73:1601–1611. [DOI] [PubMed] [Google Scholar]
- 46. Grigoratos C, Barison A, Ivanov A, Andreini D, Amzulescu MS, Mazurkiewicz L, De Luca A, Grzybowski J, Masci PG, Marczak M, et al. Meta‐analysis of the prognostic role of late gadolinium enhancement and global systolic impairment in left ventricular noncompaction. JACC Cardiovasc Imaging. 2019;12:2141–2151. [DOI] [PubMed] [Google Scholar]
- 47. Stollberger C, Gerecke B, Finsterer J, Engberding R. Refinement of echocardiographic criteria for left ventricular noncompaction. Int J Cardiol. 2013;165:463–467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Video S1
Video S2


