Abstract
BACKGROUND
Stable coronary artery disease is caused by a variable combination of organic coronary stenosis and functional coronary abnormalities, such as coronary artery spasm. Thus, we examined the clinical importance of comorbid significant coronary stenosis and coronary spasm.
METHODS AND RESULTS
We enrolled 236 consecutive patients with suspected angina who underwent acetylcholine provocation testing for coronary spasm and fractional flow reserve (FFR) measurement. Among them, 175 patients were diagnosed as having vasospastic angina (VSA), whereas the remaining 61 had no VSA (non‐VSA group). The patients with VSA were further divided into the following 3 groups based on angiography and FFR: no organic stenosis (≤50% luminal stenosis; VSA‐alone group, n=110), insignificant stenosis of FFR>0.80 (high‐FFR group, n=36), and significant stenosis of FFR≤0.80 (low‐FFR group, n=29). The incidence of major adverse cardiovascular events, including cardiovascular death, nonfatal myocardial infarction, urgent percutaneous coronary intervention, and hospitalization attributed to unstable angina was evaluated. All patients with VSA received calcium channel blockers, and 28 patients (95%) in the low‐FFR group underwent a planned percutaneous coronary intervention. During a median follow‐up period of 656 days, although the incidence of major adverse cardiovascular events was low and comparable among non‐VSA, VSA‐alone, and high‐FFR groups, the low‐FFR group had an extremely poor prognosis (non‐VSA group, 1.6%; VSA‐alone group, 3.6%; high‐FFR group, 5.6%; low‐FFR group, 27.6%) (P<0.001). Importantly, all 8 patients with major adverse cardiovascular events in the low‐FFR group were appropriately treated with percutaneous coronary intervention and calcium channel blockers.
CONCLUSIONS
These results indicate that patients with VSA with significant coronary stenosis represent a high‐risk population despite current guideline‐recommended therapies, suggesting the importance of routine coronary functional testing in this population.
Keywords: coronary artery disease, coronary atherosclerosis, coronary spasm, fractional flow reserve, percutaneous coronary intervention
Subject Categories: Coronary Artery Disease
Nonstandard Abbreviations and Acronyms
- CMD
coronary microvascular dysfunction
- DES
drug‐eluting stent
- MACE
major adverse cardiac event
- VSA
vasospastic angina
CLINICAL PERSPECTIVE
What Is New?
-
・
There were no serious complications for acetylcholine provocation testing even in patients with significant coronary stenosis of fractional flow reserve ≤0.80.
-
・
When appropriately treated with calcium channel blockers, the long‐term prognosis of patients with vasospastic angina (VSA) and insignificant organic coronary stenosis of fractional flow reserve >0.80 was good and comparable with patients with VSA without organic coronary stenosis or patients with non‐VSA.
-
・
Despite complete revascularization with percutaneous coronary intervention and optimal medical therapies with calcium channel blockers, patients with VSA with significant coronary stenosis had an extremely poor prognosis.
What Are the Clinical Implications?
-
・
Acetylcholine provocation testing for coronary spasm can be safely performed even in patients with significant coronary stenosis and suggest the importance of routine coronary functional testing in this population.
-
・
Patients with VSA with significant organic coronary stenosis of fractional flow reserve ≤0.80 represent a high‐risk population despite current guideline‐recommended therapies with fractional flow reserve–guided percutaneous coronary intervention and optimal medical therapies with calcium channel blockers, suggesting the importance of routine coronary functional testing in this population.
Stable coronary artery disease (CAD) is caused by a variable combination of organic coronary stenosis and functional coronary abnormalities, such as coronary artery spasm and coronary microvascular dysfunction (CMD). 1 , 2 , 3 , 4 , 5 , 6 To the surprise of the world, the recent ISCHEMIA Trial (international study of comparative health effectiveness with medical and invasive approaches) demonstrated that as compared with optimal medical therapy alone, additional coronary revascularization therapies, such as percutaneous coronary intervention (PCI) or coronary artery bypass grafting, have no prognostic benefit in patients with stable CAD and moderate to severe ischemia, 7 making us hypothesize whether the presence of inherent coronary functional abnormalities may potentially influence the outcome. This may be because coronary artery spasm is considered to play important roles in the pathogenesis of a wide range of ischemic heart disease. 6 , 8 Indeed, patients with vasospastic angina (VSA) frequently have a various degree of organic coronary stenosis, 9 , 10 and coronary spasm at angiographically significant coronary stenosis is an independent adverse prognostic factor of patients with VSA. 10
Fractional flow reserve (FFR) is an epicardial lesion‐specific parameter for organic coronary stenosis and is widely used for coronary revascularization. 11 Thus, the current practice guidelines strongly recommend the use of FFR measurement for therapeutic strategy in patients with organic coronary stenosis. 12 , 13 However, FFR provides no information on coronary functional abnormalities. Furthermore, provocation testing for coronary spasm, which had been demonstrated to be safe and useful for the diagnosis of VSA, 14 , 15 is not usually performed in patients with obstructive CAD even in Japan. Thus, not only the incidence and clinical importance of comorbid coronary functional abnormalities but also safety of spasm provocation in this population remain unclear. In the present study, we thus examined the importance of functional coronary abnormalities in patients with stable CAD using FFR and acetylcholine provocation testing.
METHODS
The present study was conducted following the ethics principles in the Declaration of Helsinki and approved by the Ethics Committees of Tohoku University (No. 2018‐1‐826). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Subjects
In the present study, we retrospectively evaluated a total of 299 consecutive patients with acetylcholine provocation testing for coronary artery spasm for rest angina and/or marked diurnal variation in symptom onset or exercise tolerance, regardless of the presence or absence of organic coronary stenosis, from November 2014 to July 2017. We defined organic coronary stenosis as luminal narrowing >50% by coronary angiography. When coronary stenosis remained angiographically in response to intracoronary nitrate administration after acetylcholine provocation testing, functional severity of the stenosis was assessed by FFR measurement. After excluding 63 patients with proven cardiomyopathy, end‐stage renal disease on hemodialysis, in‐stent restenotic lesions in a major coronary artery, history of PCI within 3 months, or incomplete acetylcholine provocation testing or measurement of FFR or lost to follow‐up, we finally enrolled 236 consecutive patients in a retrospective manner (Figure 1). Among them, based on the results of acetylcholine provocation testing, 175 patients (74%) were diagnosed as having VSA, whereas patients without VSA were regarded as controls (non‐VSA group, n=61). We further divided the patients with VSA into the following 3 groups based on angiographical findings and FFR values: patients with VSA and no organic coronary stenosis (≤50% luminal stenosis on coronary angiography; VSA‐alone group, n=110), insignificant organic coronary stenosis (>50% luminal stenosis on coronary angiography and high FFR>0.80; high‐FFR group, n=36), and significant organic coronary stenosis (>50% luminal stenosis on coronary angiography and low‐FFR≤0.80; low‐FFR group, n=29) (Figure 1). After diagnosis, all patients with VSA were treated with optical medical therapy including calcium channel blockers (CCBs). Furthermore, for those with significant organic coronary stenosis of FFR≤0.80, we performed planned PCIs after the initiation of dual antiplatelet therapy with aspirin and a P2Y12 receptor inhibitor (Figure 1). Because patient information was collected anonymously, institutional review boards waived the need for individual informed consent.
Figure 1. Study flow chart.
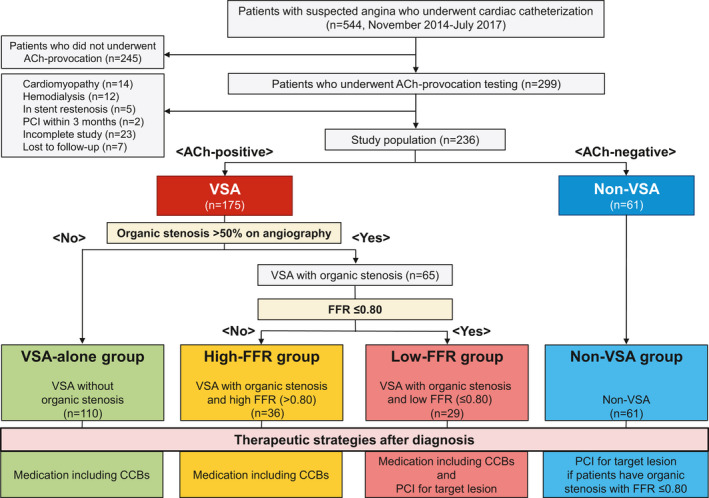
Patients were classified into 4 groups based on the result of acetylcholine provocation testing, presence or absence of organic stenosis, and FFR value. CCBs indicates calcium channel blockers; FFR, fractional flow reserve; PCI, percutaneous coronary intervention; and VSA, vasospastic angina.
Acetylcholine Provocation Testing and FFR Measurement
Acetylcholine provocation testing was performed based on the Guidelines by the Japanese Circulation Society. 16 Briefly, acetylcholine was administered into the coronary artery in a cumulative manner with continuous monitoring of arterial pressure and 12‐lead ECG and serial coronary angiograms at 1‐minute intervals. CCBs, long‐acting nitrates, and nicorandil were discontinued at least 48 hours before the provocation testing. We first performed acetylcholine provocation testing for the left coronary artery in a cumulative manner (20, 50, and 100 µg). If the test for the left coronary artery was negative or acetylcholine‐induced spasm in the left coronary artery resolved spontaneously, we then injected acetylcholine into the right coronary artery in a cumulative manner (20 and 50 µg). When coronary spasm was induced, 5 mg of isosorbide dinitrate was injected into the responsible coronary artery. The positive response for coronary spasm was defined as the development of >90% narrowing accompanied by chest pain and ischemic ECG changes. 16
Measurements of FFR were performed as previously described. 17 A catheter without side holes and a pressure sensor–tipped guidewire (Certus Pressure Wire, Abbott Laboratories, North Chicago, IL) were used. After intracoronary administration of isosorbide dinitrate (5 mg) following acetylcholine provocation testing, the sensor was positioned at a site that was as far as possible in the target vessel. Hyperemia was induced by intravenous infusion of adenosine (140 μg/kg/min) via a peripheral vein.
End Points and Follow‐Up
Primary end point was defined as major adverse cardiac event (MACE), including cardiovascular death, nonfatal myocardial infarction (MI), urgent PCI, or hospitalization for unstable angina. Cardiovascular death was defined as any death attributable to cardiac origin (eg, acute coronary syndrome, decompensated heart failure, fatal arrhythmia), unwitnessed death, or sudden death of unknown cause. 11 MI was defined as an increase in cardiac biomarkers with supporting evidence of acute myocardial ischemia such as symptoms or ECG changes, but PCI‐related MI was excluded. 18 Urgent PCI was defined as unscheduled and performed as a result of exacerbation of angina or myocardial ischemia. 11 Secondary end point was defined as a composite of cardiovascular death and nonfatal MI. Long‐term follow‐up was performed using a questionnaire that was sent to patients and primary physicians in addition to the information available in the medical records.
Statistical Analysis
Continuous variables were expressed as mean±SD or median with interquartile range as appropriate and were compared by the Welch t test. Categorical variables were expressed as numeral with percentage and were compared by the Fisher exact test. The standardized mean difference d for continuous variables and the φ statistic for categorical variables were calculated as measures of the effect size index. A value of 0.3 to 0.5 of the effect size index would be interpreted as “medium” size. 19 Survival rate from cardiac events was analyzed by Kaplan–Meier method with log‐rank tests. Comparison between groups was performed by Cox proportional hazard model and hazard ratio (HR) and 95% CI were calculated. To examine the determinants of cardiac events, we used univariable and multivariable Cox proportional hazard models and calculated HR and 95% CI by using a stepwise variable selection procedure. When performing subgroup analysis, the interaction between VSA with low‐FFR and predefined clinical subgroups in their effects on cardiac event was assessed by the Cox model with interaction terms. A P value of <0.05 and a P value for interaction of <0.10 were considered to be statistically significant. All statistical analysis was performed using IBM SPSS Statistics 26.0 (IBM, Armonk, NY) and R software (version 3.0.3; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics
Baseline patient characteristics are summarized in Table 1. Overall average age of patients was 63.1±12.0 years. When dividing them into 4 groups according to coronary angiographic findings and FFR values, the high‐FFR group was characterized by older age and the low‐FFR group by higher prevalence of men. VSA‐alone group had symptoms at rest more frequently, whereas patients with VSA with organic coronary stenosis (high‐FFR and low‐FFR groups) had higher prevalences of diabetes mellitus, dyslipidemia, and previous PCI as compared with those of in the VSA‐alone and non‐VSA groups. Laboratory data, such as serum levels of creatinine, low‐density lipoprotein cholesterol, troponin‐T, and B‐type natriuretic peptide, and echocardiographic left ventricular ejection fraction were comparable among the 4 groups. Angiographic characteristics including spasm types and sites were comparable among VSA‐alone, high‐FFR, and low‐FFR groups. Organic coronary stenosis was mainly noted at the left ascending artery, whereas multivessel organic stenoses were more commonly noted in the low‐FFR group. SYNTAX (synergy between PCI with taxus and cardiac surgery) score was not so high and comparable between the high‐FFR and low‐FFR groups. Coronary spasm at the site of organic stenosis was documented in 72% of high‐FFR and 83% of low‐FFR patients. In the non‐VSA group, 14 patients (23%) had organic coronary stenosis (FFR>0.80 in 10 and FFR≤0.80 in 4). Importantly, no serious complications related to acetylcholine provocation testing (eg, MI, sustained cardiogenic shock or death) were noted in patients with VSA with organic coronary stenosis.
Table 1.
Baseline and Angiographic Characteristics
| Overall (n=236) | VSA Alone (n=110) | High FFR (n=36) | Low‐FFR (n=29) | Non‐VSA (n=61) | |
|---|---|---|---|---|---|
| Age, y | 63.1±12.0 | 62.0±11.5 | 67.5±8.1 | 62.9±11.2 | 62.7±14.7 |
| Male | 148 (62.7) | 63 (57.3) | 24 (66.7) | 24 (82.8) | 37 (60.7) |
| Hypertension | 133 (56.4) | 55 (50.0) | 27 (75.0) | 16 (55.2) | 35 (57.4) |
| Diabetes mellitus | 63 (26.7) | 23 (20.9) | 16 (44.4) | 10 (34.5) | 14 (23.0) |
| Dyslipidemia | 103 (43.6) | 43 (39.1) | 18 (50.0) | 19 (65.5) | 23 (37.7) |
| Current smoking | 70 (29.7) | 32 (29.1) | 8 (22.2) | 12 (41.4) | 18 (29.5) |
| Chronic kidney disease | 15 (6.4) | 8 (7.3) | 1 (2.8) | 3 (10.3) | 3 (4.9) |
| Previous MI | 19 (8.1) | 8 (7.3) | 6 (16.7) | 3 (10.3) | 2 (3.3) |
| Previous PCI | 24 (10.2) | 4 (3.6) | 10 (27.8) | 4 (13.8) | 6 (9.8) |
| Atrial fibrillation | 12.7 (30) | 12 (10.9) | 7 (19.4) | 4 (13.8) | 7 (11.5) |
| Clinical status of angina attack | |||||
| Effort angina | 72 (30.5) | 27 (24.5) | 15 (41.7) | 11 (37.9) | 19 (31.1) |
| Rest angina | 153 (64.8) | 83 (75.5) | 21 (58.3) | 16 (55.2) | 33 (54.1) |
| Effort and rest angina | 22 (9.4) | 12 (10.9) | 5 (13.9) | 2 (7.1) | 3 (4.9) |
| Laboratory data | |||||
| Creatinine, mg/dL | 0.79±0.20 | 0.78±0.20 | 0.81±0.18 | 0.86±0.19 | 0.76±0.21 |
| LDL cholesterol, mg/dL | 105.9±30.3 | 107.5±29.4 | 104.8±29.6 | 102.4±32.0 | 102.4±32.0 |
| HDL cholesterol, mg/dL | 55.8±18.8 | 57.3±19.8 | 54.7±13.1 | 51.0±17.4 | 56.1±20.4 |
| Triglyceride, mg/dL | 146.0±97.5 | 149.7±95.0 | 155.3±104.1 | 156.6±85.2 | 128.5±103.5 |
| HbA1C, % | 6.1±0.9 | 6.0±1.0 | 6.1±0.5 | 6.3±0.9 | 6.0±0.9 |
| BNP, pg/mL | 20.5 (9.6–43.9) | 17.7 (7.9–32.2) | 20.2 (13.4–57.0) | 20.7 (10.3–70.5) | 26.7 (11.3–44.4) |
| Troponin T ng/mL | 0.007 (0.005–0.011) | 0.006 (0.004–0.009) | 0.008 (0.005–0.011) | 0.007 (0.005–0.021) | 0.007 (0.004–0.013) |
| LVEF, % | 65.6±10.7 | 66.9±8.8 | 66.7±8.3 | 58.9±17.4 | 65.8±10.30 |
| Angiographical characteristics | |||||
| Organic stenosis | 79 (33.1) | 0 (0) | 36 (100) | 29 (100) | 14 (23.0) |
| Organic stenosis of FFR≤0.80 | 33 (14.0) | 0 (0) | 0 (0) | 29 (100) | 4 (6.6) |
| FFR at organic stenosis | 0.80±0.10 | … | 0.87±0.05 | 0.69±0.07 | 0.84±0.08* |
| Spasm type | |||||
| Diffuse spasm | 124 (70.9) | 83 (75.5) | 23 (63.9) | 18 (62.1) | … |
| Focal spasm | 27 (15.4) | 13 (11.8) | 8 (22.2) | 6 (20.7) | … |
| Mixed spasm | 24 (13.7) | 14 (12.7) | 5 (13.9) | 5 (17.2) | … |
| Spasm site | |||||
| LAD | 159 (90.9) | 100 (90.9) | 30 (83.3) | 29 (100) | … |
| LCX | 67 (38.3) | 39 (35.5) | 17 (47.2) | 11 (37.9) | … |
| RCA | 52 (29.7) | 39 (35.5) | 12 (33.3) | 7 (24.1) | … |
| Multivessel | 90 (51.4) | 54 (49.1) | 18 (50.0) | 18 (62.1) | … |
| Organic stenotic site | |||||
| LAD | 65 (82.3) | … | 26 (72.2) | 26 (89.7) | 13 (92.9)* |
| LCX | 18 (22.8) | … | 7 (19.4) | 9 (31.0) | 2 (14.3)* |
| RCA | 8 (10.1) | … | 5 (13.9) | 2 (6.9) | 1 (7.1)* |
| Multivessel | 14 (17.7) | … | 3 (8.3) | 9 (31.0) | 2 (14.3)* |
| Spasm at organic stenosis | 50 (76.9) | … | 26 (72.2) | 24 (82.8) | … |
| SYNTAX score | 6.3±5.4 | … | 4.5±4.6 | 9.6±5.8 | 4.1±3.2 |
Values are expressed as mean±SD, median with interquartile range, or number (percentage). Chronic kidney disease was defined as an estimated glomerular filtration rate <60 mL/min per 1.73 m2. BNP indicates B‐type natriuretic peptide; FFR, fractional flow reserve; HDL, high‐density lipoprotein; LAD, left ascending artery; LCX, left circumflex artery; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; SYNTAX, synergy between PCI with taxus and cardiac surgery; and VSA, vasospastic angina.
Percentage of organic stenotic site, FFR value, and SYNTAX score are those in 14 patients with non‐VSA and organic stenosis in the non‐VSA group.
Treatments After Diagnosis of VSA and Long‐term Prognosis
Although all patients with VSA received CCBs after the diagnosis, those in the low‐FFR group more frequently received angiotensin‐converting enzyme inhibitors (ACEI), statins, and aspirin (Table 2). β‐blockers were more frequently used for patients with VSA and organic coronary stenosis (high‐FFR and low‐FFR groups) compared with those without it (VSA‐alone and non‐VSA groups). At a median of 20 days after acetylcholine provocation testing, 28 patients (95%) in the low‐FFR group successfully underwent planned PCI with coronary stents (new generation drug‐eluting stent [DES] in 27, bare‐metal stent in 1 case), whereas no patient underwent PCI in the VSA‐alone or high‐FFR groups. Among the patients in the non‐VSA group, 4 patients (7%) with significant organic stenosis and FFR≤0.80 also underwent PCI. During a median follow‐up period of 656 days, MACE occurred in 15 patients (cardiovascular death, 5; nonfatal MI, 1; urgent PCI, 3; unstable angina, 6) (Table 3). The Kaplan–Meier survival curves showed that the low‐FFR group had worse event‐free survival rates from MACE compared with the other 3 groups (Figure 2A) and also a worse composite of cardiovascular death and nonfatal MI compared with the VSA‐alone group (Figure 2B). Furthermore, a breakdown of 8 MACEs developed in the low‐FFR group during the follow‐up period was comparable (cardiovascular death, 2; nonfatal MI, 1; urgent PCI, 3; unstable angina, 2) (Table 3). In contrast, in the high‐FFR and non‐VSA groups, the incidences of primary and secondary end points were comparable with those in the VSA‐alone group (Figure 2A and 2B). All 14 non‐VSA patients with organic stenosis (FFR>0.80 in 10 and FFR≤0.80 in 4) had no MACE during follow‐up (Figure S1). Furthermore, multivariable Cox regression analysis identified current smoking (adjusted HR, 3.25; 95% CI, 1.04–10.11) and low‐FFR group (patients with VSA with significant coronary stenosis of FFR≤0.80) (adjusted HR, 3.94; 95% CI, 1.14–13.59) as significant predictors for MACE in this cohort (Table 4). When performing the subgroup analysis stratified by age, sex, diabetes mellitus, current smoking, previous MI, and left ventricular ejection fraction, low‐FFR group was consistently correlated with the poor prognosis (Figure 3). Table 5 compares the clinical profiles of the patients in the low‐FFR group according to the presence or absence of subsequent development of MACE. Importantly, all 8 patients with MACE in the low‐FFR group underwent successful complete revascularization by scheduled PCI for the target lesion and received CCBs for the prevention of coronary spasm (Table 5), indicating that they represent a high‐risk population despite guideline‐recommended conventional therapies. In contrast, in the low‐FFR group, a significant difference in the prescription rate of ACEIs was noted between the patients with and those without MACE (0% versus 48%, P=0.02), whereas that of other medications, including angiotensin II receptor blockers, β‐blockers, statins, nitrates, and nicorandil, were comparable. Regarding PCI procedural characteristics, patients with MACE in the low‐FFR group tended to undergo multivessel PCI more frequently compared with those without MACE (38% versus 5%, P=0.052), whereas stent diameter, stent length, and number of stents were comparable between the 2 groups.
Table 2.
Treatment After Acetylcholine Provocation Testing
| VSA Alone (n=110) | High FFR (n=36) | Low‐FFR (n=29) | Non‐VSA (n=61) | |
|---|---|---|---|---|
| Medication | ||||
| CCB | 110 (100) | 36 (100) | 29 (100) | 45 (73.8) |
| ACEI | 13 (11.8) | 4 (11.1) | 10 (34.5) | 7 (11.5) |
| ARB | 28 (25.5) | 16 (44.4) | 9 (31.0) | 21 (34.4) |
| β‐blocker | 23 (20.9) | 15 (41.7) | 12 (41.4) | 17 (27.9) |
| Statin | 43 (33.9) | 25 (69.4) | 27 (93.1) | 32 (52.5) |
| Nitrate | 17 (15.5) | 6 (16.7) | 6 (20.7) | 5 (8.2) |
| Nicorandil | 17 (15.5) | 8 (22.2) | 3 (10.3) | 2 (3.3) |
| Aspirin | 19 (20.2) | 25 (69.4) | 29 (100) | 21 (34.4) |
| Diuretics | 9 (8.2) | 5 (13.9) | 5 (17.2) | 4 (6.6) |
| PCI | 0 (0) | 0 (0) | 28 (95.2) | 4 (6.6) |
| DES | … | … | 27 (93.1) | 4 (6.6) |
| BMS | … | … | 1 (3.6) | 0 (0) |
| Multivessel lesion | … | … | 4 (14.3) | 1 (1.6) |
| Stent diameter (mm) | … | … | 3.0±0.4 | 3.3±0.3 |
| Stent length (mm) | … | … | 31.6±13.9 | 24.8±7.0 |
| Number of stents | … | … | 1.2±0.4 | 1.0±0.0 |
| Interval from acetylcholine provocation test to PCI, days | … | … | 20 (4–45) | 6 (1–7) |
Values are expressed as mean±SD, median with interquartile range, or number (percentage). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMS, bare‐metal stent; CCB, calcium channel blocker; DES, drug‐eluting stent; FFR, fractional flow reserve; PCI, percutaneous coronary intervention; and VSA, vasospastic angina.
Table 3.
Incidence of Major Adverse Cardiac Events in Study Population
| VSA Alone (n=110) | High FFR (n=36) | Low‐FFR (n=29) | Non‐VSA (n=61) | P Value | |
|---|---|---|---|---|---|
| MACE | 4 (3.6) | 2 (5.6) | 8 (27.6) | 1 (1.6) | <0.01 |
| Cardiovascular death or nonfatal MI | 1 (0.9) | 1 (2.8) | 3 (10.3) | 1 (1.6) | 0.047 |
| Cardiovascular death | 1 (0.9) | 1 (2.8) | 2 (6.9) | 1 (1.6) | 0.15 |
| Nonfatal MI | 0 (0) | 0 (0) | 1 (3.4) | 0 (0) | 0.13 |
| Target vessel for PCI | 0 | 0 | 1 | 0 | |
| Nontarget vessel for PCI | 0 | 0 | 0 | 0 | |
| Urgent PCI | 0 (0) | 0 (0) | 3 (10.3) | 0 (0) | <0.01 |
| Target vessel for PCI | 0 | 0 | 1 | 0 | |
| Nontarget vessel for PCI | 0 | 0 | 2 | 0 | |
| UAP | 3 (2.7) | 1 (2.8) | 2 (6.9) | 0 (0) | 0.20 |
Values are expressed as number (percentage). MACE was defined a composite of cardiovascular death, nonfatal MI, urgent PCI, or UAP. UAP was defined hospitalization for unstable angina pectoris. FFR indicates fractional flow reserve; MACE, major adverse cardiac event; MI, myocardial infarction; PCI, percutaneous coronary intervention; UAP, unstable angina pectoris; and VSA, vasospastic angina.
Figure 2. Cardiac event‐free survival during follow‐up.
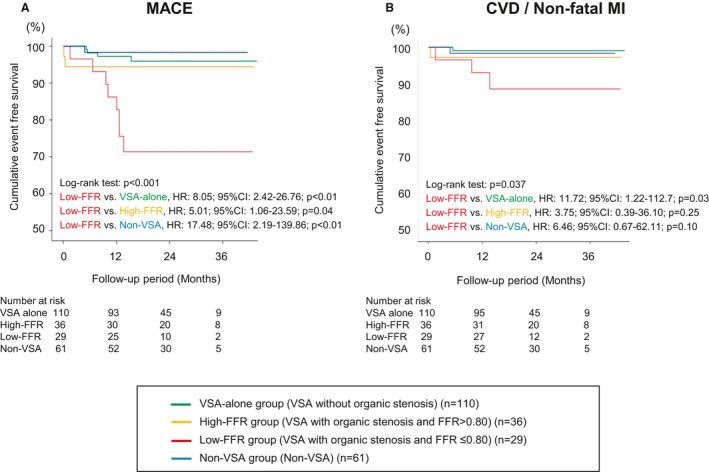
The Kaplan–Meier survival curves showed that the low‐FFR group had worse event‐free survival rates from MACE compared with other 3 groups (A) and also a worse composite of cardiovascular death and nonfatal MI compared with the VSA‐alone group (B). FFR indicates fractional flow reserve; HR, hazard ratio; MACE, major adverse cardiac event; MI, myocardial infarction; and VSA, vasospastic angina.
Table 4.
Factors Correlating with Major Adverse Cardiac Events During Follow‐Up
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Unadjusted HR | 95%CI | P Value | Adjusted HR | 95%CI | P Value | |
| Age >75 y | 0.47 | 0.06 to 3.55 | 0.46 | |||
| Male | 8.82 | 1.16 to 67.11 | 0.04 | |||
| Hypertension | 1.60 | 0.55 to 4.70 | 0.39 | |||
| Diabetes mellitus | 1.30 | 0.44 to 3.80 | 0.63 | |||
| Dyslipidemia | 1.88 | 0.67 to 5.29 | 0.23 | |||
| Current smoking | 5.01 | 1.71 to 14.67 | <0.01 | 3.25 | 1.04 to 10.11 | 0.04 |
| Chronic kidney disease | 2.27 | 0.51 to 10.06 | 0.28 | |||
| Atrial fibrillation | 0.51 | 0.07 to 3.87 | 0.51 | |||
| Previous MI | 1.69 | 0.38 to 7.51 | 0.49 | |||
| BNP >100 pg/mL | 2.54 | 0.72 to 9.01 | 0.15 | |||
| LVEF <50% | 0.82 | 0.11 to 0.63 | 0.85 | |||
| VSA | 4.96 | 0.65 to 37.74 | 0.12 | |||
| Multivessel spasm | 2.53 | 0.90 to 7.11 | 0.08 | |||
| Multivessel organic stenosis | 12.03 | 4.27 to 33.90 | <0.01 | 3.40 | 0.91 to 12.74 | 0.07 |
| Spasm at organic stenosis | 4.41 | 1.60 to 12.15 | <0.01 | |||
| Low‐FFR group | 8.53 | 3.09 to 23.6 | <0.01 | 3.94 | 1.14 to 13.59 | 0.03 |
BNP indicates B‐type natriuretic peptide; CI, confidence interval; FFR, fractional flow reserve; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; and VSA, vasospastic angina.
Figure 3. Subgroup analysis for MACE between low‐FFR group vs other 3 groups.
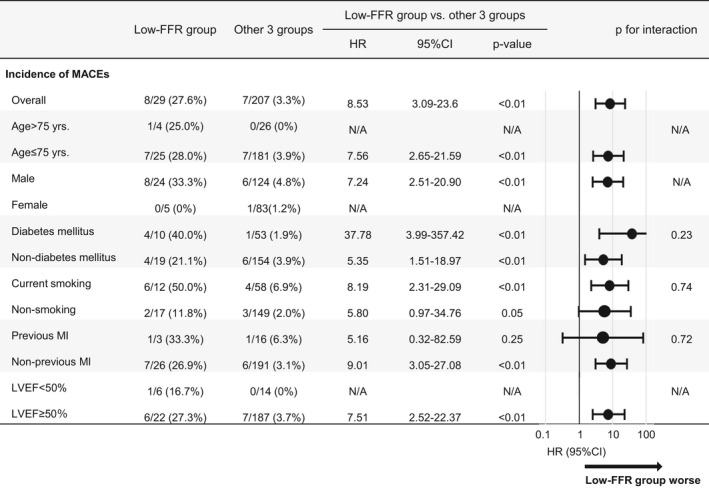
The subgroup analysis for MACE stratified by age, sex, diabetes mellitus, current smoking, previous MI, and LVEF showed consistently worse prognosis in the low‐FFR group compared with other 3 groups except for subgroups with patients aged >75 years, women, or LVEF<50%. FFR indicates fractional flow reserve; HR, hazard ratio; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac event; MI, myocardial infarction; and VSA, vasospastic angina.
Table 5.
Treatment and MACE
| Low‐FFR Group | Effect Size | P Value | Other 3 Groups | Effect Size | P Value | |||
|---|---|---|---|---|---|---|---|---|
| Patients With MACE (n=8) | Patients Without MACE (n=21) | Patients With MACE (n=7) |
Patients Without MACE (n=200) |
|||||
| Medication | ||||||||
| CCB | 8 (100) | 21 (100) | … | N/A | 7 (100) | 184 (96.3) | 0.05 | 0.57 |
| ACEI | 0 (0) | 10 (47.6) | 0.45 | 0.02 | 1 (14.3) | 23 (11.5) | 0.02 | 0.58 |
| ARB | 4 (50.0) | 5 (23.8) | 0.25 | 0.18 | 3 (42.9) | 62 (31.0) | 0.05 | 0.38 |
| β‐blocker | 2 (25.0) | 10 (47.6) | 0.21 | 0.25 | 2 (28.6) | 53 (26.0) | 0.01 | 0.60 |
| Statin | 7 (87.5) | 20 (95.2) | 0.14 | 0.48 | 3 (42.9) | 97 (48.5) | 0.02 | 0.54 |
| Nitrate | 3 (37.5) | 3 (14.3) | 0.26 | 0.19 | 0 (0) | 28 (14.0) | 0.07 | 0.36 |
| Nicorandil | 2 (25.0) | 1 (4.8) | 0.30 | 0.18 | 1 (14.3) | 26 (13.0) | 0.01 | 0.63 |
| Aspirin | 8 (100) | 21 (100) | … | N/A | 2 (28.6) | 63 (31.5) | 0.01 | 0.62 |
| Diuretics | 1 (12.5) | 4 (19.0) | 0.08 | 0.58 | 0 (0) | 18 (9.0) | 0.06 | 0.52 |
| PCI | 8 (100) | 20 (95.2) | 0.12 | 0.72 | 0 (0) | 4 (2.0) | 0.03 | 0.87 |
| DES | 7 (87.5) | 20 (95.2) | 0.14 | 0.48 | … | 4 (2.0) | … | |
| BMS | 1 (12.5) | 0 (0) | 0.31 | 0.28 | … | 0 (0) | … | |
| Stent diameter (mm) | 3.0±0.5 | 3.0±0.4 | 0.04 | 0.94 | … | 3.3±0.3 | … | |
| Stent length (mm) | 35.9±17.6 | 29.9±12.3 | 0.43 | 0.40 | … | 24.8±7.0 | … | |
| Number of stents | 1.5±0.5 | 1.2±0.4 | 0.79 | 0.12 | … | 1.0±0.0 | ||
| Target lesion | … | |||||||
| LAD | 8 (100) | 17 (81.0) | 0.25 | 0.55 | … | 4 (2.0) | … | |
| LCX | 3 (37.5) | 3 (14.3) | 0.26 | 0.30 | … | 0 (0) | … | |
| RCA | 0 (0) | 1 (4.8) | 0.12 | 0.72 | … | 1 (0.5) | … | |
| Multivessel | 3 (37.5) | 1 (4.8) | 0.42 | 0.052 | … | 1 (0.5) | … | |
| Interval from acetylcholine provocation testing to PCI (days) | 8 (1–27) | 26 (7–49) | 0.44 | 0.11 | … | 6 (1–7) | … | |
| SYNTAX score | 13.0±7.1 | 8.3±4.7 | 0.81 | 0.12 | … | 7.0±1.4 | … | |
| FFR | 0.66±0.07 | 0.71±0.07 | 0.73 | 0.08 | … | 0.74±0.03 | … | |
Values are expressed as mean±SD, median with interquartile range, or number (percentage).
PCI procedural data in other 3 groups are those in 4 patients who underwent PCI in the non‐VSA group. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMS, bare‐metal stent; CCB, calcium channel blocker; DES, drug‐eluting stent; FFR, fractional flow reserve; LAD, left ascending artery; LCX, left circumflex artery; MACE, major adverse cardiac event; N/A, not applicable; PCI, percutaneous coronary intervention; and RCA, right coronary artery; and SYNTAX, synergy between PCI with taxus and cardiac surgery.
DISCUSSION
The major findings of the present study were as follows: (1) acetylcholine provocation testing for coronary artery spasm can be safely performed even in patients with significant coronary stenosis; (2) under appropriate treatment with CCBs, the prognosis of patients with VSA with hemodynamically insignificant coronary stenosis (FFR>0.80) was good and comparable with those without organic coronary stenosis or non‐VSA patients; and (3) despite complete revascularization with PCI and the use of CCBs, patients with VSA with hemodynamically significant coronary stenosis (FFR≤0.80) had extremely worse prognosis. To the best of our knowledge, this is the first study demonstrating the prognostic impacts of comorbid significant coronary stenosis and coronary spasm in patients with stable CAD and the importance of routine provocation testing for the spasm in this population.
Importance of Functional Coronary Abnormalities in Patients With Stable CAD
Stable CAD is caused by anatomical coronary abnormalities such as flow‐limiting epicardial coronary artery disease and functional coronary abnormalities including coronary artery spasm and CMD. 1 , 2 , 3 , 20 In a nationwide large‐scale registry of patients with suspected angina who underwent coronary angiography, obstructive coronary stenosis was noted in only 38%, 21 whereas a recent all‐comer population study with computed tomography coronary angiography showed that its prevalence was relatively low (29.9% in males, and 11.5% in females). 2 Recent studies also reported that among patients with angina without obstructive CAD, functional coronary abnormalities including coronary artery spasm and CMD were frequently noted (59%–89%). 3 , 4 , 5 , 22 Furthermore, recent studies have provided evidence that functional coronary abnormalities substantially overlap and may contribute to angina even in patients with obstructive epicardial CAD. 3 , 5 , 9 , 10 Dynamic changes in coronary vessel tone and propensity to vasoconstriction at the site of obstructive stenosis are important and may cause rest angina that is frequently overlooked in patients with obstructive CAD. 6 , 8 , 23
Stable CAD is considered to be caused by a variable combination of anatomical and functional coronary abnormalities. Importantly, the recent ISCHEMIA Trial demonstrated that additional coronary revascularization with PCI or coronary artery bypass grafting has no prognostic benefits in patients with stable CAD and moderate to severe ischemia, 7 indicating the importance of coronary functional abnormalities in this population. In particular, coronary artery spasm, which is frequently and equally noted in both White and Asian patients with angina‐like chest pain, 3 , 15 , 22 plays an important role in the pathogenesis of a wide range of ischemic heart disease. 6 , 8 Thus, a significant epicardial stenosis may reflect more extensive vascular dysfunction where PCI alone may be ineffective, as demonstrated in the ISCHEMIA Trial. 7 However, provocation testing for coronary spasm is not usually performed in patients with stable obstructive CAD even in Japan, and thus the incidence and importance of comorbid functional coronary abnormalities among them remain to be elucidated. In contrast, FFR measurement is widely performed to assess the physiological significance of coronary organic stenosis and determine the therapeutic strategy in patients with stable CAD, as recommended by the current practice guidelines. 12 , 13 However, persistence or recurrence of angina after PCI is well recognized and may affect about 20% to 40% of patients, even if FFR‐guided PCI is performed. 24 Ong et al 25 demonstrated that significant vasoconstriction of epicardial coronary arteries at or distal to the PCI site is a potential cause of recurrent angina. Thus, in the present study, we examined the prognostic impacts of coronary functional abnormalities from both coronary anatomy and functional aspects in patients with stable CAD using a combination of FFR and acetylcholine provocation testing.
In the present study, we were able to demonstrate that patients with VSA with hemodynamically significant organic stenosis (FFR≤0.80) had a very poor prognosis with occurrence of MACE, despite guideline‐recommended therapies, whereas those with hemodynamically insignificant organic coronary stenosis (FFR>0.80) had a favorable prognosis with medications including CCBs. Moreover, the multivariable regression analysis also confirmed the negative prognostic impacts of comorbid VSA and hemodynamically significant organic coronary stenosis. These results indicate the importance and usefulness of evaluation of coronary vasomotion abnormality using acetylcholine provocation testing to identify the high‐risk population that is resistant to the established treatment among patients with chest pain and angiographic organic stenosis. Thus, it might be considered to routinely perform acetylcholine provocation testing for coronary spasm even in patients with stable CAD.
Therapeutic Approach for Patients With Anatomical and Functional Coronary Abnormalities
In the JCS(Japanese Circulation Society) guidelines, PCI in combination with adequate coronary vasodilators for patients with VSA with severe organic coronary stenosis is recommended as class IIa. 16 Thus, we actively performed PCI for patients with coronary spasm and significant organic stenosis of FFR≤0.80. However, surprisingly, even after successful PCI and optimal medications including CCBs, they had an extremely high incidence of MACE after PCI during the follow‐up. Indeed, their composite event rate of cardiovascular death, nonfatal MI, and urgent PCI for 2 years after PCI was 27.6%, which was markedly higher than in patients treated with FFR‐guided PCI (8.1%) in the FAME‐2 Trial (fractional flow reserve versus angiography for multivessel evaluation). 11 Such poor prognosis of patients with VSA with low‐FFR (FFR≤0.80) may be caused by 2 mechanisms. First, as shown in our recent study, 26 high‐risk patients with VSA may have increased Rho‐kinase activity, which plays a key role in the pathogenesis of coronary spasm and progression/instability of coronary plaques. 8 , 27 We also have recently demonstrated that the combination of epicardial coronary spasm and CMD, both of which are caused by Rho‐kinase activation, is associated with worse prognosis compared with each mechanism alone. 3 Thus, the present study suggests that the accumulation of coronary anatomical and functional instabilities attributable to enhanced Rho‐kinase activity, which is unresolved by coronary recanalization with stent implantation, could worsen the prognosis of patients with stable CAD. Second, newly implanted coronary stents could exacerbate coronary functional disorders in patients with VSA with organic coronary stenosis. As we have previously demonstrated, DES implantation could cause coronary perivascular inflammation and enhance Rho‐kinase activity with resultant coronary hyperconstricting responses. 28 We also demonstrated that coronary spasm is associated with inflammation of coronary adventitia and perivascular adipose tissue through Rho‐kinase activation. 29 Furthermore, it was reported that even after successful PCI, DES itself could cause CMD. 30 Taken together, in patients with VSA, DES implantation could further enhance inflammation and Rho‐kinase activity at the stented coronary artery, resulting in atherosclerotic plaque instability and coronary circulatory dysregulation, including increased coronary microcirculatory resistance.
Importantly, in the non‐VSA group, all 4 patients who underwent PCI with DES had no MACE during follow‐up, suggesting the importance of inherent coronary functional abnormalities for long‐term prognosis after PCI with DES. Thus, we consider that unidentified coronary functional abnormalities may be involved in the pathogenesis of stable CAD as suggested in the ISCHEMIA Trial. 7 Moreover, we previously demonstrated that long‐acting nifedipine could ameliorate coronary vasomotion abnormalities after DES implantation. 31 , 32 Nevertheless, the present results indicate that the therapeutic approach with a combination of new generation DES and long‐acting CCBs is not sufficient to improve long‐term prognosis of patients with VSA with significant organic coronary stenosis of FFR≤0.80. The present study also suggests that ACEIs in addition to CCBs are beneficial for those high‐risk patients. It was previously reported that renin‐angiotensin system inhibitors, including ACEIs and angiotensin II receptor blockers, were associated with improved long‐term outcomes of patients with VSA. 33 Unlike angiotensin II receptor blockers, ACEIs are known to enhance accumulation of bradykinin, exerting a number of cardiovascular protective effects, inhibit Rho‐kinase pathway, and improve CMD. 34 , 35 , 36 , 37 Thus, it is conceivable that ACEIs are beneficial for the treatment of patients with VSA with obstructive coronary stenosis undergoing PCI with DES.
Study Limitations
Several limitations should be mentioned for the present study. First, the present study was a nonrandomized, single‐center, and retrospective study with a relatively small sample size. Notably, the number of non‐VSA patients with organic coronary stenosis was too small to compare clinical outcomes with patients with VSA with organic coronary stenosis. However, in the present study, because all patients with VSA received long‐acting CCBs and almost all patients underwent the cutting‐edge FFR‐guided PCI, we were able to precisely examine the long‐term prognosis of patients with VSA with organic coronary stenosis treated with the guideline‐recommended conventional therapies. Second, although CMD is important in patients with stable CAD, 38 , 39 , 40 we did not address this point in the present study. CMD and obstructive CAD can coexist and contribute, perhaps synergistically, to the development of myocardial ischemia. 40 However, no reliable approach is currently available to precisely assess the impact of downstream CMD on coronary microcirculation apart from epicardial organic stenosis. Thus, this important issue remains to be examined in future studies. Third, because the decision of performance of acetylcholine provocation testing was left to the discretion of individual experienced cardiologists in our institution, the selection bias could be involved. Fourth, in the present study, treatment strategies with PCI and medical treatments, including the use of ACEIs or β‐blockers, was also individualized at the discretion of each attending physician. Thus, there might be a selection bias involved that could affect the present results. Fifth, we have no data regarding the changes in medical therapy, adherence to the therapy, or symptom and/or quality of life during follow‐up. Sixth and finally, the precise mechanisms for the worse prognosis of patients with VSA with significant coronary stenosis even after successful PCI and optimal medical therapy remain to be elucidated. Further large‐scale studies are needed to establish an effective therapeutic approach for patients with coronary spasm and obstructive CAD, including the use of ACEIs in addition to CCBs and PCI.
CONCLUSIONS
In the present study, we were able to demonstrate that provocation testing for coronary spasm can be safely performed even in patients with significant coronary stenosis and that patients with VSA with hemodynamically significant coronary stenosis represent a high‐risk population despite current guideline‐recommended therapies, suggesting the importance of routine coronary functional testing.
Source of Funding
This study was supported in part by the grants‐in‐aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Disclosures
Dr. Yasuda has received grants support from Takeda and Abbott and lecture fees from Daiichi Sankyo and Bristol‐Myers Squibb. Dr. Shimokawa has received lecture fees from Bayer Yakuhin and Daiichi Sankyo. The remaining authors have no disclosures to report.
Supporting information
Figure S1
(J Am Heart Assoc. 2021;10:e017831 DOI: 10.1161/JAHA.120.017831.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017831.
For Sources of Funding and Disclosures, see page 12.
See Editorial by Kaski
References
- 1. Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, et al. Stratified medical therapy using invasive coronary function testing in angina: CorMicA Trial. J Am Coll Cardiol. 2018;72:2841–2855. [DOI] [PubMed] [Google Scholar]
- 2. Mangion K, Adamson PD, Williams MC, Hunter A, Pawade T, Shah ASV, Lewis S, Boon NA, Flather M, Forbes J, et al. Sex associations and computed tomography coronary angiography‐guided management in patients with stable chest pain. Eur Heart J. 2020;41:1337–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suda A, Takahashi J, Hao K, Kikuchi Y, Shindo T, Ikeda S, Sato K, Sugisawa J, Matsumoto Y, Miyata S, et al. Comprehensive evaluation of coronary functional abnormalities in patients with angina and non‐obstructive coronary artery disease. J Am Coll Cardiol. 2019;74:2350–2360. [DOI] [PubMed] [Google Scholar]
- 4. Aziz A, Hansen HS, Sechtem U, Prescott E, Ong P. Sex‐related differences in vasomotor function in patients with angina and unobstructed coronary arteries. J Am Coll Cardiol. 2017;70:2349–2358. [DOI] [PubMed] [Google Scholar]
- 5. Ford TJ, Yii E, Sidik N, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, et al. Ischemia and no obstructive coronary artery disease: prevalence and correlates of coronary vasomotion disorders. Circ Cardiovas Interv. 2019;12:e008126. DOI: 10.1161/CIRCINTERVENTIONS.119.008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ong P, Aziz A, Hansen HS, Prescott E, Athanasiadis A, Sechtem U. Structural and functional coronary artery abnormalities in patients with vasospastic angina pectoris. Circ J. 2015;79:1431–1438. [DOI] [PubMed] [Google Scholar]
- 7. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O'Brien SM, Boden WE, Chaitman BR, Senior R, Lopez‐Sendon J, Alexander KP, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shimokawa H. 2014 Williams Harvey Lecture: importance of coronary vasomotion abnormalities‐from bench to bedside. Eur Heart J. 2014;35:3180–3193. [DOI] [PubMed] [Google Scholar]
- 9. Takagi Y, Takahashi J, Yasuda S, Miyata S, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, et al. Prognostic stratification of patients with vasospastic angina: a comprehensive clinical risk score developed by the Japanese Coronary Spasm Association. J Am Coll Cardiol. 2013;62:1144–1153. [DOI] [PubMed] [Google Scholar]
- 10. Ishii M, Kaikita K, Sato K, Tanaka T, Sugamura K, Sakamoto K, Izumiya Y, Yamamoto E, Tsujita K, Yamamuro M, et al. Acetylcholine‐provoked coronary spasm at site of significant organic stenosis predicts poor prognosis in patients with coronary vasospastic angina. J Am Coll Cardiol. 2015;66:1105–1115. [DOI] [PubMed] [Google Scholar]
- 11. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius‐Winckler S, Rioufol G, Witt N, et al. Fractional flow reserve‐guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. [DOI] [PubMed] [Google Scholar]
- 12. Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212–2241. [DOI] [PubMed] [Google Scholar]
- 13. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 14. Takagi Y, Yasuda S, Takahashi J, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, et al. Clinical implications of provocation tests for coronary artery spasm: safety, arrhythmic complications, and prognostic impact: multicentre registry study of the Japanese Coronary Spasm Association. Eur Heart J. 2013;34:258–267. [DOI] [PubMed] [Google Scholar]
- 15. Ong P, Athanasiadis A, Borgulya G, Vokshi I, Bastiaenen R, Kubik S, Hill S, Schaufele T, Mahrholdt H, Kaski JC, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129:1723–1730. [DOI] [PubMed] [Google Scholar]
- 16. JCS joint working group . Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779–2801. [DOI] [PubMed] [Google Scholar]
- 17. Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary‐artery stenoses. N Engl J Med. 1996;334:1703–1708. [DOI] [PubMed] [Google Scholar]
- 18. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138:e618–e651. [DOI] [PubMed] [Google Scholar]
- 19. Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Routledge, New York: 1988. 10.4324/97802037715 [DOI] [Google Scholar]
- 20. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 21. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. New Engl J Med. 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol. 2012;59:655–662. [DOI] [PubMed] [Google Scholar]
- 23. Gould KL. Dynamic coronary stenosis. Am J Cardiol. 1980;45:286–292. [DOI] [PubMed] [Google Scholar]
- 24. Crea F, Bairey Merz CN, Beltrame JF, Berry C, Camici PG, Kaski JC, Ong P, Pepine CJ, Sechtem U, Shimokawa H. Mechanisms and diagnostic evaluation of persistent or recurrent angina following percutaneous coronary revascularization. Eur Heart J. 2019;40:2455–2462. [DOI] [PubMed] [Google Scholar]
- 25. Ong P, Athanasiadis A, Perne A, Mahrholdt H, Schaufele T, Hill S, Sechtem U. Coronary vasomotor abnormalities in patients with stable angina after successful stent implantation but without in‐stent restenosis. Clin Res Cardiol. 2014;103:11–19. [DOI] [PubMed] [Google Scholar]
- 26. Nihei T, Takahashi J, Hao K, Kikuchi Y, Odaka Y, Tsuburaya R, Nishimiya K, Matsumoto Y, Ito K, Miyata S, et al. Prognostic impacts of Rho‐kinase activity in circulating leucocytes in patients with vasospastic angina. Eur Heart J. 2018;39:952–959. [DOI] [PubMed] [Google Scholar]
- 27. Kandabashi T, Shimokawa H, Mukai Y, Matoba T, Kunihiro I, Morikawa K, Ito M, Takahashi S, Kaibuchi K, Takeshita A. Involvement of Rho‐kinase in agonists‐induced contractions of arteriosclerotic human arteries. Arterioscler Thromb Vasc Biol. 2002;22:243–248. [DOI] [PubMed] [Google Scholar]
- 28. Shiroto T, Yasuda S, Tsuburaya R, Ito Y, Takahashi J, Ito K, Ishibashi‐Ueda H, Shimokawa H. Role of Rho‐kinase in the pathogenesis of coronary hyperconstricting responses induced by drug‐eluting stents in pigs in vivo. J Am Coll Cardiol. 2009;54:2321–2329. [DOI] [PubMed] [Google Scholar]
- 29. Ohyama K, Matsumoto Y, Takanami K, Ota H, Nishimiya K, Sugisawa J, Tsuchiya S, Amamizu H, Uzuka H, Suda A, et al. Coronary adventitial and perivascular adipose tissue inflammation in patients with vasospastic angina. J Am Coll Cardiol. 2018;71:414–425. [DOI] [PubMed] [Google Scholar]
- 30. Hokimoto S, Tabata N, Yamanaga K, Sueta D, Akasaka T, Tsujita K, Sakamoto K, Yamamoto E, Yamamuro M, Izumiya Y, et al. Prevalence of coronary macro‐ and micro‐vascular dysfunctions after drug‐eluting stent implantation without in‐stent restenosis. Int J Cardiol. 2016;222:185–194. [DOI] [PubMed] [Google Scholar]
- 31. Tsuburaya R, Yasuda S, Shiroto T, Ito Y, Gao JY, Aizawa K, Kikuchi Y, Ito K, Takahashi J, Ishibashi‐Ueda H, et al. Long‐term treatment with nifedipine suppresses coronary hyperconstricting responses and inflammatory changes induced by paclitaxel‐eluting stent in pigs in vivo: possible involvement of Rho‐kinase pathway. Eur Heart J. 2012;33:791–799. [DOI] [PubMed] [Google Scholar]
- 32. Tsuburaya R, Takahashi J, Nakamura A, Nozaki E, Sugi M, Yamamoto Y, Hiramoto T, Horiguchi S, Inoue K, Goto T, et al. Beneficial effects of long‐acting nifedipine on coronary vasomotion abnormalities after drug‐eluting stent implantation: the NOVEL study. Eur Heart J. 2016;37:2713–2721. [DOI] [PubMed] [Google Scholar]
- 33. Choi BG, Jeon SY, Rha SW, Park SH, Shim MS, Choi SY, Byun JK, Li H, Choi JY, Park EJ, et al. Impact of renin‐angiotensin system inhibitors on long‐term clinical outcomes of patients with coronary artery spasm. J Am Heart Assoc. 2016;5:e003217. DOI: 10.1161/JAHA.116.003217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen JW, Hsu NW, Wu TC, Lin SJ, Chang MS. Long‐term angiotensin‐converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol. 2002;90:974–982. [DOI] [PubMed] [Google Scholar]
- 35. Witherow FN, Helmy A, Webb DJ, Fox KA, Newby DE. Bradykinin contributes to the vasodilator effects of chronic angiotensin‐converting enzyme inhibition in patients with heart failure. Circulation. 2001;104:2177–2181. [DOI] [PubMed] [Google Scholar]
- 36. Probstfield JL, O'Brien KD. Progression of cardiovascular damage: the role of renin‐angiotensin system blockade. Am J Cardiol. 2010;105:10A–20A. [DOI] [PubMed] [Google Scholar]
- 37. Brunova A, Bencze M, Behuliak M, Zicha J. Acute and chronic role of nitric oxide, renin‐angiotensin system and sympathetic nervous system in the modulation of calcium sensitization in Wistar rats. Physiol Res. 2015;64:447–457. [DOI] [PubMed] [Google Scholar]
- 38. van de Hoef TP, Bax M, Damman P, Delewi R, Hassell ME, Piek MA, Chamuleau SA, Voskuil M, van Eck‐Smit BL, Verberne HJ, et al. Impaired coronary autoregulation is associated with long‐term fatal events in patients with stable coronary artery disease. Circ Cardiovas Interv. 2013;6:329–335. [DOI] [PubMed] [Google Scholar]
- 39. Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL, Osborne MT, Seidelmann SB, Vita T, Bibbo CF, et al. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation. 2017;136:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


