Abstract
Background
Short ECG P‐wave duration has recently been demonstrated to be associated with higher risk of atrial fibrillation (AF). The aim of this study was to assess the rate of AF recurrence after pulmonary vein isolation in patients with a short P wave, and to mechanistically elucidate the observation by computer modeling.
Methods and Results
A total of 282 consecutive patients undergoing a first single‐pulmonary vein isolation procedure for paroxysmal or persistent AF were included. Computational models studied the effect of adenosine and sodium conductance on action potential duration and P‐wave duration (PWD). About 16% of the patients had a PWD of 110 ms or shorter (median PWD 126 ms, interquartile range, 115 ms–138 ms; range, 71 ms–180 ms). At Cox regression, PWD was significantly associated with AF recurrence (P=0.012). Patients with a PWD <110 ms (hazard ratio [HR], 2.20; 95% CI, 1.24–3.88; P=0.007) and patients with a PWD ≥140 (HR, 1.87, 95% CI, 1.06–3.30; P=0.031) had a nearly 2‐fold increase in risk with respect to the other group. In the computational model, adenosine yielded a significant reduction of action potential duration 90 (52%) and PWD (7%). An increased sodium conductance (up to 200%) was robustly accompanied by an increase in conduction velocity (26%), a reduction in action potential duration 90 (28%), and PWD (22%).
Conclusions
One out of 5 patients referred for pulmonary vein isolation has a short PWD which was associated with a higher rate of AF after the index procedure. Computer simulations suggest that shortening of atrial action potential duration leading to a faster atrial conduction may be the cause of this clinical observation.
Keywords: Atrial fibrillation, computer modelling, electrophysiology, P wave, pulmonary vein isolation
Subject Categories: Atrial Fibrillation, Electrophysiology
Nonstandard Abbreviations and Acronyms
- PVI
pulmonary vein isolation
- PWD
P‐wave duration
- AP
action potential
- APD
action potential duration
- CV
conduction velocity
- 3D
3‐dimensional
- CS
coronary sinus
Clinical Perspective
What Is New?
A short P‐wave duration is a marker of a higher rate of atrial fibrillation recurrences after pulmonary vein isolation procedure.
A short P wave was reproduced in computer cardiac simulation pointing to the presence, most likely, of a so‐far unknown condition that an increase in sodium channel conductance shortens the P‐wave duration.
What Are the Clinical Implications?
This condition might be associated with lower responsiveness to class I antiarrhythmic compounds for patients with paroxysmal and persistent atrial fibrillation but also for asymptomatic individuals who present the atrial phenotype.
A prolonged duration of P wave on the standard 12‐lead ECG is commonly used as a non‐invasive marker of abnormal atrial conduction time; 1 , 2 , 3 it is associated with increased risk of atrial fibrillation (AF) development. 1 , 2 , 3 , 4 , 5 , 6 In contrast to this well‐established pathophysiological relationship between P‐wave duration (PWD) and AF, a recent study conducted in a large primary care population indicated that also a PWD ≤105 ms is robustly associated with an increased risk of AF. 7 These results were consistent with past smaller case control studies. 8 , 9 Based on the results by Nielsen et al., one may hypothesize that increased atrial conduction velocity (CV) 10 , 11 is involved in the higher frequency of AF onset in a sizable group of patients (about 20%) who show a short P wave and subsequently develop AF. 7 An alternative explanation for the association between AF and a short P wave is a shortening of the atrial action potential duration (APD), which may also affect PWD. Conclusive evidence is, however, missing. Whether a short P wave is the result of increased CV, shortening of atrial refractory period, or a combination of both can nowadays be tested in sophisticated 3‐dimensional (3D) computational model. 12 , 13 Furthermore, a computational model may enable to identify causative ionic and cellular mechanisms possibly involved in the finding. The observation that a short P wave is associated with increased risk of AF may have major therapeutic implications when antiarrhythmic drug therapy is selected and, even more, when pulmonary vein isolation (PVI) is considered. The prevalence of patients undergoing PVI presenting with a short P wave, and whether the recurrence rate of AF after a single PVI procedure in these patients is as high as those with much prolonged PWD 14 , 15 is currently unknown.
We aimed to study the distribution of PWD in patients undergoing PVI, with particular emphasis on those presenting with a short P wave. Secondly, we tested the hypothesis that patients with a short PWD may have an increased recurrence rate after PVI. Finally, we aimed to reproduce in silico a short P wave, thus providing a mechanistic understanding of the clinical observation.
Methods
Study Population
All consecutive patients undergoing a first single‐PVI procedure (irrespective of the ablation technology used) for symptomatic paroxysmal or persistent AF at Fondazione Cardiocentro Ticino (Lugano, Switzerland), and at Vrije Universiteit Brussel (Universitair Ziekenhuis Brussel, Brussels, Belgium) between March 1, 2015 and June 30, 2018 were retrospectively included in this study. Patients with a history of paroxysmal or persistent AF being in AF at the time of the PVI procedure, permanent AF, aged <18 years, or those who did not consent to study participation were excluded.
Patient history including comorbidities and medication, PVI procedural data, and any events during follow‐up were prospectively collected. AF recurrence after a PVI procedure was defined as a documented arrhythmia of a duration >30 s after a blanking period of 3 months.
Each patient provided oral and written informed consent for the PVI procedure which was performed in general anesthesia. The study was approved by the internal review boards of each respective institution and by the local ethics committee. Because of the sensitive nature of the data collected for this study, request to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Dr. Angelo Auricchio at Cardiocentro Ticino, Lugano, Switzerland.
P‐Wave Duration Measurement
After placement of a steerable decapolar catheter in the coronary sinus (CS) (as deep as possible) and before trans‐septal puncture was performed, a 12‐lead standard ECG was recorded. PWD was manually measured after placement of the CS catheter on a simultaneous 12‐lead ECG using an electronic calliper of the electrophysiology recording system (Boston Scientific LabSystem PRO or CardioTek B.V., EP‐Tracer) at a sweep speed of 200 mm/s, a scale between 20 and 50 mm/mV, and a recording bandwidth at 0.05 Hz–150 Hz. PWD measurement was independently and blindly performed by 3 experienced electrophysiologists, and in case of disagreement the final PWD was adjudicated. The PWD was defined as the duration from the earliest deflection to the latest deflection in any lead (Figure 1). To precisely detect the end of a P wave, the atrial deflection of the most distal bipolar electrodes of the steerable decapolar catheter inserted into the CS was taken as reference in all cases (Figure 1).
Figure 1. Electroanatomical mapping of a left atrial in a patient with a short P wave (86 ms) in anterio‐posterior, postero‐anterior, and left anterior oblique view.
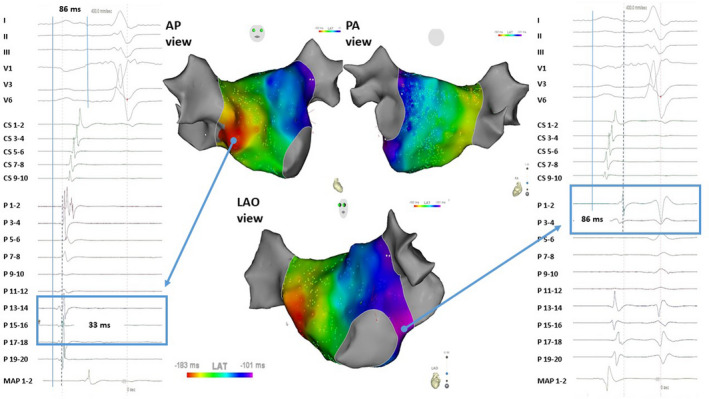
The earliest left atrial breakthrough occurred in the mid septum 33 ms after P wave onset as determined on surface ECG; the local electrogram and activation is visible on the catheter mapping (Pentaray – P 15‐16) of the right panel. Latest left atrial activation is on the lateral side of the left atrium close to the mitral valve ring; the local electrogram and activation is visible on the catheter mapping (Pentaray – P 1‐12) of the left panel. AP indicates antero posterior view; LAO, left anterior oblique view; MAP 1‐2, distal electrode pair of the ablation catheter; P, Pentaray mapping; PA, postero anterior view.
Furthermore, in those patients in whom ablation procedure was assisted by an ultra‐density mapping system (Rhythmia HDx mapping system and IntellaMap ORION Mapping Catheter, Boston Scientific, Cambridge, MA, USA) or by a conventional 3D electroanatomical mapping system (Carto 3 mapping system and Pentaray mapping catheter, Biosense Webster, Diamond Bar, CA), a complete left atrial endocardial mapping was obtained during sinus rhythm. Bipolar electrograms were recorded via the mapping catheter. Caution was taken to avoid displacement of the reference electrode within the coronary sinus during the entire procedure. An effort was made to acquire complete endocardial surface of left atrium using a pre‐procedural left atrial anatomy acquired by computed tomography or a cardiac magnetic resonance imaging. A bipolar atrial electrogram from the CS with maximum slope was taken as the time reference to calculate local activation time, which was defined as the time point of the maximal slope on bipolar electrograms. Visual inspection and, if necessary, manual correction of the local activation time at each point were performed. Recordings of premature beats were excluded. Color‐coded, 3D activation sequence maps were reconstructed, with the red color identifying the earliest activation area and the purple the latest. (Figure 1).
The P wave was a priori categorized in 3 groups, based upon clinical cut‐off points: <110 ms, 110 to 139 ms, and finally ≥140 ms.
Adenosine Pharmacological Testing
To evaluate the change in PWD by adenosine, a bolus of the drug was administered before PVI (to avoid the confounding effect by PVI, i.e., change of left atrial mass and propagation) in all patients undergoing PVI at one institution (Cardiocentro Ticino) starting on April 2018 as part of a clinical protocol. Adenosine was administered at increasing dosage (starting at 6 mg to a maximum dose of 18 mg) until complete atrioventricular block was noticed or significant prolongation of the PR interval was achieved. Patients who refused to be part of the clinical protocol, in whom frequent atrial premature beats or atrial fibrillation were triggered by adenosine or presenting with atrial fibrillation at resting or with noise on ECG were excluded. The ECG of the remaining 12 patients (out of 35 patients), in which a clear P wave during complete atrioventricular block was visible, was considered.
In‐Silico Action Potential Propagation Simulation
A 1‐dimensional (1D), 5‐cm long fiber strand was considered for simulating atrial action potential (AP) propagation and refractory period. Simulations were performed with the monodomain model, coupled with a physiologically detailed ionic membrane model. The electric conductivity, the surface‐to‐volume ratio, and the membrane capacitance of the strand were respectively set to 3 mS/cm, 1000 cm−1 and 1 uF/cm2. A current stimulus was applied on the right hand of the strand to initiate propagation. CV was evaluated by taking distance over the arrival time difference at 1 cm and 4 cm from the stimulus point. AP shape was extracted at the midpoint of the strand, and APD was the difference between the time of 90% recovery of resting potential and the upstroke time.
In the control case, the conductivities of the ionic channels were set to their reference value as described elsewhere. 13 The effect of adenosine was modeled by increasing the conductivity of IK1 to 200% of the normal value. 16 Finally, a progressive increase of INa conductance (up to 200%) from the control case was also studied.
In‐Silico 3‐Dimensional Atrial Activation and P‐Wave Duration Simulation
A generic computer model of the human heart and torso was developed by our group and extensively reported in previous publications. 12 , 17 In brief, the reference model was segmented from magnetic resonance imaging data from a normal subject. Following histological and anatomical studies, several key anatomical features of the atria such as Bachmann’s bundle, the crista terminalis, the interatrial bundles, the pectinate muscles, and fiber distribution were eventually embedded into the model. A computational 3D grid with uniform spacing of 0.2 mm was obtained from the anatomical model, with roughly 5 million voxels in total. Each voxel was automatically marked with the corresponding anatomical region for the electrophysiological model parameterization.
The sinus rhythm activation of the atria was modeled with a propagation model, for which the action potential geometrically spreads from the sinus node to the rest of the tissue with given CV. The corresponding 12‐lead surface ECG was simulated by combining the AP shape translated at the right activation time with the lead fields computed from the torso. The AP shape and CV were extrapolated from 1‐dimensional simulations described above. A detailed description of the in‐silico model can be found in Data S1 and in a previous publication by our group. 17
Statistical Analysis
All analyses were performed using Stata, version 15 (StataCorp, College Station, TX, USA). A 2‐sided P value <0.05 was considered statistically significant. Continuous data were described with the mean and SD or the median and interquartile range, categorical variables as counts and percentages. They were compared between groups with the Kruskal‒Wallis test and the Fisher exact test. As the sole contrast of interest was the group with PWD <110 ms vs ≥140 ms, no correction for multiple comparisons was applied. Paired comparisons during adenosine test were performed using the sign test.
Lin concordance correlation coefficient and Cohen coefficient (for categorical PWD) were used to estimate reproducibility. The correlation of PWD and total endocardial activation time was assessed with Spearman R. Median follow‐up (25th–75th percentiles) was computed with the reverse Kaplan‒Meier method. The rate of recurrence (95% CI) per 100‐person year was reported for each PWD group. Kaplan‒Meier AF recurrence‐free curves were plotted; the log‐rank test was also reported. The association of PWD with AF recurrence was assessed with the Cox model. Hazard ratios (HR) and 95% CI were computed for the group with a PWD <110 ms and ≥140 ms with respect to the group with a PWD ranging from >110 to 139 ms. Fractional polynomials were used within the Cox model to assess the shape of the risk of AF recurrence. The derived linear predictor was plotted against PWD.
Results
A total of 282 consecutive patients were included in the analysis. As indicated in Table 1, the demographic and clinical variables were typical of patients undergoing a first PVI procedure. They were middle‐aged male patients suffering a drug‐resistant paroxysmal atrial fibrillation, most of them undergoing a cryoballoon ablation, and having a normal left atrial diameter (Table 1).
Table 1.
Clinical and Demographic Characteristics of Patients Included According to P‐Wave Duration
|
PWD <110 ms |
PWD ≥110 to 139 ms |
PWD ≥140 ms |
P Value | |
|---|---|---|---|---|
| No. (%) | 44 (16) | 174 (62) | 64 (23) | |
| Age, y | 56±14 | 60±11 | 68±10 | <0.001 |
| Women, n (%) | 27 (61) | 58 (33) | 17 (27) | <0.001 |
| Body mass index, Kg/mq | 25±4 | 27±5 | 28±5 | <0.001 |
| CHADS‐VASc | 1.6±1.4 | 1.7±1.4 | 2.8±1.5 | <0.001 |
| Non‐ischemic heart disease, n (%) | 1 (2.3) | 9 (5.2) | 7 (11) | 0.138 |
| History | ||||
| Paroxysmal AF, n (%) | 36 (82) | 147 (85) | 38 (59) | 0.020 |
| Diabetes mellitus, n (%) | 5 (11) | 14 (8.1) | 9 (14) | 0.363 |
| Hypertension | 19 (43) | 97 (56) | 49 (77) | 0.001 |
| Stroke | 1 (2.3) | 16 (9.2) | 9 (14) | 0.046 |
| Medication, n (%) | ||||
| Beta‐blocking agents | 10 (23) | 67 (39) | 35 (55) | 0.001 |
| Antiarrhythmic drug class IC | 10 (23) | 51 (29) | 18 (28) | 0.656 |
| Sotalol | 11 (25) | 47 (27) | 12 (19) | 0.479 |
| Amiodarone | 2 (4.6) | 19 (11) | 4 (6.3) | 1.000 |
| Non‐Vitamin K oral anticoagulant | 19 (51) | 100 (70) | 41 (79) | 0.011 |
| Vitamin K oral anticoagulant | 3 (8.1) | 9 (6.3) | 7 (14) | 0.513 |
| Echocardiographic measurements | ||||
| Left ventricular ejection fraction, % | 61±4 | 59±7 | 56±7 | <0.001 |
| Left atrial diameter, mm | 36±7 | 40±6 | 42±6 | <0.001 |
| Pulmonary vein isolation by cryoablation, n (%) | 36 (82) | 159 (91) | 60 (94) | 0.066 |
AF indicates atrial fibrillation; n, absolute number; and PWD, P‐wave duration.
In the overall population, the median PWD was 126 (interquartile range, 115 ms–138 ms; range, 71 ms–180 ms). Concordance correlation coefficient for P measurement was as high as 0.987 (95% CI, 0.976–0.998); reproducibility of PWD (assessed as a categorical variable) was 0.91. About 16% of the patients had a PWD of 110 ms or shorter (Figure 1). Demographic characteristics and clinical presentation differed among the 3 groups (Table 1). Patients with a shorter PWD were younger, were more frequently women, had a lower body mass index, presented with a lower CHADS‐VASC score, had a smaller atrial size, and had a higher left ventricular ejection fraction (Table 1).
Risk of Atrial Fibrillation Recurrence
Over a median follow‐up of 14 months (95% CI, 12 months–16 months), 70 patients (24.8%) had a symptomatic episode of AF. After a blanking time of 3 months, a greater proportion of patients (19 out of 44 patients, 43.2%) with a PWD <110 ms and of patients with a PWD ≥140 ms (19 out of 64, 29.7%) experienced a symptomatic AF episode compared with patients with an intermediate PWD (32 out of 174 patients, 18.4%).
A second PVI procedure was performed in 39 patients (56%). The rate of pulmonary vein reconnections was, however, similar among the 3 groups: 43% in the group of patients with a PWD <110 ms, 50% in the group of patients with a PWD of 110–139 ms, and 43% in the group of patients with a PWD ≥140 ms.
The risk of AF recurrences associated with PWD had a U‐shaped distribution (Figure 2) being the highest in patients with a PWD <110 ms or ≥140 ms. Patients with a PWD >110 and <139 ms, had the lowest recurrence rate (18 recurrences per 100 patient years; 95% CI, 13‒25). The recurrence rate was highest in the group with a PWD <110 ms (Figure 2) with a recurrence rate of 40 per 100 patient years (95% CI, 25–62). A similarly high recurrence rate (33 per 100 patient years; 95% CI, 21–51) was found in the group of patients with a PWD of ≥140 ms. At Cox regression, PWD was significantly associated with AF recurrence (P=0.012). Patients with PWD <110 ms (HR, 2.20; 95% CI, 1.24–3.88; P=0.007) and patients with a PWD ≥140 (HR, 1.87; 95% CI, 1.06–3.30; P=0.031) had a nearly 2‐fold increase in risk with respect to the other group. The Kaplan‒Meier AF‐free survival curve for each group of patients is shown in Figure 3.
Figure 2. Atrial fibrillation recurrence according to P‐wave duration.
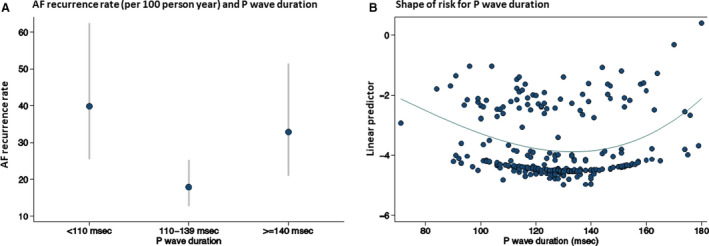
A, Atrial fibrillation recurrence rate per 100 person‐year and P‐wave duration (grouped according to predefined cut‐offs of P wave); dots indicate the rate per 100 person years, whiskers indicate the 95% CIs of the rates. B, Shape of the risk of atrial fibrillation after pulmonary vein ablation in relationship to P‐wave duration. The shape of the risk of recurrence of AF over P‐wave duration is shown, computed using fractional polynomial. For this purpose, the linear predictor (y axis) derived by a Cox model using fractional polynomial is plotted against P‐wave duration (x axis). The best fit for this model includes a power 2 and a power 3 term. There is no physical interpretation of the values on the y axis. AF indicates atrial fibrillation.
Figure 3. Atrial fibrillation recurrence‐free survival after pulmonary vein isolation (blanking period of 3 months) according to the P‐wave duration.
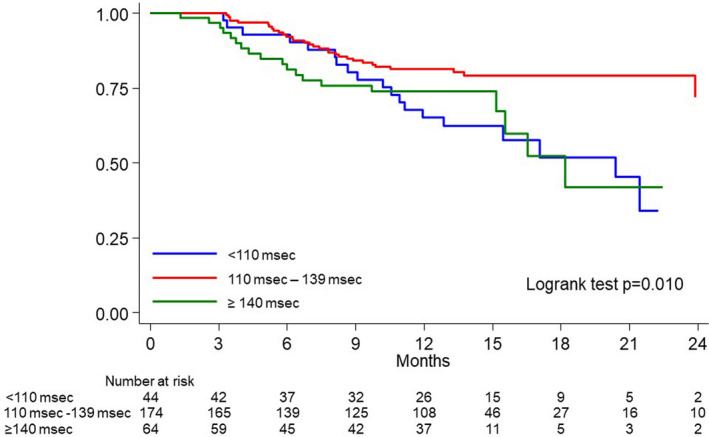
Blue line: P‐wave duration <110 ms; red line: P‐wave duration ≥110 to 139 ms; green line: P‐wave duration ≥140 ms.
The association of PWD and AF recurrence remained after adjusting for age and left atrial diameter or left ventricle ejection fraction (P=0.012 and P=0.009, respectively). HRs of PWD <110 ms versus 110 to 139 was 2.91 (95% CI, 1.43‒5.94; P<0.003) when accounting for left atrial dimension, and 2.39 (95% CI, 1.344‐4.25; P<0.003) when accounting for left ventricular ejection fraction. HRs of PWD ≥140 ms versus 110 to 139 was 1.64 (95% CI, 0.74–3.68; P<0.225) when adjusting for left atrial dimension, and 1.66 (95% CI, 0.92‒2.99; P<0.092) when adjusting for left ventricular ejection fraction.
Effect of Adenosine Administration on P‐Wave Duration
The adenosine testing performed in a limited number of patients (n=12) showed a decrease in the PWD from 124±11 ms to 113±11 ms (P<0.003), or on average by 8.2%, ranging from 4.8% to 11.3% (Figure 4). The time from P onset on surface ECG to distal CS decreased from 92±13 ms to 85±10 ms (P<0.02).
Figure 4. Effect of adenosine administration on P‐wave duration. A 9% reduction of P‐wave duration was observed on the beat preceding a complete atrioventricular block.
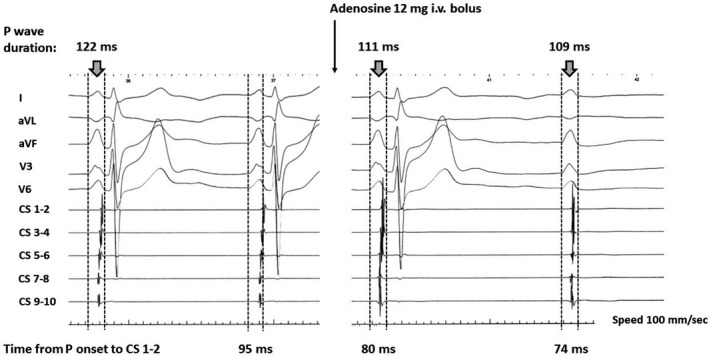
The non‐conducted P wave was slightly shorter than the previous one however, its morphology was marginally different as well. Also, the time from P‐wave onset to most distal coronary sinus was shorter during the adenosine challenge.
In‐Silico P‐Wave Simulation and P‐Wave Shortening
The control simulation in a 1‐dimensional fiber strand model (Figure 5A) showed a triangular AP with APD90 of 312 ms and a resting potential of −75 mV, whereas in the presence of adenosine, AP maintained a triangular shape but with a significant reduction in APD90 (149 ms, 52% reduction) and with a lower resting potential (−81 mV). No noticeable difference was observed in CV (only 1% faster in the presence of adenosine). In the 3D model (Figure 5B), PWD shortened from 103 ms down to 96 ms (7%). An incremental increase of sodium channel conductance from the baseline value up to 200% (Figure 5C and 5E) led to a smooth decrease in APD90 (312 ms to 224 ms) and an increase in CV (68 cm/s to 87 cm/s) in the 1‐dimensional experimental setup. In the 3D model (Figure 5F), the PWD robustly decreased from 103 ms to 81 ms.
Figure 5. In‐silico simulation of the effect of adenosine.
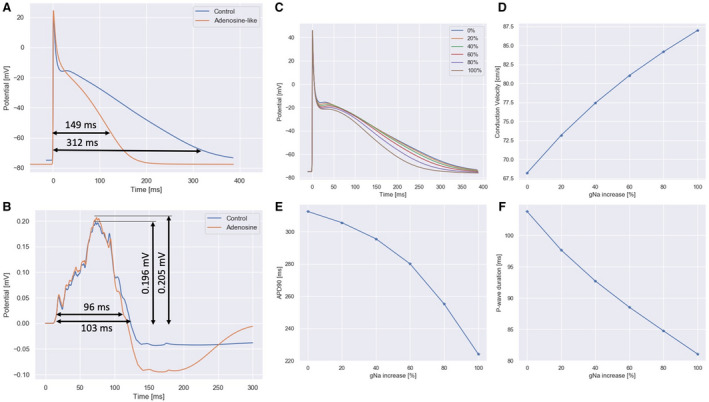
A, action potential in control and adenosine showed a triangular shape, with action potential duration 90 reducing from 312 ms to 149 ms from control to adenosine case (52% reduction). B, simulated P wave in lead II showed a reduction in duration from 103 ms to 96 ms (7% reduction). C through F, effect of an increase of sodium channels conductance from 0% to 100%. C, resting action potential shape. D, conduction velocity in a 1‐dimensional fiber strand. E, Action potential duration 90. F, P‐wave duration in lead II. A 100% increase in sodium conductance yielded a 26% increase of conduction velocity, a 28% reduction in action potential duration 90 and a 22% reduction in P‐wave duration.
Discussion
The main finding of our study is that AF recurrence after PVI is significantly higher in patients having a shorter than normal PWD despite a similar left atrial size and a similar frequency of pulmonary vein reconnection. This observation suggests the existence of a non‐pulmonary vein‐dependent AF trigger and/or mechanism for AF maintenance in this subgroup of patients, a hypothesis strongly supported by our computer modeling study. The 1D‐ and 3D‐simulations are pointing to the presence, most likely, of a so‐far unknown condition that (1) either increases sodium channel conductance thus resulting into shortening of the P wave or (2) because of shortening of the atrial AP itself might cause a shortening of the P wave, or (3) both increase in sodium channel conductance and shortening of AP leading to higher AF propensity including lower responsiveness to class I antiarrhythmic compounds. These findings are novel, significantly expand previous knowledge and may have important clinical consequences.
Nielsen et al. identified 55 305 out of 285 633 patients (19%) with a short P wave (<100 ms) in a large primary care cohort who, over a median follow‐up time of 6.7 years, developed AF. 7 Interestingly, patients with a short P wave were more frequently younger women than patients with a normal or prolonged PWD. Differently from the Copenhagen study, all other studies reporting on short PWD including ours, were conducted in patients with a history of AF. 7 , 8 , 9 In a recent prospectively‐designed large cohort registry of 2415 patients affected by different types of AF – the Swiss‐AF Study Cohort, 14% of those patients in sinus rhythm showed a PWD of ≤100 ms. 18 More consistent with the latter study, the prevalence of short PWD in our patients who had history of AF was 16%. Compared with the Copenhagen study, our patients with a short P wave and history of AF were on average 8‐ to 10‐year older and were slightly less female patients. 7 , 18 Although the prevalence of patients with a short P wave in the various studies differed, which may be in part because of the cut‐off used, the mean/median age and proportion of female sex, it consistently indicates the existence of patients at risk of developing AF or who already have documented AF having this atrial phenotype.
Based on our current understanding of AF, the association between increased PWD and the risk of AF is frequently explained by the progression of atrial cellular remodeling, structural abnormalities (e.g., fibrosis) in combination with atrial dilation. 19 , 20 , 21 , 22 An abnormal PWD and morphology, also referred to as Bayes syndrome, is determined by intra‐atrial and interatrial conduction delay or block, and associated with a high risk of AF in healthy individuals as well as in patients with history of AF. 7 , 23 Intra‐ or interatrial conduction delay/block were common (up to 85% of cases) in patients with a PWD ≥140 ms; in contrast, in our limited group of patients of patients with a short PWD, none showed intra‐ and interatrial conduction delay. Although the relationship between left atrial end‐diastolic volume, PWD and AF occurrence has been frequently reported, Nielsen et al’s recent observations show the lack of an association especially in those patients with a short P wave. 7 They reported that, in an ancillary population of >2000 individuals, atrial size explained only ≈ 16% of the variation in PWD. This observation is also in line with the recent work by Laureanti et al. in patients with short P wave and either paroxysmal or permanent AF and the present data. 18
The pathogenesis of AF in the context of Bayes syndrome has been extensively reviewed by Tse et al. in a recent publication. 24 Tse et al. 24 indicated the occurrence of the following event sequence: abnormal atrial activation can lead to increased atrial pressure, with subsequent electrophysiological and structural remodeling, such as atrial dilatation and fibrosis. Furthermore, endothelial damage and dysfunction, together with impaired atrial mechanical activity, is significantly increasing the thrombogenicity. In contrast, our data present the other side of the spectrum of AF pathogenesis, i.e., a possible increase in sodium channel conductance as cause of paroxysmal or persistent AF, which has not been previously reported.
Despite the use of antiarrhythmic drugs and effective PVI, patients with short P wave presented a twice higher risk of AF recurrence than other patients with AF. Whether patients with a short PWD also have an overall greater failure rate of antiarrhythmic drug therapy is currently unknown. Our computer simulation study, however, shows results in favor of this hypothesis. In the simulations, an increase in sodium channels conductance produced a robust shortening of the PWD related to an increase in atrial CV. If enhanced CV would also be present in patients with short P wave, a reduced efficacy to inhibit Na‐channel blockers may underlie reduced responsiveness to class I antiarrhythmic compounds in these patients and explain their higher propensity to AF. A further potential explanation for increased AF susceptibility of patients with a short P wave is given by the effect of adenosine testing on P wave. Clemo and Belardinelli have extensively described the effect of adenosine on the AP of an atrial cell during antegrade conduction. 25 In the atrial cell, adenosine causes only a reduction in APD and a small decrease in its amplitude whereas in the nodal cell, adenosine progressively increased the delay for its activation, caused an overall depression of the AP, and finally, failed to excite, resulting in atrioventricular block. Although adenosine testing is frequently used in patients undergoing PVI to unmask concealed vein conduction, its effect on PWD has not been previously reported. For the first time, we demonstrated that, after the administration of adenosine, a P wave of normal duration significantly shortened on average by 11 ms or about 8% of the baseline value. Of course, in‐vivo measurements do not allow to evaluate whether the effect was mostly on the AP or atrial conduction. However, in‐silico evaluation showed that, under a simulated adenosine testing, the AP was significantly shorter (28%). Furthermore, a progressive increase in sodium channel conductance resulted in faster atrial conduction (22%). A shorter AP and faster conduction are arrhythmogenic and well explain a non‐pulmonary vein‐dependent mechanism of AF onset and maintenance in patients with short PWD. 26 , 27 , 28 Whether an increase in sodium channel conductance is the only pathological mechanism of a shorter PWD or additional cellular factors are involved cannot be entirely clarified. Interestingly, Dupont et al. 29 have demonstrated in patients who are more prone to postoperative AF an elevated expression of connexin 40, a protein important for cardiac conduction velocity.
A short P wave may be considered the simplest, and an easily assessed biomarker which may help in setting realistic expectations after an ablation therapy and probably also after the initiation of an antiarrhythmic drug therapy. Given the higher likelihood of AF recurrence in patients with a short PWD undergoing PVI, a more frequent clinical follow‐up or early implantation of a loop recorder shall be considered in these patients.
Study Limitations
This study shares many limitations and potential selection bias of any retrospective analysis. However, all consecutive patients undergoing a first single‐PVI procedure for symptomatic paroxysmal or persistent AF at both institutions were included which significantly mitigates the issue of selection bias. Unlike most of the previous studies where ECGs were analyzed digitally with the use of clinically validated software, we performed manual measurements, thus potentially prone to subjective interpretation. However, inter‐ and intra‐observer agreement was extremely high; that might be attributable to the fact that the onset of the P wave is easily detected when all 12 leads are simultaneously considered. Although detection of the P‐wave end point is a challenging task, the use of a 3D mapping system significantly helped in confirming the end of the P wave on 12‐lead ECG more precisely and consistently. PWD was always measured in general anesthesia which may modestly affect PWD. Another potential study limitation may be related to the definition of abnormal PWD. The World Health Organization and the International Society and Federation of Cardiology Task Force consider a normal duration of a P wave up to 110 ms. 30 This PWD value is consistent with the work by Nielsen et al. showing that a PWD of 110 ms represented the 40th to 60th percentile of their population 7 as well as with Eranti et al who showed that a significant proportion of healthy individuals have PWD up to 120 ms. Notably, a recent consensus report agreed on the definition of PWD >120 ms for first degree or partial intra‐atrial block. 31 , 32 Unlike the work by Eranti et al. who considered healthy individuals, our patients had history of paroxysmal or persistent atrial fibrillation thus limiting a comparison of the findings. Furthermore, Eranti et al. focused on P‐wave morphology which was not assessed in our population. In our study the reference group for estimating risk of AF recurrence was the group of patients with a PWD 110 to 139 ms, whereas the reference group in the work by Eranti et al. had a PWD <110 ms; Eranti et al. assessed a 10‐year risk of AF hospitalization compared with a documented arrhythmia recurrence occurring over a significantly shorter follow‐up period in our study.
Conclusions
One out of 5 patients referred for PVI has a short PWD which is a marker of a higher rate of AF recurrences after the index procedure. A short P wave was reproduced in computer cardiac simulation. The 1D‐ and 3D‐simulations are pointing to the presence, most likely, of a so‐far unknown condition that an increase in sodium channel conductance shortens the PWD and might be associated with lower responsiveness to class I antiarrhythmic compounds or that shorter APD itself may cause shorter P waves. These findings may have important clinical consequences for patient with paroxysmal and persistent AF but also for asymptomatic individuals who present the atrial phenotype. Larger studies are needed to confirm our findings.
Sources of Funding
This work was supported by the Netherlands Heart Foundation (CVON2014‐09, RACE V Reappraisal of Atrial Fibrillation: Interaction between hyperCoagulability, Electrical remodeling, and Vascular Destabilisation in the Progression of AF), the European Union (ITN Network Personalized Therapies for Atrial Fibrillation: a translational network: PersonalizeAF, grant 860974, CATCH ME: Characterizing Atrial fibrillation by Translating its Causes into Health Modifiers in the Elderly, No. 633196).
Disclosures
Angelo Auricchio is a consultant to Boston Scientific, Backbeat, Biosense Webster, Cairdac, Corvia, Microport CRM, EPD‐Philips, Radcliffe Publisher. He received speaker fees from Boston Scientific, Medtronic, and Microport. He participates in clinical trials sponsored by Boston Scientific, Medtronic, EPD‐Philips. He has intellectual properties with Boston Scientific, Biosense Webster, and Microport CRM. Ulrich Schotten received consultancy fees or honoraria from Roche Diagnostics (Switzerland), EP Solutions Inc. (Switzerland), and Johnson & Johnson Medical Limited, (United Kingdom). He is co‐founder and shareholder of YourRhythmics BV, a spin‐off company of the University Maastricht. Giulio Conte has received a research grant (PZ00P3_180055) from the Swiss Science National Foundation (SNF). The remaining authors have no disclosures to report.
Supporting information
Data S1
Acknowledgments
We deeply appreciate the technical support of Mr. Joël Bondietti MSc (Biosense Webster, Johnson and Johnson, Zug, Switzerland).
(J Am Heart Assoc. 2021;10:e018572. DOI: 10.1161/JAHA.120.018572.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018572.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Platonov PG. Interatrial conduction in the mechanisms of atrial fibrillation: from anatomy to cardiac signals and new treatment modalities. Europace. 2007;9(Suppl 6):10–16. [DOI] [PubMed] [Google Scholar]
- 2. Tse G, Lai ETH, Yeo JM, Yan BP. Electrophysiological mechanisms of Bayés syndrome: Insights from clinical and mouse studies. Front Physiol. 2016;7:188. d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hernandez‐Betancor I, Izquierdo‐Gómez MM, García‐Niebla J, Laynez‐Cerdeña I, García‐González MJ, Barragan‐Acea A, Irribarren‐Sarriá JL, Jimenez‐Rivera JJ, Lacalzada‐Almeida J. Bayes syndrome and imaging techniques. Curr Cardiol Rev. 2017;13:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magnani JW, Williamson MA, Ellinor PT, Monahan KM, Benjamin EJ. P wave indices current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol. 2009;2:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conte G, Luca A, Yazdani S, Caputo ML, Regoli F, Moccetti T, Kappenberger L, Vesin JM, Auricchio A. Usefulness of P‐Wave duration and morphologic variability to identify patients prone to paroxysmal atrial fibrillation. Am J Cardiol. 2017;119:275–279. [DOI] [PubMed] [Google Scholar]
- 6. Dilaveris P, Tousoulis D. P‐wave dispersion measurement: Methodological considerations. Indian Pacing Electrophysiol J. 2017;17:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nielsen JB, Kühl JT, Pietersen A, Graff C, Lind B, Struijk JJ, Olesen MS, Sinner MF, Bachmann TN, Haunsø S, et al. P‐wave duration and the risk of atrial fibrillation: Results from the Copenhagen ECG Study. Heart Rhythm. 2015;12:1887–1895. [DOI] [PubMed] [Google Scholar]
- 8. Chang IC, Austin E, Krishnan B, Benditt DG, Quay CN, Ling LH, Chenet LY. Shorter minimum p‐wave duration is associated with paroxysmal lone atrial fibrillation. J Electrocardiol. 2014;47:106–112. [DOI] [PubMed] [Google Scholar]
- 9. Hashemi JM, Amirpour A, Zavvar R, Behjati M, Gharipour M. Predictive value of P‐wave duration and dispersion in post coronary artery bypass surgery atrial fibrillation. ARYA Atheroscler. 2012;8:59–62. [PMC free article] [PubMed] [Google Scholar]
- 10. Vagos M, van Herck IGM, Sundnes J, Arevalo HJ, Edwards AG, Koivumäki JT. Computational modeling of electrophysiology and pharmacotherapy of atrial fibrillation: recent advances and future challenges. Front Physiol. 2018;9:1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Kardiol Pol. 2016;74:1359–1469. [DOI] [PubMed] [Google Scholar]
- 12. Gharaviri A, Bidar E, Potse M, Zeemering S, Verheule S, Pezzuto S, Krause R, Maessen JG, Auricchio A, Schotten U. Epicardial fibrosis explains increased endo‐epicardial dissociation and epicardial breakthroughs in human atrial fibrillation. Front Physiol. 2020;11:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skibsbye L, Jespersen T, Christ T, Maleckar MM, van den Brink J, Tavi P, Koivumäki JT. Refractoriness in human atria: time and voltage dependence of sodium channel availability. J Mol Cell Cardiol. 2016;101:26–34. [DOI] [PubMed] [Google Scholar]
- 14. Vizzardi E, Curnis A, Latini MG, Salghetti F, Rocco E, Lupi L, Rovetta R, Quinzani F, Bonadei I, Bontempi L, et al. Risk factors for atrial fibrillation recurrence: A literature review. J Cardiovasc Med (Hagerstown). 2014;15:235–253. [DOI] [PubMed] [Google Scholar]
- 15. Wang YS, Chen GY, Li XH, Zhou X, Li YG. Prolonged P‐wave duration is associated with atrial fibrillation recurrence after radiofrequency catheter ablation: A systematic review and meta‐analysis. Int J Cardiol. 2017;227:355–359. [DOI] [PubMed] [Google Scholar]
- 16. Belardinelli L, Shryock JC, Song Y, Wang D, Srinivas M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995;9:359–365. [DOI] [PubMed] [Google Scholar]
- 17. Pezzuto S, Kal'avský P, Potse M, Prinzen FW, Auricchio A, Krause R. Evaluation of a rapid anisotropic model for ECG simulation. Front Physiol. 2017;8:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laureanti R, Conte G, Corino VDA, Osswald S, Conen D, Roten L, Rodondi N, Ammann P, Meyer‐Zuern CS, Bonati L, et al. Sex‐related electrocardiographic differences in patients with different types of atrial fibrillation: Results from the SWISS‐AF study. Int J Cardiol. 2020;307:63–70. [DOI] [PubMed] [Google Scholar]
- 19. Zhuang J, Wang Y, Tang K, Li X, Peng W, Liang C, Xu Y. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: a systematic review and meta‐analysis of observational studies. Europace. 2012;14:638–645. [DOI] [PubMed] [Google Scholar]
- 20. Qureshi W, Soliman EZ, Solomon SD, Alonso A, Arking DE, Shah A, Gupta DK, Wagenknecht LE, Herrington D. Risk factors for atrial fibrillation in patients with normal versus dilated left atria (from the atherosclerosis risk in communities [ARIC] Study). Am J Cardiol. 2014;114:1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reiffel J. Intra‐Atrial Block: Definition and relationship to atrial fibrillation and other adverse outcomes. J Atr Fibrillation. 2019;12:2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tse G, Wong CW, Gong M, Wong WT, Bazoukis G, Wong SH, Li G, Wu W, Tse LA, Lampropoulos K, et al. International Health Informatics Study (IHIS) Network. Predictive value of inter‐atrial block for new onset or recurrent atrial fibrillation: A systematic review and meta‐analysis. Int J Cardiol. 2018;250:152–156. [DOI] [PubMed] [Google Scholar]
- 23. Eranti A, Carlson J, Kenttä T, Holmqvist F, Holkeri A, Haukilahti MA, Kerola T, Aro AL, Rissanen H, Noponen K, et al. Orthogonal P‐wave morphology, conventional P‐wave indices, and the risk of atrial fibrillation in the general population using data from the Finnish Hospital Discharge Register. Europace. 2020;22:1173–1181. [DOI] [PubMed] [Google Scholar]
- 24. Tse G, Lai ETH, Yeo JM, Yan BP. Electrophysiological mechanisms of Bayès syndrome: insights from clinical and mouse studies. Frontiers Physiol. 2016;7:188. 10.3389/fphys.2016.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clemo HF, Belardinelli L. Effect of adenosine on atrioventricular conduction. II: Modulation of atrioventricular node transmission by adenosine in hypoxic isolated guinea pig hearts. Circ Res. 1986;59:437–446. [DOI] [PubMed] [Google Scholar]
- 26. Skibsbye L, Poulet C, Diness JG, Bentzen BH, Yuan L, Kappert U, Matschke K, Wettwer E, Ravens U, Grunnet M, et al. Small conductance calcium activated potassium (SK) channels contribute to action potential repolarisation in human atria. Cardiovasc Res. 2014;103:156–167. [DOI] [PubMed] [Google Scholar]
- 27. Skibsbye L, Jespersen T, Christ T, Maleckar MM, van den Brink J, Tavi P, Koivumäki JT. Refractoriness in human atria: time and voltage dependence of sodium channel availability. J Mol Cell Cardiol. 2016;101:26–34. [DOI] [PubMed] [Google Scholar]
- 28. Koivumäki JT, Clark RB, Belke D, Kondo C, Fedak PW, Maleckar MM, Giles WR. Na+ current expression in human atrial myofibroblasts: identity and functional roles. Front Physiol. 2014;5:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dupont E, Ko Y, Rothery S, Coppen SR, Baghai M, Haw M, Severs NJ. The gap‐junctional protein connexin40 is elevated in patients susceptible to postoperative atrial fibrillation. Circulation. 2001;103:842–849. [DOI] [PubMed] [Google Scholar]
- 30. Willems JL, Robles de Medina EO, Bernard R, Coumel P, Fisch C, Krikler D, Mazur NA, Meijler FL, Mogensen L, Moret P, et al. Criteria for intraventricular conduction disturbances and pre‐excitation. World Health Organizational/International Society and Federation for Cardiology Task Force Ad Hoc. J Am Coll Cardiol. 1985;5:1261–1275. [DOI] [PubMed] [Google Scholar]
- 31. Bayés de Luna A, Platonov P, Cosio FG, Cygankiewicz I, Pastore C, Baranowski R, Bayés‐Genis A, Guindo J, Viñolas X, Garcia‐Niebla J, et al. Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J Electrocardiol. 2012;45:445–451. [DOI] [PubMed] [Google Scholar]
- 32. Ariyarajah V, Frisella ME, Spodick DH. Reevaluation of the criterion for interatrial block. Am J Cardiol. 1006;98:936–937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


