Abstract
Background
Acute myocardial infarction (AMI) with in‐hospital onset (AMI‐IHO) has poor prognosis but is clinically underappreciated. Whether its occurrence has changed over time is uncertain.
Methods and Results
Since 1987, the ARIC (Atherosclerosis Risk in Communities) study has conducted adjudicated surveillance of AMI hospitalizations in 4 US communities. Our analysis was limited to patients aged 35 to 74 years with symptomatic AMI. Patients with symptoms initiating after hospital arrival were considered AMI‐IHO. A total of 26 678 weighted hospitalizations (14 276 unweighted hospitalizations) for symptomatic AMI were identified from 1995 to 2014, with 1137 (4%) classified as in‐hospital onset. The population incidence rate of AMI‐IHO increased in the 4 ARIC communities from 1995 through 2004 to 2005 through 2014 (12.7—16.9 events per 100 000 people; P for 20‐year trend <0.0001), as did the proportion of AMI hospitalizations with in‐hospital onset (3.7%–6.1%; P for 20‐year trend =0.03). The 10‐year proportions were stable for patients aged 35 to 64 years (3.0%–3.4%; P for 20‐year trend =0.3) but increased for patients aged ≥65 years (4.6%–7.8%; P for 20‐year trend =0.008; P for interaction by age group =0.04). AMI‐IHO had a more severe clinical course with lower use of AMI therapies or invasive strategies and higher in‐hospital (7% versus 3%), 28‐day (19% versus 5%), and 1‐year (29% versus 12%) mortality (P<0.0001 for all).
Conclusions
In this population‐based community surveillance, AMI‐IHO increased from 2005 to 2014, particularly among older patients. Quality initiatives to improve recognition and management of AMI‐IHO should be especially focused on hospitalized patients aged >65.
Keywords: acute myocardial infarction, inpatient onset, outcomes, surveillance
Subject Categories: Myocardial Infarction, Mortality/Survival, Quality and Outcomes, Epidemiology
Non‐Standard Abbreviations and Acronyms
- AMI‐IHO
acute myocardial infarction with in‐hospital onset
- ARIC
Atherosclerosis Risk in Communities
- IHO
in‐hospital onset
Clinical Perspective
What Is New?
Although a general decline in out‐of‐hospital–onset acute myocardial infarction has been noted in the community, temporal trends in acute myocardial infarction with in‐hospital onset (AMI‐IHO) have not been previously reported.
In this community‐based surveillance spanning 20 years, both the population incidence rate of AMI‐IHO and the proportion of acute myocardial infarction hospitalizations with in‐hospital onset increased over time.
The increasing proportion of AMI‐IHO was primarily observed among patients aged ≥65 years.
What Are the Clinical Implications?
Patients with AMI‐IHO have more comorbidities, a lower likelihood of receiving guideline‐directed therapies, lower use of invasive angiography or revascularization, and worse short‐ and long‐term survival than patients with out‐of‐hospital–onset AMI
Quality initiatives should be implemented to improve the recognition and management of AMI‐IHO.
Acute myocardial infarction (AMI) with in‐hospital onset (AMI‐IHO) has a poor prognosis and is associated with lower use of guideline‐directed therapies. 1 , 2 Several quality improvement initiatives have recently been implemented to improve the recognition and management of AMI‐IHO. 3 , 4 However, our current understanding of AMI‐IHO is limited to inferences from cross‐sectional study populations. In recent years, a general decline in the incidence of out‐of‐hospital–onset AMI has been noted in the community. 5 This is suspected to be attributable to better recognition and management of traditional cardiovascular risk factors in ambulatory care settings. The temporal trends in AMI‐IHO, however, have not been described. Older patient age, high comorbidity burden, and use of both cardiac and noncardiac surgical procedures have been associated with AMI‐IHO. 2 , 6 The use of inpatient procedures is reported to increase with Medicare eligibility at the age of 65, 7 as well as across time. 8 , 9 Worsening acuity of older hospitalized patients has also been reported in recent years, 10 , 11 potentially heightening vulnerability to in‐hospital events associated with inpatient procedures. We hypothesized that the population incidence rate and proportion of AMI hospitalizations with in‐hospital onset is increasing and anticipated that patients aged ≥65 years would be at greater risk.
METHODS
The ARIC (Atherosclerosis Risk in Communities) study's data and materials are publicly available to qualified investigators with an approved manuscript proposal and data use agreement. The authors will not distribute the data, analytic methods, or study materials to other researchers for purposes of reproducing the results or replicating the procedure.
ARIC Study Community Surveillance
As previously described, 5 the ARIC study conducted community surveillance of hospitalizations for AMI in 4 geographically defined regions of the United States (Forsyth County, North Carolina; Washington County, Maryland; Jackson, Mississippi; and 8 northwest suburbs of Minneapolis, Minnesota). All surveillance protocols were approved by local institutional review boards. Informed consent was not required, because personal identifiers were redacted from the analytic data set. Hospitalizations were selected for adjudication by randomly sampling within strata based on race, sex, ARIC community, and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) discharge codes: 402, 410–414, 427, 428 and 518.4. The underlying population size of residents within the 4 ARIC communities was interpolated and extrapolated from the US Census population estimates, as previously described. 5 Community residents aged 35 to 74 years were eligible for surveillance in 1987 to 2004, with eligibility expanded to 35 to 84 years from 2005 to 2014. To assess temporal trends without confounding by age, our analysis was limited to patients aged 35 to 74 years across all years of observation.
Clinical Covariates and Demographic Data
Clinical and demographic data were collected from the hospital record by trained abstractors, using physician notes, laboratory reports, patient histories, and discharge summaries. Diabetes mellitus was defined by documented history of diabetes mellitus or antihyperglycemic therapy use. Hypertension was defined by documented hypertension in the medical record.
Electrocardiography and Biomarkers
The first, third, and the last 12‐lead ECGs over the course of hospitalization were obtained from the medical record and coded electronically at the Minneapolis ECG Reading Center. Laboratory values for biomarkers of cardiac injury were abstracted chronologically, recording up to 3 measurements per day and noting the upper limit of normal (ULN).
Acute Cardiac Symptoms and Timing of Onset
Acute cardiac symptoms lacking an obvious noncardiac etiology were abstracted from the medical record and included the following: pain, discomfort, or heaviness in the chest; pain in the neck, left arm, or sternum; syncope or collapse; shortness of breath; nausea; palpitations; and tightness of the throat. Cardiac origin of chest pain was determined from the physician notes. Temporary, procedure‐related cardiac chest pain induced during coronary angioplasty or balloon inflation was not considered acute chest pain, nor was perioperative chest pain. The timing of symptom onset, whether before arrival or after admission, was recorded, as was the date of onset. For patients with chronic angina, symptom onset was defined by change in pain prompting medical attention.
AMI Classification
As previously described, 5 events were classified by a physician panel as definite, probable, suspected, or no myocardial infarction (MI), on the basis of ECG evidence (evolving diagnostic, diagnostic, evolving ST‐segment/T‐wave changes, equivocal, or absent/uncodable), presence of chest pain, and cardiac biomarkers (which were considered “abnormal” if ≥2× ULN), and “equivocal” if exceeding the ULN but <2× the ULN). Classification of an event as definite or probable AMI required the presence of at least 1 of the following: (1) evolving diagnostic ECG pattern, (2) diagnostic ECG pattern and abnormal biomarkers (≥2× ULN), (3) cardiac pain and abnormal biomarkers (≥2× ULN), (4) cardiac pain and equivocal biomarkers (exceeding the ULN but <2× the ULN) with evolving ST‐segment/T‐wave pattern or diagnostic ECG pattern, or (5) abnormal biomarkers with evolving ST‐segment/T‐wave pattern. Classification criteria remained constant across the study period. Perioperative myocardial injury was not considered AMI by the ARIC study.
In‐Hospital–Onset Versus Out‐of‐Hospital–Onset AMI
Our analysis was limited to patients with acute cardiac symptoms who were classified with definite or probable AMI. Onset of acute cardiac symptoms after hospital admission were considered AMI‐IHO, while onset of symptoms before admission, including onset in the emergency department or hospital ambulatory care clinics, was considered out‐of‐hospital–onset AMI.
Medical Therapies
Medications were recorded if administered during hospitalization or prescribed at hospital discharge. Aspirin required routine rather than pro re nata administration for abstraction. Nonaspirin antiplatelet therapy was recorded as a single category and included P2Y12 inhibitors (cangrelor, clopidogrel, prasugrel, ticagrelor, ticlopidine), glycoprotein IIb/IIIa inhibitors (abciximab, eptifibatide, tirofiban), phosphodiesterase 3 inhibitors (cilostazol), phosphodiesterase 5 inhibitors (dipyridamole), and protease‐activated receptor‐1 antagonists (vorapaxar). Beta blockers included β1 adrenergic antagonists. Angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers were recorded as a single category. Lipid‐lowering agents included statins, niacin, and fibrates.
Cardiac Procedures
Echocardiography, stress testing, coronary angiography, and revascularization procedures were abstracted from the medical record. Echocardiography included transthoracic and transesophageal echocardiograms. Stress testing included exercise testing (treadmill or bicycle ergometer), stress echocardiography, cardiac stress magnetic resonance imaging, and nuclear stress tests. Revascularization included percutaneous coronary intervention or coronary artery bypass graft surgery.
Type 4 and Type 5 AMI Classification
The elapsed time from chest pain onset to revascularization was calculated on the basis of the timing of symptom onset and the timing of coronary revascularization. Chest pain onset occurring after revascularization was considered evidence of type 4 (percutaneous coronary intervention–related) or type 5 (coronary artery bypass graft surgery–related) AMI.
Mortality Outcomes
In‐hospital, 28‐day, and 1‐year mortality were ascertained by the ARIC study, which linked hospitalizations with the National Death Index. Cardiovascular death was defined by death attributable to “diseases of the circulatory system” (ICD‐9 codes 390–459 and International Classification of Diseases, Tenth Revision [ICD‐10] codes I00–I99). All included patients had 28‐day and 1‐year mortality outcomes.
Primary Diagnosis at Admission
The ICD‐9 codes for primary diagnosis at admission were collected by the ARIC study from 2005 onwards. For the purposes of this analysis, admission diagnosis codes were categorized as cancer (140–210); endocrine or metabolic (240–279); cardiovascular (390–459); respiratory (460–519); gastrointestinal (520–579); genitourinary (580–629); symptoms, signs, and “ill‐defined” conditions (780–799); injury (800–999); or “other.” Cardiovascular admission codes 410–414 were considered related to AMI.
In‐Hospital Surgical Procedures
The ARIC study collected ICD‐9‐CM procedure codes for hospitalizations from 2005 onwards. For the purposes of this analysis, operative procedures were identified by codes 01 through 86.99, excluding hemodialysis (39.95), puncture of a vessel (38.9x), and wound debridement (86.22). Using the first‐listed ICD‐9‐CM code, procedures were classified by operations on the nervous system (01–05); endocrine system (06–07); eye (08–16); nose, mouth, and pharynx (21–29); respiratory system (30–34); cardiovascular system (35–39); hemic and lymphatic system (40–41); digestive system (42–54); urinary system (55–59); genitalia (60–71); and musculoskeletal system (76–84). There were no first‐listed procedural codes indicating operations to the ear, integumentary system, or obstetric procedures.
Statistical Analysis
All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Statistical tests and models accounted for the stratified sampling design and were weighted by the inverse of the sampling probability. 12 Continuous variables were assessed for normality and compared using the difference in least square means from weighted linear regression. Categorical variables were compared using Rao‐Scott χ2 tests. Annual trends in the proportion of AMI hospitalizations with in‐hospital onset of acute cardiac symptoms and the population incidence of AMI‐IHO were plotted visually with trend lines fit by second‐order polynomials. Trends were plotted with stratification by age group, ARIC community, sex, and race. Significance of annual trends was analyzed using logistic regression, by regressing on the year of admission and analyzing the Cochran‐Armitage test for trend. Modification of temporal trends in AMI‐IHO by age (35–64 versus 65–74 years), race (White versus Black), and sex (men versus women) was analyzed by logistic regression, testing the multiplicative interaction between demographic group and decade of hospital admission (1995–2004 versus 2005–2014). Prevalence of comorbidities and receipt of guideline‐directed therapies were compared between patients with in‐hospital– versus out‐of‐hospital–onset AMI, both in the 20‐year aggregate and over time. The adjusted relative probabilities of patients with in‐hospital versus out‐of‐hospital onset AMI receiving guideline‐directed medications (aspirin, other antiplatelets, beta blockers, and lipid‐lowering medications) or undergoing invasive procedures (coronary angiography and revascularization) were derived by multivariable logistic regression, with odds ratios converted into relative risks (RRs) and 95% CIs, 13 adjusted for demographics (age, sex, race, geographic region, and year of admission). Models analyzing use of coronary angiography and revascularization for patients with AMI‐IHO versus out‐of‐hospital–onset AMI excluded patients identified with type 4 and type 5 AMI. In sensitivity analyses, we also stratified the models by ST‐segment–elevation myocardial infarction (STEMI) and non–ST‐segment–elevation myocardial infarction (NSTEMI) and limited the population to patients with first‐occurring AMI, those with an ICD discharge code of 410.x – 414.x, and patients known to have health insurance. RRs of in‐hospital and 28‐day mortality were similarly compared between patients with in‐hospital– versus out‐of‐hospital–onset AMI, with adjustments for demographics, and comorbidities routinely collected across all years of surveillance (smoking, hypertension, diabetes mellitus, previous AMI, and history of stroke). Hazard ratios (HRs) of 1‐year mortality comparing in‐hospital– to out‐of‐hospital–onset AMI were analyzed by multivariable Cox regression, with adjustment for demographics and comorbidities.
Results
A total of 14 276 (unweighted) hospitalizations for symptomatic AMI among patients aged 35 to 74 years were sampled from 1995 to 2014, with total weights equaling 26 678. The study population selection flowchart is shown in Figure S1. All subsequently presented results are weighted by the sampling fraction. Most patients were White (67%) and men (64%), with a mean age of 60 years. Slightly over one‐third of the patient population was aged ≥65 years (N=9702; 36%). On average, White patients were 4 years older than Black patients (61 versus 57 years; P<0.0001), and women were 2 years older than men (61 versus 59 years; P<0.0001). When aggregated across 1995 to 2014, a total of 1137 (4%) had AMI‐IHO. Among those with AMI‐IHO, 27 (2%) developed chest pain after coronary revascularization, constituting type 4 or type 5 MI. The overall proportion of AMI hospitalizations with in‐hospital onset of acute cardiac symptoms steadily increased throughout the 20‐year surveillance period (3.7% [1995–2004] to 6.1% [2005–2014]; P for trend =0.03), as did the population incidence rate of AMI‐IHO in the 4 ARIC communities, which increased from 12.7 events per 100 000 people in 1995 to 2004 to 16.9 events per 100 000 people in 2005 to 2014 (P for trend <0.0001; Figure 1). When stratified by age group, the proportion of AMI hospitalizations with in‐hospital onset was stable for patients aged 35 to 64 (3.0% [1995–2004] to 3.4% [2005–2014]; P for trend =0.3) but increased for patients aged ≥65 years (4.6% [1995–2004] to 7.8% [2005–2014]; P for trend =0.008; P for interaction by age group =0.04; Figure 1). However, the mean patient age was unchanging from 1995 through 2004 to 2005 through 2014, both for patients aged 35 to 64 (mean age, 54 years in both intervals); or for those aged ≥65 years (mean age, 70 years in both time intervals). A similar trend in AMI‐IHO among patients aged ≥65 years was observed when stratified by the 4 ARIC communities (Figure S2). Of note, the proportion of AMI‐IHO was substantially higher in Minneapolis, Minnesota, compared with the other ARIC communities, across all years of observation. When stratified by sex, a parallel upward trend in AMI‐IHO was observed both for women and men, with no evidence of modification by sex (P for interaction =0.3; Figure S3). When stratified by race, the temporal increase in AMI‐IHO was slightly accelerated for White relative to Black patients (P for interaction by race =0.12).
Figure 1. Annual population incidence rate of symptomatic AMI with in‐hospital onset among community residents aged 35 to 74 years.
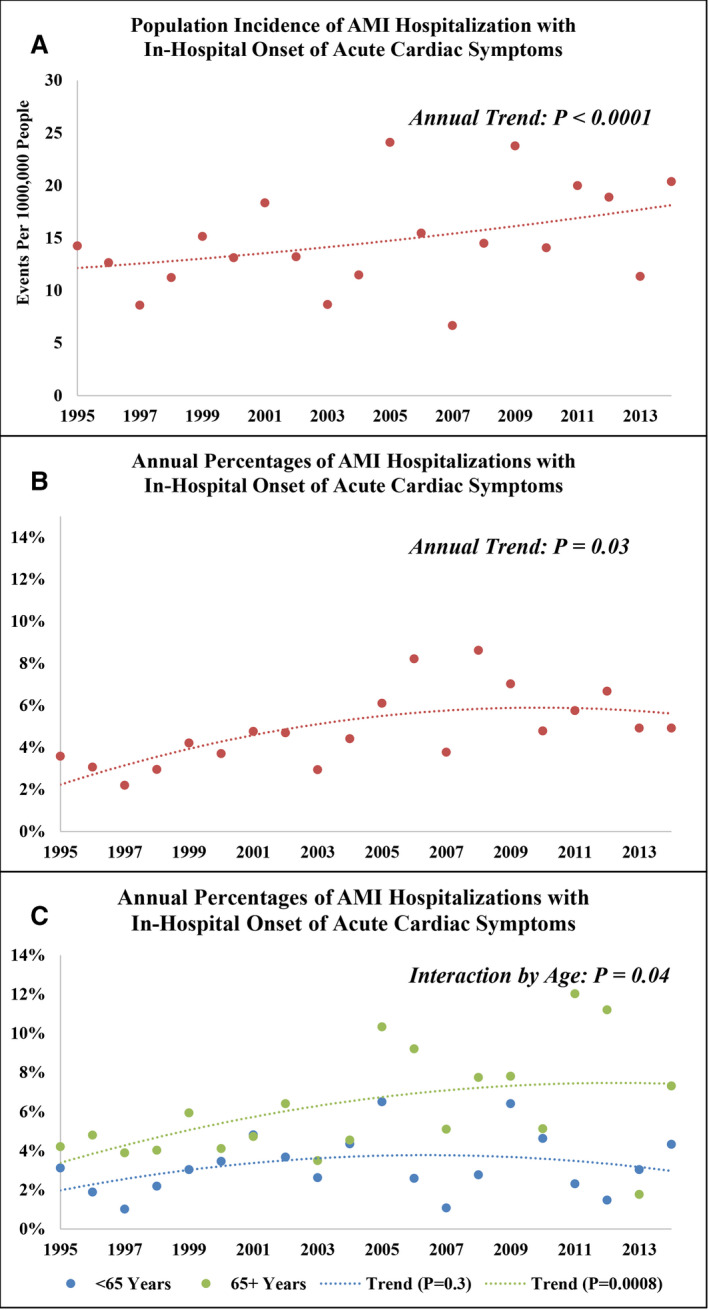
(A) Annual proportion of symptomatic AMI hospitalizations with onset occurring in‐hospital (B), and annual proportions stratified by patients aged 35 to 64 and 65 to 74 years (C). The Community Surveillance component of the Atherosclerosis Risk in Communities Study, 1995 to 2014. AMI indicates acute myocardial infarction.
When aggregated across 1995 to 2014, patients with AMI‐IHO were older than those with out‐of‐hospital–onset AMI (66 versus 61 years), and more often women (42% versus 35%), White (75% versus 66%), and insured (96% versus 88%). Diabetes mellitus (46% versus 37%), chronic kidney disease (46% versus 24%), and history of stroke (16% versus 10%) were more prevalent with AMI‐IHO; Table 1, and remained consistently more prevalent throughout the surveillance observation period (Figure 2). Acute cardiac symptoms less often presented as chest pain in patients with AMI‐IHO (52% versus 83%) and AMI‐IHO was more often classified as NSTEMI (90% versus 80%). Compared with out‐of‐hospital–onset AMI, AMI‐IHO was more often complicated by acute heart failure/pulmonary edema (41% versus 29%), cardiogenic shock (6% versus 3%), and ventricular fibrillation/cardiac arrest (20% versus 7%). When aggregated across 1995 to 2014, patients with AMI‐IHO less often received aspirin (74% versus 90%), other antiplatelets (27% versus 56%), lipid‐lowering agents (50% versus 72%), beta blockers (78% versus 82%), angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers (49% versus 62%), coronary angiography (25% versus 62%), or coronary revascularization (16% versus 45% overall, and 62% versus 72% among the subset undergoing angiography). The differential management of in‐hospital–onset versus out‐of‐hospital–onset AMI was apparent across all years of the surveillance observation (Figure 3).
Table 1.
Demographics and Clinical Characteristics of Patients Hospitalized With Symptomatic Acute Myocardial Infarction, Stratified In‐Hospital and Out‐Of‐Hospital Onset: The Community Surveillance Component of the Atherosclerosis Risk in Communities Study, 1995 to 2014
| Characteristic | In‐Hospital Onset | Out‐of‐Hospital Onset | P Value |
|---|---|---|---|
| N=1137 | N=25 541 | ||
| Demographics | |||
| Age, y median (Q1–Q3) | 66 (59–70) | 61 (53–68) | <0.0001 |
| Women, n (%) | 474 (42) | 9054 (35) | 0.04 |
| White, n (%) | 853 (75) | 16 966 (66) | 0.002 |
| Health insurance,*n (%) | 629 (96) | 11 462 (88) | 0.001 |
| Medical history, † n (%) | |||
| Smoking | 363 (33) | 9690 (39) | 0.08 |
| Hypertension | 845 (75) | 18 261 (72) | 0.7 |
| Diabetes mellitus | 528 (46) | 9472 (37) | 0.003 |
| Chronic kidney disease* | 329 (46) | 3429 (24) | <0.0001 |
| Prior myocardial infarction | 328 (30) | 7900 (32) | 0.6 |
| Prior revascularization | 387 (34) | 7691 (30) | 0.2 |
| Stroke | 178 (16) | 2579 (10) | 0.007 |
| Hospital visit, n (%) | |||
| Chest pain | 590 (52) | 21 228 (83) | <0.0001 |
| Elevated troponin (>2× ULN) | 1020 (90) | 23 021 (90) | 0.8 |
| ST‐segment elevation ‡ | 105 (10) | 4516 (20) | 0.003 |
| Acute heart failure/pulmonary edema | 468 (41) | 7481 (29) | <0.0001 |
| Cardiogenic shock | 70 (6) | 790 (3) | 0.001 |
| Ventricular fibrillation/cardiac arrest | 226 (20) | 1803 (7) | <0.0001 |
Q1, first quartile, Q3, third quartile, ULN, upper limit of normal.
Health insurance not abstracted before 2005, available for 13 618 patients. Serum creatinine not abstracted before 2004, available for 14 825 patients.
History of smoking missing for 668 patients, history of hypertension missing for 262 patients, history of myocardial infarction missing for 812 patients, history of stroke missing for 431 patients.
ST‐segment–elevation myocardial infarction/non–ST‐segment–elevation myocardial infarction classified for 24 035 patients.
Figure 2. Temporal trends in prevalence of comorbidities among patients hospitalized with symptomatic acute myocardial infarction, stratified by in‐hospital vs out‐of‐hospital onset.
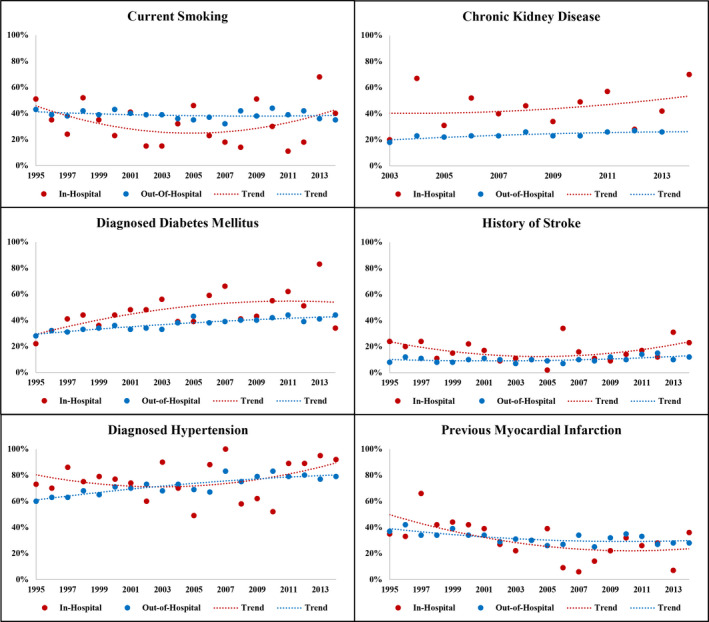
The Community Surveillance component of the Atherosclerosis Risk in Communities Study, 1995 to 2014. *Chronic kidney disease limited to 2003 to 2014.
Figure 3. Temporal trends in use of guideline‐directed therapies for among patients hospitalized with symptomatic acute myocardial infarction, stratified by in‐hospital vs out‐of‐hospital onset.
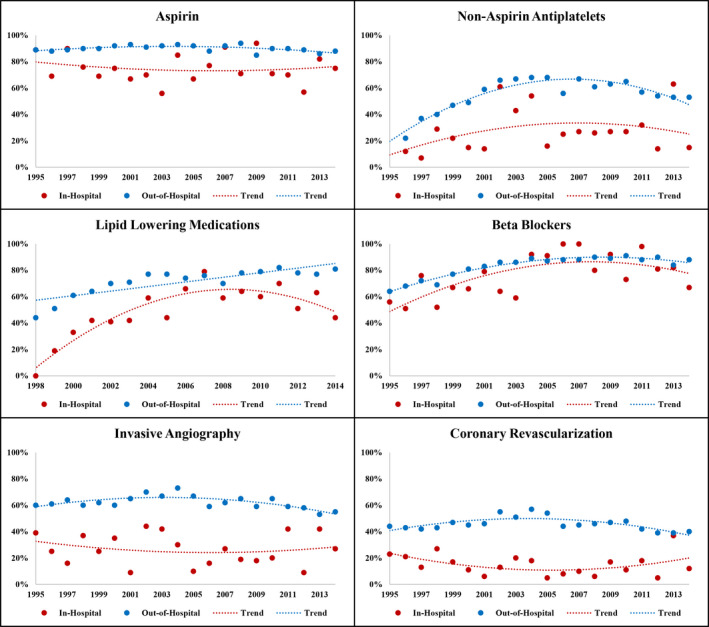
The Community Surveillance component of the Atherosclerosis Risk in Communities Study, 1995 to 2014. *Lipid‐lowering medications limited to 1998 to 2014.
In the subset of AMI‐IHO hospitalizations with available primary admission diagnosis codes (2005 onwards), a small percentage (2%) was admitted for AMI‐related codes (410–414). The overall primary causes of admission for AMI‐IHO hospitalizations are shown in Figure 4, with ICD‐9 codes 410‐414 excluded. The most prevalent causes were “ill‐defined” diagnoses (25%), followed by cardiovascular (23%), “other” (13%), injury (11%), gastrointestinal and respiratory (8% for each). The most prevalent “ill‐defined” diagnosis was malaise/vomiting (31%), followed by shortness of breath (19%), and altered mental status (11%). Among cardiovascular admissions, the most prevalent causes were cardiac arrythmias, atherosclerosis, and occluded cerebral artery (11% for each). Admission for “other” diagnoses were diverse, but the most common causes were osteomyelitis (13%) and alcohol withdrawal (10%). Among injury admissions, the most common cause was infection of a joint prosthesis or orthopedic device (35%). The leading cause of gastrointestinal admission was hemorrhage of the gastrointestinal tract (39%), and the leading causes of respiratory admissions were acute respiratory failure (36%) and pneumonia (12%). Primary causes of admission for AMI‐IHO stratified by age (35–64 and 65–74 years) are shown in Figure S4.
Figure 4. Primary admission diagnosis for patients with symptomatic acute myocardial infarction with in‐hospital onset.
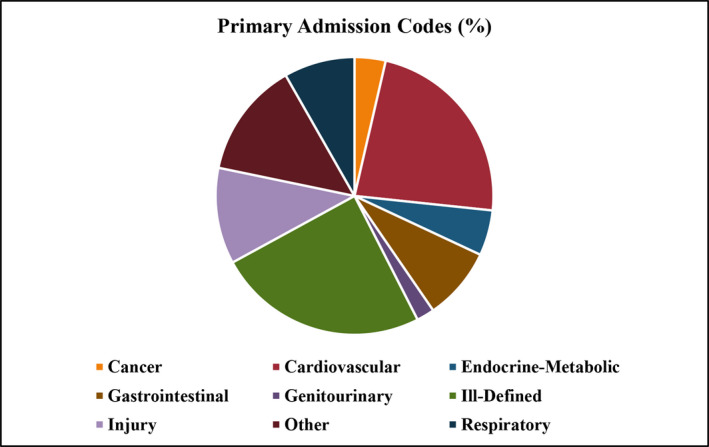
The Community Surveillance component of the Atherosclerosis Risk in Communities Study, 2005 to 2014. Admission diagnosis codes available for hospitalizations from 2005 onwards. Admission codes related to acute myocardial infarction (2%) excluded.
After adjustment for age, race, sex, geographic region, and year of admission, the relative probability of managing AMI‐IHO by guideline‐directed therapies remained significantly lower, when compared with out‐of‐hospital–onset AMI (Figure 5). Similar associations were observed among patients classified with NSTEMI and STEMI, or when limiting the population to patients with first‐occurring AMI, patients with discharge codes of 410.x–4.14.x), or among those known to have health insurance (Tables S1‐S3). Lower use of guideline‐directed therapies with AMI‐IHO relative to out‐of‐hospital–onset AMI was consistently observed, irrespective of cardiovascular or noncardiovascular primary diagnosis codes at admission (Figure S5). However, patients with AMI‐IHO accompanied by acute chest pain were more often administered guideline‐directed therapies than AMI‐IHO patients with atypical presentation (Table 2).
Figure 5. Adjusted relative probabilities of guideline‐directed therapies for patients hospitalized with symptomatic acute myocardial infarction with in‐hospital vs out‐of‐hospital onset.
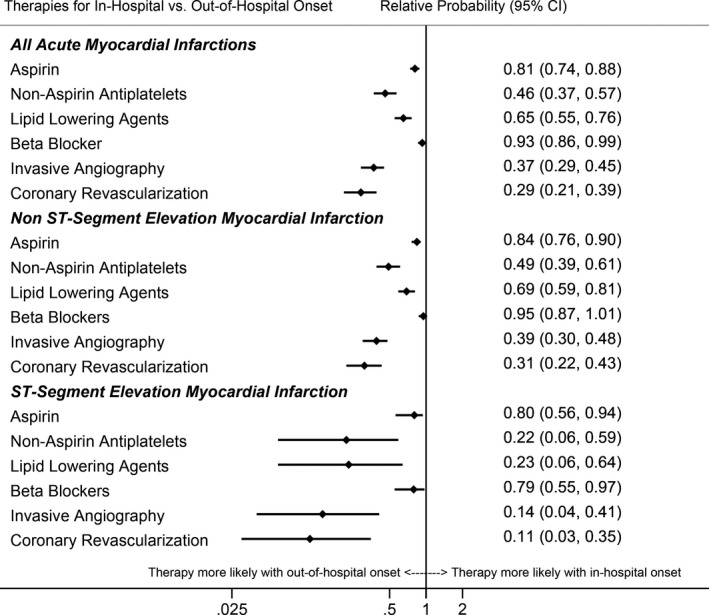
The Community Surveillance component of the Atherosclerosis Risk in Communities Study, 1995 to 2014. *Models adjusted for age, race, sex, geographic location, and year of admission. Patients with type 4/5 myocardial infarction excluded from models analyzing invasive angiography and coronary revascularization outcomes.
Table 2.
Administration of Guideline‐Directed Therapies for Acute Myocardial Infarction Among Patients With In‐Hospital–Onset Acute Myocardial Infarction, Stratified by Atypical Presentation Versus Acute Chest Pain: The Community Surveillance Component of The Atherosclerosis Risk in Communities Study, 1995 to 2014
| Therapy | Atypical Presentation | Acute Chest Pain | P Value |
|---|---|---|---|
| (N=548), n (%) | (N=590), n (%) | ||
| Aspirin | 373 (68) | 473 (80) | 0.02 |
| Antiplatelet | 95 (19) | 186 (34) | 0.001 |
| Lipid‐lowering agent* | 239 (48) | 265 (53) | 0.5 |
| Beta blocker | 415 (76) | 472 (80) | 0.4 |
| Angiography | 72 (13) | 213 (36) | <0.0001 |
| Revascularization | 38 (7) | 145 (25) | <0.0001 |
Lipid‐lowering agents limited to 1998 onwards.
Of the 637 AMI‐IHO patients with available procedural codes (2005 onwards), 66% underwent operative procedures during their hospital stay, including 8 patients classified with type 4 or 5 MI. After excluding type 4 or 5 MI, the most commonly performed operations for patients undergoing procedures were on the cardiovascular system (40%), followed by operations on the digestive, musculoskeletal, and respiratory systems (11% for each). Most cardiovascular operations were vascular (66%) rather than cardiac.
On average, patients with AMI‐IHO developed acute cardiac symptoms a median of 2 days after hospital admission (quartile 1 to quartile 3, 1–4 days). The average duration of hospital stay since onset of AMI‐IHO was 6 days ( quartile 1 to quartile 3, 3–10 days). By comparison, the average duration of hospital stay for patients with out‐of‐hospital–onset AMI was 4 days ( quartile 1 to quartile 3, 3–7 days; P<0.0001).
In total, there were 855 in‐hospital deaths, 1576 deaths within 28 days of hospitalization, and 3366 deaths within 1 year of hospitalization. Mortality was higher for AMI‐IHO than out‐of‐hospital AMI, whether in‐hospital (7% versus 3%; P<0.0001), within 28 days (19% versus 5%; P<0.0001), or within 1 year of hospitalization (29% versus 12%; P<0.0001); and remained higher throughout the surveillance period (Figure S6). Among the fatalities, a cardiovascular cause of death was less frequent with AMI‐IHO, whether in‐hospital (68% versus 81%; P=0.1), within 28 days (39% versus 58%; P=0.003), or within 1 year of hospitalization (38% versus 57%; P<0.0001). After adjustments for demographics (age, race, sex, geographic region, year of admission), and comorbidities (smoking, hypertension, diabetes mellitus, previous AMI, and history of stroke), AMI‐IHO remained strongly associated with in‐hospital death (RR, 2.30; 95% CI, 1.55–3.39), and death within 28 days (RR, 4.18; 95% CI, 2.84–6.16) and 1 year (HR, 2.00; 95% CI, 1.48–2.72) of hospitalization.
Discussion
In this community‐based surveillance of hospitalized AMI, we make the following observations: (1) The population incidence rate of AMI‐IHO and proportion of AMI hospitalizations with in‐hospital onset increased from 1995 to 2014; (2) the temporal increase in AMI‐IHO was largely driven by patients aged ≥65 years; (3) patients with AMI‐IHO were more often older, White, and women, with greater comorbidity burden; (4) patients with AMI‐IHO were less likely to be managed by invasive strategy or guideline‐directed medications usually recommended for type 1 MI; and (5) the short‐ and long‐term all‐cause mortality was higher with AMI‐IHO and more often attributable to noncardiovascular causes. To our knowledge, this is the first study examining temporal trends in AMI‐IHO from a community‐based surveillance.
AMI‐IHO has previously been described, primarily in cross‐sectional analyses of administrative claims records for STEMI hospitalizations. 2 , 14 , 15 , 16 In contrast to other study designs, the ARIC study community surveillance uses a standardized, physician‐adjudicated algorithm for the classification of AMI, allowing an analysis of temporal trends spanning several decades. From 1995 to 2014, we observed a significant increase in the proportion of AMI hospitalizations with in‐hospital onset, a temporal trend primarily driven by patients aged ≥65 years. A previous analysis from the California State Inpatient database reported a higher incidence of AMI‐IHO among patients undergoing either cardiac or noncardiac surgical procedures during their hospital stay. 2 Consistent with this report, the majority of patients with AMI‐IHO in our study population underwent operative procedures in 2005 to 2014, although procedure codes were not abstracted throughout the surveillance period from 1995 to 2004. One possible explanation for the rising trend in AMI‐IHO among patients aged ≥65 years may be temporal increases in inpatient surgical procedures provoking myocardial ischemia. 17 , 18 The use of inpatient procedures is reported to increase with Medicare eligibility at the age of 65 7 and has also increased over time for patients aged ≥65 years. 8 , 9 The rising proportion of AMI‐IHO may also be attributable to worsening acuity of older hospitalized patients in recent years, 10 , 11 which could heighten the risk of infarction either with or without inpatient procedures. On the other hand, it is also possible that AMI‐IHO constitutes an increasing proportion of AMI hospitalizations in recent years because of better recognition and management of traditional risk factors and a general community decline in out‐of‐hospital–onset AMI. 5 However, the population incidence rates of AMI‐IHO among residents in the 4 ARIC communities, which are not dependent on the number of out‐of‐hospital–onset AMI hospitalizations, also increased.
Consistent with our observations from the ARIC study community surveillance, previous studies report older age, more prevalent comorbidities, and a predominance of women among patient populations with AMI‐IHO. 2 , 14 , 15 , 16 Our analysis of adjudicated events was limited to patients with acute cardiac symptoms; however, atypical presentation was more common with AMI‐IHO, an observation also noted in previous studies. 14 In the present study, patients with AMI‐IHO were less often managed by guideline‐directed therapies, a pattern observed for both patients with STEMI and patients with NSTEMI. Previous investigations have similarly reported a lower use of invasive strategy and guideline‐directed therapies for patients with in‐hospital onset of STEMI 2 , 14 , 15 , 16 , NSTEMI, 1 , 19 and unstable angina. 20 The underlying reasons for the observed differences in management of in‐hospital–onset versus out‐of‐hospital–onset AMI are likely multifold. Patients with AMI‐IHO represent a sicker population, a category less likely to undergo an invasive strategy or receive evidence‐based therapies. 21 , 22 , 23 Given the high comorbidity burden and predominance of NSTEMI among patients with AMI‐IHO, 1 it is also plausible that AMI‐IHO largely reflects type 2 MI, a diagnosis associated with lower use of evidence‐based care, higher mortality, and diverse modes of death. 4 , 24 The lower use of guideline‐directed therapies among patients with AMI‐IHO is also likely to be influenced by atypical presentation of symptoms. Compared with out‐of‐hospital–onset AMI, AMI‐IHO was less frequently accompanied by acute chest pain, and among the subset of patients with AMI‐IHO, those with atypical presentation less often received guideline‐directed therapies.
The elevated in‐hospital mortality among patients with AMI‐IHO is likely to reflect both higher‐acuity hospitalizations and selective survival. Ventricular fibrillation/cardiac arrest was 3 times as prevalent with AMI‐IHO than with out‐of‐hospital–onset AMI. Given the poor prognosis for preadmission survival, 25 patients experiencing more severe out‐of‐hospital events with cardiac arrest would be less likely to be included in this surveillance of hospitalized patients with AMI. Beyond in‐hospital mortality, however, patients with AMI‐IHO also had higher 28‐day and 1‐year mortality. Moreover, patients with AMI‐IHO more often died of noncardiovascular causes compared with patients with out‐of‐hospital AMI.
Quality improvement programs have recently been initiated to enhance the recognition and management of inpatient‐onset STEMI. Current strategies include hospital‐wide education campaigns to promote early ECG acquisition and interpretation, adapting out‐of‐hospital systems of care for STEMI to in‐hospital–onset STEMI, and monthly review of each inpatient‐onset STEMI. 3 The Inpatient STEMI Quality Improvement Program, involving 17 participating hospitals, has also been initiated to evaluate time to recognition and time to treatment of inpatient‐onset STEMI. 6 , 26 However, few strategies have been proposed to improve recognition and management of inpatient‐onset NSTEMI, a diagnosis constituting the largest group of AMI‐IHO. 1 , 27 There is also a dearth of quality improvement initiatives aimed at the prevention of AMI‐IHO, whether type 1 or type 2 MI. Our observations from the ARIC study community surveillance suggest that quality improvement efforts should be especially focused on hospitalized patients aged >65, a group in which symptomatic AMI‐IHO appears to be increasing.
Our study has some limitations. The ARIC study community surveillance is localized to 4 US communities and may not be generalizable to the entire nation. Clinical data were limited by availability in the medical record and abstraction priority. Our descriptive analysis of surgical procedures and diagnoses at admission was limited to hospitalizations from 2005 onwards, when abstractions of ICD‐9 codes were available. We were unable to consider longitudinal outcomes of AMI hospitalization other than mortality, such as recurrent AMI or need for revascularization. Nor were we able to determine the cause of AMI‐IHO or differentiate type 1 from type 2 MI. Temporal evolution in the sensitivity of cardiac injury markers and more widespread measurement during hospitalization may have contributed to increased detection of myocardial injury; however, an upward trend in AMI‐IHO was observed only among older patients, with no accompanied increase among younger patients. This suggests that the temporal trends in AMI‐IHO were not driven solely by biomarker sensitivity. Our study also has several unique and noteworthy strengths. The ARIC study community surveillance provides a large, multiyear surveillance of 4 diverse US communities. Clinical and laboratory values were meticulously collected by certified abstractors following standardized protocols. AMI was classified by consistent criteria based on standardized physician review of the medical record, allowing an analysis of trends spanning several decades. Furthermore, mortality outcomes were verified by the National Death Index. Altogether, these protocols culminate in greater standardization of these observational data.
Conclusions
In this community‐based surveillance of patients hospitalized with symptomatic AMI from 1995 to 2014, the proportion of hospitalizations with AMI‐IHO appears to be increasing, particularly among those aged ≥65 years. Patients with in‐hospital–onset AMI have more comorbidities, a lower likelihood of receiving guideline‐directed therapies, lower use of invasive angiography or revascularization, and worse short‐ and long‐term survival. Greater recognition of AMI‐IHO and efforts to develop effective prevention and management strategies are warranted to improve outcomes for these patients.
Sources of Funding
The ARIC study was funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I.
Disclosures
Dr Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/National Center for Advancing Translational Sciences (NCATS) Award UL 1TR002541) and serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, and Relypsa. Dr Qamar is supported by institutional grant support from the NorthShore Auxiliary research scholar fund and has received funding from Daiichi‐Sankyo, American Heart Association and fees for educational activities from the American College of Cardiology, Society for Vascular Medicine, Society for Cardiovascular Angiography and Interventions, Janssen and Janssen, Pfizer, Medscape, and Clinical Exercise Physiology Association. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Figures S1–S6
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Author contributions: Drs Caughey and Arora conceptualized the study and interpreted the data. Dr Caughey performed the statistical analysis and wrote the manuscript. Drs Qamar, Chunawala, Gupta, Gupta, Vaduganathan, Pandey, Dai, Smith, and Matsushita interpreted the data and revised the manuscript critically.
(J Am Heart Assoc.2021;10:e018414. DOI: 10.1161/JAHA.120.018414.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Maynard C, Lowy E, Rumsfeld J, Sales AE, Sun H, Kopjar B, Fleming B, Jesse RL, Rusch R, Fihn SD. The prevalence and outcomes of in‐hospital acute myocardial infarction in the Department of Veterans Affairs health system. Arch Intern Med. 2006;166:1410–1416. [DOI] [PubMed] [Google Scholar]
- 2. Kaul P, Federspiel JJ, Dai X, Stearns SC, Smith SC, Yeung M, Beyhaghi H, Zhou L, Stouffer GA. Association of inpatient vs outpatient onset of ST‐elevation myocardial infarction with treatment and clinical outcomes. JAMA. 2014;312:1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dai X, Meredith D, Sawey E, Kaul P, Smith SC Jr, Stouffer GA. A quality improvement program for recognition and treatment of inpatient ST‐segment elevation myocardial infarctions. JAMA Cardiol. 2016;1:1077–1079. [DOI] [PubMed] [Google Scholar]
- 4. Arora S, Strassle PD, Qamar A, Wheeler EN, Levine AL, Misenheimer JA, Cavender MA, Stouffer GA, Kaul P. Impact of type 2 myocardial infarction (MI) on hospital‐level MI outcomes: implications for quality and public reporting. J Am Heart Assoc. 2018;7:e008661. DOI: 10.1161/JAHA.118.008661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosamond W, Chambless L, Heiss G, Mosley T, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom A. Twenty‐two year trends in incidence of myocardial infarction, CHD mortality, and case‐fatality in four US communities, 1987 to 2008. Circulation. 2012;125:1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dai X, Kaul P, Smith SC, Stouffer GA. Predictors, treatment, and outcomes of STEMI occurring in hospitalized patients. Nat Rev Cardiol. 2016;13:148–154. [DOI] [PubMed] [Google Scholar]
- 7. Lichtenberg FR. The effects of Medicare on health care utilization and outcomes. Forum Health Econ Pol. 2006;5:1028. [Google Scholar]
- 8. Anderson PL, Gelijns A, Moskowitz A, Arons R, Gupta L, Weinberg A, Faries PL, Nowygrod R, Kent KC. Understanding trends in inpatient surgical volume: vascular interventions, 1980–2000. J Vasc Surg. 2004;39:1200–1208. [DOI] [PubMed] [Google Scholar]
- 9. Oliphant SS, Ghetti C, McGough RL, Wang L, Bunker CH, Lowder JL. Inpatient procedures in elderly women: an analysis over time. Maturitas. 2013;75:349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krishnan U, Brejt JA, Schulman‐Marcus J, Swaminathan RV, Feldman DN, Goyal P, Wong SC, Minutello RM, Bergman G, Singh H, et al. Temporal trends in the clinical acuity of patients with ST‐segment elevation myocardial infarction. Am J Med. 2018;131:100.e9–100.e20. [DOI] [PubMed] [Google Scholar]
- 11. Pandey A, Vaduganathan M, Arora S, Qamar A, Mentz RJ, Shah SJ, Chang PP, Russell SD, Rosamond WD, Caughey MC. Temporal trends in prevalence & prognostic implications of comorbidities among patients with acute decompensated heart failure: the ARIC study community surveillance. Circulation. 2020;142:230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mansournia MA, Altman DG. Inverse probability weighting. BMJ. 2016;352:1–2. [DOI] [PubMed] [Google Scholar]
- 13. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. J Am Med Assoc. 1998;280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 14. Dai X, Bumgarner J, Spangler A, Meredith D, Smith SC, Stouffer GA. Acute ST‐elevation myocardial infarction in patients hospitalized for noncardiac conditions. J Am Heart Assoc. 2013;2:e000004. DOI: 10.1161/JAHA.113.000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garberich RF, Traverse JH, Claussen MT, Rodriguez G, Poulose AK, Chavez IJ, Rutten‐Ramos S, Hildebrandt DA, Henry TD. ST‐elevation myocardial infarction diagnosed after hospital admission. Circulation. 2014;129:1225–1232. [DOI] [PubMed] [Google Scholar]
- 16. Erne P, Bertel O, Urban P, Pedrazzini G, Lüscher TF, Radovanovic D. Inpatient versus outpatient onsets of acute myocardial infarction. Eur J Intern Med. 2015;26:414–419. [DOI] [PubMed] [Google Scholar]
- 17. Devereaux PJ, Biccard BM, Sigamani A, Xavier D, Chan MTV, Srinathan SK, Walsh M, Abraham V, Pearse R, Wang CY, et al. Association of postoperative high‐sensitivity troponin levels with myocardial injury and 30‐day mortality among patients undergoing noncardiac surgery. J Am Med Assoc. 2017;317:1642–1651. [DOI] [PubMed] [Google Scholar]
- 18. Puelacher C, Buse GL, Seeberger D, Sazgary L, Marbot S, Lampart A, Espinola J, Prof CK, Hammerer A, Seeberger E, et al. Perioperative myocardial injury after noncardiac surgery incidence, mortality, and characterization. Circulation. 2018;137:1221–1232. [DOI] [PubMed] [Google Scholar]
- 19. Mazzella AJ, Abisogun AA, Caughey M, Dai X. Mortality rates and length of stay in patients with acute non–ST segment elevation myocardial infarction hospitalized for noncardiac conditions on surgical versus nonsurgical services. Am J Cardiol. 2017;120:1472–1478. [DOI] [PubMed] [Google Scholar]
- 20. Zafar K, Patil N. Inpatient‐ versus outpatient‐onset acute coronary syndrome: comparison of clinical features and outcomes. Texas Hear Inst J. 2018;45:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhatt DL, Roe MT, Peterson ED, Li Y, Chen AY, Harrington RA, Greenbaum AB, Berger PB, Cannon CP, Cohen DJ, et al. Utilization of early invasive management strategies for high‐risk patients with non–ST‐segment elevation acute coronary syndromes. J Am Med Assoc. 2004;292:2096. [DOI] [PubMed] [Google Scholar]
- 22. Arora S, Stouffer GA, Kucharska‐Newton A, Vaduganathan M, Qamar A, Matsushita K, Kolte D, Reynolds HR, Bangalore S, Rosamond WD, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203. DOI: 10.1161/JAHA.118.010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steg PG, Dabbous OH, Feldman LJ, Cohen‐Solal A, Aumont MC, López‐Sendón J, Budaj A, Goldberg RJ, Klein W, Anderson FA. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation. 2004;109:494–499. [DOI] [PubMed] [Google Scholar]
- 24. Tahir K, Pauley E, Dai X, Smith SC, Sweeney C, Stouffer GA. Mechanisms of ST elevation myocardial infarction in patients hospitalized for noncardiac conditions. Am J Cardiol. 2019;123:1393–1398. [DOI] [PubMed] [Google Scholar]
- 25. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levine GN, Dai X, Henry TD, Press Calfon M, Denktas AE, Garberich RF, Jacobs AK, Jaski BE, Kaul P, Kontos MC, et al. In‐hospital ST‐segment elevation myocardial infarction improving diagnosis, triage, and treatment. JAMA Cardiol. 2018;3:527–531. [DOI] [PubMed] [Google Scholar]
- 27. Bradley SM, Borgerding JA, Wood GB, Maynard C, Fihn SD. Incidence risk factors, and outcomes associated with in‐hospital acute myocardial infarction. JAMA Netw Open. 2019;2:e187348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S6


