Abstract
Background
Evidence‐based therapies are generally underused for cardiovascular risk reduction; however, less is known about contemporary patients with type 2 diabetes mellitus and atherosclerotic cardiovascular disease.
Methods and Results
Pharmacy and medical claims data from within Anthem were queried for patients with established atherosclerotic cardiovascular disease and type 2 diabetes mellitus. Using an index date of April 18, 2018, we evaluated the proportion of patients with a prescription claim for any of the 3 evidence‐based therapies on, or covering, the index date ±30 days: high‐intensity statin, angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, and sodium glucose cotransporter‐2 inhibitor or glucagon‐like peptide‐1 receptor agonist. The potential benefit of achieving 100% adoption of all 3 evidence‐based therapies was simulated using pooled treatment estimates from clinical trials. Of the 155 958 patients in the sample, 24.7% were using a high‐intensity statin, 53.1% were using an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, and 9.9% were using either an sodium glucose cotransporter‐2 inhibitor or glucagon‐like peptide‐1 receptor agonists. Overall, only 2.7% of the population were covered by prescriptions for all 3 evidence‐based therapies, and 37.4% were on none of them. Over a 12‐month period, 70.6% of patients saw a cardiologist, while only 18% saw an endocrinologist. Increasing the use of evidence‐based therapies to 100% over 3 years of treatment could be expected to reduce 4546 major atherosclerotic cardiovascular events (myocardial infarction, stroke, or cardiovascular death) in eligible but untreated patients.
Conclusions
Alarming gaps exist in the contemporary use of evidence‐based therapies in this large population of insured patients with type 2 diabetes mellitus and atherosclerotic cardiovascular disease. These data provide a call to action for patients, providers, industry, regulators, professional societies, and payers to close these gaps in care.
Keywords: atherosclerotic cardiovascular disease, diabetes mellitus, evidence‐based
Subject Categories: Secondary Prevention, Atherosclerosis
Atherosclerotic cardiovascular disease (ASCVD) is twice as common in patients with type 2 diabetes mellitus (T2DM), where it imparts greater attributable morbidity and more frequent premature death. 1 Thus, professional societies universally recommend an aggressive approach to risk reduction among patients with T2DM.
Several classes of medications have a proven evidence base for patients with T2DM and ASCVD, including high‐intensity statins, 2 , 3 angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), 4 , 5 and sodium glucose cotransporter‐2 inhibitors (SGLT‐2is) 6 or glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs). 7 Despite a wealth of data supporting their benefit, available evidence from small studies suggests that these agents are consistently underused. 8
We sought to determine the contemporary rates and patterns of evidence‐based therapy use in a large cohort of patients with T2DM and ASCVD. Furthermore, we sought to estimate the potential treatment effect of closing the gap from observed to optimal rates of evidence‐based therapy use.
Methods
The data that support the findings of this study are available in aggregate from the corresponding author upon reasonable request and governed under strict data use agreements. Longitudinal administrative claims data (medical, pharmacy, and laboratory) of active patients from Anthem, a large commercial insurer with nationwide US representation, were analyzed. An index date of April 30, 2018, with a 1‐year baseline period from April 30, 2017, was used to determine cohort eligibility and comorbidity profile.
International Classification of Diseases, Tenth Revision (ICD‐10) codes (Table S1) were used to identify a population with T2DM and ASCVD, defined by the following conditions: (1) coronary artery disease, including prior myocardial infarction and revascularization; (2) cerebrovascular disease, including nonhemorrhagic stroke; and (3) peripheral arterial disease, including claudication, revascularization, and amputation. Patients in this cohort were considered to have indications for all 3 evidence‐based therapies (high‐intensity statins, ACEIs/ARBs, and SGLT‐2is/GLP‐1RAs). To increase the likelihood of the cohort's eligibility for ACEI/ARB and/or SGLT‐2i use, patients with estimated glomerular filtration rate <30 mL/min per 1.73 m2 (by the Chronic Kidney Disease Epidemiology Collaboration formula, in those with available serum creatinine), ICD‐10 codes for stage 4 and 5 chronic kidney disease, and/or procedural codes for dialysis were excluded.
The primary outcome was the proportion of patients with a prescription claim for each of the 3 classes of evidence‐based therapy on, or covering, the index date ±30 days (herein referred to as “use”): high‐intensity statin (atorvastatin 40–80 mg or rosuvastatin 20–40 mg), ACEI/ARB (or angiotensin receptor‐neprilysin inhibitor), and SGLT‐2i or GLP‐1RA).
An estimation of the potential benefits of increasing the use of evidence‐based therapies to 100% was performed as described previously. 9 The effect of each therapy was evaluated separately. Conservative estimates of treatment effect for reducing the composite of myocardial infarction, stroke, and cardiovascular death were derived from meta‐analyses evaluating each class (or classes) of therapy in patients with T2DM and established ASCVD. The 3‐year standardized number needed to treat was used to calculate the potential major atherosclerotic cardiovascular events (MACEs) prevented for each individual therapy and then summed to determine the overall effect. As described previously, 9 , 10 , 11 this calculation is predicated on 2 key assumptions regarding the magnitude of treatment benefit, specifically, that a therapy's benefit is linear over time and additive to each other. A range of potential benefit was determined first by increasing and decreasing the individual number needed to treat of each therapy by 20%, and second by considering each successive therapy as not fully additive by attenuating the effectiveness of each by 20%. A series of sensitivity analyses were also performed by using an average expected absolute risk reduction per component of evidence‐based therapy and estimating the effect of adding 1, 2, or 3 therapies to each of the untreated populations. As above, this was performed in a wholly additive manner and compared with successive 20% attenuation of treatment effect (Table S2).
Characteristics of patients either using or not using each of the three therapies are presented as median (Q1–Q3), mean±SD, or number (%), as appropriate. Univariate comparisons between groups were evaluated by t test or chi‐squared test, as appropriate.
The project received a waiver of informed consent by the New England Institutional Review Board. Analyses were performed using the Instant Health Data platform (Boston Health Economics, Boston, MA). Statistical analyses were performed using R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Of the 1 011 245 patients with T2DM insured with Anthem on the index date, 699 380 patients were enrolled over the preceding 12‐month baseline period (Figure 1). Of these, 155 958 had evidence of ASCVD and were >18 years of age without evidence of chronic kidney disease stage 4 or 5. Characteristics of the cohort are presented in Table 1, overall and by prescription fill of each of the 3 agents. In the preceding 12 months from index date, 70.6% of the cohort were seen by a cardiologist, while only 18% were seen by an endocrinologist.
Figure 1. CONSORT diagram of eligible cohort.
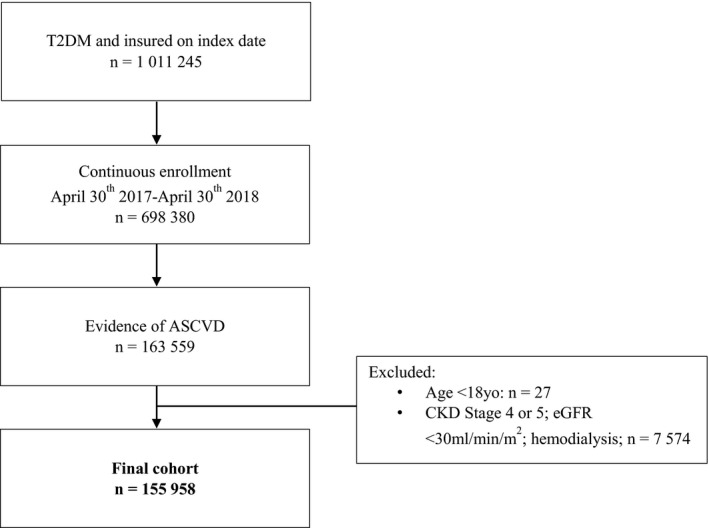
ASCVD indicates atherosclerotic cardiovascular disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; and T2DM, type 2 diabetes mellitus.
Table 1.
Eligible Cohort of Patients With Diabetes Mellitus and ASCVD; Overall and by Therapy Target
| High‐Intensity Statin | ACEI or ARB | SGLT‐2i or GLP‐1RA | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
Filled |
Not Filled |
P Value |
Filled |
Not Filled |
P Value |
Filled |
Not Filled |
P Value | |
| Population, N (%) | 38 465 (24.7) | 117 493 (75.3) | 82 782 (53.1) | 73 176 (46.9) | 15 836 (9.9) | 140 122 (90.1) | |||
| Age, y, mean±SD | 66.5±12.3 | 65.1±10.4 | <0.01 | 65.7±12.7 | 66.7±11.1 | <0.01 | 61.3±9.0 | 66.7±12.0 | <0.01 |
| Sex, % | |||||||||
| Male | 68.6 | 58.2 | <0.01 | 61.9 | 59.5 | <0.01 | 65.8 | 60.2 | <0.01 |
| Female | 31.4 | 41.8 | 38.1 | 40.5 | 34.2 | 39.8 | |||
| Region, % | |||||||||
| Midwest | 30.4 | 29.4 | All <0.01 | 29.7 | 29.4 | All <0.01 | 22.9 | 30.4 | All <0.01 |
| Northeast | 19.2 | 20.5 | 20.0 | 20.4 | 16.2 | 20.6 | |||
| South | 31.1 | 29.5 | 30.3 | 29.4 | 39.1 | 28.9 | |||
| West | 19.3 | 20.7 | 19.9 | 20.8 | 21.8 | 20.2 | |||
| Insurance, % | |||||||||
| Primary | 68.6 | 67.9 | 0.0127 | 65.3 | 71.2 | <0.01 | 85.3 | 66.1 | <0.01 |
| Medicare advantage | 31.4 | 32.1 | 34.7 | 28.8 | 14.7 | 33.9 | |||
| Cardiovascular history, % | |||||||||
| CAD | 81.7 | 65.3 | all | 70.1 | 68.5 | <0.01 | 69.5 | 69.4 | NS |
| CeVD | 19.1 | 16.9 | <0.01 | 17.4 | 17.4 | NS | 14.5 | 17.8 | <0.01 |
| PAD | 35.5 | 45.3 | 42.1 | 43.7 | <0.01 | 42.5 | 42.9 | NS | |
| Dyslipidemia, % | 93.9 | 84.9 | <0.01 | 89.6 | 84.3 | <0.01 | 92.4 | 86.5 | <0.01 |
| Hypertension, % | 94.2 | 91.1 | <0.01 | 96.8 | 86.2 | <0.01 | 93.6 | 91.6 | <0.01 |
| Heart failure, % | 22.8 | 20.2 | <0.01 | 20.5 | 21.1 | <0.01 | 15.3 | 21.5 | <0.01 |
| Obesity, % | 27.9 | 25.9 | <0.01 | 27.5 | 25.2 | <0.01 | 37.1 | 25.2 | <0.01 |
| HbA1c, mean±SD | 7.3±1.6 | 7.1±1.7 | <0.01 | 7.2±1.6 | 7.1±1.7 | <0.01 | 7.7±1.6 | 7.0±1.7 | <0.01 |
| Glycemic medications | |||||||||
| Metformin (only) | 56.6 | 43.23 | 56.5 | 35.2 | 63.9 | 44.6 | |||
| Thiazolidinedione | 5.2 | 3.8 | all | 5.2 | 3.0 | all | 9.4 | 3.6 | all |
| Sulfonylurea | 25.1 | 20.9 | <0.01 | 26.9 | 16.3 | <0.01 | 33.0 | 20.7 | <0.01 |
| Dipeptidyl peptidase‐4 | 17.5 | 13.3 | 17.4 | 10.9 | 25.6 | 13.0 | |||
| SGLT‐2i | 9.0 | 4.8 | 7.7 | 3.8 | 57.6 | 0 | |||
| GLP‐1RA | 8.2 | 4.9 | 7.5 | 3.7 | 56.3 | 0 | |||
| Insulin | 29.5 | 21.9 | 27.4 | 19.6 | 42.8 | 21.6 | |||
| Healthcare use, % | |||||||||
| Cardiology visit | 78.3 | 68.1 | <0.01 | 71.2 | 70.0 | <0.01 | 68.0 | 70.9 | <0.01 |
| Endocrinology visit | 19.9 | 17.5 | 18.6 | 17.6 | 36.8 | 16.0 | |||
Data are presented as mean±SD or median (Q1, Q3), as appropriate. Comparisons between groups (filled vs not filled) were performed with either Student t test or chi‐squared test, as appropriate. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; CeVD, cerebrovascular disease; GLP‐1RA, glucagon‐like peptide receptor‐1 agonist; HbA1c, glycosylated hemoglobin; PAD, peripheral arterial disease; and SGLT‐2i, sodium glucose cotransporter 2 inhibitor.
Patients using a high‐intensity statin were more likely to be male, have coronary ASCVD and dyslipidemia, and to have been seen by a cardiologist in the past 12 months. Patients using either an SGLT‐2i or a GLP‐1RA were generally younger and more often male. Patients prescribed one of the newer antiglycemic agents were also more likely to be located in the South than the Midwest or Northeast, have primary commercial rather than Medicare Advantage plans, and were more likely to have seen an endocrinologist in the past 12 months. While obesity was more common among patients using either an SGLT‐2i or a GLP‐1RA, those with heart failure were notably less likely to be on these agents. Although similarly likely to have filled a prescription for an ACEI or an ARB (38.1% versus 40.5%), women were less likely to be among those filling a prescription for a high‐intensity statin (31.4% versus 41.8%) or either an SGLT‐2i or a GLP‐1RA (34.2% versus 39.8%).
On the index date, 24.7% were on a high‐intensity statin (49.9% were on a non–high‐intensity statin), 53.1% were on an ACEI/ARB/angiotensin receptor–neprilysin inhibitor, and 9.9% were on either an SGLT‐2i or a GLP‐1RA. Overall, only 2.7% of the population were covered by a prescription for all 3 evidence‐based therapies, 19.4% were on 2, 40.7% were on 1, and 37.4% were on none (Figure 2).
Figure 2. Proportions of patients on all 3, 2, 1, or none of the evidence‐based therapies: high‐intensity statin, ACEI or ARB, SGLT‐2i or GLP‐1RA.
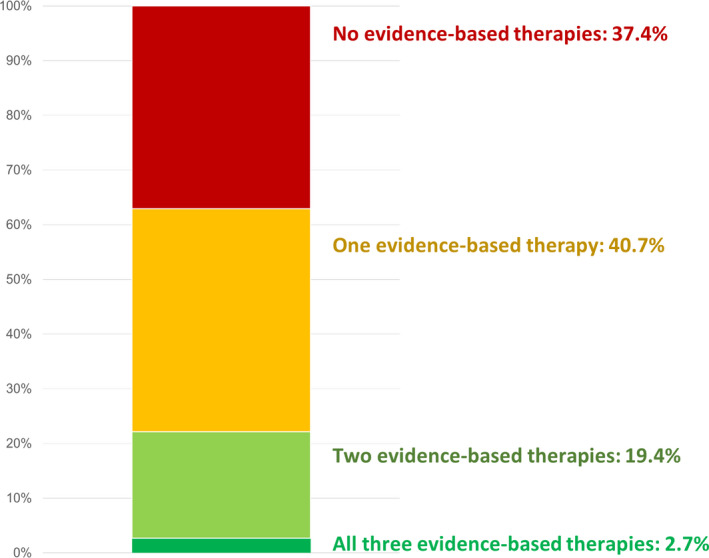
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; GLP‐1RA, glucagon‐like peptide receptor‐1 agonist; and SGLT‐2i, sodium glucose cotransporter 2 inhibitor.
The number of MACE prevented by closing the gap in the use of each respective evidence‐based therapy would be 1415 for high‐intensity statins, 871 for ACEIs/ARBs, and 2260 for SGLT‐2is or GLP‐1RAs, yielding a potential total of 4546 at 3 years (Table 2). 12 Using an analysis of extremes (±20% number needed to treat), this total MACE prevention figure ranges from 3810 to 5731 (Table 3), and by considering each therapy as not completely additive (−20% successive effectiveness), the number of MACE prevented is 3659. By assuming various estimates of treatment effect by baseline use, the number of MACE prevented ranged from 3416 to 4443 over a 3‐year period.
Table 2.
Relative and Absolute Risk Reduction and Number Needed to Treat Derived From Meta‐Analyses for Each of the Evidence‐Based Therapies
| RRR | ARR | NNT for MACE (mo) | NNT for MACE (Over 3 y) | |
|---|---|---|---|---|
| High‐intensity statin* | 15% | 0.4% per year (2.4% vs 2.8%) | 250 over 12 | 83 |
| ACEI/ARB † | 11% | 1.4% over 3.5 y (12.9% vs 14.3%) | 71 over 42 | 83 |
| SGLT‐2i/GLP‐1RA ‡ | 14% | 1.6% over 3.1 y (10.5% vs 12.1%) | 62 over 37 | 62 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARR, absolute risk reduction; GLP‐1RA, glucagon‐like peptide receptor‐1 agonist; NNT, number needed to treat; RRR, relative risk reduction; and SGLT‐2i, sodium glucose cotransporter 2 inhibitor.
High‐intensity vs moderate or low‐intensity statin; hazard ratio, 0.85 (95% CI, 0.82–0.89). 2 Conservative estimate given 25% were on no statin at all.
ACEI/ARB in normotensive participants with atherosclerotic cardiovascular disease; hazard ratio, 0.89 (95% CI, 1.85–0.93). 5 Conservative estimate given 90% of patients had hypertension and may derive greater benefit.
ARR and NNT were derived from a pooled meta‐analysis of GLP‐1RA and SGLT‐2i trials in patients with established atherosclerotic cardiovascular disease; hazard ratio, 0.86 (95% CI, 0.80–0.93). 12
Table 3.
Estimation of MACE Reduction by Applying NNT to Eligible but Untreated Population With Associated Sensitivity Analysis
| Population Untreated, N (%) | Estimated MACE Reduction Over 3 y | Sensitivity Analysis | |
|---|---|---|---|
| High‐intensity statin | 117 493 (75.3) | 1415 | (1186–1780) |
| ACEI/ARB | 73 176 (46.9) | 871 | (731–1092) |
| SGLT‐2i/GLP‐1RA | 140 122 (90.1) | 2260 | (1893–2859) |
| Total | 4546 | (3810–5731) |
The NNTs were applied to the eligible but untreated patients to calculate the number of potential MACE prevented following 3 years of therapy. A sensitivity analysis of extremes was performed increasing and decreasing the NNT by 20%. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; GLP‐1RA, glucagon‐like peptide receptor‐1 agonist; MACE, major adverse cardiovascular event; NNT, number needed to treat; and SGLT‐2i, sodium glucose cotransporter 2 inhibitor.
Discussion
These data reveal an alarming gap in the use of evidence‐based therapies for patients with T2DM and ASCVD. Among this commercially insured population, almost no one was on all 3 evidence‐based therapies, and over one‐third of these patients were on none of them. This marked underuse of evidence‐based therapies in T2DM, a condition that contributes significantly to the overall burden and growth of cardiovascular disease in the United States, provides a compelling case to address this critical gap in care.
Our estimate that only 2.7% are using all 3 evidence‐based therapies is even less than a recent analysis from the GOULD (Getting to an Improved Understanding of Low‐Density Lipoprotein Cholesterol and Dyslipidemia Management) registry. In a subgroup analysis of 1735 patients with T2DM enrolled between 2016 and 2018, 6.9% were prescribed a similar group of medications (our 3 components plus an antiplatelet), a figure that is also alarmingly low. 8 Increased rates of evidence‐based therapy use may reflect differences in end point ascertainment; specifically, medication prescription occurs upstream from prescription fill and thus GOULD data represent a more generous estimate of “use.” Furthermore, GOULD estimates of evidence‐based therapy use may be less representative of the general population because of patient selection and, given that the registry was designed to understand physician prescribing practices, may have affected prescribing behavior through a Hawthorne phenomenon on the physicians.
Despite strong evidence that has evolved over the past 5 years for SGLT‐2is 13 and GLP‐1RAs, 14 only 1 in 10 eligible patients in our cohort were using these medications. This figure is likely an overestimate for the broader US population, as our commercially insured cohort would be less likely to face cost or access barriers. That being said, the costs of these agents remain high (average monthly full cost of empagliflozin, for example, is ≈$US500 15 ). This could lead to substantial out‐of‐pocket or copay costs for patients, regardless of insurance type, which is likely one barrier to use of these effective therapies.
The identification of geographic and demographic patterns of underuse highlight both locations and subgroups in need of stronger efforts to close gaps in evidence‐based care. In particular, our findings continue to reinforce the presence of sex‐based disparities in the use of therapies for cardiovascular risk reduction. While women were overall less common in our sample, those who were included were less likely to use evidence‐based therapies—whether this reflects clinician prescribing behavior or patient adherence, or a combination of the 2, cannot be determined from claims data.
The barriers to the adoption of evidence‐based therapies have patient, clinician, and system‐level origins. Lack of clinician familiarity; variation in the content, strength, and latency of society guidelines; clinical inertia; perceived interdisciplinary boundaries; fragmented care pathways; increasing patient comorbidity; and the aforementioned cost considerations all likely contribute to the observed evidence‐to‐practice gap.
We estimate that the adoption of evidence‐based therapy use in all patients could prevent 4546 (sensitivity analyses range, 3416–5731) myocardial infarction, stroke, or cardiovascular deaths over a 3‐year period of treatment. The majority of the estimated benefit is driven by increasing the adoption of either SGLT‐2is or GLP‐1RAs given their lower overall current use (hence greater potential for improvement) and lower number needed to treat (higher absolute risk reduction). While patients with contraindications and intolerance are likely to limit complete use, these numbers provide a compelling argument to develop population health strategies to aid adoption of evidence‐based therapies. With patients 3 times more likely to see a cardiologist than an endocrinologist, our data suggest that cardiologists, along with primary care physicians, can and should play a key role in closing these gaps in care.
There are a number of limitations to these analyses. The data come from a single US managed care population that are not representative of the overall population of patients with ASCVD and T2DM, although this is a best‐case estimate compared with the less well insured general population. Assessment of eligibility has not taken into account patients with absolute contraindications beyond kidney dysfunction. While SGLT‐2is appear to have similar class‐wide efficacy, there is greater heterogeneity in the cardiovascular benefit derived from the various GLP‐1RAs; yet for this analysis, all agents in each of the classes were considered evidence‐based therapies. Importantly, it must also be acknowledged that while the evidence base for recommending these therapies has been established for several years, it would be premature to critique adherence to guideline recommendations. Indeed, one of the hurdles to effective translation of a therapy with a proven evidence base to its widespread adoption in clinical practice is the slow pace and variable strength of guideline recommendations. Notwithstanding this, the most recent updates of the American and European guidelines consistently recommend these therapies, 16 , 17 , 18 , 19 and thus this analysis, in some respects, represents a starting landscape of clinical care. The estimation of potential treatment effect makes a number of assumptions, including 100% patient eligibility, linear efficacy over time, and additive therapeutic benefit. While the validity of the additive benefit assumption may vary by baseline treatment status, the majority of the overall population‐level effect is derived from the commencement of either an SGLT‐2i or a GLP‐1RA. Given that clinical trials of these agents were performed in the context of high (≈80%) ACEI/ARB and statin use, the assumption of additive benefit is likely to be robust.
Ultimately, these data provide a much needed call to action for patients, providers, industry, regulators, professional societies, and payers to support high‐quality and rigorous research evaluating ways to close these gaps in care. Methods to overcome these barriers must comprehensively engage, empower, and incentivize patients, clinicians, and health systems to achieve the shared goals of high‐quality care.
Sources of Funding
This manuscript was funded internally by the Duke Clinical Research Institute (Durham, NC).
Disclosures
Dr Carnicelli reports research funding from the National Institutes of Health T32 training grant. Dr Nelson reports research funding from Diabetes Australia and the Royal Australasian College of Physicians. Dr Granger reports research grants from Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Janssen, Pfizer, Armetheon, AstraZeneca, US Food & Drug Administration, GlaxoSmithKline, The Medicines Company, Medtronic Foundation, Medtronic Inc., and Novartis; and consulting fees from Bayer, Boehringer Ingelheim, Boston Scientific, Bristol‐Myers Squibb, Daiichi Sankyo, Janssen, Pfizer, Abbvie, Armetheon, Astra Zeneca, Eli Lilly, GlaxoSmithKline, Hoffmann‐La Roche, The Medicines Company, National Institutes of Health, Novartis, Sirtex, Verseon, Apple, Medscape LLC, Merck, Novo Nordisk, Roche Diagnostics, and Rho Pharmaceuticals. Dr McGuire reports clinical trial leadership for AstraZeneca, Sanofi Aventis, Janssen, Boehringer Ingelheim, Merck & Co, Pfizer, NovoNordisk, Lexicon, Eisai, GlaxoSmithKline, and Esperion; consulting fees from AstraZeneca, Sanofi Aventis, Lilly US, Boehringer Ingelheim, Merck & Co, Pfizer, Novo Nordisk, Applied Therapeutics, Afimmune, and Metavant. Dr O'Brien reports research grants from Novartis, Bristol‐Myers Squibb, and Novo Nordisk. Dr Green reports research grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Sanofi; and consulting fees from AstraZeneca, Merck, Boehringer Ingelheim, Sanofi/Regeneron, and NovoNordisk. Dr Haynes is an employee of Anthem. Dr Pagidipati reports research grants from Regeneron Pharmaceuticals, Sanofi‐Aventis, Boehringer Ingelheim, NovoNordisk, and Verily Life Sciences. Dr Lopes reports research grants from Bristol‐Myers Squibb, Pfizer, Amgen, Inc., GlaxoSmithKline, Medtronic PLC, and Sanofi Aventis; and consulting fees from Bristol‐Myers Squibb, Pfizer, Boehringer Ingelheim, and Bayer AG. Shambhu is an employee of Anthem. Dr Eapen is an employee of Anthem. Ardissino has no disclosures to report.
Supporting information
Tables S1–S2
(J Am Heart Assoc.2021;10:e016835. DOI: 10.1161/JAHA.120.016835.)
For Sources of Funding and Disclosures, see page 7.
REFERENCES
- 1. Granger CB, Nelson AJ, Pagidipati NJ. Risk of total events with icosapent ethyl: can we reduce it? J Am Coll Cardiol. 2019;73:2803–2805. [DOI] [PubMed] [Google Scholar]
- 2. Cholesterol Treatment Trialists C , Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Haffner S, Hsia J, Breazna A, LaRosa J, Grundy S, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220–1226. [DOI] [PubMed] [Google Scholar]
- 4. Heart Outcomes Prevention Evaluation Study Investigators , Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med. 2000;342:145–153. [DOI] [PubMed] [Google Scholar]
- 5. Renin Angiotension System Modulator Meta‐Analysis Investigators . Angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers are beneficial in normotensive atherosclerotic patients: a collaborative meta‐analysis of randomized trials. Eur Heart J. 2012;33:505–514. [DOI] [PubMed] [Google Scholar]
- 6. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT‐2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 7. Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Kober L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–785. [DOI] [PubMed] [Google Scholar]
- 8. Arnold SV, de Lemos JA, Rosenson RS, Ballantyne CM, Liu Y, Mues KE, Alam S, Elliott‐Davey M, Bhatt DL, Cannon CP, et al. Use of guideline‐recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Circulation. 2019;140:618–620. [DOI] [PubMed] [Google Scholar]
- 9. Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence‐based heart failure therapies on mortality. Am Heart J. 2011;161:1024–1030.e1023. [DOI] [PubMed] [Google Scholar]
- 10. Alexander KP, Peterson ED, Granger CB, Casas AC, Van de Werf F, Armstrong PW, Guerci A, Topol EJ, Califf RM. Potential impact of evidence‐based medicine in acute coronary syndromes: insights from GUSTO‐IIb. Global use of strategies to open occluded arteries in acute coronary syndromes trial. J Am Coll Cardiol. 1998;32:2023–2030. [DOI] [PubMed] [Google Scholar]
- 11. Chew DP, Huynh LT, Liew D, Astley C, Soman A, Brieger D. Potential survival gains in the treatment of myocardial infarction. Heart. 2009;95:1844–1850. [DOI] [PubMed] [Google Scholar]
- 12. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Comparison of the effects of glucagon‐like peptide receptor agonists and sodium‐glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022–2031. [DOI] [PubMed] [Google Scholar]
- 13. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 14. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, Lawson FC, Ping L, Wei X, Lewis EF, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. [DOI] [PubMed] [Google Scholar]
- 15. GoodRx . Search: ‘empagliflozin’.Available at: https://www.goodrx.com/empagliflozin2020. Accessed August 10, 2020.
- 16. American Diabetes A . 10. Cardiovascular disease and risk management: standards of medical care in diabetes‐2019. Diabetes Care. 2019;42:S103–S123. [DOI] [PubMed] [Google Scholar]
- 17. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, et al. 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 18. Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL Jr, Kalyani RR, Kosiborod M, Magwire ML, Morris PB, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72:3200–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APHA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


