Abstract
Background
Statins are hypothesized to reduce the risk of cardiotoxicity associated with anthracyclines and trastuzumab. Our aim was to study the association of statin exposure with hospitalization or emergency department visits (hospital presentations) for heart failure (HF) after anthracycline‐ and/or trastuzumab‐containing chemotherapy for early breast cancer.
Methods and Results
Using linked administrative databases, we conducted a retrospective cohort study of women aged ≥66 years without prior HF who received anthracyclines or trastuzumab for newly diagnosed early breast cancer in Ontario between 2007 to 2017. Statin‐exposed and unexposed women were matched 1:1 using propensity scores. Trastuzumab‐treated women were also matched on anthracycline exposure. We matched 666 statin‐discordant pairs of anthracycline‐treated women and 390 pairs of trastuzumab‐treated women (median age, 69 and 71 years, respectively). The 5‐year cumulative incidence of HF hospital presentations after anthracyclines was 1.2% (95% CI, 0.5%–2.6%) in statin‐exposed women and 2.9% (95% CI, 1.7%–4.6%) in unexposed women (P value, 0.01). The cause‐specific hazard ratio associated with statins in the anthracycline cohort was 0.45 (95% CI, 0.24–0.85; P value, 0.01). After trastuzumab, the 5‐year cumulative incidence of HF hospital presentations was 2.7% (95% CI, 1.2%–5.2%) in statin‐exposed women and 3.7% (95% CI, 2.0%–6.2%) in unexposed women (P value 0.09). The cause‐specific hazard ratio associated with statins in the trastuzumab cohort was 0.46 (95% CI, 0.20–1.07; P value, 0.07).
Conclusions
Statin‐exposed women had a lower risk of HF hospital presentations after early breast cancer chemotherapy involving anthracyclines, with non‐significant trends towards lower risk following trastuzumab. These findings support the development of randomized controlled trials of statins for prevention of cardiotoxicity.
Keywords: anthracycline, cardiotoxicity, heart failure, statin, trastuzumab
Subject Categories: , Heart Failure
Nonstandard Abbreviations and Acronyms
- EBC
early breast cancer
- PS
propensity score
- ROS
reactive oxygen species
Clinical Perspective
What Is New?
This observational study suggests that statins may reduce the risk of heart failure following chemotherapy for women with early breast cancer.
If statins protect against cardiotoxicity, the underlying mechanisms are not limited to prevention of acute myocardial infarction by lowering cholesterol levels.
What Are the Clinical Implications?
The retrospective nature of this study precludes attribution of a causal relationship between statins and the lowered risk of heart failure following cardiotoxic chemotherapy, supporting the need for randomized controlled trials on this topic.
Statin use should be encouraged in women with established indications who are starting potentially cardiotoxic chemotherapy for early breast cancer.
The treatment of early breast cancer (EBC) frequently requires the use of anthracyclines and/ or trastuzumab. 1 Unfortunately, cardiotoxicity is a well‐recognized adverse effect of these agents, contributing to an increased risk of heart failure (HF). 2 , 3 Cardiovascular disease is a leading cause of death among elderly EBC survivors, 4 fueling widespread motivation for reducing the risk of cardiotoxicity after EBC chemotherapy. 1 Clinical trials of potentially cardioprotective agents have yielded modest and conflicting results, 5 , 6 with the exception of dexrazoxane, whose approval is limited to women with metastatic disease receiving doxorubicin doses higher than those typically used for EBC. 7 The enthusiasm for other potentially cardioprotective agents, such as angiotensin antagonists and beta‐blockers, is tempered by their hemodynamic adverse effects, which may be amplified in patients undergoing chemotherapy. Angiotensin antagonists also come with a risk of renal compromise and hyperkalemia. 8
Statins have recently emerged as promising candidates to attenuate anthracycline‐ and trastuzumab‐mediated cardiac injury based on a few small, single‐center studies. 9 , 10 , 11 , 12 Here, we present a population‐based propensity‐matched retrospective cohort study to examine the association of statin exposure with the risk of HF among older women with EBC who received anthracyclines and/ or trastuzumab. In this high‐risk population, we hypothesized that statin‐exposed women would have a lower risk of HF compared with non‐exposed patients.
METHODS
Cohort Creation
Residents of Ontario (Canada's most populous province) receive universal health coverage for medically necessary physician and hospital services through the Ontario Health Insurance Plan. Prescription medications are covered for residents aged ≥65 years through the Ontario Drug Benefit program, enabling determination of exposure to prescription medications in older adults. The Ontario Cancer Registry records data on patients diagnosed with malignancy, including breast cancer. The Cancer Activity Level Reporting database records details of systemic cancer therapy at Ontario's regional cancer centers while the New Drug Funding Program records exposure to higher‐cost systemic therapies including trastuzumab and epirubicin. These databases can be used to determine exposure to anthracyclines or trastuzumab as we previously described. 3
The Canadian Institute of Health Information's Discharge Abstract Database records hospitalization data, while the National Ambulatory Care Reporting System captures data on emergency department (ED) visits. Physician billing claims are recorded in the Ontario Health Insurance Plan claims database. The Registered Persons Database maintains vital statistics data. The Ontario Laboratory Information System is a province‐wide repository of laboratory test results, including low‐density lipoprotein (LDL) cholesterol levels. Validated algorithms 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 have been developed for determination of medical diagnoses from these databases. These data sets were linked using unique encoded identifiers and analyzed at ICES (formerly the Institute for Clinical Evaluative Sciences). The use of data in this project was authorized under section 45 of Ontario's Personal Health Information Protection Act, which does not require review by a Research Ethics Board. While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet pre‐specified criteria for confidential access, available at www.ices.on.ca/DAS.
Using these data sets, we identified all women who received anthracyclines and/ or trastuzumab within a year of being diagnosed with EBC at age ≥66 years between January 1, 2007 to December 31, 2017. Women who had hospitalizations, ED visits, or physician billings meeting criteria for a diagnosis of HF (as per validated algorithms for administrative data) 23 before starting chemotherapy were excluded. We also excluded women who were eligible for the Ontario Health Insurance Plan for less than a year before starting chemotherapy, those with previous cancer, long‐term care residents, and women whose chemotherapy details could not be ascertained (including women in clinical trials). We created a cohort of anthracycline‐exposed women, whose index date was the anthracycline start date, and a distinct cohort of trastuzumab‐exposed women, whose index date was the trastuzumab start date. Women treated with trastuzumab following anthracyclines were included in both cohorts, with the cohort‐specific index date being the start date of the specific medication under study.
For each cohort, we surveyed the Ontario Drug Benefit database to identify women who were dispensed at least 2 statin prescriptions in the 365 days preceding the index date, with the second prescription dispensed within 150% of the number of days supplied by the first prescription, and with 1 of the prescriptions' supply period containing the index date; these women were classified as statin‐exposed. Women were classified as unexposed if no statin prescriptions were dispensed for 365 days preceding the index date. We excluded women who had dispensed a statin within a year before chemotherapy initiation but did not fulfill the requirement for 2 overlapping scripts encompassing that index date. We matched statin‐exposed and unexposed women (1:1) within each of the 2 cohorts as described below. The primary outcome was a composite of hospitalizations or ED visits where HF was the most responsible diagnosis (herein referred to as hospital presentations). Death was treated as a competing risk. The date of last follow‐up was December 31, 2018.
Statistical Analysis
All analyses were conducted in parallel for the anthracycline and the trastuzumab cohorts. We summarized baseline characteristics based on statin exposure using the median (with interquartile ranges) for continuous data and counts (with percentages) for categorical data. The magnitude of difference between groups was compared using standardized differences.
We used logistic regression to model the odds of being statin‐exposed at baseline conditional on a woman's baseline characteristics. This was used to derive a propensity score (PS) for statin exposure for each woman. We developed 3 sets of propensity scores: for the anthracycline cohort, for trastuzumab‐treated women without prior anthracyclines, and for trastuzumab‐treated women with prior anthracyclines. The variables considered for inclusion in the models were age, rural residence, income quintile, year of EBC diagnosis, stage, left‐sided disease, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, atrial fibrillation, acute myocardial infarction (AMI), ischemic heart disease without prior myocardial infarction, peripheral vascular disease, non‐statin lipid‐lowering therapy, angiotensin antagonists, beta‐blockers, and the Charlson score. We used greedy nearest neighbor matching to match statin‐exposed and unexposed patients in a 1:1 ratio with a caliper distance of 0.2 of the standard deviation of the logit of the PS. 24 Women in the trastuzumab cohort were also matched on anthracycline exposure before trastuzumab initiation. Standardized differences were used to assess for residual differences in baseline characteristics of the subset of matched patients. 25
To measure crossover after the index date, we identified women who were alive 12 months after starting chemotherapy and calculated the proportion who dispensed a prescription for statins during months 6 to 12. We used cumulative incidence functions to determine the risk of HF hospital presentations over time in statin‐treated women and unexposed women while treating death as a competing risk. Statistical significance of the difference in cumulative incidence functions was assessed using a univariable Fine‐Gray model in which the sub‐distribution hazard of HF hospital presentation was regressed on a binary variable denoting treatment group. A robust variance estimator was used that accounted for the matched nature of the sample. We also used univariable cause‐specific regression models to quantify the relative rate of HF associated with statin exposure among women who were still alive, which was expressed as a hazard ratio (HR). These models also accounted for the matched nature of the sample using a robust variance estimator. Estimated 95% CIs for the cause‐specific HR were extracted from the fitted model with the robust variance estimator. For the trastuzumab cohort, we conducted stratified analyses based on prior anthracycline exposure.
Sensitivity Analyses
We repeated the analyses above while censoring women with an interim AMI hospitalization to explore whether differences in HF risk can be explained by reductions in AMI among statin‐exposed women. We also studied if the association between statins and HF risk was explained by differences in LDL levels (LDL was not included in the primary analysis because of the large proportion of subjects with missing LDL). This is described within Data S1.
All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC) in a Unix environment. Since the study used administrative data sets from a universal healthcare system encompassing the entire population, we assumed missing data were negligible unless stated otherwise. Statistical significance was defined as a 2‐tailed P value <0.05.
RESULTS
Cohort Characteristics
After exclusions (Figure S1), we retained 2545 anthracycline‐treated women (of whom 859 were statin‐exposed at the index date) and 1371 trastuzumab‐treated women (of whom 520 were statin‐exposed at the index date). Their baseline characteristics are listed in Table 1. As expected, statin‐exposed women had a higher prevalence of pre‐existing cardiovascular disease and associated risk factors. Accordingly, they were more likely to have received anti‐hypertensives and other lipid‐lowering agents before chemotherapy. In keeping with their older age and higher cardiovascular risk, statin‐exposed patients in the trastuzumab cohort were less likely to have received anthracyclines. In general, statin‐exposed women were older and more commonly residents of neighborhoods with lower income levels.
Table 1.
Baseline Characteristics of Statin‐Exposed and Unexposed Women in the Anthracycline and Trastuzumab Cohorts Before Propensity Score Matching
| Variable | Anthracycline Cohort | Trastuzumab Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Unexposed | Statin‐Exposed | Std Diff | P Value | Unexposed | Statin‐Exposed | Std Diff | P Value | |
| No. | 1686 | 859 | … | … | 851 | 520 | … | … |
| Median age, y (IQR) | 69 (67–72) | 69 (67–73) | 0.16 | <0.001 | 70 (68–74) | 71 (68–75) | 0.19 | <0.001 |
| Cohort entry, y | ||||||||
| 2007, n (%) | 142 (8.4%) | 62 (7.2%) | 0.04 | 0.12 | 42 (4.9%) | 18 (3.5%) | 0.07 | 0.42 |
| 2008, n (%) | 143 (8.5%) | 52 (6.1%) | 0.09 | 54 (6.3%) | 23 (4.4%) | 0.09 | ||
| 2009, n (%) | 119 (7.1%) | 69 (8.0%) | 0.04 | 54 (6.3%) | 32 (6.2%) | 0.01 | ||
| 2010, n (%) | 127 (7.5%) | 72 (8.4%) | 0.03 | 52 (6.1%) | 38 (7.3%) | 0.05 | ||
| 2011, n (%) | 140 (8.3%) | 76 (8.8%) | 0.02 | 63 (7.4%) | 40 (7.7%) | 0.01 | ||
| 2012, n (%) | 156 (9.3%) | 62 (7.2%) | 0.07 | 80 (9.4%) | 40 (7.7%) | 0.06 | ||
| 2013, n (%) | 154 (9.1%) | 98 (11.4%) | 0.07 | 99 (11.6%) | 54 (10.4%) | 0.04 | ||
| 2014, n (%) | 181 (10.7%) | 81 (9.4%) | 0.04 | 98 (11.5%) | 55 (10.6%) | 0.03 | ||
| 2015, n (%) | 174 (10.3%) | 90 (10.5%) | 0.01 | 99 (11.6%) | 68 (13.1%) | 0.04 | ||
| 2016, n (%) | 191 (11.3%) | 101 (11.8%) | 0.01 | 103 (12.1%) | 82 (15.8%) | 0.11 | ||
| 2017, n (%) | 159 (9.4%) | 96 (11.2%) | 0.06 | 107 (12.6%) | 70 (13.5%) | 0.03 | ||
| Nearest census based neighbourhood income quintile | ||||||||
| 1, n (%) | 267 (15.8%) | 161 (18.7%) | 0.08 | 0.003 | 136 (16.0%) | 95 (18.3%) | 0.06 | 0.006 |
| 2, n (%) | 319 (18.9%) | 183 (21.3%) | 0.06 | 169 (19.9%) | 114 (21.9%) | 0.05 | ||
| 3, n (%) | 319 (18.9%) | 179 (20.8%) | 0.05 | 161 (18.9%) | 129 (24.8%) | 0.14 | ||
| 4, n (%) | 369 (21.9%) | 185 (21.5%) | 0.01 | 168 (19.7%) | 88 (16.9%) | 0.07 | ||
| 5, n (%) | 409 (24.3%) | 150 (17.5%) | 0.17 | 215 (25.3%) | 93 (17.9%) | 0.18 | ||
| Rural residence, n (%) | 274 (16.3%) | 106 (12.3%) | 0.11 | 0.009 | 124 (14.6%) | 66 (12.7%) | 0.05 | 0.45 |
| Prior anthracycline, n (%) | … | … | … | … | 389 (45.7%) | 216 (41.5%) | 0.08 | 0.13 |
| Breast cancer stage | ||||||||
| 1, n (%) | 259 (15.4%) | 135 (15.7%) | 0.01 | 0.96 | 263 (30.9%) | 162 (31.2%) | 0.01 | 0.79 |
| 2, n (%) | 879 (52.1%) | 449 (52.3%) | <0.01 | 393 (46.2%) | 247 (47.5%) | 0.03 | ||
| 3, n (%) | 548 (32.5%) | 275 (32.0%) | 0.01 | 195 (22.9%) | 111 (21.3%) | 0.04 | ||
| Left‐sided disease*, n (%) | 851 (50.5%) | 471 (54.8%) | 0.09 | 0.04 | 468 (55.0%) | 282 (54.2%) | 0.02 | 0.95 |
| Charlson index, median (IQR) | 0 (0–6) | 1 (0–6) | 0.33 | <0.001 | 0 (0–6) | 0 (0–6) | 0.29 | <0.001 |
| Myocardial infarction, n (%) | <6 | 8 (0.9%) | 0.12 | <0.001 | <6 | 8 (1.5%) | 0.14 | 0.006 |
| Ischemic heart disease without myocardial infarction, n (%) | 58 (3.4%) | 109 (12.7%) | 0.34 | <0.001 | 40 (4.7%) | 80 (15.4%) | 0.36 | <0.001 |
| Peripheral vascular disease, n (%) | 6 (0.4%) | 23 (2.7%) | 0.19 | <0.001 | <6 | 20 (3.8%) | 0.23 | <0.001 |
| Atrial fibrillation, n (%) | 43 (2.6%) | 38 (4.4%) | 0.1 | 0.01 | 41 (4.8%) | 24 (4.6%) | 0.01 | 0.86 |
| Diabetes mellitus, n (%) | 178 (10.6%) | 317 (36.9%) | 0.65 | <0.001 | 109 (12.8%) | 199 (38.3%) | 0.61 | <0.001 |
| Hypertension, n (%) | 915 (54.3%) | 692 (80.6%) | 0.58 | <0.001 | 487 (57.2%) | 433 (83.3%) | 0.59 | <0.001 |
| Chronic kidney disease, n (%) | 26 (1.5%) | 26 (3.0%) | 0.1 | 0.01 | 18 (2.1%) | 28 (5.4%) | 0.17 | 0.001 |
| Chronic obstructive pulmonary disease, n (%) | 231 (13.7%) | 153 (17.8%) | 0.11 | 0.006 | 124 (14.6%) | 99 (19.0%) | 0.12 | 0.03 |
| Angiotensin antagonists, n (%) | 504 (29.9%) | 546 (63.6%) | 0.72 | <0.001 | 283 (33.3%) | 341 (65.6%) | 0.68 | <0.001 |
| Beta blockers, n (%) | 184 (10.9%) | 209 (24.3%) | 0.36 | <0.001 | 138 (16.2%) | 131 (25.2%) | 0.22 | <0.001 |
| Non‐statin lipid‐lowering drugs, n (%) | 45 (2.7%) | 68 (7.9%) | 0.24 | <0.001 | 34 (4.0%) | 40 (7.7%) | 0.16 | 0.003 |
| LDL level at baseline | ||||||||
| Missing, n (%) | 1035 (61.4%) | 346 (40.3%) | 0.43 | <0.001 | 497 (58.4%) | 196 (37.7%) | 0.42 | <0.001 |
| <2.0, n (%) | 36 (2.1%) | 248 (28.9%) | 0.79 | 23 (2.7%) | 169 (32.5%) | 0.85 | ||
| 2.0–3.49, n (%) | 388 (23.0%) | 232 (27.0%) | 0.09 | 220 (25.9%) | 142 (27.3%) | 0.03 | ||
| 3.5–5.0, n (%) | 217 (12.9%) | 28–32 | 0.34 | 105 (12.3%) | 8–12 | 0.39 | ||
| >5.0, n (%) | 10 (0.6%) | <6 | 0.08 | 6 (0.7%) | <6 | 0.08 | ||
In accordance with ICES privacy policies, cells with <6 counts are suppressed. LDL indicates low‐density lipoprotein; IQR, interquartile range; and Std diff indicates standardized difference.
Breast cancer laterality data missing for 7 anthracycline‐treated and <6 trastuzumab‐treated women.
The 3 logistic regression models used to estimate the PS are summarized in Table 2. The PS were used to match 666 anthracycline‐treated women who were statin‐exposed to 666 anthracycline‐treated women who did not dispense a prescription for a statin in the year before the index date. Similarly, 390 trastuzumab‐treated women who were statin‐exposed were matched on the logit of the PS and prior anthracycline exposure to 390 trastuzumab‐treated women who did not dispense statins in the year before the index date. Within the anthracycline cohort, 309 women (46.4%) used rosuvastatin, 272 (40.8%) used atorvastatin, 51 (7.7%) used simvastatin, and 27 (4.1%) used pravastatin. In the trastuzumab cohort, 182 women (46.7%) used rosuvastatin, 161 (41.3%) used atorvastatin, and 31 (8.0 %) used simvastatin.
Table 2.
Summary of the Results of the Logistic Regression Models Underlying the Propensity Scores Which Were Used to Match Statin‐Exposed and Unexposed Patients
| Variable | Anthracyclines | Trastuzumab, Prior Anthracyclines | Trastuzumab, No Prior Anthracyclines | |||
|---|---|---|---|---|---|---|
| Parameter Estimate | P value | Parameter Estimate | P Value | Parameter Estimate | P Value | |
| Age, y | 0.02 | 0.08 | 0.03 | 0.14 | 0.02 | 0.23 |
| Y (relative to 2007) | 0.03 | 0.05 | 0.06 | 0.07 | 0.02 | 0.56 |
| Income quintile (relative to lowest income quintile) | ||||||
| 2 | 0.03 | 0.85 | −0.39 | 0.23 | 0.12 | 0.64 |
| 3 | −0.01 | 0.93 | −0.08 | 0.79 | 0.29 | 0.27 |
| 4 | 0.01 | 0.95 | −0.22 | 0.47 | −0.31 | 0.3 |
| 5 | −0.25 | 0.11 | −0.07 | 0.82 | −0.52 | 0.06 |
| Missing income quintile data | −1.03 | 0.39 | 0.11 | 0.94 | … | … |
| Rural residence | −0.38 | 0.007 | −0.15 | 0.59 | −0.2 | 0.42 |
| Breast cancer stage (relative to stage 1) | ||||||
| 2 | −0.12 | 0.40 | −0.06 | 0.81 | −0.3 | 0.13 |
| 3 | −0.26 | 0.08 | −0.42 | 0.15 | −0.21 | 0.46 |
| Left‐sided breast cancer (relative to right‐sided) | 0.20 | 0.04 | 0.07 | 0.72 | −0.11 | 0.52 |
| Missing cancer laterality data (relative to right‐sided) | 1.53 | 0.06 | −11.8 | 0.99 | 0.38 | 0.83 |
| Charlson index | 0.04 | 0.02 | 0.08 | 0.02 | −0.03 | 0.36 |
| Myocardial infarction | 2.55 | 0.02 | … | … | 1.68 | 0.05 |
| Ischemic heart disease without myocardial infarction | 1.08 | <0.0001 | 0.75 | 0.1 | 1.42 | <0.001 |
| Peripheral vascular disease | 0.18 | 0.75 | … | … | 0.72 | 0.29 |
| Atrial fibrillation | 0.08 | 0.76 | 0.14 | 0.8 | −0.89 | 0.02 |
| Diabetes mellitus | 1.20 | <0.0001 | 1.16 | <.001 | 1.14 | <0.001 |
| Hypertension | 0.51 | <0.0001 | 0.98 | 0.001 | 0.41 | 0.08 |
| Chronic kidney disease | 0.23 | 0.47 | −0.48 | 0.48 | 0.54 | 0.19 |
| Chronic obstructive pulmonary disease | 0.20 | 0.11 | 0.36 | 0.2 | 0.22 | 0.31 |
| Angiotensin antagonist | 0.85 | <0.0001 | 0.71 | 0.002 | 0.71 | 0.0005 |
| Beta blockers | 0.49 | 0.00 | −0.07 | 0.8 | −0.08 | 0.72 |
| Non‐statin lipid‐lowering drugs | 0.44 | 0.05 | 1.61 | 0.01 | −0.57 | 0.11 |
The baseline characteristics of the matched anthracycline and trastuzumab cohorts are listed in Table 3. The median age was 69 (interquartile range, 67–73) years for the anthracycline PS‐matched cohort and 71 (interquartile range, 68–75) years for the trastuzumab PS‐matched cohort. The standardized differences for most variables included in the PS were ≤0.1, indicating that the matched cohorts were well balanced on these characteristics. There was a greater proportion of missing LDL levels among statin‐unexposed women. Among women with available LDL data, statin‐exposed patients had lower LDL levels. Among anthracycline‐treated women, 179 statin‐exposed women (26.9%) later received trastuzumab, compared with 162 (24.3%) non‐exposed women (P value, 0.29; standardized difference, 0.06).
Table 3.
Baseline Characteristics of Statin‐Exposed and Unexposed Women in the Anthracycline and Trastuzumab Cohorts After Propensity Score Matching
| Variable | Anthracycline Cohort | Trastuzumab Cohort | ||||
|---|---|---|---|---|---|---|
| Unexposed | Exposed | Std Diff | Unexposed | Exposed | Std Diff | |
| No. | 666 | 666 | … | 390 | 390 | … |
| Median age, y (IQR) | 69 (67–72) | 69 (67–73) | 0.02 | 71 (68–75) | 71 (68–75) | <0.01 |
| Cohort entry, y | ||||||
| 2007, n (%) | 60 (9.0%) | 49 (7.4%) | 0.06 | 23 (5.9%) | 16 (4.1%) | 0.08 |
| 2008, n (%) | 48 (7.2%) | 44 (6.6%) | 0.02 | 25 (6.4%) | 18 (4.6%) | 0.08 |
| 2009, n (%) | 49 (7.4%) | 50 (7.5%) | 0.01 | 20 (5.1%) | 28 (7.2%) | 0.09 |
| 2010, n (%) | 48 (7.2%) | 52 (7.8%) | 0.02 | 19 (4.9%) | 31 (7.9%) | 0.13 |
| 2011, n (%) | 49 (7.4%) | 63 (9.5%) | 0.08 | 29 (7.4%) | 28 (7.2%) | 0.01 |
| 2012, n (%) | 61 (9.2%) | 43 (6.5%) | 0.1 | 31 (7.9%) | 30 (7.7%) | 0.01 |
| 2013, n (%) | 68 (10.2%) | 71 (10.7%) | 0.01 | 45 (11.5%) | 44 (11.3%) | 0.01 |
| 2014, n (%) | 67 (10.1%) | 65 (9.8%) | 0.01 | 48 (12.3%) | 45 (11.5%) | 0.02 |
| 2015, n (%) | 70 (10.5%) | 70 (10.5%) | <0.01 | 46 (11.8%) | 48 (12.3%) | 0.02 |
| 2016, n (%) | 80 (12.0%) | 75 (11.3%) | 0.02 | 48 (12.3%) | 50 (12.8%) | 0.02 |
| 2017, n (%) | 66 (9.9%) | 84 (12.6%) | 0.09 | 56 (14.4%) | 52 (13.3%) | 0.03 |
| Nearest census based neighbourhood income quintile | ||||||
| 1, n (%) | 127 (19.1%) | 123 (18.5%) | 0.02 | 73 (18.7%) | 72 (18.5%) | 0.01 |
| 2, n (%) | 141 (21.2%) | 135 (20.3%) | 0.02 | 83 (21.3%) | 88 (22.6%) | 0.03 |
| 3, n (%) | 132 (19.8%) | 140 (21.0%) | 0.03 | 88 (22.6%) | 88 (22.6%) | <0.01 |
| 4, n (%) | 149 (22.4%) | 145 (21.8%) | 0.01 | 63 (16.2%) | 69 (17.7%) | 0.04 |
| 5, n (%) | 116 (17.4%) | 122 (18.3%) | 0.02 | 83 (21.3%) | 72 (18.5%) | 0.07 |
| Rural residence, n (%) | 92 (13.8%) | 91 (13.7%) | <0.01 | 52 (13.3%) | 54 (13.8%) | 0.01 |
| Prior anthracycline, n (%) | … | … | … | 166 (42.6%) | 166 (42.6%) | <0.01 |
| Breast cancer stage | ||||||
| 1, n (%) | 111 (16.7%) | 109 (16.4%) | 0.01 | 127 (32.6%) | 121 (31.0%) | |
| 2, n (%) | 339 (50.9%) | 345 (51.8%) | 0.02 | 178 (45.6%) | 190 (48.7%) | |
| 3, n (%) | 216 (32.4%) | 212 (31.8%) | 0.01 | 85 (21.8%) | 79 (20.3%) | |
| Left‐sided disease*, n (%) | 359 (53.9%) | 360 (54.1%) | <0.01 | 210 (53.8%) | 212 (54.4%) | 0.01 |
| Charlson index, median (IQR) | 0 (0–6) | 1 (0–6) | 0.08 | 0 (0–6) | 0 (0–6) | 0.04 |
| Myocardial infarction, n (%) | <6 | <6 | <0.01 | <6 | <6 | 0.06 |
| Ischemic heart disease without myocardial infarction, n (%) | 44 (6.6%) | 54 (8.1%) | 0.06 | 29 (7.4%) | 31 (7.9%) | 0.02 |
| Peripheral vascular disease, n (%) | <6 | <6 | <0.01 | <6 | <6 | 0.08 |
| Atrial fibrillation, n (%) | 25 (3.8%) | 28 (4.2%) | 0.02 | 17 (4.4%) | 17 (4.4%) | <0.01 |
| Diabetes mellitus, n (%) | 153 (23.0%) | 155 (23.3%) | 0.01 | 94 (24.1%) | 100 (25.6%) | 0.04 |
| Hypertension, n (%) | 530 (79.6%) | 507 (76.1%) | 0.08 | 316 (81.0%) | 305 (78.2%) | 0.07 |
| Chronic kidney disease, n (%) | 18 (2.7%) | 18 (2.7%) | <0.01 | 13 (3.3%) | 14 (3.6%) | 0.01 |
| Chronic obstructive pulmonary disease, n (%) | 112 (16.8%) | 114 (17.1%) | 0.01 | 69 (17.7%) | 65 (16.7%) | 0.03 |
| Angiotensin antagonists, n (%) | 361 (54.2%) | 363 (54.5%) | 0.01 | 222 (56.9%) | 220 (56.4%) | 0.01 |
| Beta blockers, n (%) | 144 (21.6%) | 136 (20.4%) | 0.03 | 87 (22.3%) | 86 (22.1%) | 0.01 |
| Non‐statin lipid‐lowering drugs, n (%) | 34 (5.1%) | 33 (5.0%) | 0.01 | 19 (4.9%) | 24 (6.2%) | 0.06 |
| LDL level at baseline | ||||||
| Missing, n (%) | 374 (56.2%) | 283 (42.5%) | 0.28 | 219 (56.2%) | 157 (40.3%) | 0.32 |
| <2.0, n (%) | 20 (3.0%) | 162 (24.3%) | 0.65 | 16 (4.1%) | 108 (27.7%) | 0.68 |
| 2.0–3.49, n (%) | 191 (28.7%) | 193 (29.0%) | 0.01 | 113 (29.0%) | 114 (29.2%) | 0.01 |
| 3.5–5.0, n (%) | 76–80 | 28 (4.2%) | 0.28 | 37–41 | 6–10 | 0.33 |
| >5.0, n (%) | <6 | 0 | 0.1 | <6 | <6 | <0.01 |
In accordance with ICES privacy policies, cells with <6 counts are suppressed. LDL indicates low‐density lipoprotein; IQR, interquartile range; and Std diff indicates standardized difference.
Breast cancer laterality data missing for 7 anthracycline‐treated and <6 trastuzumab‐treated women.
There was minimal crossover between groups after starting chemotherapy. In the anthracycline cohort, 658 women who were initially statin‐exposed were alive at 12 months, of whom 614 (93.3%) continued to receive statins during months 6 to 12. Conversely, 648 women in the unexposed group were alive at 12 months; only 28 (4.3%) received statins during months 6 to 12. In the trastuzumab cohort, 387 patients were alive at 12 months, of whom 372 (96.1%) continued to receive statins during months 6 to 12. Conversely, 380 women in the unexposed group were alive at 12 months; only 15 (4.0%) received statins during months 6 to 12.
Heart Failure in PS‒Matched Cohorts
In the PS‒matched anthracycline cohort, there were 43 HF hospital presentations over mean follow‐up of 5.1 (SD, 3.1) years. Figure 1 illustrates the cumulative incidence of HF hospital presentations by statin exposure in the anthracycline‐treated cohort, indicating that the risk of HF hospital presentations was significantly lower in statin‐exposed women (P value, 0.01). At 5 years, the cumulative incidence of HF hospital presentation was 1.2% (95% CI, 0.5%–2.6%) in statin‐exposed women, compared with 2.9% (95% CI, 1.7%–4.6%) in unexposed women. The cause‐specific HR was 0.45 (95% CI 0.24–0.85) for statin‐exposed relative to unexposed women (P value, 0.01).
Figure 1. Cumulative incidence of hospitalization or emergency department visit for heart failure in anthracycline‐treated women according to statin‐exposure after 1:1 matching on the logit of the propensity score.
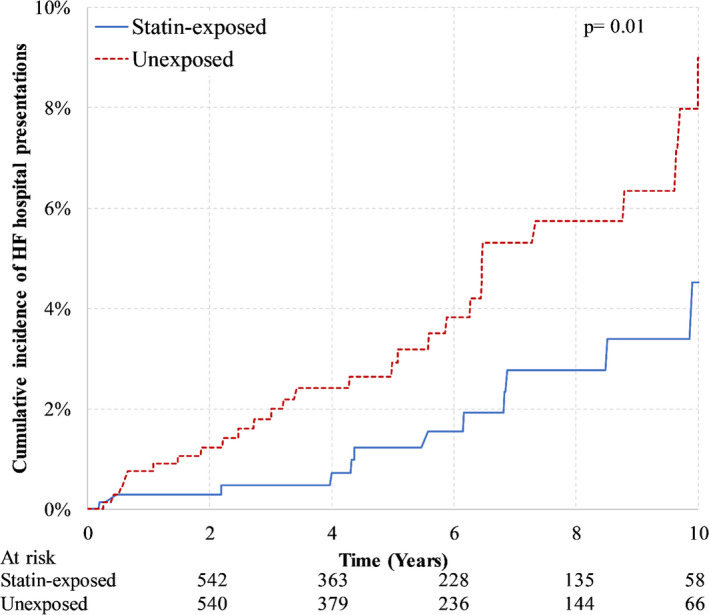
HF indicates heart failure.
In the trastuzumab‐treated cohort, there were 27 HF hospital presentations over mean follow‐up of 4.4 (SD, 2.8) years. Figure 2 illustrates a non‐significant trend towards a lower risk of HF hospital presentations in statin‐exposed women (P value, 0.09). At 5 years, the cumulative incidence of HF hospital presentation was 2.7% (95% CI, 1.2%–5.2%) in statin‐exposed women and 3.7% (95% CI, 2.0%–6.2%) in unexposed women. The cause‐specific HR associated with statin exposure was 0.46 (95% 0.20–1.07; P value, 0.07).
Figure 2. Cumulative incidence of hospitalization or emergency department visit for heart failure in trastuzumab‐treated women according to statin‐exposure after 1:1 matching on the logit of the propensity score and prior exposure to anthracyclines.
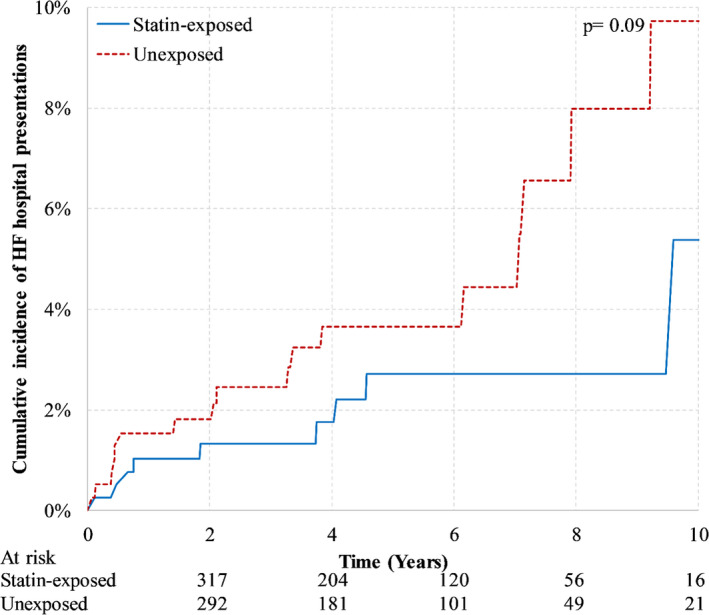
HF indicates heart failure.
There were 224 PS‐matched pairs of trastuzumab‐treated women who were anthracycline‐naïve and 166 pairs who previously received anthracyclines. Of the 166 trastuzumab‐treated women who were also exposed to anthracyclines and statins, >160 women were dispensed a statin before anthracycline initiation, while <6 women started statin therapy after receiving anthracyclines but before starting trastuzumab. There were 17 HF presentations in anthracycline‐naïve women and 10 HF presentations in anthracycline‐exposed women. The cause‐specific HR associated with statin exposure was 0.51 (95% CI, 0.19–1.41; P value, 0.19) in trastuzumab‐treated women without prior anthracyclines and 0.39 (95% CI, 0.10–1.58; P value, 0.19) in anthracycline‐exposed pairs.
Sensitivity Analyses
In the sensitivity analysis censoring women at time of interim AMI hospitalization, the 5‐year cumulative incidence of HF hospital presentations in the anthracycline cohort was 1.2% (95% CI, 0.5%–2.6%) in statin‐exposed women and 2.7% (95% CI, 1.6%–4.4%) in unexposed patients (P value, 0.02). The cause‐specific HR associated with statin exposure was 0.44 (95% CI 0.22–0.87; P value, 0.02). In the trastuzumab cohort, the 5‐year cumulative incidence was 2.7% (1.2%–5.2%) among statin‐exposed women and 3.7% (95% CI, 2.0%–6.2%) in unexposed patients (P value, 0.056). The cause‐specific HR associated with statin exposure was 0.41 (95% CI, 0.17–0.97: P value, 0.04). The sensitivity analysis with multiple imputation of LDL values yielded effect estimates were comparable to the primary analyses but did not meet threshold for statistical significance. Details are provided in Figure S2 and Table S1.
DISCUSSION
In this population‐based, PS‒matched cohort study, statin‐exposed EBC patients at high cardiovascular risk based on older age (≥65 years) had a significantly lower risk of HF hospital presentations following anthracycline‐containing chemotherapy compared with matched women who were unexposed to statins. There was a non‐significant trend towards a lower risk of HF hospital presentations associated with statin exposure among trastuzumab‐treated women compared with statin unexposed women. These results are summarized in Figure 3. We observed similar associations in sensitivity analyses that censored women who developed interim AMI and accounted for LDL levels.
Figure 3. Overall study summary.

ED indicates emergency department; HF, heart failure; and HR: hazard ratio.
Mounting evidence suggests that anthracycline‐induced cardiotoxicity is mediated by topoisomerase‐2β inhibition and oxidative stress secondary to reactive oxygen species (ROS) generation by anthracyclines. 26 , 27 , 28 , 29 , 30 , 31 Oxidative stress may also play a role in trastuzumab‐induced cardiotoxicity. Neuregulin‐1 is an epidermal growth factor produced in endocardial and myocardial microvasculature in response to stress that activates HER4 (human epidermal growth factor receptor 4) to dimerize with HER2 (human epidermal growth factor receptor 2). 26 , 32 The consequent signaling cascade regulates ROS‐induced cardiomyocyte apoptotic cell death and sarcomere organization. 26 , 33 By blocking this pathway, trastuzumab may potentiate ROS‐mediated cardiomyocyte death and interfere with myocyte–myocyte and myocyte–matrix interactions. 26 , 33 Inhibition of HMG‐CoA (3‐hydroxy‐3‐methylglutaryl coenzyme A) reductase by statins may decrease the production of isoprenoid intermediates, 34 which are required to activate Rho (Ras homologous) GTPases. Rho GTPases promote ROS production via the pro‐oxidative nicotinamide adenine dinucleotide phosphate oxidase complex 34 and affect cardiac myocyte cell size and sarcomere organization. 35 Thus, it is plausible that statins may ameliorate anthracycline‐ and trastuzumab‐induced cardiotoxicity by decreasing ROS production and promoting cardiomyocyte survival. 26 , 27 , 28 , 29 , 30 , 31 , 34 , 35
Our data corroborate prior findings of a lower risk of cardiotoxicity in statin‐exposed patients receiving anthracyclines. Seicean et al. reported that statin‐exposure was associated with a HR of 0.3 (95% CI, 0.1–0.9; P=0.03) for HF in a PS‐matched analysis of 67 statin‐exposed (8 [11.9%] received trastuzumab) and 134 unexposed women (mean age, 60 years) who received anthracycline‐based chemotherapy for breast cancer at the Cleveland Clinic Health System between January 2005 and December 2010. 12 However, 32.3% of the cohort were hospitalized for HF, which is a markedly higher rate than is expected with contemporary chemotherapy. 2 , 3 Given the small sample size, the investigators included only a limited number of potential confounders in their PS. Acar et al. randomized 40 anthracycline‐treated patients (mean age, 53 years) with hematological malignancies to 40 mg/day of atorvastatin versus control. At 6 months, they observed a significantly greater mean left ventricular ejection fraction (LVEF) decline in controls (absolute mean decline −7.9% to final mean LVEF 55.0%) without a significant change in statin‐exposed patients; LVEF declined to a final value <50% in 1 statin‐exposed patient compared with 5 in the control group. 10 Another study used cardiac magnetic resonance imaging to evaluate the association between statin exposure and LVEF among 14 statin‐exposed and 37 unexposed patients who received anthracycline‐based chemotherapy for breast (2 [14%] received trastuzumab in the statin group) or hematological malignancies. 9 They observed that the mean LVEF was unchanged in statin‐treated patients, compared with a significant absolute decline of −6.5% in non‐exposed patients to a final mean LVEF of 52.4%.
Common limitations across these studies were their single‐center design and small sample sizes involving heterogeneous cancer patients. Moreover, only 1 study evaluated clinically overt HF; the others reported small changes in LVEF with questionable long‐term clinical relevance. 36 , 37 Our study extends prior observations by studying this question in a population‐based sample encompassing a larger number of patients than prior studies. We focused on HF requiring hospital‐based care since it is a less ambiguous outcome that carries more relevance to cancer patients and their physicians. We also studied older patients, the fastest growing group of cancer survivors 38 with the highest absolute risks of clinically‐overt cardiotoxicity 38 , 39 , 40 , 41 but who remain underrepresented in clinical trials. 42 Furthermore, our study examined the relationship between statin exposure and HF while adjusting for LDL values and censoring upon development of interim AMI. Our observations suggest that the potential protective mechanism of statins against HF in older adults may be independent of their ability to prevent AMI by lowering LDL levels.
There are fewer data on the association of statin exposure with trastuzumab‐associated cardiotoxicity. Previously, we published a single‐center study of 129 trastuzumab‐treated patients with EBC 11 where statin exposure was associated with 68% lower odds of cardiotoxicity. There was no significant change in LVEF in statin‐exposed women in contrast to a significant decline in unexposed women. The population‐wide analysis reported here reveals a non‐significant association with lower risk of HF hospital presentations following trastuzumab in statin‐exposed older women. This analysis was limited by low event counts, with trastuzumab‐associated cardiotoxicity less likely to lead to decompensated HF requiring hospitalization or ED visits. However, our data suggest that this area merits further study.
The results of this study should be interpreted with caution given its retrospective design and dependence on administrative data. A key limitation is our inability to account for LVEF, diastolic function, cardiac biomarkers, or biohumoral data. Thus, we cannot determine if the HF events occurred with reduced or preserved LVEF. The study excluded women with established diagnoses of HF before chemotherapy but may have included women with asymptomatic cardiomyopathy, although this is not expected to occur more commonly in the non‐statin groups. There was a large proportion of missing LDL values, requiring us to use multiple imputation. The outcome definition (hospitalization/ED visits for HF) was chosen to prioritize specificity, but the trade‐off is that we likely underestimated the absolute risk of cardiotoxicity. We also did not account for “cross‐over” attributable to initiation or discontinuation of statins after the index date. Furthermore, we cannot exclude that the lower risk of HF in statin‐exposed patients may represent residual confounding.
CONCLUSIONS
Statin exposure was associated with a lower risk of hospitalizations and/ or ED visits for HF after chemotherapy with anthracyclines for older women with EBC. We also observed a non‐significant association with lower risk of HF following trastuzumab in statin‐exposed women. Our findings provide further support for definitive randomized controlled trials on this topic. The current data can inform the design and power calculations of such trials to determine whether pre‐treatment with statins is effective at preventing HF after anthracyclines or trastuzumab.
Sources of Funding
This work was supported by the Canadian Cardiovascular Society Atrial Fibrillation Research Award (to Dr. Abdel‐Qadir and Dr. Lee) and start‐up funds provided by the Ted Rogers Centre for Heart Research (to Dr. Abdel‐Qadir). Dr. Abdel‐Qadir is supported by a National New Investigator Award from the Heart and Stroke Foundation of Canada. Dr. Austin is supported in part by a Mid‐Career Investigator Award from the Heart and Stroke Foundation. Dr. Lee is supported by the Ted Rogers Chair in Heart Function Outcomes. Dr. Thavendiranathan is supported by the Canadian Institutes of Health Research New Investigator Award. Dr. Calvillo‐Argüelles is supported by the Hold'em for Life Oncology Clinician Scientist Award at the University of Toronto's Faculty of Medicine.
Disclosures
Dr. Abdel‐Qadir declares no conflicts of interest involving the work under consideration for publication. Relevant financial activities outside the submitted work include consultant fees from Amgen, and fees end point adjudication committee membership for the Effect of Ticagrelor on Health Outcomes in DiabEtes Mellitus Patients Intervention Study (THEMIS) trial, which was funded by Astra Zeneca. Dr. Amir declares fees for expert testimony from Genentech/Roche and fees for consulting from Sandoz, Apobiologix, Agendia, and Myriad Genetics outside the submitted work. Dr. Thavendiranathan declares consultation fees from Amgen, Takeda, and Boehringer Ingelheim. The remaining authors have no disclosures to report.
Supporting information
Data S1
Table S1
Figures S1–S2
Acknowledgments
This study was supported by the ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care. Parts of this material are based on data and/ or information compiled and provided by Canadian Institute of Health Information. Parts of this material are based on data and information provided by Cancer Care Ontario. We thank IMS Brogan Inc. for use of their Drug Information Database. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
(J Am Heart Assoc.2021;10:e018393. DOI: 10.1161/JAHA.119.018393.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.018393
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz‐Flores S, Dent S, Kondapalli L, Ky B, Okwuosa T, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137:e30–e66. 10.1161/CIR.0000000000000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdel‐Qadir H, Thavendiranathan P, Austin PC, Lee DS, Amir E, Tu JV, Fung K, Anderson GM. The risk of heart failure and other cardiovascular hospitalizations after early stage breast cancer: a matched cohort study. J Natl Cancer Inst. 2019;111:854–862. 10.1093/jnci/djy218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thavendiranathan P, Abdel‐Qadir H, Fischer HD, Camacho X, Amir E, Austin PC, Lee DS. Breast cancer therapy‐related cardiac dysfunction in adult women treated in routine clinical practice: a population‐based cohort study. J Clin Oncol. 2016;34:2239–2246. 10.1200/JCO.2015.65.1505 [DOI] [PubMed] [Google Scholar]
- 4. Abdel‐Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, Fung K, Anderson GM. A population‐based study of cardiovascular mortality following early‐stage breast cancer. JAMA Cardiol. 2017;2:88–93. 10.1001/jamacardio.2016.3841 [DOI] [PubMed] [Google Scholar]
- 5. Abdel‐Qadir H, Ong G, Fazelzad R, Amir E, Lee DS, Thavendiranathan P, Tomlinson G. Interventions for preventing cardiomyopathy due to anthracyclines: a bayesian network meta‐analysis. Ann Oncol. 2017;28:628–633. 10.1093/annonc/mdw671 [DOI] [PubMed] [Google Scholar]
- 6. Vaduganathan M, Hirji SA, Qamar A, Bajaj N, Gupta A, Zaha VG, Chandra A, Haykowsky M, Ky B, Moslehi J, et al. Efficacy of neurohormonal therapies in preventing cardiotoxicity in patients with cancer undergoing chemotherapy. JACC CardioOncol. 2019;1:54–65. 10.1016/j.jaccao.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fda statement on dexrazoxane. 2011; 2016.
- 8. Kitchlu A, McArthur E, Amir E, Booth CM, Sutradhar R, Majeed H, Nash DM, Silver SA, Garg AX, Chan CT, et al. Acute kidney injury in patients receiving systemic treatment for cancer: a population‐based cohort study. J Natl Cancer Inst. 2019;111:727–736. 10.1093/jnci/djy167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chotenimitkhun R, D'Agostino R Jr, Lawrence JA, Hamilton CA, Jordan JH, Vasu S, Lash TL, Yeboah J, Herrington DM, Hundley WG. Chronic statin administration may attenuate early anthracycline‐associated declines in left ventricular ejection function. Can J Cardiol. 2015;31:302–307. 10.1016/j.cjca.2014.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Acar Z, Kale A, Turgut M, Demircan S, Durna K, Demir S, Meric M, Agac MT. Efficiency of atorvastatin in the protection of anthracycline‐induced cardiomyopathy. J Am Coll Cardiol. 2011;58:988–989. 10.1016/j.jacc.2011.05.025 [DOI] [PubMed] [Google Scholar]
- 11. Calvillo‐Arguelles O, Abdel‐Qadir H, Michalowska M, Billia F, Suntheralingam S, Amir E, Thavendiranathan P. Cardioprotective effect of statins in patients with HER2‐positive breast cancer receiving trastuzumab therapy. Can J Cardiol. 2019;35:153–159. 10.1016/j.cjca.2018.11.028 [DOI] [PubMed] [Google Scholar]
- 12. Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol. 2012;60:2384–2390. 10.1016/j.jacc.2012.07.067 [DOI] [PubMed] [Google Scholar]
- 13. Lee DS, Donovan L, Austin PC, Gong Y, Liu PP, Rouleau JL, Tu JV. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43:182–188. 10.1097/00005650-200502000-00012 [DOI] [PubMed] [Google Scholar]
- 14. Vermeulen MJ, Tu JV, Schull MJ. Icd‐10 adaptations of the ontario acute myocardial infarction mortality prediction rules performed as well as the original versions. J Clin Epidemiol. 2007;60:971–974. 10.1016/j.jclinepi.2006.12.009 [DOI] [PubMed] [Google Scholar]
- 15. Tu K, Mitiku T, Lee DS, Guo H, Tu JV. Validation of physician billing and hospitalization data to identify patients with ischemic heart disease using data from the Electronic Medical Record Administrative Data Linked Database (EMRALD). Can J Cardiol. 2010;26:e225–e228. 10.1016/S0828-282X(10)70412-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lipscombe LL, Hwee J, Webster L, Shah BR, Booth GL, Tu K. Identifying diabetes cases from administrative data: a population‐based validation study. BMC Health Serv Res. 2018;18:316. 10.1186/s12913-018-3148-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tu K, Wang M, Young J, Green D, Ivers NM, Butt D, Jaakkimainen L, Kapral MK. Validity of administrative data for identifying patients who have had a stroke or transient ischemic attack using EMRALD as a reference standard. Can J Cardiol. 2013;29:1388–1394. 10.1016/j.cjca.2013.07.676 [DOI] [PubMed] [Google Scholar]
- 18. Tu K, Nieuwlaat R, Cheng SY, Wing L, Ivers N, Atzema CL, Healey JS, Dorian P. Identifying patients with atrial fibrillation in administrative data. Can J Cardiol. 2016;32:1561–1565. 10.1016/j.cjca.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 19. Tu K, Campbell NR, Chen ZL, Cauch‐Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1:e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 20. Fleet JL, Dixon SN, Shariff SZ, Quinn RR, Nash DM, Harel Z, Garg AX. Detecting chronic kidney disease in population‐based administrative databases using an algorithm of hospital encounter and physician claim codes. BMC Nephrol. 2013;14:81. 10.1186/1471-2369-14-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gershon AS, Warner L, Cascagnette P, Victor JC, To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011;378:991–996. 10.1016/S0140-6736(11)60990-2 [DOI] [PubMed] [Google Scholar]
- 22. Tu JV, Chu A, Donovan LR, Ko DT, Booth GL, Tu K, Maclagan LC, Guo H, Austin PC, Hogg W, et al. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8:204–212. 10.1161/CIRCOUTCOMES.114.001416 [DOI] [PubMed] [Google Scholar]
- 23. Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33:160–166. [PubMed] [Google Scholar]
- 24. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeglinski M, Ludke A, Jassal DS, Singal PK. Trastuzumab‐induced cardiac dysfunction: a 'dual‐hit'. Exp Clin Cardiol. 2011;16:70–74. [PMC free article] [PubMed] [Google Scholar]
- 27. Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. 10.1124/pr.56.2.6 [DOI] [PubMed] [Google Scholar]
- 28. Vejpongsa P, Yeh ET. Prevention of anthracycline‐induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64:938–945. 10.1016/j.jacc.2014.06.1167 [DOI] [PubMed] [Google Scholar]
- 29. Moudgil R, Yeh ET. Mechanisms of cardiotoxicity of cancer chemotherapeutic agents: cardiomyopathy and beyond. Can J Cardiol. 2016;32:863–870. 10.1016/j.cjca.2016.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cappetta D, De Angelis A, Sapio L, Prezioso L, Illiano M, Quaini F, Rossi F, Berrino L, Naviglio S, Urbanek K. Oxidative stress and cellular response to doxorubicin: a common factor in the complex milieu of anthracycline cardiotoxicity. Oxid Med Cell Longev. 2017;2017:1521020. 10.1155/2017/1521020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang S, Liu X, Bawa‐Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin‐induced cardiotoxicity. Nat Med. 2012;18:1639–1642. 10.1038/nm.2919 [DOI] [PubMed] [Google Scholar]
- 32. Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin‐1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. 2007;116:954–960. 10.1161/CIRCULATIONAHA.107.690487 [DOI] [PubMed] [Google Scholar]
- 33. Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbb2‐dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol. 2006;41:228–235. 10.1016/j.yjmcc.2006.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Henninger C, Fritz G. Statins in anthracycline‐induced cardiotoxicity: Rac and rho, and the heartbreakers. Cell Death Dis. 2017;8:e2564. 10.1038/cddis.2016.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takemoto M, Node K, Nakagami H, Liao Y, Grimm M, Takemoto Y, Kitakaze M, Liao JK. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest. 2001;108:1429–1437. 10.1172/JCI13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsao CW, Lyass A, Larson MG, Cheng S, Lam CS, Aragam JR, Benjamin EJ, Vasan RS. Prognosis of adults with borderline left ventricular ejection fraction. JACC Heart Fail. 2016;4:502–510. 10.1016/j.jchf.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. López‐Sendón J, Álvarez‐Ortega C, Zamora Auñon P, Buño Soto A, Lyon AR, Farmakis D, Cardinale D, Canales Albendea M, Feliu Batlle J, Rodríguez Rodríguez I, et al. Classification, prevalence, and outcomes of anticancer therapy‐induced cardiotoxicity: The cardiotox registry. Eur Heart J. 2020;41:1720–1729. 10.1093/eurheartj/ehaa006 [DOI] [PubMed] [Google Scholar]
- 38. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the "Silver Tsunami": prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1029–1036. 10.1158/1055-9965.EPI-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand J‐B, Ewer M, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. 10.1200/JCO.2016.70.5400 [DOI] [PubMed] [Google Scholar]
- 40. Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon AR, dos‐Santos‐Silva I, Smeeth L, Bhaskaran K. Medium and long‐term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population‐based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394:1041–1054. 10.1016/S0140-6736(19)31674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abdel‐Qadir H, Thavendiranathan P, Austin PC, Lee DS, Amir E, Tu JV, Fung K, Anderson GM. Development and validation of a multivariable prediction model for major adverse cardiovascular events after early stage breast cancer: a population‐based cohort study. Eur Heart J. 2019;40:3913–3920. 10.1093/eurheartj/ehz460 [DOI] [PubMed] [Google Scholar]
- 42. Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer‐treatment trials. N Engl J Med. 1999;341:2061–2067. 10.1056/NEJM199912303412706 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1
Figures S1–S2


