Abstract
Background
Several studies have examined hospitalizations among patients with adult congenital heart disease (ACHD). Few investigated other services or utilization patterns. Our aim was to study service utilization patterns and predictors among patients with ACHD.
Methods and Results
We identified 11 653 patients with ACHD aged ≥18 years (median, 47 years), through electronic records of 2 large Israeli healthcare providers (2007–2011). The association between patient, disease, and sociogeographic characteristics and healthcare resource utilization were modeled as recurrent events accounting for the competing death risk. Patients with ACHD had high healthcare utilization rates compared with the general population. The highest standardized service utilization ratios (SSRs) were found among patients with complex congenital heart disease including primary care visits (SSR, 1.53; 95% CI, 1.47–1.58), cardiology outpatient visits (SSR, 5.17; 95% CI, 4.69–5.64), hospitalizations (SSR, 6.68; 95% CI, 5.82–7.54), and days in hospital (SSR, 15.37; 95% CI, 14.61–16.12). Adjusted resource utilization hazard increased with increasing lesion complexity. Hazard ratios (HRs) for complex versus simple disease were: primary care (HR, 1.14; 95% CI, 1.06–1.23); cardiology outpatient visits (HR, 1.40; 95% CI, 1.24–1.59); emergency department visits (HR, 1.19; 95% CI, 1.02–1.39); and hospitalizations (HR, 1.75; 95% CI, 1.49–2.05). Effects attenuated with age for cardiology outpatient visits and hospitalizations and increased for emergency department visits. Female sex, geographic periphery, and ethnic minority were associated with more primary care visits, and female sex (HR versus men, 0.89 [95% CI, 0.84–0.94]) and periphery (HR, 0.72 [95% CI, 0.58–0.90] for very peripheral versus very central) were associated with fewer cardiology visits. Arab minority patients also had high hospitalization rates compared with the majority group of Jewish or other patients.
Conclusions
Healthcare utilization rates were high among patients with ACHD. Female sex, geographic periphery, and ethnicity were associated with less optimal service utilization patterns. Further research should examine strategies to optimize service utilization in these groups.
Keywords: adult congenital heart disease, healthcare service utilization, mortality, population‐based study
Subject Categories: Epidemiology, Congenital Heart Disease, Health Services
Nonstandard Abbreviations and Acronyms
- ACHD
adult congenital heart disease
- SSR
standardized service utilization ratio
Clinical Perspective
What Is New?
This large cohort study is among the first to report health service utilization patterns among patients with adult congenital heart disease.
Inpatient and outpatient service utilization rates are significantly higher than in the general population among all adult congenital heart disease lesion complexity groups.
While older age is associated with greater healthcare utilization (emergency department visits excepted), the increase in cardiology visits or hospital admission rates, with higher disease complexity, diminishes with age.
Female sex, geographic periphery, and ethnic minority were associated with more primary care visits, female sex and periphery were associated with fewer outpatient cardiology visits, and Arab minority were associated with high hospitalization rates.
What Are the Clinical Implications?
Adult congenital heart disease is a rapidly growing patient population and healthcare requirements of these patients are expected to further increase as the population ages.
We found less optimal service utilization patterns among female, periphery, and ethnic minority patients in a framework of universal coverage for primary to tertiary care. Greater disparities may be found in health systems based on private insurance.
Characterization of healthcare utilization patterns carries important policy‐making and infrastructure planning implications, as more experts in this field and centers of excellence for the treatment of this population will be needed in the upcoming years.
A dramatic demographic shift in the congenital heart disease patient population has taken place over the past decades. A once primarily pediatric population, now mainly consists of patients with adult congenital heart disease (ACHD), 1 , 2 , 3 , 4 , 5 presenting new challenges to health systems worldwide in terms of care organization and resource allocation. The need for lifelong follow‐up, disease‐ and procedure‐related late‐onset complications, albeit early repair, as well as age‐related acquired comorbidities, entail high health resource utilization among patients with ACHD throughout adult life. 6 , 7 A recent systematic review reported an increase in the number of hospitalizations and outpatient clinic visits among patients with ACHD over the past decade. 8 Healthcare requirements of patients with ACHD are expected to further increase, as long as the ACHD population continues to expand and age.
Data on ACHD patient healthcare utilization are paramount to informed policy making. However, most of the available information is limited to hospitalizations. 6 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Data for additional services are scarce 11 , 15 , 16 and rarely population based. 11 Moreover, most published studies originate from North America and Western Europe. Reports from other regions of the world are limited 17 , 18 and analyses of utilization patterns and associated factors are scarce.
Our aims were to assess inpatient and outpatient healthcare utilization patterns among patients with ACHD; to examine the relationship of patient, disease, and sociogeographic characteristics to health service utilization; and to compare health services utilization between patients with ACHD and the general population.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files. The study was conducted in Israel in a framework of national health insurance with universal coverage for primary to tertiary care. 19 The broad benefit package, covering physician consultations, hospitalization, imaging, laboratory services, and medication is publically funded through taxes with limited copayment or no additional charge.
We identified patients with ACHD through electronic records of the 2 largest (of 4 existing) healthcare providers (Maccabi and Clalit health services), covering 77% of the population at the time of the study. Patients aged ≥18 years, with at least 1 documented congenital heart lesion or a specific congenital heart malformation repair procedure (Table S1), insured by the participating providers between January 2007 and December 2011, were included. The institutional review boards of the Sheba Medical Center and the participating healthcare providers approved the study. No informed consent was required.
From 17 637 identified patients with ACHD, we excluded 628 patients who switched providers during the data collection period, to avoid overlap of deidentified records from different providers. We also excluded 1809 patients for whom a diagnosis of congenital heart defect could not be ascertained based on the available data, and 3547 patients for whom disease complexity could not be determined based on diagnosis codes. Eighty‐eight percent of the patients completed 5 years of follow‐up and 92% completed at least 4 follow‐up years. A unique identifier was used to link information sources (eg, chronic diagnoses, hospitalization, and clinic visits) within provider, at the patient level. All data were consolidated into a single database following coding unification and consistency checks.
The healthcare services examined included primary care and cardiology outpatient visits, emergency department (ED) visits, and hospital admissions. Maternity ward hospitalizations were excluded. Only face‐to‐face patient‐doctor encounters were included. Several visits to the same specialty clinic (general practice or cardiology) on the same day were counted once. Data limitation prevented distinction between specialized ACHD clinics and other outpatient cardiology visits.
The International Classification of Diseases, Ninth Revision (ICD‐9), was the main coding system used. Other diagnosis codes were converted to ICD‐9. Congenital heart disease complexity was categorized based on the 32nd Bethesda Conference report as simple, moderate, severe, or unclassifiable by a hierarchal algorithm according to the most severe congenital heart defect. 3
Comorbidities were summarized with the Charlson comorbidity score. 20 Geographic periphery is represented by an index based on standardized distance and accessibility of the participant residence locality, to a central economic center. 21 The index created by the central bureau of statistics (2015 version) ranges between 1 (most peripheral) and 10 (least peripheral locality). Residence in Arab localities was used as a surrogate for identification of Arab minority patients.
Statistical Analysis
Data were analyzed with SAS version 9.4 (SAS Institute Inc.). Age‐adjusted rates of baseline characteristics by sex and congenital disease complexity were calculated by a direct method with the entire cohort as the reference group. Direct adjustment was selected as a method free of assumptions inherited in multivariable models. Rates were compared while controlling for age (grouped as: 18–24, 25–44, 45–64, and >64 years) with the Cochran‐Mantel‐Haenszel general association test. 22 To account for left skewed visit and inpatient day distributions, age‐adjusted frequencies per 5 years are presented as least square geometric mean rates at mean age.
Negative binomial models with a time logarithm offset were used to assess adjusted relative rates for days in hospital. The Cox proportional hazard model was used for survival multivariable analysis. Adjusted cumulative predicted mortality rates were computed for men, intermediated complexity of congenital heart disease, and mean values of other variables in the model (healthcare provider, age, periphery index, ethnicity, noncardiac congenital abnormalities, and baseline comorbidity) unless used for stratification. The validity of the proportional hazard assumption for the variables of interest was tested by including a time‐dependent explanatory variable for each, to test the assumption of no time‐dependent effect. No violation was found.
Each service utilization was modeled as a recurrent event accounting for the competing risk of death as suggested by Andersen et al. 23 The approach combines a marginal model (based on counting process) of recurrent events with analysis of competing risk to estimate the subdistribution hazard with the PHREG procedure in SAS. The cumulative number of recurrent events was computed by the Ghosh and Lin multiplicative marginal means model. 24 The proportional hazard assumption was violated for age groups in models for primary care visits, ED visits, and hospitalizations. Stratifying these models by age yielded similar estimates as nonstratified models.
To enable model comparability, we used the same fixed variable set in all models, including age, sex, congenital heart disease complexity, health service provider, geographic periphery, ethnicity according to locality, and comorbidities.
In a separate analysis, standardized service utilization (SSR) and standardized mortality ratios were calculated in reference to the general population. The reference rates for the general population were extrapolated from the population sample of the Hadera District Study. 25 SSR and standardized mortality ratios were computed with the SAS STDRATE procedure matching for age, sex, and ethnicity among patients with ACHD aged 25 to 74 years (the age range of the reference population).
Results
The study cohort comprised 11 653 patients with ACHD (52% women). Median patient age at baseline was 47 years, with 24% of women and 20% of men >64 years. The most common congenital heart lesions were atrial septal defect among women and aortic valve stenosis or insufficiency among men (Table 1).
Table 1.
Distribution of Congenital Heart Defects Among 5551 Men and 6102 Women With ACHD
| All | Men | Women | |
|---|---|---|---|
| N (%) | n (%) | n (%) | |
| Atrial septal defect | 3539 (30.4) | 1341 (24.2) | 2198 (36.0) |
| Aortic valve stenosis/insufficiency | 3032 (26.0) | 1999 (36.0) | 1033 (16.9) |
| Anomalies of the aorta | 937 (8.0) | 512 (9.2) | 425 (7.0) |
| Ventricular septal defect | 1654 (14.2) | 714 (12.9) | 940 (15.4) |
| Mitral valve stenosis/insufficiency | 1090 (9.4) | 411 (7.4) | 679 (11.1) |
| Atrioventricular septal defect | 775 (6.7) | 274 (4.9) | 501 (8.2) |
| Pulmonary valve anomaly | 415 (3.6) | 196 (3.5) | 219 (3.6) |
| Patent ductus arteriosus | 547 (4.7) | 169 (2.3) | 378 (6.2) |
| Tetralogy of Fallot | 385 (3.3) | 201 (3.6) | 184 (3.0) |
| Common/single ventricle | 201 (1.7) | 82 (1.5) | 119 (2.0) |
| Ebstein anomaly of tricuspid valve | 146 (1.3) | 58 (1.0) | 88 (1.4) |
| Transposition of great arteries | 77 (0.7) | 35 (0.6) | 42 (0.7) |
| Other defects | 1209 (10.4) | 553 (10.0) | 654 (10.7) |
ACHD indicates adult congenital heart disease. Patients can have >1 defect; therefore, the percentage can add up to >100%.
More than 1 heart defect was recorded for 18% of the women and 15% of the men (Table 2). Seven percent of the patients had an additional noncardiac congenital anomaly. Genetic syndromes included Down (46 patients) and Marfan (22 patients) syndromes (Table S2).
Table 2.
Characteristics of 11 653 Patients With ACHD and CHD
| Sex | Disease complexity | ||||||
|---|---|---|---|---|---|---|---|
| Men | Women | P Value | Simple | Intermediate | Complex | P Value | |
| No. (%) | 5551 (48) | 6102 (52) | 8637 (74) | 2457 (21) | 559 (5) | ||
| Age, median (IQR), y | 46 (30–61) | 47 (32–64) | <0.0001 | 48 (32–63 | 44 (30–62) | 34 (25–55) | <0.0001 |
| No. of heart defects, n (%) | |||||||
| 1 | 4705 (84.8) | 5006 (82.0) | 0.0002 | 7804 (90.4) | 1589 (64.7) | 318 (56.9) | <0.0001 |
| 2 | 703 (12.7) | 874 (14.3) | 731 (8.5) | 683 (27.8) | 163 (29.2) | ||
| 3+ | 143 (2.7) | 222 (3.6) | 102 (1.2) | 185 (7.5) | 78 (14.0) | ||
| Other congenital anomaly (n of body systems affected), n (%) | |||||||
| 0 | 5229 (94.2) | 5664 (92.8) | 0.02 | 8115 (94.0) | 2272 (92.5) | 506 (90.5) | <0.0001 |
| 1 | 302 (5.4) | 405 (6.6) | 493 (5.7) | 171 (7.0) | 43 (7.7) | ||
| 2+ | 20 (0.3) | 33 (0.5) | 29 (0.3) | 14 (0.6) | 10 (1.8) | ||
| Cardiac morbidity, n (%)* | |||||||
| Arrhythmia | 916 (17.0) | 1154 (18.4) | 0.03 | 1512 (17.1) | 431 (18.0) | 127 (27.7) | <0.0001 |
| Valve disease | 387 (7.1) | 604 (9.7) | <0.0001 | 527 (6.1) | 351 (14.4) | 113 (23.8) | <0.0001 |
| Ischemic HD | 997 (18.6) | 749 (11.8) | <0.0001 | 1313 (14.8) | 364 (15.4) | 69 (16.6) | 0.42 |
| Heart failure | 472 (8.9) | 436 (6.8) | <0.0001 | 630 (7.1) | 203 (8.5) | 75 (16.9) | <0.0001 |
| Other HD | 924 (17.1) | 888 (14.2) | <0.0001 | 1176 (13.4) | 501 (20.9) | 135 (27.7) | <0.0001 |
| Other morbidity, n (%)* | |||||||
| Diabetes mellitus | 728 (13.4) | 808 (12.9) | 0.35 | 1148 (12.9) | 339 (14.4) | 49 (12.3) | 0.08 |
| Hypertension | 1773 (32.6) | 1981 (31.7) | 0.2 | 2799 (31.5) | 829 (35.1) | 126 (28.9) | 0.0004 |
| Hyperlipidemia | 1619 (29.6) | 1718 (27.8) | 0.02 | 2512 (28.4) | 712 (30.2) | 113 (26.9) | 0.07 |
| Stroke/TIA | 729 (13.5) | 846 (13.6) | 0.82 | 1289 (14.6) | 240 (10.2) | 46 (10.2) | <0.0001 |
| Kidney failure | 30 (0.6) | 14 (0.2) | 0.004 | 34 (0.4) | 9 (0.4) | 1 (0.2) | 0.9 |
| Residence locality ethnicity, n (%)* | |||||||
| Jewish/other | 3849 (69.3) | 4246 (69.9) | 0.36 | 5992 (69.6) | 1732 (70.7) | 371 (67.1) | 0.05 |
| Arab | 403 (7.1) | 389 (6.5) | 565 (6.6) | 167 (6.6) | 60 (9.1) | ||
| Mixed | 1293 (23.6) | 1444 (23.6) | 2056 (23.8) | 554 (22.7) | 127 (23.8) | ||
| Residence locality peripherality, n (%)* | |||||||
| Very peripheral | 121 (2.2) | 106 (1.8) | 0.33 | 174 (2.0) | 47 (1.9) | 6 (0.9) | 0.02 |
| Peripheral | 710 (12.7) | 749 (12.4) | 1052 (12.3) | 334 (13.5) | 73 (13.0) | ||
| Medium | 996 (17.9) | 1097 (18.1) | 1546 (18.0) | 443 (17.9) | 104 (16.6) | ||
| Central | 1140 (20.6) | 1314 (21.6) | 1890 (22.0) | 451 (18.0) | 113 (17.2) | ||
| Very central | 2576 (46.7) | 2813 (46.2) | 3950 (45.8) | 1177 (48.2) | 262 (49.3) | ||
ACHD indicates adult congenital heart disease; CHD, congenital heart disease; HD, heart disease; IQR, interquartile range; and TIA, transient ischemic attack.
Age‐adjusted percentage.
The congenital defect was complex in 5%, intermediate in 21%, and simple in 74% of the patients (Table 2). Patients with complex heart defects were younger on average, more frequently women (56% compared with 52% among patients with a simple congenital defect), and more likely to have multiple congenital heart defects and additional noncardiac anomalies (Table 2; P<0.0001 for all). Increasing congenital heart disease complexity was associated with increasing age‐adjusted prevalence of acquired cardiac morbidity (Table 2), including ischemic heart disease (17% among complex versus 15% among simple congenital heart patients), heart failure (17% versus 7%), and arrhythmia (28% versus 17%, respectively). Chronic comorbidities such as diabetes mellitus, hypertension, and hyperlipidemia, on the other hand, were more prevalent among patients classified as intermediate, whereas the prevalence of stroke or transient ischemic attack was inversely related to disease complexity (Table 2). The corresponding crude rates are presented in Table S3.
During the data collection period (mean, 5.3 years [SD, 1.0 years]; 61 354 patient‐years), 858 (7.4%) patients died, a rate 5.4 times higher (95% CI, 5.0–5.8) than expected in the general population matched by age, sex, and ethnicity. Standardized mortality ratios were 5.7 for simple and intermediate and 10.7 for complex congenital heart disease. Older age (hazard ratio [HR], 4.74 [95% CI, 2.30–9.76] for age 45–64 years, and HR, 23.8 [95% CI, 11.75–48.36] for age >64 years versus age 18–24 years), male sex (HR, 1.32 [95% CI, 1.12–1.56] versus female sex), complex congenital heart disease (HR, 1.94 [95% CI, 1.38–2.72] versus simple disease), and Arab minority (HR, 1.42 [95% CI, 0.96–2.11] versus Jewish) were associated with increased multivariable‐adjusted mortality risk. The associated cumulative adjusted mortality rates by age, sex, and disease complexity are presented in Figure 1. The adjusted 5‐year cumulative mortality probabilities were 5.5 (95% CI, 4.1–7.0) for simple, 5.2 (95% CI, 3.5–6.9) for intermediate, and 12.3 (95% CI, 7.2–17.1) for complex disease; 5.3 (95% CI, 3.6–6.9) for men and 3.6 for women (95% CI, 2.4–4.8); and ranged between 2% (95% CI, 0.5–3.2) for patients aged 18 to 24 years to 34% (95% CI, 25.2–41.7) for patients older than 64 years.
Figure 1. Cumulative multivariable‐adjusted mortality rates by age (A), sex (B), and congenital heart disease complexity (C).
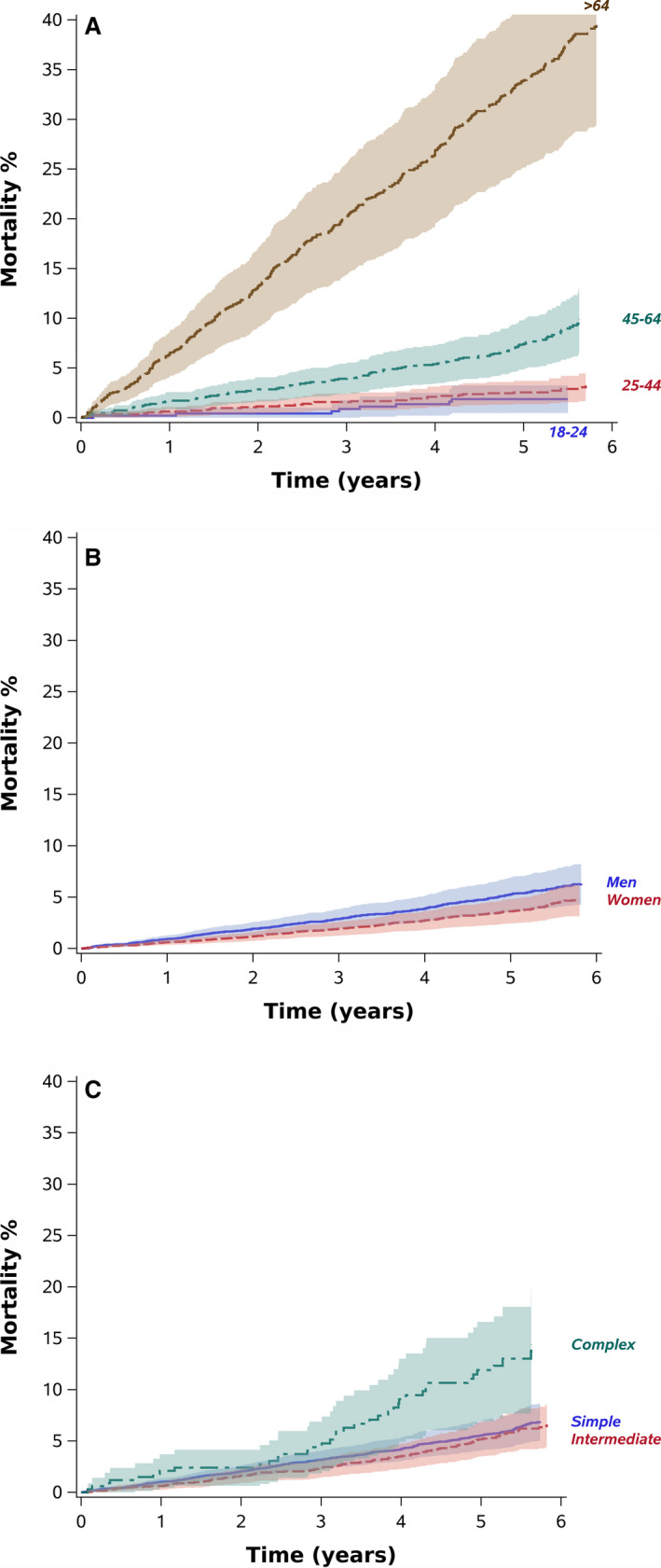
Color bands represent 95% CIs. The fixed values used included men, non‐Arab ethnicity, intermediate congenital disease complexity, and mean values of age, geographic periphery, number of noncardiac congenital defects, and baseline comorbidity score.
Health Services Utilization
Health service utilization rates are presented in Table 3. Most of the patients visited a primary care physician at least once in 5 years, with a median of 32 visits per 5 years. Cardiology outpatient visits were documented for 77% of the patients, and 53% were hospitalized during the follow‐up period.
Table 3.
Health Services Utilization by Sex and CHD Complexity
| Sex | Disease Complexity | ||||||
|---|---|---|---|---|---|---|---|
| Men | Women | P Value | Simple | Intermediate | Complex | P Value | |
| Used the service at least once,* n (%) † | |||||||
| Outpatient primary care | 5472 (98.6) | 6031 (98.8) | 0.13 | 8531 (98.8) | 2426 (98.7) | 546 (97.8) | 0.08 |
| Outpatient cardiology | 4298 (77.4) | 4717 (77.2) | 0.49 | 6643 (76.5) | 1947 (79.1) | 425 (76.0) | 0.004 |
| ED only visits | 3071 (56.2) | 3525 (57.0) | 0.36 | 4863 (55.6) | 1394 (57.7) | 339 (64.5) | <0.0001 |
| Hospital admissions | 2910 (53.3) | 3227 (52.1) | 0.17 | 4526 (51.7) | 1296 (53.6) | 315 60.3) | <0.0001 |
| Intensive care unit | 520 (9.6) | 444 (7.1) | 625 (7.1) | 249 (10.4) | 90 (16.2) | ||
| Frequency per 5 y, ‡ geometric mean (SD) | |||||||
| Primary care visits | 26.5 (2.5) | 34.1 (2.3) | 30.6 (2.4) | 29.0 (2.4) | 31.0 (2.4) | <0.0001 | |
| Outpatient cardiology visits | 4.2 (2.6) | 4.0 (2.5) | 4.0 (2.5) | 4.2 (2.5) | 5.0 (2.5) | 0.002 | |
| ED only visits | 1.9 (2.0) | 2.1 (2.1) | 2.0 (2.0) | 2.0 (2.1) | 2.2 (2.2) | <0.0001 | |
| Hospital admissions | 2.6 (2.6) | 2.5 (2.5) | 2.5 (2.5) | 2.5 (2.5) | 3.2 (2.7) | 0.26 | |
| Days in hospital | 8.7 (4.2) | 8.3 (4.2) | 8.4 (4.2) | 8.0 (4.1) | 11.5 (4.7) | 0.14 | |
| Days in intensive care unit | 7.5 (3.9) | 9.2 (4.1) | 8.8 (4.1) | 6.8 (3.6) | 8.3 (4.1) | 0.03 | |
CHD indicates congenital heart disease; and ED, emergency department visits not ending in hospitalization.
Used the service at least once in the data collection period (2007–2011).
Adjusted for age.
Age‐adjusted geometric mean per 5 years among patients who used the service.
Service utilization rates were higher among patients with ACHD than in the general population, across congenital heart disease complexity (Figure 2). The largest differences were observed for patients with a complex lesion, including primary care (SSR, 1.53; 95% CI, 1.47–1.58), cardiology outpatient visits (SSR, 5.17; 95% CI, 4.69–5.64), hospital admissions (SSR, 6.68; 95% CI, 5.82–7.54), and days in hospital (SSR, 15.37; 95% CI, 14.61–16.12). Rates of visits to the ED were higher among patients with ACHD with complex disease and lower than in the general population for patients with simple or intermediate complexity congenital heart disease (Figure 2).
Figure 2. Standardized service utilization rates (SSRs) by congenital heart disease complexity.
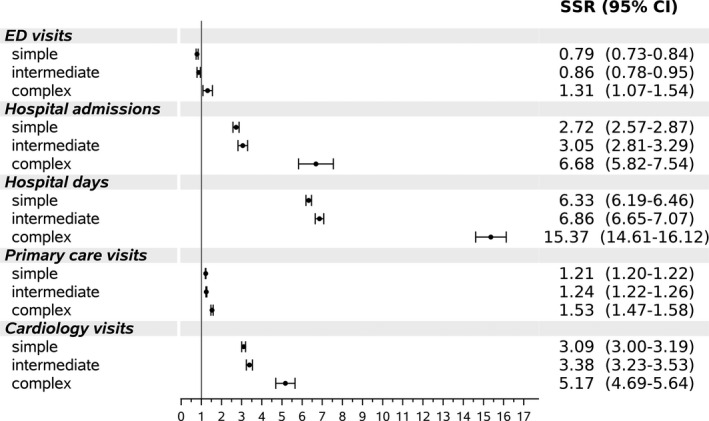
Rates matched for age, sex and ethnicity among patients with adult congenital heart disease aged 25 to 74 years compared with the general population (extrapolated from the population sample of the Hadera District Study). ED indicates emergency department.
Healthcare resource utilization among patients with ACHD increased with age in each complexity category (Figure S1), except for ED visits not resulting in hospitalization, for which the multivariable‐adjusted HR declined with increasing age (Table 4; Figure S2).
Table 4.
Multivariable‐Adjusted Recurrent Health Service HR Among Patients With ACHD
| Primary Care Visits | Outpatient Cardiology Visits | ED Only Visits* | Hospitalizations | Days in Hospital | Days in ICU | ||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | RR (95% CI) | RR (95% CI) | ||
| Age (reference=18–24 y), y † | |||||||
| 25–44 | 1.29 (1.17–1.43) | 1.26 (1.05–1.52) | 0.91 (0.37–2.22) | ||||
| 45–64 | 2.12 (1.92–2.34) | 1.93 (1.60–2.33) | 2.26 (0.90–5.74) | ||||
| >64 | 2.63 (2.36–2.93) | 4.73 (3.90–5.72) | 3.56 (1.38–9.18) | ||||
| Women (reference =men) | 1.23 (1.19–1.27) | 0.89 (0.84–0.94) | 1.25 (1.16–1.35) | 0.90 (0.83–0.97) | 0.80 (0.73–0.87) | 0.78 (0.53–1.17) | |
| Disease complexity (reference=simple) | |||||||
| Intermediate | 0.97 (0.93–1.01) | 0.99 (0.93–1.06) | 0.96 (0.87–1.06) | 1.02 (0.92–1.13) | 0.88 (0.79–0.98) | 0.60 (0.36–0.99) | |
| Complex | 1.14 (1.06–1.23) | 1.40 (1.24–1.59) | 1.19 (1.02–1.39) | 1.75 (1.49–2.05) | 1.71 (1.42–2.05) | 1.50 (0.64–3.54) | |
| Residence locality peripherality (reference=very central) | |||||||
| Central | 0.98 (0.94–1.02) | 0.94 (0.87–1.00) | 0.69 (0.62–0.77) | 1.04 (0.94–1.14) | 1.08 (0.97–1.200) | 1.64 (0.99–2.72) | |
| Medium | 1.04 (0.99–1.09) | 0.99 (0.91–1.06) | 0.93 (0.83–1.03) | 1.10 (0.99–1.22) | 1.15 (1.02–1.29) | 1.23 (0.69–2.21) | |
| Peripheral | 1.16 (1.10–1.24) | 0.89 (0.81–0.99) | 0.68 (0.60–0.77) | 1.01 (0.89–1.16) | 1.08 (0.92–1.25) | 2.58 (1.30–5.10) | |
| Very peripheral | 1.15 (1.01–1.31) | 0.72 (0.58–0.90) | 0.71 (0.56–0.92) | 1.10 (0.83–1.46) | 1.28 (0.89–1.85) | 1.04 (0.22–4.96) | |
| Residence locality ethnicity (reference=Jewish) | |||||||
| Arab | 1.38 (1.28–1.49) | 1.09 (0.96–1.25) | 1.24 (1.06–1.45) | 1.54 (1.29–1.85) | 1.02 (0.84–1.24) | 0.78 (0.31–1.93) | |
| Mixed | 0.96 (0.93–1.00) | 0.92 (0.86–0.99) | 1.15 (1.04–1.26) | 1.03 (0.94–1.13) | 0.97 (0.87–1.08) | 1.05 (0.65–1.68) | |
| Other congenital defects | 1.15 (1.10–1.20) | 1.10 (1.01–1.20) | 1.18 (1.03–1.36) | 1.03 (0.88–1.20) | 0.86 (0.76–0.99) | 1.28 (0.62–2.65) | |
| Charlson comorbidity score | 1.09 (1.08–1.10) | 1.07 (1.06–1.09) | 1.12 (1.09–1.16) | 1.19 (1.17–1.22) | 1.32 (1.28–1.35) | 1.32 (1.14–1.54) | |
Relative mean number of days in hospital or days in intensive care unit (ICU) were computed with negative binomial count models (see Methods). ACHD indicates adult congenital heart disease; HR, hazard ratio (for recurrent visits and hospitalization, computed with Cox proportional hazard models accounting for the competing risk of death) (see Methods); ICU, intensive care unit; and RR, rate ratio.
Emergency department (ED) visits—visits to the ED not leading to hospitalization.
Models were stratified by age groups when the proportional hazard assumption was violated (primary care visit, ED visits, and hospitalization). In addition to the variables in the table models were also adjusted for health service provider, geographic periphery index, ethnicity, other congenital defects, and baseline comorbidities.
Compared with simple congenital heart disease, complex lesions were associated with the highest adjusted healthcare utilization rates (Table 4; Figure S3) with hazards 14% higher for primary care visits, 40% for cardiology visits, and >70% higher for hospitalization and in‐hospital days. Further examination revealed an age and disease complexity interaction for outpatient cardiology visits (Table S4). While the relationship between congenital heart disease complexity and cardiology outpatient visits followed a dose response pattern for all age groups, the differences diminished with age, so that patients 45 years or older had a similar cardiology visit hazard across disease complexity categories. No other significant interaction was detected, although an attenuation of relative risks with age was noted among patients with complex disease for recurrent hospitalization.
Healthcare utilization patterns varied by sex (Table 3). Women were likely to visit a general practitioner more often (multivariable adjusted HR, 1.23; P<0.0001) and a cardiologist less often (adjusted HR, 0.89; P<0.0001). Women were also more likely to visit an ED (HR, 1.25; P<0.0001) but less likely to be admitted (HR, 0.90; P<0.0001), once maternity hospitalizations were excluded (Table 4; Figure S4).
Peripheral locality residence was associated with more primary care visits, fewer cardiology clinic visits (up to 28% less for very peripheral locations), fewer ED visits, and more days in intensive care (Table 4).
Compared with Jewish and other localities, residents of Arab localities were younger (median, 37 versus 47 years) and more likely to have a complex disease (6% versus 5%). Arabs had higher adjusted recurrent primary care visit rates (HR, 1.38), ED visits (HR, 1.24), and hospitalizations (HR, 1.54) (Table 4). The distance to the nearest specialized ACHD clinic, contributed little in multivariable analysis beyond periphery (HR, 1.0 for all services).
Sensitivity Analysis
As atrial septal defect and patent foramen ovale share the same ICD‐9 code (745.5), we repeated the analysis excluding 2955 (25%) patients identified solely by this code. Following exclusion, SSRs were identical and multivariable‐adjusted service utilization estimated hazards were similar to those estimated before exclusion of these patients (Table S5).
Discussion
This study based on a large ACHD patient population is among the first to report rates and patterns of both inpatient and outpatient health service utilizations and characteristics associated with utilization in this patient group. Compared with the general population, we found high healthcare utilization rates among patients with ACHD, including outpatient primary and cardiology care, ED visits, and hospitalizations, across congenital heart disease complexity. As expected, the highest SSRs were observed among patients with a complex disease, as previously reported for primary care consultations, 11 , 26 and hospital admissions. 11 , 14 Multiple congenital heart and other defects and higher age‐adjusted prevalence of ischemic heart disease, heart failure, and arrhythmia, found among patients with complex disease, likely contributed to higher healthcare utilization rates among this group. Nonetheless, in the current study, healthcare utilization rates among patients with simple or intermediate disease were also substantially higher than in the general population. This finding is in accord with the high mortality risk in the current study and previously reported increased long‐term morbidity and mortality in this patient group. 27 , 28
Most of the studies among patients with ACHD focused on inpatient services and few on outpatient care and therefore were not able to analyze utilization patterns. One exception is the study by Mackie et al, 11 which examined healthcare resource utilization in Quebec (1996–2000). Compared with that earlier study, our patients were older (median, 47 versus 43 years) with a higher proportion of men (48% versus 42%). These and health organization–related differences between the 2 studies may explain higher cardiology outpatient visit rates in our study (77% versus 55%), while rates of primary care (99% versus 91%) and hospitalizations (53% versus 51% at 5 years) were of similar magnitude.
The majority of the 11 563 patients in our study had a simple congenital heart disease and 5% had a complex congenital heart disease, a rate lower than previously reported for specialized clinics or hospitalized patients. The inclusion of patients less likely to be hospitalized in adulthood or being under follow‐up of specialized clinics in population studies leads to a higher proportion of patients with simple disease and therefore a lower proportion of patients with a complex disease. The prevalence of severe cardiac lesions reported by Mackie et al 1 was 8%, in line with our results. However, differences in classification method preclude direct comparison of this group between the studies.
Of all services examined, hospitalizations exert the highest costs. Increased survival of patients with ACHD increase hospitalization burden. 6 , 9 , 10 , 13 Based on 6 US state inpatient databases, most hospitalizations among patients with ACHD are cardiac related. 29 Acute kidney failure, bacterial infection, anemia, and procedure‐related complications were identified as the main drivers of longer hospital stay.
We found the association between congenital heart disease complexity and healthcare utilization to be age dependent. In agreement with a previous report, 11 the increase in cardiology visits or hospital admission rates, with higher disease complexity, diminished with age as acquired comorbidities play a greater role in healthcare demands.
Women visited a general practitioner more often and a cardiologist less often than men, and while they were also more likely to visit an ED, they were less likely to be hospitalized. These differences were not explained by congenital disease complexity or comorbidity in a multivariable model and may stem from sex‐related differences in healthcare utilization present in the general population. Our finding of higher mortality rates among men is supported by data from the European Heart Survey on ACHD. 30 Higher hospitalization rates among women compared with men, reported by others, 13 may be explained by the inclusion of maternity‐related hospitalizations.
Less optimal utilization patterns found in geographic periphery and minority localities, albeit universal access to care, may reflect lower accessibility of specialized ACHD care related to distance, language, and health literacy. Similar utilization patterns were reported by others for Israeli Arabs as well as for other minority and low socioeconomic population groups. 31
General cardiologists treating patients with ACHD were reported to deviate from guidelines more often and have higher rates of adverse patient sequels. 32
Strengths and Limitations
The use of electronic patient records enabled adequate sample size and population‐based assessments of healthcare utilization rates and patterns among patients with ACHD. A challenge of recurrent event analysis is the possible interaction of a death with recurrent events, which may result in biased estimates. 24 Accounting for this is one of the strengths of the current study.
A number of limitations deserve notice. First, because of data limitations, we could not distinguish between general cardiology and specialized ACHD clinic visits recommended by current guidelines. While the distance to the nearest specialized clinic, as a proxy, contributed little beyond peripheral residency in multivariable analysis, differential access to specialized clinics among minority and peripheral population sections cannot be ruled out. Evaluations of SSR and standardized mortality ratios were limited to patients aged 25 to 74 years, which hindered any conclusion regarding younger or older patients with ACHD.
Second, ICD‐9 codes have been generally used for identification and classification of patients with ACHD in studies based on administrative data (for review see reference 33), albeit recognized limitations. 34 , 35 The single code (745.5) shared by atrial septal defect and patent foramen ovale may lead to overestimation of simple congenital heart disease prevalence and healthcare resource utilization among them, when patent foramen ovale is incidentally detected. In the current study, 25% of the patients were identified solely by this code, yet results of a sensitivity analysis, excluding these patients, were similar to the main results. Additionally, the accuracy of identification and classification of patients with ACHD based on ICD‐9 codes was criticized, particularly among patients with low disease complexity. 35 Deidentified data in our study preclude direct assessment of misclassification in the current study.
Last, data were collected through December 2011. Nevertheless, the overall structure of the Israeli healthcare system, including the number of facilities and services provided, have not significantly changed since then. While diagnostic and therapeutic techniques are constantly evolving, no substantial changes have occurred over the past decade that would have rendered our results irrelevant.
Conclusions
Patients with ACHD had high healthcare utilization rates compared with the general population. Female sex, geographic periphery, and ethnic minority were associated with higher primary care visit rates, and female sex and periphery with lower outpatient cardiology visit rates. This outpatient service pattern was associated with higher hospitalization rates among Arabs and higher relative number of days in the intensive care unit for periphery. Greater disparities may be found in health systems based on private insurance. Further research should examine health policies and their implementation according to current guidelines in different parts of the patient population and in different settings.
Appendix
The Israeli Adult Congenital Heart Disease Research Group
Michal Benderly, Ofra Kalter‐Leibovici—Cardiovascular Epidemiology Unit, Gertner Institute for Epidemiology and Health Policy Research, Sheba Medical Center, Ramat‐Gan, Israel; Jonathan Buber—Division of Cardiology, Department of Medicine, University of Washington School of Medicine, Seattle, WA, USA; Leonard Blieden, Alexander Dadashev, Rafael Hirsch—Adult Congenital Heart Disease Unit, Rabin Medical Center, Petach Tikva, Israel; Avraham Lorber, Sergei Yalonetsky—Pediatric Cardiology and GUCH Unit, Rambam Health Care Campus, Haifa, Israel; Amiram Nir—Pediatric Cardiology and Adult Congenital Heart Disease Unit, Shaare Zedek Medical Center, Jerusalem, Israel; Efrat Mazor Dray—Leviev Heart Institute, Sheba Medical Center, Ramat‐Gan, Israel; Yaron Razon—Department of Pediatrics, Assuta Ashdod Medical Center, Ashdod, Israel.
Sources of Funding
This study was supported by an unrestricted grant from the Israel National Institute for Health Policy Research, Ramat‐Gan, Israel.
Disclosures
None.
Supporting information
Tables S1–S5
Figures S1–S4
Acknowledgments
The authors are grateful to Ilya Novikov, PhD, for his statistical advice and support.
(J Am Heart Assoc.2021;10:e018037. DOI: 10.1161/JAHA.120.018037.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Marelli AJ, Mackie A, Ionescu‐Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. DOI: 10.1161/CIRCULATIONAHA.106.627224 [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke‐Baerwolf C, Kaemmerer H, Kilner P, et al. Task Force on the management of grown‐up congenital heart disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG). ESC guidelines for the management of grown‐up congenital heart disease (new version 2010). Eur Heart J. 2010;31:2915–2957. [DOI] [PubMed] [Google Scholar]
- 3. Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, Somerville J, Williams RG, Webb GD. Task Force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170–1175. DOI: 10.1016/S0735-1097(01)01272-4 [DOI] [PubMed] [Google Scholar]
- 4. Webb G, Mulder BJ, Aboulhosn J, Daniels CJ, Elizari MA, Hong GU, Horlick E, Landzberg MJ, Marelli AJ, O'Donnell CP, et al. The care of adults with congenital heart disease across the globe: current assessment and future perspective: a position statement from the International Society for Adult Congenital Heart Disease (ISACHD). Int J Cardiol. 2015;195:326–333. DOI: 10.1016/j.ijcard.2015.04.230 [DOI] [PubMed] [Google Scholar]
- 5. Ntiloudi D, Giannakoulas G, Parcharidou D, Panagiotidis T, Gatzoulis MA, Karvounis H. Adult congenital heart disease: a paradigm of epidemiological change. Int J Cardiol. 2016;218:269–274. DOI: 10.1016/j.ijcard.2016.05.046 [DOI] [PubMed] [Google Scholar]
- 6. Agarwal S, Sud K, Menon V. Nationwide hospitalization trends in adult congenital heart disease across 2003–2012. J Am Heart Assoc. 2016;5:e002330. DOI: 10.1161/JAHA.115.002330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Afilalo J, Therrien J, Pilote L, Ionescu‐Ittu R, Martucci G, Marelli AJ. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol. 2011;58:1509–1515. [DOI] [PubMed] [Google Scholar]
- 8. Willems R, Werbrouck A, De Backer J, Annemans L. Real‐world healthcare utilization in adult congenital heart disease: a systematic review of trends and ratios. Cardiol Young. 2019;29:553–563. DOI: 10.1017/S1047951119000441 [DOI] [PubMed] [Google Scholar]
- 9. Islam S, Yasui Y, Kaul P, Marelli AJ, Mackie AS. Congenital heart disease hospitalizations in Canada: a 10‐year experience. Can J Cardiol. 2016;32:197–203. DOI: 10.1016/j.cjca.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 10. Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol. 2009;54:460–467. DOI: 10.1016/j.jacc.2009.04.037 [DOI] [PubMed] [Google Scholar]
- 11. Mackie AS, Pilote L, Ionescu‐Ittu R, Rahme E, Marelli AJ. Health care resource utilization in adults with congenital heart disease. Am J Cardiol. 2007;99:839–843. DOI: 10.1016/j.amjcard.2006.10.054 [DOI] [PubMed] [Google Scholar]
- 12. Gurvitz MZ, Inkelas M, Lee M, Stout K, Escarce J, Chang RK. Changes in hospitalization patterns among patients with congenital heart disease during the transition from adolescence to adulthood. J Am Coll Cardiol. 2007;49:875–882. DOI: 10.1016/j.jacc.2006.09.051 [DOI] [PubMed] [Google Scholar]
- 13. Billett J, Majeed A, Gatzoulis M, Cowie M. Trends in hospital admissions, in‐hospital case fatality and population mortality from congenital heart disease in England, 1994 to 2004. Heart. 2008;94:342–348. DOI: 10.1136/hrt.2006.113787 [DOI] [PubMed] [Google Scholar]
- 14. Verheugt CL, Uiterwaal CS, van der Velde ET, Meijboom FJ, Pieper PG, Sieswerda GT, Plokker HW, Grobbee DE, Mulder BJ. The emerging burden of hospital admissions of adults with congenital heart disease. Heart. 2010;96:872–878. DOI: 10.1136/hrt.2009.185595 [DOI] [PubMed] [Google Scholar]
- 15. Tutarel O, Kempny A, Alonso‐Gonzalez R, Jabbour R, Li W, Uebing A, Dimopoulos K, Swan L, Gatzoulis MA, Diller GP. Congenital heart disease beyond the age of 60: emergence of a new population with high resource utilization, high morbidity, and high mortality. Eur Heart J. 2014;35:725–732. DOI: 10.1093/eurheartj/eht257 [DOI] [PubMed] [Google Scholar]
- 16. Gatzoulis MA, Hechter S, Siu SC, Webb GD. Outpatient clinics for adults with congenital heart disease: increasing workload and evolving patterns of referral. Heart. 1999;81:57–61. DOI: 10.1136/hrt.81.1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koh AS, Yap BT, Le Tan J. Emergency admissions in Asians with adult congenital heart disease. Int J Cardiol. 2011;151:54–57. DOI: 10.1016/j.ijcard.2010.04.088 [DOI] [PubMed] [Google Scholar]
- 18. Negishi J, Ohuchi H, Yasuda K, Miyazaki A, Norifumi N, Yamada O. Unscheduled hospitalization in adults with congenital heart disease. Korean Circ J. 2015;45:59–66. DOI: 10.4070/kcj.2015.45.1.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosen B, Samuel H, Merkur S. Israel: health system review. World Health Organization. Regional Office for Europe, European Observatory on Health Systems and Policies; 2009.
- 20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 21. Central Bureau of Statistics . Peripheriality index—of localities and local authorities—2015. Available at https://www.cbs.gov.il/en/publications/Pages/2019/Peripheriality‐Index‐Of‐Localities‐And‐Local‐Authorities‐2015.aspx. Accessed December 15, 2020.
- 22. Woolson RF, Bean JA. Mantel‐Haenszel statistics and direct standardization. Stat Med. 1982;1:37–39. DOI: 10.1002/sim.4780010106 [DOI] [PubMed] [Google Scholar]
- 23. Andersen PK, Angst J, Ravn H. Modeling marginal features in studies of recurrent events in the presence of a terminal event. Lifetime Data Anal. 2019;25:681–695. DOI: 10.1007/s10985-019-09462-4 [DOI] [PubMed] [Google Scholar]
- 24. Ghosh D, Lin D. Marginal regression models for recurrent and terminal events. Stat Sin. 2002;12:663–688. [Google Scholar]
- 25. Benderly M, Chetrit A, Murad H, Abu‐Saad K, Gillon‐Keren M, Rogowski O, Sela B‐A, Kanety H, Harats D, Atamna A, et al. Cardiovascular health among two ethnic groups living in the same region: a population‐based study. Int J Cardiol. 2017;228:223–230. DOI: 10.1016/j.ijcard.2016.11.079 [DOI] [PubMed] [Google Scholar]
- 26. Billett J, Cowie MR, Gatzoulis MA, Vonder Muhll IF, Majeed A. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross‐sectional, population‐based study with case‐control analysis. Heart. 2008;94:1194–1199. [DOI] [PubMed] [Google Scholar]
- 27. Ávila P, Mercier LA, Dore A, Marcotte F, Mongeon FP, Ibrahim R, Asgar A, Miro J, Andelfinger G, Mondésert B, et al. Adult congenital heart disease: a growing epidemic. Can J Cardiol. 2014;30:S410–S419. DOI: 10.1016/j.cjca.2014.07.749 [DOI] [PubMed] [Google Scholar]
- 28. Diller GP, Kempny A, Alonso‐Gonzalez R, Swan L, Uebing A, Li W, Babu‐Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow‐up at a large tertiary centre. Circulation. 2015;132:2118–2125. [DOI] [PubMed] [Google Scholar]
- 29. Cedars A, Benjamin L, Burns SV, Novak E, Amin A. Clinical predictors of length of stay in adults with congenital heart disease. Heart. 2017;103:1258–1263. DOI: 10.1136/heartjnl-2016-310841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Engelfriet P, Mulder BJ. Gender differences in adult congenital heart isease. Neth Heart J. 2009;17:414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baron‐Epel O, Garty N, Green MS. Inequalities in use of health services among Jews and Arabs in Israel. Health Serv Res. 2007;42:1008–1019. DOI: 10.1111/j.1475-6773.2006.00645.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McClure RJ, Newell SJ, Edwards S. Patient characteristics affecting attendance at general outpatient clinics. Arch Dis Child. 1996;74:121–125. DOI: 10.1136/adc.74.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohen S, Gilutz H, Marelli AJ, Iserin L, Benis A, Bonnet D, Burgun A. Administrative health databases for addressing emerging issues in adults with CHD: a systematic review. Cardiol Young. 2018;28:844–853. DOI: 10.1017/S1047951118000446 [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez FH III, Ephrem G, Gerardin JF, Raskind‐Hood C, Hogue C, Book W. The 745.5 issue in code‐based, adult congenital heart disease population studies: relevance to current and future ICD‐9‐CM and ICD‐10‐CM studies. Congenit Heart Dis. 2018;13:59–64. DOI: 10.1111/chd.12563 [DOI] [PubMed] [Google Scholar]
- 35. Khan A, Ramsey K, Ballard C, Armstrong E, Burchill LJ, Menashe V, Pantely G, Broberg CS. Limited accuracy of administrative data for the identification and classification of adult congenital heart disease. J Am Heart Assoc. 2018;7:e007378. DOI: 10.1161/JAHA.117.007378 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figures S1–S4


