Abstract
Background
Ticagrelor reduces ischemic risk but increases bleeding in patients with prior myocardial infarction. Identification of patients at lower bleeding risk is important in selecting patients who are likely to derive more favorable outcomes versus risk from this strategy.
Methods and Results
PEGASUS‐TIMI 54 (Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin—Thrombolysis in Myocardial Infarction 54) randomized 21 162 patients with prior myocardial infarction in a 1:1:1 fashion to ticagrelor 60 mg or 90 mg twice daily or placebo, with ticagrelor 60 mg approved for long‐term use. TIMI major or minor bleeding was the primary end point for this analysis. Causes of bleeding were categorized by site and etiology, and independent predictors were identified. At 3 years, ticagrelor 60 mg increased the rate of TIMI major or minor bleeding by 2.0% versus placebo (1.4% placebo versus 3.4% ticagrelor). The bleeding excess was driven primarily by spontaneous gastrointestinal bleeds. A history of spontaneous bleeding requiring hospitalization and the presence of anemia were independent predictors of bleeding but not of ischemic risk. Patients with at least 1 risk predictor had 3‐fold higher rates of bleeding with ticagrelor 60 mg versus those who had neither (absolute risk increase, 4.4% versus 1.5%; P=0.01). Patients with neither predictor had a more favorable benefit profile with ticagrelor 60 mg versus placebo including lower mortality (hazard ratio, 0.79; 95% CI, 0.65–0.96; P interaction = 0.03).
Conclusions
In patients with prior myocardial infarction, bleeding with ticagrelor 60 mg twice daily is predominantly spontaneous gastrointestinal. A history of spontaneous bleeding requiring hospitalization or the presence of anemia identifies patients at higher risk of bleeding, and the absence of either identifies patients likely to have a more favorable net benefit with ticagrelor.
Registration
URL https://www.clinicaltrials.gov/. Unique identifier: NCT01225562.
Keywords: benefit‐risk ratio, bleeding, long‐term ticagrelor, myocardial infarction
Subject Categories: Acute Coronary Syndromes, Coronary Artery Disease, Thrombosis
Nonstandard Abbreviations and Acronyms
- COGENT
Clopidogrel and the Optimization of Gastrointestinal Events
- ICH
Intracranial hemorrhage
- PEGASUS
Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin
- TIMI
Thrombolysis in Myocardial Infarction
- MI
Myocardial infarction
- PPI
Proton pump inhibitor
Clinical Perspective
What Is New?
In stable patients with prior myocardial infarction on aspirin, TIMI (Thrombolysis in Myocardial Infarction) major or minor bleeding is primarily spontaneous of gastrointestinal origin and related to an underlying disease.
Ticagrelor 60 mg increases spontaneous TIMI major or minor bleeding, including gastrointestinal bleeding, but does not increase fatal bleeding, bleeding that contributed to death, or spontaneous intracranial hemorrhage.
Anemia at baseline and history of previous bleeding requiring hospitalization were independent predictors of bleeding and were not associated with ischemic risk or the benefit of ticagrelor. Patients without anemia at baseline or history of previous bleeding requiring hospitalization derived greater benefit from prolonged ticagrelor therapy.
What Are the Clinical Implications?
In a stable post–myocardial infarction population, prior bleeding and anemia may be sufficient for bleeding risk prediction and may enable clinicians to consider bleeding and ischemic risk independently.
This may be particularly helpful in groups such as the elderly in whom assessment of risks and benefits may be particularly challenging.
see Editorial by Cao et al.
Long‐term therapy with ticagrelor 60 mg twice daily added to aspirin in patients with a previous myocardial infarction (MI) reduced major adverse cardiovascular events in the PEGASUS‐TIMI 54 (Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin—Thrombolysis in Myocardial Infarction 54) trial. 1 Based on this finding, ticagrelor 60 mg twice daily has been approved in Europe and the United States for long‐term use for secondary prevention after MI. 2 However, ticagrelor also increased the risk of TIMI major and minor bleeding, although it did not increase intracranial hemorrhage (ICH) or fatal bleeding.
Serious bleeding is an important outcome and has been associated with several adverse events including hospitalizations, procedures, cessation of medications, and mortality. 3 Therefore, understanding the sites, etiologies, and outcomes after bleeding may be helpful in weighing the benefits and risks of antithrombotic therapies. In addition, identifying patients at greater risk of bleeding may enable personalization of therapy to those at lower bleeding risk who are more likely to derive greater benefit. A challenge to bleeding risk prediction, however, is that several characteristics are associated with both bleeding and ischemic risk.
In this context, a post hoc analysis of bleeding in PEGASUS‐TIMI 54 was performed, including characterization of the sites and causes of bleeding. In addition, baseline characteristics independently associated with bleeding risk, but not ischemic risk, were identified. Finally, a post hoc analysis evaluating the efficacy and safety of long‐term ticagrelor stratified by baseline bleeding risk was performed.
Methods
Study Population
The data for the analyses are held at the TIMI Study Group, and the senior author may be contacted for requests with regard to the sharing of data, methods, and materials specific to this analysis. The study protocol was approved by the relevant ethics committee at each participating site. Written informed consent was obtained from all the patients. The PEGASUS‐TIMI 54 trial, described previously, 1 , 4 randomized 21 162 patients with prior spontaneous MI occurring 1–3 years before enrollment, who had at least 1 additional atherothrombotic risk factor (age ≥65 years, diabetes mellitus requiring medication, a second prior spontaneous MI, chronic renal dysfunction, or multivessel coronary artery disease) to ticagrelor 60 mg twice daily, ticagrelor 90 mg twice daily, or placebo in a 1:1:1 fashion, all on a background of low‐dose (75 mg–150 mg) aspirin. The 60‐mg dose was approved for long‐term secondary prevention, and therefore the primary group for analysis includes patients randomized to 60 mg (N=6958) or placebo (N=6996) with additional analyses for the 90‐mg dose (N=6988) versus placebo included in the online supplement. Exclusion criteria included planned use of a P2Y12 receptor antagonist or anticoagulant therapy, a known bleeding disorder, history of stroke, a central nervous system tumor, gastrointestinal bleeding within the previous 6 months, or major surgery within the previous 30 days. Enrolling sites were requested to indicate if there was any history of bleeding leading to hospitalization. All patients had central laboratory testing for hemoglobin at baseline. Anemia was defined as a hemoglobin ≤13.5 g/dL for men and ≤ 12.0 g/dL for women.
End Points
The primary efficacy end point was major adverse cardiovascular events, consisting of the composite of cardiovascular death, MI, or stroke (3‐point major adverse cardiac event). The primary safety end point was TIMI major bleeding. Secondary safety end points were combined TIMI major and minor bleeding, as well as ICH and fatal bleeding. TIMI major bleeding was defined as any ICH, or clinically overt signs of hemorrhage associated with a reduction in hemoglobin ≥5 g/dL (or, when hemoglobin was not available, a fall in hematocrit ≥15%), or fatal bleeding (a bleeding event that directly led to death within 7 days). TIMI minor bleeding was defined as any clinically overt hemorrhage (including that detected by imaging) that was associated with a fall in hemoglobin of 3 to <5 g/dL (or, when hemoglobin was not available, a fall in hematocrit of 9 to <15%). Hemoglobin measurements were adjusted for any packed red blood cells or whole blood given between baseline and posttransfusion measurement; transfusion of 1 unit of blood was assumed to result in an increase of 1 g/dL of hemoglobin. Bleeding events leading directly to mortality were classified as fatal bleeding events. Bleeding that was not directly fatal but was in the causal pathway leading to death (eg, death attributable to a nonbleeding complication of hospitalization prompted by bleeding) were classified as “bleeding contributing to death.” All bleeding events, as well as their relationship to mortality, were adjudicated by a clinical events committee blinded to treatment allocation. A net clinical benefit analysis was defined as cardiovascular death, MI, stroke, ICH, or fatal bleeding.
Statistical Analysis
Baseline characteristics, including demographics, medical history, and clinical findings at randomization were summarized using medians and quartiles (interquartile range) for continuous variables and frequencies and percentages for categorical variables. Differences were tested with the Wilcoxon rank‐sum test for continuous variables and with the Pearson χ2 test for categorical data. The primary efficacy analysis and net clinical outcome were conducted on an intention‐to‐treat basis, whereas safety analyses included all patients who underwent randomization and received at least 1 dose of study drug. Event rates for ticagrelor and placebo were estimated by Kaplan–Meier methods from baseline to 3 years and compared with the log‐rank test. In addition, the instantaneous hazard function for TIMI major or minor bleeding was also estimated by the kernel‐based method. 5 This was examined separately by treatment arm over time.
A multivariable Cox proportional hazards model was developed by first examining univariate associations between baseline characteristics and the risk of TIMI major or minor bleeding in the overall population. Clinically meaningful covariates that met P value thresholds of 0.1 in univariable association were further retained for the pool of candidate variables. These included age, weight, systolic blood pressure, estimated glomerular filtration rate, history of spontaneous bleeding, history of diabetes mellitus, history of congestive heart failure, history of malignancy, white blood cell count, baseline anemia, and smoking status. The backward elimination method was then used to yield the final model with the reduced number of risk factors based on a P value cutoff < 0.01.
Multivariable models evaluating the association between baseline characteristics and the risk of the primary efficacy end point were evaluated. The proportional hazards assumption was examined and tested by scaled Schoenfeld residuals. The linearity assumption for continuous variables was examined by restricted cubic spline plots. Characteristics that independently predicted bleeding but were not independently associated with the risk of the primary efficacy end point were identified as those most useful for patient selection. The results of these models were described using the hazard ratio (HR) with the associated 95% CI. The discrimination index was assessed using the Harrell’s C statistic. In addition, the absolute risk differences between treatment and placebo groups were compared across high‐ and low‐bleeding‐risk groups using the Gail–Simon 2‐sided heterogeneity test. 6 A sensitivity analysis of efficacy and safety was then performed, stratified by baseline bleeding risk with patients with a history of bleeding or anemia at baseline considered to be at “high bleeding risk” and those with no predictors of bleeding considered at “low bleeding risk.”
RESULTS
A total of 20 942 patients received at least 1 dose of the study drug (safety population). Baseline characteristics in the overall safety population, according to the occurrence of TIMI major or minor bleeding during follow‐up (median, 33 months), are summarized in Table 1. Patients who experienced a TIMI major or minor bleed (n=432) were older and more likely to have hypertension, renal insufficiency, a history of malignancy, a history of a previous spontaneous bleed requiring hospitalization, and a hemoglobin value indicating anemia.
Table 1.
Baseline Characteristics by TIMI Major or Minor Bleeding During Follow‐Up
| TIMI Major or Minor Bleeding (N=432) | No TIMI Major or Minor Bleeding (N=20 510) | P Value | |
|---|---|---|---|
| Age, y, median (IQR) | 68.0 (61.0–74.0) | 65.0 (59.0–71.0) | <0.0001 |
| Female, n (%) | 100/432 (23.1) | 4903/20 510 (23.9) | 0.71 |
| Weight, kg, median (IQR) | 79.0 (68.0–90.4) | 81.0 (70.0–92.0) | 0.04 |
| History of hypertension, n (%) | 359/432 (83.1) | 15879/20 510 (77.4) | 0.005 |
| SBP, mm Hg, median (IQR) | 132.0 (120.0–145.0) | 130.0 (120.0–142.0) | 0.052 |
| History of diabetes mellitus, n (%) | 139/432 (32.2) | 6596/20 510 (32.2) | 0.99 |
| Current smoker, n (%) | 85/432 (19.7) | 3419/20 505 (16.7) | 0.10 |
| Renal dysfunction, eGFR <60 (MDRD, mL/min/1.73 m2), n (%) | 130/426 (30.5) | 4658/20 296 (23.0) | 0.0002 |
| History of CHF, n (%) | 80/432 (18.5) | 4123/20 510 (20.1) | 0.42 |
| History of malignancy, n (%) | 35/432 (8.1) | 1256/20 510 (6.1) | 0.09 |
| History of spontaneous bleeding requiring hospitalization, n (%) | 19/432 (4.4) | 245/20 510 (1.2) | <0.0001 |
| Anemia,* n (%) | 126/418 (30.1) | 3723/20 082 (18.5) | <0.0001 |
| WBC (10×9/L), median (IQR) | 6.7 (5.6–8.1) | 6.9 (5.8–8.1) | 0.10 |
| Proton pump inhibitor use, n (%) | 111/432 (25.7) | 5378/20 510 (26.2) | 0.81 |
CHF indicates congestive heart failure; eGFR, estimated glomerular filtration rate; IQR, interquartile range; MDRD, Modification of Diet in Renal Disease; TIMI, Thrombolysis in Myocardial Infarction; and WBC, white blood cell count.
Anemia was defined as hemoglobin ≤13.5 g/dL for men and ≤12.0 g/dL for women.
Type, Location, and Cause of Bleeding in All Treatment Arms
The majority of TIMI major or minor bleeding events were spontaneous (N=306; 71% of all bleeds) followed by procedural bleeding (N=65; 15%) and traumatic bleeding (N=61; 14%) (Figure 1A). Spontaneous TIMI major or minor bleeds were primarily of gastrointestinal origin (75%), followed by intracranial (12%) and genitourinary (6%, Figure 1B). The sites and causes of bleeding were similar in patients randomized to ticagrelor 60 mg twice daily or placebo (Figure S1). Of the spontaneous gastrointestinal bleeds, the most common was upper gastrointestinal ulcer or inflammation (49%), followed by diverticular bleeding or hemorrhoids (20%) and previously undiagnosed neoplasm (17%) (Figure S2).
Figure 1.
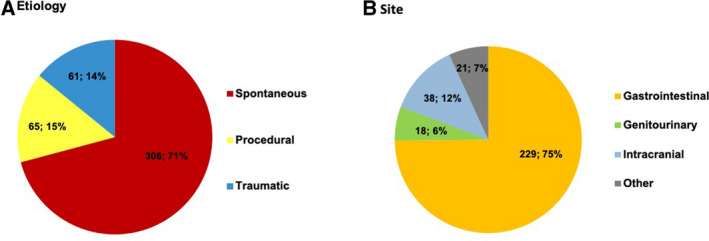
TIMI major or minor bleeding by etiology (A) and site of spontaneous bleeding (B) in the overall safety population. TIMI indicates Thrombolysis in Myocardial Infarction.
Treatment of and Outcomes After Bleeding in All Treatment Arms
Approximately half (56%) of patients who experienced a spontaneous TIMI major or minor bleed received a transfusion or underwent a nonsurgical procedure or surgery (nonsurgical procedure, 42%; surgery, 7%; transfusion only, 11%) to treat the bleeding. Of the 432 patients who experienced a TIMI major or minor bleed, 42 (9.7%) died of causes related to bleeding, with 28 having a directly fatal bleed and 14 in whom bleeding contributed to death. The median time from the bleeding event to death was 2.0 days (interquartile range, 0–5) for directly fatal bleed and 10 days (interquartile range, 2–61) for bleeding that contributed to death. Of the patients who survived their bleeding, an additional 38 died later during the study (median, 207.5 days after bleeding; interquartile range, 78–404) for causes unrelated to bleeding, including cardiovascular causes (45%), sepsis or organ failure (18%), and malignancy (37%) (Figure 2).
Figure 2.
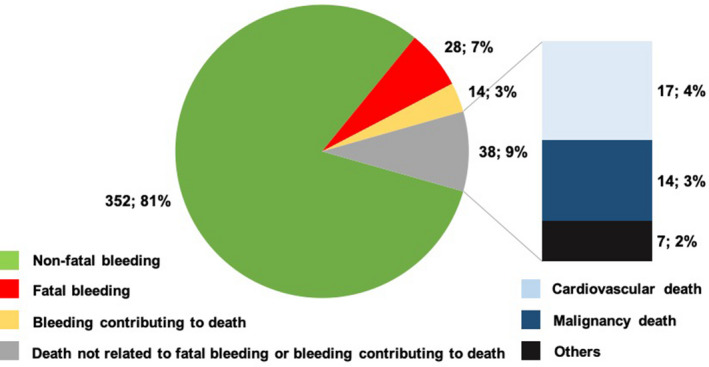
Incidence and cause of mortality among patients who experienced a TIMI major or minor bleeding (overall safety cohort). TIMI indicates Thrombolysis in Myocardial Infarction.
Predictors of Bleeding in All Treatment Arms
The following variables were identified as independently associated with TIMI major or minor bleeding in the total population: age, history of hypertension, current smoking, history of spontaneous (nontraumatic, nonprocedural) bleeding requiring hospitalization, and anemia at baseline (Table 2). Supplemental Figure S3 shows cubic splines for hemoglobin and risk of TIMI major or minor bleeding in women (a) and men (b). Age, history of hypertension, and current smoking were also independent predictors of ischemic risk. However, anemia and a history of spontaneous bleeding requiring hospitalization were not.
Table 2.
Independent Predictors of TIMI Major or Minor Bleeding in the Overall Population
| Predictors | HR (95% CI) | P Value | χ2 |
|---|---|---|---|
| Age (continuous, per 10‐y increase) | 1.47 (1.31–1.66) | <0.0001 | 40.2 |
| History of spontaneous bleeding requiring hospitalization | 3.56 (2.24–5.64) | <0.0001 | 29.1 |
| Anemia at baseline | 1.72 (1.39–2.13) | <0.0001 | 24.6 |
| Current smoker | 1.59 (1.24–2.03) | 0.0002 | 13.4 |
| History of hypertension | 1.43 (1.10–1.84) | 0.007 | 7.3 |
c‐index for variables listed 0.65 (95% CI, 0.62–0.68).
HR, hazard ratio; and TIMI, Thrombolysis in Myocardial Infarction.
Bleeding With Ticagrelor 60 mg Versus Placebo
Compared with placebo, ticagrelor 60 mg twice daily increased the risk of TIMI major or minor bleeding (HR, 2.54; 95% CI, 1.93–3.35; P < 0.001). The instantaneous hazard function for TIMI major or minor bleeding is shown in Figure S4. At 3 years, ticagrelor 60 mg twice daily increased TIMI major or minor bleeding by 2.0% (3.4% with ticagrelor compared with 1.4% with placebo). This excess was primarily in spontaneous bleeds (1.5%), traumatic (0.3%) or procedural (0.2%) (Figure 3a). The excess in spontaneous bleeds was primarily attributable to gastrointestinal bleeding (absolute risk increase, 1.1%; Figure 3b). The rate of gastrointestinal bleeding was less frequent in patients on ticagrelor 60 mg twice daily and a proton pump inhibitor (PPI), compared with patients not taking a PPI (0.4% versus 0.8%; HR, 0.47; 95% CI, 0.27–0.82; P = 0.009).
Figure 3. Etiologies (A) and sites (B) of TIMI major or minor bleeding, Ticagrelor 60 mg vs. placebo.
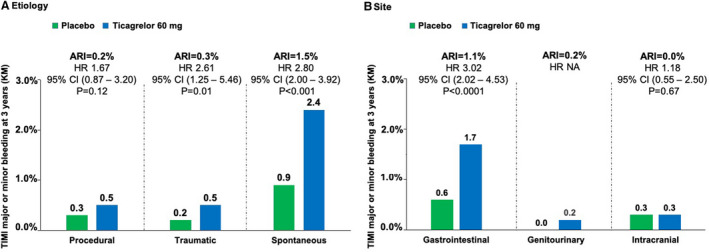
ARI indicates absolute risk increase; HR, hazard ratio, KM, Kaplan–Maier; NA, not applicable; and TIMI, Thrombolysis in Myocardial Infarction.
There was no significant increase in ICH with ticagrelor 60 mg compared with placebo (HR, 1.18; 95% CI, 0.55–2.50; P = 0.67; Figure 3b). In addition, there was no significant increase in fatal bleeding or bleeding contributing to death with ticagrelor 60 mg relative to placebo (Figure 4). Results for ticagrelor 90 mg twice daily compared with placebo were similar and are reported in Figure S5a and S5b and Figure S6.
Figure 4. Bleeding and death, ticagrelor 60 mg vs placebo.
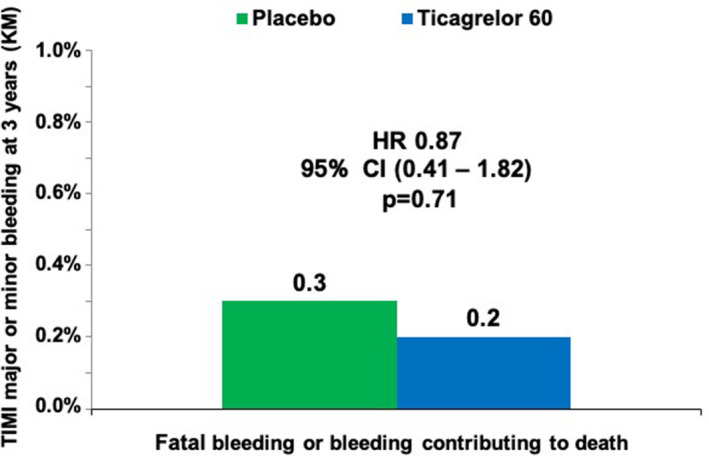
HR indicates hazard ratio; KM, Kaplan–Maier; and TIMI, Thrombolysis in Myocardial Infarction.
Efficacy and Safety of Ticagrelor 60 mg in Patients at High and Low Bleeding Risk
Based on the models of ischemic and bleeding risk, high bleeding risk was defined as either a history of spontaneous bleeding requiring hospitalization, anemia at baseline, or both (N=2714; 19% of the population), whereas low bleeding risk was defined as the absence of either characteristic (N=11 240; 81% of the population). Table S1 shows baseline characteristics stratified by high versus low bleeding risk in the overall population. When comparing the safety of ticagrelor 60 mg twice daily versus placebo, the HR for TIMI major or minor bleeding was 2.93 (95% CI, 1.80–4.78) in patients at high bleeding risk, and 2.37 (95% CI, 1.70–3.32) in patients at low bleeding risk. There was a greater absolute increase in the rate of TIMI major or minor bleeding with ticagrelor in the high‐bleeding‐risk group (increase of 4.4% at 3 years; 95% CI, 2.3%–6.4%) compared with the increase with ticagrelor in patients in the low‐bleeding‐risk group (increase of 1.5% at 3 years; 95% CI, 0.8%–2.1%) with a significant interaction based on absolute differences (P value for absolute risk difference = 0.01; Figure 5). Table S2 shows the primary end point and TIMI major bleeding rates stratified by high versus low bleeding and ischemic risk.
Figure 5. TIMI major or minor bleeding in ticagrelor 60 mg vs placebo group stratified by low (N=11 240) or high (N=2714) bleeding risk at baseline.
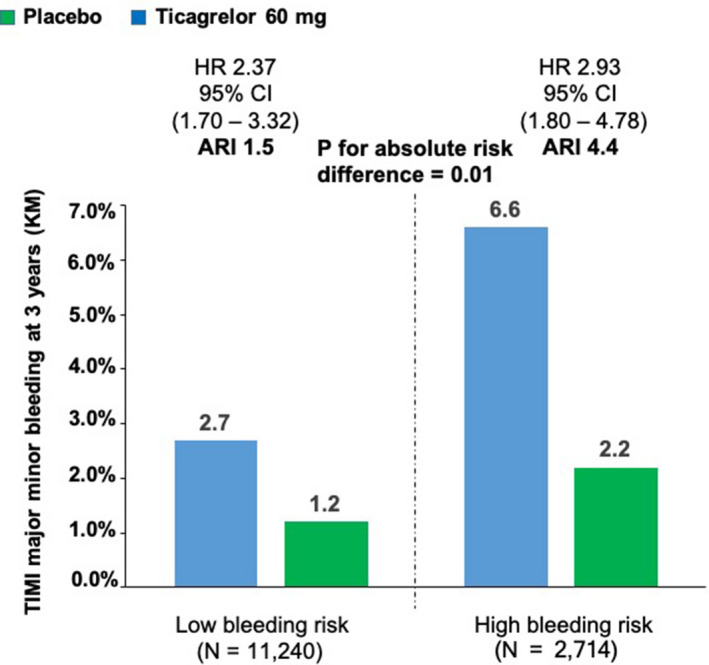
A sensitivity analysis of safety was performed based on baseline bleeding risk, defined as the presence or absence of either a history of spontaneous bleeding requiring hospitalization before the randomization or anemia (defined as hemoglobin ≤13.5 g/dL for men and ≤12.0 g/dL for women). ARI indicates absolute risk increase; HR, hazard ratio; KM, Kaplan–Maier; and TIMI, Thrombolysis in Myocardial Infarction.
In an exploratory analysis to evaluate the efficacy of ticagrelor 60 mg twice daily compared with placebo on the basis of bleeding risk, ticagrelor 60 mg reduced the risk of cardiovascular death, MI, or stroke by 20% in patients with low bleeding risk (HR, 0.80; 95% CI, 0.70–0.92; P = 0.0015; Figure 6). However, there was no apparent benefit of ticagrelor in patients at high bleeding risk (HR, 0.98; 95% CI, 0.77–1.26; P = 0.88, P interaction = 0.15). Results for the net clinical benefit showed a consistent pattern with ticagrelor 60 mg twice daily associated with favorable effects in the low‐bleeding‐risk patients (HR, 0.82; 95% CI, 0.71– 0.94; P = 0.004) but no apparent benefit in high bleeding risk (HR, 1.03; 95% CI, 0.81–1.31). Finally, there was significant heterogeneity for mortality with ticagrelor based on low versus high bleeding risk with a reduction in low bleeding risk (HR, 0.79; 95% CI, 0.65–0.96) and no benefit in high bleeding risk (HR, 1.14; 95% CI, 0.86–1.50; P interaction = 0.03; Figure 6, Table S3).
Figure 6. Efficacy and safety of ticagrelor 60 mg vs placebo stratified by high (N=2714) or low (N=11 240) bleeding risk at baseline.
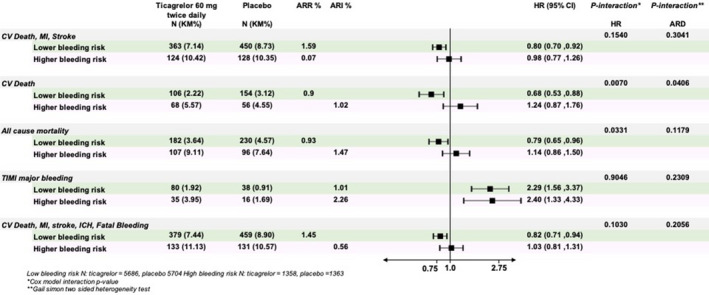
A sensitivity analysis of efficacy and safety was performed based on baseline bleeding risk, defined as the presence or absence of either a history of spontaneous bleeding requiring hospitalization before the randomization or anemia (defined as hemoglobin ≤13.5 g/dL for men and ≤12.0 g/dL for women). ARI indicates absolute risk increase; ARR, absolute risk difference; CV, cardiovascular; HR, hazard ratio; MI, myocardial infarction, ICH, intracranial hemorrhage; and TIMI, Thrombolysis in Myocardial Infarction.
Discussion
The current analysis provides three novel observations with regard to bleeding risk with ticagrelor in patients with prior MI. First, bleeding caused by ticagrelor in this population is most frequently spontaneous gastrointestinal bleeding in patients with occult sources, such as ulcer or malignancy. Second, there were 2 independent predictors of TIMI major or minor bleeding that were not independent predictors of ischemic risk, namely, prior hospitalization for bleeding and anemia. Finally, in an analysis of the efficacy of ticagrelor 60 mg twice daily stratified by bleeding risk, those at low risk appeared to have greater benefit, including lower rates of mortality, while patients at high risk appeared to have no benefit.
The observation that more potent antithrombotic therapy increases spontaneous gastrointestinal bleeding supports other recent trials evaluating bleeding risk in stable secondary prevention populations. 7 Observations regarding gastrointestinal bleeding may be helpful in considering the risks and benefits of therapy. In addition, they support strategies to reduce gastrointestinal bleeding such as PPI inhibitors, which have been shown to attenuate this risk. 8 , 9 Finally, this analysis supports the identification of anemia as a key risk marker for future bleeding. This finding suggests that hemoglobin may be a simple and widely available biomarker that may identify patients at heightened risk of gastrointestinal bleeding with more intensive antithrombotic therapy.
Predicting bleeding risk remains a challenge in clinical practice. One issue is the recognition that many factors that predict bleeding, such as advanced age and renal dysfunction, also predict ischemic events and potential benefit of risk reduction therapies. 10 The Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy score was derived to predict bleeding risk and in its validation showed a c‐index of 0.66. 11 Findings from the current analysis both support and add novel information. First, prior bleeding and anemia in the current analysis demonstrates the predictive importance of these 2 factors. In contrast, white blood cell count was not predictive of bleeding in PEGASUS‐TIMI 54, and the mechanism by which it would predict bleeding is unclear. Finally, in the current analysis, both age and renal dysfunction were associated with bleeding risk but also ischemic risk, and in the case of renal dysfunction, it has been shown previously to be associated with greater absolute benefit of ticagrelor. 11 Importantly, the current analysis shows that in a stable post‐MI population, the absence of the 2 identified bleeding risk predictors did not reduce the ability to identify high bleeding risk, and in fact, their inclusion identified only 1 additional patient as high risk for bleeding (Table S4). Therefore, in a stable post‐MI population, prior bleeding and anemia may be sufficient for bleeding risk prediction and may enable clinicians to consider bleeding and ischemic risk independently. This may be particularly helpful in groups such as the elderly in whom assessment of risk and benefit may be particularly challenging.
Although the findings of this analysis are exploratory, the greater efficacy of ticagrelor and formal interaction for all‐cause mortality in low‐ versus high‐bleeding‐risk patients helps to underscore the importance of assessment of bleeding risk. Although there was no difference in fatal bleeding or the novel outcome of bleeding contributing to death with ticagrelor, bleeding may lead to downstream consequences such as procedures and discontinuation of antithrombotic therapies, which may increase the risk of mortality. In PEGASUS‐TIMI 54, premature drug discontinuation was higher with ticagrelor than placebo and largely driven by bleeding and dyspnea. 12 In this context, the lower observed mortality in the low‐bleeding‐risk group suggests that the ischemic benefits outweigh the risks and downstream consequences of bleeding.
There are several limitations to the current analysis. The eligibility criteria for PEGASUS TIMI 54 led to a selected population that excluded some features associated with the risk of bleeding, such as low platelet count, known bleeding diathesis, active malignancy, and prior stroke. In addition, use of PPI therapy was not randomized, and therefore we cannot conclude that PPI use would have mitigated gastrointestinal bleeding caused by ticagrelor; however, the COGENT (Clopidogrel and the Optimization of Gastrointestinal Events) trial did show significantly lower gastrointestinal bleeding with PPI in patients receiving dual antiplatelet therapy. 8 It should also be noted that although apparent differences in outcomes between groups were observed, there was no formal heterogeneity by interaction testing for many outcomes, and this should be considered in interpreting the results. Finally, evaluations of efficacy and safety of ticagrelor by baseline bleeding risk were exploratory and should be viewed in this context.
CONCLUSIONS
In conclusion, in stable patients with prior MI treated with ticagrelor in addition to aspirin, TIMI major or minor bleeding is primarily spontaneous of gastrointestinal origin and related to an underlying gastrointestinal disease. Ticagrelor 60 mg twice daily did not increase fatal bleeding or ICH. Anemia and prior hospitalization for bleeding independently predict bleeding risk but not ischemic risk in this population. In patients with neither of these bleeding predictors, the balance of efficacy and safety of long‐term secondary prevention of MI with ticagrelor 60 mg twice daily appears to be favorable.
Sources of Funding
This study was supported by a grant to Brigham and Women’s Hospital from AstraZeneca.
Disclosures
The TIMI Study Group has received significant research grant support from Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi‐Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, and Zora Biosciences. Giulia Magnani reports speaking fees from AstraZeneca, Daiichi Sankyo, consultancy fee from Boehringer Ingelheim. KyungAh Im is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi‐Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, and Zora Biosciences. Andrej Budaj reports personal fees and non‐financial support from AstraZeneca, during the conduct of the study; personal fees and non‐financial support from Bristol Myers Squibb/Pfizer, personal fees and non‐financial support from Bayer, personal fees and nonfinancial support from Sanofi Aventis, personal fees from Eisai, personal fees from Novartis, and personal fees from GlaxoSmithKline, outside the submitted work. Robert F Storey reports research grants, consultancy fees and honoraria from AstraZeneca; research grants and consultancy fees from PlaqueTec; consultancy fees and honoraria from Bayer; consultancy fees and honoraria from Bristol Myers Squibb/Pfizer alliance; and consultancy fees from Avacta, Idorsia, Haemonetics, Novartis, and Thromboserin. P. Gabriel Steg reports research grants from Amarin, Bayer, Merck, Sanofi, and Servier; and speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer/Janssen, Boehringer‐Ingelheim, Bristol‐Myers‐Squibb, Idorsia, Lilly, Merck, Novartis, Novo‐Nordisk, Pfizer, Regeneron, Sanofi, and Servier. Deepak L. Bhatt reports the following relationships: Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, and Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the [Portico Re‐sheathable Transcatheter Aortic Valve System US IDE Trial, PORTICO] trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the [Efficacy of Secukinumab Compared to Adalimumab in Patients with Psoriatic Arthritis, ExCEED] trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the [Edoxaban versus standard of care and their effects on clinical outcomes in patients having undergone transcatheter aortic valve implantation, ENVISAGE] trial, funded by Daiichi Sankyo), Population Health Research Institute; honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; [Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention, RE‐DUAL] PCI clinical trial steering committee funded by Boehringer Ingelheim), Belvoir Publications (editor‐in‐chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (editor‐in‐chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor, associate editor), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national coleader, funded by Bayer), Slack Publications (chief medical editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (deputy editor), [National Cardiovascular Data Registry ACTION, NCDR‐ACTION] Registry Steering Committee (chair), [VA‐Cardiovascular Assessment Reporting and Tracking, VA CART] Research and Publications Committee (chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; royalties: Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site coinvestigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; trustee: American College of Cardiology; and unfunded research: FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, Takeda. Marc Cohen reports grants and personal fees from AstraZeneca, during the conduct of the study; personal fees from Merck, Janssen, Maquet, BMS/Pfizer, Janssen, BI, Lilly, and malpractice attorneys; and grants from Janssen and Edwards, outside the submitted work. Ton Oude Ophius reports speaker fees from AstraZeneca and Amgen and has received grant support from Medtronic and Abbott. Assen Goudev reports speaker honoraria from and served on the advisory board for AstraZeneca. Alexander Parkhomenko reports research grants and honoraria from AstraZeneca, Bristol Myers Squibb/Pfizer alliance, Bayer, Amgen, Amarin, Sanofi Aventis, Servier, and Janssen. Gabriel Kamensky participated in the PEGASUS‐TIMI 54 trial supported by AstraZeneca. Dominick J. Angiolillo reports receiving payments as an individual for (1) consulting fee or honorarium from Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Bristol‐Myers Squibb, Chiesi, Daiichi‐Sankyo, Eli Lilly, Janssen, Merck, PLx Pharma, Pfizer, Sanofi, and The Medicines Company; and (2) participation in review activities from CeloNova and St. Jude Medical. Institutional payments for grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi‐Sankyo, Eisai, Eli‐Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, and Renal Guard Solutions. Per Johanson is an employee of AstraZeneca. Eugene Braunwald reports grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consultancies with Amgen, Cardurion, MyoKardia, NovoNordisk, and Verve. Marc S. Sabatine reports research grant support through Brigham and Women’s Hospital from Abbott Laboratories, Amgen, Anthos Therapeutics, Critical Diagnostics, Daiichi‐Sankyo, Eisai, Genzyme, Gilead, GlaxoSmithKline, Intarcia, Janssen Research Development, MedImmune, Merck, Novartis, Poxel, Pfizer, Roche Diagnostics, and Takeda (all >$10 000 per year); and consulting for Alnylam, Althera, Amgen, Anthos Therapeutics, AstraZeneca, Bristol‐Myers Squibb, Cubist, CVS Caremark, Esperion, Intarcia, Ionis, The Medicines Company, MedImmune, Merck, MyoKardia, and Zeus Scientific (all ≤$10 000 per year except Amgen, Esperion, and Ionis). Marc P. Bonaca reports consulting for Aralez, Amgen, AstraZeneca, Bayer, Janssen, Merck, Novo Nordisk, Pfizer, and Sanofi. He is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women's Hospital from AstraZeneca.
Supporting information
Tables S1–S4
Figures S1–S6
(J Am Heart Assoc.2021;10:e017008. DOI: 10.1161/JAHA.120.017008.)
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Marc S. Sabatine, Email: marc.bonaca@cpcmed.org.
Marc P. Bonaca, Email: marc.bonaca@cpcmed.org.
References
- 1. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, et al. PEGASUS‐TIMI 54 Steering Committee and Investigators. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. [DOI] [PubMed] [Google Scholar]
- 2. Dellborg M, Bonaca MP, Storey RF, Steg PG, Im KA, Cohen M, Bhatt DL, Oude Ophuis T, Budaj A, Hamm C, et al. Efficacy and safety with ticagrelor in patients with prior myocardial infarction in the approved European label: insights from PEGASUS‐TIMI 54. Eur Heart J Cardiovasc Pharmacother. 2019;5:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Genereux P, Giustino G, Witzenbichler B, Weisz G, Stuckey TD, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, et al. Incidence, predictors, and impact of post‐discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015;66:1036–1045. [DOI] [PubMed] [Google Scholar]
- 4. Bonaca MP, Bhatt DL, Braunwald E, Cohen M, Steg PG, Storey RF, Held P, Jensen EC, Sabatine MS. Design and rationale for the prevention of cardiovascular events in patients with prior heart attack using ticagrelor compared to placebo on a background of aspirin‐thrombolysis in myocardial infarction 54 (PEGASUS‐TIMI 54) trial. Am Heart J. 2014;167(437–444):e5. [DOI] [PubMed] [Google Scholar]
- 5. Muller HG, Wang JL. Hazard rates estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994;50:61–76. [PubMed] [Google Scholar]
- 6. Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41:361–372. [PubMed] [Google Scholar]
- 7. Eikelboom JW, Bosch JJ, Connolly SJ, Shestakovska O, Dagenais GR, Hart RG, Leong DP, O'Donnell M, Fox KAA, Bhatt DL, et al. Major bleeding in patients with coronary or peripheral artery disease treated with rivaroxaban plus aspirin. J Am Coll Cardiol. 2019;74:1519–1528. [DOI] [PubMed] [Google Scholar]
- 8. Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, Shook TL, Lapuerta P, Goldsmith MA, Laine L, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909–1917. [DOI] [PubMed] [Google Scholar]
- 9. Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FKL, Furberg CD, Johnson DA, Mahaffey KW, Quigley EM, Harrington RA, et al. Expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;2008(52):1502–1517. [DOI] [PubMed] [Google Scholar]
- 10. Magnani G, Storey RF, Steg G, Bhatt DL, Cohen M, Kuder J, Im K, Aylward P, Ardissino D, Isaza D, et al. Efficacy and safety of ticagrelor for long‐term secondary prevention of atherothrombotic events in relation to renal function: insights from the PEGASUS‐TIMI 54 trial. Eur Heart J. 2016;37:400–408. [DOI] [PubMed] [Google Scholar]
- 11. Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, Pilgrim T, Hong M‐K, Kim H‐S, Colombo A, et al. Derivation and validation of the Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE‐DAPT) score: a pooled analysis of individual‐patient datasets from clinical trials. Lancet. 2017;389:1025–1034. [DOI] [PubMed] [Google Scholar]
- 12. Bonaca MP, Bhatt DL, Oude Ophuis T, Steg PG, Storey R, Cohen M, Kuder J, Im K, Magnani G, Budaj A, et al. Long‐term tolerability of ticagrelor for the secondary prevention of major adverse cardiovascular events: a secondary analysis of the PEGASUS‐TIMI 54 trial. JAMA Cardiol. 2016;1:425–432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S6


