Abstract
Heart transplantation remains the treatment of reference for patients experiencing end‐stage heart failure; unfortunately, graft availability through conventional donation after brain death is insufficient to meet the demand. Use of extended‐criteria donors or donation after circulatory death has emerged to increase organ availability; however, clinical protocols require optimization to limit or prevent damage in hearts possessing greater susceptibility to injury than conventional grafts. The emergence of cardiac ex situ machine perfusion not only facilitates the use of extended‐criteria donor and donation after circulatory death hearts through the avoidance of potentially damaging ischemia during graft storage and transport, it also opens the door to multiple opportunities for more sensitive monitoring of graft quality. With this review, we aim to bring together the current knowledge of biomarkers that hold particular promise for cardiac graft evaluation to improve precision and reliability in the identification of hearts for transplantation, thereby facilitating the safe increase in graft availability. Information about the utility of potential biomarkers was categorized into 5 themes: (1) functional, (2) metabolic, (3) hormone/prohormone, (4) cellular damage/death, and (5) inflammatory markers. Several promising biomarkers are identified, and recommendations for potential improvements to current clinical protocols are provided.
Keywords: biomarkers, donation after circulatory death, ex situ heart perfusion, extended‐criteria heart donors, heart transplantation
Subject Categories: Cardiovascular Surgery, Transplantation, Biomarkers
Nonstandard Abbreviations and Acronyms
- DAMP
damage‐associated molecular pattern
- DBD
donation after brain death
- DCD
donation after circulatory death
- ECD
extended‐criteria donor
- HEP
high‐energy phosphate
- H‐FABP
heart‐type fatty acid binding protein
- HSP
heat shock protein
- MP
machine perfusion
- OCS
Organ Care System Heart
Heart transplantation is the gold standard treatment for improving survival and quality of life in patients with end‐stage heart disease; however, graft availability through conventional donation after brain death (DBD) is insufficient to meet the need for all patients. 1 The number of patients awaiting cardiac transplantation has continuously increased in Europe and the United States over the past 20 years. 1 , 2
Approaches to improve cardiac graft availability include the use of extended‐criteria donors (ECDs) or donation after circulatory death (DCD). Although reports with ECDs and DCD are encouraging, clinical protocols have yet to be optimized. Improved methods of graft evaluation are of critical importance, not only for ensuring the best patient outcomes by correctly identifying suitable grafts and permitting the exclusion of grafts with excessive cellular dysfunction and damage, 3 but also for the identification and development of optimized clinical transplant protocols.
The organ shortage has also stimulated the development of ex situ organ perfusion systems as an alternative to conventional static, cold storage that can help to improve cardiac graft quality and availability, especially in situations in which grafts may be particularly susceptible to ischemic injury, such as those from ECDs or DCD, or when long transport times cannot be avoided. The Organ Care System Heart (OCS) by Transmedics has been developed for continuous, normothermic graft perfusion, and several systems, such as the Steen Heart Preservation System or the HeartPort System, are currently in development/clinical testing for hypothermic graft perfusion. 4 During ex situ, machine perfusion (MP) with the OCS, the graft is maintained in a beating, unloaded state. 3 To evaluate graft quality, variables, such as heart rate, rhythm, aortic pressure, coronary flow, and lactate profiles, are monitored during organ perfusion. 3 , 5 For standard‐criteria donor hearts preserved with the OCS or conventional cold‐static storage, 30‐day recipient and graft survival is similar, as is the incidence of cardiac allograft vasculopathy, 6 demonstrating the short‐term safety of this approach. 7 Furthermore, with organs previously not considered suitable for transplantation and/or higher‐risk recipients, MP is associated with excellent short‐term outcomes. 8 In DCD heart transplantations requiring graft transport between centers, the OCS has been used, and patient outcomes are similar to those with conventional DBD at 1‐ and 4‐year time points. 3 , 9 , 10 Thus, although still in its early stages, normothermic perfusion storage with the OCS appears promising.
To optimize patient outcomes from all donor pools, graft evaluation procedures must also evolve. Indeed, transplant criteria for conventional, DBD grafts are used; however, hearts meeting these criteria are not necessarily protected from rejection or cardiac allograft vasculopathy. Furthermore, increased use of ECDs or DCD may increase the risk of transplanting unsuitable donor hearts and lead to early graft failure. With DBD, in addition to donor inclusion/exclusion criteria, cardiac graft selection relies heavily on donor monitoring, and involves consideration of parameters such as blood pressure, electrocardiographic changes, periods of hypotension and/or cardiopulmonary resuscitation, drug history, history of hypertension, and the need for inotropic support. Up to two‐thirds of potential donor hearts are rejected before retrieval on the basis of the above criteria; however, none of these criteria alone precludes successful transplantation. 11 Furthermore, up to 50% of retrieved grafts are rejected because of heart malfunction detected only after detailed inspection. 11 Thus, on one hand, there is a pool of donor organs that is rejected because their posttransplantation function cannot be predicted with confidence, whereas on the other hand, resources are wasted in the procurement of nonsuitable organs. 11 It is therefore critical to optimize evaluation strategies to more effectively select for grafts in which adequate quality is achievable. 11 , 12
Although cardiac grafts from different types of donors are subjected to varying conditions before procurement, multiple markers of damage are likely to be relevant regardless of donor type. For example, DCD grafts are expected to undergo warm ischemia between circulatory arrest and procurement; however, ischemic damage may also occur during cardioplegia with DBD grafts, particularly with older donors. 12 Furthermore, in both DCD and DBD donors, a catecholamine surge occurs before graft procurement. This “adrenergic storm” can induce peripheral vasoconstriction and subsequently lead to transient ischemia of organs. 13
Taken together, improvement of clinical protocols to evaluate donor hearts and predict posttransplant function may not only provide better clinical outcomes, but could aid in expanding the donor pool and decreasing the number of patients awaiting a suitable graft. A greater consideration of cardiac biomarkers is of particular interest in light of recently available MP technologies that enable monitoring of multiple parameters over time during graft storage and transport. With this review, we aim to summarize the current knowledge of biomarkers that hold particular promise for cardiac graft evaluation to improve our precision and reliability in the identification of hearts for transplantation, thereby facilitating a safe increase in graft availability via expansion of the donor pool.
Methods
A systematic literature search of the PubMed database was performed with terms: “(heart or cardiac) AND (transplant or transplantation) AND biomarker AND (graft evaluation or rejection) NOT kidney NOT liver NOT lung NOT pancreas NOT (islet or islets) NOT bowel NOT pediatric NOT stem cell.” The search was limited to English. A total of 1082 (updated July 24, 2020) articles were retrieved. All abstracts were reviewed to exclude irrelevant publications.
All retained articles underwent in‐depth review and were assigned to categories of biomarkers according to the following indicators: (1) function, (2) metabolism, (3) hormone/prohormone, (4) cellular damage/death, and (5) inflammation. Additional PubMed searches were performed in each specific area to ensure that no relevant articles were overlooked. Cited references in all retained publications were carefully examined, and relevant publications were reviewed in detail.
Given that interventions to evaluate graft quality before heart procurement may not be permitted/possible with all donors, and that both DBD and DCD hearts are exposed to catecholamine storms that are potentially damaging to cardiac grafts, we have focused our attention on biomarkers monitored from the time of procurement until transplantation. Aspects considered particularly relevant for cardiac biomarkers are summarized in the tables. These include study model and design, details of biomarker sampling, outcomes, and predictive value. Predictive value was indicated as “yes” when statistically significant evidence between the measured predictor and outcome was provided (eg, statistically significant correlation). Predictive value was indicated as “no” when evidence (eg, correlation or receiver operating characteristic analysis) was provided, but no statistically significant relationship was observed. In several cases, indirect data were considered to either “support” or “not support” a predictive value, as indicated in tables.
Results and Discussion
The introduction of MP technologies holds enormous potential for optimizing graft evaluation strategies in heart transplantation. Given that cardiac MP is still in its infancy, a limited number of studies investigating graft evaluation during MP were retrieved with our literature searches. Nonetheless, evidence supporting the utility of various biomarkers measured between procurement and transplantation has been reported and is summarized in Figure 1 and in the following sections, where also suggestions for possible future strategies are presented.
Figure 1. Biomarkers with reported potential value when evaluated during graft management.
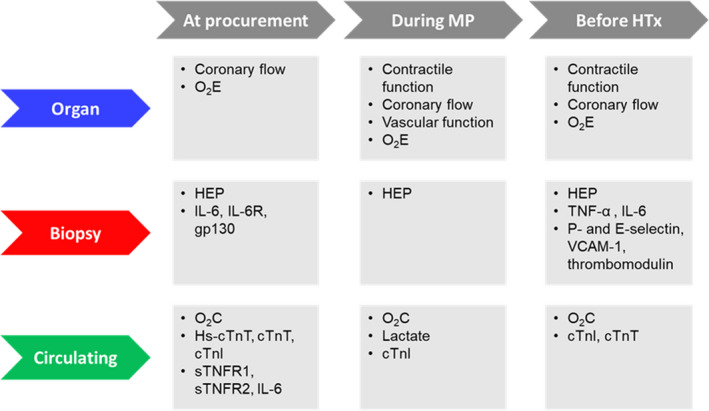
cTnI indicates cardiac troponin I; cTnT, cardiac troponin T; gp130, glycoprotein 130; HEP, high‐energy phosphates; Hs‐cTnT, high‐sensitivity cTnT; HTx, heart transplantation; IL‐6, interleukin 6; IL‐6R, IL‐6 receptor; MP, machine perfusion; O2C, cardiac oxygen consumption; O2E, cardiac oxygen efficiency; sTNFR, soluble tumor necrosis factor receptor; TNF‐α, tumor necrosis factor‐α; and VCAM‐1, vascular cell adhesion molecule 1.
Functional Markers
Contractile Function
Unlike DBD, antemortem functional graft evaluation is generally not performed in DCD, but rather during MP or normothermic regional perfusion (Figure 2). During MP, contractile function is evaluated by visual inspection. Criteria for transplantation acceptance for contractile function of the loaded DCD heart during normothermic regional perfusion are as follows: cardiac index >2.5 L/min per m2, central venous pressure <12 mm Hg, pulmonary capillary wedge pressure <12 mm Hg, and left ventricular ejection fraction >50% on transesophageal echocardiography in human DCD transplantation. 14
Figure 2. Schematic protocols for conditions before and during heart procurement and storage.

A, Hearts obtained with donation after circulatory death (DCD) are subjected to a period of warm, global ischemia, before procurement. In the direct procurement and perfusion protocol (DPP), grafts are stored and transported using normothermic machine perfusion (MP). In the normothermic, regional perfusion (NRP) protocol, the heart is reperfused in situ. Only after functional evaluation is it procured, stored, and transported using normothermic MP or cold, static storage (CSS). B, Hearts obtained with donation after brain death (DBD) remain perfused until organ procurement. Hearts are then stored and transported using CSS or normothermic MP.
Studies in both porcine and rodent DCD models support the concept that contractile evaluation during MP is of aid in graft evaluation (Table 1, 15 , 16 , 17 , 18 , 19 ). White and colleagues reported that several contractile parameters predict simultaneously measured myocardial performance (cardiac output×heart weight−1) in a porcine model, whereas end‐systolic volume and end‐systolic pressure‐volume relationship were not helpful. 16 In line with this, Ribeiro and colleagues described that invasive and noninvasive measures of left ventricle contractility strongly correlated with cardiac function following transplantation. 15 Interestingly, several parameters measured during ex vivo, unloaded perfusions in rat DCD models have been reported to correlate with cardiac functional recovery on reloading. 17 , 18 , 19
Table 1.
Contractile Function
| Biomarker | Model | Experimental Design | Biomarker Sampling | Outcome | Biomarker‐Outcome Correlation/Predictive Value |
|---|---|---|---|---|---|
| Multiple contractile function measures 15 | DCD, preclinical (pig) |
Parallel, 2‐arm study, unloaded and loaded MP at 37°C and orthotopic HTx:
|
At start of loaded MP (after 3 h unloaded MP) | Left ventricular function: | |
| Stroke work at 3 h posttransplant |
Yes: DP, dP/dt max, PRSW, NI Emax, NI PRSW (ρ=Pve, P<0.05 for all), dP/dt min, τ, EDPV relationship (ρ=Nve, P<0.05 for all) |
||||
| PRSW at 3 h posttransplant |
Yes: dP/dt max, PRSW, NI Emax (ρ=Pve, P<0.05 for all), τ, EDPV relationship (ρ=Nve, P<0.05 for both) |
||||
|
No: DP, dP/dt min, NI PRSW (P=NS for all) | |||||
| Cardiac index at 3 h posttransplant |
Yes: DP, dP/dt max, PRSW, NI Emax, NI PRSW (ρ=Pve, P<0.05 for all) |
||||
|
No: dP/dt min, τ, EDPV relationship (P=NS for all) | |||||
| Right ventricular function: | |||||
| RVSWI at 3 h posttransplant |
Yes: Stroke work (ρ=Pve, P<0.05) |
||||
|
No: DP, EDPV relationship, NI PRSW (P=NS for all) | |||||
| Cardiac index at 3 h posttransplant |
Yes: NI PRSW (ρ=Pve, P<0.05) |
||||
|
No: DP, stroke volume, EDPV relationship (P=NS for all) | |||||
| Multiple contractile function measures 16 | DCD, preclinical (pig) |
Parallel, 2‐arm study, loaded MP at 37°C for:
|
At start of loaded MP | Cardiac index (cardiac output per heart weight) at start of loaded MP (simultaneous with biomarker) |
Yes: DP (R 2=0.569, P<0.001) dP/dt max (R 2=0.537, P<0.001) dP/dt min (R 2=0.74, P<0.001) dV/dt max (R 2=0.616, P<0.001) dV/dt min (R 2=0.321, P<0.001) EDP (R 2=0.202, P<0.002) EDPV relationship (R 2=0.143, P<0.05) ejection fraction (R 2=0.80, P<0.001) ESP (R 2=0.512, P<0.001) stroke work (R 2=0.76, P<0.001) τ (R 2=0.51, P<0.001) |
|
No: EDV (R 2=0.004, P=NS) ESPV relationship (R 2=0.012, P=NS) ESV (R 2=0.081, P=NS) | |||||
| Multiple contractile function measures 17 | DCD, preclinical (rat) |
Parallel, 5‐arm study, MP (10 min unloaded+50 min loaded) at 37°C following WIT of:
(n=7–8 per group) |
At 10 min MP | LV work at 60 min MP |
Yes: LV work, DP, heart rate, dP/dt max (ρ=Pve, P<0.01 for all), dP/dt min (ρ=Nve, P<0.01) |
| TP at 60 min MP |
Yes: DP, dP/dt max, heart rate, LV work (ρ=Pve, P<0.01 for all)dP/dt min (ρ=Nve, P<0.01) |
||||
| CO at 60 min MP |
Yes: DP, dP/dt max, heart rate, LV work (ρ=Pve, P<0.01 for all) dP/dt min (ρ=Nve, P<0.01) |
||||
| dP/dt max at 60 min MP |
Yes: DP, dP/dt max, heart rate, LV work (ρ=Pve, P<0.01 for all) dP/dt min (ρ=Nve, P<0.01) |
||||
| dP/dt min at 60 min MP |
Yes: DP, dP/dt max, heart rate, LV work (ρ=Nve, P<0.01 for all) dP/dt min (ρ=Pve, P<0.01) |
||||
| Multiple contractile function measures 18 | DCD, preclinical (rat) |
Parallel, 3‐arm study in hearts subjected to WIT, as described below, followed by 10 min procurement reperfusion, cardioplegic flush, CSS for 3 h, 120 min loaded, normothermic MP:
|
(A) At 10 min procurement reperfusion (before CSS) (B) At 10 min loaded, normothermic MP (after CSS) |
CO at 120 min loaded, normothermic MP |
Yes: DP, dP/dt min, dP/dt max, heart rate, LV work (A and B; ρ=NR, P<0.05 for all) |
|
No: EDP, Pmin, PSP (A and B; P=NS for all) | |||||
| PSP at 120 min loaded, normothermic MP |
Yes: DP, dP/dt max, dP/dt min, heart rate, LV work (A and B; ρ=NR, P<0.05 for all) PSP (B only; ρ=NR, P<0.05) |
||||
|
No: EDP, Pmin (A and B; P=NS for both) | |||||
| DP at 120 min loaded, normothermic MP |
Yes: DP, dP/dt max, dP/dt min, heart rate, LV work (A and B; ρ=NR, P<0.05 for all) PSP (B only; ρ=NR, P<0.05) |
||||
|
No: EDP, Pmin (A and B; P=NS) | |||||
| HR at 120 min loaded, normothermic MP |
Yes: HR (B only; ρ=NR, P<0.05) LV work (A and B; ρ=NR, P<0.05) |
||||
|
No: EDP, DP, dP/dt max, dP/dt min, Pmin, PSP (A and B; P=NS for all) | |||||
| dP/dt max/min at 120 min loaded, normothermic MP |
Yes: DP, dP/dt max, dP/dt min, heart rate, LV work (A and B; ρ=NR, P<0.05 for all) PSP (B only; ρ=NR, P<0.05) |
||||
|
No: EDP, Pmin (A and B; P=N for both) | |||||
| LV work at 120 min loaded, normothermic MP |
Yes: DP, dP/dt max, dP/dt min, heart rate, LV work (A and B; ρ=NR, P<0.05 for all) PSP (B only; ρ=NR, P<0.05) |
||||
|
No: EDP, Pmin (A and B; P=NS for both) | |||||
| Multiple contractile function measures 19 | DCD, preclinical (rat) |
Parallel, 4‐arm study, MP (20 min unloaded+40 min loaded) at 37°C of hearts subjected to WIT of:
|
At 10 min MP | HR at 60 min MP |
Yes: DP, dP/dt min, EDP, Pmin, dP/dt max (ρ=NR, P<0.001 for all) HR (ρ=NR, P<0.05) LV work (ρ=NR, P<0.001) |
|
No: PSP (P=NS) | |||||
| PSP at 60 min MP |
Yes: DP, dP/dt min, EDP, Pmin (ρ=NR, P<0.01 for all) dP/dt max, LV work (ρ=NR, P<0.001 for both) HR (ρ=NR, P<0.05) |
||||
|
No: PSP (P=NS) | |||||
| DP at 60 min MP |
Yes: DP, HR (ρ=NR, P<0.05 for both) dP/dt min, EDP, Pmin (ρ=NR, P<0.01 for all) dP/dt max, LV work (ρ=NR, P<0.001 for both) |
||||
|
No: PSP (P=NS) | |||||
| dP/dt max at 60 min MP |
Yes: DP, EDP, HR, Pmin (ρ=NR, P<0.01 for all) dP/dt max, dP/dt min, LV work (ρ=NR, P<0.001 for all) |
||||
|
No: PSP (P=NS) | |||||
| dP/dt min at 60 min MP |
Yes: DP, EDP, HR (ρ=NR, P<0.01 for all) LV work, dP/dt max, dP/dt min (ρ=NR, P<0.001 for all) Pmin (ρ=NR, P<0.05) |
||||
|
No: PSP (P=NS) | |||||
| LV work at 60 min MP |
Yes: DP, dP/dt max, dP/dt min, EDP, LV work, Pmin (ρ=NR, P<0.001 for all) HR (ρ=NR, P<0.01) |
||||
|
No: PSP (P=NS) | |||||
| CO at 60 min MP |
No: DP, dP/dt max, dP/dt min, EDP, heart rate, LV work, Pmin, PSP (P=NS for all) |
||||
ρ indicates Spearman ρ; CO, cardiac output; CSS, cold static storage; DBD, donation after brain death; DCD, donation after circulatory death; DP, developed pressure; dP/dt max, maximal first derivative of left ventricular pressure; dP/dt min, minimal first derivative of left ventricular pressure; dV/dt max, maximal first derivative of left ventricular volume; dV/dt min, minimal first derivative of left ventricular volume; EDP, end‐diastolic pressure; EDPV, end‐diastolic pressure‐volume; EDV, end‐diastolic volume; ESP, end‐systolic pressure; ESPV, end‐systolic pressure‐volume; ESV, end‐systolic volume; HR, heart rate; HTx, heart transplantation; LV work, left ventricular work (heart rate×DP); MP, machine perfusion; NI Emax, noninvasive maximum elastance; NI PRSW, noninvasive preload recruitable stroke work; NR, not reported; NS, not significant; Nve, negative correlation; Pmin, left ventricular minimal pressure; PRSW, preload recruitable stroke work; PSP, peak systolic pressure; Pve, positive correlation; R2, correlation coefficent for linear regression; RVSWI, right ventricular stroke work index; T, Tau; TP, triple product (HR−DP−dP/dt max product); and WIT, warm ischemia time.
Notably, functional assessments of DCD grafts in clinical practice provided better correlations with myocardial performance than metabolic variables during MP in several studies. 9 , 14 , 20 Although the supremacy of functional parameters highlights the need for an MP device capable of assessing the donor heart in a physiologic, loaded mode, 16 functional assessment has not been evaluated in unloaded human hearts. It may be that functional evaluations performed in unloaded hearts also provide valuable information, which would be highly advantageous given that this type of preparation is technically much less demanding.
Vascular and Endothelial Function
Coronary vascular dysfunction is common in DBD heart recipients. Notably, the index of microvascular resistance, assessed early after heart transplantation, predicts death or retransplantation. 21 Endothelial dysfunction is an early marker for intimal thickening and graft atherosclerosis, 22 and changes in coronary endothelial function predict progression of allograft vasculopathy after transplantation. 23 Correspondingly, endothelial preservation helps to delay allograft vasculopathy. 24 As endothelial cells are more susceptible to ischemia‐reperfusion injury than cardiac myocytes, endothelial dysfunction may be present even before graft procurement, particularly in DCD hearts. 25
Preclinical studies indicate that various measures of vascular function correlate with heart recovery in ex situ preparations (Table 2, 15 , 17 , 18 , 19 , 25 , 26 , 27 , 28 ). Coronary flow and the ability of the vasculature to adapt coronary flow in response to ischemia and reperfusion (hyperemic response; coronary flow reserve) are indicators of vascular function. In preclinical models, coronary flow in loaded preparations or coronary flow and time to peak coronary flow in unloaded preparations consistently correlate with functional outcomes. These findings support the concept that assessment of vascular and/or endothelial function in both DCD and DBD grafts before transplantation may be particularly promising for graft evaluation.
Table 2.
Vascular and Endothelial Function
| Biomarker | Model | Experimental Design | Biomarker Sampling | Outcome | Biomarker‐Outcome Correlation/Predictive Value | Correlation With Other, Potential Predictive Marker |
|---|---|---|---|---|---|---|
| Coronary vascular resistance 15 | DCD, preclinical (pig) |
Parallel, 2‐arm study, unloaded and loaded MP at 37°C and orthotopic HTx:
|
(A) At 30 min MP (B) At 3 h MP |
RVSWI at 3 h posttransplant (A and B) |
Yes: A and B (ρ=Nve, P<0.05) |
|
| Coronary flow 26 | DBD, preclinical (pig) |
Parallel, 3‐arm study, 3 h brain death period, followed by in situ WIT of:
|
At time of cardioplegic flush | Post‐HTx function (details NR) | Yes (r=NR, P=NR) | NR |
| Energetic index after cardioplegic flush | Yes (R=Pve, P<0.001) | |||||
| Coronary flow 27 | DCD, preclinical (pig) |
Parallel, 5‐arm study with in situ WIT of:
|
At time of cardioplegic flush | LVDP during MP | Yes (R=0.9, P<0.001) | NR |
| Energetic index after cardioplegic flush | Yes (R=0.84, P<0.001) | |||||
| Coronary flow 25 | DCD, preclinical (rat) |
Parallel, 5‐arm study, MP (10 min unloaded+50 min loaded) at 37°C following WIT of:
|
At 3 min MP | CO, DP, LV work, TP at 60 min MP | Yes (ρ=Pve, P<0.001 for all) |
Yes: O2C (ρ=Pve, P<0.001); LDH (ρ=Nve, P<0.001); peroxynitrite (100 kDa; ρ=Nve, P<0.05); WIT (ρ=Nve, P<0.001) |
| dP/dt max at 60 min MP | Yes (ρ=Pve, P<0.05) | |||||
| dP/dt min at 60 min MP | Yes (ρ=Nve, P<0.05) | |||||
| Coronary flow 17 | DCD, preclinical (rat) |
Parallel, 5‐arm study, MP (10 min unloaded+50 min loaded) at 37°C following WIT of:
|
At 10 min MP | CO, dP/dt max, LV work, TP at 60 min MP | Yes (ρ=Pve, P<0.01) | NR |
| dP/dt min at 60 min MP | Yes (ρ=Nve, P<0.01) | |||||
| Coronary flow 18 | DCD, preclinical (rat) |
Parallel, 3‐arm study in hearts subjected to WIT, as described below, followed by 10 min procurement reperfusion, cardioplegic flush, CSS for 3 h 120 min loaded, normothermic MP:
|
(A) At 10 min procurement reperfusion (before CSS) (B) At 10 min loaded, normothermic MP (after CSS) |
CO, DP, dP/dt max, dP/dt min, LV work at 120 min loaded, normothermic MP | Yes: A and B (ρ=NR, P<0.05 for all) | NR |
| HR at 120 min loaded, normothermic MP | Yes: B only (ρ=NR, P<0.05) | |||||
| Coronary flow 19 | DCD, preclinical (rat) |
Parallel, 4‐arm study, MP (20 min unloaded+40 min loaded) at 37°C of hearts subjected to WIT of:
|
At 10 min MP | DP, dP/dt max, LV work, PSP at 60 min MP | Yes (ρ=NR, P<0.05 for all) | NR |
| dP/dt min, CO, HR at 60 min MP | No (P=NS for all) | |||||
| Coronary flow (time to peak) 28 | DCD, preclinical (rat) | Ex vivo WIT of 5–43 min, followed by 40 min MP (n=NR) | During MP | Power output recovery at 10 min MP | Yes (R=−0.86, P=0.005) | NR |
| Edema 25 | DCD, preclinical (rat) |
Parallel, 5‐arm study, 60 min MP in hearts subjected to WIT of:
|
At 60 min MP | CO, DP, LV work, TP at 60 min MP (simultaneous with biomarker) | Yes (ρ=Nve, P<0.05) |
Yes: WIT (ρ=Pve, P<0.05); LDH (ρ=Pve, P<0.05); ‐ peroxynitrite (60 kDa; ρ=Pve, P<0.05) |
| dP/dt min at 60 min MP (simultaneous with biomarker) | Yes (ρ=Pve, P<0.05) | |||||
| dP/dt max at 60 min MP (simultaneous with biomarker) | No (P=NS) | |||||
| Peroxynitrite tissue levels 25 | DCD, preclinical (rat) |
Parallel, 5‐arm study, 60 min MP in hearts subjected to WIT of:
(n=4–6 per group) |
Peroxynitrite (100 kDa) at 60 min MP | CO, LV work at 60 min MP (simultaneous with biomarker) | Yes (ρ=Nve, P<0.05 for both) | Yes: WIT (ρ=Pve, P<0.001); LDH (ρ=Pve, P<0.05); peroxynitrite (75 kDa; ρ=Pve, P<0.05); peroxynitrite (60 kDa; ρ=Pve, P<0.001) |
| DP, dP/dt max, dP/dt min, TP at 60 min MP (simultaneous with biomarker) | No (P=NS) | |||||
| Peroxynitrite (60 kDa) at 60 min MP | DP, LV work at 60 min MP (simultaneous with biomarker) | Yes (ρ=Nve, P<0.05) |
Yes: WIT (ρ=Pve, P<0.05); LDH (ρ=Pve, P<0.05); O2E (ρ=Nve, P<0.05); edema (ρ=Pve, P<0.05); peroxynitrite (100 kDa; ρ=Pve, P<0.001) |
|||
| CO, dP/dt max, dP/dt min, TP at 60 min MP (simultaneous with biomarker) | No (P=NS) | |||||
| Vascular function 25 | DCD, preclinical (rat) |
Parallel, 3‐arm study, 30 min MP in hearts subjected to WIT of:
(n=6 per group) |
Endothelial‐dependent vasodilation (bradykinin,10−8 mol/L) at 30 min MP | Surrogates (DP, dP/dt max, CO, TP at 20 min MP)* | Yes (ρ=Pve, P<0.05 for all) | Yes: WIT (ρ=Nve, P<0.05) |
| Surrogates (dP/dt min at 20 min MP)* | Yes (ρ=Nve, P<0.001) | |||||
| Surrogates (LV work at 20 min MP)* | No (P=NS) | |||||
| Endothelial‐independent vasodilati (3×10−5 mol/L SNP) at 30 min MP | Surrogates (dP/dt min at 20 min MP)* | Yes (ρ=Nve, P<0.05) | Yes: WIT (ρ=Nve, P<0.05) | |||
| Surrogates (CO, DP, dP/dt max, LV work, TP at 20 min MP)* | No (P=NS for all) |
Energetic index calculated as (ATP+0.5×ADP)/(ATP+ADP+AMP). Power output recovery expressed as ratio of reperfusion value/preischemic value for aortic flow rate×afterload pressure. ρ indicates Spearman ρ; CO, cardiac output; CSS, cold static storage; DBD, donation after brain death; DCD, donation after circulatory death; dP/dt max, maximal first derivative of left ventricular pressure; dP/dt min, minimal first derivative of left ventricular pressure; DP, developed pressure; HR, heart rate; HTx, heart transplantation; LDH, lactate dehydrogenase; LV work, left ventricular work (heart rate×DP); LVDP, left ventricular developed pressure; MP, machine perfusion; NR, not reported; NS, not significant; Nve, negative correlation; O2C, oxygen consumption; O2E, oxygen efficiency; PSP, peak systolic pressure; Pve, positive correlation; R, Pearson correlation; RVSWI, right ventricular stroke work index; SNP, sodium nitroprusside; TP, triple product (HR−DP−dP/dt max product); and WIT, warm ischemia time.
Surrogates (measured at 20 minutes MP) as indicators of LV work at 60 minutes MP.
Although vascular function is currently monitored during MP, its predictive value before transplantation remains to be determined. 29 One advantage of coronary flow is that continuous measurements can easily be obtained during MP. However, coronary flow and vascular responses are substantially influenced by many factors, such as vasodilatory agents (eg, adenosine), cardiac oxygen demand and perfusate oxygen levels, temperature, and pressure. Thus, within individual studies, when procurement and reperfusion conditions are maintained, coronary flow may correlate well with functional recovery; however, when these conditions vary, as would be expected in clinical practice, it may be less reliable. As such, these parameters must be interpreted in light of specific perfusion conditions and may be best used in combination with other predictive indicators to offset potential disadvantages related to their susceptibility to alteration by independent factors.
Metabolic Markers
Cardiac Oxygen Consumption
Cardiac oxygen consumption during MP, in both loaded and unloaded conditions, correlates with subsequent cardiac function in a small number of preclinical DCD studies (Table 3, 15 , 16 , 17 , 18 ). Similar to coronary flow, oxygen consumption is easily measurable during MP in venous and arterial perfusate samples using a standard blood gas analyzer, but is subject to perturbations by multiple factors, including cardiac function and coronary flow. One option to improve the reliability of oxygen consumption is to consider it in combination with contractile function (eg, cardiac oxygen efficiency), which correlated well with cardiac recovery when measured in unloaded preparations in a preclinical DCD model. 17
Table 3.
Cardiac Oxygen Consumption
| Biomarker | Model | Experimental Design | Biomarker Sampling | Outcome | Biomarker‐Outcome Correlation/Predictive Value | Correlation With Other, Potential Predictive Marker |
|---|---|---|---|---|---|---|
| O2C 15 | DCD, preclinical (pig) |
Parallel, 2‐arm study, unloaded and loaded MP at 37°C and orthotopic HTx:
|
At 3 h MP | RVSWI at 3 h posttransplant |
Yes: (ρ=Pve, P<0.05) |
|
| O2C 16 | DCD, preclinical (pig) |
Parallel, 2‐arm study, loaded MP at 37°C for:
|
Perfusate at start of loaded MP | Cardiac index (CO per heart weight) at start of loaded MP (simultaneous with biomarker) | Yes (R 2=0.283, P<0.001) | NR |
| O2C 17 | DCD, preclinical (rat) |
Parallel, 3‐arm study, MP (10 min unloaded+50 min loaded) at 37°C following WIT of:
(n=5–8 per group) |
At 10 min MP | Surrogates (LV work, DP, CF, and dP/dt max at 10 min MP)* | Yes (ρ=Pve, P>0.05) | Yes for WIT (ρ=Nve, P>0.01) |
| Surrogate (dP/dt min at 10 min MP)* | Yes (ρ=Nve, P>0.05) | |||||
| Surrogate (HR at 10 min MP) | No (P=NS) | |||||
| O2E 17 | DCD, preclinical (rat) |
Parallel, 3‐arm study, MP (10 min unloaded+50 min loaded) at 37°C following WIT of:
(n=5–8 per group) |
At 10 min MP | Surrogates (LV work, DP, HR, CF, and dP/dt max at 10 min MP)* | Yes (ρ=Pve, P>0.05) | Yes for WIT (ρ=Nve, P>0.01) |
| Surrogate (dP/dt min at 10 min MP)* | Yes (ρ=Nve, P>0.01) | |||||
| O2C 18 | DCD, preclinical (rat) |
Parallel, 3‐arm study in hearts subjected to WIT, as described below, followed by 10 min procurement reperfusion, cardioplegic flush, CSS for 3 h, 120 min loaded, normothermic MP:
|
(A) At 10 min procurement reperfusion (before CSS) (B) At 10 min loaded, normothermic MP (after CSS) |
CO at 120 min loaded, normothermic MP | Yes: A and B (ρ=NR, P<0.05) | NR |
| DP at 120 min loaded, normothermic MP |
Yes: A (ρ=NR, P<0.05) No: B (P=NS) |
|||||
| dP/dt max/min at 120 min loaded, normothermic MP | Yes: A and B (ρ=NR, P<0.05) | |||||
| HR at 120 min loaded, normothermic MP | No: A and B (P=NS) | |||||
| LV work at 120 min loaded, normothermic MP | Yes: A and B (ρ=NR, P<0.05) | |||||
| PSP at 120 min loaded, normothermic MP |
Yes: A (ρ=NR, P<0.05) No: B (P=NS) |
ρ indicates Spearman ρ; CF, coronary flow; CO, cardiac output; CSS, cold static storage; DBD, donation after brain death; DCD, donation after circulatory death; DP, developed pressure; dP/dt max, maximal first derivative of left ventricular pressure; dP/dt min, minimal first derivative of left ventricular pressure; HR, heart rate; HTx, heart transplantation; LV work, left ventricular work (heart rate×DP); MP, machine perfusion; NR, not reported; NS, not significant; Nve, negative correlation; O2C, cardiac oxygen consumption; O2E, cardiac oxygen efficiency; PSP, peak systolic pressure; Pve, positive correlation; R2, correlation coefficent for linear regression; RVSWI, right ventricular stroke work index; and WIT, warm ischemia time.
Surrogates (measured at 10 minutes MP) as indicators of LV work at 60 minutes MP.
Lactate
The use of lactate profiles during MP for graft evaluation has yielded varying results, but may be of greater utility in DBD compared with DCD grafts (Table 4, 15 , 16 , 17 , 18 , 19 , 20 , 30 , 31 , 32 ). Lactate extraction and/or perfusate lactate changes over time are currently used as metabolic markers for graft quality in heart transplantation during MP. 7 , 8 Inclusion criteria ([1] net lactate extraction, [2] decreasing or stable perfusate lactate levels, and [3] perfusate lactate concentration <5 mmol/L at end MP) are based on experience with DBD hearts. 7 End‐MP lactate concentration was defined in DBD hearts as a predictor of 30‐day graft failure, with a sensitivity of 0.625 and a specificity of 0.975. 31 Although these criteria have been implemented to help identify suitable human 5 and porcine 33 , 34 DCD grafts for transplantation, a lack of sensitivity for lactate in DCD cardiac graft evaluation has been reported. 14 , 20 Indeed, at least 5 of 9 DCD hearts with end‐MP lactate concentrations >5 mmol/L could be transplanted without compromising outcomes. 9 , 14 , 20 This lack of sensitivity may result from the fact that several factors can affect lactate profiles, including donor lactate levels, concentrations of other perfusate energy substrates, and erythrocyte metabolic rates. Lactate may thus be best used in combination with other biomarkers, and specific criteria/thresholds remain to be established for DCD graft evaluation. Interestingly, it has recently been reported in a preclinical study of mixed DCD and DBD donors that glucose profiles may be of greater value in predicting posttransplant heart function than those of lactate. 35
Table 4.
Lactate
| Biomarker | Model | Experimental Design | Biomarker Sampling | Outcome | Biomarker‐Outcome Correlation/Predictive Value |
|---|---|---|---|---|---|
| Lactate 30 | DBD, clinical | Randomized, prospective, parallel, 2‐arm study with 1 relevant arm: perfusion storage/MP (n=39) | Blood perfusate during MP | Rejection or acceptance for transplantation | Supported: ↑ end MP lactate in rejected vs transplanted groups (P<0.05) |
| Lactate 31 | DBD, clinical | Prospective, single‐arm study, MP (n=49) | Arterial and venous blood perfusate during MP (end‐MP lactate, rate of perfusate lactate change) | Posttransplant outcome (graft failure within 30 d) | Yes: end‐MP lactate as explanatory parameter in logistic regression model (P=0.0044) |
| Yes: rate of lactate change as explanatory parameter in logistic regression model (P=0.0065) | |||||
| Lactate 20 | DCD, clinical | Single‐arm study of grafts subjected to MP (WIT NR; n=21) | Blood perfusate at end MP | Posttransplant cardiac index, intra‐aortic balloon pump requirement, length of stay, mortality | Not supported (no difference in outcomes for hearts with lactate >5 vs <5 mmol/L (P=NR) |
| Lactate 15 | DCD, preclinical (pig) |
Parallel, 2‐arm study, unloaded and loaded MP at 37°C and orthotopic HTx:
|
(A) At 1 h MP (B) Extraction during MP (C) At 3 h MP |
RVSWI at 3 h posttransplant (A and B) |
Yes: B (ρ=Nve, P<0.05) |
|
No: A (P=NS) | |||||
| Cardiac index at 3 h posttransplant (C) |
Yes: (ρ=Nve, P<0.05) |
||||
| Lactate 32 | DCD, preclinical (pig) |
Parallel, 2‐arm study with 8–44 min warm ischemia, followed by storage conditions described below, and 1 h unloaded MP:
|
Tissue samples after 4 h storage | Myocardial function after 1 h reperfusion |
Supported: In MP vs CSS: ↓ intracellular lactate (P<0.01) and ↑ heart rate (P<0.05), P max (P<0.05), dP/dt max (P<0.05), RPP (P<0.01), and contractility index (P<0.05), ↓ dP/dt min (P<0.05) |
| Lactate 16 | DCD, preclinical (pig) |
Parallel, 2‐arm study, loaded MP at 37°C for:
|
Arterial and venous blood perfusate at start of loaded MP | Cardiac index at start of loaded MP (simultaneous with biomarker) | No (all hearts considered together): arterial lactate (R 2=0.019, P=NS), venous lactate (R 2=0.001, P=NS), venoarterial lactate difference (R 2=0.006, P=NS) |
| Lactate 17 | DCD, preclinical (rat) |
Parallel, 5‐arm study, MP (10 min unloaded+50 min loaded) at 37°C following WIT of:
(n=5–8 per group) |
Perfusate at 10 min MP | LV work, TP, CO, dP/dt max, dP/dt min at 60 min MP | No (P=NS for all) |
| Lactate 18 | DCD, preclinical (rat) |
Parallel, 3‐arm study in hearts subjected to WIT, as described below, followed by 10 min procurement reperfusion, cardioplegic flush, CSS for 3 h, 120 min loaded, normothermic MP:
|
(A) At 10 min procurement reperfusion (before CSS) (B) At 10 min loaded, normothermic MP (after CSS) |
Multiple functional parameters (CF, CO, PSP, DP, heart rate, dP/dt min, dP/dt max, RPP, TP) after 120 min loaded, normothermic MP | No, for A and B (P=NS for all) |
| Lactate 19 | DCD, preclinical (rat) |
Parallel, 4‐arm study, MP (20 min unloaded+40 min loaded) at 37°C of hearts subjected to WIT of:
|
Perfusate at 10 min MP | PSP, LV work at 60 min MP | Yes (ρ=NR, P<0.05 for both) |
| DP, heart rate, dP/dt max, dP/dt min, CO at 60 min MP | No (P=NS for all) |
ρ indicates Spearman ρ; CF, coronary flow; CO, cardiac output; CSS, cold static storage; DBD, donation after brain death; DCD, donation after circulatory death; DP, developed pressure; dP/dt max, maximal first derivative of left ventricular pressure; dP/dt min, minimal first derivative of left ventricular pressure; HTx, heart transplantation; LV work, left ventricular work (heart rate×DP); MP, machine perfusion; NR, not reported; NS, not significant; Nve, negative correlation; Pmax, maximal left ventricular pressure; PSP, peak systolic pressure; R, Pearson correlation; RPP, rate‐pressure product (heart rate×PSP); R2, correlation coefficent for linear regression; RVSWI, right ventricular stroke work index; TP, triple product (heart rate−DP−dP/dt max product); and WIT, warm ischemia time.
High‐Energy Phosphate Metabolites
Higher levels of cardiac high‐energy phosphates (HEPs), whether measured at the start, during, or after the graft storage period, are associated with better graft outcomes in both clinical and preclinical studies in DBD, as well as in preclinical DCD reports (Table 5, 32 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 ). Furthermore, preclinical reports demonstrate that partial or full replacement of conventional, cold, static graft storage with continuous perfusion provides better preservation of HEPs and enhances contractile function or recovery of function in rat, 41 , 42 , 43 dog, 39 and pig 32 , 38 models. Although fewer studies have been performed with human grafts, continuous perfusion also better preserves HEPs. 44 These findings are in agreement with the concept that better preservation of metabolic homeostasis, rather than simply limiting metabolic activity, is a superior strategy for optimal graft preservation. 45
Table 5.
HEP Metabolites
| Biomarker | Model | Experimental Design | Biomarker Sampling | Outcome | Biomarker‐Outcome Correlation/Predictive Value |
|---|---|---|---|---|---|
| MRS score (PCr/Pi+pH−7) 36 | DBD, clinical | Retrospective, parallel, 3‐arm study | Graft during static storage (MRS) |
Heart allocation to
|
Supported: MRS score progressively decreased from group 1 to group 2 and group 3 (differences among groups statistically significant; P=0.0001) |
| PCr/ATP ratio 37 | DBD, clinical | Prospective, single‐arm study (n=25) | Graft during static storage (MRS) | Cardiac index 1 wk after HTx | Yes (r=0.45, P=0.02) |
| ATP 38 | DBD, preclinical (pig) |
Parallel, 4‐arm study:
|
Tissue samples after 8 h storage | Myocardial function 2 h after HTx | Supported: ↑ ATP in group 2 vs 1 (P<0.05) with ↑ LVSP and cardiac index (P=0.00 for all) and ↑ cardiac output (P=0.001) in group 4 vs 3 |
| ATP 39 | DBD, preclinical (dog) |
Parallel, 2‐arm study with graft storage conditions, as described below, followed by 1 h unloaded, normothermic MP:
|
Tissue samples after 6 h storage | dP/dt max after 1 h normothermic MP | Yes (R=0.41, P=0.049) |
| ATP after 1 h normothermic MP |
Not supported: unchanged ATP (biomarker) after 6 h storage between groups, but ↑ ATP (outcome; P=0.003) in group 2 vs 1 |
||||
| ATP/Pi and PCr/Pi ratios 40 | DBD, preclinical (dog) |
Parallel, 4‐arm study:
|
Tissue samples after 24 h storage (MRS) | Myocardial function after HTx | Not supported: ↑ ATP/Pi and PCr/Pi ratios (P<0.05 for both) in group 2 vs 1 with unchanged recovery of CO (P=NR), LVP (P=NS), dP/dt max (P=NR), and dP/dt min (P=NS) in group 4 vs 3 |
| ATP and EC (ATP+0.5×ADP)/(ATP+ADP+AMP) 41 | DBD, preclinical (rat) |
Parallel, 6‐arm study with graft storage conditions, as described below, followed by 2 h normothermic MP: Groups 1–4: 200 min CSS (n=10) Groups 5–6: 200 min cold reperfusion (n=10) |
Tissue samples after storage period | Myocardial function after 2 h normothermic MP | Not supported: ↑ ATP and EC (biomarkers) in groups 5–6 vs groups 1–4 with unchanged RPP |
| EC (ATP+0.5×ADP)/(ATP+ADP+AMP), PCr×100/PCr+creatine and AMP/ATP ratio 32 | DCD, preclinical (pig) |
Parallel, 2‐arm study with 8–44 min WIT, followed by storage conditions described below, and 1 h unloaded, normothermic MP:
|
Tissue samples after 4 h storage | Myocardial function after 1 h normothermic MP |
Supported: ↑ EC (P<0.01), PCr×100/PCr+creatine (P<0.05) and ↓ AMP/ATP (P<0.01) with ↑ heart rate (P<0.05), Pmax (P<0.05), dP/dt max (P<0.05), RPP (P<0.01), and contractility index (P<0.05), ↓ dP/dt min (P<0.05) in group 2 (reperfusion) vs 1 (CSS) |
| EC and AMP/ATP after 1 h normothermic MP | Supported: in parallel with biomarker changes (above), ↑ EC and ↓ AMP/ATP as outcomes (P<0.001 for both) in group 2 (reperfusion) vs 1 (CSS) | ||||
| ATP 42 | DCD, preclinical (rat) |
Parallel, 7‐arm study (6 relevant arms) with 25 min global, warm ischemia, followed by storage conditions, as described below, and 1 h unloaded, normothermic MP: Group 1: 4 h CSS (n=14) Groups 2–6: 1 h reperfusion at 20°C, 25°C, 30°C, 33°C, or 37°C, respectively, and 4 h CCS (n=14) |
Tissue samples after storage period | Myocardial function after 1 h normothermic MP | Supported: ↑ ATP (P<0.0001 for all) with ↑ heart rate (P<0.0001 for all) and mean dP/dt (P=0.0125 for all) in groups 2–6 vs 1 |
| ATP after 1 h normothermic MP | Not supported: in parallel with biomarker changes (above), unchanged ATP (P=NR) as outcome in groups 2–6 vs 1 | ||||
| ATP 43 | DCD, preclinical (rat) |
Parallel, 3‐arm study (2 relevant arms) with 25 min WIT followed by storage conditions, as described below, and 1 h, unloaded, normothermic MP:
|
Tissue samples after storage period | Myocardial function after 1 h normothermic MP |
Mixed results Supported: ↑ ATP (biomarker; P<0.05) with ↑ heart rate (P=0.024) and mean dP/dt (P=0.042) in group 2 vs 1 Not supported: in parallel with biomarker changes (above), unchanged contractile index |
| ATP after 1 h normothermic MP | Supported: in parallel with biomarker changes (above) ↑ ATP (outcome; P<0.05) in group 2 vs 1 |
CO indicates cardiac output; CSS, cold static storage; DBD, donation after brain death; DCD, donation after cardiac death; dP/dt, first derivative of left ventricular pressure; dP/dt max, maximal dP/dt; dP/dt min, minimal dP/dt; EC, energy charge; EGF, early graft failure; HEP, high‐energy phosphate; HTx, heart transplantation; LVP, left ventricular pressure; LVSP, left ventricular systolic pressure; MP, machine perfusion; MRS, magnetic resonance spectroscopy; NR, not reported; NS, not significant; Pmax, maximal developed pressure; PCr, creatine phosphate; Pi, inorganic phosphate; r, correlation coefficent for linear regression; R, Pearson correlation; RPP, rate‐pressure product (heart rate×peak systolic pressure); and WIT, warm ischemia time.
HEPs are of particular interest as they can be rapidly quantified during MP by phosphorous‐31 magnetic resonance spectroscopy in graft biopsies, albeit this technology may be limited to research centers. However, any individual measurement provides only a snapshot of tissue HEP content, whereas changes in HEP profiles over time, which may prove particularly valuable, require multiple measurements that could be obtained with biopsies during MP. Further investigations are necessary to establish sensitive and reliable indicators of graft quality with myocardial HEP content.
Uric Acid
The end product of purine catabolism, uric acid, as a marker for various outcomes, when measured in recipients of DBD hearts, has been investigated in only a few studies. 46 , 47 , 48 Higher posttransplant uric acid levels are associated with increased risk of developing cardiac allograft vasculopathy, 46 and elevated serum uric acid concentrations at 1 year after heart transplant are associated with an increased risk of mortality compared with patients with uric acid levels below the upper quartile cutoff. 47 However, this may be less donor/graft related and more recipient related as hyperuricemia in recipients before heart transplant is associated with more severe rejection after transplant compared with patients with lower uric acid levels. 48
In patients with ST‐segment–elevation myocardial infarction, the prognostic value of circulating uric acid has been demonstrated, which may be particularly relevant for DCD graft evaluation. In one study, mortality in patients with myocardial infarction was reported as ≈3.7‐fold higher in patients with uric acid concentrations in the highest quartile compared with those with uric acid concentrations of the lowest quartile. 49 However, in patients with ST‐segment–elevation myocardial infarction who underwent percutaneous coronary intervention, intensive care unit complications were more prevalent in patients with higher versus lower fasting uric acid levels, and intensive care unit mortality was not statistically different. 50
Although the potential of uric acid shows promise, its utility when evaluated before transplantation has not yet been investigated.
Hormone/Prohormone Markers
Brain Natriuretic Peptide
Several clinical studies support a role for brain natriuretic peptide (BNP) as a circulating biomarker in DBD cardiac graft assessment. BNP is released from ventricular cardiomyocytes in response to various “stress” stimuli, such as mechanical stretch, neuroendocrine activation, and tachycardia. In the context of DBD, donor plasma levels of both BNP and its precursor NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), when measured around the time of brain death, are associated with cardiac function at the time of organ procurement and posttransplantation51. Furthermore, the accuracy of predicting heart function in DBD donors can be improved by combining simultaneous measurements with donor cardiac troponins. 51 In addition, as BNP and NT‐proBNP are released in acute ischemia, these molecules may be of particular utility in DCD. 52 To our knowledge, the value of BNP or NT‐proBNP as a biomarker of graft quality, when measured at the time of, or following, heart procurement has not been investigated.
Procalcitonin
Procalcitonin is a precursor of the hormone calcitonin and clinically used as a circulating marker of inflammation. To date, 3 small, cohort studies in DBD patients have reported that donor plasma procalcitonin, when measured at the start of donor management or on pericardial opening, negatively correlates with cardiac graft function both before procurement and posttransplantation. 53 , 54 , 55 Furthermore, procalcitonin, measured immediately before pericardial opening, is an independent predictor of early graft failure in DBD. 54 , 55 Although procalcitonin may be elevated as a result of brain death, the precise stimulus and cell type responsible for its release are unknown. 56 As with BNP/NT‐proBNP, the utility of procalcitonin, when measured at later stages of heart transplantation or in DCD heart transplantation, has not been investigated.
Copeptin
Copeptin may predict postoperative outcomes in heart transplant patients, 57 but to our knowledge, it has not been investigated in cardiac grafts. Copeptin is a portion of the prevasopressin‐provasopressin molecule secreted by the hypothalamus and is emerging as a biomarker in various cardiac diseases, such as heart failure and acute coronary syndrome. 58 Copeptin is measurable in donor blood; however, it is a marker not only of cardiac damage, but also of pulmonary disease, diabetes mellitus insipidus, hemorrhagic shock, and ischemic stroke. 59 Thus, copeptin assessment could contribute information about cardiac cellular damage pre–heart transplantation, but likely requires interpretation in combination with more cardiac‐specific biomarkers.
Cellular Damage/Death Markers
Although release of cellular damage/death markers can provide valuable information about cardiac injury, variable levels, which do not accurately reflect graft quality, may be present in donor blood as a result of previous defibrillation and in cardioplegia or MP perfusate solutions as a result of extended cold static storage or perioperative damage. These factors may explain the inconsistent findings for the utility of cardiac troponin T and cardiac troponin I as predictors for posttransplant graft function in clinical DBD studies (Table 6, 17 , 18 , 19 , 66 ). When investigated in this context, creatine kinase–muscle/brain isozyme levels at pericardial opening did not appear useful for human DCD graft evaluation. 63 Lack of predictive value may result from high interpatient variability for normal creatine kinase–muscle/brain isozyme values, as well as nonheart sources of circulating donor creatine kinase–muscle/brain isozyme (ie, skeletal muscle). 63 Lactate dehydrogenase was of some value in predicting functional recovery in a preclinical DCD model during MP 19 ; however, as erythrocytes may also release lactate dehydrogenase, its utility with blood/erythrocyte‐containing perfusates is likely limited. H‐FABP (heart‐type fatty acid binding protein) may also be a useful biomarker as it is rapidly released from cardiomyocytes following ischemic damage. 67 , 68 No study has addressed its potential as an indicator of cardiac damage in transplantation; however, it appears promising in acute myocardial infarction. Xu and colleagues reported a pooled sensitivity of 0.75 and a specificity of 0.81 for H‐FABP alone in diagnosis of acute myocardial infarction within 6 hours. 67 The combination of H‐FABP with high‐sensitivity troponin T improved sensitivity, but reduced specificity. 67 H‐FABP may be a particularly valuable indicator of graft damage as it can be detected earlier than troponins, from 0.5 to 1.5 hours, 68 , 69 versus 3 to 6 hours for cardiac troponin T in acute myocardial infarction. 70 Thus, the value of H‐FABP in heart transplantation graft evaluation, especially during MP, is of particular interest for future investigation.
Table 6.
Markers of Cell Death
| Biomarker | Model | Experimental Design | Biomarker Sampling | Outcome | Biomarker‐Outcome Correlation/Predictive Value | Correlation With Other, Potential Predictive Marker |
|---|---|---|---|---|---|---|
| cTnT 60 | DBD, clinical | Prospective, single‐arm study (n=64) | Donor blood before pericardial opening | PGD | No (P=NR) | NR |
| cTnI 61 | DBD, clinical |
Retrospective, exploratory study, 2 groups:
|
Preservation solution when donor heart removed from storage bag | PGD | Yes for UW (R 2=NR, P=0.031) | NR |
| Yes for Custodiol HTK solution (R 2=NR, P=0.034) | ||||||
| Ischemic duration (time graft in storage bag) | Yes for UW (ρ=0.62, P=0.004) | |||||
| No for Custodiol HTK solution (R=0.14, P=0.59) | ||||||
| cTnI 62 | DBD, clinical |
Retrospective meta‐analysis with potential heart donors, 2 groups:
|
Donor blood at varying times during management | Post‐HTx hospitalization | Supported, ↑ in group 1 vs 2 (P=0.044) | NR |
| Survival after 30 d | Not supported (OR, 0.95; P=0.96) | |||||
| Survival after 1 y | Not supported (OR, 0.81; P=0.73) | |||||
| Early graft failure | Yes (OR, 68.4; P<0.0001) | |||||
| cTnT 54 | DBD, clinical |
Retrospective study of multiorgan donors >10 y of age (n=92), 2 groups:
|
Donor blood before pericardial opening | Early graft failure | Yes for cTnI >1.6 μg/L (OR, 42.7; P<0.0001) | No for procalcitonin (R=0.12, P=0.27) |
| cTnI and cTnT 63 | DBD, clinical |
Retrospective study with multiorgan donors >10 y of age, 2 relevant groups:
|
Donor blood before pericardial opening | Early graft failure | Yes for cTnI >1.6 μg/L, sensitivity of 73% and specificity of 94% | NR |
| Yes for cTnT >0.1 μg/L, sensitivity of 64% and specificity of 98.5% | ||||||
| Acute graft failure | Yes for cTnI >1.6 μg/L (OR, 42.7; P<0.0001) | |||||
| Yes for cTnT >0.1 μg/L (OR, 56.9; P<0.0001) | ||||||
| 30 d mortality | Yes for cTnI >1.6 μg/L (OR, 6.8; P=0.006) | |||||
| Yes for cTnT >0.1 μg/L (OR, 7.2; P<0.01) | ||||||
| Yes for cTnT >0.13 μg/L (OR, 22.4; P<0.005) | ||||||
| cTnT 64 | DBD, clinical |
Prospective study (adults and children), 3 groups divided into patients who received hearts from donors with cTnT:
|
Donor blood during organ procurement | Severe ↓ in LVEFa | Yes (R=−0.59, P<0.0001) | NR |
| Grade of rejection ≤1 y post‐HTx | Yes (R=0.973, adjusted; R=0.943, P<0.001) | |||||
| cTnT 65 | DBD, clinical |
Prospective study, 3 groups:
|
Donor blood before HTx (exact time NR) | LVEFa in donor (simultaneous with biomarker) | Yes (ρ=−0.59, P<0.0001) | NR |
| cTnT 17 | DCD, preclinical (rat) |
Parallel, 5‐arm study, MP (10 min unloaded+50 min loaded) at 37°C following WIT of:
(n=5–7 per group) |
Perfusate samples at 10 min MP | LV work at 60 min MP | Yes (ρ=Nve, P<0.01) | NR |
| TP at 60 min MP | Yes (ρ=Nve, P<0.05) | |||||
| CO at 60 min MP | No (P=NR) | |||||
| dP/dt max at 60 min MP | No (P=NR) | |||||
| dP/dt min at 60 min MP | No (P=NR) | |||||
| cTnI 66 | DCD, preclinical (rat) |
Randomized, prospective, parallel study with 4 relevant arms (n=75):
Followed by MP, during which time hearts temporarily loaded mode for functional measurements |
Right atrial plasma, immediately before heart procurement | Cardiac function during MP | Not supported: unchanged cTnI in parallel with ↓ ESPVR in groups 3 and 4 vs 1, ↓ dP/dt max in group 4 vs 1, and ↑ dP/dt min in groups 2–4 vs 1 (P<0.05 for all) | NR |
| Coronary effluent at 15, 30, 45, 60 min MP | Cardiac function during MP † | Supported: ↑ cTnI in group 4 vs groups 1–3 at all sampling time points (P<0.05 for all) in parallel with functional changes (above) | ||||
| cTnT 18 | DCD, preclinical (rat) |
Parallel, 3‐arm study in hearts subjected to WIT, as described below, followed by 10 min procurement reperfusion, cardioplegic flush, CSS for 3 h 120 min loaded, normothermic MP:
|
(A) At 10 min procurement reperfusion (before CSS) (B) At 10 min loaded, normothermic MP (after CSS) |
Multiple functional parameters (CF, CO, PSP, DP, heart rate, dP/dt min, DP/dt max, RPP, TP) at 120 min loaded, normothermic MP | Yes for B: all outcomes (ρ=NR, P<0.05), except heart rate (P=NS) | NR |
| No for A (P=NS for all) | ||||||
| CK‐MB, CK‐MB/CK, and myoglobin 63 | DBD, clinical |
Retrospective study with multiorgan donors >10 y of age, 3 groups:
|
Donor blood before pericardial opening | Not applicable | Not supported, no differences in CK‐MB activity or CK‐MB/CK ratio among groups | NR |
| CK‐MB and CK‐MB/CK 65 | DBD, clinical |
Prospective study, 3 groups:
|
Donor blood before HTx (exact time NR) | LVEFa in donor (simultaneous with biomarker) | Yes for CK‐MB (ρ=−0.17, P=0.048) | NR |
| No for CK‐MB/CK (P=NS) | ||||||
| LDH 19 | DCD, preclinical (rat) |
Parallel, 4‐arm study, MP (20 min unloaded + 40 min loaded) at 37°C of hearts subjected to WIT of:
|
Perfusate samples, calculated as % change between 5 and 10 min MP | RPP, LV work at 60 min MP | Yes (ρ=NR, P<0.05) | NR |
| CO, DP, dP/dt max, dP/dt min, heart rate, PSP at 60 min MP | No (P=NS) |
ρ indicates Spearman ρ; CF, coronary flow; CK, creatine kinase; CK‐MB, CK–muscle/brain isozyme; CO, cardiac output; CSS, cold static storage; cTnI, cardiac troponin I; cTnT, cardiac troponin T; DBD, donation after brain death; DCD, donation after circulatory death; DP, developed pressure; dP/dt max, maximal first derivative of left ventricular pressure; dP/dt min, minimal first derivative of left ventricular pressure; ESPVR, end‐systolic pressure‐volume relationship; HTK, histidine‐tryptophan‐ketoglutarate; HTx, heart transplantation; LDH, lactate dehydrogenase; LVEF, left ventricular ejection fraction; LVEFa, LVEF area; LV work, left ventricular work (heart rate×DP); MP, machine perfusion; NR, not reported; NS, not significant; Nve, negative correlation; OR, odds ratio; PGD, primary graft dysfunction; PSP, peak systolic pressure; R, Pearson correlation; RPP, rate‐pressure product (heart rate×PSP); TP, triple product (heart rate−DP−dP/dt max product); UW, University of Wisconsin preservation solution; and WIT, warm ischemia time.
Impaired graft function defined as follows: intraoperative death caused by myocardial failure, intra‐aortic balloon pump use for weaning from cardiopulmonary bypass or hemodynamic support ≤12 hours postoperatively, and LVEF <30% by echocardiography ≤12 hours postoperatively.
Potentially simultaneous measurements of biomarker and outcome.
Inflammatory Markers
Endothelial Activation
Although preclinical data support the concept that endothelial activation is associated with reduced posttransplant outcomes, corroborative clinical studies are lacking (Table 7, 25 , 71 , 72 , 73 , 74 , 75 , 76 ). Stoica and colleagues demonstrated that endothelial activation (higher levels of P‐selectin and vascular cell adhesion molecule 1) was increased in biopsies of DBD cardiac grafts compared with control tissue, but that changes in endothelial activation during transplantation are not predictive for organ failure. 71 Circulating endothelial microparticles, submicroscopic membrane vesicles released from the surface of endothelial cells during activation, injury, and/or apoptosis, are also of potential interest as biomarkers. 77 Although not yet investigated in the setting of graft evaluation, these particles indicate increased endothelial apoptosis in posttransplant patients. 77 Further investigation is required to elucidate the relationships between endothelial cell activation and microparticles, rejection, and cardiac allograft vasculopathy.
Table 7.
Endothelial Activation and Inflammatory Markers
| Biomarker | Model | Experimental Design | Biomarker Sampling | Outcome | Biomarker‐Outcome Correlation/Predictive Value | Correlation With Other, Potential Predictive Marker |
|---|---|---|---|---|---|---|
| P‐ and E‐selectin, VCAM‐1, thrombomodulin 71 | DBD +/− domino, clinical |
Observational, parallel, 3‐arm study:
|
Cardiac biopsies at multiple time points from initial donor assessment up to 3 mo posttransplant Time 1: at initial donor assessment Time 2: during CSS Time 3: at end of HTx Time 4: before release of cross‐clamp Time 5: 10 min after reperfusion Time 6: 1 wk posttransplant Time 7: 1 mo posttransplant Time 8: 3 mo posttransplant |
Allograft failure (time point not specified) |
Not supported: for changes in biomarker expression (P=NS) |
NR |
| ELP expression 72 | Heterotopic HTx, preclinical (mouse) |
Parallel, 4‐arm study:
|
Not measured, on the basis of transgenic model | Rejection score | Supported: ↓ rejection score in group 2 vs 1 (P<0.01) | NR |
| Acute rejection (histologic analysis) at 3 d after HTx | Supported: ↓ mononuclear cell infiltration in group 2 vs 1 (P=NS) | |||||
| Acute rejection (histologic analysis) at 5 d after HTx | Supported: ↓ mononuclear cell infiltration and coronary artery vasculitis in group 2 vs 1 (P<0.01) | |||||
| Acute rejection (histologic analysis) at 8 d after HTx | Supported: ↓ necrosis in group 2 vs 1 (P=NR) | |||||
| Chronic rejection at 8 wk | Supported: ↓ rejection score in group 3 vs 4 (P<0.01) | |||||
| sTNFR1, sTNFR2, IL‐6 73 | DBD, clinical | Prospective, single‐arm study (n=43) | Donor blood at procurement | Recipient ICU stay | Supported: ↑ donor sTNFR1 when ICU stay ≤5 d vs >5 d (P=0.014) | NR |
| Supported: ↑ donor sTNFR2 when ICU stay ≤5 d vs >5 d (P=0.03) | ||||||
| Recipient requirement for higher NOR doses after CPB weaning and during postoperative period | Supported: ↓ donor sTNFR2 when moderate/high doses of NOR required vs not required (P=0.028) | NR | ||||
| Supported: ↓ donor IL‐6 when moderate/high doses of NOR required vs not required (P=0.001) | ||||||
| Recipient hospitalization time | Supported: ↑ donor IL‐6 when hospitalization ≤25 d vs >25 d (P=0.029) | NR | ||||
| IL‐6, IL‐6R, and gp130 74 | DBD, clinical |
Parallel, 3‐arm study with 2 relevant arms:
|
Cardiac biopsies (immediately after heart procurement for DBD group) | On the basis of study groups | Supported: mRNA expression of all biomarkers for group 1 vs 2 (P<0.005) | NR |
| TNF‐α 75 | DBD with or without domino HTx, clinical | Parallel, 2‐arm study with DBD (n=16) and DBD domino (n=10) hearts | RV graft biopsies immediately before HTx | Recipients with (A) or without (B) right heart failure* | Supported: ↑ TNF‐α mRNA expression in A vs B (P<0.005) |
Correlations not statistically significant for:
|
| Supported: ↑ TNF‐α protein expression in A vs B (P<0.05) | ||||||
| Supported: ↑ TNF‐α expression in cardiac myocytes in A vs B (P=NS) | ||||||
| TNF‐α and IL‐6 76 | DBD, clinical |
Parallel, 4‐arm study with 2 relevant arms:
|
LV graft biopsies after CSS | On the basis of study groups | Supported for TNF‐α mRNA, ↑ in 1 vs 2 (P<0.005) | NR |
| Supported for IL‐6 mRNA, ↑ in 1 vs 2 (P<0.0001) | ||||||
| Supported for TNF‐α protein expression, ↑ in 1 vs 2 (P<0.05) | ||||||
| Supported for TNF‐α in cardiac myocytes, ↑ in 1 vs 2 (P=NR) | ||||||
| Donor blood samples before procurement | On the basis of study groups | Supported for serum TNF‐α, ↑ in 1 vs 2 (P<0.05) | ||||
| Not supported for serum TNFR1, serum TNFR2, or serum IL‐6, 1 vs 2 (P=NS for all) | ||||||
| IL‐10, IL‐6, and TNF‐α 25 | DCD, preclinical (rat) |
Parallel, 5‐arm study, MP (10 min unloaded+50 min loaded) at 37°C of hearts subjected to ischemia for:
|
IL‐10 in cardiac tissue at 60 min MP | CO at 60 min MP | Yes (ρ=Nve, P<0.001) |
Yes: ‐O2C (ρ=Nve, P<0.05) ‐LDH (ρ=Pve, P<0.05) ‐Edema (ρ=Pve, P<0.05) ‐Peroxynitrite (100 kDa) (ρ=Pve, P<0.001) ‐Peroxynitrite (60 kDa) (ρ=Pve, P<0.05) ‐WIT (ρ=Pve, P<0.001) |
| LV work at 60 min MP | Yes (ρ=Nve, P<0.05) | |||||
| TP at 60 min MP | Yes (ρ=Nve, P<0.05) | |||||
| DP at 60 min MP | No (P=NS) | |||||
| dP/dt min at 60 min MP | No (P=NS) | |||||
| dP/dt max at 60 min MP | No (P=NS) | |||||
| IL‐6 in cardiac tissue at 60 min MP | LV work at 60 min MP | No (P=NS) |
Yes: ‐Peroxynitrite (100 kDa) (ρ=Pve, P<0.05) ‐Peroxynitrite (75 kDa) (ρ=Pve, P<0.05) ‐WIT (ρ=Pve, P<0.05) |
|||
| DP at 60 min MP | No (P=NS) | |||||
| dP/dt min at 60 min MP | No (P=NS) | |||||
| dP/dt max at 60 min MP | No (P=NS) | |||||
| CO at 60 min MP | No (P=NS) | |||||
| TP at 60 min MP | No (P=NS) | |||||
| TNF‐α in cardiac tissue at 60 min MP | LV work at 60 min MP | No (P=NS) | No | |||
| DP at 60 min MP | No (P=NS) | |||||
| dP/dt min at 60 min MP | No (P=NS) | |||||
| dP/dt max at 60 min MP | No (P=NS) | |||||
| CO at 60 min MP | No (P=NS) | |||||
| TP at 60 min MP | No (P=NS) |
ρ indicates Spearman ρ; CO, cardiac output; CPB, cardiopulmonary bypass; CSS, cold static storage; DBD, donation after brain death; DCD, donation after circulatory death; DP, developed pressure; dP/dt max, maximal first derivative of left ventricular pressure; dP/dt min, minimal first derivative of left ventricular pressure; EF, ejection fraction; ELP, E‐, P‐, and L‐selectin; gp130, glycoprotein 130; HTx, heart transplantation; ICU, intensive care unit; IL‐2, interleukin 2; IL‐6, interleukin 6; IL‐6R, IL‐6 receptor; IL‐10, interleukin 10; iNOS, inducible NO synthase; LDH, lactate dehydrogenase; LV, left ventricle; LV work, left ventricular work (heart rate×DP); MP, machine perfusion; NOR, noradrenaline; NR, no reported; NS, not significant; Nve, negative correlation; O2C, oxygen consumption; Pve, positive correlation; RV, right ventricle; sTNFR, soluble TNFR; TNF‐α, tumor necrosis factor‐α; TNFR, tumor necrosis factor receptor; TP, triple product (heart rate−DP−dP/dt max product); VCAM‐1, vascular cell adhesion molecule 1; WIT, warm ischemia time; and WT, wild type.
Right heart failure: dilated, poorly contracting RV, low CO, inotropic requirements >0.5 μg×kg−1×min−1, cardiac index <2, and metabolic acidosis.
Inflammatory Cytokines
Inflammatory cytokines may be involved in graft dysfunction. 75 , 76 Reports investigating the utility of tumor necrosis factor (TNF)‐α, soluble TNF receptors 1 and 2, and interleukin 6 (IL‐6) as indicators of posttransplant graft function are presented in Table 7.
TNF‐α protein levels can rapidly increase in cardiac myocytes after brain death and may have negative consequences, including activation of inducible NO synthase, disruption of excitation‐contraction coupling and systolic/diastolic function, inflammation, cardiac myocyte apoptosis, and even heart failure. 75 Although only few investigations of TNF‐α in DBD heart transplantation have been reported, they consistently demonstrate elevated levels of TNF‐α in cardiac tissue and donor serum samples for hearts with poor myocardial function, as well as for hearts that develop right heart failure early after transplantation. 75
Interleukin 6 is released by immune cells, mesenchymal cells, endothelial cells, and fibroblasts, among others, into the circulation in response to various stimuli. Interleukin 6 has been investigated only in a few heart transplantation studies; nonetheless, levels in cardiac biopsies or donor blood negatively correlate with graft quality and patient outcomes. 73 , 75
Importantly, as donor serum TNF‐α levels correlate with tissue content in biopsies, 76 it may be that biopsy measurements could be avoided. Instead, inflammatory cytokine profiles of donor blood and/or MP perfusate could be evaluated, providing potential advantages for these biomarkers.
Damage‐Associated Molecular Patterns
Tissue and cells undergoing stress or damage, such as ischemia‐reperfusion injury, release molecules collectively termed damage‐associated molecular patterns (DAMPs). DAMPs are analogous to pathogen‐associated molecular patterns, with the activation of innate immunity, which may ultimately lead to graft inflammation and early graft dysfunction. 78 DAMPs include the following cellular and extracellular components: fibrinogen, nucleic acids (RNA and DNA), high‐mobility group box 1, S100, HSPs (heat shock proteins) (HSP70/72, HSP90, and HSP60), tenascin c, and histone. Circulating levels of high‐mobility group box 1, S100, HSP70, and tenascin c in patients with signs of acute ischemic coronary disease are potential biomarkers for early diagnosis of myocardial infarction.
Similarly, damaged mitochondria also liberate mitochondrial components of the DAMP class, termed mitochondrial DAMPs. Recognized mitochondrial DAMPs include the following: mitochondrial DNA, cytochrome c, ATP, mitochondrial transcription factor A, N‐formyl peptides, succinate, and cardiolipin. 79 Importantly, increased circulating mitochondrial DAMPs in both DBD and DCD donors have been reported, and donor plasma mitochondrial DNA levels correlate with early allograft dysfunction in liver transplant recipients. 80 In an isolated rat heart model of DCD, we have recently demonstrated that cytochrome c and succinate are released during early reperfusion following warm, global ischemia, and levels negatively correlate with subsequently measured functional recovery. 17
As DAMPs are ubiquitously expressed, evaluation of release during MP, rather than donor levels, is likely to provide superior specificity for cardiac graft evaluation.
New Approaches Toward Graft Evaluation
Omics Approaches
Investigation into patterns of changes in genomics (gene expression), transcriptomics (mRNA expression), proteomics (protein expression and posttranslational modifications), and metabolomics (metabolites) in cardiac grafts may also provide valuable information about graft quality. Indeed, mRNA signatures have been reported to detect injury both at procurement and during MP in a preclinical DCD model. 66 Furthermore, the development of pharmacogenomics approaches could potentially be used to individualize and optimize drug therapy during MP. 81
Exosome Profiling
Exosomes, secreted nanovesicles of 50 to 200 nm with specific RNA, lipid, and protein content, are released into the circulation or body fluids in patients with various pathological conditions, indicating a potential role for exosomes as a diagnostic tool. Following heart transplantation, recipient dendritic cells acquire donor major histocompatibility complex molecules by capturing donor‐derived exosomes, 82 implicating exosomes in the induction of antigen‐specific effector immune responses. 67 This exosome‐mediated major histocompatibility complex “cross‐dressing” in immune homeostasis 83 may therefore be exploited as a biomarker to monitor acute rejection. 84
Before transplantation, exosomes extracted from heart perfusate may reflect the pathophysiological status of the donor cells and organ. The number of donor exosomes has been demonstrated to correlate with organ stability/rejection in a mouse heart transplantation model. 69 Importantly, cardiac myosin and vimentin are recognized as tissue‐restricted self‐antigens that are detectable on the surface of circulating exosomes and are associated with primary graft dysfunction. 85 Even before cell membrane rupture, cardiomyocytes can release cardiac myosin and vimentin in association with exosomes. Not only are structural proteins enveloped in exosomes of damaged, but nondisrupted, cardiomyocytes, recently Hu et al showed that apoptosis‐related proteins are enriched in exosomes released by cardiomyocytes subjected to oxidative stress. 86 Thus, it is plausible that following the catecholamine surge that accompanies donor death and/or graft ischemia‐reperfusion injury, damaged cells release exosomes into the circulation, which could lead to sensitization and eventually rejection in the recipient. Thus, exosomes may be of particular interest for graft evaluation not only posttransplantation to monitor rejection, but also in donor blood and/or in graft perfusate during MP.
Cardiac Imaging
Imaging techniques are already used to assess cardiac graft function in DBD donors; however, this may not be possible in all situations, particularly in DCD.
Echocardiography can assess myocardial function and anatomical structural abnormalities. With the Doppler technique to assess anatomic information, pressure gradients, and blood flow velocities, echocardiography can help in the diagnosis and grading of cardiac disease. Echocardiography is part of the standard donor heart evaluation procedure in DBD and is also used to assess DCD graft function during normothermic regional perfusion. 14 Despite strict limitations on antemortem interventions in DCD, Chew et al 10 reported the use of echocardiography to assess the function of potential donor hearts before withdrawal of life‐sustaining therapy.
X‐ray fluoroscopy is one of the most common examinations for imaging the coronary arteries. The utility of coronary angiography in donor heart assessment during MP has been demonstrated in 2 case reports. 87 , 88 Cardiac computed tomography is frequently used as a low‐radiation alternative, for noninvasive, rapid screening for both coronary artery stenosis and structural abnormalities, such as valve disease. 89
Nuclear imaging techniques have long been established, and new tracers are being developed constantly. 90 With radioactive tracers, various metabolic pathways can be investigated using single‐photon emission computed tomography or positron emission tomography. 89 Disadvantaged by poor spatial resolution, fusion imaging methods, such as positron emission tomography with computed tomography or magnetic resonance imaging, improve accuracy of tracer localization and accumulation, and of tissue characteristics themselves.
Cardiovascular magnetic resonance is the gold standard for assessment of cardiac function, especially of the right ventricle, which is harder to assess with echocardiography as it does not follow a classic geometric model. 91 Cardiovascular magnetic resonance can be considered a comprehensive examination as it can also detect many factors, including, but not limited to, structural changes, such as fibrosis and scar, iron or fat accumulation, or other pathological conditions. 92 It also allows for the detection and (semi‐) quantitative assessment of myocardial edema, which is of prognostic value in inflammatory diseases and ischemia. 93 The addition of contrast agents permits the detection of fibrosis and scar, as well as the assessment of coronary and microvascular function. More recent approaches focus on exploiting endogenous contrast and vasodilation techniques to assess microvascular function and myocardial oxygenation. 94 , 95 Furthermore, techniques, such as parametric mapping, permit quantification of tissue characteristics. 96 Mapping may be more sensitive to early‐stage pathological conditions, 97 and reader bias is eliminated as evaluations are no longer based on visual assessment.
Magnetic resonance spectroscopy has mainly been used to assess energetic inorganic phosphates as the ATP/creatine phosphate ratio noninvasively in vivo, 98 but there are also various other molecules that can be traced, permitting measurement of even the deoxygenation of heme molecules. 99 It is ideally suited to studying cardiac metabolism; however, it is restricted to research and possesses limited sensitivity. Hyperpolarized metabolic magnetic resonance allows real‐time metabolic imaging without the need for radioactive compounds 100 ; however, it is in its infancy in humans.
In combination with imaging technologies, MP opens the door to new, ex situ possibilities, with the advantages of permitting imaging after the heart has already been exposed to potentially damaging conditions, such as warm ischemia and reperfusion in DCD, and the ability to monitor multiple variables rapidly. Nonetheless, the use of contrast agents must be carefully considered, and purpose‐built perfusion systems may be required.
Conclusions
Major obstacles in heart transplantation include insufficient donor organs and high rates of primary graft dysfunction. Improved protocols for cardiac graft evaluation may not only aid in expanding the donor pool, but may also provide better clinical outcomes by helping to identify grafts at risk for primary dysfunction. A greater consideration of cardiac biomarkers, be they measured at procurement, during static storage, and/or during MP, holds potential for developing clinical protocols that allow a more precise and reliable evaluation of graft suitability for transplantation. This is of particular interest, on one hand, in light of increasing use of ECDs and DCD, which may require more sophisticated graft evaluation techniques; and, on the other hand, as cardiac MP options, which enable monitoring the profile of multiple, graft‐specific parameters, become available.
In this review, we have identified and summarized particularly promising approaches in the development of biomarkers for cardiac graft quality and provided indications for the time window of assessment (Figure 3). Importantly, MP allows monitoring of changes in biomarker release over time via perfusate sampling. This possibility should permit more precise graft evaluation. Nonetheless, identification of reliable predictive parameters will require standardization of perfusion conditions, such as perfusion temperature, composition, and pressure, as well as chronotropy and inotropy. Furthermore, techniques must permit timely biomarker measure evaluation to fit within the time frame of clinical heart transplantation protocols. Several biomarkers, such as troponins and lactate, are rapidly and routinely measured in clinical practice and could be implemented without difficulty. However, other approaches, such as HEP or exosome profiling, which may be of particular value in graft evaluation, require further development to establish rapid measurement protocols and appropriate thresholds/criteria. Importantly, the combined use of several biomarker measurements would likely provide the most robust assessment. Current imaging modalities could also be used to evaluate cardiac grafts, permitting detailed graft characterization. Of particular relevance are techniques that enable the assessment of metabolic flux and allow optimization of the perfusion environment with a high potential for translatability and graft assessment before transplant without the need for radioactive tracers. Although MP systems must be specially adapted for computed tomography and cardiovascular magnetic resonance, imaging modalities are particularly attractive as several graft characteristics can be simultaneously evaluated. Cardiovascular magnetic resonance is of particular promise as it possesses excellent spatial and temporal resolution, it is not limited by poor ultrasound windows, as may occur with echocardiography, and does not use ionizing radiation.
Figure 3. Schematic overview of markers for cardiac graft evaluation.

Markers can be assessed at the level of the whole organ (blue), tissue biopsies (red), or circulating in donor blood or ex situ perfusate (green). Cardiac metabolism: cardiac oxygen consumption and efficiency, lactate release, and high‐energy phosphate metabolites. Tissue injury: cardiac troponin I and cardiac troponin T. Endothelial activation: P‐, E‐, and L‐selectin expression, vascular cell adhesion molecule‐1, and thrombomodulin. Vascular function: coronary flow, vascular relaxation, vascular leakage (edema), and endothelial NO synthase coupling (peroxynitrite formation). Inflammation: tumor necrosis factor (TNF)‐α, interleukin 6, interleukin 10, glycoprotein 130, and soluble TNF receptors 1/2. Hormones: procalcitonin and brain natriuretic peptide.
Sources of Funding
None.
Disclosures
None.
(J Am Heart Assoc.2021;10:e018966. DOI: 10.1161/JAHA.120.018966.)
For Sources of Funding and Disclosures, see page 27.
References
- 1. Branger P, Samuel U. Annual report 2018 Eurotransplant International Foundation. 2018. Available at https://www.eurotransplant.org/cms/mediaobject.php?file=ET_Jaarv. Accessed July 22, 2019.
- 2. Colvin M, Smith JM, Hadley N, Skeans MA, Carrico R, Uccellini K, Lehman R, Robinson A, Israni AK, Snyder JJ, et al. OPTN/SRTR 2016 annual data report: heart. Am J Transplant. 2018;18:291–362. DOI: 10.1111/ajt.14561. [DOI] [PubMed] [Google Scholar]
- 3. Dhital KK, Chew HC, Macdonald PS. Donation after circulatory death heart transplantation. Curr Opin Organ Transplant. 2017;22:189–197. DOI: 10.1097/MOT.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 4. Chew HC, Macdonald PS, Dhital KK. The donor heart and organ perfusion technology. J Thorac Dis. 2019;11:S938–S945. DOI: 10.21037/jtd.2019.02.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhital KK, Iyer A, Connellan M, Chew HC, Gao L, Doyle A, Hicks M, Kumarasinghe G, Soto C, Dinale A, et al. Adult heart transplantation with distant procurement and ex‐vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385:2585–2591. DOI: 10.1016/S0140-6736(15)60038-1. [DOI] [PubMed] [Google Scholar]
- 6. Sato T, Azarbal B, Cheng R, Esmailian F, Patel J, Kittleson M, Czer L, Thottam M, Levine R, Dimbil S, et al. Does ex vivo perfusion lead to more or less intimal thickening in the first‐year post‐heart transplantation? Clin Transplant. 2019;33:e13648. DOI: 10.1111/ctr.13648. [DOI] [PubMed] [Google Scholar]
- 7. Ardehali A, Esmailian F, Deng M, Soltesz E, Hsich E, Naka Y, Mancini D, Camacho M, Zucker M, Leprince P, et al. Ex‐vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open‐label, multicentre, randomised non‐inferiority trial. Lancet. 2015;385:2577–2584. DOI: 10.1016/S0140-6736(15)60261-6. [DOI] [PubMed] [Google Scholar]
- 8. García Sáez D, Zych B, Sabashnikov A, Bowles CT, De Robertis F, Mohite PN, Popov A‐F, Maunz O, Patil NP, Weymann A, et al. Evaluation of the organ care system in heart transplantation with an adverse donor/recipient profile. Ann Thorac Surg. 2014;98:2099–2106. DOI: 10.1016/j.athoracsur.2014.06.098. [DOI] [PubMed] [Google Scholar]
- 9. Messer S, Page A, Axell R, Berman M, Hernández‐Sánchez J, Colah S, Parizkova B, Valchanov K, Dunning J, Pavlushkov E, et al. Outcome after heart transplantation from donation after circulatory‐determined death donors. J Heart Lung Transplant. 2017;36:1311–1318. DOI: 10.1016/j.healun.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 10. Chew HC, Iyer A, Connellan M, Scheuer S, Villanueva J, Gao L, Hicks M, Harkness M, Soto C, Dinale A, et al. Outcomes of donation after circulatory death heart transplantation in Australia. J Am Coll Cardiol. 2019;73:1447–1459. DOI: 10.1016/j.jacc.2018.12.067. [DOI] [PubMed] [Google Scholar]
- 11. Dronavalli VB, Banner NR, Bonser RS. Assessment of the potential heart donor. J Am Coll Cardiol. 2010;56:352–361. [DOI] [PubMed] [Google Scholar]
- 12. Reich HJ, Kobashigawa JA, Aintablian T, Ramzy D, Kittleson MM, Esmailian F. Effects of older donor age and cold ischemic time on long‐term outcomes of heart transplantation. Tex Heart Inst J. 2018;45:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Novitzky D. Detrimental effects of brain death on the potential organ donor. Transplant Proc. 1997;29:3770–3772. [DOI] [PubMed] [Google Scholar]
- 14. Messer SJ, Axell RG, Colah S, White PA, Ryan M, Page AA, Parizkova B, Valchanov K, White CW, Freed DH, et al. Functional assessment and transplantation of the donor heart after circulatory death. J Heart Lung Transplant. 2016;35:1443–1452. [DOI] [PubMed] [Google Scholar]
- 15. Ribeiro RVP, Alvarez JS, Yu F, Adamson MB, Paradiso E, Mbadjeu Hondjeu AR, Xin L, Gellner B, Degen M, Bissoondath V, et al. Comparing donor heart assessment strategies during ex situ heart perfusion to better estimate post‐transplant cardiac function. Transplantation. 2020;104:1890–1898. [DOI] [PubMed] [Google Scholar]
- 16. White CW, Ambrose E, Müller A, Li Y, Le H, Hiebert B, Arora R, Lee TW, Dixon I, Tian G, et al. Assessment of donor heart viability during ex vivo heart perfusion. Can J Physiol Pharmacol. 2015;93:893–901. [DOI] [PubMed] [Google Scholar]
- 17. Wyss RK, Méndez‐Carmona N, Sanz M‐N, Arnold M, Segiser A, Fiedler GM, Carrel TP, Djafarzadeh S, Tevaearai Stahel HT, Longnus SL. Mitochondrial integrity during early reperfusion in an isolated rat heart model of donation after circulatory death‐consequences of ischemic duration. J Heart Lung Transplant. 2019;38:647–657. [DOI] [PubMed] [Google Scholar]
- 18. Sourdon J, Dornbierer M, Huber S, Gahl B, Carrel TP, Tevaearai HT, Longnus SL. Cardiac transplantation with hearts from donors after circulatory declaration of death: haemodynamic and biochemical parameters at procurement predict recovery following cardioplegic storage in a rat model. Eur J Cardiothorac Surg. 2013;44:e87–e96. [DOI] [PubMed] [Google Scholar]
- 19. Dornbierer M, Stadelmann M, Sourdon J, Gahl B, Cook S, Carrel TP, Tevaearai HT, Longnus SL. Early reperfusion hemodynamics predict recovery in rat hearts: a potential approach towards evaluating cardiac grafts from non‐heart‐beating donors. PLoS One. 2012;7:e43642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Page A, Messer S, Axell R, Naruka V, Colah S, Fakelman S, Ellis C, Abu‐Omar Y, Ali A, Berman M, et al. Does the assessment of DCD donor hearts on the organ care system using lactate need redefining? J Heart Lung Transplant. 2017;36:S16–S17. [Google Scholar]
- 21. Yang H‐M, Khush K, Luikart H, Okada K, Lim H‐S, Kobayashi Y, Honda Y, Yeung AC, Valantine H, Fearon WF. Invasive assessment of coronary physiology predicts late mortality after heart transplantation. Circulation. 2016;133:1945–1950. DOI: 10.1161/CIRCULATIONAHA.115.018741. [DOI] [PubMed] [Google Scholar]
- 22. Vecchiati A, Tellatin S, Angelini A, Iliceto S, Tona F. Coronary microvasculopathy in heart transplantation: consequences and therapeutic implications. World J Transplant. 2014;4:93–101. DOI: 10.5500/wjt.v4.i2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hollenberg SM, Klein LW, Parrillo JE, Scherer M, Burns D, Tamburro P, Bromet D, Satran A, Costanzo MR. Changes in coronary endothelial function predict progression of allograft vasculopathy after heart transplantation. J Heart Lung Transplant. 2004;23:265–271. DOI: 10.1016/S1053-2498(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 24. Ramzy D, Rao V, Brahm J, Miriuka S, Delgado D, Ross HJ. Cardiac allograft vasculopathy: a review. Can J Surg. 2005;48:319. [PMC free article] [PubMed] [Google Scholar]
- 25. Méndez‐Carmona N, Wyss RK, Arnold M, Joachimbauer A, Segiser A, Fiedler GM, Carrel TP, Tevaearai Stahel HT, Longnus SL. Differential effects of ischemia/reperfusion on endothelial function and contractility in donation after circulatory death. J Heart Lung Transplant. 2019;38:767–777. DOI: 10.1016/j.healun.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 26. Ferrera R, Bopassa J‐C, Rodriguez C, Baverel G, Ovize M. A simple and reliable method to assess heart viability after hypothermic procurement. Transplant Proc. 2006;38:2283–2284. DOI: 10.1016/j.transproceed.2006.06.124. [DOI] [PubMed] [Google Scholar]
- 27. Ferrera R, Forrat R, Marcsek P, de Lorgeril M, Dureau G. Importance of initial coronary artery flow after heart procurement to assess heart viability before transplantation. Circulation. 1995;91:257–261. DOI: 10.1161/01.CIR.91.2.257. [DOI] [PubMed] [Google Scholar]
- 28. Houston RJ, Skotnicki SH, Heerschap A, Oeseburg B. Coronary flow response after myocardial ischemia may predict level of functional recovery. Adv Exp Med Biol. 1997;411:121–127. [DOI] [PubMed] [Google Scholar]
- 29. Haddad F, Khazanie P, Deuse T, Weisshaar D, Zhou J, Nam CW, Vu TA, Gomari FA, Skhiri M, Simos A, et al. Clinical and functional correlates of early microvascular dysfunction after heart transplantation. Circ Heart Fail. 2012;5:759–768. DOI: 10.1161/CIRCHEARTFAILURE.111.962787. [DOI] [PubMed] [Google Scholar]
- 30. Deng M, Soltesz E, Hsich E, Naka Y, Mancini D, Esmailian F, Kobashigawa J, Camacho M, Baran D, Madsen J, et al. Is lactate level during warm perfusion a predictor for post transplant outcomes? J Heart Lung Transplant. 2013;32:S156–S157. DOI: 10.1016/j.healun.2013.01.363. [DOI] [Google Scholar]
- 31. Hamed A, Tsui S, Huber J, Lin R, Poggio EC, Ardehali A. Serum lactate is a highly sensitive and specific predictor of post cardiac transplant outcomes using the organ care system. J Heart Lung Transplant. 2009;28:S71. [Google Scholar]
- 32. Van Caenegem O, Beauloye C, Bertrand L, Horman S, Lepropre S, Sparavier G, Vercruysse J, Bethuyne N, Poncelet AJ, Gianello P, et al. Hypothermic continuous machine perfusion enables preservation of energy charge and functional recovery of heart grafts in an ex vivo model of donation following circulatory death. Eur J Cardiothorac Surg. 2016;49:1348–1353. [DOI] [PubMed] [Google Scholar]
- 33. Iyer A, Gao L, Doyle A, Rao P, Cropper JR, Soto C, Dinale A, Kumarasinghe G, Jabbour A, Hicks M, et al. Normothermic ex vivo perfusion provides superior organ preservation and enables viability assessment of hearts from DCD donors. Am J Transplant. 2015;15:371–380. [DOI] [PubMed] [Google Scholar]
- 34. García Sáez D, Elbetanony A, Lezberg P, Hassanein A, Bowles CT, Popov A‐F, Zych B, Sabashnikov A, Mohite P, Simon AR. Ex vivo heart perfusion after cardiocirculatory death; a porcine model. J Surg Res. 2015;195:311–314. [DOI] [PubMed] [Google Scholar]
- 35. Xin L, Yao W, Yu F, Ribeiro RV, Alvarez J, Peng Y, Sun Y, Badiwala M. Comparison of lactate and glucose during ex situ heart perfusion as predictors of early‐stage heart transplantation outcomes. J Heart Lung Transplant. 2020;39:S180. DOI: 10.1016/j.healun.2020.01.759. [DOI] [Google Scholar]
- 36. Caus T, Kober F, Mouly‐Bandini A, Riberi A, Métras DR, Cozzone PJ, Bernard M. 31P MRS of heart grafts provides metabolic markers of early dysfunction. Eur J Cardiothorac Surg. 2005;28:576–580. DOI: 10.1016/j.ejcts.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 37. Van Dobbenburgh JO, Lahpor JR, Woolley SR, de Jonge N, KlÕpping C, Van Echteld CJA. Functional recovery after human heart transplantation is related to the metabolic condition of the hypothermic donor heart. Circulation. 1996;94:2831–2836. DOI: 10.1161/01.CIR.94.11.2831. [DOI] [PubMed] [Google Scholar]
- 38. Lin H, Mo A, Zhang F, Huang A, Wen Z, Ling S, Hu Y, Zhou Y, Lu C. Donor heart preservation in an empty beating state under mild hypothermia. Ann Thorac Surg. 2010;89:1518–1523. DOI: 10.1016/j.athoracsur.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 39. Ozeki T, Kwon MH, Gu J, Collins MJ, Brassil JM, Miller MB Jr, Gullapalli RP, Zhuo J, Pierson RN III, Griffith BP, et al. Heart preservation using continuous ex vivo perfusion improves viability and functional recovery. Circ J. 2007;71:153–159. DOI: 10.1253/circj.71.153. [DOI] [PubMed] [Google Scholar]
- 40. Tsutsumi H, Oshima K, Mohara J, Takeyoshi I, Aizaki M, Tokumine M, Matsumoto K, Morishita Y. Cardiac transplantation following a 24‐h preservation using a perfusion apparatus. J Surg Res. 2001;96:260–267. DOI: 10.1006/jsre.2001.6077. [DOI] [PubMed] [Google Scholar]
- 41. Peltz M, He T‐T, Adams GA, Koshy S, Burgess SC, Chao RY, Meyer DM, Jessen ME. Perfusion preservation maintains myocardial ATP levels and reduces apoptosis in an ex vivo rat heart transplantation model. Surgery. 2005;138:795–805. DOI: 10.1016/j.surg.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 42. Tolboom H, Olejníčková V, Reser D, Rosser B, Wilhelm MJ, Gassmann M, Bogdanova A, Falk V. Moderate hypothermia during ex vivo machine perfusion promotes recovery of hearts donated after cardiocirculatory death†. Eur J Cardiothorac Surg. 2016;49:25–31. [DOI] [PubMed] [Google Scholar]
- 43. Tolboom H, Makhro A, Rosser BA, Wilhelm MJ, Bogdanova A, Falk V. Recovery of donor hearts after circulatory death with normothermic extracorporeal machine perfusion. Eur J Cardiothorac Surg. 2015;47:173–179. DOI: 10.1093/ejcts/ezu117. [DOI] [PubMed] [Google Scholar]
- 44. Cobert ML, Merritt ME, West LM, Ayers C, Jessen ME, Peltz M. Metabolic characteristics of human hearts preserved for 12 hours by static storage, antegrade perfusion or retrograde coronary sinus perfusion. J Thorac Cardiovasc Surg. 2014;148:2310. DOI: 10.1016/j.jtcvs.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schipper DA, Marsh KM, Ferng AS, Duncker DJ, Laman JD, Khalpey Z. The critical role of bioenergetics in donor cardiac allograft preservation. J Cardiovasc Transl Res. 2016;9:176–183. DOI: 10.1007/s12265-016-9692-2. [DOI] [PubMed] [Google Scholar]
- 46. Asleh R, Prasad M, Briasoulis A, Nardi V, Adigun R, Edwards BS, Pereira NL, Daly RC, Lerman A, Kushwaha SS. Uric acid is an independent predictor of cardiac allograft vasculopathy after heart transplantation. J Heart Lung Transplant. 2018;37:1083–1092. DOI: 10.1016/j.healun.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 47. Arora S, Aukrust P, Ueland T, Broch K, Simonsen S, Gude E, Fiane AE, Geiran O, Wergeland R, Andreassen AK, et al. Elevated serum uric acid levels following heart transplantation predict all‐cause and cardiac mortality. Eur J Heart Fail. 2009;11:1005–1013. DOI: 10.1093/eurjhf/hfp115. [DOI] [PubMed] [Google Scholar]
- 48. António N, Prieto D, Antunes MJ. Uric acid: a prognostic marker not only before but also after heart transplantation. Eur J Cardiothorac Surg. 2010;38:187–191. DOI: 10.1016/j.ejcts.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 49. Kojima S, Sakamoto T, Ishihara M, Kimura K, Miyazaki S, Yamagishi M, Tei C, Hiraoka H, Sonoda M, Tsuchihashi K, et al; Japanese Acute Coronary Syndrome Study (JACSS) Investigators . Prognostic usefulness of serum uric acid after acute myocardial infarction (the Japanese Acute Coronary Syndrome Study). Am J Cardiol. 2005;96:489–495. DOI: 10.1016/j.amjcard.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 50. Lazzeri C, Valente S, Chiostri M, Picariello C, Gensini GF. Uric acid in the early risk stratification of ST‐elevation myocardial infarction. Intern Emerg Med. 2012;7:33–39. DOI: 10.1007/s11739-011-0515-9. [DOI] [PubMed] [Google Scholar]
- 51. Nicolas‐Robin A, Salvi N, Medimagh S, Amour J, Manach YL, Coriat P, Riou B, Langeron O. Combined measurements of N‐terminal pro‐brain natriuretic peptide and cardiac troponins in potential organ donors. Intensive Care Med. 2007;33:986–992. DOI: 10.1007/s00134-007-0601-7. [DOI] [PubMed] [Google Scholar]
- 52. Sabatine MS, Morrow DA, de Lemos JA, Omland T, Desai MY, Tanasijevic M, Hall C, McCabe CH, Braunwald E. Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol. 2004;44:1988–1995. DOI: 10.1016/j.jacc.2004.07.057. [DOI] [PubMed] [Google Scholar]
- 53. Venkateswaran RV, Dronavalli V, Lambert PA, Steeds RP, Wilson IC, Thompson RD, Mascaro JG, Bonser RS. The proinflammatory environment in potential heart and lung donors: prevalence and impact of donor management and hormonal therapy. Transplantation. 2009;88:582–588. DOI: 10.1097/TP.0b013e3181b11e5d. [DOI] [PubMed] [Google Scholar]
- 54. Potapov EV, Wagner FD, Loebe M, Ivanitskaia EA, Müller C, Sodian R, Jonitz B, Hetzer R. Elevated donor cardiac troponin T and procalcitonin indicate two independent mechanisms of early graft failure after heart transplantation. Int J Cardiol. 2003;92:163–167. DOI: 10.1016/S0167-5273(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 55. Wagner FD, Jonitz B, Potapov EV, Qedra N, Wegscheider K, Abraham K, Ivanitskaia EA, Loebe M, Procalcitonin HR. A donor‐specific predictor of early graft failure‐related mortality after heart transplantation. Circulation. 2001;104:I‐192–I‐196. DOI: 10.1161/hc37t1.094836. [DOI] [PubMed] [Google Scholar]
- 56. Rangeard O, Audibert G, Perrier J‐F, Loos‐Ayav C, Lalot J‐M, Agavriloaie M, Meistelman C, Grégoire H, Mertes PM, Longrois D. Relationship between procalcitonin values and infection in brain‐dead organ donors. Transplant Proc. 2007;39:2970–2974. DOI: 10.1016/j.transproceed.2007.02.101. [DOI] [PubMed] [Google Scholar]
- 57. Malyszko J, Przybylowski P, Koc‐Zorawska E, Iaina‐Levin N, Sadowski J, Mysliwiec M, Malyszko JS. Copeptin in relation to New York Heart Association class in heart transplant recipients and kidney transplant recipients. Transplant Proc. 2010;42:4259–4262. DOI: 10.1016/j.transproceed.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 58. Schurtz G, Lamblin N, Bauters C, Goldstein P, Lemesle G. Copeptin in acute coronary syndromes and heart failure management: state of the art and future directions. Arch Cardiovasc Dis. 2015;108:398–407. DOI: 10.1016/j.acvd.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 59. Lasota B, Mizia‐Stec K. Copeptin in heart failure. Res Rep Clin Cardiol. 2014;5:133–144. DOI: 10.2147/RRCC.S43427. [DOI] [Google Scholar]
- 60. Szarszoi O, Besik J, Smetana M, Maly J, Urban M, Maluskova J, Lodererova A, Hoskova L, Tucanova Z, Pirk J, et al. Biomarkers of cellular apoptosis and necrosis in donor myocardium are not predictive of primary graft dysfunction. Physiol Res. 2016;65:251–257. DOI: 10.33549/physiolres.933105. [DOI] [PubMed] [Google Scholar]
- 61. Schechter MA, Watson MJ, Feger BJ, Southerland KW, Mishra R, Dibernardo LR, Kuchibhatla M, Schroder JN, Daneshmand MA, Patel CB, et al. Elevated cardiac troponin I in preservation solution is associated with primary graft dysfunction. J Card Fail. 2016;22:158–162. DOI: 10.1016/j.cardfail.2015.08.339. [DOI] [PubMed] [Google Scholar]
- 62. Khush KK, Menza RL, Babcock WD, Zaroff JG. Donor cardiac troponin I levels do not predict recipient survival after cardiac transplantation. J Heart Lung Transplant. 2007;26:1048–1053. DOI: 10.1016/j.healun.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 63. Potapov EV, Ivanitskaia EA, Loebe M, Möckel M, Müller C, Sodian R, Meyer R, Hetzer R. Value of cardiac troponin I and T for selection of heart donors and as predictors of early graft failure. Transplantation. 2001;71:1394–1400. [DOI] [PubMed] [Google Scholar]
- 64. Vijay P, Scavo VA, Morelock RJ, Sharp TG, Brown JW. Donor cardiac troponin T: a marker to predict heart transplant rejection. Ann Thorac Surg. 1998;66:1934–1938. DOI: 10.1016/S0003-4975(98)01057-1. [DOI] [PubMed] [Google Scholar]
- 65. Riou B, Dreux S, Roche S, Arthaud M, Goarin JP, Léger P, Saada M, Viars P. Circulating cardiac troponin T in potential heart transplant donors. Circulation. 1995;92:409–414. DOI: 10.1161/01.CIR.92.3.409. [DOI] [PubMed] [Google Scholar]
- 66. Kearns MJ, Miller SD, Cheung A, Bashir J, Wong S, Seidman MA, Boyd JH. A rodent model of cardiac donation after circulatory death and novel biomarkers of cardiac viability during ex vivo heart perfusion. Transplantation. 2017;101:e231–e239. DOI: 10.1097/TP.0000000000001815. [DOI] [PubMed] [Google Scholar]
- 67. Xu L‐Q, Yang Y‐M, Tong H, Xu C‐F. Early diagnostic performance of heart‐type fatty acid binding protein in suspected acute myocardial infarction: evidence from a meta‐analysis of contemporary studies. Heart Lung Circ. 2018;27:503–512. DOI: 10.1016/j.hlc.2017.03.165. [DOI] [PubMed] [Google Scholar]
- 68. Pelsers MMAL, Hermens WT, Glatz JFC. Fatty acid‐binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352:15–35. DOI: 10.1016/j.cccn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 69. McCann CJ, Glover BM, Menown IBA, Moore MJ, McEneny J, Owens CG, Smith B, Sharpe PC, Young IS, Adgey JA. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J. 2008;29:2843–2850. DOI: 10.1093/eurheartj/ehn363. [DOI] [PubMed] [Google Scholar]
- 70. Panteghini M, Pagani F, Bonetti G. The sensitivity of cardiac markers: an evidence‐based approach. Clin Chem Lab Med. 1999;37:1097–1106. DOI: 10.1515/CCLM.1999.160. [DOI] [PubMed] [Google Scholar]
- 71. Stoica SC, Atkinson C, Satchithananda DK, Charman S, Goddard M, Redington AN, Large SR. Endothelial activation in the transplanted human heart from organ retrieval to 3 months after transplantation: an observational study. J Heart Lung Transplant. 2005;24:593–601. DOI: 10.1016/j.healun.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 72. Izawa A, Ueno T, Jurewicz M, Ito T, Tanaka K, Takahashi M, Ikeda U, Sobolev O, Fiorina P, Smith RN, et al. Importance of donor‐ and recipient‐derived selectins in cardiac allograft rejection. J Am Soc Nephrol. 2007;18:2929–2936. DOI: 10.1681/ASN.2006111261. [DOI] [PubMed] [Google Scholar]
- 73. Braulio R, Dias Sanches M, Teixeira Junior AL, Nogueira Costa PH, da Consolacao Vieira Moreira M, Rocha MA, de Andrade SA, Gelape CL. Associated clinical and laboratory markers of donor on allograft function after heart transplant. Braz J Cardiovasc Surg. 2016;31:89–97. DOI: 10.5935/1678-9741.20160025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Plenz G, Eschert H, Erren M, Wichter T, Böhm M, Flesch M, Scheld HH, Deng MC. The interleukin‐6/interleukin‐6‐receptor system is activated in donor hearts. J Am Coll Cardiol. 2002;39:1508–1512. DOI: 10.1016/S0735-1097(02)01791-6. [DOI] [PubMed] [Google Scholar]
- 75. Birks EJ, Owen VJ, Burton PBJ, Bishop AE, Banner NR, Khaghani A, Polak JM, Yacoub MH. Tumor necrosis factor‐α is expressed in donor heart and predicts right ventricular failure after human heart transplantation. Circulation. 2000;102:326–331. DOI: 10.1161/01.CIR.102.3.326. [DOI] [PubMed] [Google Scholar]
- 76. Birks EJ, Burton PB, Owen V, Mullen AJ, Hunt D, Banner NR, Barton PJ, Yacoub MH. Elevated tumor necrosis factor‐alpha and interleukin‐6 in myocardium and serum of malfunctioning donor hearts. Circulation. 2000;102:III352–III358. [DOI] [PubMed] [Google Scholar]
- 77. Garcia S, Chirinos J, Jimenez J, Del Carpio MF, Canoniero M, Jy W, Jimenez J, Horstman L, Ahn Y. Phenotypic assessment of endothelial microparticles in patients with heart failure and after heart transplantation: switch from cell activation to apoptosis. J Heart Lung Transplant. 2005;24:2184–2189. DOI: 10.1016/j.healun.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 78. Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A. Transplantation and damage‐associated molecular patterns (DAMPs). Am J Transplant. 2016;16:3338–3361. DOI: 10.1111/ajt.13963. [DOI] [PubMed] [Google Scholar]
- 79. Nakahira K, Hisata S, Choi AMK. The roles of mitochondrial damage‐associated molecular patterns in diseases. Antioxid Redox Signal. 2015;23:1329–1350. DOI: 10.1089/ars.2015.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pollara J, Edwards RW, Lin L, Bendersky VA, Brennan TV. Circulating mitochondria in deceased organ donors are associated with immune activation and early allograft dysfunction. JCI Insight. 2018;3:e121622. DOI: 10.1172/jci.insight.121622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Evans WE, Relling MV. Moving towards individualized medicine with pharmacogenomics. Nature. 2004;429:464–468. [DOI] [PubMed] [Google Scholar]
- 82. Liu Q, Rojas‐Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, Sullivan MLG, Gibson GA, Watkins SC, Larregina AT, et al. Donor dendritic cell–derived exosomes promote allograft‐targeting immune response. J Clin Invest. 2016;126:2805–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zeng F, Morelli AE. Extracellular vesicle‐mediated MHC cross‐dressing in immune homeostasis, transplantation, infectious diseases, and cancer. Semin Immunopathol. 2018;40:477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Castellani C, Burrello J, Fedrigo M, Burrello A, Bolis S, Di Silvestre D, Tona F, Bottio T, Biemmi V, Toscano G, et al. Circulating extracellular vesicles as non‐invasive biomarker of rejection in heart transplant. J Heart Lung Transplant. 2020;39:1136–1148. DOI: 10.1016/j.healun.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 85. Sharma M, Liu W, Perincheri S, Gunasekaran M, Mohanakumar T. Exosomes expressing the self‐antigens myosin and vimentin play an important role in syngeneic cardiac transplant rejection induced by antibodies to cardiac myosin. Am J Transplant. 2018;18:1626–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hu M, Guo G, Huang Q, Cheng C, Xu R, Li A, Liu N, Liu S. The harsh microenvironment in infarcted heart accelerates transplanted bone marrow mesenchymal stem cells injury: the role of injured cardiomyocytes‐derived exosomes. Cell Death Dis. 2018;9:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ghodsizad A, Bordel V, Ungerer M, Karck M, Bekeredjian R, Ruhparwar A. Ex vivo coronary angiography of a donor heart in the organ care system. Heart Surg Forum. 2012;15:E161–E163. [DOI] [PubMed] [Google Scholar]
- 88. Anthony C, Michel J, Christofi M, Wilson SH, Granger E, Cropper J, Dhital K, Macdonald P. Ex vivo coronary angiographic evaluation of a beating donor heart. Circulation. 2014;130:e341–e343. [DOI] [PubMed] [Google Scholar]
- 89. Wang Y, Osborne MT, Tung B, Li M, Li Y. Imaging cardiovascular calcification. J Am Heart Assoc. 2018;7:e008564. DOI: 10.1161/JAHA.118.008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shaw LJ, Hachamovitch R, Min JK, Di Carli M, Mieres JH, Phillips L, Blankstein R, Einstein A, Taqueti VR, Hendel R, et al. Evolving, innovating, and revolutionary changes in cardiovascular imaging: we’ve only just begun! J Nucl Cardiol. 2018;25:758–768. [DOI] [PubMed] [Google Scholar]
- 91. Tadic M. Multimodality evaluation of the right ventricle: an updated review: evaluation of the right ventricle. Clin Cardiol. 2015;38:770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Messroghli DR, Moon JC, Ferreira VM, Grosse‐Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, et al. Correction to: clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. 2018;20:9. DOI: 10.1186/s12968-017-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Stiermaier T, Thiele H, Eitel I. Early myocardial edema after acute myocardial infarction is stable and not bimodal in humans—evidence from a large CMR multicenter study. Int J Cardiol. 2017;246:87–89. DOI: 10.1016/j.ijcard.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 94. Friedrich MG, Karamitsos TD. Oxygenation‐sensitive cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:43. DOI: 10.1186/1532-429X-15-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fischer K, Yamaji K, Luescher S, Ueki Y, Jung B, von Tengg‐Kobligk H, Windecker S, Friedrich MG, Eberle B, Guensch DP. Feasibility of cardiovascular magnetic resonance to detect oxygenation deficits in patients with multi‐vessel coronary artery disease triggered by breathing maneuvers. J Cardiovasc Magn Reson. 2018;20:31. DOI: 10.1186/s12968-018-0446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Puntmann VO, Peker E, Chandrashekhar Y, Nagel E. T1 mapping in characterizing myocardial disease: a comprehensive review. Circ Res. 2016;119:277–299. DOI: 10.1161/CIRCRESAHA.116.307974. [DOI] [PubMed] [Google Scholar]
- 97. Puntmann VO, Isted A, Hinojar R, Foote L, Carr‐White G, Nagel E. T1 and T2 mapping in recognition of early cardiac involvement in systemic sarcoidosis. Radiology. 2017;285:63–72. DOI: 10.1148/radiol.2017162732. [DOI] [PubMed] [Google Scholar]
- 98. Abdurrachim D, Prompers JJ. Evaluation of cardiac energetics by non‐invasive 31 P magnetic resonance spectroscopy. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1939–1948. DOI: 10.1016/j.bbadis.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 99. Zhang J, From AHL, Ugurbil K, Bache RJ. Myocardial oxygenation and high‐energy phosphate levels during KATP channel blockade. Am J Physiol‐Heart Circ Physiol. 2003;285:H1420–H1427. [DOI] [PubMed] [Google Scholar]
- 100. Cunningham CH, Lau JYC, Chen AP, Geraghty BJ, Perks WJ, Roifman I, Wright GA, Connelly KA. Hyperpolarized 13C metabolic MRI of the human heartnovelty and significance: initial experience. Circ Res. 2016;119:1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]


