Abstract
Background
High levels of supraventricular ectopy are associated with greater risk of atrial fibrillation, stroke, and death. Little information is available about differences by race/ethnicity in the extent of supraventricular ectopy, or about whether high levels of supraventricular ectopy are associated with impaired left atrial (LA) function and LA enlargement.
Methods and Results
In the MESA (Multi‐Ethnic Study of Atherosclerosis), 1148 participants (47% men; mean age, 67 years) had cardiovascular magnetic resonance imaging in 2010 to 2012, followed by 14‐day ambulatory electrocardiographic monitoring in 2016 to 2018. We analyzed participant characteristics and cardiovascular magnetic resonance measures of LA function and structure in relation to average count of premature atrial contractions (PACs) per hour and average number of runs per day of supraventricular tachycardia. In adjusted regression analyses, older age, male sex, White race, elevated NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), and a history of clinically detected atrial fibrillation were associated with more PACs/hour. Chinese and Hispanic participants had on average fewer PACs/hour than White participants (Chinese participants, 31% less [95% CI, 8%–49%]; Hispanic participants, 38% less [95% CI, 19%–52%]). Greater LA total emptying fraction was associated with fewer PACs/hour (per SD, 16% fewer PACs/hour [95% CI, 7%–25% fewer PACs/hour]). Larger LA minimum volume was associated with more PACs/hour (per SD, 7% more PACs/hour [95% CI, 2%–13% more PACs/hour]). Associations of LA volumes with runs of supraventricular tachycardia/day were similar in direction but were weaker.
Conclusions
Impaired LA function and LA enlargement were associated with more PACs/hour on extended ambulatory electrocardiographic monitoring. Measurement of supraventricular ectopy may provide information about the extent of atrial myopathy.
Keywords: emptying fraction, left atrium, strain, supraventricular ectopy, volume
Subject Categories: Arrhythmias, Epidemiology, Race and Ethnicity
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities
- CCHS
Copenhagen City Heart Study
- CHS
Cardiovascular Health Study
- MESA
Multi‐Ethnic Study of Atherosclerosis
- SVE
supraventricular ectopy
- SVT
supraventricular tachycardia
Clinical Perspective
What Is New?
In 1148 MESA (Multi‐Ethnic Study of Atherosclerosis) participants, impaired left atrial emptying fraction and larger left atrial volume by cardiovascular magnetic resonance imaging were associated with more frequent premature atrial contractions on ambulatory cardiac monitoring, both before and after adjustment for sociodemographic and clinical characteristics and for left ventricular function and structure.
In adjusted analyses, older age, male sex, White race, elevated NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) level, and a history of clinically detected atrial fibrillation were associated with more premature atrial contractions/hour; Chinese and Hispanic participants had on average fewer premature atrial contractions/hour than White participants.
What Are the Clinical Implications?
Measurement of supraventricular ectopy may provide information about the extent of atrial myopathy.
Supraventricular ectopy (SVE), which includes premature atrial contractions (PACs) and runs of supraventricular tachycardia (SVT), is common, increases with age, 1 and has generally been considered benign when infrequent and not accompanied by symptoms. However, among patients with implanted cardiac pacemakers, greater SVE (identified by atrial high‐rate episodes) was associated with higher risk of developing clinically recognized atrial fibrillation (AF), stroke, and death. 2 An assigned diagnosis of paroxysmal SVT was associated with increased risk of subsequent stroke in a healthcare database study, 3 and frequent PACs on Holter recordings were a risk factor for subsequent development of AF. 4 In the CCHS (Copenhagen City Heart Study), high levels of SVE on Holter recordings were associated with increased risk of ischemic stroke, even in the absence of clinically recognized AF. 5 And in the 30‐day cardiac event monitor belt for recording atrial fibrillation after a cerebral ischemic event trial, among patients with recent cryptogenic stroke or transient ischemic attack, higher PAC count on 24‐hour Holter monitoring was a strong dose‐dependent predictor of AF on a subsequent 30‐day monitor. 6 These findings are consistent with the hypothesis that frequent SVE and AF may both be manifestations of underlying atrial myopathy or atrial failure, often characterized by impaired left atrial (LA) function and LA enlargement. 7 , 8 However, little or no information is available on the association of LA function and structure with extent of SVE. We hypothesized that greater impairment of LA function and greater LA volume are associated with more SVE. In the MESA (Multi‐Ethnic Study of Atherosclerosis), we examined these associations using data from cardiovascular magnetic resonance (CMR) imaging and from 14‐day ambulatory cardiac monitoring. We also examined associations of cardiovascular risk factors and race/ethnicity with extent of SVE.
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because of participant privacy issues. Investigators interested in analyzing MESA data may contact the MESA Coordinating Center at the University of Washington or use the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repositories Information Coordinating Center repository.
Study Sample
MESA is designed to investigate the pathogenesis of early cardiovascular disease and its progression; the study design has been described previously. 9 In 2000 to 2002, MESA enrolled 6814 participants, aged 45 to 84 years and free of clinically recognized cardiovascular disease, from 6 US communities. Participants self‐identified with 1 of 4 race/ethnic groups: Black participants (28%), White participants (38%), Hispanic participants (22%), and Asian participants, of Chinese descent (12%); 53% were women. After the initial baseline examination, there have been 5 follow‐up examinations.
CMR Imaging
At the 2010 to 2012 examination, consenting participants underwent CMR imaging using 1.5‐T scanners (Avanto and Espree [Siemens Medical Systems, Erlangen, Germany] and Signa LX [GE Healthcare, Waukesha, WI]) with a 6‐channel phased array coil, as previously described. 10 The cine images included coverage of the entire left ventricle (LV) and LA using short‐axis slices, 1 2‐chamber slice, and 1 4‐chamber view, scanned by steady‐state free precession sequences. LV mass and volumes were determined using CIM software version 6.2 (Auckland MRI Research Group, University of Auckland, Auckland, New Zealand). LA maximum and minimum volumes, total, active, and passive emptying fractions, and LA peak longitudinal strain were measured using Multimodality Tissue Tracking software version 6.0 (Toshiba, Japan) using 2‐ and 4‐chamber long‐axis images, as previously described. 11
Assessment of SVE
Approximately 6 years after the CMR imaging, during the 2016 to 2018 examination, we enrolled a subset of MESA participants (n=1557) in an ancillary study involving ambulatory electrocardiographic monitoring. Participants both with and without a history of heart disease or clinically recognized AF were included. Participants were not queried about a history of symptoms possibly related to arrhythmias and were not asked to record symptoms during the monitoring period. As previously described, 12 study staff applied an electrocardiographic monitoring device and asked the participant to wear it for 14 days and to return it by mail to the manufacturer for interpretation. A subset of 577 participants (37%) had 2 monitoring periods of up to 14 days each, with a median interval of 23 days between monitoring periods. The monitoring device used in this study was the Zio Patch XT (iRhythm Technologies, Inc, San Francisco, CA), a Food and Drug Administration–approved single‐channel electrocardiographic patch monitor capable of recording up to 14 days of cardiac rhythm. 13 Certified technicians at iRhythm processed and analyzed the electrocardiographic data. Reported arrhythmias were verified by the Epidemiological Cardiology Reading Center at Wake Forest University School of Medicine, Winston‐Salem, NC.
The SVE measures of interest were the average count of PACs/hour and the average number of runs per day (24 hours) of SVT, with a run defined as ≥4 consecutive PACs. For participants who wore 2 monitoring devices, the monitored time from both devices was included in quantifying SVE. The average count of PACs/hour was calculated as the sum of (the number of isolated PACs plus 2 times the number of couplets plus 3 times the number of triplets) divided by the total time during which the monitor provided a tracing adequate to determine cardiac rhythm.
Assessment of Participant Characteristics
Educational attainment, sex, and self‐identified race/ethnicity were ascertained at baseline. Other participant demographic and clinical characteristics were determined at the 2010 to 2012 examination. Height and weight were measured; smoking, current medications, and a physician diagnosis of hypertension were assessed by questionnaire. 9 Blood pressure was measured with the participant in a seated position and after a 5‐minute rest; serum glucose was measured in a fasting blood sample. Diabetes mellitus was defined by use of a diabetes mellitus medication or fasting glucose ≥126 mg/dL. The estimated glomerular filtration rate was calculated on the basis of serum creatinine using the 2012 Chronic Kidney Disease Epidemiology Collaboration equation. 14 NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) was measured using the Roche e411 special chemistry analyzer (Roche Diagnostics, Indianapolis, IN).
Cardiovascular Events During Follow‐Up
Since baseline, participants have been contacted by telephone every 9 to 12 months to identify new hospitalizations and medical diagnoses during follow‐up. Medical records were obtained; stroke, myocardial infarction, and heart failure were adjudicated by the MESA Morbidity and Mortality Committee. Clinically recognized AF, including AF and atrial flutter, was identified by an International Classification of Diseases, Ninth Revision (ICD‐9), code (427.31 or 427.32) or an International Classification of Diseases, Tenth Revision (ICD‐10), code (I48) in any position assigned at hospital discharge; by 12‐lead electrocardiographic at the 2010 to 2012 MESA examination; or, for those enrolled in fee‐for‐service Medicare, by an inpatient, outpatient, or physician claim with an AF ICD‐9 or ICD‐10 diagnosis code in any position. Cardiovascular events were ascertained up to the date of CMR imaging.
Statistical Analysis
Because LA and LV volumes and LV mass vary by body size and sex, we used an allometric approach to index these measures for height and weight by sex, as previously described. 15 , 16 An allometric method develops a model that describes how cardiac chamber measurements vary with body size. We identified a healthy subset of 192 participants with CMR at the 2010 to 2012 examination and free of obesity, hypertension, diabetes mellitus, impaired fasting glucose, stroke, myocardial infarction, heart failure, and AF. In these participants, we developed regression models that regressed each log‐transformed CMR volume or mass on log (height), log (weight), and sex. These models give an indexed value for the CMR measure (ie, the percentage of the value predicted on the basis of height, weight, and sex). For example, if the indexed LA volume is 125%, this indicates that the participant's measured LA volume is 125% of that predicted for height, weight, and sex. The derived indexes are presented in Table S1.
In unadjusted analyses, we examined distributions of sociodemographic characteristics and CMR characteristics by quantiles of the SVE measures. Because of their skewed distributions (Figure S1), we applied log transformation to the outcome variables of average PACs/hour and average runs of SVT/day. Using multivariable linear regression with robust SE estimation, 17 we examined associations of participant demographic, clinical, and CMR characteristics with log (PACs/hour) and with log (runs of SVT/day). One participant had no PACs at all, and 14% (N=164) had no runs of SVT. To allow inclusion of those participants, before log transformation, we added the smallest observed value of PACs/hour (0.017) or runs of SVT/day (0.036) to the calculated value for all participants.
Adjusted associations are expressed as the ratio of geometric means, which provides the percentage difference in, for example, the average PACs/hour, per increment of the characteristic. For characteristics that are continuous variables, the increment was ≈1 SD, except for NT‐proBNP, for which the increment was a doubling of the level. In analyses of LA strain and ejection fraction, models were adjusted for age, sex, race, height, and weight, and for the clinical variables of diabetes mellitus, current smoking, use of antihypertensive medication, systolic blood pressure, NT‐proBNP, estimated glomerular filtration rate, history of clinically recognized AF, and LV mass and end‐diastolic volume. Analyses of LA volumes and mass, which were already indexed for height and weight by sex, were adjusted for age, race, and the same list of clinical variables as above. Missing values for smoking (n=2), diabetes mellitus (n=3), glomerular filtration rate (n=4), and NT‐proBNP (n=15) were estimated using multiple imputation by chained equations. 18
In sensitivity analyses, we examined whether associations differed: (1) with additional adjustment for a history of stroke, myocardial infarction, or heart failure before CMR imaging; (2) in analyses restricted to those with no history of clinically recognized AF, stroke, myocardial infarction, or heart failure; (3) with additional adjustment for educational attainment; (4) with cardiac chamber volumes and LV mass indexed to body surface area rather than the newly derived allometric indexes; and (5) after excluding users of sympathomimetic or dopaminergic agents (N=49 excluded). We also examined models of CMR measures and PAC count fit with cubic splines to check for nonlinearity. We used Stata 14 software (StataCorp, College Station, TX) for the analysis. A 2‐sided P≤0.05 was considered statistically significant.
Approval for the study was obtained from the institutional review board on human research at the University of Washington and at each participating institution; all participants provided written informed consent. The monitoring devices were purchased for the study, and the device manufacturer had no role in the study design, statistical analysis, or interpretation of results. SRH and PNJ had full access to all the data in the study and take responsibility for their integrity and the data analysis.
RESULTS
A total of 1557 MESA participants underwent electrocardiographic monitoring. We excluded 22 participants who were monitored for <24 hours, 49 with continuous AF throughout the monitoring period (because PACs and runs of SVT are not assessed while the rhythm is AF), and 6 with paced beats (Figure 1). We further excluded 332 participants without CMR imaging, leaving 1148 participants available for analysis. The characteristics at the 2016 to 2018 examination of MESA participants included and not included in the present analysis were similar (Table S2). The median (interquartile range) duration of analyzable time on the electrocardiographic monitor(s) was 14.0 (13.6–26.6) days. The Spearman rank correlation coefficient between log‐transformed average PACs/hour and log‐transformed average runs of SVT/day was 0.59 (Figure S2).
Figure 1. Study participation and inclusion in analysis.
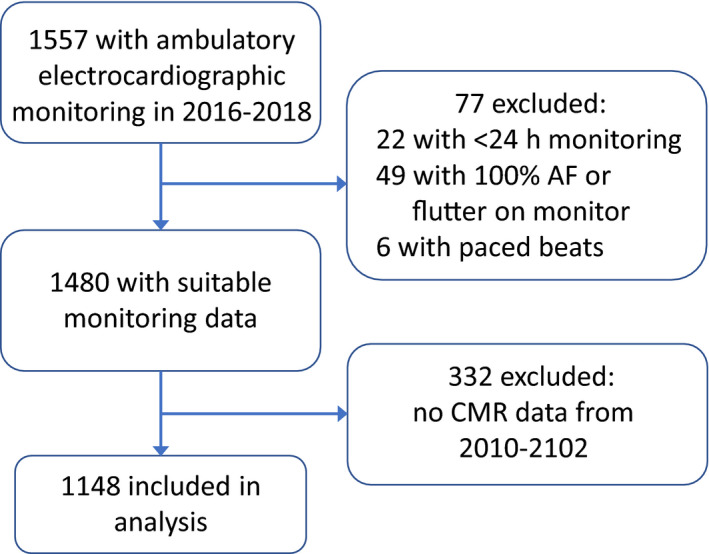
AF indicates atrial fibrillation; CMR, cardiovascular magnetic resonance.
Unadjusted and Adjusted Associations With Average PACs/Hour
In unadjusted analyses, average PACs/hour were greater in those with advanced age, male sex, White race, antihypertensive medication use, higher systolic blood pressure, higher NT‐proBNP, lower estimated glomerular filtration rate, and a history of clinically recognized AF (Table 1). CMR measures associated with greater average PACs/hour included lower LA peak longitudinal strain; lower total, passive, and active emptying fraction; larger LA maximum and minimum volumes; greater LV mass; and larger LV end‐diastolic volume (Table 1).
Table 1.
Demographic, Clinical, and CMR Imaging Characteristics of 1148 Participants by Quartiles of the Distribution of Average PACs/Hour
| Characteristic | All Participants (n=1148)* | Average PACs/h | |||
|---|---|---|---|---|---|
| 0–1.40 (n=287) | 1.41–4.25 (n=286) | 4.28–20.6 (n=286) | 20.8–1482 (n=289) | ||
| Age, mean (SD), y | 67 (8) | 62 (7) | 65 (7) | 69 (9) | 71 (8) |
| Men, % | 47 | 42 | 45 | 47 | 56 |
| Race/ethnicity, % | |||||
| White | 43 | 33 | 46 | 47 | 45 |
| Chinese | 14 | 20 | 13 | 12 | 11 |
| Black | 24 | 22 | 21 | 22 | 29 |
| Hispanic | 20 | 25 | 21 | 19 | 15 |
| BMI, mean (SD), kg/m2 | 28 (5) | 28 (5) | 28 (5) | 28 (5) | 28 (5) |
| Diabetes mellitus, % | 15 | 14 | 18 | 13 | 16 |
| Current smoking, % | 7 | 7 | 10 | 6 | 6 |
| Antihypertensive medication, % | 48 | 41 | 43 | 50 | 56 |
| Systolic BP, mean (SD), mm Hg | 120 (18) | 118 (17) | 118 (17) | 122 (20) | 123 (20) |
| NT‐proBNP, mean (SD), pg/mL | 102 (138) | 61 (63) | 83 (93) | 129 (155) | 134 (190) |
| eGFR, mean (SD), mL/min per 1.73 m2 | 82 (20) | 86 (19) | 81 (19) | 81 (19) | 79 (21) |
| History of clinical AF, % | 3 | 1 | 2 | 2 | 7 |
| Left atrial function, mean (SD), % | |||||
| Peak longitudinal strain | 33 (14) | 35 (13) | 35 (14) | 33 (14) | 31 (14) |
| Total emptying fraction | 56 (11) | 59 (9) | 58 (10) | 56 (11) | 53 (11) |
| Passive emptying fraction | 25 (8) | 27 (8) | 25 (8) | 24 (8) | 22 (8) |
| Active emptying fraction | 43 (11) | 44 (10) | 44 (11) | 42 (12) | 41 (11) |
| Left atrial structure, mean (SD), mL | |||||
| Maximum volume | 63 (20) | 60 (18) | 62 (21) | 65 (22) | 67 (21) |
| Minimum volume | 28 (13) | 25 (11) | 27 (13) | 30 (14) | 32 (14) |
| Left ventricular structure, mean (SD) | |||||
| Mass, g | 122 (33) | 117 (29) | 121 (34) | 121 (35) | 128 (34) |
| End‐diastolic volume, mL | 121 (30) | 118 (27) | 121 (30) | 121 (31) | 123 (30) |
AF indicates atrial fibrillation; BMI, body mass index; BP, blood pressure; CMR, cardiovascular magnetic resonance; eGFR, estimated glomerular filtration rate; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and PAC, premature atrial contraction.
Total number of participants included in the analysis ranged from 1091 to 1113 for measures of left atrial function and structure, and was 1147 for measures of left ventricular structure.
In multivariable analyses adjusted only for demographic characteristics, advanced age and male sex were associated with more PACs/hour (Table 2, model 1). Relative to White race, Chinese and Hispanic race/ethnicity were each associated with fewer PACs/hour. After further adjustment for clinical characteristics (Table 2, model 2), each 10‐year increment in age was associated with a 93% greater average count of PACs/hour, male sex was associated with 61% more PACs/hour, and Chinese and Hispanic race/ethnicity remained associated with fewer PACs/hour (31% and 38% fewer, respectively). The association of Black race with PACs/hour did not differ from that of White race. Among the clinical characteristics examined, higher NT‐proBNP was associated with more PACs/hour (27% more per 2‐fold increment), and a history of clinical AF was associated with 122% more PACs/hour.
Table 2.
Association of Demographic and Clinical Characteristics With Average PACs/Hour in 1148 Participants From Multivariable Linear Regression
| Characteristic | Model 1* | Model 2 † | ||
|---|---|---|---|---|
| Ratio of Geometric Means | 95% CI | Ratio of Geometric Means | 95% CI | |
| Age, per 10 y | 2.23 | 1.99–2.49 | 1.93 | 1.66–2.23 |
| Men vs women | 1.41 | 1.16–1.73 | 1.61 | 1.32–1.97 |
| Race/ethnicity | ||||
| White | Reference | Reference | ||
| Chinese | 0.57 | 0.43–0.77 | 0.69 | 0.51–0.92 |
| Black | 1.07 | 0.82–1.4 | 1.09 | 0.83–1.43 |
| Hispanic | 0.60 | 0.46–0.77 | 0.62 | 0.48–0.81 |
| BMI, per 5 kg/m2 | … | … | 1.10 | 0.98–1.22 |
| Diabetes mellitus | … | … | 0.91 | 0.69–1.21 |
| Current smoking | … | … | 1.06 | 0.73–1.52 |
| Antihypertensive medication | … | … | 1.06 | 0.86–1.31 |
| Systolic BP, per 20 mm Hg | … | … | 1.10 | 0.98–1.23 |
| NT‐proBNP, per 2‐fold increment | … | … | 1.27 | 1.16–1.38 |
| eGFR, per 20 mL/min per 1.73 m2 | … | … | 1.16 | 1.00–1.33 |
| History of clinical AF | … | … | 2.22 | 1.17–4.25 |
AF indicates atrial fibrillation; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and PAC, premature atrial contraction.
Model 1 includes only sociodemographic variables: age, sex, and race/ethnicity.
Model 2 includes sociodemographic variables and all clinical variables in the left column.
We examined each LA CMR measure in a multivariable model adjusted for sociodemographic and clinical characteristics, LV mass, LV end‐diastolic volume, and monitoring duration. Greater LA total, passive, and active emptying fractions were each associated with fewer PACs/hour. Larger LA minimum volume was associated with more PACs/hour (Figure 2 and Table S3). In these models, the association of Black race with PACs/hour did not differ from that of White race. Taller height was only weakly associated with more PACs/hour in models including indexed LA volumes and was unassociated with PACs/hour in models including LA function measures.
Figure 2. Adjusted percentage difference in average premature atrial contractions (PACs)/hour associated with left atrial (LA) function and volume from multivariable linear regression.

*Adjusted for weight, height, sex, age, race/ethnicity, diabetes mellitus, current smoking, antihypertensive medication use, systolic blood pressure, estimated glomerular filtration rate, NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), history of clinically detected atrial fibrillation, left ventricular mass and end‐diastolic volume, and monitoring duration. †The SDs of strain and each of the emptying fractions were ≈10%. ‡LA volume was indexed for height and weight by sex. A difference in indexed LA volume of 25% indicates a measured LA volume that is 25% larger than predicted for height, weight, and sex. The SD of both maximum and minimum indexed LA volumes was ≈25%.
Unadjusted and Adjusted Associations With Average Runs of SVT/Day
In unadjusted analyses, differences in the distribution of runs of SVT/day by sociodemographic and clinical characteristics were similar to those observed for average PACs/hour. However, there was little difference in CMR measures of LA function or structure by categories of runs of SVT/day (Table S4). In multivariable analyses of sociodemographic and clinical characteristics, associations with runs of SVT/day were also similar to those for PACs/hour (Table S5), except that no association was observed for male sex, and Black race was associated with fewer runs of SVT/day. In multivariable models for each CMR characteristic, adjusted for demographic and clinical characteristics, none of the LA or LV function or structure measures was significantly associated with runs of SVT/day (Table S6). In the models that included LA function or structure measures, Black race remained associated with significantly fewer runs of SVT/day than White race.
Sensitivity Analyses
The associations of CMR measures with SVE were little changed by adjustment for history of a cardiovascular event or in analyses restricted to those without a history of a cardiovascular event. Results were not materially changed after further adjustment for educational attainment, when chamber volumes were indexed to body surface area rather than the newly derived allometric indexes, or after exclusion of participants who reported use of sympathomimetic or dopaminergic agents. Examination of models fit with cubic splines did not provide evidence of departure from linearity in models examining CMR measures in relation to PAC frequency.
DISCUSSION
In the MESA cohort, impaired LA emptying fraction and larger LA volume by CMR were associated with more frequent PACs on ambulatory cardiac monitoring, both before and after adjustment for sociodemographic and clinical characteristics and for LV function and structure. We did not observe associations of LA function and structure with more frequent runs of SVT after adjustment for sociodemographic and clinical characteristics.
Our findings that greater age, higher NT‐proBNP, and history of clinically detected AF were associated with more PACs/hour are in agreement with findings from a Swiss population studied with 24‐hour Holter monitoring. 1 Also like the Swiss study, we found no association of body mass index, diabetes mellitus, or hypertension with PACs/hour in a multivariable model.
The present analysis, conducted in a multiethnic population, reveals that Chinese and Hispanic participants had fewer PACs on average than White participants, both before and after adjustment for clinical characteristics. Notably, average PACs/hour did not differ in Black compared with White participants, and this finding was unchanged in models that included the LA function and structure measures. Inconsistent findings have been reported from other studies that compared PAC frequency in White versus Black participants. In the CHS (Cardiovascular Health Study), baseline 12‐lead electrocardiographics revealed more PACs in Black participants, 19 but 24‐hour Holter monitoring 2 to 5 years after baseline showed more PACs in White participants. 20 In the ARIC (Atherosclerosis Risk in Communities) study, no difference in PACs between White and Black participants was found on 2‐minute rhythm strip recordings. 21 The reasons for these inconsistent findings are not clear; greatly differing durations of recording and different methods for identifying and verifying PACs may be partly responsible. The lack of difference in PAC frequency between White and Black MESA participants is, however, consistent with our finding of no difference in monitor‐detected AF in these same individuals. 22 We are not aware of other reports on extent of runs of SVT in Chinese, Black, or Hispanic participants. Additional investigation is needed into the reasons for differences in the association of race/ethnicity with the 2 different measures of SVE studied herein.
Results from several general population studies, including MESA, indicate that larger LA size, impaired LA function, 23 , 24 and progression in both measures over time 25 are associated with the development of incident AF. LA enlargement and impaired LA function are also associated with risk of ischemic stroke and transient ischemic attack, independent of AF. 26 These findings, taken together with the results of the present analysis, provide support for the hypothesis that LA enlargement, impaired LA function, and more frequent PACs are all manifestations of atrial remodeling that provide a substrate for AF, promote atrial ectopy that may initiate AF, and may establish an environment favoring formation of thrombi capable of producing ischemic cerebrovascular disease.
Strengths of our analysis include the large multiethnic study population, the careful measurement of cardiac function and structure by CMR, and the extended measurement of SVE through ambulatory cardiac monitoring. A limitation of the analysis is the inability to determine cause and effect from an observational study. In addition, although the assessment of LA function and structure preceded the assessment of atrial ectopy by about 6 years, we cannot be certain that the functional and structural abnormalities preceded the development of frequent atrial ectopy. Finally, LA enlargement and functional impairment may have progressed in some participants during the 6‐year interval, such that the relationship between CMR measures and the extent of ectopy might be different if they had been measured contemporaneously.
CONCLUSIONS
The findings of our study indicate that impaired LA function and LA enlargement are associated with more frequent PACs. Additional research will be needed to establish the clinical utility of LA CMR imaging and measurement of PAC frequency as potential indicators of the risk of cerebrovascular events.
Sources of Funding
This work was supported by National Institutes of Health (NIH) contracts 75N92020D00001, HHSN268201500003I, N01‐HC‐95159, 75N92020D00005, N01‐HC‐95160, 75N92020D00002, N01‐HC‐95161, 75N92020D00003, N01‐HC‐95162, 75N92020D00006, N01‐HC‐95163, 75N92020D00004, N01‐HC‐95164, 75N92020D00007, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 and grants R01 HL127659, UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420. The research reported herein is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding agencies had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Disclosures
None.
Supporting information
Tables S1–S6
Figures S1–S2
(J Am Heart Assoc. 2021;10:e018093. DOI: 10.1161/JAHA.120.018093.)
For Sources of Funding and Disclosures, see page 7.
References
- 1. Conen D, Adam M, Roche F, Barthelemy J‐C, Felber Dietrich D, Imboden M, Künzli N, von Eckardstein A, Regenass S, Hornemann T, et al. Premature atrial contractions in the general population: frequency and risk factors. Circulation. 2012;126:2302–2308. DOI: 10.1161/CIRCULATIONAHA.112.112300. [DOI] [PubMed] [Google Scholar]
- 2. Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, Cook J, Paraschos A, Love J, Radoslovich G, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107:1614–1619. DOI: 10.1161/01.CIR.0000057981.70380.45. [DOI] [PubMed] [Google Scholar]
- 3. Kamel H, Elkind MS, Bhave PD, Navi BB, Okin PM, Iadecola C, Devereux RB, Fink ME. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke. 2013;44:1550–1554. DOI: 10.1161/STROKEAHA.113.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med. 2013;159:721–728. DOI: 10.7326/0003-4819-159-11-201312030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Larsen BS, Kumarathurai P, Falkenberg J, Nielsen OW, Sajadieh A. Excessive atrial ectopy and short atrial runs increase the risk of stroke beyond incident atrial fibrillation. J Am Coll Cardiol. 2015;66:232–241. [DOI] [PubMed] [Google Scholar]
- 6. Gladstone DJ, Dorian P, Spring M, Panzov V, Mamdani M, Healey JS, Thorpe KE; for the EMBRACE Steering Committee and Investigators . Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke. 2015;46:936–941. DOI: 10.1161/STROKEAHA.115.008714. [DOI] [PubMed] [Google Scholar]
- 7. Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47:895–900.DOI: 10.1161/STROKEAHA.115.012004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bisbal F, Baranchuk A, Braunwald E, Bayes de Luna A, Bayes‐Genis A. Atrial failure as a clinical entity: JACC review topic of the week. J Am Coll Cardiol. 2020;75:222–232. DOI: 10.1016/j.jacc.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 9. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, et al. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881.DOI: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10. Liu CY, Lai S, Kawel‐Boehm N, Chahal H, Ambale‐Venkatesh B, Lima JAC, Bluemke DA. Healthy aging of the left ventricle in relationship to cardiovascular risk factors: the Multi‐Ethnic Study of Atherosclerosis (MESA). PLoS One. 2017;12:e0179947. DOI: 10.1371/journal.pone.0179947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zareian M, Ciuffo L, Habibi M, Opdahl A, Chamera EH, Wu CO, Bluemke DA, Lima JA, Venkatesh BA. Left atrial structure and functional quantitation using cardiovascular magnetic resonance and multimodality tissue tracking: validation and reproducibility assessment. J Cardiovasc Magn Reson. 2015;17:52. DOI: 10.1186/s12968-015-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heckbert SR, Austin TR, Jensen PN, Floyd JS, Psaty BM, Soliman EZ, Kronmal RA. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: the Multi‐Ethnic Study of Atherosclerosis. J Electrocardiol. 2018;51:997–1002. DOI: 10.1016/j.jelectrocard.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang MJ, Roetker NS, Folsom AR, Alonso A, Heckbert SR, Chen LY. Feasibility of using a leadless patch monitor in community cohort studies: the Multi‐Ethnic Study of Atherosclerosis. Pacing Clin Electrophysiol. 2018;41:1389–1390.DOI: 10.1111/pace.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29.DOI: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dewey FE, Rosenthal D, Murphy DJ Jr, Froelicher VF, Ashley EA. Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation. 2008;117:2279–2287. DOI: 10.1161/CIRCULATIONAHA.107.736785. [DOI] [PubMed] [Google Scholar]
- 16. Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. DOI: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. White H. Heteroskedasticity‐consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- 18. Carlin JB, Galati JC, Royston P. A new framework for managing and analyzing multiply imputed data in Stata. Stata J. 2008;8:49–67. DOI: 10.1177/1536867X0800800104. [DOI] [Google Scholar]
- 19. Nguyen KT, Vittinghoff E, Dewland TA, Dukes JW, Soliman EZ, Stein PK, Gottdiener JS, Alonso A, Chen LY, Psaty BM, et al. Ectopy on a single 12‐lead ECG, incident cardiac myopathy, and death in the community. J Am Heart Assoc. 2017;6:e006028. DOI: 10.1161/JAHA.1117.006028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Christensen MA, Nguyen KT, Stein PK, Fohtung RB, Soliman EZ, Dewland TA, Vittinghoff E, Psaty BM, Heckbert SR, Marcus GM. Atrial ectopy as a mediator of the association between race and atrial fibrillation. Heart Rhythm. 2017;14:1856–1861. DOI: 10.1016/j.hrthm.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ofoma U, He F, Shaffer ML, Naccarelli GV, Liao D. Premature cardiac contractions and risk of incident ischemic stroke. J Am Heart Assoc. 2012;1:e002519. DOI: 10.1161/JAHA.112.002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heckbert SR, Austin TR, Jensen PN, Chen LY, Post WS, Floyd JS, Soliman EZ, Kronmal RA, Psaty BM. Differences by race/ethnicity in the prevalence of clinically detected and monitor‐detected atrial fibrillation: MESA. Circ Arrhythm Electrophysiol. 2020;13:e007698. DOI: 10.1161/CIRCEP.119.007698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Habibi M, Samiei S, Ambale Venkatesh B, Opdahl A, Helle‐Valle TM, Zareian M, Almeida AL, Choi EY, Wu C, Alonso A, et al. Cardiac magnetic resonance‐measured left atrial volume and function and incident atrial fibrillation: results from MESA (Multi‐Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging. 2016;9:e004299. DOI: 10.1161/CIRCIMAGING.115.004299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sardana M, Lessard D, Tsao CW, Parikh NI, Barton BA, Nah G, Thomas RC, Cheng S, Schiller NB, Aragam JR, et al. Association of left atrial function index with atrial fibrillation and cardiovascular disease: the Framingham Offspring Study. J Am Heart Assoc. 2018;7:e008435. DOI: 10.1161/JAHA.1117.008435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lim DJ, Ambale‐Ventakesh B, Ostovaneh MR, Zghaib T, Ashikaga H, Wu C, Watson KE, Hughes T, Shea S, Heckbert SR, et al. Change in left atrial function predicts incident atrial fibrillation: the Multi‐Ethnic Study of Atherosclerosis. Eur Heart J Cardiovasc Imaging. 2019;20:979–987. DOI: 10.1093/ehjci/jez176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Habibi M, Zareian M, Ambale Venkatesh B, Samiei S, Imai M, Wu C, Launer LJ, Shea S, Gottesman RF, Heckbert SR, et al. Left atrial mechanical function and incident ischemic cerebrovascular events independent of AF: insights from the MESA study. JACC Cardiovasc Imaging. 2019;12:2417–2427. DOI: 10.1016/j.jcmg.2019.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figures S1–S2


