Abstract
Background
Anti‐Sjögren's syndrome‐related antigen A‐antibodies (anti‐Ro/SSA‐antibodies) are responsible for a novel form of acquired long‐QT syndrome, owing to autoimmune‐mediated inhibition of cardiac human ether‐a‐go‐go‐related gene‐potassium channels. However, current evidence derives only from basic mechanistic studies and relatively small sample‐size clinical investigations. Hence, the aim of our study is to estimate the risk of QTc prolongation associated with the presence of anti‐Ro/SSA‐antibodies in a large population of unselected subjects.
Methods and Results
This is a retrospective observational cohort study using the Veterans Affairs Informatics and Computing Infrastructure. Participants were veterans who were tested for anti‐Ro/SSA status and had an ECG. Descriptive statistics and univariate and multivariate logistic regression analyses were performed to identify risk factors for heart rate‐corrected QT interval (QTc) prolongation. The study population consisted of 7339 subjects (61.4±12.2 years), 612 of whom were anti‐Ro/SSA‐positive (8.3%). Subjects who were anti‐Ro/SSA‐positive showed an increased prevalence of QTc prolongation, in the presence of other concomitant risk factors (crude odds ratios [OR], 1.67 [1.26–2.21] for QTc >470/480 ms; 2.32 [1.54–3.49] for QTc >490 ms; 2.77 [1.66–4.60] for QTc >500 ms), independent of a connective tissue disease history. Adjustments for age, sex, electrolytes, cardiovascular risk factors/diseases, and medications gradually attenuated QTc prolongation estimates, particularly when QT‐prolonging drugs were added to the model. Nevertheless, stepwise‐fully adjusted OR for the higher cutoffs remained significantly increased in anti‐Ro/SSA‐positive subjects, particularly for QTc >500 ms (2.27 [1.34–3.87]).
Conclusions
Anti‐Ro/SSA‐antibody positivity was independently associated with an increased risk of marked QTc prolongation in a large cohort of US veterans. Our data suggest that within the general population individuals who are anti‐Ro/SSA‐positive may represent a subgroup of patients particularly predisposed to ventricular arrhythmias/sudden cardiac death.
Keywords: anti‐Ro/SSA, connective tissue diseases, general population, QTc prolongation, sudden death risk
Subject Categories: Arrhythmias, Biomarkers, Sudden Cardiac Death
Nonstandard Abbreviations and Acronyms
- Anti‐Ro/SSA‐antibodies
anti‐Sjögren's syndrome‐related antigen A‐antibodies
- CTD
connective tissue disease
- hERG
human ether‐a‐go‐go‐related gene
- IHD
ischemic heart disease
- LQTS
long QT‐syndrome
- QTc
heart rate‐corrected QT interval
- SCD
sudden cardiac death
- TdP
torsades de pointes
- VA
ventricular arrhythmia
Clinical Perspective
What Is New?
In a large cohort of US veterans, subjects who were anti‐Sjögren's syndrome‐related antigen A‐antibodies positive (anti‐Ro/SSA), independent of a history of connective tissue disease, showed an increased prevalence of heart rate‐corrected QT interval prolongation, in the presence of other concomitant risk factors.
Circulating anti‐Ro/SSA‐antibodies were independently associated with a ≈2‐times higher risk of marked QTc prolongation.
Anti‐Ro/SSA positivity was a significant contributor to marked heart rate‐corrected QT interval prolongation occurrence, even representing one of the most important risk factors involved; a synergy between anti‐Ro/SSA‐antibodies and most of the traditional risk factors was demonstrated.
What Are the Clinical Implications?
Our data suggest that within the general population subjects who are anti‐Ro/SSA‐positive may represent a subgroup with an increased predisposition to life‐threatening ventricular arrhythmias, particularly when other QT‐prolonging risk factors are concomitantly present.
It is proposed that subjects who are anti‐Ro/SSA‐positive should receive counseling about drugs and management of other risk factors that may increase the risk for malignant arrhythmias; at the same time, specific anti‐Ro/SSA testing should be considered in patients with “idiopathic” rhythm disturbances, as it could intriguingly open novel avenues in antiarrhythmic treatment, primarily immunomodulatory therapies.
The QT‐interval on the ECG reflects the duration of the ventricular action potential, in turn generated by transmembrane flow of ions, mainly inward depolarizing currents through sodium and calcium channels, and outward repolarizing currents through potassium channels. 1 Heart rate‐corrected QT‐interval (QTc) prolongation, currently defined as QTc >470 ms for men and 480 ms for women, 2 is a well‐recognized risk factor for arrhythmic events and sudden cardiac death (SCD) in the general population. 3 Specifically, the term long‐QT syndrome (LQTS) designates a clinical condition characterized by the presence of a prolonged QTc, and an increased predisposition to develop life‐threatening ventricular arrhythmias (VAs), particularly torsades de pointes (TdP). 2 , 4 Notably, the more the QTc prolongs, the greater the TdP risk, becoming significantly high when QTc >500 ms. 2
Although LQTS may be congenital or acquired, inherited forms are relatively rare (≈1:2000 apparently healthy newborns). 5 Conversely, acquired LQTS is rather common, most frequently associated with electrolyte imbalances, medications, and structural heart diseases. 6 Other well‐recognized risk factors for acquired LQTS/TdP include female sex, older age, and bradycardia. 2 It should be noted that in most cases multiple simultaneous risk factors need to be present for TdP occurrence or to develop QTc prolongation. 2 , 7 Because numerous often‐redundant ion channel mechanisms are implicated in preserving the normal action potential, multiple QT‐prolonging factors are required to significantly disrupt ventricular repolarization (multihit theory). 7
Immune‐inflammatory activation is increasingly recognized as an emerging risk factor for QTc prolongation and TdP. 7 , 8 , 9 , 10 In particular, recent evidence demonstrates that anti‐Ro/SSA‐antibodies are responsible for a novel form of acquired LQTS because of an autoimmune‐mediated inhibition of cardiac human‐ether‐à‐go‐go‐related gene (hERG)‐potassium channels. 8 , 11 , 12
Anti‐Ro/SSA‐antibodies, including anti‐Ro/SSA‐52 kDa and anti‐Ro/SSA‐60 kDa subtypes, are frequently detected in patients with connective tissue diseases (CTD). 13 Several studies provided evidence that in these patients, and their children, anti‐Ro/SSA‐antibodies (specifically anti‐Ro/SSA‐52 kDa) can promote LQTS 14 , 15 , 16 , 17 , 18 and VAs 19 , 20 , 21 by blocking the hERG‐potassium current. 11 Such a block is caused by the anti‐Ro/SSA‐antibody direct binding to the hERG‐potassium pore region, acutely interfering with gating properties and chronically resulting in reduced expression of the channel. 11 , 12 , 22
Notably, anti‐Ro/SSA‐antibodies are also detected in a significant percentage of apparently healthy subjects (0.5–2.7%) 23 , 24 , 25 , 26 and are associated with TdP occurrence regardless of the presence or absence of a manifest CTD. 22 , 27 In fact, in a cohort of 25 consecutive patients with TdP prospectively collected from the general population, anti‐Ro/SSA‐52 kDa positivity was found in 60% of cases, always in the absence of a concomitant CTD. 22 These findings intriguingly suggest that anti‐Ro/SSA‐antibodies may be also silently involved in promoting VAs and SCD in a percentage of subjects of the general population.
Although these data point to anti‐Ro/SSA‐antibodies as a novel cause of LQTS/TdP, current evidence derives only from basic experimental studies and clinical investigations involving relatively small samples of patients. Moreover, no population data are presently available on the percentage of carriers of anti‐Ro/SSA who actually manifest the LQTS and thereby are at increased risk of TdP and SCD when other QT‐prolonging risk factors are concomitantly present.
In order to address this gap in knowledge, we undertook an observational cohort study using US Veterans Affairs Informatics and Computing Infrastructure to estimate the risk of QTc prolongation associated with the presence of anti‐Ro/SSA‐antibodies in a large population of unselected subjects.
METHODS
The authors declare that all supporting data are available within the article (and its supplementary files).
Study Design and Setting
This is a retrospective cross‐sectional study using Veterans Affairs Informatics and Computing Infrastructure, which provides a secure, central analytic platform for performing research using data collected from veterans at 1400 points of care. Veterans Affairs Informatics and Computing Infrastructure Clinical Data Warehouse, which is a relational database, was interrogated using structured query language. Logical Observation Identifiers Names and Codes were used to search for all patients who have been tested for anti‐Ro/SSA‐antibodies and who had at least 1 ECG from October 1999 until December 2016. Using a unique patient identifier, we obtained the following data: demographic data, laboratory data including anti‐Ro/SSA status, electrolytes, thyrotropin, C‐reactive protein, medications, diagnoses, and ECG. QTc measurement was automated using the Bazett's formula (QT/RR interval1/2) and confirmed by a cardiologist who was independent of the study and blinded to anti‐Ro/SSA status. Patient‐related QTc and laboratory values were computed by averaging multiple available measurements (Table S1).
Participants
We included all subjects who were tested for anti‐Ro/SSA‐antibodies and underwent ECG. Exclusion criteria were complete bundle branch block (QRS interval >120 ms), ventricular paced rhythm, atrial fibrillation or flutter, and profound brady‐ and tachycardia (<50 bpm and >120 bpm, respectively).
Anti‐Ro/SSA‐Antibody Status
The presence of total circulating anti‐Ro/SSA‐antibodies was assessed by an immune‐enzymatic method or by immunoblotting. In the large majority of the cases, the results were provided qualitatively (positive/negative), whereas a quantitative result was available in only few subjects (4%). Moreover, the cutoff level used was not always the same, depending on the single point of care. For this reason, the subjects were classified in a categorical manner as anti‐Ro/SSA‐positive or ‐negative based on the specific reference cutoff used for each anti‐Ro/SSA determination.
Study Objectives
The primary study objective was to estimate the risk of QTc prolongation associated with circulating anti‐Ro/SSA‐antibodies. The secondary study objective was to investigate the sinergy between anti‐Ro/SSA‐antibodies and other concomitant risk factors in prolonging QTc.
Data Analyses
The definition of QTc prolongation was based on current recommendations by the American Heart Association/American College of Cardiology. In particular, we employed 3 different, progressively more stringent cutoffs: (1) >470 ms in men or >480 in women, representing ≈99th percentile QTc values for otherwise healthy postpubertal individuals (American Heart Association/American College of Cardiology: abnormal QTc prolongation); (2) >490 ms; and (3) >500 ms, representing a marker of significant arrhythmic risk for both sexes (American Heart Association/American College of Cardiology: marked or highly abnormal QTc prolongation). 2
Statistical Analysis
Descriptive statistics were computed, including mean and SD for quantitative variables, frequency count and percentage for qualitative variables. Kolmogorov‐Smirnov test was applied to verify normality distribution of the quantitative variables, that is, age, electrolytes (potassium, calcium, magnesium), thyrotropin, and C‐reactive protein. When normality was accepted/refused, Student t or Mann‐Whitney tests were respectively used to compare groups.
Fisher exact test was applied to 2×2 contingency tables to evaluate the association between frequencies of qualitative variables; Mantel‐Hantzel chi‐square test for trend was used for the association of dichotomous and ordinal variables (2×n tables). When the association was between a binary risk factor and a long QTc condition (identified by a QTc cutoff value), the odds ratio (OR) and its 95% CI were also evaluated.
Multivariate stepwise logistic regression analysis was carried out to identify, among all risk factors included in the model (independent variables), a statistically significant minimum subset of factors with the highest possible accuracy to predict QTc interval. In the stepwise process, 1 independent variable was added to or removed from the discriminant model at each step based on the criterion of maximum likelihood ratio. The process stops when no more statistical significant variables can be entered or removed. OR was calculated for significant variables. Statistical significance of OR was assessed by evaluating the 95% CI of sample estimates. To minimize the impact of confounding factors on the relationship between anti‐Ro/SSA‐positivity and QTc prolongation, a subgroup of the overall cohort was selected by the nearest neighbor matching procedure based on propensity score. Specifically, the variables used for propensity score calculation were age, sex, potassium, calcium, history of tobacco use, diabetes mellitus, ischemic heart disease (IHD), anesthetics, antiarrhythmics, antibiotics, antidepressants, antiemetics, antihistaminics, antipsychotics, bronchodilators, cholinesterase inhibitors, diuretics, H2‐histamine receptor antagonists, inotropes, proton pump inhibitors, and vasoconstrictors. As a result, for each subject who was anti‐Ro/SSA‐positive, 3 subjects who were anti‐Ro/SSA‐negative were selected, in order to obtain a significant sample size and at the same time maintain satisfactory patient matching. A balance check of the matched cohorts was performed by statistically evaluating for each variable the significance of the difference, as well as the changes in the standardized mean difference before and after matching. A statistical significance level of 95% (P<0.05) was always considered. SPSS software, release 10, was used for all statistical computations.
Ethics
The data were de‐identified and the study was approved by the Veterans Affairs New York Harbor Healthcare System Institutional Review Board under ID#01443.
RESULTS
Characteristics of the Overall Cohort
We found 8522 subjects in the database who were tested for anti‐Ro/SSA status and underwent ECG. However, 1183 were excluded for the presence of complete bundle brunch block, ventricular paced rhythm, profound brady‐ or tachycardia, or atrial fibrillation/flutter patterns. As a result, 7339 subjects were eventually included in the study, with a mean age of 61.4±12.2 years. Most of them were male (79.1%), and White (69.3%). In the whole population, 612 individuals were anti‐Ro/SSA‐positive (8.3%), and 6727 tested negative (Table 1). Depending on the specific cutoff used, QTc prolongation was found in 6.5% (QTc >470 [men]/480 [women] ms; n=479), 2.3% (QTc >490 ms; n=170), and 1.3% (QTc >500 ms; n=96) of subjects, respectively (Table 1).
Table 1.
Demographic and Health Characteristics of the Overall Cohort
| Characteristic | |
|---|---|
| Total, n | 7339 |
| Demography | |
| Age, y | 61.4±12.2 |
| Men, n | 5803 (79.1%) |
| White, n | 5084 (69.3%) |
| Black, n | 1277 (17.4%) |
| Others/unknown, n | 978 (13.3%) |
| Body mass index | 29.5±6.2 |
| ECG findings | |
| QTc, ms | 431.3±25.9 |
| QTc >470 men/480 women | 479 (6.5%) |
| QTc >490 ms, n | 170 (2.3%) |
| QTc >500 ms, n | 96 (1.3%) |
| Laboratory data | |
| Anti‐Sjögren's syndrome‐related antigen A‐antibodies positive | 612 (8.3%) |
| Potassium, mEq/L | 4.2±0.3 |
| Calcium, mg/dL | 9.3±0.4 |
| Magnesium, mg/dL | 2.0±0.2 |
| Thyrotropin, µUI/L | 2.4±4.3 |
| C‐reactive protein, mg/L | 13.0±32.3 |
| Cardiovascular risk factors/diseases | |
| History of tobacco use, n | 518 (7.1%) |
| Hypertension, n | 405 (5.5%) |
| Diabetes mellitus, n | 2100 (28.6%) |
| Ischemic heart disease, n | 1218 (16.6%) |
| Drugs | |
| Anesthetics, n | 85 (1.2%) |
| Antiarrhythmics, n | 82 (1.1%) |
| Antibiotics, n | 3893 (53.0%) |
| Antidepressants, n | 3870 (52.7%) |
| Antiemetics, n | 1449 (19.7%) |
| Antifungals, n | 1362 (18.6%) |
| Antihistaminics, n | 2160 (29.4%) |
| Antimalarials, n | 718 (9.8%) |
| Antimanias, n | 110 (1.5%) |
| Antipsychotics, n | 1630 (22.2%) |
| Bronchodilators, n | 2617 (35.7%) |
| Cholinesterase inhibitors, n | 182 (2.5%) |
| Diuretics, n | 2918 (39.8%) |
| H2‐histamine receptor antagonists, n | 102 (1.4%) |
| Immunosuppressants, n | 145 (2.0%) |
| Inotropes, n | 32 (0.4%) |
| Opiates, n | 358 (4.9%) |
| Proton pump inhibitors, n | 1322 (18.0%) |
| Phosphodiesterase inhibitors, n | 1248 (17.0%) |
| Vasoconstrictors, n | 183 (2.5%) |
For continuous variables, the data are expressed as mean±SD. For categorical variables, the data are expressed as n (%). QTc indicates heart rate‐corrected QT interval.
In the univariate analysis, a number of variables were found to be consistently associated throughout the different cutoffs with an increased risk of QTc prolongation in the whole population, including demographic factors (age, sex), laboratory data (anti‐Ro/SSA positivity, potassium, calcium, thyrotropin, C‐reactive protein, cardiovascular risk factors/diseases (history of tobacco use, diabetes mellitus, IHD, and drugs [anesthetics, antiarrhythmics, antibiotics, antidepressants, antiemetics, antihistaminics, antimalarials, antipsychotics, bronchodilators, cholinesterase inhibitors, diuretics, H2‐histamine receptor antagonists, inotropes, proton pump inhibitors, and vasoconstrictors]) (data not shown). Notably, although mean QTc values were higher in the presence versus absence of each risk factor, in all cases they were within the normal range (ie, <470 ms men/480 ms women) (Table S2), thereby in some way supporting the view that no one factor alone is able to prolong QTc interval over limits (multihit theory). 7
Association Between Anti‐Ro/SSA Positivity and QTc: Univariate Analysis
Empirical distribution of QTc values by the presence or absence of anti‐Ro/SSA‐antibodies is presented in the Figure 1. Subjects who were anti‐Ro/SSA‐positive showed a significantly longer mean QTc than individuals who were anti‐Ro/SSA‐negative (436.8±28.2 versus 430.8±25.6 ms; P<0.001), a difference persisting when the subjects were analyzed by race (Table 2). Although within the range of normal, mean values of QTc in subjects who were anti‐Ro/SSA‐positive were comparable or higher than those observed in the presence of most of the other QT‐prolonging risk factors (including several well‐recognized factors, such as older age, female sex, hypokalemia, hypocalcemia, diabetes mellitus, IHD, use of antibiotics, antidepressants, antihistaminics, antipsychotics, diuretics, opiates, diuretics, antiemetics) (Table S2). Subjects who were anti‐Ro/SSA‐positive were at higher risk of presenting with QTc prolongation, which gradually increased as the cutoff used was progressively more stringent. Specifically, in the group who were anti‐Ro/SSA‐positive the absolute prevalence of QTc prolongation ranged from 10% (versus 6.2% in the group who were negative; P=0.001) for abnormal QTc prolongation (>470 [men]/480 [women] ms), to 3.1% (versus 1.1%; P<0.001), for highly abnormal QTc prolongation (>500 ms). The crude ORs in subjects who weree anti‐Ro/SSA‐positive versus ‐negative were 1.67 (1.26–2.21) for QTc >470/480 ms, 2.32 (1.54–3.49) for QTc >490 ms, and 2.77 (1.66–4.60) for QTc >500 ms, respectively (Table 2). Notably, a significant increase in the OR was already present for lower cutoffs (440–460 ms); however, the OR value remained substantially the same (≈1.5) until the 470/480 ms cutoff, then showing a sharp rise from 490 ms onwards (Figure S1). Accordingly, a parallel increase in the percentage of individuals who were anti‐Ro/SSA‐positive was observed in the groups with prolonged QTc, until reaching a value ≈20% among subjects with highly abnormal QTc prolongation (whole population: 8.3%; >470/480 ms: 12.7%; >490 ms: 17.1%; >500 ms: 19.8%; P<0.001, chi‐square test for trend).
Figure 1. Empirical distribution of QTc values in the overall cohort.
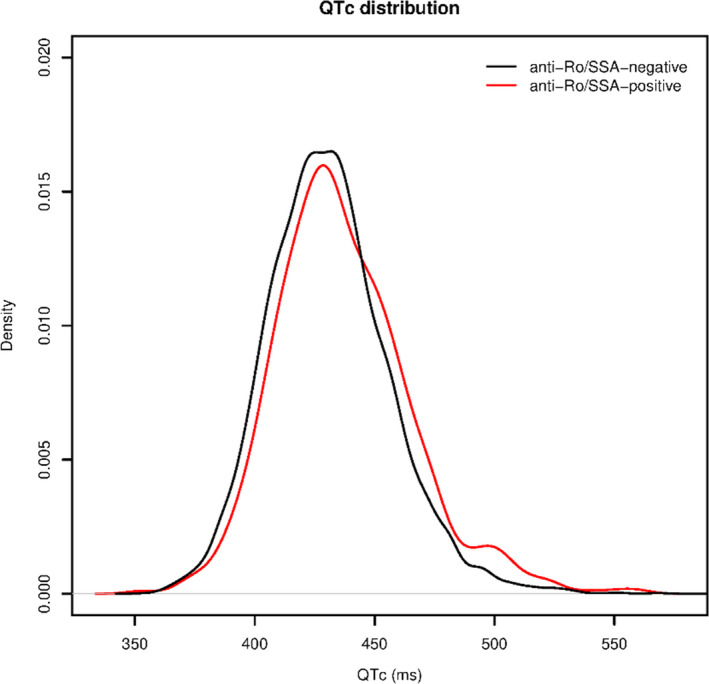
Anti‐Ro/SSA‐antibodies indicates anti‐Sjögren's syndrome‐related antigen A‐antibodies; and QTc, heart rate‐corrected QT interval.
Table 2.
Mean QTc Values and QTc Prolongation Frequencies in Anti‐Ro/SSA‐Positive (n=612) Versus Anti‐Ro/SSA‐Negative (n=6727) Subjects of the Study Population
| QTc | n | Positive | n | Negative | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Mean values, ms | ||||||
| All subjects | 612 | 436.8±28.2 | 6727 | 430.8±25.6 | … | <0.001* |
| White | 390 | 437.1±28.4 | 4694 | 430.5±25.4 | … | <0.001* |
| Black | 41 | 437.3±28.6 | 1236 | 431.0±26.0 | … | 0.010* |
| QTc prolongation frequencies, % | ||||||
| QTc >470 ms Men/480 ms women | 612 | 10.0 | 6727 | 6.2 | 1.67 (1.26–2.21) | 0.001* |
| QTc >490 ms | 612 | 4.7 | 6727 | 2.1 | 2.32 (1.54–3.49) | <0.001* |
| QTc >500 ms | 612 | 3.1 | 6727 | 1.1 | 2.77 (1.66–4.60) | <0.001* |
For continuous variables, the data are expressed as mean±SD. For categorical variables, the data are expressed as n (%). Differences in quantitative variables were evaluated by the 2‐tail Mann‐Whitney test or unpaired t test. Differences in categorical variables were evaluated by the 2‐sided Fisher's exact test. Anti‐Ro/SSA indicates anti‐Sjögren's syndrome‐related antigen A‐antibodies; Negative, anti‐Ro/SSA‐negative subjects; Positive, anti‐Ro/SSA‐positive subjects; and QTc, heart rate‐corrected QT interval.
P<0.05.
QT‐Prolonging Risk Factors in Subjects Who Were Anti‐Ro/SSA‐Positive Versus ‐Negative
In subjects who were anti‐Ro/SSA‐positive, the global burden of QT‐prolonging risk factors was significantly increased when compared with those who were anti‐Ro/SSA‐negative. Subjects who were anti‐Ro/SSA‐positive were older and more frequently females; had lower potassium and calcium levels; were more frequently affected with IHD and treated with drugs with QT‐prolonging potential (specifically, antiarrhythmics, antibiotics, antidepressants, antiemetics, antihistaminics, antimalarials, antipsychotics, bronchodilators, cholinesterase inhibitors, diuretics, inotropes, proton pump inhibitors, and vasoconstrictors), when compared with subjects who were negative (Table 3).
Table 3.
QTc Prolonging Risk Factors in Anti‐Ro/SSA‐Positive (n=612) Versus Anti‐Ro/SSA‐Negative (n=6727) Subjects of the Study Population
| Risk factor | n | Positive | n | Negative | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Quantitative variables | ||||||
| Age | 606 | 63.5±11.8 y | 6654 | 61.2±12.2 y | 1.016 (1.009–1.023) | <0.001* |
| Potassium | 611 | 4.1±0.3 mEq/L | 6701 | 4.1±0.3 mEq/L | 0.681 (0.510–0.909) | 0.023* |
| Calcium | 602 | 9.2±0.4 mg/dL | 6438 | 9.3±0.4 mg/dL | 0.435 (0.352–0.536) | <0.001* |
| Thyrotropin | 550 | 2.6±3.4 µUI/mL | 5783 | 2.4±4.4 µUI/mL | 1.009 (0.994–1.024) | 0.22 |
| C‐reactive protein | 154 | 12.5±29.1 mg/L | 1176 | 13.0±32.7 mg/L | 0.999 (0.994–1.005) | 0.83 |
| Categorical variables | ||||||
| Demographic factors | ||||||
| Female sex | 612 | 26.3% | 6727 | 20.4% | 1.39 (1.15–1.68) | 0.001* |
| Cardiovascular risk factors/diseases | ||||||
| Smoking | 612 | 8.8% | 6727 | 7.7% | 1.16 (0.87–1.56) | 0.31 |
| Diabetes mellitus | 612 | 29.1% | 6727 | 26.8% | 1.12 (0.93–1.34) | 0.24 |
| Ischemic heart disease | 612 | 24.7% | 6727 | 15.9% | 1.74 (0.43–2.11) | <0.001* |
| Drugs | ||||||
| Anesthetics | 612 | 1.6% | 6727 | 1.1% | 1.47 (0.76–2.86) | 0.23 |
| Antiarrhythmics | 612 | 5.1% | 6727 | 0.8% | 6.98 (4.43–11.0) | <0.001* |
| Antibiotics | 612 | 62.9% | 6727 | 52.1% | 1.56 (1.31–1.85) | <0.001* |
| Antidepressants | 612 | 62.4% | 6727 | 51.9% | 1.54 (1.30–1.83) | <0.001* |
| Antiemetics | 612 | 29.1% | 6727 | 18.9% | 1.76 (1.46–2.12) | <0.001* |
| Antihistaminics | 612 | 40.8% | 6727 | 28.4% | 1.74 (1.47–2.06) | <0.001* |
| Antimalarials | 612 | 48.9% | 6727 | 6.2% | 14.38 (11.93–17.33) | <0.001* |
| Antipsychotics | 612 | 32.0% | 6727 | 21.3% | 1.74 (1.45–2.08) | <0.001* |
| Bronchodilators | 612 | 48.7% | 6727 | 34.5% | 1.80 (1.53–2.13) | <0.001* |
| Cholinesterase inhibitors | 612 | 4.2% | 6727 | 2.3% | 1.87 (1.22–2.85) | 0.006* |
| Diuretics | 612 | 44.4% | 6727 | 39.3% | 1.23 (1.04–1.45) | 0.014* |
| H2‐histamine receptor antagonists | 612 | 1.1% | 6727 | 1.4% | 0.81 (0.37–1.75) | 0.71 |
| Inotropes | 612 | 1.0% | 6727 | 0.4% | 2.55 (1.05–6.22) | 0.046* |
| Opiates | 612 | 4.9% | 6727 | 4.9% | 1.01 (0.68–1.47) | 0.92 |
| Proton pump inhibitors | 612 | 22.4% | 6727 | 17.6% | 1.35 (1.10–1.65) | 0.004* |
| Vasoconstrictors | 612 | 1.8% | 6727 | 0.7% | 2.66 (1.37–5.16) | 0.007* |
For continuous variables, the data are expressed as mean±SD. For categorical variables, the data are expressed as n (%). Differences in quantitative variables were evaluated by the 2‐tail Mann‐Whitney test or unpaired t test. Difference in categorical variables were evaluated by the 2‐sided Fisher's exact test. Anti‐Ro/SSA indicates anti‐Sjögren's syndrome‐related antigen A‐antibodies; Negative, anti‐Ro/SSA‐negative subjects; Positive, anti‐Ro/SSA‐positive subjects; and QTc, heart rate‐corrected QT interval.
P<0.05.
Relationship Between Anti‐Ro/SSA Positivity, History of Connective Tissue Disease, and QTc
In our veteran population, a history of CTD, including systemic lupus erythematosus, discoid lupus, Sjögren syndrome, systemic sclerosis, idiopathic inflammatory myositis, mixed CTD, undifferentiated CTD, and rheumatoid arthritis, was present in 993 subjects (13.5%). As expected, it was more frequently found in subjects who were anti‐Ro/SSA‐positive (288/612; 47.1%) than anti‐Ro/SSA‐negative (705/6727: 10.5%), with an OR >7 (Table S3). However, a positive history for CTD was not a necessary condition for the occurrence of anti‐Ro/SSA‐associated QTc prolongation. In fact, when individuals who were anti‐Ro/SSA‐positive without history of CTD (ie, ≈50% of cases) were selectively considered, the mean QTc and frequencies of QTc prolongation persisted substantially unmodified and significantly higher with respect to subjects who were anti‐Ro/SSA‐negative (Table S4). Accordingly, the percentage of subjects who were anti‐Ro/SSA‐positive without CTD remained stable around 50% throughout the different cutoffs (whole population: 52.9%; >470/480 ms: 52.5%; >490 ms: 51.7%; >500 ms: 47.4%; P=0.97, chi‐square test for trend).
There was a trend, although not statistically significant, for an increase in QTc values in subjects who were anti‐Ro/SSA‐positive with CTD when compared with those who were anti‐Ro/SSA‐positive but without CTD (Table S3). In this regard, it is important to note that subjects who were anti‐Ro/SSA‐positive with CTD showed a higher burden of QT‐prolonging risk factors than anti‐Ro/SSA‐positive without CTD, specifically an increased prevalence of female sex and a more frequent use of some medications (ie, antibiotics, antidepressants, antihistaminics, antimalarials, diuretics, proton pump inhibitors) (Table S5).
To further improve the confidence that anti‐Ro/SSA‐antibodies are the cause of QTc prolongation, rather than merely serving as a surrogate marker of an autoimmune state, we evaluated the impact of 7 other autoantibodies (all well recognized to be associated with CTDs, ie, antibodies anti‐cyclic citrullinated peptide, anti‐centromere, anti‐double stranded DNA, anti‐Sjögren syndrome‐related antigen B, anti‐ribonucleoprotein, anti‐topoisomerase‐I, and anti‐Smith) 28 on the prevalence of QTc prolongation in the population who were anti‐Ro/SSA‐negative. As shown in Table S6, none of these autoantibodies showed any significant association with the risk of QTc prolongation, regardless of the specific cutoff used.
Association Between Anti‐Ro/SSA Positivity and QTc Prolongation: Multivariate Analysis
The role of anti‐Ro/SSA as a risk factor for long QTc was evaluated using a multivariate logistic regression analysis by gradually adding potential adjustment or confounding risk factors. Such factors were selected by means of the aforementioned preliminary analyses, sequentially performed to identify (1) which variables were crudely associated in the whole population with a significant risk of QTc prolongation, throughout different cutoffs, and then (2) which among these selected variables were also significantly different when populations who were anti‐Ro/SSA‐positive versus ‐negative were specifically compared. The variables selected by the first step were age, sex, potassium, calcium, thyrotropin, C‐reactive protein, history of tobacco use, diabetes mellitus, IHD anesthetics, antiarrhythmics, antibiotics, antidepressants, antiemetics, antihistaminics, antimalarials, antipsychotics, bronchodilators, cholinesterase inhibitors, diuretics, H2‐histamine receptor antagonists, inotropes, proton pump inhibitors, and vasoconstrictors. Among these, the variables selected by the second step were age, sex, potassium, calcium, IHD antiarrhythmics, antibiotics, antidepressants, antiemetics, antihistaminics, antimalarials, antipsychotics, bronchodilators, cholinesterase inhibitors, diuretics, inotropes, proton pump inhibitors, vasoconstrictors.
Based on this final set of selected variables, the following multivariate models were performed:
Model 1: anti‐Ro/SSA, age (as continuous variable), and sex;
Model 2: model 1 plus electrolytes (potassium and calcium levels, as continuous variables);
Model 3: model 2 plus cardiovascular risk factors/diseases (IHD);
Model 4: model 3 plus use of any drug with QT‐prolonging potential (including antiarrhythmics, antibiotics, antidepressants, antiemetics, antihistaminics, antimalarials, antipsychotics, bronchodilators, cholinesterase inhibitors, diuretics, inotropes, proton pump inhibitors, and vasoconstrictors).
The association between anti‐Ro/SSA and QTc prolongation was progressively attenuated by the 4 levels of adjustment, particularly when QT‐prolonging drugs were added to the model (Tables 4, 5, 6). Nevertheless, fully adjusted OR for the highest cutoff remained significantly increased in subjects who were anti‐Ro/SSA‐positive. Particularly, OR for QTc >500 ms was 1.92 (1.02–3.62) (Table 6), indicating a ≈2‐times higher risk of developing a highly abnormal QTc prolongation in the presence of circulating anti‐Ro/SSA antibodies. The role of anti‐Ro/SSA‐positivity as an independent predictor of QTc prolongation was further supported and better characterized by additionally applying a stepwise method to the regression logistic analysis (in which all the variables selected by the aforementioned 2‐step selection process were entered to identify a minimum subset of factors with the highest possible prediction accuracy for QTc prolongation). In fact, with this approach, we additionally demonstrated that the presence of QTc prolongation >490 and >500 ms was explained by only a few variables (ie, age, calcium, potassium, anti‐Ro/SSA, diuretic use) among which anti‐Ro/SSA positivity was in both cases significantly included (>500 ms; OR 2.27 [1.34–3.87]) (Table 7; Figure S2), thereby pointing to circulating anti‐Ro/SSA‐antibodies as one of the most important risk factors for marked QTc prolongation in this population.
Table 4.
Multivariate Logistic Regression Analysis Showing the Odds Ratio of Anti‐Ro/SSA‐Positivity for QTc Interval Prolongation >470 ms Men/480 ms Women, After Adjustment for Confounders
| Adjustment Factors | n | Odds Ratio | 95% CI | P Value |
|---|---|---|---|---|
| None | 7339 | 1.67 | 1.26–2.21 | 0.001* |
| Age, sex | 7260 | 1.58 | 1.18–2.12 | 0.002* |
| Age, sex, electrolytes † | 6949 | 1.40 | 1.04–1.88 | 0.025* |
| Age, sex, electrolytes † , cardiovascular risk factors/diseases ‡ | 6949 | 1.35 | 1.00–1.81 | 0.049* |
| Age, sex, electrolytes † , cardiovascular risk factors/diseases ‡ , Drugs § | 6949 | 1.06 | 0.75–1.51 | 0.73 |
Anti‐Ro/SSA indicates anti‐Sjögren's syndrome‐related antigen A‐antibodies; and QTc, heart rate‐corrected QT interval.
P<0.05.
Including potassium, calcium.
Including ischemic heart disease.
Including antiarrhythmics, antibiotics, antidepressants, antiemetics, antihistaminics, antimalarials, antipsychotics, bronchodilators, cholinesterase inhibitors, diuretics, inotropes, proton pump inhibitors, vasoconstrictors.
Table 5.
Multivariate Logistic Regression Analysis Showing the Odds Ratio of Anti‐Ro/SSA‐Positivity for QTc Interval Prolongation >490 ms, After Adjustment for Confounders
| Adjustment Factors | n | Odds Ratio | 95% CI | P Value |
|---|---|---|---|---|
| None | 7339 | 2.32 | 1.54–3.49 | <0.001* |
| Age, sex | 7260 | 2.09 | 1.37–3.17 | 0.001* |
| Age, sex, electrolytes † | 6949 | 1.85 | 1.20–2.83 | 0.005* |
| Age, sex, electrolytes † , cardiovascular risk factors/diseases ‡ | 6949 | 1.77 | 1.15–2.72 | 0.009* |
| Age, sex, electrolytes † , cardiovascular risk factors/diseases ‡ , drugs § | 6949 | 1.63 | 0.98–2.70 | 0.060 ‖ |
Anti‐Ro/SSA indicates anti‐Sjögren's syndrome‐related antigen A‐antibodies; and QTc, heart rate‐corrected QT interval.
P<0.05.
Including potassium, calcium.
Including ischemic heart disease.
Including antiarrhythmics, antibiotics, antidepressants, antiemetics, antihistaminics, antimalarials, antipsychotics, bronchodilators, cholinesterase inhibitors, diuretics, inotropes, proton pump inhibitors, vasoconstrictors.
P<0.07
Table 6.
Multivariate Logistic Regression Analysis Showing the Odds Ratio of Anti‐Ro/SSA Positivity for QTc Interval Prolongation >500 ms, After Adjustment for Confounders
| Adjustment Factors | n | Odds Ratio | 95% CI | P Value |
|---|---|---|---|---|
| None | 7339 | 2.77 | 1.66–4.60 | <0.001* |
| Age, sex | 7260 | 2.48 | 1.46–4.20 | 0.001* |
| Age, sex, electrolytes † | 6949 | 2.19 | 1.28–3.73 | 0.004* |
| Age, sex, electrolytes † , cardiovascular risk factors/diseases ‡ | 6949 | 2.09 | 1.22–3.57 | 0.007* |
| Age, sex, electrolytes † , cardiovascular risk factors/diseases ‡ , drugs § | 6949 | 1.92 | 1.02–3.62 | 0.045* |
Anti‐Ro/SSA indicates anti‐Sjögren's syndrome‐related antigen A‐antibodies; and QTc, heart rate‐corrected QT interval.
P<0.05.
Including potassium, calcium.
Including ischemic heart disease.
Including antiarrhythmics, antibiotics, antidepressants, antiemetics, antihistaminics, antimalarials, antipsychotics, bronchodilators, cholinesterase inhibitors, diuretics, inotropes, proton pump inhibitors, vasoconstrictors.
Table 7.
Demographic, Clinical, Laboratory, and Therapeutic Variables Independently Associated With QTc Interval Prolongation >490 and >500 ms in the Study Population: A Stepwise Multivariate Logistic Regression Analysis
| Dependent Variable | Independent Variables | Model Coefficients (β) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| QTc >490 ms | Age | 0.052 | 1.05 (1.04–1.07) | <0.001* |
| Calcium | −0.804 | 0.45 (0.31–0.66) | <0.001* | |
| Diuretics | 0.623 | 1.86 (1.34–2.66) | <0.001* | |
| Anti‐Ro/SSA | 0.639 | 1.90 (1.24–2.90) | 0.003* | |
| Potassium | −0.755 | 0.47 (0.27–0.83) | 0.009* | |
| QTc >500 ms | Age | 0.044 | 1.05 (1.03–1.07) | <0.001* |
| Calcium | −0.903 | 0.41 (0.25–0.67) | <0.001* | |
| Anti‐Ro/SSA | 0.821 | 2.27 (1.34–3.87) | 0.002* | |
| Diuretics | 0.539 | 1.71 (1.11–2.64) | 0.015* |
Variables entered in the multivariate analysis include age, sex, potassium, calcium, ischemic heart disease, antiarrhythmics, antibiotics, antidepressants, antiemetics, antihistaminics, antimalarials, antipsychotics, bronchodilators, cholinesterase inhibitors, diuretics, inotropes, proton pump inhibitors, vasoconstrictors. Anti‐Ro/SSA indicates anti‐Sjögren's syndrome‐related antigen A‐antibodies; and QTc, heart rate‐corrected QT interval.
P<0.05.
To further support these findings, several additional sensitivity analyses were conducted. A first sensitivity analysis was performed on a subgroup of 2384 subjects selected by nearest neighbor matching procedure, in which the impact of confounding factors was minimized. As shown in Table S7, this population included 596 subjects who were anti‐Ro/SSA‐positive and 1788 anti‐Ro/SSA‐negative, strictly matched for all QT‐prolonging risk factors, except for antiarrhythmic and antimalarial use (because of the small sample size of treated subjects and clinical considerations 29 ; more details are provided in the Online‐Data S1). Also in this case, stepwise multivariate logistic regression analysis (taking into account all variables, including antiarrhythmic and antimalarial use) confirmed anti‐Ro/SSA‐positivity as an independent predictor of QTc prolongation >500 (OR 2.18 [1.18–4.02]), along with age and calcium levels (Table S8). Notably, the evidence that both antimalarials and antiarrhythmics were removed during the stepwise analysis process ruled out a significant influence of these variables on the relationship between anti‐Ro/SSA‐antibodies and QTc prolongation. As an additional confirmation, by stratifying these subjects according the antiarrhythmics and antimalarials use, we found that even in the absence of these drugs subjects who were anti‐Ro/SSA‐positive showed a prevalence of QTc >500 ms significantly higher than in those who were anti‐Ro/SSA‐negative (antiarrhythmic untreated subjects, n=2308: OR 2.04 [1.03–3.94], P<0.05; antimalarial untreated subjects, n=1953: OR 2.26 [1.00–4.79], P<0.05).
Moreover, a further sensitivity analysis was conducted to evaluate the impact of confounding factors on anti‐Ro/SSA‐associated QTc prolongation by comparing the mean QTc and the prevalence of QTc prolongation with different cutoffs in patients who were anti‐Ro/SSA‐positive with or without a specific confounding factors versus subjects who were anti‐Ro/SSA negative. Again, an increase in QTc parameters with respect to subjects who were anti‐Ro/SSA‐negative persisted in all cases when subjects who were anti‐Ro/SSA‐positive without the specific risk factor were considered, in most cases the differences remained statistically significant (16/19, 84%, in 1 or more parameters) (Table S9).
Furthermore, 2 additional sensitivity analyses were performed to exclude that temporality, that is, the fact that ECG acquisition, anti‐Ro/SSA testing, and biochemical parameters measured had not been always performed at the same time point may have biased our results. First, by analyzing the whole cohort of the 612 patients who were anti‐Ro/SSA‐positive, we found that in most cases anti‐Ro/SSA test preceded ECG (n=494, Ro‐PRE group, ie, the group in which ECG was taken after anti‐Ro/SSA‐antibodies were discovered); whereas in 72 subjects the 2 examinations were concomitant (Ro‐CC group, ie, the group in which ECG and anti‐Ro/SSA testing were performed at the same time), and in only 46 the anti‐Ro/SSA testing followed the ECG (Ro‐POST group, that is, the group in which ECG was taken before anti‐Ro/SSA‐antibodies were discovered). Interestingly, the mean QTc found in the Ro‐PRE and Ro‐CC groups were very similar (437.5±28.6 and 436.1±23.8, respectively) and overlapping that found in the whole population who were anti‐Ro/SSA‐positive (436.8±28.2; Table 2). Conversely, in the Ro‐POST group (in which there was an increased risk of including subjects underwent ECG when circulating anti‐Ro/SSA antibodies were still not present) the mean QTc was significantly shorter (430.7±29.9), and overlapping that observed in the whole population who were anti‐Ro/SSA‐negative (430.8±25.6; Table 2). Based on these findings, stepwise multivariate logistic regression analysis was repeated after excluding the Ro‐POST group subjects, without any substantial change in the ORs for anti‐Ro/SSA (>490: 1.93 [1.23–2.93]; >500: 2.27 [1.28–3.82]).
Moreover, the impact of temporality of electrolyte measurements on ECG findings was also evaluated. Given that, by nature, their influence on QTc is transient, we focused on the subjects in whom ECG and electrolytes were evaluated concomitantly. In this group, including the majority of subjects (potassium: n=5432; calcium: n=4873), the mean QTc in the presence or absence of the risk factor (ie, hypokaliemia or hypocalcemia, respectively) (Table S10) was similar to that found in the whole population, thereby providing evidence that temporality of electrolyte measurements did not significantly bias the results of our study.
Synergy Between Anti‐Ro/SSA Positivity and Other Concomitant QT‐Prolonging Risk Factors
A single QT‐prolonging risk factor per se is frequently not able to induce significant QTc prolongation. To evaluate whether circulating anti‐Ro/SSA antibodies can synergistically act with other concomitant risk factors to prolong QTc, we compared mean QTc values in subjects who were anti‐Ro/SSA‐positive versus ‐negative in the presence of various recognized QT‐prolonging risk factors. We found that for any of the 15 risk factors analysed with a sample size of at least 50 subjects for each category, subjects who were anti‐Ro/SSA‐positive showed an increased QTc value and/or QTc prolongation prevalence when compared with the subjects who were anti‐Ro/SSA‐negative, in most cases such a difference being statistically significant (10/15) (Table S9).
DISCUSSION
The key findings of the present study are the following: (1) in a large cohort of US veterans, subjects who were anti‐Ro/SSA‐positive, independent of a history of CTD, showed an increased prevalence of QTc prolongation, in the presence of other concomitant risk factors; (2) circulating anti‐Ro/SSA‐antibodies were independently associated with a ≈2‐times higher risk of marked QTc prolongation; (3) anti‐Ro/SSA positivity was a significant contributor to marked QTc prolongation occurrence, even representing one of the most important risk factors involved; and (4) a synergy between anti‐Ro/SSA‐antibodies and most of the traditional risk factors was demonstrated. Altogether, our study provides evidence that anti‐Ro/SSA positivity is a strong and independent risk factor for marked QTc prolongation in a large cohort of US veterans. These data suggest that within the general population subjects who were anti‐Ro/SSA‐positive may represent a subgroup with an increased predisposition to life‐threatening VAs, particularly when other QT‐prolonging risk factors are concomitantly present.
Autoimmune cardiac channelopathies represent a recently recognized novel mechanism for cardiac arrhythmias. 8 In fact, the existence of a number of arrhythmogenic autoantibodies cross‐reacting with specific cardiac ion channels involved in action potential genesis has been demonstrated. Specifically, by targeting calcium, potassium, or sodium channels, these autoantibodies lead to significant electrophysiological changes promoting conduction disturbances and/or life‐threatening tachyarrhythmias, both in patients with manifest autoimmune diseases and in apparently healthy individuals. 7 , 8 , 10 Anti‐Ro/SSA is the most investigated anti‐ion channel antibody and its role in the pathogenesis of the congenital heart block via a molecular mimicry‐driven interaction with calcium channels of the fetal conduction system is well established. 8 , 30 , 31 , 32 More recently, a growing body of evidence demonstrated that anti‐Ro/SSA‐antibodies can also target the hERG‐potassium channel leading to a novel form of acquired LQTS of autoimmune origin. 8 , 11 Several clinical studies in adults and newborns provided evidence that subjects who were anti‐Ro/SSA‐positive frequently show a prolonged QTc, 14 , 15 , 16 , 17 , 18 , 19 whose length correlates with circulating autoantibody levels 16 , 17 , 33 and incidence of complex VAs, 19 , 20 including TdP. 22 , 27 From a molecular point of view, we established that purified IgG/anti‐Ro/SSA‐52 kDa from patients who were antibody positive with LQTS/TdP acutely inhibited the rapid component of the delayed rectifier potassium current in human embryonic kidney‐293 cells and guinea‐pig ventricular myocytes. 11 , 22 Such effects, found in both subjects with LQTS with CTD and in patients with TdP without any sign of autoimmune disease, were a result of a specific interaction of the autoantibody with the extracellular loop of the hERG‐potassium channel, between segments S5 and S6 of the pore‐forming subunit, where a significant homology with the Ro/SSA‐52 kDa antigen is present. 11 , 22 Other authors demonstrated that this region is also critical for the occurrence of the chronic effects of anti‐Ro/SSA‐52 kDa on the channel, that is, reduced expression by triggering channel internalization. 12 , 27 Finally, we provided evidence that immunization of adult guinea‐pigs with the Ro/SSA‐52 kDa antigens reproduced QTc prolongation in these animals, because of the autoantibody‐mediated prolonging effect on ventricular action potential duration via the rapid component of the delayed rectifier potassium current inhibition. 11 Although these data strongly support the role of anti‐Ro/SSA as a novel, nontraditional, risk factor for LQTS/TdP in patients with CTD, information regarding its actual clinical impact on the general population is substantially missing as only studies on relatively small groups of selected patients are currently available. 14 , 15 , 16 , 17 , 18 , 19 , 22 , 27 , 33
In the present study, we provide evidence for the first time that in a large population of over 7000 US veterans, including 612 with circulating anti‐Ro/SSA, subjects who were autoantibody positive showed a longer mean QTc duration (+6.0 ms) along with a higher prevalence of QTc prolongation when compared with subjects who were anti‐Ro/SSA‐negative. In the univariate analysis, the greater the QTc cutoff used, the greater was the estimated OR for QTc prolongation, with an OR close to 3 for QTc >500 ms. By progressively setting a higher QTc cutoff, a parallel rise in the percentage of subjects who were anti‐Ro/SSA‐positive among those with QTc prolongation was observed. In the highest cutoff of QTc >500 ms, ≈1 in every 5 subjects with marked QTc prolongation (19.8%) showed circulating anti‐Ro/SSA. Although these data are remarkable, it should be considered that in the group who were anti‐Ro/SSA‐positive a significantly higher burden of QT‐prolonging risk factors was found. It is well known that anti‐Ro/SSA‐antibodies are strongly associated with the presence of CTDs, 13 frequently characterized by a multisystemic involvement, cardiovascular (particularly IHD) and noncardiovascular, 34 leading to an increased probability of needing medications, including QT‐prolonging drugs. Accordingly, in our cohort, the prevalence of CTD in the group who were anti‐Ro/SSA‐positive was over 7‐times higher than in those who were anti‐Ro/SSA‐negative. Moreover, among subjects who were anti‐Ro/SSA‐positive, those with a history of CTD were more frequently treated with antibiotics, antidepressants, antihistaminics, antimalarials, diuretics, and proton pump inhibitors when compared with those without. In addition, large population studies have demonstrated that the likelihood of presenting circulating anti‐Ro/SSA is higher in women and in advanced age, 23 , 25 both also representing a recognized risk factor for QTc prolongation. As mentioned earlier, subjects who were anti‐Ro/SSA‐positive in our population were older and more frequently female when compared with those who were negative. The concomitant factors partly responsible for QTc prolongation in these subjects are likely synergistic with anti‐Ro/SSA in promoting QTc prolongation. Indeed, according to the multihit theory, 7 more than 1 factor/hit is necessary in most cases to significantly disturb ventricular repolarization and induce a clinically measurable lengthening of QTc. 7 , 35 In this view, anti‐Ro/SSA may intriguingly represent a risk factor for QTc prolongation both directly by inhibiting the hERG‐potassium channel and indirectly by affecting the probability that other QT‐prolonging risk factor are at the same time present in the individual subject.
The results obtained from the multivariable analysis robustly supported this hypothesis. In fact, although adjustments for concomitant risk factors progressively reduced the magnitude of the OR associated with the presence of circulating anti‐Ro/SSA, a ≈2‐times increased risk of marked QTc prolongation was still observed in subjects who were anti‐Ro/SSA‐positive even after full adjustment. Moreover, quantitative assessment by stepwise analysis demonstrated that anti‐Ro/SSA positivity was included in the small subset of critical variables explaining the occurrence of QTc prolongation >490 and >500 ms in our cohort. These findings provide evidence for a strong and independent role of anti‐Ro/SSA in increasing the risk for having a prolonged QTc, thereby, confirming that the direct electrophysiologic effects of these autoantibodies have a clinically relevant impact also in a large population of unselected individuals. The multivariate analysis also demonstrated how the anti‐Ro/SSA‐associated QT‐prolonging potential was significantly enhanced when other traditional risk factors are present. By introducing age, sex, electrolytes, IHD, and medications step by step in the model, a parallel reduction in ORs for QTc prolongation was observed (even resulting in no significant independent effect for the 470/480 ms cutoff). Moreover, by specifically focusing on the bivariate interaction existing between anti‐Ro/SSA and each of the other risk factors present in the US veteran population, we found that in most cases the anti‐Ro/SSA positivity copresence with other risk factors was associated with additional and significant QT‐prolonging effects.
Another important finding of the present study is that anti‐Ro/SSA represents a risk factor for QTc prolongation in the US veteran population regardless of the presence or absence of a manifest CTD. In fact, a significant increase of both mean QTc duration and QTc prolongation prevalence was also observed in subjects who were anti‐Ro/SSA‐positive without a history of CTD when compared with those who were anti‐Ro/SSA‐negative. In addition, none of the other CTD‐associated autoantibodies evaluated (as surrogate markers of an autoimmune disease state) showed any significant association with the QTc prolongation risk. This evidence further supports the view that anti‐Ro/SSA‐antibodies are per se able to promote LQTS by exerting direct electrophysiological effects on the heart, thereby suggesting that these autoantibodies are a risk factor for QTc prolongation in the whole population. Thus, the clinical implications extend not only to patients with clinical symptoms of CTD but more widely in all subjects showing anti‐Ro/SSA positivity, even though asymptomatic. Notably, in accordance with a previous large population study, 23 the fraction of subjects who were anti‐Ro/SSA‐positive without CTD in our cohort were elevated, ≈50% of cases. The key implication of these results is that in the general population, a number of subjects who carry a concealed anti‐Ro/SSA may be predisposed to QT‐related malignant arrhythmias, and in whom only specific testing can reveal the presence of the risk factor. Concordant with this view is a study cohort of patients with TdP consecutively enrolled from the general population, more than a half showed circulating anti‐Ro/SSA, and in all cases without any history of CTD. 22 Importantly, the number of subjects potentially exposed to this risk is not negligible. In fact, previous studies in the general population demonstrated that circulating anti‐Ro/SSA are found in up to ≈2.5% of the cases, 23 , 25 a percentage who rise to ≈4% when people older than 50 years are specifically considered. 23 , 25 In our population, such a proportion further increases to ≈8%, at least in part owing to the fact that US veterans are older, with a mean age >60 years. Thus, it can be estimated that 1 individual of every ≈40 in the general population (≈15 in elderly) may be anti‐Ro/SSA‐positive. In this scenario, our data suggest that 1 in every 5 older US veterans with marked QTc prolongation have circulating anti‐Ro/SSA. This evidence provides strong support to the hypothesis that these autoantibodies might be silently involved in a number of unexplained arrhythmias or SCDs in the general population. To this end, specific autoantibody testing in patients with “idiopathic” rhythm disturbances could lead to novel treatment and prophylactic opportunities. 8
The study has several limitations and strengths. First, our population includes US veterans who are predominantly male, White, and older, a fact that might in some way limit the generalizability of the results to a wider population. On the other hand, the use of national large‐scale data, collected during routine medical care from a network of integrated healthcare systems, significantly minimized the selection bias. Moreover, previous clinical and experimental data strongly suggest that the hERG‐blocking activity of anti‐Ro/SSA‐antibodies is restricted to the anti‐52 kDa subtype 11 , 12 , 22 and that relatively high antibody levels are necessary to promote QTc prolongation. 33 However, no adequate information is available in our cohort regarding the subtype specificities or plasma concentrations of anti‐Ro/SSA‐antibodies in subjects who were positive, and this may represent another limitation. Still, such limitation may conversely represents a strength of the study. In fact, based on the general population data demonstrating that among subjects who were anti‐Ro/SSA‐positive more than 40% tested negative for anti‐Ro/SSA‐52 kDa‐antibodies, 25 it is expected that a significant number of subjects in our cohort labelled as anti‐Ro/SSA‐positive actually did not present the anti‐52 kDa subtype. In addition, among those anti‐Ro/SSA‐52 kDa‐positive, it is plausible that only a fraction had circulating antibody levels as high as necessary to exert a clinically relevant effect. Thus, the testing method used in our cohort probably underestimated the potential impact of anti‐Ro/SSA‐antibodies on QTc. The evidence that an independent association was found, further supports the hypothesis that the anti‐Ro/SSA‐associated QTc prolongation has a relevant impact in the clinical setting. Further population studies are warranted to expressly evaluate the impact of subtype specificity and concentration on QTc prolongation risk. Patients' selection modality and measurement temporality represent other potential limitations to highlight. In fact, the retrospective enrolment of patients in whom an anti‐Ro/SSA test had been done (possibly because of a preexisting clinical suspicion of CTD) may have increased the probability that the test was positive, and that a CTD was present, when compared with that expected in the general population. However, as discussed previously, the percentage of subjects who were anti‐Ro/SSA‐positive reported by large studies in the elderly general population 23 , 25 is similar to what we found, suggesting a relatively small bias impact. Moreover, the influence of a possible higher CTD prevalence in our population has been minimized by the sensitivity analysis we performed demonstrating no significant differences in QTc parameters by comparing subjects who were anti‐Ro/SSA‐positive with or without a history of CTD. Regarding the temporal bias, it was attenuated by the fact that for each variable, for a specific patient, we used the mean value resulting from multiple measurements performed throughout the time points. Nevertheless, the sensitivity analyses focused on the temporal relationship between ECG execution and anti‐Ro/SSA‐antibody or electrolyte testing provided evidence that temporality of measurements did not significantly bias the results of our study.
In conclusion, anti‐Ro/SSA positivity was independently associated with an increased risk of marked QTc prolongation in a large cohort of US veterans, regardless of the presence or absence of a manifest CTD. Our findings suggest that within the general population subjects with circulating anti‐Ro/SSA may be predisposed to VAs/SCD. It is proposed that subjects who were anti‐Ro/SSA‐positive should receive counseling about drugs and management of other risk factors that may increase the risk for malignant arrhythmias. At the same time, specific anti‐Ro/SSA testing should be considered in patients with “idiopathic” rhythm disturbances, as it could intriguingly open novel avenues in antiarrhythmic treatment, primarily immunomodulatory therapies.
Sources of Funding
This work was partially funded by a Merit Review grant (I01 BX002137) from the Biomedical Laboratory Research & Development Service of Veterans Affairs Office of Research and Development (to Boutjdir).
Disclosures
Dr Lazzerini received a grant from Roche Italia S.p.A. outside the submitted work, in 2018. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S10
Figures S1–S2
(J Am Heart Assoc.2021;10:e018735. DOI: 10.1161/JAHA.120.018735.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018735
For Sources of Funding and Disclosures, see page 13.
Contributor Information
Pietro Enea Lazzerini, Email: lazzerini7@unisi.it.
Deana Lazaro, Email: deana.lazaro@va.gov.
References
- 1. Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009;2:185–194. DOI: 10.1161/CIRCEP.108.789081. [DOI] [PubMed] [Google Scholar]
- 2. Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology tCoCN, and the American College of Cardiology Foundation . Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121:1047–1060. DOI: 10.1161/CIRCULATIONAHA.109.192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary. Circulation. 2018;138:e210–e271. DOI: 10.1161/CIR.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 4. Moss AJ. Long QT syndrome. JAMA. 2003;289:2041–2044. DOI: 10.1001/jama.289.16.2041. [DOI] [PubMed] [Google Scholar]
- 5. Schwartz PJ, Stramba‐Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, Gabbarini F, Goulene K, Insolia R, Mannarino S, et al. Prevalence of the congenital long‐QT syndrome. Circulation. 2009;120:1761–1767. DOI: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. El‐Sherif N, Turitto G, Boutjdir M. Acquired long QT syndrome and torsade de pointes. Pacing Clin Electrophysiol. 2018;41:414–421. DOI: 10.1111/pace.13296. [DOI] [PubMed] [Google Scholar]
- 7. Lazzerini PE, Capecchi PL, El‐Sherif N, Laghi‐Pasini F, Boutjdir M. Emerging arrhythmic risk of autoimmune and inflammatory cardiac channelopathies. J Am Heart Assoc. 2018;7:e010595. DOI: 10.1161/JAHA.118.010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lazzerini PE, Capecchi PL, Laghi‐Pasini F, Boutjdir M. Autoimmune channelopathies as a novel mechanism in cardiac arrhythmias. Nat Rev Cardiol. 2017;14:521–535. DOI: 10.1038/nrcardio.2017.61. [DOI] [PubMed] [Google Scholar]
- 9. Lazzerini PE, Capecchi PL, Laghi‐Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J. 2017;38:1717–1727. [DOI] [PubMed] [Google Scholar]
- 10. Lazzerini PE, Laghi‐Pasini F, Boutjdir M, Capecchi PL. Cardioimmunology of arrhythmias: the role of autoimmune and inflammatory cardiac channelopathies. Nat Rev Immunol. 2019;19:63–64. DOI: 10.1038/s41577-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 11. Yue Y, Castrichini M, Srivastava U, Fabris F, Shah K, Li Z, Qu Y, El‐Sherif N, Zhou Z, January C, et al. Pathogenesis of the novel autoimmune‐associated long‐QT syndrome. Circulation. 2015;132:230–240. DOI: 10.1161/CIRCULATIONAHA.115.009800. [DOI] [PubMed] [Google Scholar]
- 12. Szendrey J, Lamothe SM, Vanner S, Guo J, Yang T, Li W, Davis J, Joneja M, Baranchuk A, Zhang S. Anti‐Ro52 antibody acts on the S5‐pore linker of hERG to chronically reduce channel expression. Cardiovasc Res. 2019;115:1500–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franceschini F, Cavazzana I. Anti‐Ro/SSA and La/SSB antibodies. Autoimmunity. 2005;38:55–63. DOI: 10.1080/08916930400022954. [DOI] [PubMed] [Google Scholar]
- 14. Cimaz R, Stramba‐Badiale M, Brucato A, Catelli L, Panzeri P, Meroni PL. QT interval prolongation in asymptomatic anti‐SSA/Ro‐positive infants without congenital heart block. Arthritis Rheum. 2000;43:1049–1053. DOI: . [DOI] [PubMed] [Google Scholar]
- 15. Lazzerini PE, Acampa M, Guideri F, Capecchi PL, Campanella V, Morozzi G, Galeazzi M, Marcolongo R, Laghi‐Pasini F. Prolongation of the corrected QT interval in adult patients with anti‐Ro/SSA‐positive connective tissue diseases. Arthritis Rheum. 2004;50:1248–1252. DOI: 10.1002/art.20130. [DOI] [PubMed] [Google Scholar]
- 16. Bourré‐Tessier J, Clarke AE, Huynh T, Bernatsky S, Joseph L, Belisle P, Pineau CA. Prolonged corrected QT interval in anti‐Ro/SSA‐positive adults with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2011;63:1031–1037. DOI: 10.1002/acr.20470. [DOI] [PubMed] [Google Scholar]
- 17. Cimaz R, Meroni PL, Brucato A, Fesstovà V, Panzeri P, Goulene K, Stramba‐Badiale M. Concomitant disappearance of electrocardiographic abnormalities and of acquired maternal autoantibodies during the first year of life in infants who had QT interval prolongation and anti‐SSA/Ro positivity without congenital heart block at birth. Arthritis Rheum. 2003;48:266–268. DOI: 10.1002/art.10700. [DOI] [PubMed] [Google Scholar]
- 18. Boutjdir M, Lazzerini PE, Capecchi PL, Laghi‐Pasini F, El‐Sherif N. Potassium channel block and novel autoimmune‐associated long QT syndrome. Card Electrophysiol Clin. 2016;8:373–384. DOI: 10.1016/j.ccep.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 19. Lazzerini PE, Capecchi PL, Guideri F, Bellisai F, Selvi E, Acampa M, Costa A, Maggio R, Garcia‐Gonzalez E, Bisogno S, et al. Comparison of frequency of complex ventricular arrhythmias in patients with positive versus negative anti‐Ro/SSA and connective tissue disease. Am J Cardiol. 2007;100:1029–1034. DOI: 10.1016/j.amjcard.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 20. Duke C, Stuart G, Simpson JM. Ventricular tachycardia secondary to prolongation of the QT interval in a fetus with autoimmune mediated congenital complete heart block. Cardiol Young. 2005;15:319–321. DOI: 10.1017/S1047951105000673. [DOI] [PubMed] [Google Scholar]
- 21. Wang B, Hu S, Shi D, Bing Z, Li Z. Arrhythmia and/or cardiomyopathy related to maternal autoantibodies: descriptive analysis of a series of 16 cases from a single center. Front Pediatr. 2019;7:465. DOI: 10.3389/fped.2019.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lazzerini PE, Yue Y, Srivastava U, Fabris F, Capecchi PL, Bertolozzi I, Bacarelli MR, Morozzi G, Acampa M, Natale M, et al. Arrhythmogenicity of anti‐Ro/SSA antibodies in patients with torsades de pointes. Circ Arrhythm Electrophysiol. 2016;9:e003419. DOI: 10.1161/CIRCEP.115.003419. [DOI] [PubMed] [Google Scholar]
- 23. Hayashi N, Koshiba M, Nishimura K, Sugiyama D, Nakamura T, Morinobu S, Kawano S, Kumagai S. Prevalence of disease‐specific antinuclear antibodies in general population: estimates from annual physical examinations of residents of a small town over a 5‐year period. Mod Rheumatol. 2008;18:153–160. DOI: 10.3109/s10165-008-0028-1. [DOI] [PubMed] [Google Scholar]
- 24. Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, Jusko TA, Walker NJ, Germolec DR, Whitt IZ, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64:2319–2327. DOI: 10.1002/art.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo YP, Wang CG, Liu X, Huang YQ, Guo DL, Jing XZ, Yuan CG, Yang S, Liu JM, Han MS, et al. The prevalence of antinuclear antibodies in the general population of China: a cross‐sectional study. Curr Ther Res Clin Exp. 2014;76:116–119. DOI: 10.1016/j.curtheres.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lazzerini PE, Capecchi PL, Laghi‐Pasini F. Anti‐Ro/SSA antibodies and cardiac arrhythmias in the adult: facts and hypotheses. Scand J Immunol. 2010;72:213–222. DOI: 10.1111/j.1365-3083.2010.02428.x. [DOI] [PubMed] [Google Scholar]
- 27. Nakamura K, Katayama Y, Kusano KF, Haraoka K, Tani Y, Nagase S, Morita H, Miura D, Fujimoto Y, Furukawa T, et al. Anti‐KCNH2 antibody‐induced long QT syndrome: novel acquired form of long QT syndrome. J Am Coll Cardiol. 2007;50:1808–1809. DOI: 10.1016/j.jacc.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 28. Goldblatt F, O'Neill SG. Clinical aspects of autoimmune rheumatic diseases. Lancet. 2013;382:797–808. DOI: 10.1016/S0140-6736(13)61499-3. [DOI] [PubMed] [Google Scholar]
- 29. Murphy G, Lisnevskaia L, Isenberg D. Systemic lupus erythematosus and other autoimmune rheumatic diseases: challenges to treatment. Lancet. 2013;382:809–818. DOI: 10.1016/S0140-6736(13)60889-2. [DOI] [PubMed] [Google Scholar]
- 30. Boutjdir M, Chen L, Zhang ZH, Tseng CE, DiDonato F, Rashbaum W, Morris A, el‐Sherif N, Buyon JP. Arrhythmogenicity of IgG and anti‐52‐kD SSA/Ro affinity‐purified antibodies from mothers of children with congenital heart block. Circ Res. 1997;80:354–362. DOI: 10.1161/01.RES.80.3.354. [DOI] [PubMed] [Google Scholar]
- 31. Xiao GQ, Hu K, Boutjdir M. Direct inhibition of expressed cardiac L‐ and T‐type calcium channels by IgG from mothers whose children have congenital heart block. Circulation. 2001;103:1599–1604. DOI: 10.1161/01.CIR.103.11.1599. [DOI] [PubMed] [Google Scholar]
- 32. Karnabi E, Boutjdir M. Role of calcium channels in congenital heart block. Scand J Immunol. 2010;72:226–234. DOI: 10.1111/j.1365-3083.2010.02439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lazzerini PE, Capecchi PL, Acampa M, Morozzi G, Bellisai F, Bacarelli MR, Dragoni S, Fineschi I, Simpatico A, Galeazzi M, et al. Anti‐Ro/SSA‐associated corrected QT interval prolongation in adults: the role of antibody level and specificity. Arthritis Care Res (Hoboken). 2011;63:1463–1470. DOI: 10.1002/acr.20540. [DOI] [PubMed] [Google Scholar]
- 34. Radner H. Multimorbidity in rheumatic conditions. Wien Klin Wochenschr. 2016;128:786–790. DOI: 10.1007/s00508-016-1090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roden DM. Repolarization reserve: a moving target. Circulation. 2008;118:981–982. DOI: 10.1161/CIRCULATIONAHA.108.798918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S10
Figures S1–S2


