Abstract
Background
Commonly used cardiovascular risk calculators do not provide risk estimation of stroke, a major postoperative complication with high morbidity and mortality. We developed and validated an accurate cardiovascular risk prediction tool for stroke, major cardiac complications (myocardial infarction or cardiac arrest), and mortality after non‐cardiac surgery.
Methods and Results
This retrospective cohort study included 1 165 750 surgical patients over a 4‐year period (2007–2010) from the American College of Surgeons National Surgical Quality Improvement Program Database. A predictive model was developed with the following preoperative conditions: age, history of coronary artery disease, history of stroke, emergency surgery, preoperative serum sodium (≤130 mEq/L, >146 mEq/L), creatinine >1.8 mg/dL, hematocrit ≤27%, American Society of Anesthesiologists physical status class, and type of surgery. The model was trained using American College of Surgeons National Surgical Quality Improvement Program data from 2007 to 2009 (n=809 880) and tested using data from 2010 (n=355 870). Risk models were developed using multivariate logistic regression. The outcomes were postoperative 30‐day stroke, major cardiovascular events (myocardial infarction, cardiac arrest, or stroke), and 30‐day mortality. Major cardiac complications occurred in 0.66% (n=5332) of patients (myocardial infarction, 0.28%; cardiac arrest, 0.41%), postoperative stroke in 0.25% (n=2005); 30‐day mortality was 1.66% (n=13 484). The risk prediction model had high predictive accuracy with area under the receiver operating characteristic curve for stroke (training cohort=0.869, validation cohort=0.876), major cardiovascular events (training cohort=0.871, validation cohort=0.868), and 30‐day mortality (training cohort=0.922, validation cohort=0.925). Surgery types, history of stroke, and coronary artery disease are significant risk factors for stroke and major cardiac complications.
Conclusions
Postoperative stroke, major cardiac complications, and 30‐day mortality can be predicted with high accuracy using this web‐based predictive model.
Keywords: myocardial infarction, prediction, stroke, surgery
Subject Categories: Cerebrovascular Disease/Stroke, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- ACS‐NSQIP
American College of Surgeons National Surgical Quality Improvement Program Database
- EVAR
endovascular aneurysm repair
Clinical Perspective
What Is New?
A non‐cardiac surgery risk prediction tool newly includes 30‐day postoperative stroke risk, along with major cardiac complications and mortality.
This new risk model was developed based on >1 million patients from the American College of Surgeons National Surgical Quality Improvement Program Database.
The risk model includes 9 predictors and has excellent predictive performance.
What Are the Clinical Implications?
The important clinical risk of postoperative stroke can now be stratified along with standard cardiac complications and mortality risk using this web‐based risk model during preoperative evaluation.
Individualized risk assessment of stroke, cardiac complications, and mortality helps clinicians better counsel patients and inform surgeons and possibly implement measures to lower the risk.
Stroke is a rare, life threatening complication of surgery with high morbidity and mortality. 1 Mortality from postoperative stroke was reported to be 26% after general surgery procedures and can increase to 60% in those with prior stroke. 2 , 3 Furthermore, patients experiencing in‐hospital stroke have worse outcomes compared with community‐onset stroke, where nearly 50% of the in‐patient strokes in 1 cohort were perioperative. 4 Because stroke in the postoperative period is associated with a delay to diagnostic imaging and lower use of thrombolysis given the risks of recent surgery, surgical patients are at higher risk for poor outcomes. 4
Accurate assessment of postoperative risk using readily available clinical information helps determine the need for change in management in the perioperative period and timing of surgery. Consequently, the American Heart Association and American College of Cardiology guidelines emphasize the use of clinical risk factors for preoperative cardiac evaluation. 5
A major drawback of commonly used risk estimators is a lack of assessment for postoperative stroke risk. The Revised Cardiac Risk Index and Gupta Myocardial Infarction or Cardiac Arrest (MICA) risk calculator have been used extensively to predict postoperative cardiac complications. 6 Revised Cardiac Risk Index is limited by relatively moderate predictive accuracy (area under the curve [AUC], 0.759). 7 , 8 The Gupta MICA risk calculator is only limited to myocardial infarction or cardiac arrest. 6 These calculators do not estimate the risk of stroke, and mortality. The Universal American College of Surgeons National Surgical Quality Improvement Program (ACS‐NSQIP) Surgical Risk Calculator includes 21 preoperative factors to predict 8 outcomes. 9 This calculator also does not assess the risk of postoperative stroke and its inclusion of 21 predictors limits its ease of use and utility by clinicians.
The aim of this study is to identify risk factors for stroke, major cardiovascular complications, and 30‐day mortality.
Methods
Study Participants
All data in this study are available from the ACS‐NSQIP. Data used in the analysis were extracted from the ACS‐NSQIP database from 2007 to 2010. The ACS‐NSQIP data set is a multicenter, prospective database with >500 US and international hospitals (academic, community) participating in data collection. 10 , 11 Trained surgical clinical reviewers at each participating hospital compiled the data. Each patient was followed for 30 days after surgery and complications within this period were included in the database. 12 Variables examined in the analysis included age, sex, smoking, alcohol use (>2 drinks/day in 2 weeks before admission), history of coronary artery disease (defined as myocardial infarction within 6 months of surgery, history of angina within 1 month before surgery, previous percutaneous coronary intervention), previous cardiac surgery, history of congestive heart failure, history of stroke, hypertension medication use, peripheral vascular disease, chronic obstructive pulmonary disease, dyspnea, weight loss, bleeding disorder, dialysis, steroid use, rest pain, preoperative sepsis, chemotherapy, disseminated cancer, esophageal varices, ascites, emergency procedure, diabetes mellitus, acute renal failure (defined by increase in blood urea nitrogen and a rising creatinine of >3 mg/dL, within 24 hours before surgery), hemiplegia, quadriplegia, paraplegia, American Society of Anesthesiologists physical status class, and preoperative functional status. Risk factors are further described in Table 1. Preoperative laboratory testing included in the analysis were serum sodium, creatinine, white blood cell, platelet, and hematocrit. Vascular surgery was divided into carotid endarterectomy, endovascular aneurysm repair (EVAR), thoracic EVAR, suprainguinal bypass, infrainguinal bypass, open aortic aneurysm repair, other vascular surgery as described previously. 13 , 14 Figure S1 shows the study participant flowchart.
Table 1.
Characteristics of the Patients (Patients From 2007–2009)
| No MI/Arrest (n=804 548) | MI/Arrest (n=5332) | Unadjusted Odds Ratio (95% CI) for MI/Arrest | No Stroke (n=807 875) | Stroke (n=2005) | Unadjusted Odds Ratio (95% CI) For Stroke | |
|---|---|---|---|---|---|---|
| Age, y (mean±SD) | 55.4 (±17.1) | 69.8 (±13.0) | 1.059 (1.057–1.061) | 55.4 (±17.2) | 70.0 (±13.6) | 1.06 (1.056–1.063) |
| Sex (men) (%) | 42.52 | 57.58 | 1.83 (1.74–1.94) | 42.6 | 48.8 | 1.29 (1.18–1.4) |
| ASA class* 1 (%) (Ref=1) | 9.90 | 0.24 | 9.86 | 0.40 | ||
| 2 | 46.00 | 7.71 | 6.82 (3.93–11.85) | 45.83 | 10.9 | 5.89 (2.91–11.93) |
| 3 | 37.62 | 48.9 | 52.83 (30.63–91.11) | 37.65 | 56.26. | 36.94 (18.43–74.04) |
| 4 | 6.20 | 38.14 | 248.78 (144.19–429.24) | 6.35 | 30.22 | 117.58 (58.53–236.21) |
| 5 | 0.27 | 5.09 | 767.5 (439.25–1341.04) | 0.30 | 2.19 | 182.6 (85.87–388.26) |
| Diabetes mellitus (%) | 14.79 | 32.15 | 2.73 (2.58–2.89) | 14.9 | 26.78 | 2.09 (1.9–2.31) |
| Alcohol use >2 drinks/day in 2 wks before admission (%) | 2.61 | 3.98 | 1.54 (1.34–1.77) | 2.6 | 4.84 | 1.89 (1.54–2.32) |
| Chemotherapy (%) | 1.06 | 1.82 | 1.73 (1.42–2.12) | 1.06 | 1.35 | 1.27 (0.87–1.86) |
| Disseminated cancer (%) | 1.93 | 4.24 | 2.25 (1.97–2.57) | 1.94 | 2.74 | 1.42 (1.09–1.86) |
| Paraplegia (%) | 0.49 | 0.99 | 2.04 (1.56–2.68) | 0.49 | 0.75 | 1.53 (0.92–2.54) |
| Quadriplegia (%) | 0.13 | 0.43 | 3.38 (2.24–5.12) | 0.13 | 0.15 | 1.15 (0.37–3.58) |
| Hemiplegia (%) | 0.92 | 2.87 | 3.17 (2.70–3.73) | 0.92 | 6.93 | 8.01 (6.74–9.54) |
| Smoker within 1 y (%) | 20.68 | 24.46 | 1.24 (1.17–1.32) | 20.69 | 26.43 | 1.38 (1.25–1.52) |
| Pack‐year of smoking | 10.59 | 23.73 | 1.02 (1.01–1.02) | 10.64 | 23.44 | 1.01 (1.01–1.02) |
| Esophageal varices (%) | 0.12 | 0.32 | 2.72 (1.68–4.4) | 0.12 | 0.05 | 0.42 (0.06–2.98) |
| Chronic obstructive pulmonary disease (%) | 4.73 | 16.97 | 4.12 (3.83–4.42) | 4.79 | 14.11 | 3.27 (2.88–3.71) |
| Ascites (%) | 1.01 | 5.29 | 5.46 (4.83–6.17) | 1.04 | 2.19 | 2.14 (1.59–2.89) |
| Congestive heart failure (%) | 0.83 | 7.28 | 9.41 (8.46–10.46) | 0.86 | 4.49 | 5.41 (4.38–6.7) |
| Myocardial infarction (%) | 0.58 | 5.61 | 10.2 (9.04–11.5) | 0.60 | 3.84 | 6.57 (5.22–8.26) |
| Previous cardiac surgery (%) | 5.73 | 21.23 | 4.43 (4.15–4.74) | 5.80 | 18.45 | 3.67 (3.28–4.11) |
| Previous percutaneous coronary intervention (%) | 5.33 | 17.74 | 3.83 (3.57–4.11) | 5.40 | 12.77 | 2.56 (2.25–2.93) |
| Angina (%) | 0.68 | 4.05 | 6.12 (5.33–7.04) | 0.70 | 2.84 | 4.14 (3.18–5.4) |
| Peripheral vascular disease (%) | 4.00 | 17.18 | 4.98 (4.63–5.35) | 4.06 | 13.17 | 3.58 (3.14–4.08) |
| Hypertension on medication (%) | 45.65 | 77.83 | 4.18 (3.92–4.46) | 45.8 | 77.61 | 4. 1 (3.69–4.56) |
| Acute renal failure (%) | 0.55 | 5.98 | 11.47 (10.2–12.89) | 0.58 | 3.14 | 5.55 (4.31–7.14) |
| Dialysis (%) | 1.97 | 12.23 | 6.93 (6.38–7.54) | 2.03 | 5.79 | 2.97 (2.46–3.58) |
| Steroid (%) | 3.05 | 7.48 | 2.57 (2.32–2.85) | 3.08 | 5.99 | 2.01 (1.67–2.41) |
| Stroke with neurologic deficit (%) | 2.30 | 7.95 | 3.67 (3.32–4.05) | 2.31 | 15.91 | 8.02 (7.11–9.04) |
| Stroke with no neurologic deficit (%) | 2.01 | 6.06 | 3.14 (2.81–3.52) | 2.02 | 9.93 | 5.35 (4.62–6.2) |
| Transient ischemic attack (%) | 2.89 | 7.69 | 2.8 (2.53–3.09) | 2.89 | 15.31 | 6.07 (5.37–6.86) |
| Weight loss (%) | 2.23 | 6.98 | 3.29 (2.96–3.66) | 2.26 | 4.19 | 1.89 (1.52–2.36) |
| Bleeding disorder (%) | 5.52 | 20.46 | 4.4 (4.11–4.71) | 5.58 | 20.75 | 4.43 (3.97–4.93) |
| Emergency (%) | 12.39 | 34.02 | 3.65 (3.44–3.86) | 12.50 | 26.63 | 2.54 (2.3–2.81) |
| Rest Pain (%) | 2.28 | 11.07 | 5.33 (4.89–5.82) | 2.32 | 8.13 | 3.72 (3.17–4.37) |
| White blood count, (109/L) | 8.44 | 10.69 | 8.45 | 9.72 | ||
| Hematocrit (%) | 38.98 | 35.02 | 38.95 | 36.61 | ||
| Platelet (109/L) | 261.5 | 240.72 | 261.4 | 246.67 | ||
| Partial thromboplastin time | 30.79 | 34.61 | 30.82 | 33.91 | ||
| International normalized ratio | 1.11 | 1.28 | 1.11 | 1.19 |
ASA class indicates American Society of Anesthesiologists Physical Status Classification; and MI, myocardial infarction.
Outcome
The outcomes were 30‐day postoperative stroke, major cardiovascular events (myocardial infarction, cardiac arrest, or stroke), or 30‐day postoperative mortality.
Stroke is defined by the ACS‐NSQIP as "An embolic, thrombotic, or hemorrhagic vascular accident or stroke with motor, sensory, or cognitive dysfunction (eg, hemiplegia, hemiparesis, aphasia, sensory deficit, impaired memory) that persists for 24 or more hours". 15
Cardiac arrest is defined by the ACS‐NSQIP as “The absence of cardiac rhythm or presence of chaotic cardiac rhythm which results in a cardiac arrest requiring the initiation of CPR. Patients are included who are in a pulseless ventricular tachycardia or ventricular fibrillation in which defibrillation is performed and pulseless electrical activity arrest requiring chest compressions.” 15 Myocardial infarction was defined in the ACS‐NSQIP database as “A new transmural acute myocardial infarction occurring during surgery or within 30 days as manifested by new Q‐waves on ECG”. 15
Statistical Analysis
A risk prediction model was built based on the data set from 2007 to 2009 (n=809 880). The data set from 2010 (n=355 870) was used to test and validate the predictive model. Patient risk factors were compared using χ 2 for categorical variables and Wilcoxon for continuous variables. Bivariate odds ratio with 95% CI were calculated.
Clinical predictors were selected using backwards elimination using Akaike information criterion for cardiac complications, stroke complications, and mortality. Predictors that were significant in cardiac, stroke, and mortality were included in the model. Even though coronary artery disease history was not selected based on the Akaike information criterion for stroke model, it was included in the final model because of its clinical importance in cardiac risk prediction.
The logistic regression models were constructed with 30‐day postoperative cardiac complications, stroke complications, and death as the dependent variables. Multivariate logistic regression analysis was performed for predictor coefficient and adjusted odds ratio. AUC was used for model evaluation. Best model parameters were selected using GridSearchCV package with 5‐fold cross validation. Missing laboratory values (hematocrit, creatinine, and sodium) had separate categorical levels.
Statistical analysis was performed with Python (version 3.6.6.), Statsmodels (version 0.9.0), r programming (RStudio version 1.1.463). Scikit‐learn was used for machine learning modeling. 16 This study was approved by Thomas Jefferson University institutional review board, and informed consent from study participants was waived.
Results
Study Population
A total of 1 165 750 patients from 2007 to 2010 were included in the analysis and 57.4% were women. A risk prediction model was developed based on 809 880 participants from 2007 to 2009. Table 1 shows characteristics of study participants from the years 2007 to 2009. Major cardiac complications were prevalent in 0.66% (n=5332) of patients (myocardial infarction, 0.28%; cardiac arrest, 0.41%). Postoperative stroke was found in 0.25% (n=2005) of patients. The stroke rate was similar to prior studies reporting an incidence of strokes between 0.1% and 0.7% in patients undergoing non‐cardiac surgery; 17 , 18 , 19 , 20 1.66% (n=13 484) of patients died within 30 days after surgery. Patients with postoperative cardiac complications and stroke were significantly older than patients who did not have these complications (P<0.001). History of stroke and transient ischemic attack are also significantly associated with a high rate of postoperative stroke (odds ratio [OR]=8.02, 6.07; P<0.001). Patients with major cardiac complications had higher prevalence of preoperative coronary artery disease. A history of percutaneous coronary intervention, prior cardiac surgery, and prior myocardial infarction were likewise significantly associated with major cardiac events (OR=3.83, 4.43, 10.2; P<0.001). Preoperative renal dysfunction including acute renal failure was significantly associated with postoperative major cardiac complications (OR=11.47; P<0.001) and stroke (OR=5.55; P<0.001) with dialysis as a strong predictor of cardiovascular morbidity (cardiac events OR=6.93; stroke OR=2.97; P<0.001).
Stroke, Myocardial Infarction, and Mortality Rate
Patients with a postoperative stroke had a high 30‐day unadjusted mortality rate (23.8%), at a rate similar to prior studies. 2 , 17 , 19 Postoperative stroke had an OR of 19.08 (95% CI, 17.19–21.17) for death within 30 days postoperatively. The unadjusted mortality rate associated with stroke is similar to that for postoperative myocardial infarction (24.4%, OR=19.88; 95% CI, 18.05–21.90). Our study showed 15.8% of myocardial infarctions, 10.9% of cardiac arrests, and 25.0% of strokes occurred after the initial hospital stay.
Prediction of Postoperative Stroke
Figure 1 and Table S1 show predictors of postoperative stroke in a multivariate model. History of stroke, including transient ischemic attack, is significantly associated with postoperative stroke complications (P<0.001; OR=2.3). Emergency surgery is also highly significant (P<0.001; OR=1.89). Three other predictors (age, preoperative serum hematocrit, creatinine) were also found to be significant. Figure S2 shows unadjusted stroke complication rates according to preoperative serum sodium, creatinine, and hematocrit levels. Anemia (hematocrit ≤27%) is associated with increased risk of stroke (OR=1.29; P<0.001). The risk of stroke also increases with renal dysfunction (serum creatinine >1.8 mg/dL; OR=1.41; P<0.001). Specific types of high‐risk surgery such as carotid endarterectomy, thoracic EVAR, vascular‐aorta surgery, and brain surgery are significantly associated with stroke. However, there is no significant association with history of coronary artery disease (P=0.73). The model has excellent AUC (training cohort=0.869, validation cohort=0.876) and brier score (0.002). Figure S3A shows excellent calibration.
Figure 1. Forest plot and adjusted odds ratio of predictors for 30‐day postoperative stroke complication.
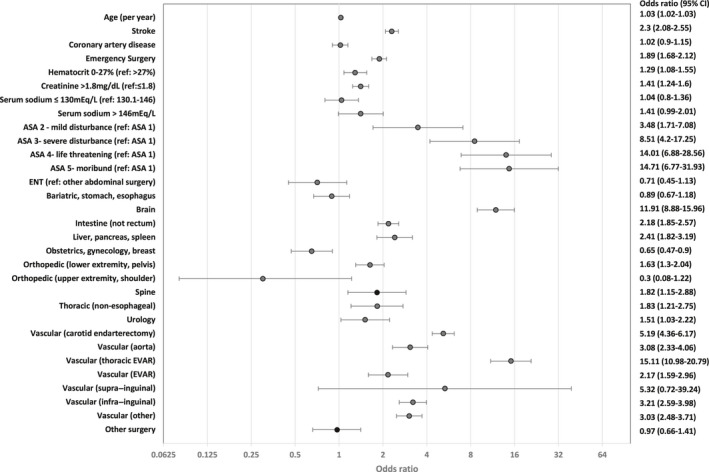
ASA indicates American Society of Anesthesiologists Physical Status Classification; ENT‐ear, nose, throat; and EVAR‐endovascular aneurysm repair.
Prediction of Postoperative Major Cardiac and Cerebrovascular Complications — Myocardial Infarction, Cardiac Arrest, and/or Stroke
Figure 2 and Table S2 show predictors of major cardiovascular complications in a multivariate model. The major complications include the occurrence of myocardial infarction, cardiac arrest, or stroke within 30 days after surgery. History of coronary artery disease and stroke were significant predictors with high adjusted odds of major complications (adjusted OR=1.43, 1.46). Hypernatremia (serum sodium >146 mEq/L) was associated with increased risk of major complications (OR=1.67; P<0.001). Hyponatremia (serum sodium ≤130 mEq/L) also had increased risk (OR=1.2; P<0.001). Compared with reference renal function (serum creatinine, Cr [0–1.8 mg/dL]), renal dysfunction (>1.8 mg/dL) has increased adjusted odds of complications (OR=1.96; P<0.001). Anemia was another predictor that had high adjusted odds of major complications. Serum hematocrit (≤27%) has increased odds (1.42 [95% CI, 1.31–1.55]). Figure S4 shows unadjusted major cardiovascular complication rates according to serum sodium, creatinine, and hematocrit from the patient cohort in 2007 to 2009. The model for major cardiovascular complications had excellent AUC (training cohort=0.871, validation cohort=0.868) and brier score (0.008). Figure S3B shows excellent calibration.
Figure 2. Forest plot and adjusted odds ratio of predictors for 30‐day postoperative major cardiovascular complications (myocardial infarction, cardiac arrest, and/or stroke).
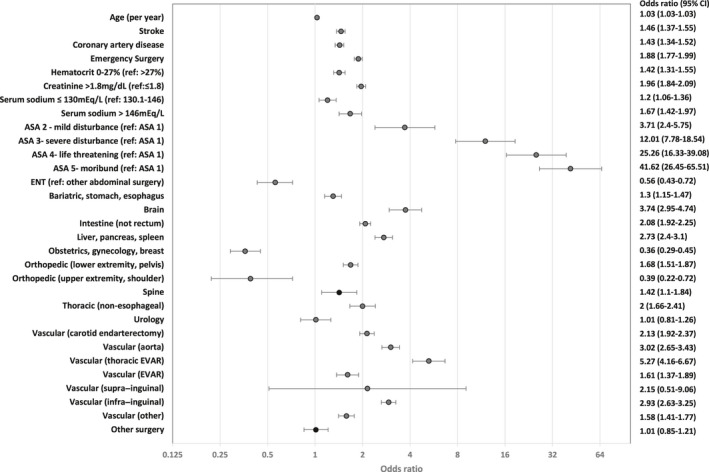
ASA indicates American Society of Anesthesiologists Physical Status Classification; ENT‐ear, nose, throat; and EVAR‐endovascular aneurysm repair.
In predicting postoperative 30‐day myocardial infarction, stroke and death, this model has greater predictive power in the validation cohort (AUC, 0.904) compared with the Dakik cardiovascular index (AUC, 0.82). 21
Prediction of Postoperative Myocardial Infarction and Cardiac Arrest
The cardiac risk model with 9 predictors has excellent AUC (training cohort=0.879, validation cohort=0.872), and brier score (0.006). Tables S3 and S4 show predictors of major cardiac complications in a multivariate model.
Prediction of Postoperative 30‐Day Mortality
The mortality risk model with 9 predictors had outstanding AUC (training cohort=0.922, validation cohort=0.925). Figure S3C shows excellent calibration.
Table S5 shows predictors of 30‐day mortality in the model.
Validation of Prediction Model
The model derived in the training cohort was validated and tested by applying the model on the validation cohort of 355 870 patients from 2010. Figure 3 shows the model performance (receiver operating characteristic curve) of the validation cohort. The prediction model also used a 5‐fold cross validation method for the training cohort from 2007 to 2009. AUC from 5‐fold cross validation also showed high predictive performance for major cardiovascular complications (mean AUC, 0.870), stroke (mean AUC, 0.866), cardiac risk (mean AUC, 0.877), and death (mean AUC, 0.922).
Figure 3. Model performance (receiver operating characteristic curve) for stroke and 30‐day mortality in the validation group.
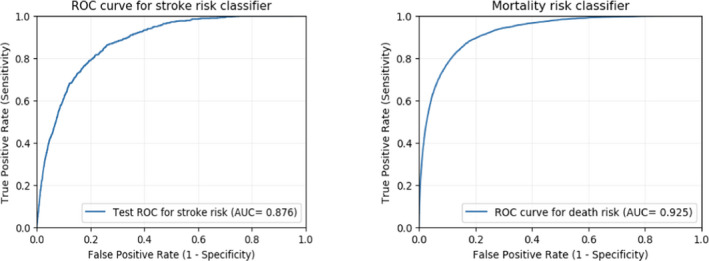
AUC indicates area under the receiver operating characteristic curve.
Stroke and Major Cardiac Complications According to Surgery Types
Figure S5 shows the occurrences of 30‐day postoperative stroke and cardiac complications (myocardial infarction and stroke) according to surgery types. Vascular (thoracic‐EVAR), brain surgery, and carotid endarterectomy had the highest rates of stroke. Open aorta surgery has a higher incidence of stroke compared with EVAR (1.12% versus 0.5%). In regard to cardiac complications, open aorta surgery had the highest risk (4.51%) followed by vascular (thoracic‐EVAR, 3.79%; suprainguinal bypass, 3.46%; EVAR, 1.67%), intestinal surgery (1.51%), and liver/pancreas/spleen (1.4%) surgery.
Practical Application of Risk Model
A probability (%) of 30‐day postoperative stroke, major cardiovascular events, and mortality can be calculated using coefficients of predictors from multivariate logistic regression models. Figure 4 shows a web‐based risk model. A mobile web‐based risk prediction model was built using a machine learning library, Scikit‐Learn (https://www.predictionmodel.org/).
Figure 4. Mobile web‐based cardiac, stroke, mortality risk model.
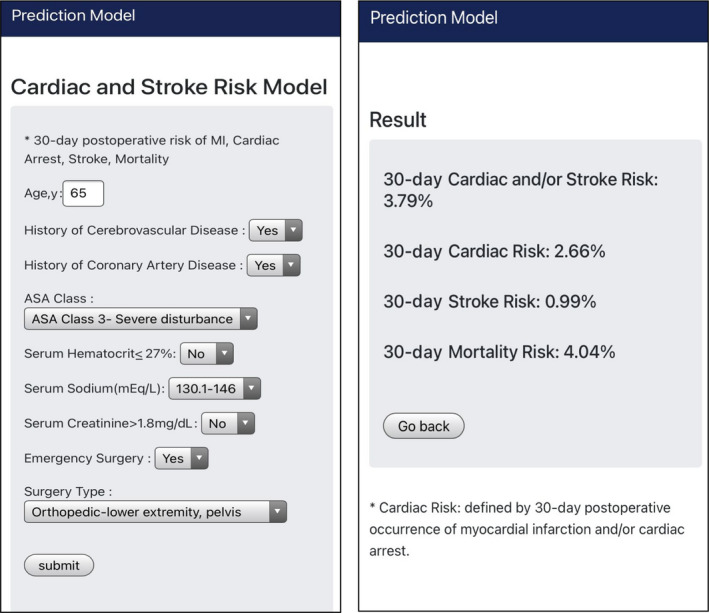
MI indicates myocardial infarction.
Here are 2 examples of the practical application of stroke, the major cardiovascular (myocardial infarction, cardiac arrest, or stroke), cardiac risk (MI, cardiac arrest), and mortality risk calculator in the clinical setting.
A 65‐year‐old man with a history of coronary artery disease, a history of stroke was admitted to a hospital for emergency hip fracture surgery. The patient has American Society of Anesthesiologists (ASA) physical status class 3, hematocrit 30%, serum sodium 143 mEq/L, serum creatinine 1.5 mg/dL; 30‐day postoperative cardiac or stroke risk 3.79%, cardiac risk 2.66%, stroke risk 0.99%, 30‐day mortality risk 4.04%.
A 70‐year‐old woman with a history of stroke, no history of coronary artery disease is scheduled to have elective spine surgery. The patient has ASA physical status class 3, hematocrit 33%, serum sodium 128 mEq/L, serum creatinine 2.5 mg/dL.; 30‐day postoperative cardiac or stroke risk 3.27%, cardiac risk 2.09%, stroke risk 0.96%, 30‐day mortality risk 3.91%.
Discussion
Our study demonstrated a well‐validated prediction model built on 1.16 million patients over 4 years for postoperative stroke, major cardiovascular complications (myocardial infarction, cardiac arrest, or stroke), and death. Although it is well established that stroke is a major complication of surgery with a high mortality rate, 2 , 17 , 19 to our knowledge there are no effective prediction models for individualized estimation of postoperative stroke and cardiac risk. Using 9 clinical variables in a national cohort of patient data from the robust ACS‐NSQIP database collected over 4 years, we developed and validated a clinically useful risk assessment model for stroke, cardiac, and mortality risk.
Our stroke predictive model has excellent discriminative ability. Wilcox et al 22 compared existing cardiovascular risk scores in stroke prediction and our model had higher predictive power (AUC, 0.876) for postoperative stroke compared with Gupta MICA (AUC, 0.833), American College of Surgeons surgical risk calculator (AUC, 0.836), Mashour (AUC, 0.773), CHA2DS2‐VASc (AUC, 0.744), and Revised Cardiac Risk Index (AUC, 0.743). 18,22
Our study found that even small increases of serum sodium level are associated with an increased risk of major cardiovascular complications. Anemia, prior stroke, age, renal dysfunction, and ASA class (class 4 and 5) were also associated with increased risk of postoperative stroke.
In addition to the stroke prediction, our model has excellent major cardiovascular complication prediction (validation AUC, 0.868). History of stroke and coronary artery disease were significant predictors of postoperative cardiovascular complications similar to the Revised Cardiac Risk Index. In the Gupta MICA model, these cardiovascular predictors are not included in the risk calculator. 6 The Gupta MICA and ACS‐NSQIP risk calculators do not use history of coronary artery disease such as prior myocardial infarction or angina in their calculators for cardiac complications. We think it is important to include history of coronary artery disease in the model as coronary artery disease history was shown to be a significant risk factor for future cardiac events. 23
Renal dysfunction is a significant risk factor for both stroke and major cardiac events in our study. The association with stroke and cardiac complications may be accounted for by the accelerated atherosclerosis associated with renal disease. 24 , 25 Renal disease was previously reported as a risk factor for perioperative stroke in cardiac surgery. 26
We differentiated specific types of surgery to further delineate specific risk factors for stroke and cardiac complications. Risk estimates based on the type of procedure are clinically relevant. In terms of stroke risk, brain, and vascular surgery (carotid endarterectomy, aorta, thoracic endovascular aneurysm repair) demonstrate the highest risk. Upper vascular and aortic procedures demonstrate a much higher risk than other lower or peripheral vascular procedures, consistent with prior studies. 27
Other studies showed preoperative hyponatremia and hypernatremia are associated with significant postoperative mortality and morbidity such as major coronary events, pneumonia, and venous thromboembolism. 28 , 29 , 30 Anemia is known to be associated with postoperative cardiac events, and increased hematocrit is associated with pulmonary embolism and deep vein thrombosis. 31 , 32 , 33
In our analysis, a significant proportion of postoperative myocardial infarctions (15.8%), cardiac arrests (10.9%), and strokes (25.0%) occurred after discharge. The Revised Cardiac Risk Index is limited in that the model does not predict the occurrence of cardiac complications after discharge unlike our model. 8 , 34
Our model (AUC, 0.789) performs as well as Geriatrics‐Sensitive Cardiac Index (AUC, 0.76) in geriatrics population (aged ≥65 years) for cardiac risk prediction. 35 In addition, this model has greater predictive ability in postoperative myocardial infarction, stroke, and death (validation AUC, 0.904) compared with Dakik cardiovascular index (AUC, 0.82). 21
Our study has several strengths. First, a large number of patients were included in the analysis (n=1 165 750) compared with Revised Cardiac Risk Index (n=4315) and Gupta (n=468 795). Second, our study included more recent data over a longer period of time from years 2007 to 2010 compared with Revised Cardiac Risk Index (years 1989–1994, from single hospital) and Gupta (years 2007–2008). 6 , 7 Third, our model may help clinicians not only assess the surgical risk but potentially reduce the risk by optimizing serum sodium and hematocrit levels preoperatively, a concept requiring further study. The Revised Cardiac Index, Gupta model, and ACS‐NSQIP risk calculator do not include these potentially modifiable risk factors.
Despite its many strengths, this study has some limitations. First, the timing of prior stroke and history of atrial fibrillation are important considerations in risk assessment of stroke before surgery. 36 In addition, new postoperative atrial fibrillation with low rates of anticoagulation initiation within 30 days of discharge, has been found to be associated with increased postoperative risk of stroke after non‐cardiac surgery. 37 However, these variables were not included in the model because such information was not available for analysis. We used database analysis from 2007 to 2010 in our study because more recent data did not include history of coronary artery disease, or stroke in the data sets. In conclusion, our study developed a clinically relevant web‐based postoperative stroke, major cardiovascular, and mortality risk prediction model. Our model allows for more individualized risk prediction and assessment for postoperative stroke and cardiac complications.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S5
Figs S1–S5
Acknowledgments
The authors thank Dr Inna Chervoneva for suggestions with statistical analysis. The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
(J Am Heart Assoc.2021;10:e018013. DOI: 10.1161/JAHA.120.018013.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018013
For Sources of Funding and Disclosures, see page 9.
References
- 1. Wong GY, Warner DO, Schroeder DR, Offord KP, Warner MA, Maxson PM, Whisnant JP. Risk of surgery and anesthesia for ischemic stroke. Anesthesiology. 2000;92:425–432. [DOI] [PubMed] [Google Scholar]
- 2. Parikh S, Cohen JR. Perioperative stroke after general surgical procedures. N Y State J Med. 1993;93:162–165. [PubMed] [Google Scholar]
- 3. Landercasper J, Merz BJ, Cogbill TH, Strutt PJ, Cochrane RH, Olson RA, Hutter RD. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125:986–989. [DOI] [PubMed] [Google Scholar]
- 4. Saltman AP, Silver FL, Fang J, Stamplecoski M, Kapral MK. Care and outcomes of patients with in‐hospital stroke. JAMA Neurol. 2015;72:749–755. [DOI] [PubMed] [Google Scholar]
- 5. Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila‐Roman VG, Gerhard‐Herman MD, Holly TA, Kane GC, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e278–e333. [DOI] [PubMed] [Google Scholar]
- 6. Gupta PK, Gupta H, Sundaram A, Kaushik M, Fang X, Miller WJ, Esterbrooks DJ, Hunter CB, Pipinos II, Johanning JM, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–387. [DOI] [PubMed] [Google Scholar]
- 7. Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. [DOI] [PubMed] [Google Scholar]
- 8. Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152:26–35. [DOI] [PubMed] [Google Scholar]
- 9. Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833–842.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen ME, Ko CY, Bilimoria KY, Zhou L, Huffman K, Wang X, Liu Y, Kraemer K, Meng X, Merkow R, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217:336–346.e1. [DOI] [PubMed] [Google Scholar]
- 11. Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250:363–376. DOI: 10.1097/SLA.0b013e3181b4148f . [DOI] [PubMed] [Google Scholar]
- 12. American College of Surgeons NSQIP . User guide for the 2012 participant use data file.
- 13. Record M . Society for vascular surgery.
- 14. Bertges DJ, Neal D, Schanzer A, Scali ST, Goodney PP, Eldrup‐Jorgensen J, Cronenwett JL. Vascular quality initiative. The vascular quality initiative cardiac risk index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411–1421.e4. DOI: 10.1016/j.jvs.2016.04.045 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American College of Surgeons NSQIP . User guide for the 2011 participant use data file.
- 16. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, et al. Scikit‐Learn: machine learning in python. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 17. Bateman BT, Schumacher HC, Wang S, Shaefi S, Berman MF. Perioperative acute ischemic stroke in noncardiac and nonvascular surgery: incidence, risk factors, and outcomes. Anesthesiology. 2009;110:231–238. DOI: 10.1097/ALN.0b013e318194b5ff . [DOI] [PubMed] [Google Scholar]
- 18. Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114:1289–1296. DOI: 10.1097/ALN.0b013e318216e7f4 . [DOI] [PubMed] [Google Scholar]
- 19. Vasivej T, Sathirapanya P, Kongkamol C. Incidence and risk factors of perioperative stroke in noncardiac, and nonaortic and its major branches surgery. J Stroke Cerebrovasc Dis. 2016;25:1172–1176. DOI: 10.1016/j.jstrokecerebrovasdis.2016.01.051 . [DOI] [PubMed] [Google Scholar]
- 20. El‐Saed A, Kuller LH, Newman AB, Lopez O, Costantino J, McTigue K, Cushman M, Kronmal R. Factors associated with geographic variations in stroke incidence among older populations in four US communities. Stroke. 2006;37:1980–1985. DOI: 10.1161/01.STR.0000231454.77745.d9 . [DOI] [PubMed] [Google Scholar]
- 21. Dakik HA, Chehab O, Eldirani M, Sbeity E, Karam C, Abou Hassan O, Msheik M, Hassan H, Msheik A, Kaspar C, et al. A new index for pre‐operative cardiovascular evaluation. J Am Coll Cardiol. 2019;73:3067–3078. DOI: 10.1016/j.jacc.2019.04.023 . [DOI] [PubMed] [Google Scholar]
- 22. Wilcox T, Smilowitz NR, Xia Y, Berger JS. Cardiovascular risk scores to predict perioperative stroke in noncardiac surgery. Stroke. 2019;50:2002–2006. DOI: 10.1161/STROKEAHA.119.024995 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smolina K, Wright FL, Rayner M, Goldacre MJ. Long‐term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5:532–540. DOI: 10.1161/CIRCOUTCOMES.111.964700 . [DOI] [PubMed] [Google Scholar]
- 24. Schiffrin EL, Lipman ML, Mann JFE. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. DOI: 10.1161/CIRCULATIONAHA.106.678342 . [DOI] [PubMed] [Google Scholar]
- 25. Lamprea‐Montealegre JA, McClelland RL, Grams M, Ouyang P, Szklo M, de Boer IH. Coronary heart disease risk associated with the dyslipidaemia of chronic kidney disease. Heart. 2018;104:1455–1460. DOI: 10.1136/heartjnl-2017-312794 . [DOI] [PubMed] [Google Scholar]
- 26. D’Ancona G, Saez de Ibarra JI, Baillot R, Mathieu P, Doyle D, Metras J, Desaulniers D, Dagenais F. Determinants of stroke after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2003;24:552–556. DOI: 10.1016/S1010-7940(03)00440-8 . [DOI] [PubMed] [Google Scholar]
- 27. Axelrod DA, Stanley JC, Upchurch GR, Khuri S, Daley J, Henderson W, Demonner S, Henke PK. Risk for stroke after elective noncarotid vascular surgery. J Vasc Surg. 2004;39:67–72. DOI: 10.1016/j.jvs.2003.08.028 . [DOI] [PubMed] [Google Scholar]
- 28. Leung AA, McAlister FA, Finlayson SRG, Bates DW. Preoperative hypernatremia predicts increased perioperative morbidity and mortality. Am J Med. 2013;126:877–886. DOI: 10.1016/j.amjmed.2013.02.039 . [DOI] [PubMed] [Google Scholar]
- 29. Mc Causland FR, Wright J, Waikar SS. Association of serum sodium with morbidity and mortality in hospitalized patients undergoing major orthopedic surgery. J Hosp Med. 2014;9:297–302. DOI: 10.1002/jhm.2168 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122:857–865. DOI: 10.1016/j.amjmed.2009.01.027 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta PK, Sundaram A, MacTaggart JN, Johanning JM, Gupta H, Fang X, Forse RA, Balters M, Longo GM, Sugimoto JT, et al. Preoperative anemia is an independent predictor of postoperative mortality and adverse cardiac events in elderly patients undergoing elective vascular operations. Ann Surg. 2013;258:1096–1102. DOI: 10.1097/SLA.0b013e318288e957 . [DOI] [PubMed] [Google Scholar]
- 32. Wu W‐C, Schifftner TL, Henderson WG, Eaton CB, Poses RM, Uttley G, Sharma SC, Vezeridis M, Khuri SF, Friedmann PD. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297:2481–2488. DOI: 10.1001/jama.297.22.2481 . [DOI] [PubMed] [Google Scholar]
- 33. Musallam KM, Porter JB, Sfeir PM, Tamim HM, Richards T, Lotta LA, Peyvandi F, Jamali FR. Raised haematocrit concentration and the risk of death and vascular complications after major surgery. Br J Surg. 2013;100:1030–1036. DOI: 10.1002/bjs.9176 . [DOI] [PubMed] [Google Scholar]
- 34. Cohn SL, Fernandez RN. Comparison of 4 cardiac risk calculators in predicting postoperative cardiac complications after noncardiac operations. Am J Cardiol. 2018;121:125–130. DOI: 10.1016/j.amjcard.2017.09.031 . [DOI] [PubMed] [Google Scholar]
- 35. Alrezk R, Jackson N, Al Rezk M, Elashoff R, Weintraub N, Elashoff D, Fonarow GC. Derivation and validation of a geriatric‐sensitive perioperative cardiac risk index. J Am Heart Assoc. 2017;6:e006648. DOI: 10.1161/JAHA.117.006648 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jørgensen ME, Torp‐Pedersen C, Gislason GH, Jensen PF, Berger SM, Christiansen CB, Overgaard C, Schmiegelow MD, Andersson C. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA. 2014;312:269–277. DOI: 10.1001/jama.2014.8165 . [DOI] [PubMed] [Google Scholar]
- 37. Butt JH, Olesen JB, Havers‐Borgersen E, Gundlund A, Andersson C, Gislason GH, Torp‐Pedersen C, Køber L, Fosbøl EL. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol. 2018;72:2027–2036. DOI: 10.1016/j.jacc.2018.07.088 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figs S1–S5


