Abstract
Background
Restless legs syndrome (RLS) is associated with higher cardiovascular disease (CVD) risk. However, it remains unknown whether treatment of RLS lowers the cardiovascular risk associated with RLS.
Methods and Results
All data were collected retrospectively, but subjects were prospectively followed forward in time to determine outcomes of interest. We used the Truven Health MarketScan Commercial Claims and Encounters database from January 1, 2006, through December 31, 2014. Participants were 169 393 individuals, which included 24 199 nonpregnant participants with an RLS diagnosis (16 694 receiving treatments for RLS and 7505 without treatment) during 2006 to 2008 and 145 194 age‐ and sex‐matched participants without RLS. All participants were free of CVD before January 1, 2009 (analysis baseline). Incident CVD cases (myocardial infarction, angina, stroke, atrial fibrillation, and heart failure) were identified. We adjusted for potential confounders, such as presence of chronic conditions and medication use. We identified 16 574 incident CVD cases during 2009 to 2014. Relative to the non‐RLS group, the adjusted hazard ratio (HR) for future CVD was 1.26 (95% CI, 1.20–1.32) (P<0.001) for the RLS with treatment group, and 1.53 (95% CI, 1.42–1.65) (P<0.001) for the RLS without treatment group. Significant lower CVD risk was observed for all different RLS treatments, including dopaminergics, anticonvulsants, benzodiazepines, and opiates (adjusted HRs range, 0.71‐0.84; P<0.001 for all), except for ergot‐dopamine use.
Conclusions
RLS was associated with higher future CVD risk. However, RLS was associated with statistically significantly less future cardiovascular risk in RLS patients with treatment than in those without treatment.
Keywords: cardiovascular disease, cohort study, restless legs syndrome, stroke, treatment
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors, Primary Prevention, Cerebrovascular Disease/Stroke
Nonstandard Abbreviations and Acronyms
- NHS
Nurses’ Health Study
- PLMS
periodic limb movements in sleep
- RLS
restless legs syndrome
Clinical Perspective
What Is New?
Restless legs syndrome (RLS) previously has been shown to be associated with high cardiovascular risk.
There is no previous study to determine whether treatment of RLS with RLS medications lowers cardiovascular risk.
In this epidemiological cohort study in which retrospective data were followed forward in time, treatment with well‐proven medications for RLS is significantly associated with lower future cardiovascular risk among those with RLS, and this was observed for all treatments studied, except ergotamine‐based dopamine agonists; the wide variety of agents showing improvement of cardiovascular disease in RLS suggests that this effect is specific to the improvement of RLS.
What Are the Clinical Implications?
The results of this study add further evidence to the premise that RLS may lead to cardiovascular disease.
Treatment of RLS symptoms may decrease the cardiovascular risk associated with RLS, but further studies are needed to more fully explore this possibility.
The restless legs syndrome (RLS) is characterized by 4 obligate criteria: (1) an urge to move the legs, usually in response to discomforting sensations in the legs; (2) worsening of symptoms later in the evening or at night; (3) worsening of the symptoms while at rest (ie, lying or sitting); and (4) at least partial and temporary relief by activity. 1 A survey indicates that the quality of life in patients with RLS is as low as that seen in diabetes mellitus, depression, or osteoarthritis with hypertension. 2 Even after treatment, it is estimated that up to one third of those who seek medical attention are getting incomplete relief. 3
Treatments include the following: dopaminergic agents with 3 of these, pramipexole, ropinirole, and rotigotine, Food and Drug Administration approved for use in RLS; α‐2‐Δ calcium channel anticonvulsant drugs, such as pregabalin and gabapentin enacarbil, with the latter Food and Drug Administration approved for use 1 , 3 ; and opioids, such as the agonist/antagonist combination drug oxycodone/naloxone, approved for use in refractory RLS in 20 countries throughout Europe, including France, Germany, and the United Kingdom. 4 Iron therapy, particularly intravenous iron therapy in the form of ferric carboxymaltose, is effective in double‐blind studies; and benzodiazepines can be helpful in selective cases, particularly when insomnia is involved. 1 , 3
Over the past 15 years, there have been several publications suggesting a link between RLS and heart disease, stroke, and hypertension. Patients with RLS also have a nondipping pattern for nocturnal blood pressure, a known risk factor for cardiovascular disease (CVD), 5 and studies of vascular endothelial function in RLS have also shown abnormalities. 6 Magnetic resonance imaging studies of the brain have shown increased microvascular disease in RLS, which is a prestroke condition. 7 The relationship of RLS to heart disease, hypertension, and stroke could be a reciprocal one, with some epidemiological studies indicating that RLS can be a risk factor for the development of CVD and other epidemiological studies indicating that CVD could be a risk factor for the development of RLS. 8 , 9 , 10 , 11 Yet other epidemiological studies indicate no relationship whatsoever when comorbidities are taken into account. 12 In an analysis based on 57 417 female participants of the NHS (Nurses’ Health Study), we found that physician‐diagnosed RLS was significantly associated with 43% increased future CVD mortality. 10 On the other hand, we did not observe this same relationship in men. 13 Direct observational studies do indicate that RLS can occur acutely after stroke in a large series of patients, presumably because of anatomic considerations. 14
If RLS is a risk factor for the development of CVD, a major question in the field has been whether treatment of the RLS/periodic limb movements in sleep (PLMS) can defray this increased cardiovascular risk. The current study is an exploration of this nexus with 24 199 nonpregnant participants with RLS diagnosis (16 694 receiving RLS‐related treatment and 7505 without treatment) during 2006 to 2008 and 145 194 age‐ and sex‐matched participants without RLS.
Methods
Data, Materials, and Code Disclosure Statement
The data that support the findings of this study are available from Xiang Gao, MD, PhD, Nutritional Epidemiology Lab, Department of Nutritional Sciences, The Pennsylvania State University, 109 Chandlee Lab, University Park, PA 16802; telephone 814‐867‐5959; fax 814‐863‐6103.
Source of Data
This is a prospective cohort study. All data were collected retrospectively, but subjects were followed forward in time to determine outcomes of interest. The study was based on the IBM Truven Health MarketScan Commercial Claims and Encounters data (IBM Truven Health Analytics) from January 1, 2006, through December 31, 2014, which contains individual‐level, longitudinal, and comprehensive healthcare use data for a sample of privately insured people, aged 0 to 64 years, in the United States from all 50 states and the District of Columbia, as detailed elsewhere. 15 The IBM Truven Health Analytics (MarketScan) database was obtained through agreement with Penn State.
This study protocol was submitted to the Pennsylvania State College of Medicine institutional review board and was not considered to be human subject research. Because data of the study participants were deidentified, informed consent was waived.
Cohort Derivation and Assessment of Exposure
We identified patients with RLS, aged ≥20 years, with the RLS International Classification of Diseases, Ninth Revision (ICD‐9), code (333.94) during January 1, 2006, through December 31, 2008. To increase statistical power, a 1:6 matching ratio was used for participants with RLS versus controls. For each patient with RLS, we randomly matched 6 individuals without RLS, who had the same birth year and sex. All participants met the following criteria: (1) free of CVD (including coronary heart disease, stroke, atrial fibrillation, and heart failure; see Table S1 for codes used), malignancy (ICD‐9 codes: 140–172, 174–199.1, and 200–208), and end‐stage renal disease (ICD‐9 code 585.6) before January 1, 2009 (the analysis baseline), (2) not pregnant at least a year before the RLS diagnosis data (the date of the first RLS ICD‐9 code), (3) having a 3‐year (2006–2008) continuous enrollment in a medical and pharmacy benefits program, and (4) having complete data on the exposure, outcome, and covariates. Finally, 24 199 participants with RLS and 145 194 age‐ and sex‐matched participants without RLS (average age, 49 years; 31% men) were included in the current analysis. We identified 16 694 patients with RLS with any prescription of RLS‐related medications during 2006 to 2008, including non–ergot‐dopaminergic agents, ergot‐dopaminergic agents, benzodiazepines, anticonvulsants, and opiates (Table S2), and 7505 patients with RLS without treatment. We used the first date of receiving RLS treatment as the index date for the patients with RLS with treatment and the first date of RLS diagnosis for patients with RLS without treatment in the current analysis.
Participants were divided into 3 categories in our primary analysis, according to the RLS and treatment status: non‐RLS, RLS with treatment, and RLS without treatment. Because different RLS treatments may have different impact on cardiovascular health, 16 , 17 we also examined whether these different treatments were specifically associated with altered CVD risk in the secondary analysis.
Assessment of Outcomes
The primary outcome was the composite end of any CVDs (coronary heart disease, stroke, atrial fibrillation, and heart failure) that occurred during 2009 to 2014. Incident CVD cases were identified by searching the ICD‐9 codes and Current Procedural Terminology (Table S1). We also created an alternate composite CVD outcome, in which we did not include atrial fibrillation, coronary atherosclerosis, and other unspecified forms of chronic ischemic heart disease. As the secondary outcome, association of RLS and related treatment with these individual CVDs was explored.
Assessment of Covariates
On the basis of a comprehensive literature review, we derived 4 groups of potential confounders: demographics (age, sex, and region), lifestyle (smoking, alcohol consumption, and obesity), presence of chronic conditions, and medications from the baseline period. Because the MarketScan data did not contain detailed information on smoking status, presence of chronic obstructive pulmonary disease was used as a surrogate, as done previously. 18 We assessed the chronic conditions, including depression, obstructive sleep apnea, insomnia, diabetes mellitus, hypertension, peripheral neuropathy, arthritis, iron‐deficiency anemia, Parkinson disease, and chronic kidney disease. We evaluated the medications, including antiplatelets, anticoagulants, statins, other lipid‐lowering drugs, and antihypertensive and hypoglycemic agents. We selected these factors because they could be associated with both exposure (RLS status) and outcome (CVD risk). However, for some factors, such as smoking and social economic status factors, although the current literature does not support that they are well‐established risk factors for RLS (thus, do not meet the criteria for confounder), 19 , 20 we still included their surrogate factors as covariates because they are associated with CVD risk.
Statistical Analysis
The person‐time for each participant was accumulated from the index data to the first occurrence of an outcome of interest, inpatient death, end of enrollment, or end of study period (December 31, 2014), whichever took place first. Statistical comparisons were made across 3 RLS categories (non‐RLS, RLS with treatment, and RLS without treatment), and an ANOVA test was used to for continuous variables and χ2 analysis was used for categorical variables.
We used the Kaplan‐Meier method to estimate the cumulative CVD rate, with between‐group comparisons of cumulative event rates calculated by means of the log‐rank test. Cox proportional hazards regression analysis was used to calculate hazards ratios (HRs) and corresponding 95% CIs for the risk of developing CVD across the 3 RLS categories, with the “non‐RLS” as reference group, adjusted for age, sex, region, alcohol consumption, obesity, presence of chronic obstructive pulmonary disease, depression, obstructive sleep apnea, insomnia, diabetes mellitus, hypertension, peripheral neuropathy, arthritis, iron‐deficiency anemia, Parkinson disease, chronic kidney disease, and use of antiplatelets, anticoagulants, statins, and antihypertensive and hypoglycemic medications. The proportional hazards assumption was violated (P<0.001), which could be because of the large sample size of the current study. We thus further reported HRs, stratified by follow‐up duration (<1, 1–1.99, and ≥2 years).
We also conducted an analysis to examine whether RLS treatment was associated with altered CVD risk, in which the RLS without treatment group was used as reference group (P for proportional hazards assumption=0.11). Interactions of RLS, with age (years) and sex (the 2 most important determinants for risk of RLS and CVD), in relation to CVD risk were assessed by likelihood ratio testing, adjusting for aforementioned covariates. Subgroup analyses were further conducted when the significant interaction was observed.
We conducted a sensitivity analysis with inverse probability of treatment weighting using the propensity score as a different approach, which could significantly reduce the effects of confounders and achieve a better balance between the 3 RLS groups. 21 To reduce the possible misclassification for RLS ascertainment caused by errors in ICD‐9 coding, record entry, or diagnosis, we repeated analyses by restricting the RLS cases with ≥2 RLS ICD‐9 codes. Because some RLS‐related medicines are not specific, which could be used for other conditions, we further excluded 50 981 participants without RLS who used any RLS‐related medications. Because individuals with RLS were more likely to have other sleep disorders, we conducted another sensitivity analysis by excluding individuals with insomnia and obstructive sleep apnea. Finally, we restricted our analyses among an apparently healthy population, who were free of sleep apnea, insomnia, depression, obesity, chronic obstructive pulmonary disease, diabetes mellitus, hypertension, hyperlipidemia, peripheral neuropathy, rheumatic arthritics, osteoarthritis, chronic kidney disease, iron‐deficiency anemia, and Parkinson disease, and did not use common medications (ie, antiplatelets, anticoagulants, statins, and antihypertensives and hypoglycemics). This could minimize the residual confounding attributable to uneven distribution of these factors across 3 RLS groups.
Results
During 661 215 person‐years of follow‐up, we identified 16 574 incident CVD cases. Individuals with RLS, particularly those without treatment, had higher likelihood of having chronic conditions, such as metabolic abnormities, sleep disorders, iron‐deficiency anemia, depression, and Parkinson disease, relative to those without RLS (Table 1). Of note, individuals in the RLS without treatment group also had lower prevalence of use of other medications, such as lipid‐lowering, antihypertensive, and hypoglycemic medications, although they experienced higher prevalence of chronic conditions (Table 1).
Table 1.
Baseline Basic Characteristics, According to RLS Status
| Characteristic | No RLS (n=145 194) | RLS | P Value for Difference* | |
|---|---|---|---|---|
| With Treatment (n=16 694) | Without Treatment (n=7505) | |||
| Age, mean±SD, y | 49.4±9.1 | 49.4±8.9 | 49.4±9.6 | 0.99 |
| Male sex, % | 31.5 | 30.9 | 32.9 | 0.008 |
| Rural residence, % | 19.4 | 24.5 | 25.5 | <0.001 |
| Region of United States, % | <0.001 | |||
| South | 40.0 | 47.0 | 59.9 | |
| West | 22.6 | 14.9 | 8.2 | |
| Midwest | 27.6 | 31.8 | 25.9 | |
| Northeast | 0.2 | 0.3 | 0.1 | |
| Unknown | 9.6 | 6.0 | 5.9 | |
| Diabetes mellitus, % | 7.3 | 9.6 | 10.2 | <0.001 |
| Hypertension, % | 19.2 | 26.4 | 29.5 | <0.001 |
| Obesity, % | 1.5 | 4.5 | 5.4 | <0.001 |
| Hyperlipidemia, % | 2.7 | 4.2 | 5.5 | <0.001 |
| Obstructive sleep apnea, % | 1.7 | 13.5 | 12.6 | <0.001 |
| Depression, % | 3.0 | 9.0 | 6.5 | <0.001 |
| Insomnia, % | 1.1 | 6.8 | 7.0 | <0.001 |
| Peripheral neuropathy, % | 0.4 | 2.6 | 2.6 | <0.001 |
| Rheumatologic disease, % | 1.1 | 2.0 | 2.0 | <0.001 |
| Osteoarthritis, % | 4.9 | 12.6 | 12.0 | <0.001 |
| Iron‐deficiency anemia, % | 1.0 | 2.7 | 2.7 | <0.001 |
| CKD, % | 0.3 | 0.5 | 0.6 | <0.001 |
| Parkinson disease, % | 0.1 | 0.2 | 0.2 | <0.001 |
| COPD, % | 6.0 | 13.2 | 14.6 | <0.001 |
| Alcohol drinking, % | 0.29 | 0.65 | 0.55 | <0.001 |
| Antiplatelets, % (n) | 0.2 (284) | 0.43 (71) | 0.07 (5) | <0.001 |
| Anticoagulants, % (n) | 0.02 (30) | 0.01 (3) | 0 (1) | 0.38 |
| Statins, % (n) | 13.9 (20 228) | 14.9 (2459) | 3.8 (313) | <0.001 |
| Other lipid‐lowering drugs, % (n) | 4.7 (6755) | 6.1 (1014) | 1.4 (117) | <0.001 |
| Antihypertensive drugs, % (n) | 27.8 (40 559) | 31.9 (5286) | 8.3 (648) | <0.001 |
| Hypoglycemic drugs, % (n) | 10.9 (15 903) | 11.9 (1970) | 3.1 (243) | <0.001 |
CKD indicates chronic kidney disease; COPD, chronic obstructive pulmonary disease; and RLS, restless legs syndrome.
On the basis of comparison between any 2 of the 3 RLS groups.
Presence of RLS was associated with higher risk of developing CVD (Figure 1 and Table 2). Relative to the non‐RLS group, the adjusted HR was 1.26 (95% CI, 1.20–1.32; P<0.001) for the RLS with treatment group, and 1.53 (95% CI, 1.42–1.65; P<0.001) for the RLS without any treatment group, after adjusting for the potential confounders (Table 2). Stratified analysis based on follow‐up duration showed a similar pattern (Table 2). Propensity score adjustment or restricting to patients with RLS with at least 2 RLS ICD‐9 codes did not materially change the observed results (Table 2). Significant results were consistently obtained when we excluded individuals without RLS but using any RLS treatment (Table 2). We obtained similar results when we excluded atrial fibrillation and other nonischemic diseases from the primary composite CVD outcome: the adjusted HR was 1.29 (95% CI, 1.22–1.35) for those with RLS treatment and 1.51 (95% CI, 1.39–1.63) for RLS without treatment. Excluding participants with other common chronic conditions (eg, sleep disorders and depression) and use of common medications (eg, statins and antihypertensives) also generated similar results (Table 2). A similar pattern was observed when individual CVDs were examined as outcome (Table S3). Slightly stronger association was observed in women, relative to men (P‐interaction=0.04) (Table S4). The interaction between RLS and age was not significant (P‐interaction=0.07).
Figure 1. The Kaplan‐Meier survival curves for cardiovascular disease (CVD) during 2009 to 2014, according to baseline restless legs syndrome (RLS) status.
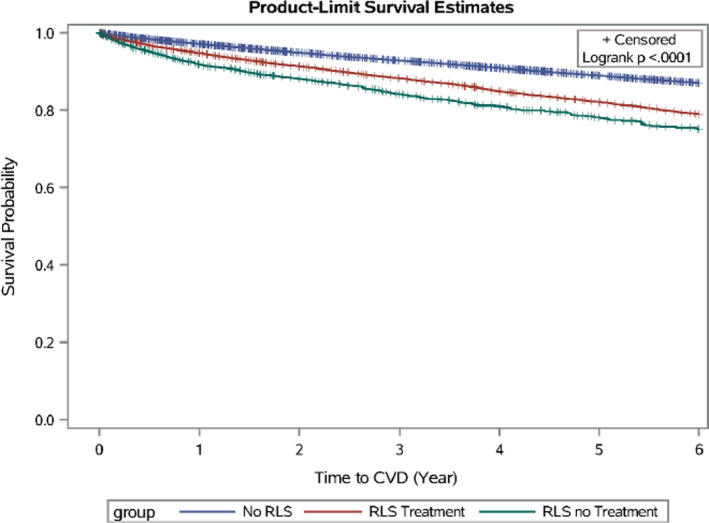
Table 2.
Hazard ratios for the Development of Future CVD Among 169 393 Participants When the RLS With Treatment Group and the RLS Without Treatment Group Were Each Individually Compared With the Group With No RLS
| Variable | No RLS | RLS With Treatment | RLS Without Treatment |
|---|---|---|---|
| Person‐years | 580 070 | 60 287 | 20 858 |
| CVD case, n | 13 659 | 2128 | 787 |
| Incident rate, per 1000 person‐years | 24 | 35 | 38 |
| Model 1 | 1 (Reference) | 1.51 (1.44–1.58) | 1.61 (1.49–1.73) |
| Model 2 | 1 (Reference) | 1.42 (1.35–1.48) | 1.44 (1.34–1.55) |
| Model 3 | 1 (Reference) | 1.26 (1.20–1.32) | 1.53 (1.42–1.65) |
| Stratified by follow‐up duration, y | |||
| <1 | 1 (Reference) | 1.04 (0.92–1.17) | 1.06 (0.89–1.27) |
| 1–1.9 | 1 (Reference) | 1.22 (1.10–1.35) | 1.53 (1.33–1.75) |
| ≥2 | 1 (Reference) | 1.37 (1.29–1.46) | 1.80 (1.33–1.75) |
| Sensitivity analyses* | |||
| Propensity score adjustment | 1 (Reference) | 1.46 (1.41–1.52) | 1.72 (1.65–1.80) |
| Alternate composite CVD outcome ‡ | 1 (Reference) | 1.29 (1.22–1.35) | 1.51 (1.39–1.63) |
| Restricting to 6946 patients with RLS with at least 2 RLS ICD‐9 codes during 2006–2008 | 1 (Reference) | 1.30 (1.19–1.41) | 1.75 (1.52–2.01) |
| Excluding 50 981 participants without RLS but using any RLS‐related treatments | 1 (Reference) | 1.38 (1.31–1.46) | 1.68 (1.55–1.82) |
| Excluding 16 362 participants with insomnia or obstructive sleep apnea | 1 (Reference) | 1.32 (1.25–1.39) | 1.59 (1.46–1.73) |
| Excluding 78 528 participants with common chronic conditions and use of antiplatelets, anticoagulants, statins, antihypertensives, and hypoglycemics § | 1 (Reference) | 1.31 (1.19–1.45) | 1.58 (1.38–1.80) |
Data are given as HR (95% CI), unless otherwise indicated. Model 1, age and sex adjusted. Model 2, further adjustment for residence (rural vs urban), region (east, south, Midwest, and unknown), alcohol consumption, obesity, and chronic obstructive pulmonary disease (surrogate for smoking). Model 3, further adjustment for presence of diabetes mellitus, hypertension, hyperlipidemia, sleep apnea, depression, insomnia, peripheral neuropathy, rheumatic arthritics, osteoarthritis, chronic kidney disease, iron‐deficiency anemia, or Parkinson disease (each yes/no), and use of antiplatelets, anticoagulants, statins, antihypertensives, and hypoglycemics (each yes/no). P<0.001 for all comparisons in the table. CVD indicates cardiovascular disease; HR, hazard ratio; ICD‐9, International Classification of Diseases, Ninth Revision; and RLS, restless legs syndrome.
On the basis of model 3.
P values for proportional hazard assumption were 0.01 for follow‐up duration <1 years, 0.07 for follow‐up duration 1 to 1.9 years, and 0.33 for follow‐up duration ≥2 years.
Excluding atrial fibrillation and other forms of chronic ischemic heart disease. Alternate CVD outcome included myocardial infarction, angina, coronary artery bypass grafting, percutaneous coronary intervention, heart failure, ischemic stroke, hemorrhagic stroke, and other strokes.
Common chronic conditions include obesity, chronic obstructive pulmonary disease, diabetes mellitus, hypertension, hyperlipidemia, sleep apnea, depression, insomnia, peripheral neuropathy, rheumatoid arthritis, osteoarthritis, chronic kidney disease, iron‐deficiency anemia, and Parkinson disease. These variables were thus not included in the model.
Among patients with RLS, treatment was associated with 13% lower CVD risk (95% CI, 4%–20%; P<0.001), relative to those without RLS treatment (Figure 2). Excluding those with major chronic conditions and use of common medications generated similar results (adjusted HR, 0.81; 95% CI, 0.69–0.95). Using alternate CVD outcome, excluding atrial fibrillation, atherosclerosis, and forms of chronic ischemic heart disease other than myocardial infarction and angina, generated similar significant results (adjusted HR, 0.90; 95% CI, 0.82–0.99; P=0.03). Similarly, RLS treatment was associated with significantly lower risk of atrial fibrillation (adjusted HR, 0.69; 95% CI, 0.56–0.87; P<0.001) but not for other unspecified chronic ischemic heart disease (adjusted HR, 0.92; 95% CI, 0.79–1.07; P=0.25). Significant lower CVD risk was observed for all different RLS treatments (dopaminergics, anticonvulsants, benzodiazepines, or opiates; adjusted HR range, 0.71–0.84; P<0.001 for all), except for ergot‐dopamine use (adjusted HR, 1.01; 95% CI, 0.48–2.15; P=0.98) (Figure 2). Specifically, the association between RLS treatment and low CVD risk was statistically significant only for patients with RLS who received combinations of different medications for the treatment of RLS (n=10 559; adjusted HR, 0.73; 95% CI, 0.67–0.80) but not for those receiving a single medication for the treatment of RLS (n=6135; adjusted HR, 1.09; 95% CI, 0.99–1.21), relative to those without any treatment.
Figure 2. Adjusted hazard ratios of cardiovascular disease, according to restless legs syndrome (RLS) treatment status (RLS with treatment vs RLS without treatment), among 24 199 participants with RLS.
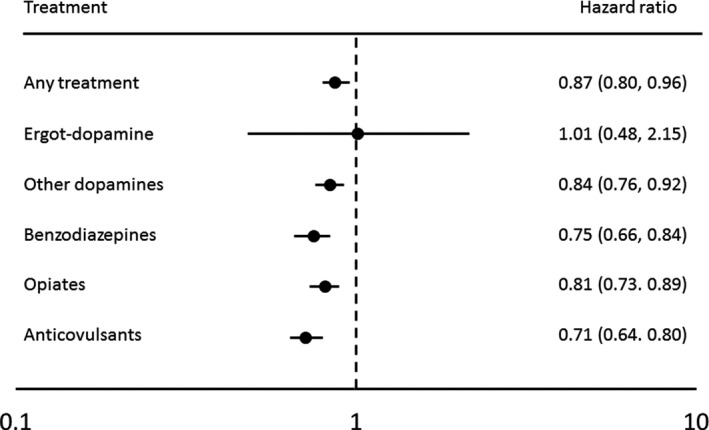
Adjusted for age, sex, residence (rural vs urban), region (east, south, Midwest, and unknown), alcohol consumption, presence of obesity, chronic obstructive pulmonary disease, diabetes mellitus, hypertension, hyperlipidemia, sleep apnea, depression, insomnia, peripheral neuropathy, rheumatic arthritics, osteoarthritis, chronic kidney disease, iron‐deficiency anemia, or Parkinson disease (each yes/no), and use of antiplatelets, anticoagulants, statins, antihypertensives, and hypoglycemics (each yes/no). P values were <0.001 for all, except for ergot‐dopamine (P=0.98).
Discussion
In this large‐scale national claim’s data set, physician‐diagnosed RLS was associated with higher future CVD risk, as has been documented in much of the previous literature. 8 , 9 , 10 , 11 , 12 , 14 Treating RLS appeared to alleviate this potential harmful impact. This was true of all the agents commonly used for the treatment of RLS, except for ergot‐based dopaminergic alkaloids, a result compatible with the known deleterious effect of ergot alkaloids on cardiovascular health (eg, cardiac valvular fibrosis). 16 , 17 The wide variety of agents showing improvement of CVD risk in RLS suggests that this effect is specific to the improvement of RLS. To our knowledge, our study represents the first attempt to determine if treatment of RLS lowers the increased cardiovascular risk associated with RLS. Using RLS treatment statistically significantly lowers the cardiovascular risk associated with RLS but not to the level of subjects without RLS. One of the major strengths of this study is its prospective nature. All of the data were collected prospectively, and subjects were followed forward in time to determine outcomes of interest. Another one of the major strengths of this study is that it is not an industry‐sponsored study and thus multiple treatments well known to be effective in the treatment of RLS were able to be evaluated.
Because this study is based on claims data, we do not have before and after ratings of the severity of RLS and thus we do not know for sure that the improvement in RLS‐related CVD with RLS treatment is really paralleled by an improvement in RLS. However, the numerous large clinical trials showing a positive benefit on RLS from the medications chosen for this study would suggest that this is so. 1 , 3 Another limitation is that we did not have information on why some patients with RLS use medications and why some patients with RLS did not receive any treatment. One potential explanation is that this difference could be because of RLS severity. However, we found that patients with RLS with treatment had lower future CVD risk, relative to those without treatment. Using ICD‐9 codes to identify patients with RLS could lead to misclassification of the non‐RLS group, as RLS is an underdiagnosed disease. Therefore, the observed association between presence of RLS and CVD could be underestimated. For the same reason, measurement errors could be introduced or other sleep disorders (eg, insomnia and obstructive sleep apnea) could be assessed via searching ICD‐9 codes, which could introduce residual confounding. Our study is also limited by lack of direct assessment on several covariates, such as smoking (we used the presence of chronic obstructive pulmonary disease as a surrogate measure of smoking) and social economic status (we used region and residence as surrogates). However, although these factors are associated with CVD risk, whether they are risk factors for RLS remains unclear. 19 , 20 Furthermore, sensitivity analyses excluding participants with major chronic diseases, which are closely related to smoking and social economic status, generated similar results. This suggests that the impact of residual confounding attributable to these factors on the RLS‐CVD relationship could be small.
The reason why a putative improvement in RLS would cause an improvement in RLS‐related cardiovascular risk is unknown. An intermediary mechanism may be an improvement in sleep, as it is well known that RLS causes severe insomnia 1 and it is also well known that insomnia alone can increase cardiovascular risk. However, again, because our current study was based on claims data, we did not have access to detailed sleep schedule histories. On the other hand, it is likely, if there is an impact of the medications on sleep, that this is as a result of the improvement in RLS symptoms rather than a direct impact on sleep because none of the medications studied are used as hypnosedatives, minus the benzodiazepines. 1 , 3 One could also consider the possibility that the dopaminergic agents could lower cardiovascular risk by their hypotensive effect. However, this antihypertensive effect is not characteristic of the other therapeutic agents in this study. Again, this suggests that the improvement in CVD could be as a result of the direct impact of the various therapeutic modalities in this study on the RLS symptoms themselves.
The agents in this study have been shown to ameliorate the symptoms of RLS in both short‐term and long‐term studies. 1 , 3 , 4 In some cases, however, long‐term treatment with dopaminergic therapy may lead to a paradoxical worsening for the symptoms called augmentation. 1 , 3 , 4 As this study was based on claims data, we did not have any long‐term measure of augmentation. However, despite the fact that we did not have this measure, our study shows that long‐term the dopaminergic agents were associated with lower cardiovascular risk.
One of the weaknesses of the current study was that we did not include the effect of iron therapy on future cardiovascular risk. However, at the time of the initiation of the study, we believed that iron therapy is so frequently used as an adjunct to other medications in RLS that it would be difficult to exclude it as a confounding factor in the determination of the cardiovascular lowering risk of other individual drugs. In addition, despite the fact that iron deficiency has a strong pathophysiologic link to RLS, the therapeutic response of RLS to oral iron is rather low and dependent on months of treatment for therapeutic benefit to be obtained, as opposed to the pharmacological agents where improvement is immediate once the proper doses are obtained. We believed that this also would interfere with the proper determination of the impact of iron on CVD.
Because this study was a secondary data analysis based on existing data, we did not have the opportunity to bring patients into the laboratory and do an overnight polysomnography to determine whether our subjects had PLMS. Although not necessary for the diagnosis of RLS, PLMS or repetitive involuntary leg movements occur during sleep in ≈80% of patients with RLS, although PLMS may also occur in isolation. 1 There is also a strong literature connecting PLMS to CVD. More specifically, early studies showed a link of PLMS to congestive heart failure, and in a separate study, the number of PLMS were shown to parallel the level of daytime hypertension. 22 , 23 , 24 Echocardiographic abnormalities have been shown to be related to PLMS. 25 , 26 Patients with PLMS also have a higher prevalence of cardiac arrhythmias than would be expected by chance alone. 27 Magnetic resonance imaging studies of the brain have shown that the level of PLMS is also related to transient ischemic attack or minor stroke. 28 Studies also show that marked increases in pulse and blood pressure accompany PLMS in patients with RLS. 29 , 30 , 31 , 32 Among the mechanisms proposed for the connection between RLS/PLMS and heart disease/hypertension and stroke are the nocturnal hypertension generated by the PLMS. In general, all the medications that improve RLS also improve PLMS. 1 In this light, it is interesting that the dopamine agonist rotigotine, which is one of the main treatments for RLS, lowers the pulse and blood pressure elevations seen with PLMS in patients with RLS. 33
In patients with RLS, there is a nondipping pattern observed in nocturnal blood pressure, which is a well‐known risk factor the development of CVD. 5 Other mechanisms for the development of CVD in RLS have been reviewed in detail and include daytime hypertension, sympathetic overactivity, increased atherosclerotic plaque formation and rupture, insomnia, hypoxia, and upregulation of inflammatory markers. 34 , 35
A randomized controlled trial of a single agent for the treatment of RLS, where controls as well as patients receive drug and placebo, is necessary to definitively prove that RLS treatment lowers cardiovascular risk. However, the large number of participants and the length of follow‐up that would generally be needed for such a study are challenges that would need to be overcome with unique experimental paradigms.
Conclusions
In conclusion, this study shows that RLS is associated with increased cardiovascular risk, consistent with the previous literature, and we show for the first time that RLS treatment with various known effective treatments for RLS lowers this increased cardiovascular risk. The large number and type of medications used suggest that the effect is specific to the amelioration of RLS, but large randomized clinical trials will be needed to further delineate this effect.
Sources of Funding
This is not an industry‐sponsored study. The IBM Truven Health Analytics (MarketScan) database was obtained through agreement with Penn State. This research is supported by the start‐up grant from the College of Health and Human Development and the Department of Nutritional Sciences, Penn State University, and the Institute for CyberScience Seed Grant Program, Penn State University.
Disclosures
Dr Walters reports receiving funding from the National Institutes of Health for the development of a broad complex‐tramtrack‐bric‐a‐brac‐domain 9 (BTBD‐9) knockout mouse as a model of restless legs syndrome and separate funding from the National Institutes of Health for investigating the role of manganese in the restless legs syndrome. Dr Walters also reports receiving funding from MundiPharma to develop a µ opiate receptor knockout mouse as a model of restless legs syndrome and funding from Xenoport/Arbor Pharma for pharmacotherapeutic studies of gabapentin enacarbil in adolescent restless legs syndrome. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
(J Am Heart Assoc. 2021;10:e018674. DOI: 10.1161/JAHA.120.018674.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018674
For Sources of Funding and Disclosures, see page 8.
References
- 1. Trenkwalder C, Allen R, Hogl B, Hogl B, Clemens S, Patton S, Schormair B, Winkelmann J. Comorbidities, treatment, and pathophysiology in restless legs syndrome. Lancet Neurol. 2018;17:994–1005. DOI: 10.1016/S1474-4422(18)30311-9. [DOI] [PubMed] [Google Scholar]
- 2. Allen RP, Walters AS, Montplaisir J, Hening W, Myers A, Bell TJ, Ferini‐Strambi L. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–1292. DOI: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 3. Rinaldi F, Galbiati A, Marelli S, Ferini Strambi L, Zucconi M. Treatment options in intractable restless legs syndrome/Willis‐Ekbom disease (RLS/WED). Curr Treat Options Neurol. 2016;18:7. DOI: 10.1007/s11940-015-0390-1. [DOI] [PubMed] [Google Scholar]
- 4. Silber MH, Becker PM, Buchfuhrer MJ, Earley CJ, Ondo WG, Walters AS, Winkelman JW. The appropriate use of opioids in the treatment of refractory restless legs syndrome. Mayo Clin Proc. 2018;93:59–67. DOI: 10.1016/j.mayocp.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 5. Erden EC, Erden L, Tucker Y, Sivri N, Dikici S, Ozsahin M. Incremental effects of restless legs syndrome on nocturnal blood pressure in hypertensive patients and normotensive individuals. Blood Press Monit. 2012;17:231–234. DOI: 10.1097/MBP.0b013e32835b5a39. [DOI] [PubMed] [Google Scholar]
- 6. Koh SY, Kim MS, Lee SM, Hong JM, Yoon JH. Impaired vascular endothelial function in patients with restless legs syndrome: a new aspect of the vascular pathophysiology. J Neurol Sci. 2015;359:207–210. DOI: 10.1016/j.jns.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 7. Ferri R, Cosentino FII, Moussouttas M, Lanuzza B, Aricò D, Bagai L, Wang L, McLaughlin B, Walters AS. Silent cerebral small vessel disease in restless legs syndrome. Sleep. 2016;39:1371–1377. DOI: 10.5665/sleep.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Molnar MZ, Lu JL, Kalantar‐Zadeh K, Kovesdy CP. Association of incident restless legs syndrome with outcomes in a large cohort of US veterans. J Sleep Res. 2016;25:47–56. DOI: 10.1111/jsr.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Y, Walters AS, Chiuve SE, Rimm EB, Winkelman JW, Gao X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation. 2012;126:1689–1694. DOI: 10.1161/CIRCULATIONAHA.112.112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y, Li Y, Winkelman JW, Walters AS, Han J, Hu FB, Gao X. Prospective study of restless legs syndrome and total and cardiovascular mortality among women. Neurology. 2018;90:e135–e141. DOI: 10.1212/WNL.0000000000004814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Szentkiralyi A, Volzke H, Hoffmann W, Happe S, Berger K. A time sequence analysis of the relationship between cardiovascular risk factors, vascular diseases and restless legs syndrome in the general population. J Sleep Res. 2013;22:434–442. DOI: 10.1111/jsr.12040. [DOI] [PubMed] [Google Scholar]
- 12. Katsanos AH, Kosmidou M, Konitsiotis S, Tsivgoulis G, Fiolaki A, Kyritsis AP, Giannopoulos S. Restless legs syndrome and cerebrovascular/cardiovascular events: systematic review and meta‐analysis. Acta Neurol Scand. 2018;137:142–148. DOI: 10.1111/ane.12848. [DOI] [PubMed] [Google Scholar]
- 13. Li Y, Wang W, Winkelman JW, Malhotra A, Ma J, Gao X. Prospective study of restless legs syndrome and mortality among men. Neurology. 2013;81:52–59. DOI: 10.1212/WNL.0b013e318297eee0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SJ, Kim JS, Song IU, An JY, Kim YL, Lee KS. Poststroke restless legs syndrome and lesion location: anatomical considerations. Mov Disord. 2009;24:77–84. DOI: 10.1002/mds.22303. [DOI] [PubMed] [Google Scholar]
- 15. Truven Health Analytics, part of the IBM Watson Health business. The Truven Health MarketScan databases for health services researchers. Available at: https://www.ibm.com/downloads/cas/6KNYVVQ2. Accessed February 1, 2020.
- 16. Steiger M, Jost W, Grandas F, Van Camp G. Risk of valvular heart disease associated with the use of dopamine agonists in Parkinson's disease: a systematic review. J Neural Transm (Vienna). 2009;116:179–191. DOI: 10.1007/s00702-008-0179-4. [DOI] [PubMed] [Google Scholar]
- 17. Horvath J, Fross RD, Kleiner‐Fisman G, Lerch R, Stalder H, Liaudat S, Raskoff WJ, Flachsbart KD, Rakowski H, Pache J‐C, et al. Severe multivalvular heart disease: a new complication of the ergot derivative dopamine agonists. Mov Disord. 2004;19:656–662. DOI: 10.1002/mds.20201. [DOI] [PubMed] [Google Scholar]
- 18. Zhuang S, Na M, Winkelman JW, Ba D, Liu C, Liu G, Gao X. Association of restless legs syndrome with risk of suicide and self‐harm. JAMA Netw Open. 2019;2:e199966. DOI: 10.1001/jamanetworkopen.2019.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yeh P, Walters AS, Tsuang JW. Restless legs syndrome: a comprehensive overview on its epidemiology, risk factors, and treatment. Sleep Breath. 2012;16:987–1007. DOI: 10.1007/s11325-011-0606-x. [DOI] [PubMed] [Google Scholar]
- 20. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:286–295. DOI: 10.1016/j.smrv.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32:3388–3414. DOI: 10.1002/sim.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hanly PJ, Zuberi‐Khokhar N. Periodic limb movements during sleep in patients with congestive heart failure. Chest. 1996;109:1497–1502. DOI: 10.1378/chest.109.6.1497. [DOI] [PubMed] [Google Scholar]
- 23. Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients: the final report. Int J Cardiol. 2006;106:21–28. DOI: 10.1016/j.ijcard.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 24. Espinar‐Sierra J, Vela‐Bueno A, Luque‐Otero M. Periodic leg movements in sleep in essential hypertension. Psychiatry Clin Neurosci. 1997;51:103–107. DOI: 10.1111/j.1440-1819.1997.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 25. Giannaki CD, Zigoulis P, Karatzaferi C, Hadjigeorgiou GM, George KP, Gourgoulianis K, Koutedakis Y, Stefanidis I, Sakkas GK. Periodic limb movements in sleep contribute to further cardiac structure abnormalities in hemodialysis patients with restless legs syndrome. J Clin Sleep Med. 2013;9:147–153. DOI: 10.5664/jcsm.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mirza M, Shen WK, Sofi A, Jahangir A, Mori N, Tajik AJ, Jahangir A. Frequent periodic leg movement during sleep is associated with left ventricular hypertrophy and adverse cardiovascular outcomes. J Am Soc Echocardiogr. 2013;26:783–790. DOI: 10.1016/j.echo.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mirza M, Shen WK, Sofi A, Tran C, Jahangir A, Sultan S, Khan U, Viqar M, Cho C, Jahangir A. Frequent periodic leg movement during sleep is an unrecognized risk factor for progression of atrial fibrillation. PLoS One. 2013;8:e78359. DOI: 10.1371/journal.pone.0078359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boulos MI, Murray BJ, Muir RT, Gao F, Szilagyi GM, Huroy M, Kiss A, Walters AS, Black SE, Lim AS, et al. Periodic limb movements and white matter hyperintensities in first‐ever minor stroke or high‐risk transient ischemic attack. Sleep. 2017;40:zsw080. DOI: 10.1093/sleep/zsw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22:575–580. DOI: 10.1093/sleep/22.5.575. [DOI] [PubMed] [Google Scholar]
- 30. Ali NJ, Davies RJ, Fleetham JA, Stradling JR. Periodic movements of the legs during sleep associated with rises in systemic blood pressure. Sleep. 1991;14:163–165. [PubMed] [Google Scholar]
- 31. Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–1218. DOI: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 32. Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–1930. DOI: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 33. Bauer A, Cassel W, Benes H, Kesper K, Rye D, Sica D, Winkelman JW, Bauer L, Grieger F, Joeres L, et al. Rotigotine's effect on PLM‐associated blood pressure elevations in restless legs syndrome: an RCT. Neurology. 2016;86:1785–1793. DOI: 10.1212/WNL.0000000000002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walters AS, Rye DB. Review of the relationship of restless legs syndrome/periodic limb movements in sleep to hypertension, heart disease and stroke. Sleep. 2009;32:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferini‐Strambi L, Walters AS, Sica D. The relationship among restless legs syndrome (Willis‐Ekbom disease), hypertension, cardiovascular disease and cerebrovascular disease. J Neurol. 2014;261:1051–1068. DOI: 10.1007/s00415-013-7065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


