Abstract
Background
To explore the pathophysiological features of ischemic stroke in patients with atrial fibrillation (AF), we evaluated the association between 268 plasma proteins and subsequent ischemic stroke in 2 large AF cohorts receiving oral anticoagulation.
Methods and Results
A case‐cohort sample of patients with AF from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial, including 282 cases with ischemic stroke or systemic embolism and a random sample of 4124 without these events, during 1.9 years of follow‐up was used for identification. Validation was provided by a similar case‐cohort sample of patients with AF from the RE‐LY (Randomized Evaluation of Long‐Term Anticoagulation Therapy) trial, including 149 cases with ischemic stroke/systemic embolism and a random sample of 1062 without these events. In plasma obtained before randomization, 268 unique biomarkers were measured with OLINK proximity extension assay panels (CVD II, CVD III, and Inflammation) and conventional immunoassays. The association between biomarkers and outcomes was evaluated by random survival forest and adjusted Cox regression. According to random survival forest or Cox regression analyses, the biomarkers most strongly and consistently associated with ischemic stroke/systemic embolism were matrix metalloproteinase‐9, NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), osteopontin, sortilin, soluble suppression of tumorigenesis 2, and trefoil factor‐3. The corresponding hazard ratios (95% CIs) for an interquartile difference were as follows: 1.18 (1.00–1.38), 1.55 (1.28–1.88), 1.28 (1.07–1.53), 1.19 (1.02–1.39), 1.23 (1.05–1.45), and 1.19 (0.97–1.45), respectively.
Conclusions
In patients with AF, of 268 unique biomarkers, the 6 biomarkers most strongly associated with subsequent ischemic stroke/systemic embolism represent fibrosis/remodeling (matrix metalloproteinase‐9 and soluble suppression of tumorigenesis 2), cardiac dysfunction (NT‐proBNP), vascular calcification (osteopontin), metabolism (sortilin), and mucosal integrity/ischemia (trefoil factor‐3).
Registration
URL: https://www.clinicaltrials.gov. Unique Identifiers: NCT00412984 and NCT00262600.
Keywords: atrial fibrillation, biomarkers, ischemic stroke, pathophysiological features, screening
Subject Categories: Atrial Fibrillation, Biomarkers
Nonstandard Abbreviations and Acronyms
- ADAMTS13
a disintegrin and metalloproteinase with thrombospondin motifs 13
- ARISTOTLE
Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation
- cTnT‐hs
high‐sensitivity cardiac troponin T
- MMP
matrix metalloproteinase
- PEA
proximity extension assay
- RE‐LY
Randomized Evaluation of Long‐Term Anticoagulation Therapy
- SE
systemic embolism
- sST2
soluble suppression of tumorigenesis 2
- ST2
suppression of tumorigenesis 2
- TFF3
trefoil factor 3
Clinical Perspective
What Is New?
In this study, the novel proximity extension assay protein screening technology was for the first time used for mass screening to identify biomarkers associated with ischemic stroke or systemic embolism during ongoing anticoagulation treatment in patients with atrial fibrillation.
The identified biomarkers represent fibrosis/remodeling (matrix metalloproteinase‐9 and soluble suppression of tumorigenesis 2), cardiac dysfunction (NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide]), vascular calcification (osteopontin), metabolism (sortilin), and mucosal integrity/ischemia (trefoil factor‐3).
What Are the Clinical Implications?
The results represent an important contribution in the step toward a better mechanistic understanding of ischemic stroke in patients with atrial fibrillation.
These markers could help guide research into new therapeutic targets beyond anticoagulation in patients with atrial fibrillation at risk for stroke, and potentially even identify the population of patients who are at risk for thromboembolic stroke.
Atrial fibrillation (AF) is a common arrhythmia and constitutes a major health problem worldwide mainly because of its associated increased risk of stroke. 1 In individuals with AF, the risk of ischemic stroke is substantially reduced by oral anticoagulation; however, residual risk remains. 2 , 3 Several clinical characteristics are associated with an increased risk of ischemic stroke in patients with AF regardless of oral anticoagulation, most importantly older age, prior stroke, and cardiovascular comorbidity. 4 , 5 In recent years, protein biomarkers, such as cardiac troponin, reflecting myocardial damage, and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), reflecting cardiac stress and dysfunction, have been shown to be more important for prediction of ischemic stroke than all clinical information, except prior stroke, in patients with AF. 4 , 6 Biomarkers reflecting pathways of inflammation, renal function, coagulation, and platelet activity have also been investigated, although without consistent evidence of association with risk. 7 To better understand the remaining risk of ischemic stroke in anticoagulated patients with AF, there is a need to further explore additional mechanisms.
Recently, a highly sensitive proteomic assay platform has been developed, the proximity extension assay (PEA), which allows high‐throughput multiplex screening of proteins in a resource‐efficient procedure using small amounts of plasma. 8 We explored this new biomarker screening approach to identify plasma biomarkers associated with subsequent risk of ischemic stroke or systemic embolism (SE) and to improve the mechanistic understanding of ischemic stroke risk in patients with AF on oral anticoagulation.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patient Population
Identification Cohort
Details of the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial have been published previously. 3 , 9 Briefly, the ARISTOTLE trial was a double‐blind, double‐dummy, randomized clinical trial that enrolled 18 201 patients with AF and at least 1 risk factor for stroke or SE between December 2006 and April 2010. Patients were randomized to warfarin (n=9081) or apixaban (n=9120). Exclusion criteria included conditions other than AF that required anticoagulation (eg, prosthetic heart valve) and severe renal insufficiency (serum creatinine >2.5 mg/dL [>221 µmol/L] or calculated creatinine clearance <25 mL/min).
Validation Cohort
The RE‐LY (Randomized Evaluation of Long‐Term Anticoagulation Therapy) trial was a prospective, multicenter, randomized trial comparing 2 blinded doses of dabigatran with open‐label warfarin that enrolled 18 113 patients with AF between December 2005 and March 2009. 2 , 10 Exclusion criteria included severe heart valve disorder, recent stroke, creatinine clearance of <30 mL/min, or active liver disease.
In both trials, patients at certain centers participated in biomarker substudies and provided, before randomization, venous blood samples into vacutainer tubes containing EDTA, which were centrifuged immediately. Plasma was frozen in aliquots and stored at –70°C until analyzed centrally at the Uppsala Clinical Research Center, an academic platform for analyses of biomarkers at the Uppsala University Hospital, Uppsala, Sweden. In both trials, ethics committee approval was obtained for all investigational sites, and all patients provided written informed consent.
Multimarker Screening Study Design
The inclusion of patients in this multimarker substudy was based on an unstratified case‐cohort design. The ARISTOTLE trial multimarker subset consisted of all 282 cases with ischemic stroke or SE during follow‐up of patients in the biomarker substudy, which is compared with a random sample of 4124 without these events. The RE‐LY trial multimarker subset consisted of all 149 cases with ischemic stroke or SE and a random sample of 1062 without these events during follow‐up of patients in the RE‐LY trial biomarker substudy. As such, and in accordance with the traditional case‐cohort method, individuals with events may be selected to the random sample of controls. The median follow‐up time in both multimarker substudy cohorts was 1.9 years.
Outcome Assessment
All strokes were adjudicated by an international team of adjudicators blinded to treatment assignment. 2 , 3 , 9 , 10 Stroke was defined as the sudden onset of a focal neurologic deficit in a location consistent with the territory of a major cerebral artery and categorized as ischemic, hemorrhagic, or unspecified. SE was defined as an acute vascular occlusion of an extremity or organ, documented by means of imaging, surgery, or autopsy. The predefined primary outcome event for these substudies was ischemic stroke (including unspecified) or SE.
Biochemical Analyses
The plasma concentrations of high‐sensitivity cardiac troponin T (cTnT‐hs), NT‐proBNP, and growth differentiation factor 15 (precommercial assay) were determined by Roche immunoassays using a Cobas Analytics e601 (Roche Diagnostics, Penzberg, Germany) and interleukin 6 high‐sensitivity sandwich ELISA immunoassays (R&D Systems Inc, Minneapolis, MN). Cystatin C was analyzed with the ARCHITECT system ci8200 (Abbott Laboratories, Abbott Park, IL) using the particle‐enhanced turbidimetric immunoassay from Gentian (Moss, Norway). 11 Estimated glomerular filtration rate was calculated on the basis of centrally determined creatinine levels using the Chronic Kidney Disease Epidemiology Collaboration equation. 12
The proteomic analyses were performed at the Clinical Biomarkers Facility, Science for Life Laboratory, Uppsala University, without information on any other data. The determinations were performed using a high‐throughput technique using the OLINK Proteomics Multiplex CVD II96x96, CVD III96x96, and Inflammation96x96 panels, which together simultaneously measured 276 selected proteins in plasma potentially related to cardiovascular disease and inflammation. The PEA technology uses a homogeneous assay that uses pairs of antibodies equipped with DNA reporter molecules. In the kits, 92 oligonucleotide‐labeled antibody probe pairs are allowed to bind to their respective target if present in the sample. 8 , 13 As only the correctly matched antibody pairs produce a signal, the technology has an exceptionally high specificity. When binding to their correct targets, they produce new DNA amplicons, with each identifier barcoding its respective antigens. The amplicons are subsequently quantified using a Fluidigm BioMark HD real‐time polymerase chain reaction platform. The analyses were run using the internal controls for the PEA, including 2 incubation controls and extension and detection controls. For sample control in each plate, there is an interplate control used for normalization and it compensates for interplate variation. For each plate, a negative control, buffer without antigen, and 2 positive controls, pooled plasma, are used. All samples were analyzed in one set. Interplate variability was adjusted by intensity normalization. The resulting relative values, normalized protein expression data, were log2 transformed and a high value corresponded to a high protein concentration. For data analysis, the OLINK wizard was used and all statistical analyses were performed at Uppsala Clinical Research Center. The PEA assays have shown high reproducibility and repeatability, with mean intra‐assay and interassay coefficients of variation around 8% and 12%, respectively; average intersite variation has been reported at 15%. 8 Prior validation studies have also showed that biomarkers analyzed with the PEA technique have an adequate concordance with conventional immunoassays. 14 The protein markers in the identification cohort included 3 panels, CVD II, CVD III, and Inflammation, and are detailed in Table S1 and S2. Of the 276 PEA proteins, 10 were available on >1 panel, resulting in 266 unique markers. As initial results in the identification cohort identified biomarkers from the CVD II and CVD III panels as more strongly associated with the outcome, the Inflammation panel was omitted in the external validation.
Statistical Analysis
The pairwise association between PEA biomarkers and established conventional biomarkers was assessed by the Spearman correlation.
A random survival forest algorithm 15 was used to evaluate the simultaneous association between ischemic stroke/SE and biomarkers. The evaluation included levels of 263 PEA markers, 4 conventional markers (NT‐proBNP, cTnT‐hs, growth differentiation factor 15, and interleukin 6), renal function, and 13 clinical characteristics (randomized treatment, age, sex, body mass index, smoking, hypertension, diabetes mellitus, hemoglobin, previous myocardial infarction, stroke/transient ischemic attack, peripheral artery disease, heart failure, and bleeding). The number of trees was 5000, splits were done according to a maximally selected statistic criterion, and the variables were ranked according to their permutation variable importance. Subjects with all PEA markers missing were excluded. There were only a few partially missing values, and these were singly imputed using multivariate imputations by chained equations. 16 Three PEA markers were omitted as they were also measured as conventional markers. An identical approach was used in the RE‐LY trial evaluation, with a total of 184 PEA markers.
Cox regression analyses were performed, including each of the established standard immunoassays (naturally log transformed) and the PEA biomarkers, one at a time, assuming a linear association with the log hazard rate. Sampling weights were used to account for the case‐cohort design. The randomly sampled controls were given weights equal to 1/0.2945632, corresponding to the reciprocal of the sampling probability of being selected for the PEA substudy. The Cox regression analyses were performed in 2 steps, first unadjusted and second adjusting for baseline characteristics (age, sex, body mass index, smoking, hypertension, diabetes mellitus, prior myocardial infarction, prior stroke/transient ischemic attack, peripheral artery disease, heart failure, and randomized treatment), renal function (cystatin C in ARISTOTLE trial and Chronic Kidney Disease Epidemiology Collaboration equation in RE‐LY trial), and established biomarkers (NT‐proBNP and cTnT‐hs). Time to event was defined as the time since randomization until the occurrence of an ischemic stroke/SE or, if no ischemic stroke/SE occurs, the event is censored at last day of follow‐up at the end of the study or death. Results were presented as the relative hazard for an interquartile difference of each marker with corresponding 95% CIs and P values. Thus, the hazard ratio (HR) can be interpreted as the relative hazard comparing the 2 biomarker values defining the inner 50% of the distribution (ie, the third versus the first quartile). On the inflammation panel, 16 of the proteins had >80% of the measurements below the limit of detection and these were not included in the Cox regression models.
Because of the large number of biomarkers evaluated, only biomarkers of high ranking in the random survival forest analysis (top 20) or with significant association in the adjusted Cox regression analysis, in both the identification and external validation cohorts, were considered to have confirmed association with the risk of ischemic stroke/SE.
All analyses were done using the R environment for statistical computing, version 3.3.1, 17 using the ranger 18 package.
Results
Baseline Characteristics and Distribution of Biomarkers
The baseline characteristics of the multimarker substudy identification and validation cohorts are presented in Tables 1 and 2, respectively, according to occurrence of ischemic stroke/SE during follow‐up. Baseline characteristics were similar between the identification and screening cohort, with only slight differences in regard to age and renal function (Tables 1 and 2). Patients with ischemic stroke/SE events were slightly older and to a larger extent had a history of stroke/transient ischemic attack. There were substantial differences in the levels of conventional biomarkers at baseline (eg, higher concentrations of NT‐proBNP and cardiac troponin and lower estimated glomerular filtration rate in cases versus noncases). The relative concentration (normalized protein expression values) and limit of detection of all 266 biomarkers in the identification cohort are shown in Tables S1 and S2 for the external validation cohort. The biomarkers in general showed a low correlation with the established cardiovascular biomarkers. Correlation data for the biomarkers are presented in Tables S3 and S4, which show that several biomarkers were correlated with renal function.
Table 1.
Baseline Characteristics for the Identification Cohort
| Variable | No Event (n=4124) | Ischemic Stroke/SE (n=282) |
|---|---|---|
| Age, y | 70.0 (62.0–76.0) | 71.0 (66.0–77.0) |
| Women | 36.3 (1499) | 41.8 (118) |
| Body mass index, kg/m2 | 28.6 (25.4–32.7) [20] | 27.7 (24.4–31.6) [1] |
| Current smoker | 8.9 (367) [1] | 8.2 (23) [0] |
| Hypertension | 87.5 (3610) | 88.3 (249) |
| Diabetes mellitus | 25.1 (1035) | 27.7 (78) |
| Prior myocardial infarction | 13.1 (539) | 16.3 (46) |
| Prior stroke/TIA | 18.0 (743) | 37.2 (105) |
| Peripheral arterial disease | 4.7 (194) | 8.2 (23) |
| Heart failure | 31.1 (1282) | 35.1 (99) |
| Hemoglobin, g/dL | 14.2 (13.1–15.2) [22] | 14.3 (13.2–15.2) [2] |
| NT‐proBNP, ng/L | 692.0 (363.0–1253.0) [3] | 1014.0 (565.0–1773.2) [0] |
| cTnT‐hs, ng/L | 10.8 (7.4–16.6) | 12.8 (9.0–20.6) |
| GDF‐15, ng/L | 1369.5 (965.0–2062.8) | 1591.0 (1128.5–2318.2) |
| Cystatin C, mg/L | 1.0 (0.8–1.2) [4] | 1.1 (0.9–1.3) [0] |
| IL‐6, ng/L | 2.3 (1.5–4.0) [1] | 2.7 (1.6–3.9) [0] |
| eGFR, CKD‐EPI equation, mL/min | 75.1 (57.1–96.8) [4] | 67.6 (52.0–88.1) [0] |
Continuous variables presented as median (quartile 1–quartile 3). Categorical variables presented as percentage (frequency). Number of missing values presented in brackets, and does not include cases where biomarker concentrations were below detection limit. CKD‐EPI indicates Chronic Kidney Disease Epidemiology Collaboration; CRP, C‐reactive protein; cTnT‐hs, high‐sensitivity cardiac troponin T; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor 15; IL‐6, interleukin 6; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SE, systemic embolism; and TIA, transient ischemic attack.
Table 2.
Baseline Characteristics for the External Validation Cohort
| Variable | No Event (n=1062) | Ischemic Stroke/SE (n=149) |
|---|---|---|
| Age, y | 72.0 (67.0–77.0) | 74.0 (69.0–80.0) |
| Women | 37.1 (394) | 37.6 (56) |
| Body mass index, kg/m2 | 27.9 (25.0–31.3) [2] | 27.1 (24.3–30.5) [0] |
| Current smoker | 7.4 (79) | 12.1 (18) |
| Hypertension | 79.4 (843) | 77.9 (116) |
| Diabetes mellitus | 21.4 (227) | 27.5 (41) |
| Prior myocardial infarction | 16.9 (179) | 18.1 (27) |
| Prior stroke/TIA | 20.0 (212) | 33.6 (50) |
| Peripheral arterial disease | 3.6 (38) | 4.7 (7) |
| Heart failure | 28.9 (307) | 34.9 (52) |
| Hemoglobin, g/dL | 14.2 (13.1–15.3) [22] | 14.1 (13.0–15.3) [3] |
| NT‐proBNP, ng/L | 839.5 (390.2–1457.5) | 1156.0 (583.0–2127.0) |
| cTnT‐hs, ng/L | 12.1 (7.7–19.4) | 14.2 (9.8–23.3) |
| GDF‐15, ng/L | 1470.0 (1095.2–2150.5) | 1711.0 (1334.0–2618.0) |
| Cystatin C, mg/L | 1.0 (0.8–1.2) [348] | 1.1 (0.9–1.3) [41] |
| IL‐6, ng/L | 2.4 (1.5–3.9) [349] | 2.8 (1.9–4.5) [41] |
| eGFR, CKD‐EPI equation, mL/min | 65.7 (54.9–76.9) [8] | 61.8 (50.8–75.0) [1] |
Continuous variables presented as median (quartile 1–quartile 3). Categorical variables presented as percentage (frequency). Number of missing values presented in brackets, and does not include cases where biomarker concentrations were below detection limit. CKD‐EPI indicates Chronic Kidney Disease Epidemiology Collaboration; CRP, C‐reactive protein; cTnT‐hs, high‐sensitivity cardiac troponin T; eGFR, estimated glomerular filtration rate; GDF‐15, growth differentiation factor 15; IL‐6, interleukin 6; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SE, systemic embolism; and TIA, transient ischemic attack.
Evaluation of Prognostic Biomarkers for Ischemic Stroke/SE
The median normalized protein expression values in both cohorts, divided by cases and noncases, are presented in Table S1 and S2.
In the identification cohort, of the 268 unique biomarkers and 13 clinical variables, the variables most strongly associated with ischemic stroke/SE in the random survival forest analysis are presented in Figure 1A. Among the biomarkers most strongly associated with the outcome, most were from the OLINK CVD II and CVD III panels. Thus, the Inflammation panel was not analyzed in the external validation cohort. The results from the validation process in the external cohort, on the basis of 186 biomarkers and 12 clinical variables, are presented in Figure 2B. Among the top 20 markers in both cohorts, 5 variables (prior stroke, NT‐proBNP, trefoil factor 3 [TFF3], soluble suppression of tumorigenesis 2 (sST2), and osteopontin) were consistently associated with ischemic stroke/SE.
Figure 1. Variable importance for ischemic stroke/systemic embolism, according to random survival forest.

A, Identification cohort. Red indicates biomarkers analyzed on CVD II panel; green, biomarkers analyzed on CVD III panel; and blue, biomarkers analyzed on Inflammation panel. Biomarkers listed in black were analyzed with conventional immunoassays. Only the top 50 variables are shown. The evaluation included 263 proximity extension assay (PEA) markers, 4 conventional markers (NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide], high‐sensitivity cardiac troponin T [cTnT‐hs], growth differentiation factor 15 [GDF‐15], and interleukin 6 [IL‐6]), renal function, and 13 clinical characteristics. Protein names and UniProt numbers are found in Table S1. B, Validation cohort. Red indicates biomarkers analyzed on CVD II panel; green, biomarkders analyzed on CVD III panel; and blue, biomarkers analyzed on Inflammation panel. Biomarkers listed in black were analyzed with conventional immunoassays. Only the top 50 variables are shown. The evaluation included levels of 182 PEA markers, 4 conventional markers (NT‐proBNP, cTnT‐hs, GDF‐15, and IL‐6), renal function, and 13 clinical characteristics. Protein names and UniProt numbers are found in Table S2. MI indicates myocardial infarction; ST2, suppression of tumorigenesis 2; and TIA, transient ischemic attack.
Figure 2. Forest plot of biomarkers associated with ischemic stroke or systemic embolism, according to adjusted Cox regression analysis.
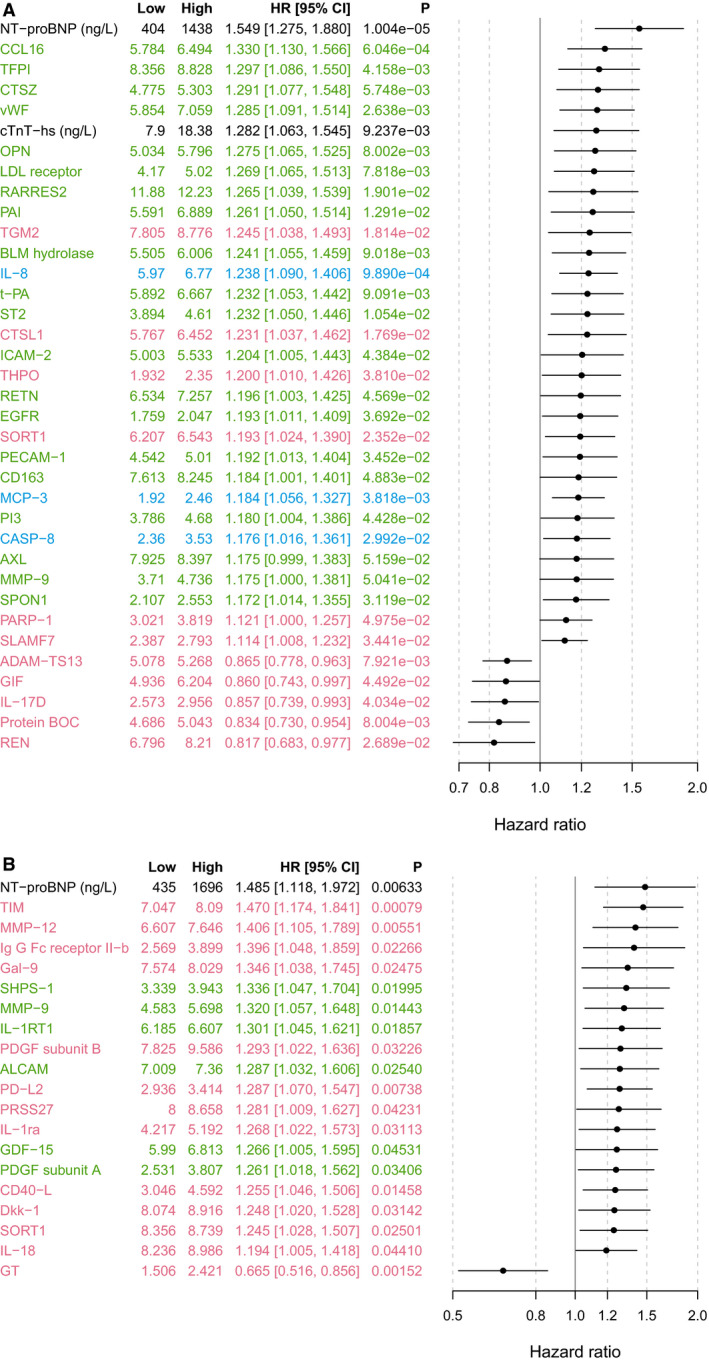
A, Identification cohort. A forest plot showing all 255 biomarkers is available in Figure S1. Red indicates biomarkers analyzed on CVD II panel; green, biomarkers analyzed on CVD III panel; and blue, biomarkers analyzed on Inflammation panel. Biomarkers listed in black were analyzed with conventional immunoassays. Model adjusted for baseline characteristics, renal function, and cardiac biomarkers (NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide] and high‐sensitivity cardiac troponin T [cTnT‐hs]). Low and High correspond to the first and third sample quartiles of the respective biomarkers. Protein names and UniProt numbers are found in Table S1. B, Validation cohort. Red indicates biomarkers analyzed on CVD II panel; and green, biomarkers analyzed on CVD III panel. Biomarkers listed in black were analyzed with conventional immunoassays. Model adjusted for baseline characteristics, renal function, and cardiac biomarkers (NT‐proBNP and cTnT‐hs). Low and High correspond to the first and third sample quartiles of the respective biomarkers. Protein names and UniProt numbers are found in Table S2. HR indicates hazard ratio; and ST2, suppression of tumorigenesis 2.
The Cox analyses, evaluating each of the individual 255 biomarkers, identified 35 as statistically significantly associated with ischemic stroke/SE when adjusting for clinical characteristics, renal function (cystatin C), and cardiac biomarkers (NT‐proBNP and cTnT‐hs) in the identification cohort (Figure 2A, with unadjusted results in Figure S1A), and 20 in the validation cohort (Figure 2B, with unadjusted results in Figure S1B). Of these, 3 biomarkers were consistently significantly associated with ischemic stroke/SE in both cohorts (Table 3): NT‐proBNP, sortilin, and matrix metalloproteinase (MMP)‐9 (Table 3). For these biomarkers, the HR (95% CI) per interquartile range in the Cox regression analyses adjusted for clinical variables, renal function, and the cardiac biomarkers, was 1.55 (1.28–1.88) for NT‐proBNP, 1.19 (1.02–1.39) for sortilin, and 1.18 (1.00–1.38) for MMP9. The corresponding HRs in the external validation cohort are presented in Table 3.
Table 3.
Biomarkers Consistently Associated With Ischemic Stroke/SE, According to RF or Adjusted Cox Regression Analyses
| Variable | RF Ranking | Low | High | Cox Model A | Cox Model B | ||
|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | ||||
| Identification cohort | |||||||
| NT‐proBNP | 1 | 404 | 1438 | 1.646 (1.379–1.963) | 3.19E‐08* | 1.549 (1.275–1.880) | 1.00361E‐05 |
| TFF3 | 3 | 5.338 | 6.043 | 1.278 (1.098–1.488) | 0.00153 | 1.188 (0.974–1.450) | 0.0899 |
| ST2 | 10 | 3.894 | 4.61 | 1.419 (1.231–1.635) | 1.44E‐06 | 1.232 (1.050–1.446) | 0.0105 |
| Osteopontin | 14 | 5.034 | 5.796 | 1.574 (1.347–1.838) | 1.09E‐08 | 1.275 (1.065–1.525) | 0.0080 |
| ADAMTS13 | 16 | 5.078 | 5.268 | 0.856 (0.779–0.941) | 0.00130 | 0.865 (0.778–0.963) | 0.00792 |
| Sortilin | 116 | 6.207 | 6.543 | 1.257 (1.089–1.451) | 0.00182 | 1.193 (1.024–1.390) | 0.0235 |
| MMP9 | 183 | 3.71 | 4.736 | 1.213 (1.036–1.420) | 0.0165 | 1.175 (1.000–1.381) | 0.0504 |
| External validation cohort | |||||||
| NT‐proBNP | 3 | 435 | 1696 | 1.736 (1.342–2.246) | 2.65E‐05 | 1.485 (1.118–1.972) | 0.00633 |
| Osteopontin | 14 | 7.127 | 7.936 | 1.426 (1.130–1.798) | 0.00276 | 1.156 (0.878–1.521) | 0.302 |
| TFF3 | 19 | 4.856 | 5.551 | 1.354 (1.142–1.606) | 0.0005 | 1.041 (0.783–1.384) | 0.780 |
| ST2 | 20 | 4.16 | 4.887 | 1.179 (0.954–1.457) | 0.128 | 1.020 (0.803–1.296) | 0.869 |
| MMP9 | 28 | 4.583 | 5.698 | 1.297 (1.051–1.601) | 0.01547 | 1.320 (1.057–1.648) | 0.01443 |
| Sortilin | 76 | 8.356 | 8.739 | 1.310 (1.090–1.574) | 0.00405 | 1.245 (1.028–1.507) | 0.02501 |
Biomarkers identified as top markers by 2 different statistical methods, an RF (top 20 biomarkers) or an adjusted Cox regression analysis (model B; P≤0.05), were included in the table.
Analyses based on 4075 patients and 261 events, with all covariates available in the identification cohort, and 1196 patients and 129 events in the external validation.
Model A: Cox regression model adjusted for baseline characteristics: age, sex, body mass index, smoking, hypertension, diabetes mellitus, prior myocardial infarction, prior stroke/transient ischemic attack, peripheral artery disease, heart failure, and randomized treatment. Model B: same as A with addition of renal and cardiac biomarkers (NT‐proBNP and high‐sensitivity cardiac troponin T).
Hazard ratios per interquartile range. Figure S2 shows unadjusted nonlinear associations of these biomarkers with ischemic stroke/SE.
ADAMTS13 indicates a disintegrin and metalloproteinase with thrombospondin motifs 13; MMP, matrix metalloproteinase; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; RF, random survival forest; SE, systemic embolism; ST2, suppression of tumorigenesis 2; and TFF3, trefoil factor‐3.
Among the few biomarkers associated with decreased risk of ischemic stroke/SE, according to both random survival forest and adjusted Cox regression analysis, was a disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS13); however, this finding was not consistent in the validation cohort (Table 3).
The correlation between these top candidate prognostic biomarkers and established cardiovascular (NT‐proBNP and cTnT‐hs) and renal biomarkers (cystatin C) is shown in Table 4. TFF3 was moderately correlated with renal function (ρ, 0.59). Beyond that, no strong patterns of correlation were seen (ρ, <0.5). The baseline concentrations of these top candidate prognostic biomarkers are summarized in Table S5, and their associations with ischemic stroke/SE, by using splines, are shown in Figure S2.
Table 4.
Spearman Correlation Between the Top Novel Prognostic Candidate PEA Biomarkers and Established Biomarkers
| Biomarker | Cystatin C | NT‐proBNP | Troponin T |
|---|---|---|---|
| MMP9 | 0.14 | 0.03 | 0.06 |
| Osteopontin | 0.37 | 0.283 | 0.34 |
| Sortilin | 0.10 | 0.17 | 0.13 |
| ST2 | 0.13 | 0.23 | 0.23 |
| TFF3 | 0.59 | 0.34 | 0.44 |
| ADAMTS13 | ‐0.13 | ‐0.11 | ‐0.14 |
On the basis of the random identification cohort only (N=4075), excluding enriched cases.
PEA biomarkers in normalized protein expression values. Established biomarkers (cystatin C, NT‐proBNP, and high‐sensitivity troponin T) in logarithmic transformation of the ng/L level. Additional data on correlation for PEA biomarkers, identified in adjusted Cox analyses, are presented in Table S3 and S4.
Table S5 shows the baseline concentrations of the identified PEA biomarkers. ADAMTS13 indicates a disintegrin and metalloproteinase with thrombospondin motifs 13; MMP, matrix metalloproteinase; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PEA, proximity extension assay; ST2, suppression of tumorigenesis 2; and TFF3, trefoil factor‐3.
Discussion
In the present study, on the basis of 2 separate cohorts with anticoagulated patients with AF, a comprehensive and systematic screening of 268 protein biomarkers to identify those associated with ischemic stroke/SE was performed. An unbiased evaluation of the most important prognostic variables by a random survival forest approach and traditional Cox regression analyses identified NT‐proBNP and 5 novel biomarkers (MMP9, osteopontin, sortilin, sST2, and TFF3) as having independent association with ischemic stroke/SE in patients with AF on oral anticoagulation. Furthermore, one additional biomarker may be of potential interest, as it was associated with reduced risk: ADAMTS13. These findings provide new opportunities to improve the mechanistic understanding of ischemic stroke in patients with AF as well as further refining risk assessment and clinical decision making in these patients.
Candidate Biomarkers and Potential Mechanisms
NT‐proBNP demonstrated the strongest and most consistent association with ischemic stroke/SE. NT‐proBNP is a previously established risk marker in cardiovascular disease and widely integrated in healthcare systems worldwide. 6 This study provides strong evidence that the association is not only robust, but it is independent of a comprehensive set of other protein cardiovascular biomarkers. Natriuretic peptides have repeatedly shown independent association with stroke, other cardiovascular outcomes, and death in many patient settings, including AF. 6 NT‐proBNP has been suggested to be of atrial origin in AF because of myocyte stress in the atria, reflecting atrial dysfunction, which is an established risk factor for thrombosis formation in AF. This may be one plausible mechanism for the relation between NT‐proBNP and thrombosis.17 However, the exact mechanism behind the association with ischemic stroke in AF is still elusive, although several possible concepts have been suggested previously and revolve around pathways of myocardial damage. 19
Recently, the biomarker was also included in a biomarker‐based risk score for stroke in AF and has also been proposed for possible use for further refinement of stroke risk in international AF guidelines. 4 , 5
Among the 5 newly identified biomarkers showing consistent association with ischemic stroke, TFF3 and osteopontin were moderately correlated and sortilin, sST2, and MMP9 were weakly correlated with established cardiovascular biomarkers, such as troponin and natriuretic peptides. Thus, they appear to represent different pathways, including metabolism, mucosal integrity, remodeling/fibrosis, and vascular calcification (Figure 3).
Figure 3. Illustration of putative pathways for the novel candidate biomarkers in relation to a model based on the Virchow triad for thrombus formation.
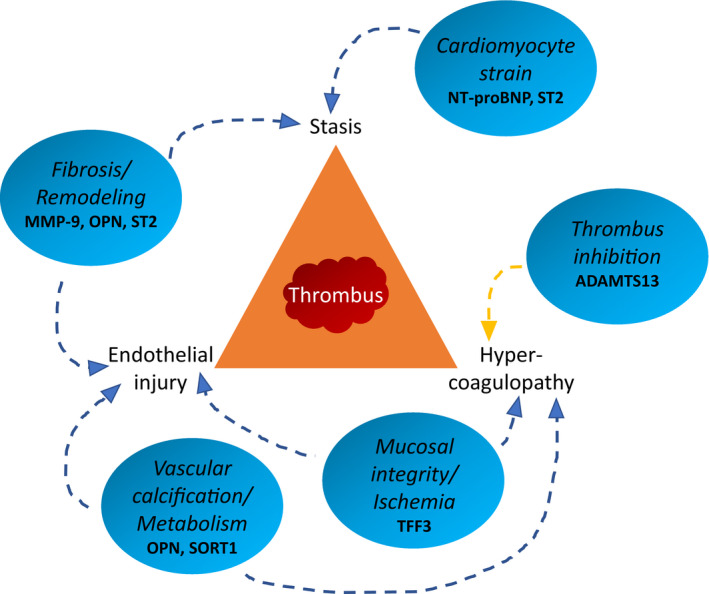
ADAMTS13 indicates a disintegrin and metalloproteinase with thrombospondin motifs 13; MMP‐9, matrix metalloproteinase‐9; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; OPN, osteopontin; SORT1, sortilin; ST2, suppression of tumorigenesis 2; and TFF3, trefoil factor 3.
MMP9 belongs to a family of zinc‐dependent endopeptidases involved in tissue remodeling. 20 MMP9, previously known as collagenase or gelatinase B, directly degrades extracellular matrix proteins and has been widely studied. It is secreted by several cells, most prominently neutrophils, fibroblasts, and macrophages. 20 In cardiovascular disease, MMP9 has been associated with hypertension, arterial stiffness, and cardiac hypertrophy. 20 , 21 MMPs have often been used as markers for myocardial fibrosis and have been associated with poorer prognosis in several cardiovascular settings. 22 , 23 , 24 However, the role of MMPs in AF has been less studied. Concentrations of MMP9 and other MMPs have been described to be higher in patients with persistent AF compared with controls in sinus rhythm. 25 , 26 The present results, to our knowledge, describe for the first time the prognostic role of MMP9 as a risk marker for ischemic stroke in patients with AF. The relation may be through potential direct mechanisms because these extracellular matrix degradation proteinases have previously been associated with left atrial dilatation, or potentially by less specific pathways reflecting a general burden of cardiac fibrosis and cardiomyopathy. 22
Osteopontin is a noncollagenous bone matrix protein synthesized by osteoblasts and osteocytes. 27 , 28 It is, however, also expressed and secreted by a large number of other cells, such as neutrophils, fibroblasts, and myoblasts. 27 , 28 Osteopontin has been described to be a multifunctional cytokine involved in many physiological and pathological processes, including inflammation. 27 , 28 In cardiology, osteopontin has been described to be a mediator of cardiac fibrosis, with higher concentrations in the presence of cardiac fibrosis. 27 Osteopontin has also been suggested to be involved in vascular calcification and coronary atherosclerosis and associated with cardiovascular events, such as myocardial infarction and cardiovascular death. 29 Not much is known about osteopontin in the setting of AF. However, recently, osteopontin was described to be associated with atrial fibrosis 30 and identified as a prognostic biomarker for incident AF in community‐dwelling adults in a protein profiling program. 31 The association of osteopontin with ischemic stroke/SE in patients with AF is novel.
Sortilin is expressed in several tissues and belongs to a family of Vps10p‐domain receptors. 32 Sortilin has been described to be involved in the hepatic metabolism of low‐density lipoproteins. 33 In several genome‐wide association studies, the sortilin locus has consistently been associated with low‐density lipoprotein concentrations. 34 , 35 Sortilin has therefore been suggested as a lipoprotein receptor that mediates the uptake of low‐density lipoprotein particles into cells. 35 In addition, sortilin has been indicated to play a role in vascular calcification and potentially also in neuronal apoptosis. 32 , 33 The role of sortilin in AF has, to our knowledge, not been investigated previously. The present results show sortilin to be a consistent risk marker of ischemic stroke in AF. It is unclear if it plays a casual role through its involvement in calcification or exhibits secondary relations by pathways of atherosclerotic disease via lipid metabolism. Potentially, multiple effects may also be involved as a soluble form of sortilin is released from activated platelets. 36
Among the newly identified biomarkers associated with ischemic stroke/SE, sST2 is probably the most recognized and most explored in the cardiovascular field. 37 Similar to the natriuretic peptides, the main focus of research on sST2 has been in heart failure. Suppression of tumorigenesis 2 (ST2) is a member of the interleukin 1 receptor family, with 2 main isoforms: transmembrane or cellular and soluble or circulating (sST2) forms. sST2 is considered to be a marker of myocardial stress, fibrosis, and remodeling. 38 Interleukin 33 binds to the transmembrane form of ST2 and has antihypertrophic and antifibrotic effects. The soluble form of ST2 (sST2), however, acts as a decoy receptor and blocks the cardioprotective effects. It is plausible that sST2 is associated with stroke in patients with AF in part via a similar pathophysiologic pathway as for the natriuretic peptides. However, beyond its myocardial role, the interleukin 33/ST2 system has recently also been shown to induce tissue factor, the main initiator of blood coagulation, expression and activity, in a subset of human monocytes and monocyte‐derived procoagulant microvesicles and thus possibly represents another pathway for ischemic stroke/SE in patients with AF. 39 Recently, sST2 was shown to be associated with incident stroke in ambulatory individuals from the Framingham Offspring cohort. 40 The present results thus for the first time extend these observations to a population with AF.
TFF3, a member of the trefoil family, is mainly secreted from goblet cells in the gastrointestinal system and is involved in supporting mucosal integrity. 41 TFF3 has been associated with inflammation and different types of malignancies, as both conditions carry higher TFF3 concentrations. Studies of TFF3 in cardiovascular disease are limited. 41 , 42 Some studies have demonstrated increased concentrations in states of myocardial ischemia, and TFF3 has also been associated with cardioprotective effects in experimental animal models. 43 Its potential role in AF is thus novel. It may, however, be possible that TFF3 is simply a marker of underlying myocyte damage/ischemia rather than possessing direct causal effects in thrombus formation and ischemic stroke/SE in AF.
Of the 255 biomarkers in the identification cohort, few were associated with a reduced risk of ischemic stroke/SE. Among the biomarkers most strongly associated with lower risk of ischemic stroke/SE in this cohort with AF was ADAMTS13, also known as von Willebrand factor–cleaving protease. ADAMTS13 cleaves von Willebrand factor multimers into smaller less procoagulant forms and thereby exerts an inhibitory effect on platelet thrombus formation. 44 Low concentration of ADAMTS13 has also been associated with increased atherosclerosis in animal models. Some smaller case‐control studies in humans have shown low concentrations of ADAMTS13 to be associated with higher risk of ischemic stroke; however, inconsistencies exist. 44 However, the result concerning ADAMTS13 needs to be interpreted with caution because it could not be confirmed in the external validation cohort. Still, the rather unique association of this biomarker with lower risk of stroke may merit it for more in‐depth studies with quantitative assays in prospective materials in the efforts to further understand and mitigate the risk of stroke in AF.
Implications
In this study, the novel PEA protein screening technology was, for the first time, used for mass screening to identify biomarkers associated with ischemic stroke or SE during ongoing anticoagulation treatment in patients with AF. Several biomarkers, reflecting different pathophysiological pathways for ischemic stroke/SE in AF, were consistently identified. The present results thus represent an important contribution in the step toward a better mechanistic understanding of ischemic stroke in patients with AF. These markers could help guide research into new therapeutic targets beyond anticoagulation in patients with AF at risk for stroke, and potentially even identify a population of patients (including those without clinical AF) who are at risk for thromboembolic stroke. The novel candidate biomarkers therefore need to be further evaluated using quantitative assays in prospective materials, and evaluated with mendelian randomization analyses and functional studies to better understand their pathophysiological links and causality to the disease processes.
Strengths and Limitations
The use of a well‐defined clinical cohort with complete follow‐up data and a stringent method of statistical evaluation applied, using 2 separate cohorts for an identification and validation process, strengthens the results. This approach therefore provides a large degree of certitude about the identified novel prognostic biomarkers. Also, 2 different statistical methods were used. The random survival forest treated all variables simultaneously and allowed, inherently, for nonlinear associations and complex interactions among the variables. The Cox regression, on the other hand, assumed a linear association between the log relative hazard of ischemic stroke/SE and each marker one at a time, making it possible to estimate average adjusted HRs in a more conventional way. The 2 methods thus captured different aspects of the possibly complex relationship between the biomarkers and the risk for ischemic stroke/SE and by that complemented each other in the process of screening for top biomarkers. Because of the high number of biomarkers in relation to number of events, the analyses focused on taking advantage of the availability of 2 separate AF cohorts instead of using specific methods to account for multiple testing, such as Bonferroni. There were slight differences between the 2 cohorts in regard to age and renal function. This may influence the results to some degree. Likewise, 3 panels were initially used in the identification cohort, and only 2 panels, containing more prognostically relevant biomarkers, were used in the external validation cohort. Thus, it is possible that some additional biomarkers of potential interest remained unconfirmed despite the thorough evaluation. For instance, this includes ADAMTS13 or spondin‐1 in the identification cohort and T‐cell immunoglobulin mucin receptor 1 or MMP12 in the validation cohort, as these biomarkers only showed strong associations with ischemic stroke in 1 of the 2 cohorts. Also, all patients were on oral anticoagulation and the results may thus not be fully generalizable to patients without antithrombotic treatment, although this data set may be especially valuable to explore “residual risk.” For the PEA method, a limitation is the lack of absolute values; however, prior validation studies have shown that biomarkers analyzed with the PEA technique have an adequate concordance with conventional immunoassays. 14
Conclusions
In patients with AF on oral anticoagulation, of 268 biomarkers, 1 established biomarker, NT‐proBNP, and 5 novel biomarkers, representing different pathophysiological pathways, showed consistent association with the risk of ischemic stroke/SE; MMP9 and sST2 (remodeling/fibrosis), osteopontin (vascular calcification), sortilin (metabolism), and TFF3 (mucosal integrity). Further evaluation of these novel biomarkers and pathways associated with ischemic stroke in patients with AF is warranted.
Sources of Funding
The ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial was funded by Bristol‐Myers Squibb Co, Princeton, NJ, and Pfizer Inc, New York, NY, and coordinated by the Duke Clinical Research Institute, Durham, NC, and Uppsala Clinical Research Center, Uppsala, Sweden. The RE‐LY (Randomized Evaluation of Long‐Term Anticoagulation Therapy) trial was funded by Boehringer Ingelheim, Ingelheim, Germany. The analyses were supported by The Swedish Foundation for Strategic Research (grant RB13‐0197), the Swedish Heart‐Lung Foundation (grant 20090183), and Science for Life Laboratory, Uppsala University, Uppsala, Sweden. Dr Hijazi reports research support from the Swedish Heart‐Lung Foundation (20170718) and the Swedish Society for Medical Research (S17‐0133). Roche Diagnostics, Rotkreuz, Switzerland, provided the precommercial assay of growth differentiation factor 15.
Disclosures
Dr Hijazi has received lecture fees from Boehringer Ingelheim, Roche, Bristol‐Myers Squibb, and Pfizer, and consulting fees from Merck Sharp & Dohme, Roche, Bristol‐Myers Squibb, and Pfizer. The growth differentiation factor 15 (GDF‐15) assays were provided by Roche. Dr Wallentin has received institutional research grants, consultancy fees, lecture fees, and travel support from Bristol‐Myers Squibb/Pfizer, AstraZeneca, GlaxoSmithKline, and Boehringer Ingelheim. Dr Wallentin also received institutional research grants from Merck & Co and Roche, received consultancy fees from Abbott, and holds 2 patents involving GDF‐15. J. Lindbäck received institutional research grants from Boehringer Ingelheim and Bristol‐Myers Squibb/Pfizer. Dr Alexander has received institutional research grants, consulting fees, and honoraria from Bristol‐Myers Squibb, Regado Biosciences, and Merck, and consulting fees and honoraria from Pfizer, AstraZeneca, Boehringer Ingelheim, Ortho‐McNeil‐Janssen, Polymedix, and Bayer. Dr Connolly has received consulting fees, speaker fees, and research grants from Boehringer Ingelheim, Bristol‐Myers Squibb, Bayer, and Portola, consulting fees and research grants from Sanofi‐Aventis, and research grants from Boston Scientific. Dr Eikelboom has received institutional research grants and honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb/Pfizer, Daiichi‐Sankyo, Eli Lilly, Glaxo Smith Kline, Janssen, and Sanofi. Dr Ezekowitz has received grants and consultant fees from Boehringer Ingelheim, Bristol Myers‐Squibb, and Pfizer, and consultant fees from Boston Scientific, Anthos Therapeutic, and Alta Therapeutics. Dr Granger has received grants and personal fees from GlaxoSmithKline, Boehringer Ingelheim, Bristol‐Myers Squibb, Pfizer, Sanofi‐Aventis, Takeda, The Medicines Company, Janssen, Bayer, and Hoffmann‐La Roche, grants from Medtronics Foundation, Merck & Co, and Armetheon, and personal fees from Lilly, AstraZeneca, Daiichi Sankyo, Ross Medical Corporation, Salix Pharmaceuticals, and Gilead. Dr Lopes has received an institutional research grant and consulting fees from Bristol‐Myers Squibb, an institutional research grant from GlaxoSmithKline, and consulting fees from Bayer, Boehringer Ingleheim, Pfizer, Merck, and Portola. Dr Yusuf has received grants, speaker fees, and paid travel expenses from Boehringer Ingelheim. Dr Oldgren has received consulting and lecture fees from Boehringer Ingelheim, Bayer, Bristol‐Myers Squibb, and Pfizer. Dr Siegbahn has received institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb/Pfizer, GlaxoSmithKline, and Roche Diagnostics, and consulting fees from OLINK Proteomics. Dr Pol has no disclosures to report.
Supporting information
Tables S1–S5
Figures S1–S2
(J Am Heart Assoc. 2020;9:e018984. DOI: 10.1161/JAHA.120.018984.)
Supplementary material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018984
For Sources of Funding and Disclosures, see pages 11 and 12.
REFERENCES
- 1. Kirchhof P, Breithardt G, Aliot E, Al Khatib S, Apostolakis S, Auricchio A, Bailleul C, Bax J, Benninger G, Blomstrom‐Lundqvist C, et al. Personalized management of atrial fibrillation: proceedings from the fourth Atrial Fibrillation competence NETwork/European Heart Rhythm Association consensus conference. Europace. 2013;15:1540–1556. 10.1093/europace/eut232 [DOI] [PubMed] [Google Scholar]
- 2. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 3. Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 4. Hijazi Z, Lindbäck J, Alexander JH, Hanna M, Held C, Hylek EM, Lopes RD, Oldgren J, Siegbahn A, Stewart RAH, et al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker‐based risk score for predicting stroke in atrial fibrillation. Eur Heart J. 2016;37:1582–1590. 10.1093/eurheartj/ehw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 6. Hijazi Z, Oldgren J, Siegbahn A, Granger CB, Wallentin L. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J. 2013;34:1475–1480. 10.1093/eurheartj/eht024 [DOI] [PubMed] [Google Scholar]
- 7. Hijazi Z, Oldgren J, Siegbahn A, Wallentin L. Application of biomarkers for risk stratification in patients with atrial fibrillation. Clin Chem. 2017;63:152–164. 10.1373/clinchem.2016.255182 [DOI] [PubMed] [Google Scholar]
- 8. Assarsson E, Lundberg M, Holmquist G, Björkesten J, Bucht Thorsen S, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, et al. Homogenous 96‐plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9:e95192. 10.1371/journal.pone.0095192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopes RD, Alexander JH, Al‐Khatib SM, Ansell J, Diaz R, Easton JD, Gersh BJ, Granger CB, Hanna M, Horowitz J, et al. Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J. 2010;159:331–339. 10.1016/j.ahj.2009.07.035 [DOI] [PubMed] [Google Scholar]
- 10. Ezekowitz MD, Connolly S, Parekh A, Reilly PA, Varrone J, Wang S, Oldgren J, Themeles E, Wallentin L, Yusuf S. Rationale and design of RE‐LY: randomized evaluation of long‐term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805–810.e2. 10.1016/j.ahj.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 11. Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, Keltai M, Lanas F, Lopes RD, Lopez‐Sendon J, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33:2821–2830. 10.1093/eurheartj/ehs274 [DOI] [PubMed] [Google Scholar]
- 12. Hijazi Z, Hohnloser SH, Andersson U, Alexander JH, Hanna M, Keltai M, Parkhomenko A, López‐Sendón JL, Lopes RD, Siegbahn A, et al. Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. 2016;1:451–460. 10.1001/jamacardio.2016.1170 [DOI] [PubMed] [Google Scholar]
- 13. Ebai T, Kamali‐Moghaddam M, Landegren U. Parallel protein detection by solid‐phase proximity ligation assay with real‐time PCR or sequencing. Curr Protoc Mol Biol. 2015;109:21–25. 10.1002/0471142727.mb2010s109 [DOI] [PubMed] [Google Scholar]
- 14. Siegbahn A, Eriksson S, Lindbäck J, Wallentin L. A comparison of the proximity extension assay with established immunoassays. In Advancing Precision Medicine: Current and Future Proteogenomic Strategies for Biomarker Discovery and Development. Science 2017:22–25. [Google Scholar]
- 15. Ishwaran H, Kogalur UB, Blackstone EH, Lauer MS. Random survival forests. Annuals Appl Stat. 2008;2:841–860. [Google Scholar]
- 16. van Buuren S, Groothuis‐Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 17. R_Core_Team Computing . A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 18. Wright MN, Ziegler A. ranger: A Fast Implementation of Random forests for High Dimensional Data in C++ and R. J Stat Softw. 2017;77:1–17. [Google Scholar]
- 19. Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, Reilly PA, Vinereanu D, Siegbahn A, Yusuf S, et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long‐term Anticoagulation Therapy (RE‐LY) substudy. Circulation. 2012;125:1605–1616. [DOI] [PubMed] [Google Scholar]
- 20. Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase‐9: many shades of function in cardiovascular disease. Physiology (Bethesda). 2013;28:391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dhingra R, Pencina MJ, Schrader P, Wang TJ, Levy D, Pencina K, Siwik DA, Colucci WS, Benjamin EJ, Vasan RS. Relations of matrix remodeling biomarkers to blood pressure progression and incidence of hypertension in the community. Circulation. 2009;119:1101–1107. 10.1161/CIRCULATIONAHA.108.821769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collier P, Watson CJ, Voon V, Phelan D, Jan A, Mak G, Martos R, Baugh JA, Ledwidge MT, McDonald KM. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011;13:1087–1095. 10.1093/eurjhf/hfr079 [DOI] [PubMed] [Google Scholar]
- 23. Kelly D, Khan SQ, Thompson M, Cockerill G, Ng LL, Samani N, Squire IB. Plasma tissue inhibitor of metalloproteinase‐1 and matrix metalloproteinase‐9: novel indicators of left ventricular remodelling and prognosis after acute myocardial infarction. Eur Heart J. 2008;29:2116–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lopez‐Andrès N, Rossignol P, Iraqi W, Fay R, Nuee J, Ghio S, Cleland JG, Zannad F, Lacolley P. Association of galectin‐3 and fibrosis markers with long‐term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: insights from the CARE‐HF (Cardiac Resynchronization in Heart Failure) trial. Eur J Heart Fail. 2012;14:74–81. [DOI] [PubMed] [Google Scholar]
- 25. Polyakova V, Miyagawa S, Szalay Z, Risteli J, Kostin S. Atrial extracellular matrix remodelling in patients with atrial fibrillation. J Cell Mol Med. 2008;12:189–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stanciu AE, Vatasescu RG, Stanciu MM, Serdarevic N, Dorobantu M. The role of pro‐fibrotic biomarkers in paroxysmal and persistent atrial fibrillation. Cytokine. 2018;103:63–68. [DOI] [PubMed] [Google Scholar]
- 27. Collins AR, Schnee J, Wang W, Kim S, Fishbein MC, Bruemmer D, Law RE, Nicholas S, Ross RS, Hsueh WA. Osteopontin modulates angiotensin II‐induced fibrosis in the intact murine heart. J Am Coll Cardiol. 2004;43:1698–1705. [DOI] [PubMed] [Google Scholar]
- 28. Zhao H, Chen Q, Alam A, Cui J, Suen KC, Soo AP, Eguchi S, Gu J, Ma D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018;9:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Minoretti P, Falcone C, Calcagnino M, Emanuele E, Buzzi MP, Coen E, Geroldi D. Prognostic significance of plasma osteopontin levels in patients with chronic stable angina. Eur Heart J. 2006;27:802–807. [DOI] [PubMed] [Google Scholar]
- 30. Lin R, Wu S, Zhu D, Qin M, Liu X. Osteopontin induces atrial fibrosis by activating Akt/GSK‐3beta/beta‐catenin pathway and suppressing autophagy. Life Sci. 2020;245:117328. [DOI] [PubMed] [Google Scholar]
- 31. Molvin J, Jujic A, Melander O, Pareek M, Råstam L, Lindblad U, Daka B, Leosdottir M, Nilsson P, Olsen M, et al. Exploration of pathophysiological pathways for incident atrial fibrillation using a multiplex proteomic chip. Open Heart. 2020;7:e001190. 10.1136/openhrt-2019-001190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, et al. Sortilin is essential for proNGF‐induced neuronal cell death. Nature. 2004;427:843–848. 10.1038/nature02319 [DOI] [PubMed] [Google Scholar]
- 33. Schmidt V, Willnow TE. Protein sorting gone wrong–VPS10P domain receptors in cardiovascular and metabolic diseases. Atherosclerosis. 2016;245:194–199. 10.1016/j.atherosclerosis.2015.11.027 [DOI] [PubMed] [Google Scholar]
- 34. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, et al. A comprehensive 1,000 Genomes‐based genome‐wide association meta‐analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeller T, Blankenberg S, Diemert P. Genomewide association studies in cardiovascular disease–an update 2011. Clin Chem. 2012;58:92–103. 10.1373/clinchem.2011.170431 [DOI] [PubMed] [Google Scholar]
- 36. Ogawa K, Ueno T, Iwasaki T, Kujiraoka T, Ishihara M, Kunimoto S, Takayama T, Kanai T, Hirayama A, Hattori H. Soluble sortilin is released by activated platelets and its circulating levels are associated with cardiovascular risk factors. Atherosclerosis. 2016;249:110–115. 10.1016/j.atherosclerosis.2016.03.041 [DOI] [PubMed] [Google Scholar]
- 37. Januzzi JL Jr. ST2 as a cardiovascular risk biomarker: from the bench to the bedside. J Cardiovasc Transl Res. 2013;6:493–500. 10.1007/s12265-013-9459-y [DOI] [PubMed] [Google Scholar]
- 38. de Boer RA, Daniels LB, Maisel AS, Januzzi JL Jr. State of the art: newer biomarkers in heart failure. Eur J Heart Fail. 2015;17:559–569. 10.1002/ejhf.273 [DOI] [PubMed] [Google Scholar]
- 39. Stojkovic S, Thulin Å, Hell L, Thaler B, Rauscher S, Baumgartner J, Gröger M, Ay C, Demyanets S, Neumayer C, et al. IL‐33 stimulates the release of procoagulant microvesicles from human monocytes and differentially increases tissue factor in human monocyte subsets. Thromb Haemost. 2017;117:1379–1390. 10.1160/TH16-10-0784 [DOI] [PubMed] [Google Scholar]
- 40. Andersson C, Preis SR, Beiser A, DeCarli C, Wollert KC, Wang TJ, Januzzi JL Jr, Vasan RS, Seshadri S. Associations of circulating growth differentiation factor‐15 and ST2 concentrations with subclinical vascular brain injury and incident stroke. Stroke. 2015;46:2568–2575. 10.1161/STROKEAHA.115.009026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide‐triggered signals promoting mucosal restitution. Cell Mol Life Sci. 2005;62:2932–2938. 10.1007/s00018-005-5481-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–732. 10.1038/nrm1203 [DOI] [PubMed] [Google Scholar]
- 43. Liu SQ, Tefft BJ, Roberts DT, Zhang LQ, Ren Y, Li YC, Huang Y, Zhang D, Phillips HR, Wu YH. Cardioprotective proteins upregulated in the liver in response to experimental myocardial ischemia. Am J Physiol Heart Circ Physiol. 2012;303:H1446–1458. 10.1152/ajpheart.00362.2012 [DOI] [PubMed] [Google Scholar]
- 44. Sonneveld MA, de Maat MP, Leebeek FW. Von Willebrand factor and ADAMTS13 in arterial thrombosis: a systematic review and meta‐analysis. Blood Rev. 2014;28:167–178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figures S1–S2


