Abstract
Background
The aim of this study was to determine whether frailty is associated with increased admission and mortality risk in the setting of heart failure.
Methods and Results
This retrospective cohort analysis included patients treated within the Veterans Affairs Health System who had International Classification of Diseases, Ninth Revision (ICD‐9) codes for heart failure on 2 or more dates over a 2‐year period. The clinical variables identifiable in claims data, such as demographic variables and markers of physical and cognitive dysfunction, were used to identify patients meeting the frailty phenotype. Of 388 785 extracted patients with coding of heart failure between 2015 and 2018, 163 085 patients (41.9%) with ejection fraction (EF) measurement were included in the present analysis (38.3% with reduced EF and 61.7% with preserved EF). There were 16 660 patients (10.2%) who were identified as frail (9.1% in heart failure with reduced EF and 10.9% in heart failure with preserved EF). Frail patients were older, more often depressed, and were likely to have been admitted in the previous year. One‐year all‐cause mortality rate was 9.7% and 28.1%, and admission rate was 58.1% and 79.5% for nonfrail and frail patients, respectively. Frailty was associated with mortality and admission risk compared with the nonfrail group (adjusted odds ratio [OR], 1.71; 95% CI, 1.65–1.77 for mortality; adjusted OR, 1.29; 95% CI, 1.24–1.34 for admission) independent of EF.
Conclusions
Frailty based on diagnostic coding was associated with particularly higher risk of mortality despite adjustment for known clinical variables. Our findings underscore the importance of nontraditional parameters in the prognostic assessment.
Keywords: diagnostic coding, frailty, heart failure
Subject Categories: Heart Failure, Mortality/Survival
Heart failure (HF) is a leading cause of hospitalization and is associated with poor prognosis and increased medical costs. 1 , 2 As the population ages, HF is becoming increasingly common among the elderly population, and frailty has become a high‐priority issue. 3 Geriatric risk assessment is an area that has seen a great deal of progress in recent years. 4 Although traditional models of risk assessment have aided in the management of patients with HF, there is strong evidence that assessment of frailty is useful to predict outcomes in patients with cardiovascular disease. 5 , 6 , 7
There still is a paucity of effort to include frailty assessment for patients with HF. For instance, Get With the Guidelines Heart Failure Registry, the largest registry collecting patient characteristics and outcomes related to HF admissions in the United States, collects only a few frailty markers, such as albumin and hemoglobin. 8 , 9 Moreover, identifying frailty based on the clinical score in acute decompensated phase, such as the Clinical Frailty Scale, tend to overestimate its prevalence, because symptoms during acute heart failure frequently overlap with the clinical variables associated with frailty. 10
In the absence of prospectively collected data, the ability to identify frailty in diagnostic‐coding records may allow for enhanced mortality prediction. Over the past years, the Department of Veterans Affairs (VA) has attempted to measure and improve healthcare quality of HF, but outcome measures evaluating HF mortality have not included makers of frailty. 11 Herein, we evaluated patients with HF in a VA administrative database to determine whether the incorporation of claims‐based measures of frailty might augment mortality prediction compared with using comorbidities alone. The ability to identify frail patients through identification of multimorbidity will not only allow for enhanced mortality prediction but will also have important implications for better care decisions and design of future clinical studies.
METHODS
The data and study materials cannot be made available by the authors to other researchers for purposes of reproducing the results or replicating the procedure, per VA policy. However, all data used in the analyses are available to VA researchers through the VA Informatics and Computing Infrastructure.
Study Population
The VA contains a national integrated healthcare system with a comprehensive all‐electronic medical record called Veterans Information Systems and Technology Architecture across all VA healthcare facilities. Within the VA healthcare system there are more than 1700 hospitals, clinics, and nursing homes. The VA has provided health care to millions of veterans over the past 2 decades and most of the care is recorded through Veterans Information Systems and Technology Architecture. These data are aggregated into the VA corporate data warehouse that contains billions of records for over 20 million patients starting October 1999.
The study population consisted of randomly extracted adult patients with outpatient or inpatient visits within the VA Health System with established HF and left ventricular ejection fraction (EF) measurement, identified between 2015 and 2018. We obtained administrative healthcare data from the VA's corporate data warehouse, which contains detailed information on all inpatient, outpatient, laboratory, and pharmacy encounters throughout the VA healthcare system. We also obtained comprehensive Medicare Fee‐for‐Service administrative claims from the Outpatient, Carrier, and Medicare Provider Analysis and Review files for each veteran in the cohort during this period. The study was approved by the human subjects' research committee of the Stanford University School of Medicine, which waived the need for patient consent.
Veterans were identified as having HF if they had at least 1 administrative record with the following International Classification of Diseases, Ninth Revision (ICD‐9) codes over 2 different dates in the VA system during the study period and during the previous 2 years: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 425.4, 428.0, 428.1, 428.20–428.23, 428.30–428.33, 428.40–428.43, or 428.9. All patients in the cohort had a minimum of 2 year of prior healthcare data to permit accurate coding of comorbidities and antecedent health events.
Defining Frailty With Claims‐Based Diagnostic Coding
There are several ways to measure frailty. Most measurements of frailty require in‐person or patient‐reported functional measurements, which make widespread implementation impractical. In this study, previously described claims‐based diagnostic coding was used to identify frailty. Several indices that identify frailty using administrative data from health insurance claims have been published in the past several years, albeit there still is little evidence of the association between frailty as identified by claims data and patient outcomes in HF. The clinical variables identifiable in claims data, such as demographic variables and markers of physical and cognitive dysfunction, were used to identify patients meeting the Fried frailty phenotype: 781.2 (abnormality of gait:), 783.2 (abnormal loss of weight and underweight), 783.7 (adult failure to thrive), 799.4 (cachexia), 799.3 (debility), 719.7 (difficulty in walking), V15.88 (fall), 780.7 (malaise and fatigue), 728.2 (muscular wasting and disuse atrophy), 728.87 (muscle weakness), 707.0, 707.2 (pressure ulcer), and 797 (senility without mention of psychosis). For the present analysis, we excluded the list on the durable medical equipment (E01XX) and nursing or personal care services (T10XX) because our main aim was to test “diagnostic” coding based on medical conditions. Based on the previously published work, we defined frailty as the presence of at least 2 of these diagnoses. 12 A cutoff value of 2 was chosen because incorporating lower values (cutoff of ≥1) would lead to overdiagnoses, and higher values are not adequately sensitive. All covariates were ascertained using primary or secondary diagnosis codes that were coded as present during the study period and during the previous 2 years.
Demographics
We obtained each veteran's age, race/ethnicity, and sex from the VA's enrollment database. Comorbidities (eg, hypertension, diabetes mellitus, coronary artery disease, valve disease, chronic liver and obstructive pulmonary diseases, malignancy) were assessed using diagnosis codes in either VA or Medicare administrative claims. Each patient's comorbidity vector of binary predictor variables was updated quarterly based on the VA and Medicare healthcare encounters occurring during that quarter. Each patient's chronic comorbid conditions (eg, diabetes mellitus) were assumed to be present perpetually after their onset, unless the condition and a related condition were mutually exclusive. Age was categorized into several groups: 18 years or younger, 18 to 34, 35 to 39, and 5‐year intervals thereafter, up to 90 years or older. For the regression models, the group aged 45 to 49 was used as the reference.
Cutoff value of EF for definition of HF with reduced EF (HFrEF) versus preserved EF (HFpEF) was 40% for the present analysis. For the subanalysis on HF with midrange EF (HFmrEF), HFpEF was defined EF ≧50%, HFmrEF EF 40% to 49%, and HFrEF <40%, respectively. Baseline use and dose of the following medication categories were examined at the baseline for the patients with reduced EF: angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, evidence‐based beta blocker, and mineral corticosteroid receptor antagonists. For each medication class, the presence and absence of absolute contraindications were determined based on vital signs and laboratory values at baseline. Patients who were considered eligible for guideline‐based medical therapy were those with a left ventricular EF measurement ≦40% according to imaging performed within the study period and systolic blood pressure ≧100 mm Hg. In addition, heart rate ≧55/min was a prerequisite for the use of beta blockers, and glomerular filtration rate ≧30 and serum potassium level ≦5.2 mEq/L for angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers.
Outcomes
The primary outcome was 1‐year all‐cause mortality and 1‐year hospital admission for any cause. We assessed the VA's vital status master file to ascertain deaths occurring from 2015 through 2018. This data set incorporates death records from the VA's Beneficiary Identification and Records Locator Subsystem database as well as the Social Security Death Master File; it is considered a highly complete record of deaths among VA‐enrolled veterans. 13
Statistical Analysis
Baseline characteristics of participants were compared by presence or absence of frailty. Two‐sample t test for continuous variables and chi‐square test for categorical variables were used to compare the baseline patient‐level characteristics between the frail and nonfrail cohorts (Table 1). We used a logistic regression model to identify predictors of frailty (presence of 2 or more diagnoses that indicate frail condition). All of the variables listed in Table 2 were included as a covariate. We then performed a fully adjusted analysis that controlled for all patient characteristics described previously. All statistical testing was 2 sided at a significance level of P<0.05. Analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc, Cary, NC).
Table 1.
Baseline Characteristics by Presence or Absence of Frailty
| Nonfrail | Frail | P Value | |
|---|---|---|---|
|
N=146 425 (89.78%) |
N=16 660 (10.22%) | ||
| Age, y | 71.78±10.46 | 77.51±10.61 | |
| Age (y) above 75, % | 33.32 | 55.07 | <0.001 |
| Male, % | 97.48 | 97.27 | 0.1039 |
| Race/Ethnicity | <0.001 | ||
| Hispanic, % | 4.93 | 5.11 | |
| Black, % | 19.94 | 21.69 | |
| White, % | 72.61 | 70.79 | |
| Race/Ethnicity other than Black, White, Hispanic, % | 2.52 | 2.41 | |
| Vital signs at the baseline visit | |||
| Systolic blood pressure | 128.35±20.03 | 127.14±20.57 | <0.001 |
| Diastolic blood pressure | 72.23±11.44 | 69.81±11.10 | <0.001 |
| Heart rate | 75.71±14.73 | 76.42±14.56 | <0.001 |
| Oxygen saturation | 95.87±2.64 | 95.87±2.68 | <0.001 |
| Respiration rate | 18.27±2.18 | 18.38±2.31 | <0.001 |
| Body temperature | 97.77±0.69 | 97.77±0.74 | <0.001 |
| Height | 69.46±3.09 | 69.30±3.29 | <0.001 |
| Weight | 211.6±53.61 | 190.65±55.75 | <0.001 |
| Medical history | |||
| Coronary artery disease, % | 71.03 | 75.32 | <0.001 |
| Valve disease, % | 22.07 | 25.83 | <0.001 |
| Hypertension, % | 92.64 | 94.98 | <0.001 |
| Diabetes mellitus, % | 55.93 | 60.49 | <0.001 |
| Chronic pulmonary disease, % | 47.63 | 56.57 | <0.001 |
| Liver disease, % | 10.97 | 13.29 | <0.001 |
| Malignancy, % | 17.72 | 26.33 | <0.001 |
| Depression, % | 33.88 | 47.29 | <0.001 |
| Psychiatric disease, % | 4.23 | 7.67 | <0.001 |
| Any admission in previous year, % | 58.13 | 79.47 | <0.001 |
| Serum creatinine within 6 mo | 1.54±1.25 | 1.69±1.37 | <0.001 |
| 0.0–0.7 mg/dL, % | 5.7 | 7.23 | |
| 0.8–0.9 mg/dL, % | 19.28 | 15.53 | |
| 1.0–1.4 mg/dL, % | 45.19 | 40.04 | |
| 1.5–1.9 mg/dL, % | 15.4 | 18.05 | |
| 2.0–2.4 mg/dL, % | 12.48 | 17.59 | |
| >2.5 mg/dL, % | 1.95 | 1.57 | |
| BNP or NT‐proBNP within 6 mo | <0.001 | ||
| ≦100 [BNP] or ≦400 [NT‐pro], % | 13.89 | 10.73 | |
| 101–200 or 401–1000, % | 11.14 | 11.02 | |
| 201–700 or 1001–4000, % | 22.35 | 26.21 | |
| 701–1000 or 4001–6000, % | 5.07 | 7.07 | |
| >1000 or >6000, % | 12.7 | 19.66 | |
| Missing, % | 34.86 | 25.31 | |
| LVEF >40, % | 61.2 | 65.97 | <0.001 |
| LVEF | <0.001 | ||
| <20, % | 4.93 | 4.51 | |
| 20–29, % | 14.51 | 12.98 | |
| 30–39, % | 19.36 | 16.54 | |
| 40–49, % | 21.04 | 21.04 | |
| 50–59, % | 23.42 | 26.13 | |
| 60–69, % | 14.51 | 16.33 | |
| ≧70, % | 2.23 | 2.46 | |
| Use of guideline‐based medications | |||
| Beta blocker, % | 73.63 | 71.61 | 0.002 |
| Angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, % | 74.18 | 67.44 | <0.001 |
| Mineral corticosteroid receptor antagonists, % | 29.16 | 24.33 | <0.001 |
BNP indicates brain natriuretic peptides; LVEF, left ventricular ejection fraction; and NT‐proBNP, N‐terminal pro–brain natriuretic peptide.
Table 2.
Clinical Predictors of Coding‐Based Frailty
| Predictors of Frailty | Adjusted Odds Ratio | 95% CI | P Value | |
|---|---|---|---|---|
| Registered year (vs 2018) | ||||
| 2015 vs 2108 | 1.04 | 0.96 | 1.12 | 0.005 |
| 2016 vs 2018 | 0.97 | 0.91 | 1.04 | 0.612 |
| 2017 vs 2018 | 0.91 | 0.86 | 0.97 | <0.001 |
| Age, y (vs 45–59) | ||||
| 18–34 | 0.34 | 0.08 | 1.39 | 0.77 |
| 35–39 | 0.51 | 0.18 | 1.43 | 0.85 |
| 40–44 | 0.66 | 0.34 | 1.29 | 0.90 |
| 50–54 | 1.30 | 0.93 | 1.81 | 0.96 |
| 55–59 | 1.86 | 1.37 | 2.52 | 0.88 |
| 60–64 | 2.11 | 1.57 | 2.83 | 0.86 |
| 65–69 | 2.68 | 2 | 3.59 | 0.81 |
| 70–74 | 3.46 | 2.58 | 4.63 | 0.76 |
| 75–79 | 4.63 | 3.45 | 6.22 | 0.69 |
| >80 | 8.68 | 6.47 | 11.64 | 0.58 |
| Sex (vs male) | ||||
| Female | 1.16 | 1.04 | 1.29 | 0.008 |
| Race/Ethnicity (vs White) | ||||
| Hispanic | 0.90 | 0.83 | 0.98 | 0.18 |
| Black | 1.26 | 1.20 | 1.31 | <0.001 |
| Asian | 1.09 | 0.89 | 1.32 | 0.41 |
| Native American | 1.02 | 0.85 | 1.22 | 0.79 |
| Pacific Islander | 1.15 | 0.94 | 1.40 | 0.18 |
| Refused | 0.57 | 0.36 | 0.90 | 0.009 |
| Missing | 1.16 | 0.51 | 2.61 | 0.68 |
| Vital signs at the baseline | ||||
| Systolic blood pressure | 0.99 | 0.98 | 1.00* | 0.029 |
| Diastolic blood pressure | 0.93 | 0.91 | 0.95 | <0.001 |
| Heart rate | 1.02 | 1.00 | 1.03 | 0.013 |
| Oxygen saturation | 1.02 | 1.01 | 1.03 | <0.001 |
| Respiration rate | 1.00 | 0.99 | 1.01 | 0.68 |
| Body temperature | 1.02 | 0.99 | 1.04 | 0.072 |
| Height | 1.09 | 1.07 | 1.10 | <0.001 |
| Weight | 0.94 | 0.94 | 0.95 | <0.001 |
| Pain | 1.05 | 1.04 | 1.06 | <0.001 |
| Medical history | ||||
| Coronary artery disease | 1.04 | 1.00 | 1.08 | 0.067 |
| Valvular disease | 0.97 | 0.93 | 1.01 | 0.148 |
| Hypertension | 1.15 | 1.07 | 1.25 | <0.001 |
| Diabetes mellitus | 1.35 | 1.31 | 1.40 | <0.001 |
| Chronic pulmonary disease | 1.17 | 1.13 | 1.21 | <0.001 |
| Liver disease | 1.23 | 1.17 | 1.30 | <0.001 |
| Malignancy | 1.23 | 1.18 | 1.28 | <0.001 |
| Depression | 1.97 | 1.90 | 2.04 | <0.001 |
| Psychiatric disease | 1.64 | 1.54 | 1.76 | <0.001 |
| Any admission in the previous year | 2.14 | 2.04 | 2.25 | <0.001 |
| Kidney function (creatinine) (vs <0.8 mg/dL) | ||||
| 0.8–0.9 mg/dL | 0.68 | 0.63 | 0.73 | <0.001 |
| 1.0–1.4 mg/dL | 0.65 | 0.60 | 0.69 | <0.001 |
| 1.5–1.9 mg/dL | 0.71 | 0.66 | 0.77 | <0.001 |
| 2.0–2.4 mg/dL | 0.81 | 0.75 | 0.88 | <0.001 |
| >2.5 mg/dL | 0.63 | 0.54 | 0.73 | <0.001 |
| BNP (vs ≦100 [BNP] or ≦400 [N‐terminal pro]) | ||||
| 101–200 or 401–1000 | 1.04 | 0.97 | 1.11 | 0.26 |
| 201–700 or 1001–4000 | 1.01 | 0.95 | 1.07 | 0.90 |
| 701–1000 or 4001–6000 | 1.07 | 0.98 | 1.17 | 0.36 |
| >1000 or >6000 | 1.08 | 1.01 | 1.16 | <0.001 |
| Missing | 0.88 | 0.82 | 0.93 | <0.001 |
| Left ventricular ejection fraction (vs 50–59%) | ||||
| <20% | 0.85 | 0.78 | 0.92 | <0.001 |
| 20–29% | 0.81 | 0.77 | 0.86 | <0.001 |
| 30–39% | 0.80 | 0.76 | 0.85 | <0.001 |
| 40–49% | 0.91 | 0.86 | 0.95 | <0.001 |
| 60–69% | 1.02 | 0.96 | 1.07 | 0.77 |
| ≧70% | 0.96 | 0.86 | 1.07 | 0.21 |
BNP indicates brain natriuretic peptides.
Rounded to the nearest hundredth.
RESULTS
Of 388 785 randomly extracted patients with coding of HF, 163 085 patients (41.9%) with EF measurement were included in the present analysis (52 484 [38.3%] patients with HFrEF, and 100 601 [61.7%] with HFpEF). There were 16 660 patients (10.2%) who were identified as frail (9.1% in HFrEF, and 10.9% in HFpEF). The baseline characteristics of patients are shown in Table 1.
Predictors of Frailty
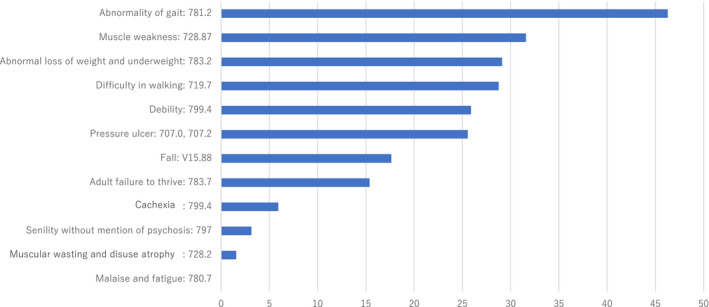
The most prevalent markers of frailty were “abnormality of gait” (46.3%) and “muscle weakness” (31.6%) (Figure 1). The predictors of frailty are presented in Table 2. The risk of frailty rose exponentially with age after 70.
Figure 1. Diagnostic coding‐based indicators of frailty and its prevalence.
Outcomes
One‐year all‐cause mortality rate was 9.7% and 28.1%, and incident admission rate was 58.1% and 79.5% for nonfrail and frail patients, respectively. Event rates for other clinical events or time intervals are shown in Figure 2. The adjusted odds ratios (ORs) for 1‐year mortality and admission in all patients (OR, 1.71; 95% CI, 1.65–1.77; and OR, 1.29; 95% CI, 1.24–1.34, respectively), and in the subgroup of patients with HFpEF (OR, 1.64; 95% CI, 1.56–1.72; and OR, 1.31; 95% CI, 1.26–1.37, respectively) and patients with HFrEF (OR, 1.71; 95% CI, 1.60–1.83; and OR, 1.23; 95% CI, 1.16–1.31, respectively) are presented in Figure 3. Further, when patients with HFmrEF were subcategorized within the HFpEF subgroup, the adjusted ORs for frailty were 1.63 (95% CI, 1.54–1.73), 1.87 (95% CI, 1.72–2.03), and 1.72 (95% CI, 1.61–1.83) for HFpEF, HFmrEF, and HFrEF, respectively (P for interaction, 0.010).
Figure 2. Observed event rate for all heart failure patients, and patients with reduced or preserved ejection fraction by presence or absence of frailty.
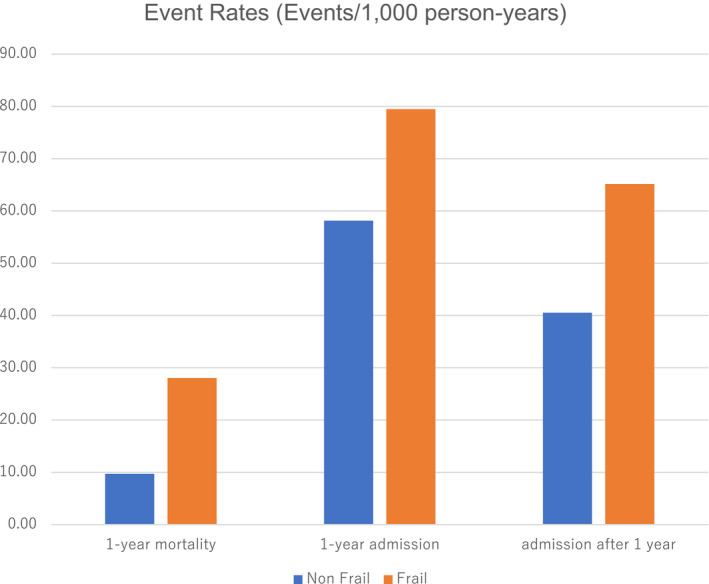
Figure 3. Adjusted odds ratio (95% CI) for 1‐year mortality and admission in all heart failure patients and in patients with reduced or preserved ejection fraction.
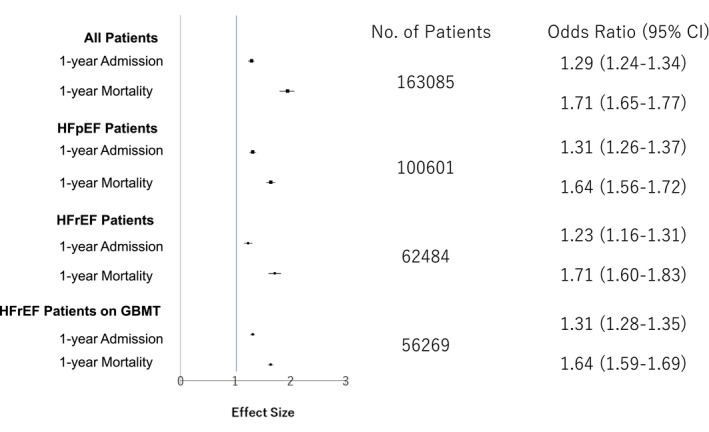
GBMT indicates guideline‐based medical therapy; HFpEF, heart failure with preserved ejection fraction; and HFrEF, heart failure with reduced ejection fraction.
The stratified analysis data looking at the OR for 1‐year mortality with frailty were 1.73 (95% CI, 1.64–1.83) and 1.69 (95% CI, 1.60–1.78) for inpatients and outpatients, respectively (P for interaction, 0.95). The interaction was also tested for age group (P=0.32) and sex (P=0.36). The P value for interaction was P=0.005 for race group, although the only significant individual interaction was with Pacific Islanders (P=0.04, frailty was more predictive of death in Pacific Islanders than in other races).
The claims‐based predictors of long‐term mortality used in the current study are similar to those defined in clinical studies; other than frailty, the covariates that were most strongly associated with increased long‐term mortality were age, chronic liver disease, malignancy, any previous admission in the previous year, and advanced kidney disease (serum creatinine above 2.0 mg/dL).
DISCUSSION
In the present study, ≈10% of the patients with HF in the database were categorized as frail. We also found frailty was independently associated with adverse outcomes such as 1‐year readmission and mortality after adjustment for other clinical characteristics. Furthermore, our results demonstrated that the impact of frailty was similar across HF phenotype (reduced, midrange versus preserved EF) and use of guideline‐recommended medications.
There is a paucity of clinical studies for patients with HF that evaluate risk factors for frailty, although multiple frailty assessments are available. Previous studies have primarily assessed frailty using clinical scores during periods of acute decompensation. However, evaluation for frailty during a period of acute illness may overestimate its true prevalence. Additionally, the hospital environment itself and physiologic stressors associated with hospitalization can accelerate functional decline and preexisting frailty. Frailty is closely associated with cognitive impairment, which can also vary during acute hospitalization. The primary advantage of our VA analysis is the inclusion of a large, generalized population with long‐term mortality data. The adjusted risk estimates for incident mortality (OR, 1.71) and hospital admission (OR, 1.29) in our study are also generally in line with the literature and a recently published systematic review. 14
Association of adverse outcomes and frailty may differ by HF phenotypes. Indeed, within our study, a significant interaction effect was noted for the HF phenotype. HFmrEF and HFpEF are highly heterogeneous and influenced by a range of comorbidities (eg, hypertension, diabetes mellitus, and atrial fibrillation) typically experienced by elderly patients. These comorbidities may lead to systematic microvascular inflammation, which adversely affects the adjacent cardiomyocytes, and lead to impaired myocardial energetics and decreased nutritional status. 15 , 16 Additionally, because of the increasing number of patients with HFmrEF and HFpEF in contemporary cardiovascular practice and the neutral results of large‐scale, randomized controlled trials that tested conventional HF therapies, the results of this study provide crucial evidence that could lead to guidelines that enable better care for these patients.
Our study determined frailty score based on diagnostic coding, rather than questionnaire surveys. This is a valuable approach given the ability to efficiently identify frailty across large patient cohorts in which survey or direct functional testing is unavailable. 17 , 18 , 19 , 20 , 21 Professional societies also emphasize the importance of recognizing frailty to identify patients with frailty who are at greater risk of adverse outcomes and who might benefit from treatment optimization. 22 Appropriate early intervention, such as aerobic exercise, nutrition education, and patient education reversibly improves functional capacity in frail patients with HF. 23
Frailty can also aid in how healthcare providers assess about their patients with HF; the patients may, at times, be overly reassured by the patient's chronologic youth when they are frail and phenotypically older.
Limitations
Our study has several strengths. The data set was extracted from a large, national, well‐characterized patient cohort at the VA and offered an opportunity to study both administrative and clinical variables, including laboratory values and vital signs. Unlike prior studies that have focused on short‐term outcomes, we assessed the clinical information on longer follow‐up. Our study also has important limitations. Because of the observational nature of our study, we could not fully account for residual confounding that may explain the association between frailty and mortality. We based our frailty assessment on available elements that differ from prior classification schemes using physical measurements, although this algorithm has been extensively validated and shown to predict poor health outcomes. 21 An original model initially proposed by Fried et al was an operational definition of frailty as a biologic syndrome based on physical factors, whereas contemporary frailty indices tend to focus on the accumulation of deficits in physical, cognitive, and functional domains. Because prior studies demonstrated that the risk of mortality, and other adverse outcomes, increases with the burden of health deficits and not a specific deficit type, a scoring system was chosen over a traditional Fried‐based model. 24 However, the risk of decreased specificity needs to be balanced with the user‐friendliness of this approach. Finally, we did not have all necessary variables to calculate traditional HF risk scores, such as the Seattle Heart Failure Model or Meta‐Analysis Global Group in Chronic Heart Failure Score. However, the claims‐based predictors of long‐term mortality used in the current study are similar to those defined in prior studies, and the final model had strong discrimination. 25 , 26
Conclusions
In a national sample of VA patients with HF, ≈1 in 10 patients were frail. We found an association between frailty and all‐cause mortality and admission, regardless of HF phenotype. Estimation of frail status may facilitate clinical management and shared decision‐making.
Sources of Funding
Part of the study was funded by the Japan Society for the Promotion of Science (Grant No. 16KK0186 and 20H03915).
Disclosures
Kohsaka reports investigator‐initiated grant funding from Bayer and Daiichi Sankyo and personal fees from Bristol‐Myers Squibb. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2020;9:e016502. DOI: 10.1161/JAHA.120.016502.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Christiansen MN, Køber L, Weeke P, Vasan RS, Jeppesen JL, Smith JG, Gislason GH, Torp‐Pedersen C, Andersson C. Age‐specific trends in incidence, mortality, and comorbidities of heart failure in Denmark, 1995 to 2012. Circulation. 2017;135:1214–1223. [DOI] [PubMed] [Google Scholar]
- 2. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, et al. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet. 2018;391:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joseph SM, Rich MW. Targeting frailty in heart failure. Curr Treat Options Cardiovasc Med. 2017;19:31. 10.1007/s11936-017-0527-5. [DOI] [PubMed] [Google Scholar]
- 4. Bieniek J, Wilczyński K, Szewieczek J. Fried frailty phenotype assessment components as applied to geriatric inpatients. Clin Interv Aging. 2016;11:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanchis J, Bonanad C, Ruiz V, Fernández J, García‐Blas S, Mainar L, Ventura S, Rodríguez‐Borja E, Chorro FJ, Hermenegildo C, et al. Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am Heart J. 2014;168:784–791.e2. [DOI] [PubMed] [Google Scholar]
- 6. Dodson JA, Hochman JS, Roe MT, Chen AY, Chaudhry SI, Katz S, Zhong H, Radford MJ, Udell J, Bagai A, et al. The association of frailty with in‐hospital bleeding among older adults with acute myocardial infarction: insights from the ACTION Registry. JACC Cardiovasc Interv. 2018;11:2287–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Magnani JW, Wang N, Benjamin EJ, Garcia ME, Bauer DC, Butler J, Ellinor PT, Kritchevsky S, Marcus GM, Newman A, et al. Atrial fibrillation and declining physical performance in older adults: the Health, Aging, and Body Composition Study. Circ Arrhythm Electrophysiol. 2016;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shiraishi Y, Kohsaka S, Abe T, Mizuno A, Goda A, Izumi Y, Yagawa M, Akita K, Sawano M, Inohara T, et al. Validation of the Get With The Guideline‐Heart Failure risk score in Japanese patients and the potential improvement of its discrimination ability by the inclusion of B‐type natriuretic peptide level. Am Heart J. 2016;171:33–39. [DOI] [PubMed] [Google Scholar]
- 9. Smaha LA. The American Heart Association get with the guidelines program. Am Heart J. 2004;148:S46–S48. [DOI] [PubMed] [Google Scholar]
- 10. Theou O, Squires E, Mallery K, Lee JS, Fay S, Goldstein J, Armstrong JJ, Rockwood K. What do we know about frailty in the acute care setting? A scoping review. BMC Geriatr. 2018;18:139. DOI: 10.1186/s12877-018-0823-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Groeneveld PW, Medvedeva EL, Walker L, Segal AG, Richardson DM, Epstein AJ. Outcomes of care for ischemic heart disease and chronic heart failure in the Veterans Health Administration. JAMA Cardiol. 2018;3:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Figueroa JF, Maddox KEJ, Beaulieu N, Wild RC, Jha AK. Concentration of potentially preventable spending among high‐cost medicare subpopulations. Ann Intern Med. 2017;167:706–713. [DOI] [PubMed] [Google Scholar]
- 13. Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang X, Lupón J, Vidán MT, Ferguson C, Gastelurrutia P, Newton PJ, Macdonald PS, Bueno H, Bayés‐Genís A, Woo J, et al. Impact of frailty on mortality and hospitalization in chronic heart failure: a systematic review and meta‐analysis. J Am Heart Assoc. 2018;7:e008251. DOI: 10.1161/JAHA.117.008251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 16. Lam CSP, Voors AA, De Boer RA, Solomon SD, Van Veldhuisen DJ. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J. 2018;39:2780–2792. [DOI] [PubMed] [Google Scholar]
- 17. Vidán MT, Blaya‐Novakova V, Sánchez E, Ortiz J, Serra‐Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non‐dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18:869–875. [DOI] [PubMed] [Google Scholar]
- 18. Davidoff AJ, Zuckerman IH, Pandya N, Hendrick F, Ke X, Hurria A, Lichtman SM, Hussain A, P.Weiner J, Edelman MJ. A novel approach to improve health status measurement in observational claims‐based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Segal JB, Chang HY, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims‐based frailty indicator anchored to a well‐established frailty phenotype. Med Care. 2017;55:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Faurot KR, Jonsson Funk M, Pate V, Brookhart MA, Patrick A, Hanson LC, Castillo WC, Stürmer T. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim DH, Schneeweiss S. Measuring frailty using claims data for pharmacoepidemiologic studies of mortality in older adults: evidence and recommendations. Pharmacoepidemiol Drug Saf. 2014;23:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kernick D, Chew‐Graham CA, O’Flynn N. Clinical assessment and management of multimorbidity: NICE guideline. Br J Gen Pract. 2017;67:235–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mitnitski A, Song X, Skoog I, Broe GA, Cox JL, Grunfeld E, Rockwood K. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. [DOI] [PubMed] [Google Scholar]
- 25. Kundi H, Valsdottir LR, Popma JJ, Cohen DJ, Strom JB, Pinto DS, Shen C, Yeh RW. Impact of a claims‐based frailty indicator on the prediction of long‐term mortality after transcatheter aortic valve replacement in Medicare beneficiaries. Circ Cardiovasc Qual Outcomes. 2018;11:e005048. DOI: 10.1161/CIRCOUTCOMES.118.005048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shashikumar SA, Luke AA, Johnston KJ, Joynt Maddox KE. Assessment of HF outcomes using a claims‐based frailty index. JACC Heart Fail. 2020;8:481–488. [DOI] [PubMed] [Google Scholar]


