Abstract
Background
Guideline recommendations for oral anticoagulation (OAC) in patients with atrial fibrillation (AF) are based on CHA2DS2‐VASc score alone. Patients with cardiac implantable electronic devices provide an opportunity to assess how the interaction between AF duration and CHA2DS2‐VASc score influences OAC prescription rates.
Methods and Results
Data from the Optum de‐identified Electronic Health Record data set were linked to the Medtronic CareLink database of cardiac implantable electronic devices. An index date was assigned as the later of 6 months after device implant or 1 year after Electronic Health Record data availability. Maximum daily AF duration (no AF, 6 minutes–23.5 hours, and >23.5 hours) was assessed for 6 months before index date. OAC prescription rates were computed as a function of both AF duration and CHA2DS2‐VASc score. A total of 35 779 patients with CHA2DS2‐VASc scores ≥1 were identified, including 27 198 not prescribed OAC. Overall OAC prescription rate among the 12 938 patients with device‐detected AF >6 minutes was 36.7% and significantly higher in those with a maximum daily AF duration >23.5 hours (45.4%) compared with those with 6 minutes to 23.5 hours (28.7%). OAC prescription rates increased monotonically with both increasing AF duration and CHA2DS2‐VASc score, reaching a maximum of 67.2% for patients with AF >23.5 hours and a CHA2DS2‐VASc score ≥5.
Conclusions
Real‐world prescription of OAC increased with both increasing duration of AF and CHA2DS2‐VASc score. This highlights the need for further research into the role of AF duration, stroke risk, and the need for anticoagulation in patients with devices capable of long‐term AF monitoring.
Keywords: anticoagulation, atrial fibrillation, device‐detection, subclinical
Subject Categories: Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- OAC
oral anticoagulation
Clinical Perspective
What Is New?
This retrospective study in a large database of patients with cardiac implantable electronic devices describes rates of oral anticoagulation prescription in patients with device‐detected atrial fibrillation (AF).
Oral anticoagulation prescription rates increased monotonically with both increasing AF duration and CHA2DS2‐VASc score, reaching a maximum of 67.2% for patients with AF >23.5 hours and a CHA2DS2‐VASc score ≥5.
There is geographic variation in rate of oral anticoagulation prescription for patients of similar AF duration and CHA2DS2‐VASc score.
What Are the Clinical Implications?
Over a range of CHA2DS2‐VASc scores and AF duration, oral anticoagulation is underused.
Physicians often consider AF duration in addition to CHA2DS2‐VASc score when making decisions about anticoagulation.
Current US guidelines strongly support the use of chronic oral anticoagulation (OAC) in patients with atrial fibrillation (AF) and ≥2 stroke risk factors, without regard of AF duration or burden. 1 Device‐detected AF of various durations, often referred to as subclinical AF, has been shown to be associated with increased stroke risk, though at a rate less than that of clinically detected AF. 2 , 3 , 4 Anticoagulation recommendations for clinical AF are based on studies of stroke risk in the clinical AF population. 1 While patients may have both clinical and subclinical AF, the increasing use of implantable and consumer‐grade technologies capable of detecting AF will likely increase the latter population significantly. There is, however, considerable clinical equipoise about the management of these episodes of subclinical AF, particularly those that are paroxysmal and of short duration. Even the 2019 update of the American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines noted that it is not yet clear what the optimal management strategy is in these patients with regards to anticoagulation. 1 There are trials currently being conducted to answer this question, but in the meantime, clinicians are presented with such clinical scenarios daily. 5 , 6
A recent statement from the American Heart Association noted the considerable evidence gap in answering this clinical question. 7 Given the uncertainty of anticoagulation management in device‐detected AF and the absence of randomized trial results for guidance, it is of interest to determine the practice patterns of anticoagulation use in patients with device‐detected AF among US physicians.
METHODS
Because of contractual arrangements between Medtronic, Inc. and Optum, the data cannot be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Cohort
Data were obtained from the Optum Electronic Health Record (EHR) database, which contains de‐identified data from patients collected in EHR systems during 2007 to 2017 from multiple health provider networks within the United States. Patients were included if they had a cardiovascular diagnosis code in their medical records or they had a cardiovascular‐related procedure performed during the data collection period. In this study, the analysis‐cohort was formed by linking Optum EHR data to the Medtronic CareLink database of cardiac implantable electronic devices (CIEDs) capable of continuous AF monitoring (dual and triple chamber pacemakers, implantable cardioverter defibrillators, and cardiac resynchronization therapy devices with an atrial lead). The Institutional Review Board at Northwestern University determined that the study was not human research and that no approval was indicated. Patients were included in the analysis only if they had medications recorded in the EHR database to ensure that anticoagulation status was correctly assigned. Patients with a CHA2DS2‐VASc score of zero were excluded.
Derivation of Clinical Data
A patient was considered to have a clinical history of a specific stroke risk factor if there was a diagnosis code date associated with the patient that occurred before the index date. The index date was defined as the later of either 6 months after device implantation or 1 year after EHR data availability. Therefore, CHA2DS2‐VASc scores were assessed via EHR based on the patient's clinical history before the index date using the diagnosis codes provided in Table S1. Female sex was treated as a risk modifier in the computation of the CHA2DS2‐VASc score. 1 Consequently, women with no other CHA2DS2‐VASc risk factors were assigned a score of zero. Anticoagulation status was determined from medication data in the year before the index date. Geographical regions were divided based on the United States Census Bureau designations of West, South, Midwest, and Northeast. 8
Derivation of Device Data
Previous studies have shown that the detection algorithms used in this study are capable of quantifying AF duration with >95% accuracy. 9 Patients were categorized into 1 of 3 groups depending on the maximum daily AF duration observed over the 180‐day period before the index date: No AF, 6 minutes to 23.5 hours, and >23.5 hours. The thresholds of 6 minutes and 23.5 hours were based on previous studies which demonstrated an increased risk of thromboembolic events with AF episodes of these durations. 2 , 3 , 4 , 10 Rates of anticoagulation were then determined based on combinations of AF duration and CHA2DS2‐VASc score.
Statistical Analysis
Rates of anticoagulation were determined by dividing the number of patients in each group on anticoagulation by the total number in that group. Continuous variables are reported as mean and SD or median (interquartile range) while discrete variables are reported as counts and percentages, as appropriate. Continuous variables were compared using a t test and discrete variables were compared using a Chi‐square test. Statistical significance was assigned for P<0.05. All analyses were performed with SAS software version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
There were 212 816 patients identified by the merger of the clinical and device databases. Of these, 35 779 patients with CIED were identified for the study based on the presence of data in available databases, a device capable of recording episodes of AF, and documented anticoagulation prescriptions (Figure 1). In this group, 27 198 patients (76.0%) were not on anticoagulation. Demographics of patients who were prescribed and who were not prescribed anticoagulation are compared in Table 1. The average age of the patients was 71.8±10.3 years with 65% men.
Figure 1. Development of the study cohort.
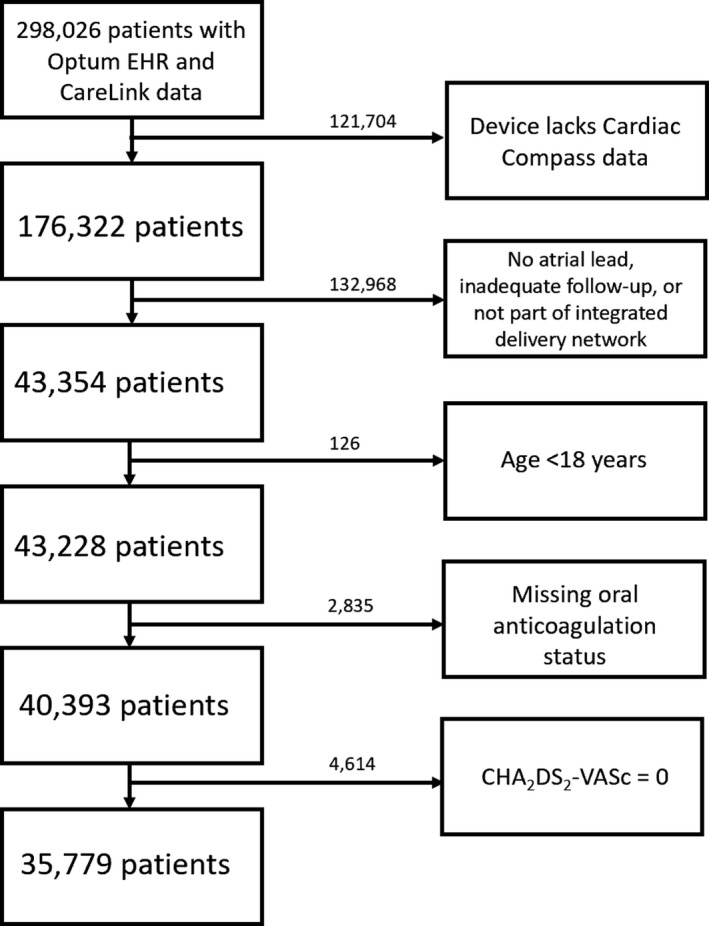
EHR indicates Electronic Health Record.
Table 1.
Baseline Demographics
| No Anticoagulation (n=27 198) | Anticoagulation (n=8581) | Total (n=35 779) | |
|---|---|---|---|
| Mean age (SD), y | 71.7 (10.3) | 71.9 (10.4) | 71.8 (10.3) |
| Age ≥75 | 12 491 (46%) | 3991 (47%) | 16 482 (46%) |
| Age ≥65 | 21 945 (81%) | 6722 (78%) | 28 667 (80%) |
| Male sex | 17 607 (65%) | 5587 (65%) | 23 194 (65%) |
| CHA2DS2‐VASc Score | |||
| Mean (SD) | 3.3 (1.7) | 4.4 (1.8) | 3.6 (1.8) |
| 1 | 3612 (13%) | 352 (4%) | 3964 (11%) |
| 2 | 7080 (26%) | 1006 (12%) | 8086 (23%) |
| 3–4 | 10 034 (37%) | 3225 (38%) | 13 259 (37%) |
| ≥5 | 6472 (24%) | 3998 (47%) | 10 470 (29%) |
| CHADS2 Score | |||
| Mean (SD) | 1.9 (1.5) | 2.9 (1.4) | 2.2 (1.5) |
| 0 | 4088 (15%) | 259 (3.0%) | 4347 (12%) |
| 1 | 8567 (32%) | 1213 (14%) | 9780 (27%) |
| 2 | 5848 (22%) | 2151 (25%) | 7999 (22%) |
| 3 | 4647 (17%) | 2214 (26%) | 6861 (19%) |
| ≥4 | 4048 (15%) | 2744 (32%) | 6792 (19%) |
| Hypertension | 15 008 (55%) | 7067 (82%) | 22 075 (62%) |
| Heart failure | 10 310 (38%) | 5637 (66%) | 15 947 (45%) |
| Stroke/TIA | 3884 (14%) | 2474 (29%) | 6358 (18%) |
| Diabetes mellitus | 7037 (26%) | 3245 (38%) | 10 282 (29%) |
| Vascular disease | 1933 (7%) | 1298 (15%) | 3231 (9%) |
| Myocardial infarction | 4831 (18%) | 2624 (31%) | 7455 (21%) |
| Atrial fibrillation* | 6124 (23%) | 6787 (79%) | 12 911 (36%) |
| Device type | |||
| CRT‐D | 8720 (32%) | 2711 (32%) | 11 431 (32%) |
| CRT‐P | 848 (3%) | 611 (7%) | 1459 (4%) |
| ICD | 8396 (31%) | 1996 (23%) | 10 392 (29%) |
| IPG | 9234 (34%) | 3263 (38%) | 12 497 (35%) |
CRT‐D indicates cardiac resynchronization therapy‐defibrillator; CRT‐P, cardiac resynchronization therapy‐pacemaker; ICD, implantable cardioverter defibrillators; IPG, implantable pulse generator; and TIA, transient ischemic attack.
Indicates atrial fibrillation diagnosed before the index date of this study.
The percentages of patients receiving anticoagulation for each combination of AF duration and CHA2DS2‐VASc score are shown in Figure 2. Rates of anticoagulation increased significantly with both increasing AF duration and increasing CHA2DS2‐VASc score (P<0.001) among the groups. The highest rate of anticoagulation (67.2%) was seen in the group with the longest AF duration (>23.5 hours) and highest CHA2DS2‐VASc score (≥5). Even in patients with a CHA2DS2‐VASc score of 1 where OAC is a Class IIB indication, prescription rates reached 19.5% for those with AF >23.5 hours. A similar analysis of percentage of patients receiving OAC at each AF duration using CHADS2 score rather than CHA2DS2‐VASc is presented in Figure S1.
Figure 2. Percentage of patients on oral anticoagulation.
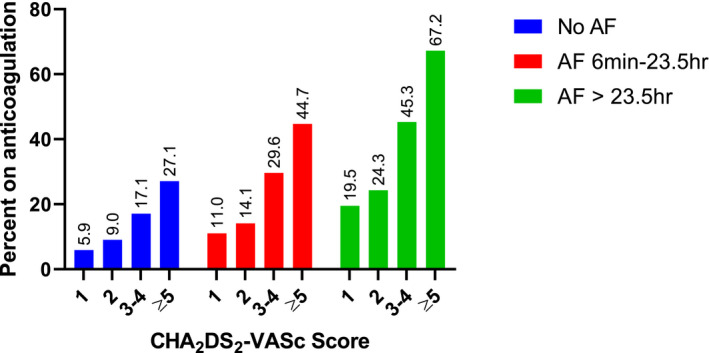
AF indicates atrial fibrillation.
Rates of OAC usage for each component of the CHA2DS2‐VASc score are presented in Table 2. For each comorbidity, its presence was associated with higher use of OAC. However, in the case of sex itself, rates of OAC were similar between men and women. Younger patients (aged <65 years) had the highest rate of OAC while the oldest group (aged >75 years) had the second highest rate of OAC. Even for patients who had a prior stroke or TIA, just 38.9% were on OAC. While that number includes patients without AF, a prior stroke is sufficient to warrant anticoagulation in someone with AF and without any additional risk factors. Even among patients with AF and prior stroke or TIA, only 60.1% were receiving OAC. While the cohort was predominantly White in race (88.1%), those patients who were Black had significantly lower rates of OAC.
Table 2.
Rates of Oral Anticoagulation Based on the Occurrence of Individual Risk Factors
| Total | Anticoagulation (%) | P Value | |
|---|---|---|---|
| Age, y | <0.001 | ||
| Age <65 | 7112 | 1859 (26.1%) | |
| Age 65–74 | 12 185 | 2731 (22.4%) | |
| Age ≥75 | 16 482 | 3991 (24.2%) | |
| Sex | 0.529 | ||
| Men | 23 194 | 5587 (24.1%) | |
| Women | 12 585 | 2994 (23.8%) | |
| Hypertension | <0.001 | ||
| No | 13 704 | 1514 (11.0%) | |
| Yes | 22 075 | 7067 (32.0%) | |
| Heart failure | <0.001 | ||
| No | 19 382 | 2944 (14.8%) | |
| Yes | 15 947 | 5637 (35.3%) | |
| Stroke/TIA (Transient ischemic attack) | <0.001 | ||
| No | 29 421 | 6107 (20.8%) | |
| Yes | 6358 | 2474 (38.9%) | |
| Diabetes mellitus | <0.001 | ||
| No | 25 497 | 5336 (20.9%) | |
| Yes | 10 282 | 3245 (31.6%) | |
| Vascular disease | <0.001 | ||
| No | 32 548 | 7283 (22.4%) | |
| Yes | 3231 | 1298 (40.2%) | |
| Race | <0.001 | ||
| Black | 2355 | 480 (20.4%) | |
| Asian | 140 | 41 (29.3%) | |
| White | 31 537 | 7683 (24.4%) | |
| Other (includes Native American/Alaskan Native, Pacific Islander/Native Hawaiian/unknown) | 1747 | 377 (21.6%) | |
| Geography | <0.001 | ||
| Northeast | 1898 | 402 (21.2%) | |
| South | 13 564 | 3219 (23.7%) | |
| Midwest | 17 643 | 4271 (24.2%) | |
| West | 2023 | 548 (27.1%) | |
| Other/unknown | 651 | 141 (21.7%) |
TIA indicates transient ischemic attack.
There were 6124 patients who were not prescribed OAC despite a documented history of clinical AF. Of those, 40% had a CHA2DS2‐VASc score of at least 5 and 55% had a CHA2DS2‐VASc score of 2 or 3. These patients had a mean (SD) CHA2DS2‐VASc score of 4.1 (1.8). A distribution of this cohort incorporating AF duration is shown in Table 3. About half of this group also had device‐detected AF (48.7%).
Table 3.
Distribution of CHA2DS2‐VASc Scores and Atrial Fibrillation Duration in Patients Not on Anticoagulation But With a Clinical History of Atrial Fibrillation
| AF Duration Group | CHA2DS2‐VASc Group | No. Subjects (%) |
|---|---|---|
| No AF (n=3138) | 1 | 155 (3%) |
| 2 | 418 (7%) | |
| 3–4 | 1199 (20%) | |
| ≥5 | 1366 (22%) | |
| AF 6 min–23.5 h (n=1536) | 1 | 94 (2%) |
| 2 | 228 (4%) | |
| 3–4 | 649 (11%) | |
| ≥5 | 565 (9%) | |
| AF >23.5 h (n=1450) | 1 | 94 (2%) |
| 2 | 213 (3%) | |
| 3–4 | 637 (10%) | |
| ≥5 | 506 (8%) |
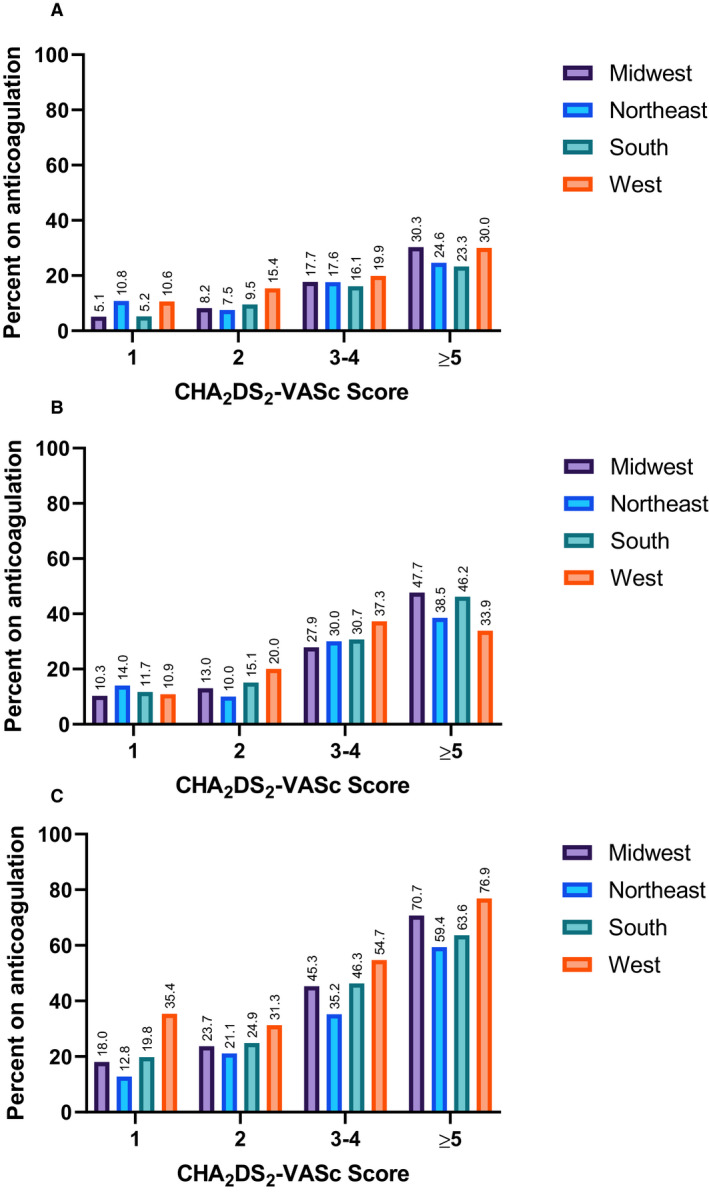
Use of OAC also varied across geographical regions of the United States. Highest rates of overall OAC were seen in the West region with the lowest rates in the Northeast. Rates of anticoagulation for specific combinations of CHA2DS2‐VASc score and AF duration compared across geographical regions are shown in Figure 3. Even for patients with AF duration >23.5 hours and CHA2DS2‐VASc score of at least 3, rates of OAC were as low as 35.2% in the South and up to 54.7% in the West. A similar analysis of OAC across geographical regions by AF duration and CHADS2 score is presented in Figure S2.
Figure 3. Rates of oral anticoagulation across geographical regions for patients with no atrial fibrillation (A), AF duration 6 minutes–23.5 hours (B), and AF duration >23.5 hours (C).
DISCUSSION
In a large population of patients with CIEDs, rates of OAC use increase with increasing AF duration and increasing CHA2DS2‐VASc score, indicating that clinicians are considering both factors when making decisions about initiation of anticoagulation for device‐detected AF.
Oral anticoagulation has been demonstrated to be underused in patients with clinical AF in numerous prior studies. 11 , 12 , 13 Even in large registries of patients with clinical AF, rates of OAC in patients with CHA2DS2‐VASc ≥2 are around 50%. Our data further emphasize this important underuse of OAC in patients with AF. 14 As the utility of OAC for stroke prevention in subclinical device‐detected AF is not yet elucidated, it is not surprising that there is substantial heterogeneity in practice patterns. Even within the groups of patients with >23.5 hours of AF in a day, the rates of anticoagulation usage ranged from 24.3% for a CHA2DS2‐VASc score of 2 to 67.2% for a CHA2DS2‐VASc score of ≥5. At present, American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines recommend anticoagulation based on CHA2DS2‐VASc score alone, however studies of real‐world use even in a clinical AF population have demonstrated lower rates in paroxysmal AF compared with persistent AF. 1 , 15 Such a pattern appears to be extended in practice with device‐detected AF as well.
The variability and underuse of anticoagulation in device‐detected AF was also demonstrated in a study by Perino et al of a large number of patients with CIED in the Veterans Affairs (VA) healthcare system. 16 Our findings expand upon this point in patients more reflective of the general AF population in the United States. Whereas 98% of the VA patients in that study were men, our population is 65% men. Additionally, the vast majority of the patients in the VA study had implantable cardioverter‐defibrillators (82%) compared with 62% in the present study, likely reflecting the increased morbidities common in the VA population, as well as the greater use of remote monitoring for implantable cardioverter defibrillators rather than pacemakers at the time of the VA study. 16
We have previously demonstrated that rates of stroke and systemic embolism follow a similar pattern of increasing with both increasing AF duration and increasing CHA2DS2‐VASc score. 17 These findings will help interpret future results from trials of anticoagulation in device‐detected AF, as these factors may identify groups of patients who benefit more than others. Such a strategy is already included in the European Heart Rhythm Association guidelines from 2017, recommending anticoagulation with a CHA2DS2‐VASc of at least 2 in men or 3 in women and AF duration >5.5 hours but also noting that anticoagulation should be considered with a lower burden if additional risk factors are present. 18 As the present study demonstrates, many clinicians appear to be considering these factors in making their own decisions about anticoagulation for their patients, even without specific recommendations from the American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines. 1 Future guidelines should address the interaction of AF burden/duration with clinical risk factors in discussing anticoagulation indications. Randomized controlled trials that are currently in process will likely provide a higher level of evidence on this issue. 5 , 6
Our data also reveal variation in OAC usage by geographical regions. While practice patterns have been shown to vary by hospital site in a study of VA hospitals, the large regional groupings in the present study include a variety of different types of practice settings (eg, community hospitals and academic medical centers). 16 However there continues to be variations in OAC usage which in some combinations of CHA2DS2‐VASc score and AF duration reach a 15% difference between regions. Interestingly, the highest rates were not in the regions with the highest density of academic medical centers.
While higher rates of OAC were seen in the presence of each comorbidity that contributes to the CHA2DS2‐VASc score, rates of OAC were highest in the youngest patients, in whom age does not add any points to their CHA2DS2‐VASc score. Interestingly, OAC rates did not decrease with increasing age but rather were higher in the oldest patients compared with those in the middle range of 65 to 74 years old. Differences were also seen in usage of OAC by racial group with the lowest rate of OAC in Black patients. Such disparities have been previously demonstrated, even after adjustment for insurance status and income. 19 These data further support the need to address not just suboptimal levels of OAC in all patients, but specifically in groups that have been receiving even lower rates of guideline‐indicated therapy.
Several major strengths of this study include the large data set with representation from across the United States. Additionally, with CIEDs in every patient, each with an atrial lead, the accuracy of AF quantification is quite high. While randomized controlled trials are the gold standard of evidence in medicine, it is also acknowledged that trial populations tend to be different from real‐world populations. 20 Therefore, real‐world studies such as this one can be quite informative in bridging the gap from guidelines to practice. There are several limitations given the retrospective nature of this study as well as the limitations inherent to the use of the EHR for research. First, we recognize that there are other uses of OAC beyond stroke prevention in AF. Therefore, some of the OAC use, particularly in those with no AF, could have been in response to other indicated uses of these medications. About a third of patients had a clinical diagnosis of AF, and so even without any documented episodes during the study period, they might have been on OAC based on historical episodes. In addition, we do not have information on appropriateness of anticoagulation dosing or time in therapeutic range for patients treated with warfarin. Importantly, reasons for not prescribing an anticoagulant are not available in our database. While individual AF episodes were not adjudicated, the accuracy of the AF algorithms, particularly for episodes lasting at least a few minutes as required by this study, is quite high. 21 However, it should be noted that all devices in this study were manufactured by Medtronic, Inc. and thus used their algorithm.
In conclusion, in a large database of patients with CIED across the United States, rates of oral anticoagulation increased with increasing AF duration and increasing CHA2DS2‐VASc score. Even in the highest AF duration and CHA2DS2‐VASc group, only two thirds of the patients were on OAC. The ongoing randomized trials of OAC for subclinical AF will provide clarity to the necessity of OAC in these patients.
Sources of Funding
None.
Disclosures
Ms Koehler, Dr Sarkar, Dr Landman, and Mr Ziegler are employees and shareholders of Medtronic, Inc. Dr Passman receives research support and consulting fees from Medtronic, Inc. and Abbott Inc., and royalties from UpToDate. Dr Kaplan has no disclosures to report.
Supporting information
Table S1
Figures S1–S2
(J Am Heart Assoc. 2020;9:e018378. DOI: 10.1161/JAHA.120.018378.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018378
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 2. Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, Cook J, Paraschos A, Love J, Radoslovich G, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107:1614–1619. [DOI] [PubMed] [Google Scholar]
- 3. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 4. Van Gelder IC, Healey JS, Crijns H, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau CP, Morillo CA, Hobbelt AH, et al. Duration of device‐detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 5. Kirchhof P, Blank BF, Calvert M, Camm AJ, Chlouverakis G, Diener HC, Goette A, Huening A, Lip GYH, Simantirakis E, et al. Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the Non‐vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH‐AFNET 6) trial. Am Heart J. 2017;190:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopes RD, Alings M, Connolly SJ, Beresh H, Granger CB, Mazuecos JB, Boriani G, Nielsen JC, Conen D, Hohnloser SH, et al. Rationale and design of the apixaban for the reduction of thrombo‐embolism in patients with device‐detected sub‐clinical atrial fibrillation (ARTESiA) trial. Am Heart J. 2017;189:137–145. [DOI] [PubMed] [Google Scholar]
- 7. Noseworthy PA, Kaufman ES, Chen LY, Chung MK, Elkind MSV, Joglar JA, Leal MA, McCabe PJ, Pokorney SD, Yao X; American Heart Association Council on Clinical Cardiology E, Arrhythmias C, Council on Arteriosclerosis T, Vascular B, Council on C, Stroke N and Stroke C . Subclinical and device‐detected atrial fibrillation: pondering the knowledge gap: a scientific statement from the American Heart Association. Circulation. 2019;140:e944–e963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Census Regions and Divisions of the United States . https://www2.census.gov/geo/pdfs/maps‐data/maps/reference/us_regdiv.pdf. Accessed July 30, 2020.
- 9. Purerfellner H, Gillis AM, Holbrook R, Hettrick DA. Accuracy of atrial tachyarrhythmia detection in implantable devices with arrhythmia therapies. Pacing Clin Electrophysiol. 2004;27:983–992. [DOI] [PubMed] [Google Scholar]
- 10. Capucci A, Santini M, Padeletti L, Gulizia M, Botto G, Boriani G, Ricci R, Favale S, Zolezzi F, Di Belardino N, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46:1913–1920. [DOI] [PubMed] [Google Scholar]
- 11. Hsu JC, Maddox TM, Kennedy KF, Katz DF, Marzec LN, Lubitz SA, Gehi AK, Turakhia MP, Marcus GM. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE Registry. JAMA Cardiol. 2016;1:55–62. [DOI] [PubMed] [Google Scholar]
- 12. Turakhia MP, Hoang DD, Xu X, Frayne S, Schmitt S, Yang F, Phibbs CS, Than CT, Wang PJ, Heidenreich PA. Differences and trends in stroke prevention anticoagulation in primary care vs cardiology specialty management of new atrial fibrillation: the Retrospective Evaluation and Assessment of Therapies in AF (TREAT‐AF) study. Am Heart J. 2013;165:93–101.e1. [DOI] [PubMed] [Google Scholar]
- 13. Perino AC, Fan J, Schmitt SK, Askari M, Kaiser DW, Deshmukh A, Heidenreich PA, Swan C, Narayan SM, Wang PJ, et al. Treating specialty and outcomes in newly diagnosed atrial fibrillation: from the TREAT‐AF Study. J Am Coll Cardiol. 2017;70:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marzec LN, Wang J, Shah ND, Chan PS, Ting HH, Gosch KL, Hsu JC, Maddox TM. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69:2475–2484. [DOI] [PubMed] [Google Scholar]
- 15. Hsu JC, Chan PS, Tang F, Maddox TM, Marcus GM. Differences in anticoagulant therapy prescription in patients with paroxysmal versus persistent atrial fibrillation. Am J Med. 2015;128:654.e1–654.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perino AC, Fan J, Askari M, Heidenreich PA, Keung E, Raitt MH, Piccini JP, Ziegler PD, Turakhia MP. Practice variation in anticoagulation prescription and outcomes after device‐detected atrial fibrillation: insights from the Veterans Health Administration. Circulation. 2019;139:2502–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke risk as a function of atrial fibrillation duration and CHA2DS2‐VASc Score. Circulation. 2019;140:1639–1646. [DOI] [PubMed] [Google Scholar]
- 18. Gorenek B, Pelliccia A, Benjamin EJ, Boriani G, Crijns HJ, Fogel RI, Van Gelder IC, Halle M, Kudaiberdieva G, Lane DA, et al. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). Europace. 2017;19:190–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tedla YG, Schwartz SM, Silberman P, Greenland P, Passman RS. Racial disparity in the prescription of anticoagulants and risk of stroke and bleeding in atrial fibrillation patients. J Stroke Cerebrovasc Dis. 2020;29:104718. [DOI] [PubMed] [Google Scholar]
- 20. Najafzadeh M, Schneeweiss S. From trial to target populations—calibrating real‐world data. N Engl J Med. 2017;376:1203–1205. [DOI] [PubMed] [Google Scholar]
- 21. Passman RS, Weinberg KM, Freher M, Denes P, Schaechter A, Goldberger JJ, Kadish AH. Accuracy of mode switch algorithms for detection of atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 2004;15:773–777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1–S2


