Abstract
Background
Hypergravity may promote human hemostasis thereby increasing thrombotic risk. Future touristic suborbital spaceflight will expose older individuals with chronic medical conditions, who are at much higher thromboembolic risk compared with professional astronauts, to hypergravity. Therefore, we tested the impact of hypergravity on hemostasis in healthy volunteers undergoing centrifugation.
Methods and Results
We studied 20 healthy seated men before and after 15 minutes under 3 Gz hypergravity on a long‐arm centrifuge. We obtained blood samples for hemostasis testing before, immediately after, and 30 minutes after centrifugation. Tests included viscoelastic thromboelastometry, platelet impedance aggregometry, endothelial activation markers, blood rheology testing, microparticle analyses, and clotting factor analysis. Exposure to hypergravity reduced plasma volume by 12.5% (P=0.002) and increased the red blood cell aggregation index (P<0.05). With hypergravity, thrombelastographic clotting time of native blood shortened from 719±117 seconds to 628±89 seconds (P=0.038) and platetet reactivity increased (P=0.045). Hypergravity shortened partial thromboplastin time from 28 (26–29) seconds to 25 (24–28) seconds (P<0.001) and increased the activity of coagulation factors (eg, factor VIII 117 [93–134] versus 151 [133–175] %, P<0.001). Tissue factor concentration was 188±95 pg/mL before and 298±136 pg/mL after hypergravity exposure (P=0.023). Antithrombin (P=0.005), thrombin‐antithrombin complex (P<0.001), plasmin‐alpha2‐antiplasmin complex (0.002), tissue‐plasminogen activatior (P<0.001), and plasminogen activator inhibitor‐1 (P=0.002) increased with centrifugation. Statistical adjustment for plasma volume attenuated changes in coagulation.
Conclusions
Hypergravity triggers low‐level hemostasis activation through endothelial cell activation, increased viscoelasticity, and augmented platelet reactivity, albeit partly counteracted through endogenous coagulation inhibitors release. Hemoconcentration may contribute to the response.
Keywords: astronaut, blood coagulation, commercial spaceflight, human spaceflight, thrombotic risk
Subject Categories: Endothelium/Vascular Type/Nitric Oxide, Hemodynamics, Physiology, Platelets, Thrombosis
Nonstandard Abbreviations and Acronyms
- FV
coagulation factor V
- FVIII
coagulation factor VIII
- FXIII
coagulation factor XIII
- Gz
force of weight in head‐to‐foot direction
- y at dIsc min
shear rate balancing red blood cell aggregation and disaggregation y at dIsc min
Clinical Perspective
What Is New?
Altered gravity may pose an increased thrombotic risk on astronauts; we report the first systematic physiological data on the effects of hypergravity on healthy human hemostasis.
We observed red blood cells, platelets, plasmatic coagulation, and endothelium to be affected by hypergravity.
Hypergravity transiently elicits a subtle procoagulatory phenotype in healthy people, which may be partly mediated through hemoconcentration.
What Are the Clinical Implications?
These findings should prompt clinical studies on hemostasic alterations and the thrombotic risk of hypergravity exposure in aviators, professional astronauts, as well as individuals with chronic medical conditions applying for a touristic spaceflight.
The gravitational challenge imposed on the human cardiovascular system during standing on Earth elicits venous stasis, hemoconcentration, orthostatic hypercoagulability, and vascular endothelial activation. 1 Moreover, orthostatic presyncope induces blood hypercoagulability 2 lasting at least 20 minutes. 3 The response may be exacerbated following bedrest immobilization 4 and in patients recovering from strokes. 5 In addition to classical components of the coagulation cascade, red blood cells appear to contribute to hemostasis and thrombosis. Red blood cell aggregation and deformability, which affect blood viscosity, respond to gravity, exercise, temperature, and hypoxia. 6 Fighter pilots and astronauts during take off and landing are exposed to much greater gravity forces compared with standing on Earth. Presyncopal symptoms or overt syncope may ensue. We hypothesized that hypergravity may elicit hypercoagubility. Indeed, cell and animal studies revealed platelet and endothelial cell activation along with excess red blood cell aggregation with hypergravity. 7 , 8 Furthermore, serious thromboembolic conditions have been reported during hypergravity centrifuge training and during rollercoster rides. 9 , 10 However, studies on the effects of hypergravity on human blood rheology and hemostasis are scarce. The issue is relevant for professional astronauts, particularly during deep space exploration and for recreational astronauts. 11 The latter group will comprise individuals with preexisting thromboembolic risk factors. Therefore, we tested effects of exposure to a 15‐minute cycle of 3 Gz hypergravity stress on the hemostasic system in healthy men.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Participants
We included 20 healthy, nonsmoking, men (33±8 years, 1.83±0.06 cm, 82±6 kg). None had a history of thromboembolic events or orthostatic intolerance. They did not ingest platelet inhibiting drugs for at least 14 days prior to the study. Each participant successfully passed a flight medical examination before participation. We obtained written informed consent prior to inclusion, and the study was approved by the ethics committee of the North Rhine Medical Association (protocol No. 2013060). The participant shown on Figure 1 provided written informed consent for this publication. All research was performed in accordance with the Declaration of Helsinki.
Figure 1. Evolution of the estimated arterial pressure at the level of the feet during centrifugation.
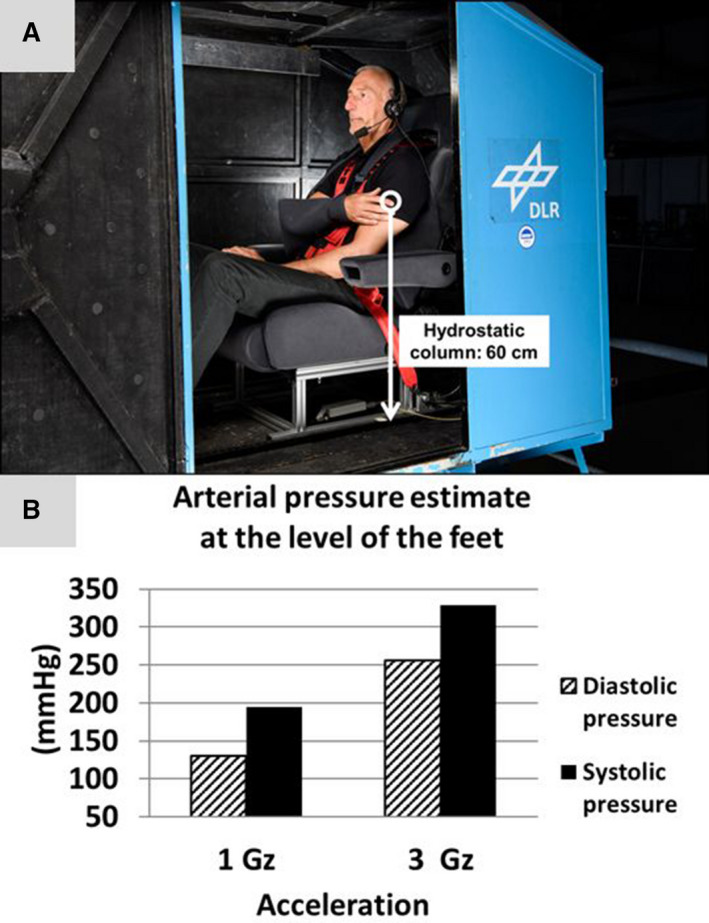
A, A representative participant sitting inside the centrifuge gondola with the right arm fixated at the level of the heart for beat‐to‐beat blood pressure measurements. The white arrow indicates the hydrostatic column of interest for arterial pressure estimates at the level of the feet. B, Graphs indicate averaged diastolic (shaded columns) and systolic (black columns) pressure at the level of the feet in 18 participants.
Experimental Hypergravity
We conducted the study at the Deutsches Zentrum für Luft‐ und Raumfahrt Institute of Aerospace Medicine in Cologne, Germany. Experimental hypergravity challenge was executed using a human long‐arm centrifuge. Centrifuge runs were performed between 8:00 am and 11:00 am. Ambient temperature and humidity during centrifuge runs were held at 26°C and 56%, respectively. Heart rate and beat‐to‐beat finger blood pressure at heart level were continuously monitored (Finometer MIDI Device; Finapres Medical Systems, Amsterdam, The Netherlands) and stored using BIOPAC‐software (BIOPAC Systems Inc., Goleta, CA, USA). Arterial pressure at the level of the feet was subsequently estimated by adding a hydrostatic pressure component to the systolic arterial pressure at the heart level (Figure 1). The pressure of the hydrostatic column from the heart to the foot (P) was calculated as P=h×g×ρ. h is the height of the level of the heart above the ground (0.6 m), g is the acceleration acting on the participant inside the centrifuge gondola resulting from 3g centrifugal acceleration and 1g Earth gravity (3.16g), and ρ is the density of the blood (1.0621 g/cm3). 12
Participants were placed in sitting position in a fully closed swing‐out cabin attached to the 5 m long centrifuge arm (Figure 1A). During centrifugation, the G‐load acted strictly caudally. Centrifuge runs included a 15‐minute plateau at 3 Gz (22 rpm) and a 0.1 g/s acceleration and decelaration phase (Figure 2A). For baseline recordings, participants remained seated for 30 minutes inside the centrifuge gondola. Blood samples were drawn from antecubital veins through puncture without tourniquet after the 30‐minute baseline phase had elapsed (pre), immediately after the centrifuge run (post), and after 30 minutes ambulation in the centrifuge facility (post+30) (Figure 2A).
Figure 2. Effects of 15 minutes of 3 Gz hypergravity on the cardiovascular system, red blood cell function, and cell‐based blood coagulation.
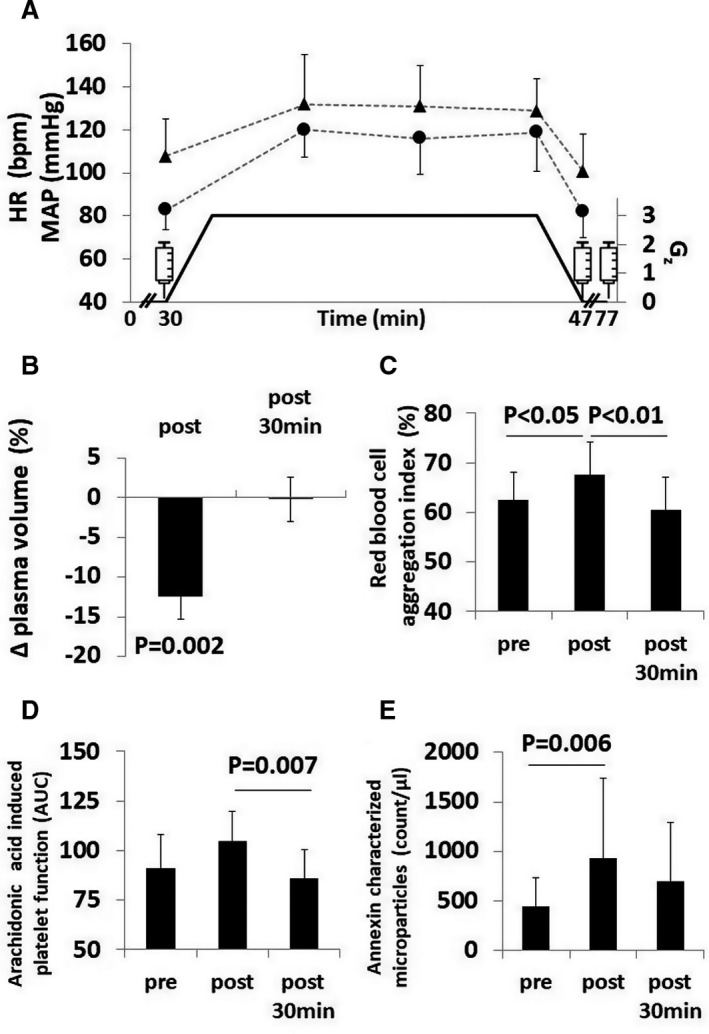
A, Black triangles are medians±interquartile range and black dots are means±SD indicating heart rate (HR) and mean arterial pressure (MAP) responses with respect to gravity load shown by black graph. One‐minute intervals were averaged at pre and post and 5‐minute intervals were averaged during centrifugation. Syringes indicate blood draws (pre, post, post+30); (B) black columns indicate medians of relative plasma changes±interquartile range at post and post+30 compared to pre; (C) black columns show mean±SD aggregation index of red blood cells before, after and 30 minutes after hypergravity exposure; (D) black columns give median±interquartile range of the area under the curve (AUC) of platelet activation with arachidonic acid before, after, and 30 minutes after hypergravity; (E) black columns give median±interquartile range of the counts of microparticles presenting externalized phosphatidylserine characterized by annexin surface markers before, after, and 30 minutes after hypergravity.
Red Blood Cell Aggregation
We calculated relative blood and plasma volume changes from hemoglobin and hematocrit measurements. 13 For red blood cell rheology measurements, sampled blood was anticoagulated using sodium heparin vacutainer. Measured red blood cell parameters (Sysmex Digitana KX‐21N, Sysmex, Switzerland) included red blood cell number, hemoglobin concentration, hematocrit, and mean cellular hemoglobin concentration, a marker for intracellular viscosity. For red blood cell aggregation measurements, we adjusted hematocrit to 40% and fully oxygenated samples for 15 minutes with a Roller Mixer (Karl Hecht KG, Germany). We measured aggregation index (%) and shear rate balancing red blood cell aggregation and disaggregation (y at dIsc min [1/s]) using a laser‐assisted optical rotational cell analyzer (RR Mechatronics, Zwaag, The Netherlands). 14 We assessed red blood cell deformability by ektacytometry using the same device. 15 The samples were sheared in a Couette system at 9 consecutive shear stresses ranging from 0.3 and 50 Pa. We calculated the ratio of maximum deformability and shear stress required for one‐half of maximum deformability with lower values representing higher red blood cell deformability.
Functional Viscoelastic Assays and Platelet Function
We conducted whole point‐of‐care coagulation testing within 30 minutes after each blood draw from citrated blood (ROTEM; Tem International GmbH, Munich, Germany) and from hirudine blood using an impendance aggregometer (Multiplate; Verum Diagnostica GmbH, Munich, Germany). We performed native, extrinsic, intrinsic, and under platelet inhibition activated viscoelastic tests. Moreover, we determined coagulation time, clot formation time, and maximum clot firmness. We analyzed platelet function by impedance aggregometry after stimulation with adenosine diphosphate, arachidonic acid, thrombin receptor activating peptide‐6, ristocetin, or collagen.
Extended Coagulation Assays
We centrifuged blood samples for subsequent laboratory coagulation assays 20 minutes at 1550g and 20°C and stored them at −80°C. Flow cytometry with cell‐specific markers was used to determine the microparticle quantity and their cellular origin. We analyzed microparticles after samples had undergone ultracentrifugation. We added cell‐specific antibodies for thrombocytes to 30 μL plasma and incubated 20 minutes at room temperature, as described previously. 16 For the determination of platelet origin, we used fluorescein isothiocyanate labelled anti‐CD42b against glycoprotein Ib and peridinin chlorophyll Cy5.5‐labelled anti‐CD61 against glycorpotein IIIa. For microparticles derived from activated platelets, we applied phyocerythrin labelled anti‐CD62p against p‐selectin. Additionally, allophycocyanin labelled annexin V served as marker of procoagulant phosphatidylserine.
We analyzed the concentration of fibrinogen, the activity of coagulation factors V, VIII, and XIII, the concentration of soluble tissue factor, coagulation inhibitiors (protein C activity, antithrombin activity, thrombomodulin concentration), activation markers thrombin‐antithrombin III complex, d‐dimer concentration, prothrombin fragment F1+2 concentration, plasminogen activator inhibitor concentration), plasmin‐alpha2‐antiplasmin complex concentration, and tissue plasminogen activator concentration at the Institutes of Hematology of the University Hospital Bonn and the Cologne‐Merheim Medical Center (Germany). The results of proteins examined by concentration measures were corrected for changes in plasma volume to indicate a hemoconcentration‐driven effect on the concentrations of large circulating molecules. 17
Statistical Analysis
A Shapiro‐Wilk test was used to test for normality. Normally and nonnormally distributed data are expressed as means± SD and median±interquartile range (IQR), respectively. We compared groups (blood samples: pre, post, post+30; cardiovascular data: phases 1–5) by 1‐way repeated measures analysis of variance with Tukey's post hoc test. Data with nonnormal distribution were analyzed with Wilcoxon and Friedman test, respectively. Individual differences between pre and post measurements of data sets with high interindividual variance were plotted as waterfall charts for a visual test for clinically relevant extreme subgroups. We then tested for significant subgroup effects by quantile regression. Calculations were performed with IBM SPSS Statistics version 26. A value for P<0.05 was considered as statistically significant.
Results
Hypergravity Elicits Cardiovascular Stress, Plasma Volume Loss, and Hemoconcentration
Eighteen out of 20 participants completed the entire centrifuge run. We excluded 2 participants from the analysis because centrifugation had to be terminated prematurely because of severe motion sickness. During centrifugation, heart rate increased from 83 (IQR, 76–95) to 119 bpm (IQR, 101–137) (P<0.001) and mean arterial pressure at the level of the heart from 108±17 to 129±15 mm Hg (P<0.001) (Figure 1A). The estimated average of the mean arterial pressure at the level of the feet rose from 251 mm Hg in 1 Gz to 277 mm Hg in 3 Gz. Hypergravity elicited substantial reduction in plasma volume and hemoconcentration (Figure 2B and Table 1).
Table 1.
Hypergravity‐Induced Changes of the Cellular Hemostatic System
| Pre | Post | Post+30 | P Value | |
|---|---|---|---|---|
| Plasma volume change (%) | … | −12.5 (−14.5 to −8.9) | −0.2 (−2.9 to 2.8) | 0.002 |
| Blood volume change (%) | … | −6.9 (−7.8 to −6.1) | −0.1 (−1.4 to 1.9) | 0.003 |
| Blood count | ||||
| Hematocrit, % | 43.2±3.1 | 46.3±3.6 | 43.5±3.2 | <0.001 |
| Hemoglobin, g/dL | 15.4±1.0 | 16.4±1.2 | 15.4±1.1 | <0.001 |
| Red blood cells, ×106/µL | 5.0±0.4 | 5.3±0.4 | 5.0±0.4 | <0.001 |
| Mean corpuscular hemoglobin concentration, g/dL | 35.6±0.4 | 35.5±0.3 | 35.4±0.4 | 0.015 |
| Platelets, ×109/L | 219±35 | 242±35 | 213±29 | 0.023 |
| 228±34 † | 214±31 † | 0.431 † | ||
| White blood cells, ×109/L | 5.1±1.2 | 6.5±2.0 | 5.0±1.3 | 0.006 |
| 6.1±1.9 † | 5.0±1.3 † | 0.045 *,† | ||
| Blood rheology | ||||
| Red blood cell aggregation index, % | 62.4±5.9 | 67.7±6.5 | 60.4±6.8 | 0.001 |
| Shear rate balancing red blood cell aggregation and disaggregation (y at dIsc min [1/s]) | 75.8±18.7 | 320.0±349.7 | 119.7±172.0 | 0.003 |
| Red blood cell deformability (SS1/2:EImax) | 3.69±0.4 | 3.68±0.6 | 3.80±0.6 | 0.668 |
| Platelet function | ||||
| ADP induced, velocity | 16 (14–21) | 18 (14–22) | 17 (12–21) | 0.022 |
| ADP, induced aggregation | 163±28 | 172±31 | 163±32 | 0.622 |
| ADP induced, AUC (U) | 81±18 | 90±21 | 82±22 | 0.387 |
| Arachidonic acid induced, velocity | 19 (17–22) | 21 (19–24) | 18 (15–21) | 0.021 |
| Arachidonic acid induced, aggregation | 188 (149–219) | 199 (162–218) | 175 (143–200) | 0.182 |
| Arachidonic acid induced, AUC (U) | 91 (79–113) | 105 (81–111) | 86 (75–104) | 0.045 |
| Thrombin receptor activating peptide‐6 induced, velocity | 25±4 | 26±5 | 26±5 | 0.852 |
| Thrombin receptor activating peptide‐6 induced, aggregation | 221±35 | 226±34 | 218±35 | 0.799 |
| Thrombin receptor activating peptide‐6 induced, AUC (U) | 119±2 | 124±20 | 119±21 | 0.736 |
| Ristocetin induced, velocity | 30 (24–38) | 30 (23–38) | 31 (26–32) | 0.846 |
| Ristocetin induced, aggregation | 223±49 | 226±43 | 221±52 | 0.951 |
| Ristocetin induced, AUC (U) | 100±28 | 101±26 | 99±32 | 0.963 |
| Collagen induced, velocity | 18 (17–21) | 20 (17–23) | 19 (16–22) | 0.278 |
| Collagen induced, aggregation | 166±38 | 174±34 | 174±44 | 0.762 |
| Collagen induced, AUC (U) | 75±18 | 80±18 | 78±21 | 0.658 |
| Microparticles | ||||
| CD42 (events/µL plasma) | 984 (468–2043) | 1216 (753–3382) | 957 (633–2098) | 0.327 |
| CD61 (events/µL plasma) | 539 (145–1442) | 1363 (420–2368) | 784 (212–1451) | 0.494 |
| CD62P (events/µL plasma) | 462 (205–975) | 1075 (345–2274) | 615 (174–1032) | 0.113 |
| Annexin (events/µL plasma) | 447 (235–811) | 933 (338–1952) | 704 (207–1387) | 0.028 |
Pre, baseline values registered after 30 minutes of sitting in the centrifuge gondola; post, values registered immediately after the centrifuge had stopped; post+30, values registered 30 minutes after the centrifuge run. During this half hour the participants were allowed to move freely inside the centrifuge facility. EImax, the ratio of maximum deformability of red blood cells, SS1/2: EImax, shear stress required for one‐half of EImax with lower values representing higher red blood cell deformability. Normal distributed values are shown as mean±SD. Nonnormal distributed values are shown as median (first–third percentile). ADP indicates adenosine diphosphate; AUC, area under the curve.
Concentration measures corrected for plasma volume changes.
Hypergravity Worsens Red Blood Cell Rheology
Both aggregation index and minimal shear rate needed to dissociate red blood cell aggregates (y at dIsc min) increased after hypergravity compared with baseline (both P<0.05) (Figure 2C, Table 1). A subset of 6 out of the 20 participants (33%, 77th percentile) showed an above average increase of y at dIsc min to 728±8 s−1. However red blood cell deformability was unaffected by hypergravity.
Platelet and Endothelial Cell Activation With Hypergravity
Circulating platelet and endothelial microparticle concentrations increased after centrifugation. Ex vivo platelet function testing revealed temporal changes in aggregability after exposure to hypergravity. Arachidonic acid induced platelet function increased in overall strength from 87 (IQR, 79–113) to 94 (IQR, 81–111) area under the curve units (P=0.045) (Figure 2D, Table 1). In addition, microparticle counts, characterized by annexin surface marker, displayed a temporal increase during hypergravity (P=0.028) (Figure 2E, Table 1). The finding is consistent with in vivo platelet and endothelial cell activation.
Integrated Blood Coagulability Is Augmented With Hypergravity
The thrombelastographic clotting time of native blood decreased from 719±117 seconds to 628±89 seconds (P=0.038) following centrifugation indicating increased blood coagulability. Remarkably, despite normalization of cardiovascular regulation, plasma volume, and hemoconcentration, the response was sustained after 30 minutes of recovery (Figure 3B). Compared with baseline, hypergravity altered further viscoelastic properties of blood clots (Table 2).
Figure 3. Effects of 15 minutes of 3 Gz hypergravity on the cellular and plasmatic components of the hemostatic system.
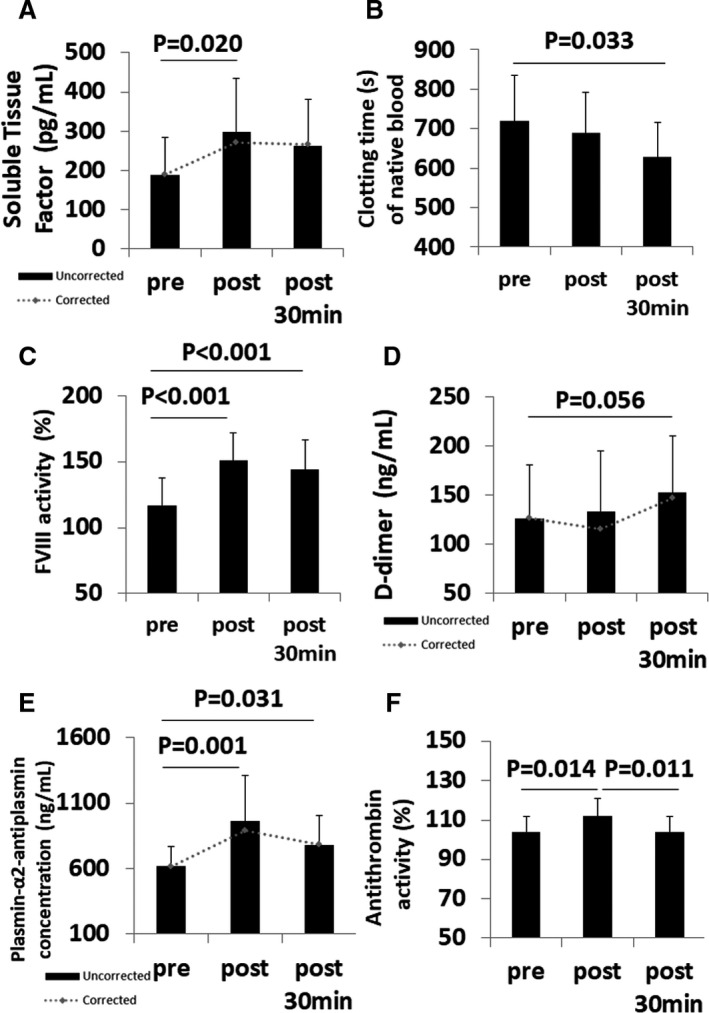
A, Black columns represent means of soluble tissue factor concentrations±SD before, after, and 30 minutes after hypergravity. Grey dotted graph represents means corrected for plasma volume; (B) black columns give means±SD of clotting times of native blood before, after, and 30 minutes after hypergravity; (C) medians±interquartile range (IQR) of activity percentage of coagulation factor VIII (F VIII) are given as black column before, after, and 30 minutes after hypergravity; (D) black columns indicate medians of d‐dimer concentrations±interquartile range (IQR) before, after, and 30 minutes after hypergravity. Grey dotted graph represents medians corrected for plasma volume; (E) black columns show median concentrations±interquartile range of plasmin‐alpha2‐antiplasmin complex before, after, and 30 minutes after hypergravity exposure. Grey dotted graph represents medians corrected for plasma volume; (F) means±SD of antithrombin activity before, after, and 30 minutes after hypergravity are given as black columns.
Table 2.
Hypergravity‐Induced Changes of the Plasmatic Hemostasis System
| Pre | Post | Post+30 | P Value | |
|---|---|---|---|---|
| Thrombelastography | ||||
| Clotting time of native blood, s | 719±117 | 688±104 | 628±89 | 0.038 |
| Clot formation time of native blood, s | 273±61 | 268±59 | 258±55 | 0.716 |
| Mean clot firmness of native blood, mm | 46±5 | 48±4 | 47±5 | 0.498 |
| Extrinsic activation, clotting time, s | 51 (44–54) | 47 (40–56) | 48 (42–54) | 0.959 |
| Extrinsic activation, clot formation time, s | 102±21 | 98±18 | 102±19 | 0.616 |
| Extrinsic activation, mean clot firmness, mm | 55±5 | 57±4 | 55±4 | 0.464 |
| Intrinsic activation, clotting time, s | 162±17 | 153±17 | 157±15 | 0.319 |
| Intrinsic activation, clot formation time, s | 83±14 | 84±15 | 88±14 | 0.603 |
| Intrinsic activation, mean clot firmness, mm | 55±3 | 55±4 | 54±4 | 0.467 |
| Extrinsic activation with platelet inhibition, mean clot firmness, mm | 11 (10–13) | 11 (10–14) | 11 (10–13) | 0.101 |
| Inflammation/global coagulation testing | ||||
| C‐reactive protein, mg/dL | 0.09 (0.04–0.17) | 0.11 (0.04–0.18) | … | 0.001 |
| 0.11 (0.04–0.17) † | 0.396 † | |||
| Prothrombin time, % | 97±13 | 103±13 | 95±11 | 0.173 |
| Partial thromboplastin time, s | 28 (26–29) | 25 (24–28) | 26 (24–28) | <0.001 |
| Procoagulation | ||||
| Fibrinogen, mg/dL | 303 (285–345) | 315 (303–366) | 301 (282–341) | <0.001 |
| 275 (285–345) † | 298 (282–336) † | 0.016 † | ||
| Tissue factor, pg/mL | 188±95 | 298±136 | 263±119 | 0.023 |
| 270±134 † | 265±122 † | 0.076 † | ||
| Coagulation factor V (%) | 86 (73–91) | 88 (78–101) | 88 (80–95) | 0.015 |
| Coagulation factor VIII (%) | 117 (93–134) | 151 (133–175) | 144 (127–173) | <0.001 |
| Coagulation factor XIII (%) | 116 (106–159) | 137 (120–167) | 115 (106–163) | <0.001 |
| D‐dimer, µg/L | 127 (107–214) | 134 (103–226) | 153 (103–220) | 0.056 |
| 116 (95–208) † | 147 (106–225) † | 0.801 † | ||
| Activated protein C resistance [ratio] | 2.7 (2.6–2.8) | 2.7 (2.6–2.7) | 2.7 (2.6–2.9) | 0.617 |
| F1,F2 prothrombin fragment, nmol/mL | 0.09±0.03 | 0.10±0.03 | 0.09±0.03 | 0.250 |
| 0.09 (0.06–0.13) † | 0.08 (0.07–0.12) † | 0.513 † | ||
| Thrombin‐antithrombin complex, ng/mL | 2.2 (2.0–2.5) | 2.5 (2.2–3.2) | 2.4 (2.0–2.6) | <0.001 |
| 2.4 (2.0–2.8) † | 2.4 (2.1–2.6) † | 0.128 † | ||
| Anticoagulation | ||||
| Antithrombin, % | 104±8 | 112±9 | 104±8 | 0.005 |
| Protein C, % | 98±13 | 106±14 | 98±14 | 0.132 |
| Thrombomodulin, ng/mL | 1.9±0.4 | 2.0±0.6 | 1.8±0.5 | 0.471 |
| 1.8±0.5 † | 1.8±0.6 † | 0.658 † | ||
| Plasminogen activator inhibitor antigen, ng/mL | 11.3 (8.1–16.6) | 21.7 (19.0–34.8) | 13.3 (8.7–17.1) | <0.001 |
| 20.0 (17.3–32.1) † | 12.9 (8.6–17.6) † | <0.001 † | ||
| Plasmin‐α2‐antiplasmin complex, ng/mL | 618 (476–774) | 961 (549–1246) | 783 (500–950) | 0.002 |
| 892 (467–1135) † | 783 (492–945) † | 0.024 † | ||
| Tissue plasminogen activator antigen, µg/L | 1.0 (0.8–1.4) | 3.3 (2.7–4.5) | 1.9 (1.7–2.3) | <0.001 |
| 3.0 (2.5–3.8) † | 1.9 (1.7–2.3) † | <0.001 † | ||
Pre, baseline values registered after 30 minutes of sitting in the centrifuge gondola; post, values registered immediately after the centrifuge had stopped; post+30, values registered 30 minutes after the centrifuge run. During this half hour the participants were allowed to move freely inside the centrifuge facility. Normal distributed values are shown as mean±SD. Nonnormal distributed values are shown as median (first–third percentile).
Concentration measures corrected for plasma volume changes.
Hypergravity Augments Plasmatic Procoagulation Pathways
Concentration measures corrected for plasma volume are shown by italicized numbers in the tables and by grey dotted graphs in the figures. D‐dimer concentrations tended to increase modestly after centrifugation (134 ng/mL, IQR, 103–226) with respect to baseline (127 ng/mL, IQR, 107–215) (P=0.056) (Figure 3D). Thrombin‐antithrombin complex, a marker of prothrombotic state, increased from 2.2 (IQR, 2.0–2.2) at baseline to 2.5 ng/mL (IQR, 2.2–3.2) after hypergravity (P<0.001). We observed significant influences of hypergravity on the intrinsic and common coagulation pathways. Activated partial thromboplastin time was reduced from 28 seconds (IQR, 26–29) to 25 seconds (IQR, 24–28) (P<0.001). Circulating levels of fibrinogen, and the activity of the accelerators of the common pathway coagulation factors V, VIII, and XIII increased with centrifugation compared with baseline (Figure 3C, Table 2). Soluble tissue factor, the primary trigger of the coagulation cascade through the extrinsic pathway was increased after hypergravity exposure (P=0.023) (Figure 3A). Plasmin‐alpha2‐antiplasmin complex was higher after centrifugation (961 ng/mL, IQR, 549–1246) compared with baseline (618, IQR, 476–774) (P=0.002) (Figure 3E).
Coagulation Inhibitors May Attenuate Hypercoagulability During Hyper Gravity
Neither the protein C activity nor the thrombomodulin concentration were affected by hypergravity. In contrast hypergravity increased the activity of antithrombin from 104±8% to 112±9% compared with baseline (P=0.005) (Figure 3F). Additionally, tissue‐plasminogen activator, which is involved in breakdown of blood clots, was higher after hypergravity exposure compared with baseline (P<0.001) (Table 2).
Discussion
The important finding of our study is that centrifugation‐induced hypergravity resembling gravity loads during suborbital spaceflight elicits significant hemostatic changes in healthy people. Hypergravity accelerated blood clot formation ex vivo, as indicated by increased soluble tissue factor concentrations and modestly elevated d‐dimer levels. The response was associated with hemoconcentration, platelet activation, increased microparticle formation, and red blood cell aggregation. However, coagulation inhibitors and fibrinolytic factors increased to a similar degree after exposure to hypergravity and no participant displayed clinical signs of thromboembolism. Thus, hypergravity transiently promotes a mild prothrombotic phenotype in healthy people, which should prompt adequately powered clinical studies on hemostasis in aviators, professional astronauts, and candidates for touristic spaceflight.
Following hypergravity exposure, we observed augmented integrated blood coagulation by thrombelastography indicated by shortened clotting time of native blood. A shortening of clotting time indicates accelerated thrombin generation, which can be attributed to an increased tissue factor expression on circulating cells caused by traumatic tissue destruction, inflammation, 18 circulating tumor cells, or infection. 19 , 20 Several thrombelastographic parameters indicate hypercoagulability, namely shortened clotting time and clot formation time, increased mean clot firmness, and shutdown of fibrinolysis. The potency of thrombelastometry to reveal hypercoagulability has been intensively investigated. 21 , 22 , 23
Externalization of platelet membrane phosphatidylserine following hypergravity exposure, which likely results from the so‐called flip‐flop process, is consistent with platelet activation. 24 Exposure of cell membrane phosphatidylserine is required for clot formation via vitamin‐K dependent clotting factors and thrombin generation. Platelet‐derived microparticles, which are formed following platelet activation, are strong procoagulants and have been linked with clinical thromboembolic risk. 25 Although platelets appeared to be activated, we did not observe an increase in platelet‐derived microparticles with hypergravity.
Hypergravity induced rapid reductions in blood and plasma volumes by ≈7% and 13%, respectively, with accompanying increases in blood viscosity and platelet counts. Similar plasma volume changes occur within 1 hour standing still on Earth. 1 Changes in coagulation measurements in our study were attenuated after adjusting for plasma volume suggesting that hemoconcentration may have contributed to the response. Indeed, hemoconcentrations has been implicated in altered hemostasis associated with hypoxia, heat exposure, and psychological, physical, or orthostatic stress. A procoagulatory state, exemplified by clotting time (Figure 3B), persisted 30 minutes after centrifugation while intravascular volume had recovered. Moreover, hydration does not attenuate orthostatic hypercoagulability. 26 Red blood cell aggregation and shear forces required to separate the aggregates were observed even after adjusting hematocrit. The findings are qualitatively in line with previous findings of increased red blood cell aggregation after 30 minutes of short‐arm centrifugation at +2 Gz. 6 Overall, changes in coagulability with hypergravity may not be solely explained by hypergravity‐induced changes in plasma volume.
Increases in tissue factor concentrations could also be mediated through hypergravity‐driven vascular endothelium and monocyte activation. Activated circulating monocytes account for more than 90% of soluble tissue factor activity. 27 Monocytes are activated by cyclic pressure changes via a pathway engaging PIEZO1 (Piezo1 channel protein) and hypoxia‐inducible factor proteins. 28 Furthermore, endothelial cells are sensitive to hypergravity‐induced shear forces to the vascular wall. 7 Orthostatic stress also stimulates endothelial tissue factor expression, tissue plasminogen activator, and plasminogen activator inhibitor‐1 release. 1 During centrifugation, our participants were exposed to ≈330 mm Hg systolic arterial pressure at the level of the feet, which is well above the pressure causing failure of endothelial barrier function. 29 A mismatch in d‐dimers and prothrombin fragments F1+2 concentrations is a known phenomenon likely resulting from higher thrombin generation rates than expected by prothrombin fragment plasma levels. 30 , 31 The fact that the endothelial derived biomarkers thrombomodulin and protein C were unchanged after hypergravity exposure is unexpected but may be explained by the brevity of hypergravity exposure and the type of endothelial stress. Thrombomodulin is a marker for endothelial injury secondary to vascular inflammation rather than mechanical stress. 32 Similarly, 51 minutes of combined orthostatic and heat stress while promoting systemic inflammation and coagulation did not affect thrombomodulin and protein C concentrations. 33
Biomarkers for fibrinolytic activity, such as plasmin‐alpha2‐antiplasmin, increased after hypergravity exposure indicating the fibrinolytic activation of endothelial cells. Plasmin‐alpha2‐antiplasmin levels represent the in vivo plasmin generation. 34 Tissue‐type plasminogen activator and plasminogen activator inhibitor type 1 release after centrifugation points toward vulnerability of the fibrinolytic system to hypergravity. Sympathoadrenal activation, which occurs during 3 Gz exposures, 35 is known to increase tissue plasminogen activator concentrations. 36
Limitations
One potential limitation of our study is that we studied only young to middle‐aged men although sex and age are relevant factors affecting hemostasis. 37 Yet, in women, oral contraception could interact with hypergravity‐induced prothrombotic responses. 38 Because of safety concerns, we exposed our participants to moderate hypergravity load of modest duration. Animal studies suggest that higher gravity loads, which can occur in high performance aircrafts and during spaceflight, may induce more pronounced hemostasis. 8 Because the follow‐up phase of our study was limited to 30 minutes, we may have missed delayed but relevant hemostatic responses to hypergravity as suggested by a low though rapid increase of thrombin‐antithrombin complex and discordant prothrombin fragments F1+2 and d‐dimer responses, respectively. For comparison, the so‐called economy class syndrome can occur hours to days after air travel. Moreover, we did not analyze von Willebrand factor in its role as a blood plasma marker for endothelial activation. 39 Although waterfall plots revealed extreme individual responses in some cell‐based measurements, quantile regression analysis failed to uncover any concordant extreme reaction across several related variables. Given the limited sample size, we cannot exclude that subgroups with extreme hemostatic responses to hypergravity exist. Finally, although changes in intravascular volumes may have contributed to our findings, there is no consensus on how to correct for such changes. 40 , 41 , 42 , 43 Plasma volume correction reduced the magnitude of alterations of parameters examined by concentration means. However, given nonlinear kinetics and dynamic interactions between coagulation factors, simple arithmetic correction for plasma volume changes may not suffice.
Conclusions
In healthy people, hypergravity augments blood coagulability within physiological limits through increased red blood cell adherence, endothelial cell activation, increased viscoelasticity, and augmented platelet reactivity. For example, thrombin‐antithrombin complex concentrations increased only from 2.2 to 2.5 ng/mL. The response may be partly compensated by elevated levels of coagulation inhibitors. Furthermore, venous pooling in the dependent part of the body may produce venous stasis and increased intravascular pressure. Thus, hypergravity affects all components of Virchow's triad, which could predispose to thrombus formation (Figure 4). Yet, whether the modest response may increase thromboembolic risk in people exposed to hypergravity stays highly speculative. Previous studies of the economy class syndrome suggested that modest alterations in classical coagulation factors may promote thromboembolic risk. 44 , 45 The relevance of this issue for governmental and touristic spaceflight should be pursued in further adequately powered studies. To uncover whether subsets of indivduals with extreme hemostatic responses to hypergravity exist should be a priority. Initiatives to fly tourists to the International Space Station and the development of commercial spaceflight programs will lead to an increasing number of individuals exposed to hypergravity. Only recently, the first 2 cases of venous thromboembolism during a space mission have been reported. 46 To date, only ≈600 highly selected and trained individuals at a considerably young age have flown into space with no fatal thromboembolic event but this may lack generalizability. 47 Space tourists, in contrast, will be less well selected, older, and less fit compared with professional astronauts, and a medical history of minor to moderate thromboembolic or cardiovascular disease will most likely not disqualify them from flight.
Figure 4. Effects of hypergravity on the categories of the Virchow's triad.
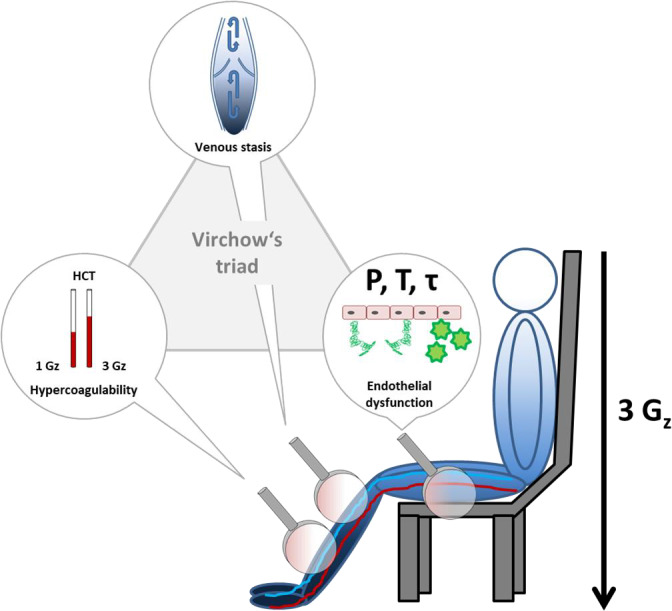
The illustration shows an individual sitting in the centrifuge gondola. Blue color gradient symbolizes hypergravity driven blood volume shift towards the dependent parts of the body. Middle magnifier symbolizes reduced venous blood flow and stasis in the dependent body during hypergravity. Left magnifier shows two hematocrit capillaries indicating hemoconcentration caused by plasma volume loss leading to hypercoagulability after centrifugation. Right magnifier symbolizes activation of the vascular endothelium during centrifugation through increased hydrostatic pressure (P), vessel diameter dependent wall stress (T) and blood flow mediated shear stress (τ) acting on the walls of the vascular tree of the lower body. Activated endothelium induces platelet activation and adhesion (green stars) and tissue factor release (green curled objects).
Sources of Funding
This study was supported by the programmatic funding of the German Aerospace Center (Deutsches Zentrum für Luft‐ und Raumfahrt), by internal funding of the Witten/Herdecke University and German Sport University Cologne, and by a grant from the European Space Agency.
Disclosures
Dr Limper and Dr Ahnert report grants from the Ground Based Facility program of the European Space Agency, during the conduct of the study. Dr Ulrich Limper received funding from the internal grant program (project IFF 2020‐26) of the Faculty of Health at Witten/Herdecke University, Germany, during the conduct of this study. Dr Goerlinger works as the medical director of Tem Innovations, Munich, Germany. The remaining authors have no disclosures to report.
Acknowledgments
We thank the sponsor of this study, the European Space Agency, Verum Diagnostica GmbH (Munich, Germany) for providing the multiplate device and reagents, and TEM International (Munich, Germany) for providing the Rotem® delta device. We also thank the participants for their time and patience.
Author contributions: Dr Limper: conceptualization, methodology, formal analysis, investigation, writing—original draft, funding acquisition; Dr Ahnert: formal analysis, investigation, writing—review and editing, project administration, Professor Marc Maegele: investigation, project administration, provision of study materials, writing—review and editing; Dr Froehlich: formal analysis, investigation, resources, writing—review and editing; Dr Grau: formal analysis, investigation, resources, writing—review and editing; Mr Gauger: provision and design of study materials, visualization, investigation, writing—review and editing; Dr Bauerfeind: investigation, resources, writing—review and editing; Professor Goerlinger: resources, writing—review and editing; Professor Poetzsch: investigation, resources, writing—review and editing; Professor Jordan: methodology, supervision, writing—review and editing. The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
(J Am Heart Assoc. 2020;9:e016479. DOI: 10.1161/JAHA.120.016479.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Masoud M, Sarig G, Brenner B, Jacob G. Orthostatic hypercoagulability: a novel physiological mechanism to activate the coagulation system. Hypertension. 2008;51:1545–1551. DOI: 10.1161/HYPERTENSIONAHA.108.112003. [DOI] [PubMed] [Google Scholar]
- 2. Hamrefors V, Fedorowski A, Strandberg K, Sutton R, Isma N. Procoagulatory changes induced by head‐up tilt test in patients with syncope: observational study. Thromb J. 2017;15:16. DOI: 10.1186/s12959-017-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cvirn G, Schlagenhauf A, Leschnik B, Koestenberger M, Roessler A, Jantscher A, Vrecko K, Juergens G, Hinghofer‐Szalkay H, Goswami N. Coagulation changes during presyncope and recovery. PLoS One. 2012;7:e42221. DOI: 10.1371/journal.pone.0042221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cvirn G, Waha JE, Ledinski G, Schlagenhauf A, Leschnik B, Koestenberger M, Tafeit E, Hinghofer‐Szalkay H, Goswami N. Bed rest does not induce hypercoagulability. Eur J Clin Invest. 2015;45:63–69. DOI: 10.1111/eci.12383. [DOI] [PubMed] [Google Scholar]
- 5. Cvirn G, Kneihsl M, Rossmann C, Paar M, Gattringer T, Schlagenhauf A, Leschnik B, Koestenberger M, Tafeit E, Reibnegger G, et al. Orthostatic challenge shifts the hemostatic system of patients recovered from stroke toward hypercoagulability. Front Physiol. 2017;8:12. DOI: 10.3389/fphys.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grau M, Abeln V, Vogt T, Bloch W, Schneider S. Erythrocyte deformability and aggregation responses to intermittent and continuous artificial gravity exposure. Life Sci Space Res (Amst). 2017;12:61–66. DOI: 10.1016/j.lssr.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 7. Maier JA, Cialdai F, Monici M, Morbidelli L. The impact of microgravity and hypergravity on endothelial cells. Biomed Res Int. 2015;2015:434803. DOI: 10.1155/2015/434803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li S, Shi Q, Liu G, Zhang W, Wang Z, Wang Y, Dai K. Mechanism of platelet functional changes and effects of anti‐platelet agents on in vivo hemostasis under different gravity conditions. J Appl Physiol (1985). 2010;108:1241–1249. [DOI] [PubMed] [Google Scholar]
- 9. Cayce WR, Zerull RG. Myocardial infarction occurring at the conclusion of centrifuge training in a 37‐year‐old aviator. Aviat Space Environ Med. 1992;63:1106–1108. [PubMed] [Google Scholar]
- 10. Pelletier AR, Gilchrist J. Roller coaster related fatalities, United States, 1994–2004. Inj Prev. 2005;11:309–312. DOI: 10.1136/ip.2005.008425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Limper U, Tank J, Ahnert T, Maegele M, Grottke O, Hein M, Jordan J. The thrombotic risk of spaceflight: has a serious problem been overlooked for more than half of a century? Eur Heart J. 2020:ehaa359. May 18 [epub ahead of print]. DOI: 10.1093/eurheartj/ehaa359. [DOI] [PubMed] [Google Scholar]
- 12. Trudnowski RJ, Rico RC. Specific gravity of blood and plasma at 4 and 37°C. Clin Chem. 1974;20:615–616. DOI: 10.1093/clinchem/20.5.615. [DOI] [PubMed] [Google Scholar]
- 13. Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–248. DOI: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- 14. Baskurt OK, Uyuklu M, Ulker P, Cengiz M, Nemeth N, Alexy T, Shin S, Hardeman MR, Meiselman HJ. Comparison of three instruments for measuring red blood cell aggregation. Clin Hemorheol Microcirc. 2009;43:283–298. DOI: 10.3233/CH-2009-1240. [DOI] [PubMed] [Google Scholar]
- 15. Hardeman MR, Dobbe JG, Ince C. The laser‐assisted optical rotational cell analyzer (LORCA) as red blood cell aggregometer. Clin Hemorheol Microcirc. 2001;25:1–11. [PubMed] [Google Scholar]
- 16. Frohlich M, Schafer N, Caspers M, Bohm JK, Sturmer EK, Bouillon B, Maegele M. Temporal phenotyping of circulating microparticles after trauma: a prospective cohort study. Scand J Trauma Resusc Emerg Med. 2018;26:33. DOI: 10.1186/s13049-018-0499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alis R, Sanchis‐Gomar F, Primo‐Carrau C, Lozano‐Calve S, Dipalo M, Aloe R, Blesa JR, Romagnoli M, Lippi G. Hemoconcentration induced by exercise: revisiting the Dill and Costill equation. Scand J Med Sci Sports. 2015;25:e630–e637. DOI: 10.1111/sms.12393. [DOI] [PubMed] [Google Scholar]
- 18. Türk SM, Cansu D, Teke H, Kaşifoğlu T, Meltem Akay O, Bilgin M, Korkmaz C. Can we predict thrombotic tendency in rheumatoid arthritis? A thromboelastographic analysis (with ROTEM). Clin Rheumatol. 2018;37:2341–2349. DOI: 10.1007/s10067-018-4134-y. [DOI] [PubMed] [Google Scholar]
- 19. Kasuda S, Sakurai Y, Tatsumi K, Takeda T, Kudo R, Yuui K, Hatake K. Experimental hypercoagulable state induced by tissue factor expression in monocyte‐derived dendritic cells and its modulation by C1 inhibitor. J Thromb Thrombolysis. 2018;46:219–226. DOI: 10.1007/s11239-018-1688-0. [DOI] [PubMed] [Google Scholar]
- 20. Adamzik M, Schäfer S, Frey UH, Becker A, Kreuzer M, Winning S, Frede S, Steinmann J, Fandrey J, Zacharowski K, et al. The NFKB1 promoter polymorphism (‐94ins/delATTG) alters nuclear translocation of NF‐κB1 in monocytes after lipopolysaccharide stimulation and is associated with increased mortality in sepsis. Anesthesiology. 2013;118:123–133. [DOI] [PubMed] [Google Scholar]
- 21. Davies NA, Harrison NK, Sabra A, Lawrence MJ, Noble S, Davidson SJ, Evans VJ, Morris R, Hawkins K, Williams PR, et al. Application of rotem to assess hypercoagulability in patients with lung cancer. Thromb Res. 2015;135:1075–1080. DOI: 10.1016/j.thromres.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 22. Koch L, Hofer S, Weigand MA, Frommhold D, Poeschl J. Lipopolysaccharide‐induced activation of coagulation in neonatal cord and adult blood monitored by thrombelastography. Thromb Res. 2009;124:463–467. DOI: 10.1016/j.thromres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 23. Hartmann M, Ozlügedik S, Peters J. Thiopental inhibits lipopolysaccharide‐induced tissue factor expression. Anesth Analg. 2009;109:109–113. DOI: 10.1213/ane.0b013e3181a27cfb. [DOI] [PubMed] [Google Scholar]
- 24. Halliez M, Fouassier M, Robillard N, Ternisien C, Sigaud M, Trossaert M, Bene MC. Detection of phosphatidyl serine on activated platelets' surface by flow cytometry in whole blood: a simpler test for the diagnosis of scott syndrome. Br J Haematol. 2015;171:290–292. DOI: 10.1111/bjh.13391. [DOI] [PubMed] [Google Scholar]
- 25. Owens AP III, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–1297. DOI: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masoud M, Sarig G, Brenner B, Jacob G. Hydration does not prevent orthostatic hypercoagulability. Thromb Haemost. 2010;103:284–290. DOI: 10.1160/TH09-06-0370. [DOI] [PubMed] [Google Scholar]
- 27. Bogdanov VY, Versteeg HH. "Soluble tissue factor" in the 21st century: definitions, biochemistry, and pathophysiological role in thrombus formation. Semin Thromb Hemost. 2015;41:700–707. DOI: 10.1055/s-0035-1556049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Solis AG, Bielecki P, Steach HR, Sharma L, Harman CCD, Yun S, de Zoete MR, Warnock JN, To SDF, York AG, et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019;573:69–74. DOI: 10.1038/s41586-019-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prystopiuk V, Fels B, Simon CS, Liashkovich I, Pasrednik D, Kronlage C, Wedlich‐Söldner R, Oberleithner H, Fels J. A two‐phase response of endothelial cells to hydrostatic pressure. J Cell Sci. 2018;131:jcs206920. DOI: 10.1242/jcs.206920. [DOI] [PubMed] [Google Scholar]
- 30. Lee D, Nayak S, Martin SW, Heatherington AC, Vicini P, Hua F. A quantitative systems pharmacology model of blood coagulation network describes in vivo biomarker changes in non‐bleeding subjects. J Thromb Haemost. 2016;14:2430–2445. [DOI] [PubMed] [Google Scholar]
- 31. Friedrich MJ, Schmolders J, Rommelspacher Y, Strauss A, Rühl H, Mayer G, Oldenburg J, Wirtz DC, Müller J, Pötzsch B. Activity pattern analysis indicates increased but balanced systemic coagulation activity in response to surgical trauma. TH Open. 2018;2:e350–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boehme MW, Galle P, Stremmel W. Kinetics of thrombomodulin release and endothelial cell injury by neutrophil‐derived proteases and oxygen radicals. Immunology. 2002;107:340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meyer MA, Ostrowski SR, Overgaard A, Ganio MS, Secher NH, Crandall CG, Johansson PI. Hypercoagulability in response to elevated body temperature and central hypovolemia. J Surg Res. 2013;185:e93–e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rühl H, Müller J, Wäschenbach J, Oldenburg J, Dewald O, Pötzsch B. Short‐term venous stasis induces fibrinolytic activation but not thrombin formation. J Atheroscler Thromb. 2014;21:1260–1270. [DOI] [PubMed] [Google Scholar]
- 35. Schneider S, Guardiera S, Kleinert J, Steinbacher A, Abel T, Carnahan H, Struder HK. Centrifugal acceleration to 3Gz is related to increased release of stress hormones and decreased mood in men and women. Stress. 2008;11:339–347. [DOI] [PubMed] [Google Scholar]
- 36. Otowa K, Takamura M, Murai H, Maruyama M, Nakano M, Ikeda T, Kobayashi D, Ootsuji H, Okajima M, Furushou H, et al. Altered interaction between plasminogen activator inhibitor type 1 activity and sympathetic nerve activity with aging. Circ J. 2008;72:458–462. [DOI] [PubMed] [Google Scholar]
- 37. Roeloffzen WW, Kluin‐Nelemans HC, Mulder AB, Veeger NJ, Bosman L, de Wolf JT. In normal controls, both age and gender affect coagulability as measured by thrombelastography. Anesth Analg. 2010;110:987–994. [DOI] [PubMed] [Google Scholar]
- 38. Jain V, Wotring VE. Medically induced amenorrhea in female astronauts. NPJ Microgravity. 2016;2:16008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karampini E, Bierings R, Voorberg J. Orchestration of primary hemostasis by platelet and endothelial lysosome‐related organelles. Arterioscler Thromb Vasc Biol. 2020;40:1441–1453. [DOI] [PubMed] [Google Scholar]
- 40. Scharrer I, Vigh Z. Release of von Willebrand factor after venous occlusion—the importance of standardization of venous occlusion tests. Thromb Haemost. 1993;70:880–881. [PubMed] [Google Scholar]
- 41. Fall L, Evans KA, Lewis MH, Bailey DM. Haemostatic response to hypoxaemic/exercise stress: the dilemma of plasma volume correction. J Clin Pathol. 2011;64:269–271. [DOI] [PubMed] [Google Scholar]
- 42. Fall L, Bailey DM. Interpretive limitations associated with plasma volume shifts in the clinical assessment of hemostasis. Psychosom Med. 2013;75:222–223. DOI: 10.1097/PSY.0b013e318286f799. [DOI] [PubMed] [Google Scholar]
- 43. Matomäki P, Kainulainen H, Kyröläinen H. Corrected whole blood biomarkers—the equation of Dill and Costill revisited. Physiol Rep. 2018;6:e13749. DOI: 10.14814/phy2.13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scurr JH, Machin SJ, Bailey‐King S, Mackie IJ, McDonald S, Smith PD. Frequency and prevention of symptomless deep‐vein thrombosis in long‐haul flights: a randomised trial. Lancet. 2001;357:1485–1489. DOI: 10.1016/S0140-6736(00)04645-6. [DOI] [PubMed] [Google Scholar]
- 45. Schut AM, Venemans‐Jellema A, Meijers JC, Middeldorp S, de Groot PG, Rosendaal FR, Roest M, Lisman T, Cannegieter SC. Coagulation activation during air travel is not initiated via the extrinsic pathway. Br J Haematol. 2015;169:903–905. DOI: 10.1111/bjh.13257. [DOI] [PubMed] [Google Scholar]
- 46. Marshall‐Goebel K, Laurie SS, Alferova IV, Arbeille P, Auñón‐Chancellor SM, Ebert DJ, Lee SMC, Macias BR, Martin DS, Pattarini JM, et al. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw Open. 2019;2:e1915011. DOI: 10.1001/jamanetworkopen.2019.15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kovacs GTA, Shadden M. Analysis of age as a factor in nasa astronaut selection and career landmarks. PLoS One. 2017;12:e0181381. DOI: 10.1371/journal.pone.0181381. [DOI] [PMC free article] [PubMed] [Google Scholar]


