Abstract
Background
Coronary artery disease (CAD) is increasing among young adults. We aimed to describe the cardiovascular risk factors and long‐term prognosis of premature CAD.
Methods and Results
Using the Duke Databank for Cardiovascular Disease, we evaluated 3655 patients admitted between 1995 and 2013 with a first diagnosis of obstructive CAD before the age of 50 years. Major adverse cardiovascular events (MACEs), defined as the composite of death, myocardial infarction, stroke, or revascularization, were ascertained for up to 10 years. Cox proportional hazard regression models were used to assess associations with the rate of first recurrent event, and negative binomial log‐linear regression was used for rate of multiple event recurrences. Past or current smoking was the most frequent cardiovascular factor (60.8%), followed by hypertension (52.8%) and family history of CAD (39.8%). Within a 10‐year follow‐up, 52.9% of patients had at least 1 MACE, 18.6% had at least 2 recurrent MACEs, and 7.9% had at least 3 recurrent MACEs, with death occurring in 20.9% of patients. Across follow‐up, 31.7% to 37.2% of patients continued smoking, 81.7% to 89.3% had low‐density lipoprotein cholesterol levels beyond the goal of 70 mg/dL, and 16% had new‐onset diabetes mellitus. Female sex, diabetes mellitus, chronic kidney disease, multivessel disease, and chronic inflammatory disease were factors associated with recurrent MACEs.
Conclusions
Premature CAD is an aggressive disease with frequent ischemic recurrences and premature death. Individuals with premature CAD have a high proportion of modifiable cardiovascular risk factors, but failure to control them is frequently observed.
Keywords: cholesterol, heterozygous familial hypercholesterolemia, long‐term evolution, premature coronary artery disease
Subject Categories: Cardiovascular Disease, Risk Factors, Secondary Prevention
Nonstandard Abbreviations and Acronyms
- APOB
apolipoprotein B
- DDCD
Duke Databank for Cardiovascular Disease
- HeFH
heterozygous familial hypercholesterolemia
- LDLR
low‐density lipoprotein receptor
- MACE
major adverse cardiovascular event
- PCSK9
proprotein convertase subtilisin/kexin type 9
Clinical Perspective
What Is New ?
Patients with premature coronary artery disease (CAD) have a high rate of concomitant modifiable cardiovascular risk factors, and premature CAD is a fast‐evolving disease with a high rate of major adverse cardiovascular events and a 10‐year mortality of 21%.
Factors of subsequent ischemic event were as follows: female sex, diabetes mellitus, chronic inflammatory disease, chronic kidney disease, and absence of revascularization; around one third of patients with premature CAD continued smoking, >80% had low‐density lipoprotein cholesterol above target goals, and 16% of patients developed diabetes mellitus.
Less than 1% of patients had mutations associated with heterozygous familial hypercholesterolemia in the subset of patients with genetic data.
What Are the Clinical Implications ?
Education should be provided to young individuals with several cardiovascular risk factors about their risk of developing CAD and the long‐term prognostic of premature CAD; specific primary prevention strategies targeting active smoking, lifestyle, and statins should be provided to young patients with several concomitant risk factors.
Long‐term secondary prevention strategies should be provided to young patients with early CAD, including cessation of smoking, high‐intensity lipid‐lowering therapy, and extended dual antiplatelet therapy.
Specific attention should be brought to the high risk of developing diabetes mellitus in this young population, and more research is needed to improve care in women with premature CAD, as they are at higher risk to develop subsequent ischemic events than men.
Despite improvements in preventive therapies, recent North American registries have indicated an increase in young patients admitted for premature myocardial infarction (MI). 1 , 2 This trend was more commonly observed in women <50 years old, for whom the incidence of hospitalization for acute MI has nearly doubled over the past 20 years. Little evidence exists about the characteristics of individuals with premature coronary artery disease (CAD) as well as the contribution of traditional cardiovascular risk factors and the efficacy of modern secondary prevention. 3 , 4
Recent studies have described the association between active smoking and other nontraditional risk factors, such as ethnicity or inflammation, on the prognosis of patients with premature CAD. 5 Other studies have linked heterozygous familial hypercholesterolemia (HeFH) with the diagnosis of atherothrombosis in these young individuals. 6 Overall, more efforts are needed to understand the risk profile and behaviors of young individuals, as well as the natural history of their atherosclerotic heart disease. This is of paramount importance, in terms of life years saved for a population who is professionally active and for whom theoretical life expectancy exceeds 20 years.
We hypothesized that patients with young premature MI had a high rate of multiple ischemic events and an unfavorable control of cardiovascular risk factors along time. Thus, using the Duke Databank for Cardiovascular Disease (DDCD), a large longitudinal registry of patients undergoing cardiovascular testing at Duke University Health System, our objective was to describe the baseline and follow‐up risk factors of patients admitted with premature (<50 years old) CAD in North Carolina, and to evaluate their long‐term prognosis.
METHODS
Study Design and Population
The data that support the findings of this study are available from the corresponding author on reasonable request. The study population consisted of patients enrolled in the DDCD for a first manifestation of obstructive CAD before the age of 50 years from 1995 to 2013. The design of the DDCD has been previously described; in brief, it is a prospective registry with a prespecified data collection of clinical and angiographic baseline characteristics of all patients undergoing cardiac catheterization at Duke University Medical Center. 7 , 8 , 9
Obstructive CAD was defined as a coronary stenosis >50% in an epicardial vessel. Patients with prior documented CAD and those previously treated with percutaneous coronary intervention (PCI) or coronary artery bypass grafting were excluded, as were patients who did not have follow‐up for >1 year.
This retrospective observational study was approved by the Duke Institutional Review Board under a waiver of informed consent and had Health Insurance Portability and Accountability Act of 1996 approval.
Data Collection and Follow‐Up
Baseline clinical characteristics, risk factors, and angiographic findings were collected from both DDCD and Duke Health System records according to prespecified methods. 7 , 10 Baseline clinical characteristics included traditional cardiovascular risk factors, kidney function, comorbid conditions, such as HIV, cancer, and connective tissue disease, and medications at discharge. Baseline angiographic descriptions included coronary dominance, coronary anatomical features, and burden of disease, including number and localization of vessels with significant stenosis. Genetic data about mutations related to familial hypercholesterolemia were obtained in a subset of 9300 individuals via a linkage with CATHGEN (Catherization Genetics), which is a subregistry of DDCD. 11 Coding variants within the apolipoprotein B (APOB), low‐density lipoprotein receptor (LDLR), and proprotein convertase subtilisin/kexin type 9 (PCSK9) genes were annotated and determined as pathogenic or likely pathogenic on the basis of the American College of Medical Genetics criteria.
Follow‐up of vital status, ischemic events, and cardiovascular risk factors was performed in DDCD until 2014. Using a prespecified protocol, DDCD consistently ascertained follow‐up of patients with significant CAD, including both Duke and non‐Duke hospitalization adverse events and smoking exposure (usual number of cigarettes smoked/day in past 6 weeks), via an annual follow‐up telephone call or mailed survey. Mortality follow‐up was supplemented by a query of the National Death Index for patients with unknown vital status. The Duke Health System records were also extracted for analysis of follow‐up low‐density lipoprotein cholesterol (LDL‐C) values and new occurrences of diabetes mellitus, in patients with follow up at Duke.
Follow‐Up of Patients and Events in DDCD
Clinical End Points
The primary objective was to determine the rate of major adverse cardiovascular events (MACEs) in patients with premature CAD, during up to 10 years of follow‐up. MACEs were defined as the composite of all‐cause death, nonfatal MI, revascularization by PCI and CABG, or stroke. The second objective was to determine the factors associated with time to a first recurrent MACE and the factors associated with the rate of multiple recurrent MACE events. A third objective was to evaluate the control of traditional cardiovascular risk factors after index catheterization in the population of patients with discrete follow‐up data. Patients surveyed as smoking >0 cigarettes/day were classified as currently smoking at survey date. Cholesterol control was defined as LDL‐C <70 mg/dL, according to the latest American Heart Association/American College of Cardiology 2018 Cholesterol Clinical Practice Guidelines. 12 A sensitivity analysis was performed using an LDL‐C threshold of 100 mg/dL. New diagnosis of diabetes mellitus was determined by presence of a relevant billing diagnostic code (International Classification of Diseases, Ninth Revision [ICD‐9], code 250.x) in Duke Health System records.
Statistical Analysis
Participants were classified according to age categories at presentation (extremely premature [≤35 years old], very premature [>35–45 years old], and premature [>45–<50 years old]). Continuous variables are presented as median and interquartile ranges and compared across groups using a Kruskal‐Wallis test. Categorical variables are presented as counts and percentages and compared using a χ2 test or Fisher exact test in the case of low number of events. Except for death, nonfatal ischemic events occurring within 30 days of the diagnosis of premature CAD were not counted as recurrent events. Similarly, nonfatal ischemic events were not counted if they occurred within 30 days before death, and if 2 subsequent nonfatal ischemic events occurred within 30 days, only the first one was considered.
For the primary objective, the cumulative percentage of patients developing a first, second, and third recurrent ischemic event was estimated using the Kaplan‐Meier method with follow‐up censored at 10 years. The cumulative incidence of the first event type (treating other first events as competing risks) was also presented. The average rates of recurrence of the composite end point and individual components were calculated by fitting a log‐linear Poisson model, and expressed as the number of new cardiovascular events divided by the number of patient‐years of follow‐up (number of events per 100 patient‐years) with 95% CI, for the overall population and stratified by sex. For the log‐linear Poisson model, the outcome variable was the patient‐level count of new cardiovascular events during follow‐up. To adjust for differential follow‐up, the model included an offset term equal to the logarithm of the years of follow‐up for each patient. For estimating the event rate in the overall cohort, the model included only an intercept parameter, which was estimated by maximum likelihood. The resulting point estimate and 95% confidence limits were exponentiated and multiplied by 100 to provide the estimated recurrence rate per 100 patient‐years. The third set of analyses assessed associations of prespecified patient characteristics and risk factors evaluated at time of CAD diagnosis, with the rate of future ischemic event recurrence. These variables were age, sex, body mass index, ethnic group, chronic inflammation, cardiovascular risk factors, presentation, estimated glomerular filtration rate, LDL‐C level, triglyceride levels, multivessel disease, treatment with statin at discharge, and type of treatment (PCI or CABG). Cox proportional hazard regression models were used to assess associations with the rate of first recurrent event, and negative binomial log‐linear regression was used for rate of multiple event recurrences. For the Cox proportional hazards models, the outcome variable was the time to first MACE event recurrence. In patients without an event, time to event was censored at end of follow‐up or 10 years if earlier. Associations with the predictor variables were expressed as hazard ratios (HRs) for first MACE event recurrence. For the negative binomial log‐linear regression, the outcome variable was the count of the number of recurrent MACE events per patient. To adjust for differential follow‐up, the model included an offset term equal to the logarithm of the years of follow‐up for each patient. Associations with predictor variables were expressed as rate ratios for recurrent MACE events. For both multivariable regression models, the variables included as independent variables were identified a priori on the basis of clinical relevance.
Available data on risk factor prevalence (smoking and LDL‐C ≥70 mg/dL) during follow‐up were summarized by time after CAD diagnosis (0–1, 1–3, 3–5, and 5–10 years). Within each interval, the number and percentage of patients with smoking status or with LDL‐C ≥70 mg/dL was summarized. We stratified the description of ongoing smoking according to baseline status (overall, never, former, or current) and LDL‐C elevation by whether baseline LDL‐C was controlled (<70 mg/dL) or not. In patients without diabetes mellitus at baseline, we estimated the cumulative incidence of patients newly diagnosed with diabetes mellitus during 10 years of follow‐up.
Descriptive statistics were calculated using observed data only. For regression models, missing data were imputed using a multiple imputation approach (n=25). No adjustment was made for multiple comparisons. All statistical analyses were performed using SAS software, Version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Baseline Characteristics
Of 101 061 patients who presented to the Duke University Adult Cardiac Catheterization Laboratory between 1995 and 2013, 3655 (3.6%) aged <50 years had a first manifestation of obstructive CAD (Figure S1 shows study flowchart). The median age of these individuals was 45 years (interquartile range, 41–47 years), 27.5% of patients were women, 26.0% were Black individuals, and 6.5% presented before 35 years of age (Table 1). The main presentations were ST‐segment–elevation MI (38.6%) and non–ST‐segment–elevation MI (36.0%). Most patients had single‐vessel obstructive CAD (59.6%). Of interest, 9.5% of patients had kidney impairment with an estimated glomerular filtration rate <60 mL/min, including 2.5% who required dialysis at baseline. Few patients had a history of HIV, connective tissue disease, or malignancy.
Table 1.
Baseline Characteristics, Stratified by Age Groups
| Characteristic | Overall (N=3655) | Those Aged ≤35 y (N=239) | Those Aged >35–≤45 y (N=1772) | Those Aged >45–<50 y (N=1644) | P Value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, median (IQR), y | 45 (41–47) | 33 (30–34) | 42 (40–44) | 48 (47–49) | ||
| Women | 1005 (27.5) | 55 (23.0) | 492 (27.8) | 458 (27.9) | 0.2749 | |
| BMI, median (IQR), kg/m2 | 30 (26–34) | 30 (27–34) | 30 (26–34) | 29 (26–33) | 0.0339 | |
| BMI >30 kg/m2 | 1714 (47.1) | 125 (53.2) | 866 (49.1) | 723 (44.2) | 0.0026 | |
| Race/Ethnicity | 0.2989 | |||||
| White | 2376 (67.0) | 147 (63.9) | 1128 (65.6) | 1101 (68.9) | ||
| Black | 921 (26.0) | 63 (27.4) | 460 (26.7) | 398 (24.9) | ||
| Native American | 160 (4.5) | 13 (5.7) | 87 (5.1) | 60 (3.8) | ||
| Other (eg, Hispanics, Pacific Islander) | 90 (2.5) | 7 (3.0) | 45 (2.6) | 38 (2.4) | ||
| Index presentation | 0.0016 | |||||
| STEMI | 1410 (38.6) | 120 (50.2) | 687 (38.8) | 603 (36.7) | ||
| NSTEMI or unstable angina | 1315 (36.0) | 76 (31.8) | 636 (35.9) | 603 (36.7) | ||
| Other | 930 (25.4) | 43 (18.0) | 449 (25.3) | 438 (26.6) | ||
| Risk factors, comorbidity, and clinical history | ||||||
| Smoking status | 0.0005 | |||||
| Never | 1434 (39.2) | 103 (43.1) | 699 (39.4) | 632 (38.4) | ||
| Former | 417 (11.4) | 20 (8.4) | 168 (9.5) | 229 (13.9) | ||
| Current | 1804 (49.4) | 116 (48.5) | 905 (51.1) | 783 (47.6) | ||
| Hypertension | 1930 (52.8) | 90 (37.7) | 920 (51.9) | 920 (56.0) | <0.0001 | |
| Diabetes mellitus | 871 (23.8) | 43 (18.0) | 415 (23.4) | 413 (25.1) | 0.0459 | |
| Family history of coronary artery disease | 1456 (39.8) | 90 (37.7) | 719 (40.6) | 647 (39.4) | 0.5956 | |
| Hyperlipidemia | 1696 (46.4) | 79 (33.1) | 850 (48.0) | 767 (46.7) | <0.0001 | |
| No. of cardiovascular risk factors | ||||||
| 0 | 344 (9.4) | 37 (15.5) | 167 (9.4) | 140 (8.5) | 0.0026 | |
| At least 1 | 3311 (90.6) | 202 (84.5) | 1605 (90.6) | 1504 (91.5) | 0.0026 | |
| At least 2 | 2657 (72.7) | 145 (60.7) | 1299 (73.3) | 1213 (73.8) | <0.0001 | |
| ≥3 | 1564 (42.8) | 71 (29.7) | 771 (43.5) | 722 (43.9) | 0.0001 | |
| Comorbidities | ||||||
| Chronic obstructive pulmonary disease | 97 (2.7) | 4 (1.7) | 37 (2.1) | 56 (3.4) | 0.0353 | |
| Peripheral vascular disease | 132 (3.6) | 3 (1.3) | 60 (3.4) | 69 (4.2) | 0.0581 | |
| Cerebrovascular disease | 121 (3.3) | 4 (1.7) | 55 (3.1) | 62 (3.8) | 0.1894 | |
| Connective tissue disease | 24 (0.7) | 3 (1.3) | 10 (0.6) | 11 (0.7) | 0.4611 | |
| Kidney disease | 162 (4.4) | 8 (3.3) | 86 (4.9) | 68 (4.1) | 0.4177 | |
| Dialysis | 93 (2.5) | 5 (2.1) | 53 (3.0) | 35 (2.1) | 0.2508 | |
| Liver disease | 26 (0.7) | 0 (0.0) | 13 (0.7) | 13 (0.8) | 0.3923 | |
| HIV/AIDS | 29 (0.8) | 0 (0.0) | 19 (1.1) | 10 (0.6) | 0.1121 | |
| History of cancer | 45 (1.2) | 1 (0.4) | 17 (1.0) | 27 (1.6) | 0.0973 | |
| History of ETOH | 192 (5.3) | 16 (6.7) | 104 (5.9) | 72 (4.4) | 0.0876 | |
| Chronic inflammatory disease | 85 (2.3) | 4 (1.7) | 38 (2.1) | 43 (2.6) | 0.5190 | |
| Laboratory results | ||||||
| LDL‐C, median (IQR), mg/dL | 117 (92–145) | 121 (95–158) | 117 (90–145) | 117 (92–143) | 0.0538 | |
| LDL‐C 70–189 mg/dL | 2642 (84.1) | 171 (79.9) | 1267 (84.1) | 1204 (84.7) | 0.2070 | |
| LDL‐C ≥190 mg/dL | 171 (5.4) | 24 (11.2) | 80 (5.3) | 67 (4.7) | 0.0005 | |
| HDL‐C, median (IQR), mg/dL | 37 (31–44) | 37 (30–45) | 36 (30–44) | 37 (31–45) | 0.0358 | |
| Triglycerides, median (IQR), mg/dL | 141 (91–218) | 136 (91–207) | 141 (91–223) | 141 (90–214) | 0.6175 | |
| Triglycerides ≥175 mg/dL | 1166 (35.8) | 69 (31.5) | 572 (36.6) | 525 (35.6) | 0.3245 | |
| Creatinine, median (IQR), μmol/L | 1 (0.80–1.10) | 1 (0.80–1.10) | 1 (0.80–1.10) | 1 (0.80–1.10) | 0.2188 | |
| eGFR, median (IQR), mL/min | 93 (79.5–107.1) | 101 (87.1–116.6) | 95 (81.2–108.7) | 89 (78.1–103.2) | <0.0001 | |
| eGFR <60 mL/min | 342 (9.5) | 16 (6.8) | 152 (8.7) | 174 (10.8) | 0.0396 | |
| Genetic testing for HeFH (n=632) | ||||||
|
Patient had exome data in CATHGEN |
632 (17.3) | 39 (16.3) | 311 (17.6) | 282 (17.2) | 0.8765 | |
| APOB pathogenic, likely pathogenic mutation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| LDLR pathogenic, likely pathogenic mutation | 4 (0.6) | 2 (5.1) | 2 (0.6) | 0 (0.0) | 0.0107 | |
| PCSK9 pathogenic, likely pathogenic mutation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Angiographic findings | ||||||
| No. of diseased vessels | <0.0001 | |||||
| 1 | 2180 (59.6) | 171 (71.5) | 1084 (61.2) | 925 (56.3) | ||
| 2 | 882 (24.1) | 43 (18.0) | 422 (23.8) | 417 (25.4) | ||
| 3 | 593 (16.2) | 25 (10.5) | 266 (15.0) | 302 (18.4) | ||
| Left main ≥50% | 191 (5.2) | 9 (3.8) | 81 (4.6) | 101 (6.1) | 0.0686 | |
| Left anterior descending ≥70% | 2120 (58.0) | 133 (55.6) | 1038 (58.6) | 949 (57.7) | 0.6582 | |
| Left circumflex ≥70% | 1473 (40.3) | 81 (33.9) | 702 (39.6) | 690 (42.0) | 0.0422 | |
| Right coronary artery ≥70% | 1734 (47.4) | 87 (36.4) | 807 (45.5) | 840 (51.1) | <0.0001 | |
| Subsequent treatment, within 30 d of catheterization | ||||||
| PCI | 2049 (56.1) | 135 (56.5) | 1003 (56.6) | 911 (55.4) | 0.7756 | |
| CABG | 429 (11.7) | 28 (11.7) | 195 (11.0) | 206 (12.5) | 0.3835 | |
| PCI and CABG | 34 (0.9) | 1 (0.4) | 20 (1.1) | 13 (0.8) | 0.4099 | |
| Medical treatment only | 1143 (31.3) | 75 (31.4) | 554 (31.3) | 514 (31.3) | 0.9993 | |
| Medications, within 30 d of catheterization | ||||||
| Statin | 2577 (70.5) | 168 (70.3) | 1257 (70.9) | 1152 (70.1) | 0.8557 | |
| Aspirin | 3455 (94.5) | 230 (96.2) | 1672 (94.4) | 1553 (94.5) | 0.4823 | |
| Blood pressure medications | 3432 (93.9) | 224 (93.7) | 1660 (93.7) | 1548 (94.2) | 0.8360 | |
| P2Y12 inhibitor medications | 2484 (68.0) | 176 (73.6) | 1220 (68.8) | 1088 (66.2) | 0.0373 | |
| β Blockers | 3191 (87.3) | 207 (86.6) | 1559 (88.0) | 1425 (86.7) | 0.4933 | |
| ACE inhibitors/ARBs | 2498 (68.3) | 173 (72.4) | 1203 (67.9) | 1122 (68.2) | 0.3715 | |
| Calcium channel blockers | 652 (17.8) | 34 (14.2) | 299 (16.9) | 319 (19.4) | 0.0497 | |
| Diuretics | 1874 (51.3) | 126 (52.7) | 891 (50.3) | 857 (52.1) | 0.5019 | |
Data are given as number (percentage), unless otherwise indicated. ACE indicates angiotensin‐converting enzyme; APOB, apolipoprotein B; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CATHGEN, Catheterization Genetics; eGFR, estimated glomerular filtration rate; ETOH, ethyl alcohol consumption; HDL‐C, high‐density lipoprotein cholesterol; HeFH, heterozygous familial hypercholesterolemia; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; LDLR, low‐density lipoprotein receptor; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; PCSK9, proprotein convertase subtilisin/kexin type 9; and STEMI, ST‐segment–elevation myocardial infarction.
Traditional Cardiovascular Risk Factors
Approximately 73% of individuals had at least 2 cardiovascular risk factors and 43% had ≥3 (Table 1). Active or prior smoking was the most frequent risk factor (60.8%), followed by hypertension (52.8%), family history of CAD (39.8%), and diabetes mellitus (23.8%). Obesity (body mass index >30 kg/m2) was prevalent in 47.1% of this premature CAD population. Within 12 months of the index procedure, the median LDL‐C was 117 (interquartile range, 92–145) mg/dL, the median HDL‐C was 37 (interquartile range, 31–34) mg/dL, and the median triglycerides level was 141 (interquartile range, 91–218) mg/L. Of note, the baseline LDL‐C was >190 mg/dL in 5.4% of patients and triglycerides ≥175 mg/dL in 35.8% of patients. When comparing age categories, individuals who presented very prematurely (≤35 years) were more frequently obese compared with the population between 35 and 45 years and >45 years (53.2% versus 49.1% versus 44.2%; P<0.01), and these individuals had a greater prevalence of LDL‐C levels >190 mg/dL (11.2% versus 5.3% versus 4.7%; P<0.01). The trend in cardiovascular risk factors at baseline is displayed in Table S1: between 1995 and 2013, the rate of smokers and median LDL‐C decreased among young patients with a first diagnosis of obstructive CAD. In contrast, the rate of diabetes mellitus and hypertension among young adults increased.
Prevalence of HeFH
To ensure that premature CAD in this population was not caused by monogenic disease, we evaluated the prevalence of familial hypercholesterolemia in a subset of our population. Of the 3655 patients in the present study, 632 (17.3%) had whole exome sequence data available. Only 4 of these patients harbored pathogenic or likely pathogenic mutations, all within LDLR.
First Recurrent Ischemic Event
By 10 years, 52.9% of patients had developed at least one recurrent ischemic event, defined by the composite of all‐cause death, recurrent MI, revascularization, or stroke. After premature CAD onset, the most frequent first events were follow‐up revascularization PCI or CABG (15.3% and 6.3%, respectively), recurrent MI (14.4%), or death (13.4%). Stroke was the first event in 3.5% of patients (Figure 1).
Figure 1. Time to first subsequent major adverse cardiovascular event within 10 years after premature coronary artery disease diagnosis.
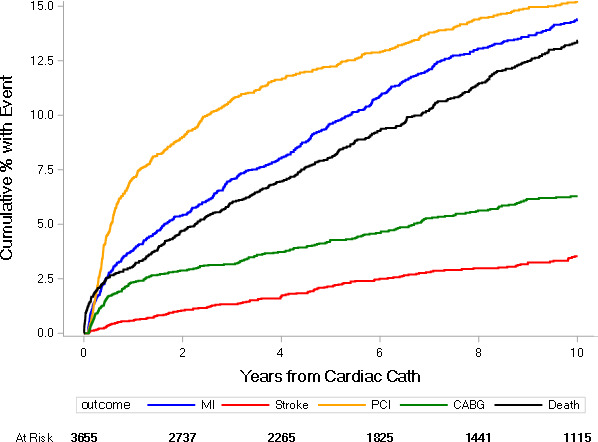
CABG indicates coronary artery bypass grafting; Cath, catheterization; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
Multiple Recurrent Ischemic Events
The time to first, second, and third event is displayed in Figure 2. By 10 years, 18.6% of patients had experienced a second event, and 7.9% had experienced a third event. The all‐cause mortality rate was 20.9% at 10 years. Overall, the present population experienced MACEs at a rate of 10.0 (95% CI, 9.6–10.4) events per 100 patient‐years, with nonfatal MI and death occurring at respective rates of 2.7 (95% CI, 2.5–2.9) and 2.3 (95% CI, 2.2–2.5) per 100 patient‐years. Women and men had respective rates of MACEs of 12.2 (95% CI, 11.4–13.0) and 9.2 (95% CI, 8.8–9.6) per 100 patient‐years.
Figure 2. Time to first, second, or third major adverse cardiovascular event within 10 years after premature coronary artery disease diagnosis.
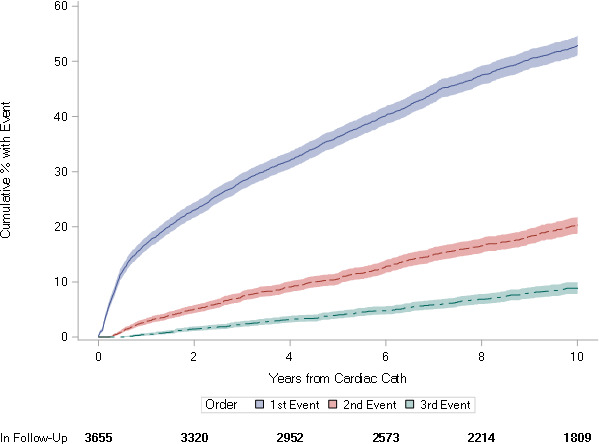
Cath indicates catheterization.
Factors Associated With Time to First Recurrent Ischemic Event
The factors associated with subsequent events following premature CAD onset are displayed in Table 2. Women with premature CAD were at a higher risk of a subsequent ischemic event compared with men (adjusted HR, 1.15; 95% CI, 1.03–1.28; P=0.01). Among traditional risk factors, baseline diabetes mellitus was the only variable independently associated with a first ischemic recurrence. Although infrequently observed, chronic inflammatory disease was strongly associated with a first ischemic recurrence (adjusted HR, 1.61; 95% CI, 1.23–2.11; P<0.001). Baseline chronic kidney disease (estimated glomerular filtration rate <60 mL/min), multivessel disease, and absence of revascularization after a first event were also associated with poor outcomes.
Table 2.
Factors Associated With Time to First Ischemic Recurrence
| Covariate | Univariable | Univariable P Value | Multivariable | Multivariable P Value |
|---|---|---|---|---|
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | |||
| Age (per 10‐y increase) | 1.06 (0.96–1.16) | 0.282 | 1.03 (0.93–1.14) | 0.565 |
| Sex (women vs men) | 1.32 (1.20–1.47) | <0.001 | 1.15 (1.03–1.28) | 0.010 |
| BMI ≥27 kg/m2 (per 5‐kg/m2 increase) | 1.06 (1.02–1.10) | 0.002 | 1.04 (1.00–1.09) | 0.052 |
| Racial/Ethnic group (White is the reference) | <0.001 | 0.054 | ||
| Black | 1.29 (1.16–1.44) | 1.10 (0.98–1.23) | ||
| Native American and other (eg, Hispanic, Pacific Islander) | 1.16 (0.97–1.39) | 1.16 (0.96–1.39) | ||
| Chronic inflammation | 1.69 (1.29–2.20) | <0.001 | 1.61 (1.23–2.11) | <0.001 |
| Family history of coronary disease | 1.04 (0.95–1.15) | 0.364 | 1.06 (0.96–1.17) | 0.213 |
| Admission with ACS vs no ACS | 0.83 (0.75–0.93) | <0.001 | 1.10 (0.99–1.23) | 0.080 |
| Current/former smoker | 0.96 (0.87–1.06) | 0.431 | 1.03 (0.93–1.13) | 0.627 |
| History of hypertension | 1.27 (1.15–1.39) | <0.001 | 1.03 (0.93–1.14) | 0.521 |
| Diabetes mellitus | 1.68 (1.52–1.86) | <0.001 | 1.35 (1.21–1.51) | <0.001 |
| eGFR per 20 mL/min per 1.73 m2 decrease | 1.34 (1.28–1.40) | <0.001 | 1.19 (1.13–1.25) | <0.001 |
| LDL‐C ≥120 mg/dL per 20‐mg/dL increase | 0.97 (0.93–1.02) | 0.280 | 1.02 (0.96–1.08) | 0.523 |
| Triglycerides per 50‐mg/dL increase | 1.01 (0.99–1.04) | 0.237 | 1.00 (0.98–1.03) | 0.909 |
| Multivessel disease | 1.39 (1.27–1.53) | <0.001 | 1.47 (1.33–1.63) | <0.001 |
| Statin at discharge | 0.80 (0.72–0.88) | <0.001 | 1.01 (0.91–1.13) | 0.823 |
| Subsequent treatment within 30 d | <0.001 | <0.001 | ||
| PCI vs medical treatment | 0.41 (0.37–0.45) | 0.45 (0.41–0.50) | ||
| CABG vs medical treatment | 0.29 (0.24–0.34) | 0.25 (0.21–0.30) |
ACS indicates acute coronary syndrome; BMI, body mass index; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; LDL‐C, low‐density lipoprotein cholesterol; and PCI, percutaneous coronary intervention.
Factors Associated With the Rate of Multiple Recurrent Events
The factors associated with the rate of multiple recurrent events are displayed in Table 3. Baseline diabetes mellitus, kidney impairment, and multivessel disease were associated with increased rates of multiple ischemic events. The absence of revascularization at the time of index diagnosis was also associated with the occurrence of multiple ischemic events.
Table 3.
Factors Associated With Multiple Ischemic Recurrences
| Covariate | Univariable | Univariable P Value | Multivariable | Multivariable P Value |
|---|---|---|---|---|
| Rate Ratio (95% CI) | Rate Ratio (95% CI) | |||
| Age (per 10‐y increase) | 0.95 (0.65–1.41) | 0.817 | 0.88 (0.62–1.25) | 0.470 |
| Sex (women vs men) | 1.45 (0.96–2.17) | 0.075 | 1.13 (0.77–1.65) | 0.535 |
| BMI ≥27 kg/m2 (per 5‐kg/m2 increase) | 1.07 (0.91–1.25) | 0.410 | 1.05 (0.90–1.22) | 0.531 |
| Racial/Ethnic group (White is the reference) | 0.175 | 0.512 | ||
| Black | 1.51 (0.98–2.34) | 1.27 (0.85–1.90) | ||
| Native American and other (eg, Hispanic, Pacific Islander) | 1.09 (0.52–2.30) | 1.09 (0.56–2.11) | ||
| Chronic inflammation | 2.05 (0.59–7.10) | 0.255 | 1.87 (0.63–5.52) | 0.259 |
| Family history of coronary disease | 0.98 (0.67–1.43) | 0.904 | 1.05 (0.74–1.48) | 0.803 |
| Admission with ACS vs no ACS | 0.82 (0.53–1.26) | 0.365 | 1.09 (0.73–1.62) | 0.673 |
| Current/former smoker | 0.90 (0.62–1.33) | 0.610 | 1.04 (0.72–1.49) | 0.842 |
| History of hypertension | 1.47 (1.01–2.14) | 0.044 | 1.11 (0.77–1.58) | 0.581 |
| Diabetes mellitus | 1.99 (1.29–3.06) | 0.002 | 1.47 (0.99–2.20) | 0.058 |
| eGFR per 20 mL/min per 1.73 m2 decrease | 1.48 (1.24–1.77) | <0.001 | 1.29 (1.06–1.55) | 0.010 |
| LDL‐C ≥120 mg/dL per 20‐mg/dL increase | 0.92 (0.77–1.10) | 0.375 | 0.96 (0.80–1.16) | 0.677 |
| Triglycerides per 50‐mg/dL increase | 1.01 (0.92–1.10) | 0.875 | 1.00 (0.92–1.09) | 0.969 |
| Multivessel disease | 1.50 (1.03–2.17) | 0.034 | 1.44 (1.00–2.06) | 0.049 |
| Statin at discharge | 0.84 (0.57–1.23) | 0.365 | 1.01 (0.70–1.48) | 0.943 |
| Subsequent treatment within 30 d | <0.001 | <0.001 | ||
| PCI vs medical treatment | 0.42 (0.28–0.61) | 0.48 (0.33–0.69) | ||
| CABG vs medical treatment | 0.30 (0.14–0.61) | 0.27 (0.14–0.52) |
ACS indicates acute coronary syndrome; BMI, body mass index; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; LDL‐C, low‐density lipoprotein cholesterol; and PCI, percutaneous coronary intervention.
Evolution of Cardiovascular Risk Factors After Diagnosis of Obstructive CAD
Across different time points of follow‐up, 31.7% to 37.2% of patients continued smoking after premature CAD onset (Figure 3). Although most of these ongoing smokers were active smokers at baseline, >10% of patients who had never smoked before reported smoking during each interval assessed. LDL‐C was rarely within latest guideline recommendation in this young population, with 89.3% of patients having LDL‐C ≥70 mg/dL during the first year after CAD diagnosis and 84.3% having at least one LDL‐C value ≥70 mg/dL between 5 and 10 years after diagnosis. Only half of patients had a level of LDL‐C within 100 mg/dL along follow‐up (Figure S2 and Table S2). Eventually, the cumulative incidence of new‐onset diabetes mellitus in patients without history of diabetes mellitus at baseline was 16% (95% CI, 15%−18%) within 10 years after premature CAD onset (Figure 4).
Figure 3. Smoking continuation after premature coronary artery disease (CAD) onset, stratified by baseline smoking status.

Figure 4. Cumulative rate of new‐onset diabetes mellitus after premature coronary artery disease diagnosis.

Cath indicates catheterization.
DISCUSSION
In this analysis of DDCD, there were multiple important findings. First, we observed that young patients with premature obstructive CAD often had numerous modifiable traditional cardiovascular risk factors. Second, premature CAD is a chronic evolving disease with half of patients experiencing a substantial evolution of coronary atherosclerosis within 10 years, and 1 of 5 patients dying prematurely. Women, Black and Hispanic patients, and patients with diabetes mellitus or chronic inflammatory disease were at high risk of ischemic recurrences. Modification of cardiovascular risk factors over time was also poor in these young individuals as the rate of smoking remained high and LDL‐C levels were above goal at follow‐up.
Our study showed that smoking was the most common risk factor among patients with premature CAD. Prior literature has shown that the accumulation of active smoking with an additional cardiovascular risk factor is frequent, and contributes to aggressive atherosclerosis and premature death. 13 Nearly half of the study population was obese, compared with 10% to 30% in the US population reported between 2007 and 2014, suggesting the contribution of obesity as a risk enhancer for early atherosclerosis. 14 Obesity predisposes patients to a metabolic syndrome, such as insulin resistance and hypertriglyceridemia, which is associated with increased cardiovascular morbidity and mortality beyond traditional risk factors in middle‐aged individuals. 15 , 16 , 17 Of importance, the rate of diabetes mellitus in this young population with CAD was also elevated, and as frequent as what is usually observed in an average population with CAD in the United States. This comes in contrast with findings from a prospective cohort of French individuals with premature CAD, for whom obesity and diabetes mellitus were rare, but active smoking and high LDL‐C levels were much more frequent. 5 Overall, the present study supports the notion that there are several modifiable risk factors in individuals with premature CAD. Furthermore, our findings emphasize the importance of early intervention through either pharmacologic treatments to aid in smoking cessation and reducing LDL‐C or lifestyle modifications for weight loss and a healthy diet. 18 , 19 , 20
Conversely, nonmodifiable risk factors were not as apparent in our findings compared with modifiable ones, as genetic mutations related to HeFH were found in 1% of the subset of tested individuals. There were 5% of individuals with baseline LDL‐C >190 mg/dL; this proportion increased to 11% in patients who presented with CAD before the age of 35 years. Previous reports have shown a proportion of up to 20% of HeFH among young adults with MIs. 6 One explanation could be that only 17% of our study population had genetic testing, and complete family history information was not readily available to calculate a Dutch Lipid Score. Finally, chronic diseases responsible for clinical or subclinical inflammation, such as HIV, cancer, or connective tissue diseases, are present in a small proportion of patients, but significantly contribute to poor outcomes. Such findings highlight the potential benefits of innovative anti‐inflammatory therapies to prevent cardiovascular events in certain subgroups of patients with premature CAD. 21 , 22
Of 5 patients with premature CAD, 1 died within 10 years of follow‐up. These young individuals also had multiple recurrences of events, especially atherosclerotic progression leading to stable angina or recurrent MIs. With almost 10 events per 100 patient‐years, including recurrent MI or death, patients with premature CAD display worse outcomes than those reported in patients >50 years of age, even including higher‐risk groups. 23 , 24 Such a pejorative prognosis highlights the need for more aggressive primary and secondary prevention, with appropriate smoking cessation strategies and low LDL‐C target goals in young patients at risk. Of importance, more than half of patients continued smoking despite early CAD; this observation is consistent with the findings of the Partners YOUNG‐MI registry, which displayed a clear association with subsequent mortality. 25 This also demonstrates the need for specific trials to test innovative strategies, like early PCSK9 inhibitors, as soon as premature CAD is diagnosed, for these individuals with a life‐long atherosclerotic burden. Attention should also be given to risk enhancers, such as biomarkers like lipoprotein (a), which are hereditary and can also lead to premature cardiovascular disease. 26 , 27 Extended dual antiplatelet therapy duration is another path requiring evaluation, in this population with a low bleeding risk with early recurrent ischemic events. The other unmet need is a specific consideration of these individuals in scientific guidelines, to provide a specific cardiovascular risk assessment and to guide intensive therapies for which they are currently rarely eligible. 28 , 29 , 30 In a recent evaluation of the recent 2018 American College of Cardiology/American Heart Association blood cholesterol guidelines in DDCD, we found that <50% of patients admitted for a first MI before the age of 55 years would have been eligible for primary prevention statins before this event, in comparison with 75% to 85% of older patients with MI. 31 The major explanation is the important contribution of age in the risk stratification by the 10‐year atherosclerotic cardiovascular disease risk score. 32
Our findings also show that women with premature CAD have higher rates of ischemic recurrences, including all‐cause death and MI, when compared with men with premature CAD. These sex‐specific findings for prognosis have been reported across a broad spectrum of cardiovascular diseases, and have been mostly associated with less‐than‐optimal secondary prevention and absence of intensive therapy. 33 , 34 , 35 The failure to provide adequate prevention strategies in women has been attributed to the misconception that they do not exhibit classic signs of atherothrombosis. 36 The present study demonstrates that the proportion of women with premature atherosclerotic CAD is high, as they represent >1 of 4 patients of the cohort. Black and Hispanic individuals also display a higher risk of recurrences than White individuals. Socioeconomic factors, absence of tailored secondary prevention, and less access to health care and subsequent prevention strategies have been reported as factors contributing to disparate outcomes, according to differences in sex, race, and ethnicity. 37
The control of cardiovascular risk factors was suboptimal during follow‐up in our study, in a population for whom life expectancy in the United States should exceed 30 years. Although great advancements have been made in the contribution of genetic polymorphisms and gene scores to risk stratify early onset of CAD and its poor prognosis, 38 , 39 the present observations display a clear need to improve strategies aimed at modifiable cardiovascular risk factors. This effort is paramount, as genetic predispositions and environmental factors frequently overlap and carry a combined high‐risk burden on cardiovascular outcomes. 40 The efforts to promote a healthy lifestyle in young patients can only be successful via multimodal and multifactorial interventions involving long‐term social, psychological, and medical assessment. New strategies are needed to promote primary and secondary prevention in this young and active population.
Limitations
We acknowledge several limits of this observational study. This is a single‐center study with a population referred to a quaternary care hospital. We included subsequent PCI or CABG as a clinical end point; although the performance of PCI and CABG can be subjective, these are meaningful events related to the progression of atherosclerosis requiring interventional treatments, and are important to the broad patient population. The events that occurred outside of Duke hospital were patient reported, suggesting a possible underestimation of the rate of events. There was only a small proportion of patients for whom genetic data were reported. The important difference between the proportion of mutations and HeFH according to Dutch Lipid Score warrants further investigations. The associations displayed in the multivariable models should be interpreted as hypothesis generating, because of the potential for confounding and unmeasured bias. For instance, the compliance to medication, blood pressure control, recovery of left ventricular fraction, and possible social and psychological bias were not measured or collected for the model. 41 , 42 Furthermore, the model could not evaluate how risk factor changes during follow‐up might relate to outcomes, because of the heterogeneity in the collection and completeness of data in time.
CONCLUSIONS
Premature CAD is an aggressive disease with a high rate of ischemic recurrences and premature death. Younger individuals have a high proportion of modifiable cardiovascular risk factors, with suboptimal control of smoking and LDL‐C cholesterol over time being frequent. This highlights the need to implement early, multimodal, and innovative prevention strategies for younger patients in both primary and secondary prevention.
Sources of Funding
Analysis was independently funded by Dr Jones. The Duke Cardiovascular Databank was funded by the Duke Clinical Research Institute.
Disclosures
Dr Zeitouni has received research grants from Institut Servier and Fédération Française de Cardiologie and lecture fees from Bristol Myers Squibb/Pfizer. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Figures S1–S2
(J Am Heart Assoc. 2020;9:e017712. DOI: 10.1161/JAHA.120.017712.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017712
For Sources of Funding and Disclosures, see page 13.
REFERENCES
- 1. Arora S, Stouffer GA, Kucharska‐Newton A, Qamar A, Vaduganathan M, Pandey A, Porterfield DS, Blankstein R, Rosamond WD, Bhatt DL, et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction: the ARIC Community Surveillance Study. Circulation. 2019;139:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde‐Price C, D’Onofrio G, Lichtman JH, Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mohammad AM, Jehangeer HI, Shaikhow SK. Prevalence and risk factors of premature coronary artery disease in patients undergoing coronary angiography in Kurdistan, Iraq. BMC Cardiovasc Disord. 2015;15:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharma M, Ganguly NK. Premature coronary artery disease in Indians and its associated risk factors. Vasc Health Risk Manag. 2005;1:217–225. [PMC free article] [PubMed] [Google Scholar]
- 5. Collet J‐P, Zeitouni M, Procopi N, Hulot J‐S, Silvain J, Kerneis M, Thomas D, Lattuca B, Barthelemy O, Lavie‐Badie Y, et al. Long‐term evolution of premature coronary artery disease. J Am Coll Cardiol. 2019;74:1868–1878. [DOI] [PubMed] [Google Scholar]
- 6. Singh A, Gupta A, Collins BL, Qamar A, Monda KL, Biery D, Lopez JAG, de Ferranti SD, Plutzky J, Cannon CP, et al. Familial hypercholesterolemia among young adults with myocardial infarction. J Am Coll Cardiol. 2019;73:2439–2450. [DOI] [PubMed] [Google Scholar]
- 7. Harris PJ, Harrell FE, Lee KL, Behar VS, Rosati RA. Survival in medically treated coronary artery disease. Circulation. 1979;60:1259–1269. [DOI] [PubMed] [Google Scholar]
- 8. Hirji SA, Stevens SR, Shaw LK, Campbell EC, Granger CB, Patel MR, Sketch MH, Wang TY, Ohman EM, Peterson ED, et al. Predicting risk of cardiac events among ST‐segment elevation myocardial infarction patients with conservatively managed non–infarct‐related artery coronary artery disease: an analysis of the Duke Databank for Cardiovascular Disease. Am Heart J. 2017;194:116–124. [DOI] [PubMed] [Google Scholar]
- 9. O’Connor CM, Velazquez EJ, Gardner LH, Smith PK, Newman MF, Landolfo KP, Lee KL, Califf RM, Jones RH. Comparison of coronary artery bypass grafting versus medical therapy on long‐term outcome in patients with ischemic cardiomyopathy (a 25‐year experience from the Duke Cardiovascular Disease Databank). Am J Cardiol. 2002;90:101–107. [DOI] [PubMed] [Google Scholar]
- 10. Rosati RA, McNeer JF, Starmer CF, Mittler BS, Morris JJ, Wallace AG. A new information system for medical practice. Arch Intern Med. 1975;135:1017–1024. [PubMed] [Google Scholar]
- 11. Kraus WE, Granger CB, Sketch MH, Donahue MP, Ginsburg GS, Hauser ER, Haynes C, Newby LK, Hurdle M, Dowdy ZE, et al. A guide for a cardiovascular genomics biorepository: the CATHGEN experience. J Cardiovasc Transl Res. 2015;8:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;73:25709. [Google Scholar]
- 13. Hu Y, Zong G, Liu G, Wang M, Rosner B, Pan A, Willett WC, Manson JE, Hu FB, Sun Q. Smoking cessation, weight change, type 2 diabetes, and mortality. N Engl J Med. 2018;379:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shin D, Kongpakpaisarn K, Bohra C. Trends in the prevalence of metabolic syndrome and its components in the United States 2007–2014. Int J Cardiol. 2018;259:216–219. [DOI] [PubMed] [Google Scholar]
- 15. Lakka H‐M, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA. 2002;288:2709–2716. [DOI] [PubMed] [Google Scholar]
- 16. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;56:1113–1132. [DOI] [PubMed] [Google Scholar]
- 17. Divakaran S, Singh A, Biery D, Yang J, DeFilippis EM, Collins BL, Ramsis M, Qamar A, Hainer J, Klein J, et al. Diabetes is associated with worse long‐term outcomes in young adults after myocardial infarction: the Partners YOUNG‐MI Registry. Diabetes Care. 2020;43:1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pack QR, Rodriguez‐Escudero JP, Thomas RJ, Ades PA, West CP, Somers VK, Lopez‐Jimenez F. The prognostic importance of weight loss in coronary artery disease: a systematic review and meta‐analysis. Mayo Clin Proc. 2014;89:1368–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 20. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 21. Tardif J‐C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. Efficacy and safety of low‐dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 22. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 23. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, et al. A prospective natural‐history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. [DOI] [PubMed] [Google Scholar]
- 24. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, et al. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. [DOI] [PubMed] [Google Scholar]
- 25. Biery DW, Berman AN, Singh A, Divakaran S, DeFilippis EM, Collins BL, Gupta A, Fatima A, Qamar A, Klein J, et al. Association of smoking cessation and survival among young adults with myocardial infarction in the Partners YOUNG‐MI Registry. JAMA Netw Open. 2020;3:e209649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Langsted A, Kamstrup PR, Benn M, Tybjærg‐Hansen A, Nordestgaard BG. High lipoprotein(a) as a possible cause of clinical familial hypercholesterolaemia: a prospective cohort study. Lancet Diabetes Endocrinol. 2016;4:577–587. [DOI] [PubMed] [Google Scholar]
- 27. Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A‐I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. [DOI] [PubMed] [Google Scholar]
- 28. Singh A, Collins BL, Gupta A, Fatima A, Qamar A, Biery D, Baez J, Cawley M, Klein J, Hainer J, et al. Cardiovascular risk and statin eligibility of young adults after an MI: Partners YOUNG‐MI Registry. J Am Coll Cardiol. 2018;71:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akosah KO, Schaper A, Cogbill C, Schoenfeld P. Preventing myocardial infarction in the young adult in the first place: how do the National Cholesterol Education Panel III guidelines perform? J Am Coll Cardiol. 2003;41:1475–1479. [DOI] [PubMed] [Google Scholar]
- 30. Zeitouni M, Sabouret P, Kerneis M, Silvain J, Collet J‐P, Bruckert E, Montalescot G. 2019 ESC/EAS guidelines for management of dyslipidaemia: strengths and limitations. Eur Heart J Cardiovasc Pharmacother. 2020:pvaa077. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31. Zeitouni M, Nanna MG, Sun J‐L, Chiswell K, Peterson ED, Navar AM. Performance of guideline recommendations for prevention of myocardial infarction in young adults. J Am Coll Cardiol. 2020;76:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Navar‐Boggan AM, Peterson ED, D’Agostino RB, Pencina MJ, Sniderman AD. Using age‐ and sex‐specific risk thresholds to guide statin therapy: one size may not fit all. J Am Coll Cardiol. 2015;65:1633–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the Women’s Health and Aging Study. Circulation. 2000;101:1007–1012. [DOI] [PubMed] [Google Scholar]
- 34. Bots SH, Peters SAE, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health. 2017;2:e000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chichareon P, Modolo R, Kerkmeijer L, Tomaniak M, Kogame N, Takahashi K, Chang C‐C, Komiyama H, Moccetti T, Talwar S, et al. Association of sex with outcomes in patients undergoing percutaneous coronary intervention: a subgroup analysis of the GLOBAL LEADERS randomized clinical trial. JAMA Cardiol. 2020;5:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lichtman JH, Leifheit EC, Safdar B, Bao H, Krumholz HM, Lorenze NP, Daneshvar M, Spertus JA, D’Onofrio G. Sex differences in the presentation and perception of symptoms among young patients with myocardial infarction: evidence from the VIRGO Study (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients). Circulation. 2018;137:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, et al. Socioeconomic status and cardiovascular outcomes. Circulation. 2018;137:2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Inouye M, Abraham G, Nelson CP, Wood AM, Sweeting MJ, Dudbridge F, Lai FY, Kaptoge S, Brozynska M, Wang T, et al. Genomic risk prediction of coronary artery disease in 480,000 adults. J Am Coll Cardiol. 2018;72:1883–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thériault S, Lali R, Chong M, Velianou JL, Natarajan MK, Paré G. Polygenic contribution in individuals with early‐onset coronary artery disease. Circ Genom Precis Med. 2018;11:e001849. [DOI] [PubMed] [Google Scholar]
- 40. Said MA, Verweij N, van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank Study. JAMA Cardiol. 2018;3:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. DeFilippis EM, Singh A, Divakaran S, Gupta A, Collins BL, Biery D, Qamar A, Fatima A, Ramsis M, Pipilas D, et al. Cocaine and marijuana use among young adults with myocardial infarction. J Am Coll Cardiol. 2018;71:2540–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu WY, Biery DW, Singh A, Divakaran S, Berman AN, Ayuba G, DeFilippis EM, Nasir K, Januzzi JL, Di Carli MF, et al. Recovery of left ventricular systolic function and clinical outcomes in young adults with myocardial infarction. J Am Coll Cardiol. 2020;75:2804–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S2


