The global prevalence of latent tuberculosis infection (LTBI) exceeds 1.7 billion. 1 Clinically, tuberculosis is categorized as either active or latent. Active tuberculosis is characterized by persistent infection and clinical symptoms such as chronic cough, weight loss, night sweats, and fatigue and has significant associated acute and subacute morbidity. 1 , 2 Conversely, LTBI is characterized by chronic, clinically asymptomatic infection in the setting of robust inflammatory signaling, marked by production of tumor necrosis factor‐α, interferons, and interleukins. 2 Despite clear evidence from clinical and experimental models on the role of amplified and dysregulated inflammation in the pathogenesis of cardiovascular disease (CVD) and associated risk factors, 3 , 4 data relating LTBI to CVD risk factors and overt CVD are sparse. Given the link between LTBI and proinflammatory immune dysregulation, the role of inflammation in CVD and its risk factors, and recent cross‐sectional evidence suggesting heightened myocardial infarction risk among people with LTBI, 5 we investigated associations of LTBI with major CVD risk factors: diabetes mellitus (DM) and hypertension.
Our study was exempt by institutional board review. Data were de‐identified by the Northwestern Medicine Enterprise Data Warehouse team before our access. The data that support our findings in this study are available from the corresponding author upon reasonable request.
Using the Northwestern Medicine Enterprise Data Warehouse, a comprehensive data repository on over 6.6 million patients in a large metropolitan healthcare system, adults aged 18 to 75 with LTBI and frequency‐matched on age, sex, race, and geographic region with non‐LTBI controls were identified. Patients meeting these conditions who received care (defined as at least 1 face‐to‐face encounter every 2 years between baseline and censoring date) between January 1, 2000 and January 1, 2020 were included. Of 7298 total patients, 5185 had neither hypertension nor DM at baseline and were eligible for analyses (2679 LTBI and 2506 LTBI‐free controls). LTBI was defined by both diagnosis of LTBI and either a positive tuberculin skin test and/or interferon‐γ release assay (T Spot, QuantiFERON). Comorbid conditions were identified based on International Classification of Diseases, Ninth Revision and Tenth Revision (ICD‐9 and ICD‐10) codes. Multivariable Cox regression analysis, adjusting for age, sex, and race, was performed to evaluate the incidence of DM and hypertension. A sensitivity analysis was performed with additional adjustment for baseline body mass index, total cholesterol level, and HIV serostatus. Smoking status was not widely available, precluding additional adjustment for smoking. Statistical significance was defined as P<0.05. Statistical analyses were performed using R (https://R‐project.org/) survival and mice packages.
People with LTBI had a significantly higher risk of developing hypertension (hazard ratio [HR], 2.0; 95% CI, 1.6–2.5; P<0.001) than controls without LTBI, whereas there was no significant difference in risk for DM (HR, 1.2; CI, 0.7–1.8; P=0.53) (Figure). To determine whether LTBI treatment (N=406 out of 2679 patients with LTBI were treated) was associated with any change in risk for hypertension, we performed a secondary analysis stratifying by treatment status. The risk of hypertension (versus controls without LTBI) was attenuated among patients with treated LTBI (HR, 1.4; 95% CI, 0.9–2.2; P=0.10), whereas patients with untreated LTBI had a significantly higher risk of hypertension than patients without LTBI (HR, 2.1; 95% CI, 1.6–2.7; P<0.001). Findings, including the gradient of association by LTBI treatment status, were similar in sensitivity analyses after additional adjustment for baseline body mass index, total cholesterol, and HIV serostatus: the HRs (95% CI) for incident hypertension for people with untreated and treated LTBI versus controls without LTBI were 2.3 (1.8–3.0) and 1.6 (1.0–2.5).
Figure 1. Cumulative hazards of (A) incident hypertension and (B) diabetes mellitus among patients with latent tuberculosis infection (LTBI) compared with matched patients without latent tuberculosis (control) (N=5185).
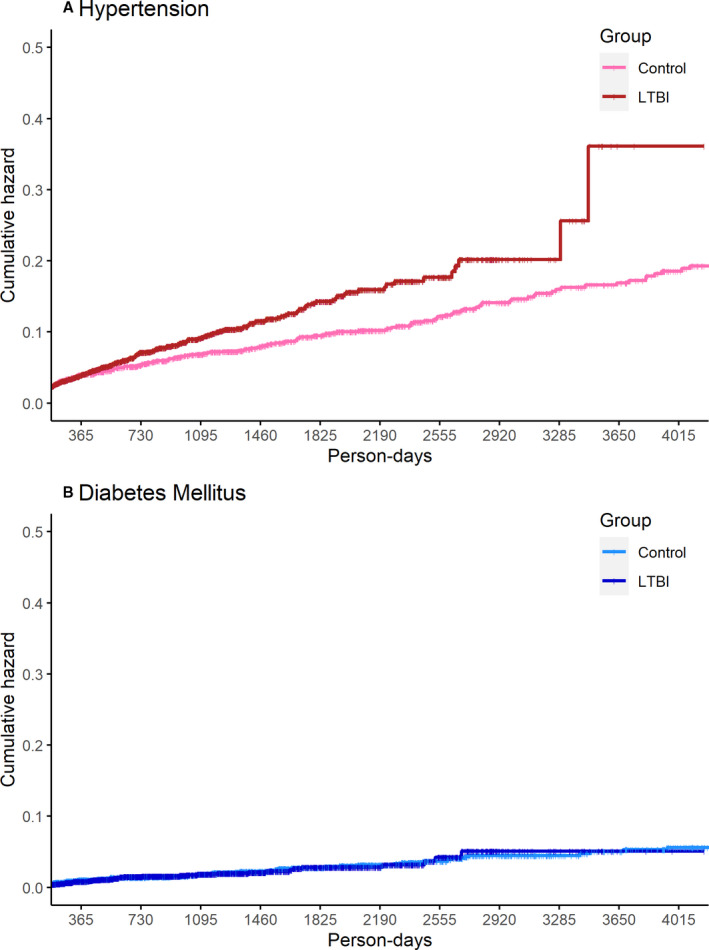
In conclusion, we observed that LTBI is associated with a significantly elevated incidence of hypertension after multivariable adjustment. DM was not associated with LTBI. We also observed in secondary analyses that treatment of LTBI was associated with lower risk for developing hypertension. One potential mechanism for this finding—as observed in other chronic infectious conditions such as HIV—related to chronic low‐level pathogen‐mediated immune activation and inflammation, with associated endothelial activation. However, the observational nature of this study precludes definitive mechanistic insights, which warrant further study. Overall, given the global prevalence of LTBI and hypertension, these observations warrant confirmation in other care settings and geographic regions and may inform future efforts to identify and treat LTBI.
Sources of Funding
This work was supported by an American Heart Association Fellow‐to‐Faculty Award (16FTF31200010; PI: Feinstein).
Disclosures
None.
(J Am Heart Assoc. 2020;9:e019144. DOI: 10.1161/JAHA.120.019144.)
For Sources of Funding and Disclosures, see page 3.
References
- 1. WHO . World Health Organization Global tuberculosis report 2019. 2019.
- 2. Singhania A, Wilkinson RJ, Rodrigue M, Haldar P, O'Garra A. The value of transcriptomics in advancing knowledge of the immune response and diagnosis in tuberculosis. Nat Immunol. 2018;19:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Miguel C, Rudemiller NP, Abais JM, Mattson DL. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep. 2015;17:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation, and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huaman MA, Ticona E, Miranda G, Kryscio RJ, Mugruza R, Aranda E, Rondan PL, Henson D, Ticona C, Sterling TR, et al. The relationship between latent tuberculosis infection and acute myocardial infarction. Clin Infect Dis. 2018;66:886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]


