Abstract
Background
Global fractional flow reserve (FFR) (ie, the sum of the FFR values in the 3 major coronary arteries) is a physiologic correlate of global atherosclerotic burden. The objective of the present study was to investigate the value of global FFR in predicting long‐term clinical outcome of patients with stable coronary artery disease but no ischemia‐inducing stenosis.
Methods and Results
We studied major adverse cardiovascular events (MACEs: all‐cause death, myocardial infarction, and any revascularization) after 5 years in 1122 patients without significant stenosis (all FFR >0.80; n=275) or with at least 1 significant stenosis successfully treated by percutaneous coronary intervention (ie, post–percutaneous coronary intervention FFR >0.80; n=847). The patients were stratified into low, mid, or high tertiles of global FFR (≤2.80, 2.80–2.88, and ≥2.88). Patients in the lowest tertile of global FFR showed the highest 5‐year MACE rate compared with those in the mid or high tertile of global FFR (27.5% versus 22.0% and 20.9%, respectively; log‐rank P=0.040). The higher 5‐year MACE rate was mainly driven by a higher rate of revascularization in the low global FFR group (16.4% versus 11.3% and 11.8%, respectively; log‐rank P=0.038). In a multivariable model, an increase in global FFR of 0.1 unit was associated with a significant reduction in the rates of MACE (hazard ratio [HR], 0.988; 95% CI, 0.977–0.998; P=0.023), myocardial infarction (HR, 0.982; 95% CI, 0.966–0.998; P=0.032), and revascularization (HR, 0.985; 95% CI, 0.972–0.999; P=0.040).
Conclusions
Even in the absence of ischemia‐producing stenoses, patients with a low global FFR, physiologic correlate of global atherosclerotic burden, present a higher risk of MACE at 5‐year follow‐up.
Keywords: coronary atherosclerosis, fractional flow reserve, percutaneous coronary intervention
Subject Categories: Coronary Artery Disease
Nonstandard Abbreviations and Acronyms
- FFR
fractional flow reserve
- MACE
major adverse cardiovascular event
Clinical Perspective
What Is New?
Even in the absence of ischemia‐producing stenoses, patients with a low global fractional flow reserve (ie, the sum of fractional flow reserve in the 3 coronary arteries) present a higher risk of major adverse cardiovascular events at 5‐year follow‐up.
What Are the Clinical Implications?
Global fractional flow reserve might be proposed as an additional metric for risk stratification of patients undergoing either computed tomography angiography or conventional angiography.
More stringent preventive measures could be proposed in patients with a low global fractional flow reserve even in the absence of overt reversible myocardial ischemia.
Percentage diameter stenosis by conventional angiography 1 , 2 (with a usual cutoff of 50%) remains the cornerstone of the definition of significant coronary artery disease. 3 In addition to a marked inaccuracy, diameter stenosis on angiography is limited to gauge the impact of diffuse atherosclerosis. Fractional flow reserve (FFR) is often proposed as a natural complement of angiography. For the sake of clinical decision making, FFR is frequently dichotomized, even though it has been shown that a continuum exists between the actual value of FFR and clinical outcomes. 4
Yet, coronary events still occur in patients with FFR values >0.80, usually, and incorrectly, referred to as “negative.” In these patients, the sum of the FFR values in the 3 major epicardial arteries (ie, global FFR) by reflecting hyperemic epicardial conductance to the whole myocardium can be considered a hemodynamic correlate of the global coronary atherosclerotic burden.
The present study aims at investigating whether, beyond the absence of hemodynamically significant lesions, the global value of FFR in the 3 major epicardial coronary arteries correlates with clinical outcome.
METHODS
Anonymized data will be made available by the corresponding author for reasonable requests.
Patient Population
The present study population consists of patients included in the prospective controlled randomized FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) 1 (NCT00267774) and FAME 2 (NCT01132495) trials. 5 , 6 , 7 , 8 , 9 In brief, in the FAME 1 trial, 1005 patients with multivessel coronary artery disease in Europe and in the United States were randomly assigned to undergo percutaneous coronary intervention (PCI) guided by angiography alone (with PCI of all lesions identified before randomization) or guided by fractional flow reserve (FFR) (with PCI of lesions with an FFR ≤0.80). In the FAME 2 trial, 1220 patients with chronic coronary syndromes were randomized to receive medical therapy only or to undergo FFR‐guided PCI plus medical therapy when at least 1 stenosis had an FFR ≤0.80. When all stenoses had an FFR >0.80, patients were not randomized, but followed in a registry. Both trials were approved by the institutional review board at the participating centers, and all patients provided written informed consent. In patients in whom PCI was performed, the post‐PCI FFR value was used for the calculation of the global FFR.
In the present study, patients from the FFR group of FAME 1 trial, patients from the FFR‐guided PCI group of FAME 2 trial, and patients from the registry group of FAME 2 trial were considered for inclusion. Patients were included in the present analysis only if the FFR values in all 3 major coronary arteries were >0.80.
Physiologic and Angiographic Measurements
Angiography and pressure measurements were obtained through 6 Fr guide catheters. After administration of isosorbide dinitrate a pressure monitoring guide wire (PressureWire Certus or PressureWire Aeris; Abbott Vascular, St. Paul, MN) was advanced in the distal part of the artery. Hyperemic proximal aortic pressure and distal coronary arterial pressure were obtained and FFR was calculated by the mean of distal coronary arterial pressure/proximal aortic pressure during hyperemia.
Global FFR consisted of the sum of the FFR values measured in the 3 major coronary arteries. Accordingly, for the present analysis, global FFR ranged from 2.43 to 3.0. When a PCI was performed, the post‐PCI value of the stented artery was used to calculate the global FFR. If the post PCI‐FFR value was not available, an FFR value of 0.90 was used (it was the case in 841 patients). This value was chosen as being the median value of all post‐PCI FFR values obtained in FAME 1 and FAME 2 trials. 10 When the vessel was angiographically “normal,” an FFR value of 1 was imputed (it was the case in 206 patients).
The angiographic extent of atherosclerosis was expressed as the number of lesions with a diameter stenosis of >50% by visual estimate.
Outcomes
The primary outcome of the present study was a composite of major adverse cardiovascular events (MACEs), including overall death, myocardial infarction (MI), and any revascularization. The events were adjudicated by an independent clinical event committee blinded to the treatment allocation and to the global FFR values. Follow‐up was censored at the time of the first event or, at the latest, 5 years after patient's enrollment in the studies.
Statistical Analysis
Continuous variables are expressed as mean±SD or median (Percentile 25–Percentile 75) as appropriate, whereas categorical variables are reported as frequencies and percentages. Student t test was used to compare normally distributed continuous variables as appropriate, whereas Kruskal‐Wallis test was used to compare nonnormally distributed continuous variables. Comparisons between categorical variables were evaluated using the Pearson χ2 test. The global FFR, corresponding to the sum of FFR in the 3 major epicardial vessels, was calculated. Patients were then classified into 3 groups of global FFR according to tertiles of global FFR. Characteristics of the lesions between the groups were compared, and all parameters with a P≤0.15 were included in a Cox regression analysis. 11 Kaplan‐Meier curves for survival were used to compare the 3 groups of global FFR with the log‐rank test. To analyze the relationship between global FFR and outcomes, we used nonparametric regression based on Cox regression analysis used to calculate the hazard ratio (HR) and 95% CI for events for each 0.1 absolute value of global FFR. Significance was defined as a P<0.05. Statistical analysis was performed using SPSS 24.0 software (SPSS Inc, Chicago, IL), and figures were performed with Prism 6.0h (GraphPad Software, La Jolla, CA).
RESULTS
Population
The population of this study encompassed 1122 patients, 509 from FAME 1 trial and 613 from FAME 2 trial. Among the FAME 2 trial patients, 166 belonged to the registry (no hemodynamically significant lesion after evaluation by FFR) and 447 to the FFR‐guided PCI plus medical therapy group.
Tertiles of Global FFR
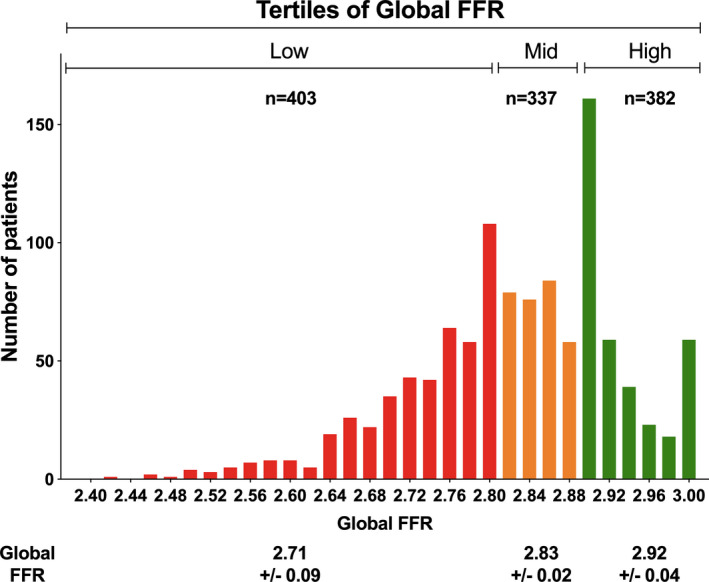
Frequency distribution of global FFR with classification into 3 tertiles is represented in Figure 1. The lowest tertile of global FFR encompassed 403 patients (35.9%) with a global FFR ≤2.80; the middle tertile of global FFR encompassed 337 patients (30.0%) with a global FFR >2.80 and ≤2.88; and the highest tertile of global FFR encompassed 382 patients (34.1%) with a global FFR >2.88.
Figure 1. Frequency distribution of global fractional flow reserve (FFR) with classification into 3 tertiles.
Clinical Characteristics
Clinical and angiographic characteristics of the patients among the 3 tertiles of global FFR are presented in Table 1. The proportion of patients with arterial hypertension was significantly higher in the high global FFR group (75.9% versus 70.3% in the mid global FFR group and 67.5% in the low global FFR group; P=0.031), and the patients in the low global FFR group had a higher rate of PCI (85.4% versus 66.8% and 72.8%, respectively; P<0.001) and a higher number of vessels with ≥50% diameter stenosis (P<0.001). All other baseline clinical and angiographic characteristics were similar.
Table 1.
Baseline and Angiographic Characteristics
| Characteristic |
Lowest Global FFR (N=403) |
Mid Global FFR (N=337) |
Highest Global FFR (N=382) |
P Value |
|---|---|---|---|---|
| Age, y | 64 (58–71) | 64 (57–70) | 64 (57–70) | 0.522 |
| Current smoker | 302 (74.9) | 263 (78) | 293 (76.7) | 0.722 |
| Any diabetes mellitus | 115 (28.5) | 83 (24.6) | 92 (24.1) | 0.301 |
| Hypertension | 272 (67.5) | 237 (70.3) | 290 (75.9) | 0.031 |
| History of MI | 247 (61.3) | 211 (62.6) | 238 (62.3) | 0.809 |
| Dyslipidemia | 304 (76) | 243 (72.1) | 274 (71.7) | 0.330 |
| Male sex | 318 (78.9) | 247 (73.3) | 286 (74.9) | 0.177 |
| Underwent PCI | 344 (85.4) | 225 (66.8) | 278 (72.8) | <0.001 |
| Vessels with ≥50% stenosis at discharge | <0.001 | |||
| 0 | 206 (51.1) | 173 (51.3) | 259 (67.8) | |
| 1 | 147 (36.5) | 150 (44.5) | 109 (28.5) | |
| 2 | 48 (11.9) | 14 (4.2) | 14 (3.5) | |
| 3 | 2 (0.5) | 0 | 0 |
Data are given as median (Percentile 25–Percentile 75) or number (percentage). FFR indicates fractional flow reserve; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
Clinical Outcomes and Global FFR Value
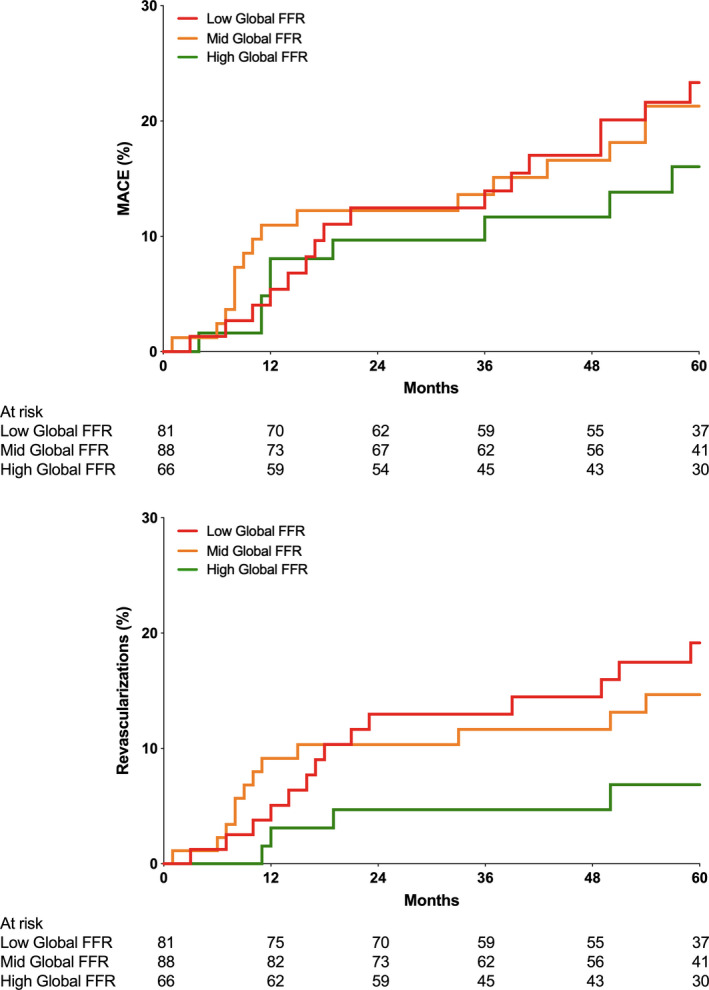
Patients in the lowest tertile of global FFR showed the highest 5‐year MACE rate compared with those in the highest tertile of global FFR (27.5% versus 22.0% and 20.9% in the 2 other tertiles, respectively; log‐rank P=0.040; Table 2 and Figure 2A). The higher 5‐year MACE rate was mainly driven by a higher rate of revascularization (Figure 2B) in the lowest global FFR group (16.4% versus 11.3% and 11.8%, respectively; log‐rank P=0.038). The rate of all‐cause mortality and the rate of MI were comparable between tertiles. The same findings were also observed among patients in whom FFR was measured in the 3 vessels (Figure 3) and among patients without any PCI (registry group of FAME 2 trial; Figure S1).
Table 2.
Outcomes per Tertile at 5 Years of Follow‐Up
| Outcome at 5 y | Low Global FFR (N=403) | Mid Global FFR (N=337) | High Global FFR (N=382) | Log‐Rank P Value |
|---|---|---|---|---|
| Any MACE | 111 (27.5) | 74 (22) | 80 (20.9) | 0.040 |
| All‐cause mortality | 27 (6.7) | 17 (5) | 27 (7.1) | 0.490 |
| MI | 38 (9.4) | 35 (10.4) | 25 (6.5) | 0.179 |
| Any revascularization | 66 (16.4) | 38 (11.3) | 45 (11.8) | 0.038 |
Data are given as number (percentage). FFR indicates fractional flow reserve; MACE, major adverse cardiovascular event; and MI, myocardial infarction.
Figure 2. Clinical outcomes up to 5 years.
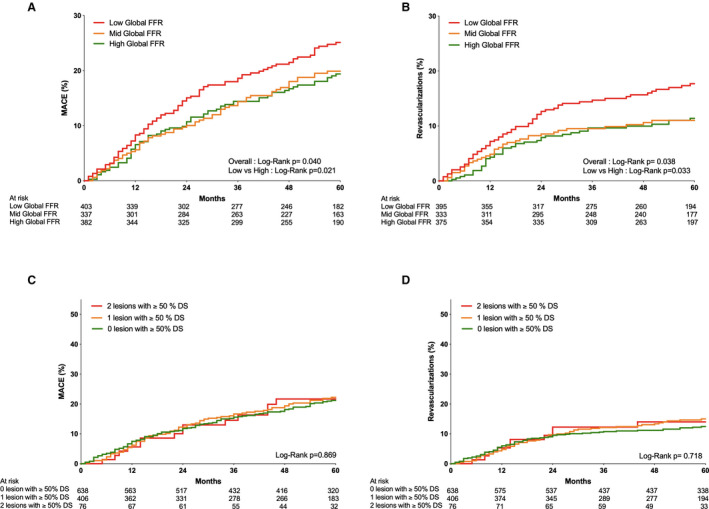
A, Kaplan‐Meier graph reporting the cumulative incidence of major adverse cardiovascular events (MACEs) up to 5 years in the low global fractional flow reserve (FFR) group (green), mid global FFR group (red), and high global FFR group (blue). B, Kaplan‐Meier graph reporting the cumulative incidence of revascularization up to 5 years in the low global FFR group (green), mid global FFR group (red), and high global FFR group (blue). C, Kaplan‐Meier graph reporting the cumulative incidence of MACEs up to 5 years in patients with 0, 1, or 2 ≥50% stenoses at discharge (blue, red, and green, respectively). D, Kaplan‐Meier graph reporting the cumulative incidence of revascularization up to 5 years in patients with 0, 1, or 2 ≥50% stenoses at discharge (blue, red, and green, respectively).
Figure 3. Kaplan‐Meier graph reporting the cumulative incidence of major adverse cardiovascular events (MACEs) (top) and revascularizations (bottom) up to 5 years in the low global fractional flow reserve (FFR) group (green) and in the high global FFR group (blue) for patients in whom FFR was measured in the 3 vessels.
In a Cox regression analysis investigating the relationship between global FFR and events (Figure 4), an increase of 0.1 of global FFR was associated with a statistically significant reduction of MACE (absolute reduction of MACE of 1.2%; HR, 0.89 [95% CI, 0.80–0.98]; P=0.019), MI (HR, 0.83 [95% CI, 0.70–0.97]; P=0.016), and revascularization (HR, 0.87 [95% CI, 0.76–0.99]; P=0.038). In contrast, global FFR was not associated with death. Adjusting for baseline characteristics (hypertension, PCI, and number of vessels with at least 50% stenosis at discharge), global FFR remained associated with a statistically significant reduction of MACE (HR, 0.98 [95% CI, 0.977–0.998]; P=0.023), MI (HR, 0.982 [95% CI, 0.966–0.998]; P=0.032), and revascularization (HR, 0.985 [95% CI, 0.972–0.999]; P=0.040).
Figure 4. Hazard ratio (HR) with 95% CI of the different outcomes per increase of global fractional flow reserve (FFR) of 0.1.
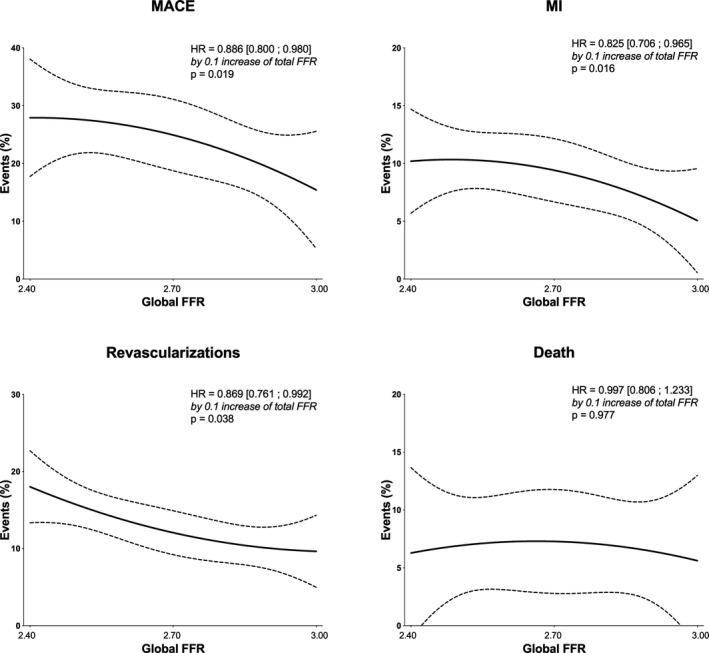
MACE indicates major adverse cardiovascular event; and MI, myocardial infarction.
Clinical Outcomes and Angiography
Clinical outcome was similar in patients with 0, 1, or 2 arteries with 1 stenosis of >50% at discharge in terms of MACE (23.8%, 23.4%, and 23.7%, respectively; log‐rank=0.869) and in terms of revascularizations rates (12.5%, 14.3%, and 14.5%, respectively; log‐rank=0.718) (Figure 2C and 2D).
DISCUSSION
Summary of Results
The present study analyzed the importance of global epicardial conductance assessed using FFR on the 5‐year clinical outcome in patients with coronary atherosclerosis and nonfunctionally significant epicardial stenosis. Patients with a low global FFR had an ≈30% higher MACE rate than patients with a high global FFR. Global FFR was an independent predictor for the occurrence of MACE. In contrast, no relationship was found between the presence of angiographically significant lesions and 5‐year clinical outcome.
Previous Studies
In clinical practice, FFR is used in a dichotomous way. This is based on numerous outcome studies showing that lesions with an FFR of >0.80 do not benefit from revascularization, whereas stenoses with an FFR of <0.80 might do. Yet, beyond this dichotomous interpretation, previous studies suggested a “dose‐effect” relationship between the hemodynamic impact of atherosclerosis and clinical outcome. In a large meta‐analysis, Johnson et al demonstrated a continuous relationship between the numeric value of FFR and subsequent outcomes modulated by the treatment strategy. 12 Similarly, Barbato et al showed that in patients with chronic coronary syndromes and not undergoing revascularization, FFR shows an independent, nonlinear, and inverse risk continuum of lesion‐related events. 4 In a study in which FFR was measured in the 3 vessels, Lee et al also found that the lower the sum of the FFR values, the poorer the outcome. 13 Yet, in this study, stenoses with a whole range of FFR values were included. The MACE rate after 2 years reached 3.8% and 7.1%, which is approximately half of what was encountered in the present report, possibly because of potential bias attributable to underreporting in registry data. The present data extend the findings of previous studies to the 5‐year outcome in patients without ischemia‐producing lesions.
Ischemia Versus Global Atherosclerotic Burden
The treatment strategy and the risk stratification of patients with coronary artery disease are traditionally based on the presence or absence and the extent of myocardial ischemia. Yet, in addition to ischemia, the total atherosclerotic burden and its metabolic activity have been shown to determine outcome. In the PROSPECT (Prospective Natural‐History Study of Coronary Atherosclerosis) study, plaque burden, as assessed at plaque level by intravascular ultrasound, was the main predictor of plaque progression and events in nonintervened coronary segments. 14 Several large clinical data sets, based on both conventional and computed tomography angiography, have demonstrated that patients with “nonobstructive” coronary atherosclerosis carry a risk of MI and death, similar to that of patients with single‐vessel obstructive disease. 15 , 16 , 17 , 18 Yet, in these studies, the absence of ischemia was not ascertained, whereas coronary atherosclerosis, even with none or mild obstruction, is frequently associated with abnormal FFR values. 1 , 19 , 20 In the present study, only patients without ischemia‐producing lesions were included. Within this narrow range of FFR values, the global FFR, a metric of physiologic total epicardial plaque burden, was associated with clinical outcome despite the absence of ischemia, whereas the number of stenoses of >50% diameter was not, thus suggesting that a global physiologic metric is superior to an angiographic approach.
Mechanisms
The mechanistic link between a low global FFR and outcome in patients without ischemia remains speculative. It is likely that the accumulation of small wall irregularities creates serial resistances that lead to pressure losses, especially during high flow states. Recently, however, it was suggested that, in addition to plaque burden, some adverse plaque characteristics could be associated with a decreased myocardial perfusion independently of the luminal narrowing. 21 , 22 Whether this disruptive hypothesis can play a role in vessels with moderate luminal narrowing not severe enough to lead to ischemia remains to be established. The pattern of the physiological disease has been also implicated in the pathogenesis of adverse events in patients with atherosclerosis without ischemia. Focal lesions, with high translational gradients (eg, ΔFFR >0.06), have been independently associated with plaque rupture, 23 but some adverse plaque characteristics might lead to plaque rupture even in the absence of any detectable hemodynamic abnormality. 24
Limitations
Several limitations should be taken into account. First, in an important proportion of post‐PCI segments and in angiographically strictly normal arteries, an arbitrary value was given. This might have led to an overestimation of the value of global FFR. Yet, a sensitivity analysis incorporating only patients in whom FFR had actually been measured in the 3 coronary arteries showed similar results as shown in Figure 3. Second, a large proportion of patients underwent PCI in one or more arteries. Therefore, the present data do not represent the “natural history” of diffuse atherosclerosis, but the outcome of patients with atherosclerosis as modulated by PCI. Third, in the FAME trials, clinicians in charge of the patients were not blinded to either the treatment group or the final hemodynamic results, introducing a risk of bias to the end point of any revascularization. Accordingly, the decision to send (or not) the patient for control angiography might have been influenced by the knowledge of the details of the index procedure. Yet, global FFR was not available as it was calculated offline for the present analysis. Finally, the present study does not account for the respective distribution of the FFR in the different arteries and their respective myocardial mass. For example, it is likely that an FFR value of 0.80 in the left anterior descending coronary artery will have more prognostic importance that the same value in a nondominant left circumflex coronary artery.
Clinical Implication
The concept of global atherosclerotic plaque burden has emerged as an independent determinant of clinical outcome in patients with coronary artery disease. The present data suggest that global FFR, used as a physiological surrogate of atherosclerotic burden, carries prognostic information, even in the absence of ischemia. Patients with a low global FFR had an ≈30% higher MACE rate than patients with a high global FFR. Yet, proposing an invasive measurement of coronary hemodynamics in all 3 vessels to improve risk stratification is not realistic. However, the value of global FFR is frequently at hand thanks to approaches that quantify FFR in the entire coronary tree, as derived from coronary computed tomography 25 or from the conventional invasive angiography. 26
Accordingly, global FFR might be proposed as an additional metric for risk stratification of patients undergoing either computed tomography angiography or conventional angiography. More stringent preventive measures could be proposed in patients with a low global FFR even in the absence of overt reversible myocardial ischemia.
Conclusions
Even in the absence of ischemia‐producing stenoses, patients with a lower global FFR present a higher risk of MACE at 5‐year follow‐up.
Sources of Funding
Dr Fournier and Dr Di Gioia were supported by a research grant from the CardioPaTh PhD Program, and Dr Fournier was supported by the Swiss National Science Foundation and by the Fondation Vaudoise de Cardiologie.
Disclosures
Dr Fournier reports consultancy fees from Cathworks, serves as member of the advisory board of Bayer, and reports speaker fees from Bayer, Amgen and Biotronik. Dr Collet reports grants from Heartflow, Abbott Vascular, Biosensors, and Pie Medical and consultancy fees from HeartFlow and Philips. He serves as member of the advisory board of Abbott Vascular, Pie Medical, and Opsens. Dr Pijls reports grants from Abbott, grants from Hexacath, personal fees from Abbott, personal fees from Opsens, other from GE, other from Philips, other from Heartflow, other from ASML, and personal fees from GE, during the conduct of the study. Dr Fearon reports grants from Abbott Vascular, grants from Medtonic, grants from CathWorks, grants from ACIST Medical, personal fees from Boston Scientific, and other from HeartFlow, outside the submitted work. Dr De Bruyne reports grants from Abbott, grants from Boston Scientific, grants from Biotronic AG, personal fees from Abbott, personal fees from Opsens, personal fees from Boston Scientific, other from Siemens, other from GE, other from Bayer, other from Philips, other from Heartflow, other from Edwards Life Sciences, and other from Ceyliad, during the conduct of the study.
Supporting information
Figure S1
(J Am Heart Assoc. 2020;9:e017729. DOI: 10.1161/JAHA.120.017729.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017729
For Sources of Funding and Disclosures, see pages 8 and 9.
REFERENCES
- 1. Toth G, Hamilos M, Pyxaras S, Mangiacapra F, Nelis O, De Vroey F, Di Serafino L, Muller O, Van Mieghem C, Wyffels E, et al. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J. 2014;35:2831–2838. [DOI] [PubMed] [Google Scholar]
- 2. Marzilli M, Merz CN, Boden WE, Bonow RO, Capozza PG, Chilian WM, DeMaria AN, Guarini G, Huqi A, Morrone D, et al. Reply: to PMID 22954239. J Am Coll Cardiol. 2013;61:388. [DOI] [PubMed] [Google Scholar]
- 3. Genders TS, Steyerberg EW, Alkadhi H, Leschka S, Desbiolles L, Nieman K, Galema TW, Meijboom WB, Mollet NR, de Feyter PJ, et al. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J. 2011;32:1316–1330. [DOI] [PubMed] [Google Scholar]
- 4. Barbato E, Toth GG, Johnson NP, Pijls NH, Fearon WF, Tonino PA, Curzen N, Piroth Z, Rioufol G, Juni P, et al. A prospective natural history study of coronary atherosclerosis using fractional flow reserve. J Am Coll Cardiol. 2016;68:2247–2255. [DOI] [PubMed] [Google Scholar]
- 5. van Nunen LX, Zimmermann FM, Tonino PA, Barbato E, Baumbach A, Engstrom T, Klauss V, MacCarthy PA, Manoharan G, Oldroyd KG, et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5‐year follow‐up of a randomised controlled trial. Lancet. 2015;386:1853–1860. [DOI] [PubMed] [Google Scholar]
- 6. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius‐Winckler S, Rioufol G, Witt N, et al. Fractional flow reserve‐guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. [DOI] [PubMed] [Google Scholar]
- 7. De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius‐Winkler S, Rioufol G, Witt N, et al. Fractional flow reserve‐guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 8. Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van't Veer M, Klauss V, Manoharan G, Engstrøm T, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2‐year follow‐up of the FAME (fractional flow reserve versus angiography for multivessel evaluation) study. J Am Coll Cardiol. 2010;56:177–184. [DOI] [PubMed] [Google Scholar]
- 9. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van' t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. [DOI] [PubMed] [Google Scholar]
- 10. Piroth Z, Toth GG, Tonino PAL, Barbato E, Aghlmandi S, Curzen N, Rioufol G, Pijls NHJ, Fearon WF, Juni P, et al. Prognostic value of fractional flow reserve measured immediately after drug‐eluting stent implantation. Circ Cardiovasc Interv. 2017;10:e005233. 10.1161/CIRCINTERVENTIONS.116.005233. [DOI] [PubMed] [Google Scholar]
- 11. Cox DR. Regression models and life‐tables. J Roy Stat Soc Ser B (Methodol). 1972;34:187–202. [Google Scholar]
- 12. Johnson NP, Toth GG, Lai D, Zhu H, Acar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen SL, et al. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64:1641–1654. [DOI] [PubMed] [Google Scholar]
- 13. Lee JM, Koo BK, Shin ES, Nam CW, Doh JH, Hwang D, Park J, Kim KJ, Zhang J, Hu X, et al. Clinical implications of three‐vessel fractional flow reserve measurement in patients with coronary artery disease. Eur Heart J. 2018;39:945–951. [DOI] [PubMed] [Google Scholar]
- 14. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, et al. A prospective natural‐history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. [DOI] [PubMed] [Google Scholar]
- 15. Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 16. Min JK, Dunning A, Lin FY, Achenbach S, Al‐Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, et al. Age‐ and sex‐related differences in all‐cause mortality risk based on coronary computed tomography angiography findings results from the international multicenter confirm (coronary CT angiography evaluation for clinical outcomes: an international multicenter registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–860. [DOI] [PubMed] [Google Scholar]
- 17. Bittencourt MS, Hulten E, Ghoshhajra B, O'Leary D, Christman MP, Montana P, Truong QA, Steigner M, Murthy VL, Rybicki FJ, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7:282–291. [DOI] [PubMed] [Google Scholar]
- 18. Mushtaq S, De Araujo GP, Garcia‐Garcia HM, Pontone G, Bartorelli AL, Bertella E, Campos CM, Pepi M, Serruys PW, Andreini D. Long‐term prognostic effect of coronary atherosclerotic burden: validation of the computed tomography‐Leaman score. Circ Cardiovasc Imaging. 2015;8:e002332. 10.1161/CIRCIMAGING.114.002332. [DOI] [PubMed] [Google Scholar]
- 19. De Bruyne B, Hersbach F, Pijls NH, Bartunek J, Bech JW, Heyndrickx GR, Gould KL, Wijns W. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but "normal" coronary angiography. Circulation. 2001;104:2401–2406. [DOI] [PubMed] [Google Scholar]
- 20. Gould KL, Nakagawa Y, Nakagawa K, Sdringola S, Hess MJ, Haynie M, Parker N, Mullani N, Kirkeeide R. Frequency and clinical implications of fluid dynamically significant diffuse coronary artery disease manifest as graded, longitudinal, base‐to‐apex myocardial perfusion abnormalities by noninvasive positron emission tomography. Circulation. 2000;101:1931–1939. [DOI] [PubMed] [Google Scholar]
- 21. Driessen RS, de Waard GA, Stuijfzand WJ, Raijmakers PG, Danad I, Bom MJ, Min JK, Leipsic JA, Ahmadi A, van de Ven PM, et al. Adverse plaque characteristics relate more strongly with hyperemic fractional flow reserve and instantaneous wave‐free ratio than with resting instantaneous wave‐free ratio. JACC Cardiovasc Imaging. 2020;13:746–756. [DOI] [PubMed] [Google Scholar]
- 22. Driessen RS, Stuijfzand WJ, Raijmakers PG, Danad I, Min JK, Leipsic JA, Ahmadi A, Narula J, van de Ven PM, Huisman MC, et al. Effect of plaque burden and morphology on myocardial blood flow and fractional flow reserve. J Am Coll Cardiol. 2018;71:499–509. [DOI] [PubMed] [Google Scholar]
- 23. Collet C, Miyazaki Y, Ryan N, Asano T, Tenekecioglu E, Sonck J, Andreini D, Sabate M, Brugaletta S, Stables RH, et al. Fractional flow reserve derived from computed tomographic angiography in patients with multivessel CAD. J Am Coll Cardiol. 2018;71:2756–2769. [DOI] [PubMed] [Google Scholar]
- 24. Park JB, Choi G, Chun EJ, Kim HJ, Park J, Jung JH, Lee MH, Otake H, Doh JH, Nam CW, et al. Computational fluid dynamic measures of wall shear stress are related to coronary lesion characteristics. Heart. 2016;102:1655–1661. [DOI] [PubMed] [Google Scholar]
- 25. Min JK, Taylor CA, Achenbach S, Koo BK, Leipsic J, Norgaard BL, Pijls NJ, De Bruyne B. Noninvasive fractional flow reserve derived from coronary CT angiography: clinical data and scientific principles. JACC Cardiovasc Imaging. 2015;8:1209–1222. [DOI] [PubMed] [Google Scholar]
- 26. Fearon WF, Achenbach S, Engstrom T, Assali A, Shlofmitz R, Jeremias A, Fournier S, Kirtane AJ, Kornowski R, Greenberg G, et al. Accuracy of fractional flow reserve derived from coronary angiography. Circulation. 2019;139:477–484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


