Abstract
Background
Atherosclerosis in >1 vascular bed (ie, polyvascular disease), often a feature of peripheral artery disease (PAD), is associated with high morbidity and mortality. We sought to identify risk factors for polyvascular involvement in patients with PAD.
Methods and Results
We performed 2‐sample Mendelian randomization using an inverse‐variance‐weighted approach, to assess 60 exposures including size and lipid content of atherogenic lipoproteins, blood pressure, glycated hemoglobin, and smoking as causal mediators for polyvascular disease in patients with PAD. Genetic instruments for these exposures were obtained from prior genome‐wide association studies. Patients with PAD were from the Mayo Vascular Disease Biorepository, and polyvascular disease (ie, concomitant coronary heart disease, cerebrovascular disease, and/or abdominal aortic aneurysm) was ascertained by validated phenotyping algorithms. Of 3279 patients with PAD, 61% had polyvascular disease. Genetically predicted levels of the lipid content and/or particle measures of very small and small size very low‐density lipoprotein, intermediate‐density lipoprotein, and large low‐density lipoprotein were associated with polyvascular disease: odds ratios (OR) of 1.80 (95% CI, 1.23–2.61), 1.70 (95% CI, 1.17–2.61), and 1.40 (95% CI, 1.09–1.80) per 1 SD increase in genetically determined levels, respectively. Both genetically predicted diastolic and systolic blood pressure were associated with polyvascular disease; OR per 10 mm Hg genetic increase in diastolic and systolic blood pressure were 1.66 (95% CI, 1.19–2.33) and 1.31 (95% CI, 1.07–1.60), respectively.
Conclusions
Lifetime exposure to increased lipid content and levels of very small and small very low‐density lipoprotein, intermediate‐density lipoprotein, and large low‐density lipoprotein particles as well as elevated blood pressure are associated with polyvascular involvement in patients with PAD. Reduction in levels of such exposures may limit progression of atherosclerosis in patients with PAD.
Keywords: atherosclerosis, blood pressure, lipoproteins, Mendelian randomization, peripheral artery disease, polyvascular disease
Subject Categories: Lipids and Cholesterol, High Blood Pressure, Pathophysiology, Translational Studies, Risk Factors
Peripheral artery disease (PAD), defined as atherosclerotic disease of the arteries supplying the lower limbs, 1 affects >200 million individuals worldwide and is associated with greatly increased risk of adverse cardiovascular events and death. 1 , 2 PAD is often associated with atherosclerosis in other vascular beds (ie, polyvascular disease) with prior studies reporting that nearly 60% of patients have concomitant coronary and/or cerebral artery involvement. 3 In comparison with patients with PAD alone, patients with PAD and polyvascular disease are at higher risk of adverse cardiovascular events and need for lower extremity revascularization. 4 Although it has been suggested that oxidative damage from smoking, vessel wall permeability of lipoprotein particles, hemodynamic factors, and serum glycated hemoglobin levels may contribute to vascular bed specificity of atherosclerosis, 5 factors contributing to diffuse atherosclerosis across multiple vascular beds are unclear. Furthermore, lipoprotein subclasses beyond low‐density lipoprotein (LDL) may confer residual risk not addressed with statin medications. 6 However, it remains unknown if any of these factors specifically confer risk for the development of polyvascular disease in addition to PAD. To address this gap in knowledge, we performed Mendelian randomization (MR) analyses to assess the association of genetically predicted levels of different atherogenic lipoprotein subclasses, blood pressure (BP), glycated hemoglobin, and genetic predisposition to smoking with the presence of polyvascular disease in patients with PAD.
METHODS
The authors declare that all supporting data are available within the article.
Genetic Instruments for Atherosclerotic Cardiovascular Disease Risk Factors
We performed 2‐sample MR to assess 60 exposures related to conventional risk factors for atherosclerotic cardiovascular disease (ASCVD) as causal mediators for polyvascular disease in patients with PAD: measurements of total lipid, total cholesterol, cholesterol ester, free cholesterol, triglyceride, phospholipid content, and particle concentration of different sizes (very large, large, medium, small, very small) of atherogenic lipoprotein subclasses (lipoprotein[a], LDL, intermediate‐density lipoprotein [IDL], and very low‐density lipoprotein [VLDL]) as available, systolic and diastolic BP, glycated hemoglobin, ever being a smoker, smoking initiation age, smoking cessation, and smoking quantity. Independent genetic instruments for these exposures were obtained by clumping publicly available summary statistics from previous European‐ancestry genome‐wide association studies 7 , 8 , 9 , 10 , 11 (Table S1) using a linkage disequilibrium reference panel of 503 European samples from 1000 Genomes phase 3 version 5 (distance=10 000 kb, linkage disequilibrium r 2<0.001, P<5×10−8). Please see Table S2 for a list of genetic instruments for each exposure and their respective R 2 and F statistics quantifying the instrument strength.
Ascertaining PAD and Related Comorbidities
Patients with PAD were identified from the Mayo Vascular Disease Biorepository, which enrolled patients referred for noninvasive vascular evaluation and exercise stress testing at the Mayo Clinic Gonda Vascular Center from January 14, 2006 to July 24, 2020. We restricted the study cohort to genetically unrelated adult participants (≥18 years) of European ancestry given the low proportion of non‐European ancestry individuals. ASCVD phenotypes and related comorbidities were ascertained using previously validated electronic phenotyping algorithms based on both structured data elements, including International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes, current procedural terminology codes, laboratory measurements, and natural language processing of unstructured data elements such as vascular laboratory and imaging reports in the electronic health record. 12 , 13 , 14
PAD was defined as either an ankle‐brachial index ≤0.9 at rest or 1‐minute postexercise, presence of poorly compressible arteries, or history of lower extremity revascularization. Coronary heart disease was defined based on history of myocardial infarction, coronary atherosclerosis/chronic ischemic heart disease, or coronary revascularization. Cerebrovascular disease was ascertained based on the history of ischemic stroke, carotid artery disease, or carotid revascularization. Abdominal aortic aneurysm was defined as having an infrarenal abdominal aortic diameter ≥3 cm or a history of open/endovascular abdominal aortic aneurysm repair. Polyvascular disease was deemed present when 1 or more of these vascular territories (coronary heart disease, cerebrovascular disease, or abdominal aortic aneurysm) were involved in the setting of PAD any time before enrollment and up to 1 year after (Table 1). All participants gave written informed consent, and the study protocol was approved by the institutional review board at Mayo Clinic, Rochester, MN.
Table 1.
Patient Characteristics* (n=3279)
| PAD alone (n=1270) | PAD With Polyvascular Involvement (n=2009) | |
|---|---|---|
| Age at enrollment, y, mean±SD | 65.5±12.0 | 71.0±9.7 |
| Female, n (%) | 587 (46.2) | 590 (29.4) |
| Hypertension, n (%) | 831 (65.4) | 1803 (89.7) |
| Diabetes mellitus, n (%) | 283 (22.3) | 663 (33.0) |
| Dyslipidemia, n (%) | 1012 (79.7) | 1925 (95.8) |
| Statin use, n (%) | 756 (59.5) | 1545 (76.9) |
| Ever smoker, n (%) | 907 (71.4) | 1611 (80.2) |
PAD indicates peripheral artery disease.
All baseline characteristics differed significantly between cases and controls (P<0.001).
Statistical Analysis
We extracted DNA sequence variants for the genetic instruments of the exposures from the Mayo Vascular Disease Biorepository imputed array data set after applying the following quality control filters: minor allele frequency >1%, Hardy–Weinberg equilibrium P>1×10−5, imputation quality r 2>0.3.
We performed multivariable logistic regression with polyvascular disease as the outcome and genetic instrument as the predictor, adjusting for age at enrollment, sex, electronic health record duration, and the first 2 genetic principal components. Age at enrollment and electronic health record duration were modeled flexibly by including restricted cubic splines with 4 knots to allow for nonlinear effects.
Association statistics of the genetic instruments for the exposures and the outcome were harmonized to conduct 2‐sample MR with a multiplicative random effects inverse‐variance‐weighted approach. Given the correlated nature of the exposures, we accounted for multiple hypothesis testing by considering 2‐sided P‐values at a Benjamini–Hochberg false discovery rate <0.1 as significant. For significant MR associations, we additionally performed sensitivity analyses using other MR methods such as weighted median estimator and MR–Egger regression, which have lower statistical power than the inverse‐variance‐weighted MR but provide more robust estimates in the presence of horizontal pleiotropy. Further details regarding MR assumptions and sensitivity analyses are provided in Data S1. All analyses were performed using "rms" and "TwoSampleMR" 15 packages in R version 3.6.2 (R Foundation for Statistical Computing).
RESULTS
Of the 53 blood lipoprotein concentrations and 7 nonlipid traits related to ASCVD risk factors, there were 18 significant associations with polyvascular disease across measures of 3 lipoprotein subclasses of different sizes and BP traits (Table 2). Genetically predicted levels of the lipid content and/or particle concentration measures of very small and small VLDL, IDL, and large LDL increased the odds of polyvascular involvement in the setting of PAD; odds ratios (OR) of 1.80 (95% CI, 1.23–2.61), 1.70 (95% CI, 1.17–2.61), and 1.40 (95% CI, 1.09–1.80) per 1 SD increase in genetically determined levels, respectively. Atherogenic lipoproteins significantly associated with polyvascular disease were within a specific particle size range, and the strength of association for the measures of lipoprotein components correlated with the particle diameter; OR per 1 SD increase in genetically determined levels of very small VLDL and small VLDL particle measures were the highest, and ORs were progressively lower for IDL and large LDL particles, respectively (Figure and Table 2).
Table 2.
Mendelian Randomization Results
| Comorbidity/Lipid Fraction | Exposure | Number of SNVs | Units | Nexposure * | OR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Nonlipid exposure | ||||||
| Hypertension | Systolic blood pressure | 443 | 10 mm Hg | 745 820 | 1.31 (1.07–1.60) † | 0.008 † |
| Diastolic blood pressure | 444 | 10 mm Hg | 757 601 | 1.66 (1.19–2.33) † | 0.003 † | |
| Smoking | Smoking initiation ‡ | 87 | Log odds | 1 232 091 | 0.81 (0.47–1.38) | 0.432 |
| Smoking cessation § | 8 | Log odds | 547 219 | 1.53 (0.57–4.12) | 0.400 | |
| Smoking age ‖ | 7 | Years | 341 427 | 0.80 (0.07–9.21) | 0.857 | |
| Smoking quantity ¶ | 22 | Cigarettes per day | 337 334 | 1.40 (0.90–2.15) | 0.132 | |
| Diabetes mellitus | HbA1C—glycated hemoglobin | 37 | % | 123 665 | 0.85 (0.28–2.65) | 0.786 |
| Lipid exposures (in ascending order of lipid subclass particle size) | ||||||
| Lp(a) | Lp(a)—lipoprotein(a) | 5 | SD | 6440 | 1.15 (0.92–1.44) | 0.213 |
| S.LDL | S.LDL.C—total cholesterol in small LDL | 10 | SD | 21 556 | 1.22 (0.88–1.70) | 0.234 |
| S.LDL.L—total lipids in small LDL | 12 | SD | 19 273 | 1.32 (0.91–1.92) | 0.141 | |
| S.LDL.P—concentration of small LDL particles | 8 | SD | 19 273 | 1.41 (0.96–2.08) | 0.078 | |
| M.LDL | M.LDL.C—total cholesterol in medium LDL | 13 | SD | 21 559 | 1.29 (0.95–1.76) | 0.103 |
| M.LDL.CE—cholesterol esters in medium LDL | 14 | SD | 19 273 | 1.33 (0.99–1.78) | 0.057 | |
| M.LDL.L—total lipids in medium LDL | 13 | SD | 19 273 | 1.36 (1.00–1.84) | 0.047 | |
| M.LDL.P—concentration of medium LDL particles | 13 | SD | 19 273 | 1.37 (1.01–1.86) | 0.046 | |
| M.LDL.PL—phospholipids in medium LDL | 11 | SD | 19 273 | 1.21 (0.82–1.79) | 0.330 | |
| L.LDL | L.LDL.C—total cholesterol in large LDL | 14 | SD | 21 552 | 1.33 (1.04–1.70) † | 0.025 † |
| L.LDL.CE—cholesterol esters in large LDL | 14 | SD | 19 273 | 1.35 (1.05–1.74) † | 0.021 † | |
| L.LDL.FC—free cholesterol in large LDL | 11 | SD | 21 555 | 1.33 (1.04–1.71) † | 0.022 † | |
| L.LDL.L—total lipids in large LDL | 15 | SD | 19 273 | 1.37 (1.07–1.75) † | 0.012 † | |
| L.LDL.P—concentration of large LDL particles | 14 | SD | 19 273 | 1.40 (1.09–1.80) † | 0.008 † | |
| L.LDL.PL—phospholipids in large LDL | 13 | SD | 21 550 | 1.35 (1.05–1.75) † | 0.020 † | |
| IDL | IDL.C—total cholesterol in IDL | 13 | SD | 19 273 | 1.47 (1.10–1.95) † | 0.008 † |
| IDL.FC—free cholesterol in IDL | 12 | SD | 21 559 | 1.53 (1.18–1.99) † | 0.001 † | |
| IDL.L—total lipids in IDL | 14 | SD | 19 273 | 1.48 (1.13–1.95) † | 0.005 † | |
| IDL.P—concentration of IDL particles | 13 | SD | 19 273 | 1.56 (1.20–2.03) † | 0.001 † | |
| IDL.PL—phospholipids in IDL | 12 | SD | 21 559 | 1.49 (1.14–1.94) † | 0.004 † | |
| IDL.TG—triglycerides in IDL | 11 | SD | 19 273 | 1.70 (1.13–2.56) † | 0.010 † | |
| XS.VLDL | XS.VLDL.L—total lipids in very small VLDL | 12 | SD | 19 273 | 1.80 (1.23–2.61) † | 0.002 † |
| XS.VLDL.P—concentration of very small VLDL particles | 12 | SD | 19 273 | 1.75 (1.17–2.61) † | 0.007 † | |
| XS.VLDL.PL—phospholipids in very small VLDL | 13 | SD | 19 273 | 1.59 (1.19–2.11) † | 0.001 † | |
| XS.VLDL.TG—triglycerides in very small VLDL | 10 | SD | 19 273 | 1.39 (0.89–2.17) | 0.150 | |
| S.VLDL | S.VLDL.C—total cholesterol in small VLDL | 10 | SD | 21 557 | 1.70 (1.10–2.62) † | 0.017 † |
| S.VLDL.FC—free cholesterol in small VLDL | 10 | SD | 21 559 | 1.68 (1.02–2.75) | 0.040 | |
| S.VLDL.L—total lipids in small VLDL | 10 | SD | 19 273 | 1.60 (1.00–2.57) | 0.051 | |
| S.VLDL.P—concentration of small VLDL particles | 12 | SD | 19 273 | 1.22 (0.79–1.87) | 0.366 | |
| S.VLDL.PL—phospholipids in small VLDL | 10 | SD | 21 551 | 1.49 (0.93–2.37) | 0.097 | |
| S.VLDL.TG—triglycerides in small VLDL | 11 | SD | 21 558 | 1.15 (0.73–1.81) | 0.542 | |
| M.VLDL | M.VLDL.C—total cholesterol in medium VLDL | 10 | SD | 21 551 | 1.17 (0.81–1.69) | 0.403 |
| M.VLDL.CE—cholesterol esters in medium VLDL | 11 | SD | 19 273 | 1.34 (0.89–1.99) | 0.157 | |
| M.VLDL.FC—free cholesterol in medium VLDL | 7 | SD | 21 240 | 1.08 (0.65–1.80) | 0.767 | |
| M.VLDL.L—total lipids in medium VLDL | 9 | SD | 19 273 | 1.29 (0.81–2.04) | 0.282 | |
| M.VLDL.P—concentration of medium VLDL particles | 11 | SD | 19 273 | 1.29 (0.88–1.88) | 0.194 | |
| M.VLDL.PL—phospholipids in medium VLDL | 11 | SD | 21 240 | 1.00 (0.64–1.57) | 0.988 | |
| M.VLDL.TG—triglycerides in medium VLDL | 10 | SD | 21 241 | 0.99 (0.63–1.53) | 0.947 | |
| L.VLDL | L.VLDL.C—total cholesterol in large VLDL | 8 | SD | 21 235 | 1.14 (0.63–2.07) | 0.657 |
| L.VLDL.CE—cholesterol esters in large VLDL | 9 | SD | 18 960 | 1.50 (0.96–2.35) | 0.078 | |
| L.VLDL.FC—free cholesterol in large VLDL | 9 | SD | 21 238 | 0.96 (0.54–1.68) | 0.874 | |
| L.VLDL.L—total lipids in large VLDL | 9 | SD | 18 960 | 0.92 (0.48–1.78) | 0.806 | |
| L.VLDL.P—concentration of large VLDL particles | 7 | SD | 18 960 | 1.05 (0.55–2.00) | 0.884 | |
| L.VLDL.PL—phospholipids in large LDL | 8 | SD | 21 239 | 1.24 (0.68–2.25) | 0.477 | |
| L.VLDL.TG—triglycerides in large VLDL | 8 | SD | 21 239 | 0.83 (0.44–1.57) | 0.574 | |
| XL.VLDL | XL.VLDL.L—total lipids in very large VLDL | 8 | SD | 19 273 | 0.95 (0.50–1.80) | 0.879 |
| XL.VLDL.P—concentration of very large VLDL particles | 7 | SD | 18 960 | 0.86 (0.48–1.55) | 0.612 | |
| XL.VLDL.PL—phospholipids in very large VLDL | 7 | SD | 21 237 | 0.91 (0.46–1.78) | 0.777 | |
| XL.VLDL.TG—triglycerides in very large VLDL | 6 | SD | 21 548 | 0.74 (0.42–1.33) | 0.314 | |
| XXL.VLDL | XXL.VLDL.L—total lipids in chylomicrons and extremely large VLDL | 7 | SD | 18 960 | 0.86 (0.45–1.64) | 0.641 |
| XXL.VLDL.P—concentration of chylomicrons and extremely large VLDL particles | 7 | SD | 18 960 | 0.77 (0.43–1.38) | 0.385 | |
| XXL.VLDL.PL—phospholipids in chylomicrons and extremely large VLDL | 7 | SD | 21 542 | 0.98 (0.44–2.18) | 0.970 | |
| XXL.VLDL.TG—triglycerides in chylomicrons and extremely large VLDL | 8 | SD | 21 540 | 0.95 (0.51–1.77) | 0.866 | |
IDL indicates intermediate‐density lipoprotein; L.LDL, large low‐density lipoprotein; LDL, low‐density lipoprotein; M.LDL, medium low‐density lipoprotein; M.VLDL, medium very low‐density lipoprotein; OR, odds ratio; S.LDL, small low‐density lipoprotein; S.VLDL, small very low‐density lipoprotein; SNVs, single nucleotide variants; VLDL, very low‐density lipoprotein; XL.VLDL, very large very low‐density lipoprotein; XS.VLDL, very small very low‐density lipoprotein; and XXL.VLDL, extremely large very low‐density lipoprotein.
Number of participants included in the original genome‐wide association study to identify genetifc instruments.
Significant associations at false discovery rate <0.1.
Age at which an individual started smoking cigarettes regularly.
Current smokers in reference to former smokers.
Ever smokers in reference to nonsmokers.
Average number of cigarettes per day.
Figure 1. Lipid fractions significantly associated with polyvascular involvement in patients with peripheral artery disease.
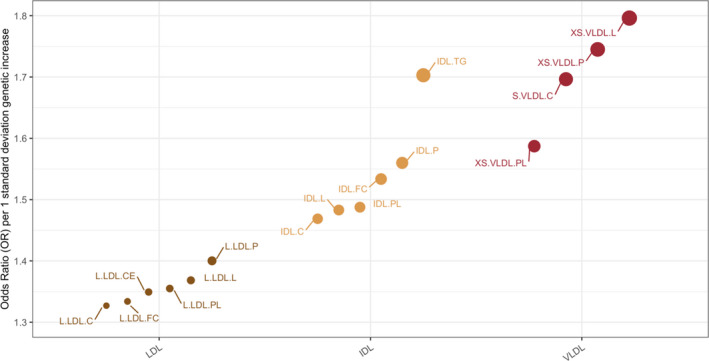
Odds ratios per 1 SD increase in genetically determined levels of individual particle components of three lipoprotein subclasses; low‐density lipoprotein (brown), intermediate‐density lipoprotein (yellow), very low‐density lipoprotein (red) in the ascending order of particle diameter. Size of the dots representing each measurement is proportional to the magnitude of odds ratios obtained from inverse‐variance weighted mendelian randomization. .C indicates total cholesterol; .CE, cholesterol ester; .FC, free cholesterol; .L, total lipid; .P, particle concentration; .PL, phospholipid; .TG, triglycerides; IDL, Intermediate‐density lipoprotein; L.LDL, large low‐density lipoprotein; S.VLDL, small very low‐density lipoprotein; and XS.VLDL, very small very low‐density lipoprotein.
Both genetically predicted diastolic and systolic BP were significantly associated with polyvascular disease; a 10 mm Hg genetic increase in diastolic BP resulted in a 1.66‐fold higher risk (95% CI, 1.19–2.33), and a 10 mm Hg genetic increase in systolic BP resulted in a 1.31‐fold higher risk (95% CI, 1.07–1.60) of polyvascular involvement.
There were no significant associations of genetically predicted levels of other lipoprotein subclasses and sizes, glycated hemoglobin, or the genetic predisposition to smoking with polyvascular involvement in patients with PAD. In sensitivity analyses for the significant associations, we did not find any evidence of significant horizontal pleiotropy based on the Cochran Q test and MR–Egger intercept term. Effect estimates across different MR methods were all directionally concordant (Table 3).
Table 3.
Sensitivity Analyses for Significant Associations
| Comorbidity/Lipid Fraction | Exposure | MR Method | Number of SNVs | Units | OR (95% CI) | P Value of OR | Q* | P Value of Q* | QR † | EI | P Value of EI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypertension | Systolic blood pressure | IVW | 443 | 10 mm Hg | 1.31 (1.07–1.60) | 0.008 | 452.12 | 0.359 | 0.998 | N/A | N/A |
| Systolic blood pressure | Weighted median | 443 | 10 mm Hg | 1.20 (0.89–1.64) | 0.234 | N/A | N/A | N/A | N/A | N/A | |
| Systolic blood pressure | MR Egger | 443 | 10 mm Hg | 1.59 (0.96–2.65) | 0.074 | 451.44 | 0.355 | 0.998 | −0.01 | 0.415 | |
| Diastolic blood pressure | IVW | 444 | 10 mm Hg | 1.66 (1.19–2.33) | 0.003 | 449.89 | 0.4 | 0.995 | N/A | N/A | |
| Diastolic blood pressure | Weighted median | 444 | 10 mm Hg | 1.52 (0.90–2.57) | 0.121 | N/A | N/A | N/A | N/A | N/A | |
| Diastolic blood pressure | MR Egger | 444 | 10 mm Hg | 2.96 (1.31–6.72) | 0.010 | 447.57 | 0.417 | 0.995 | −0.01 | 0.131 | |
| L.LDL | L.LDL.C—total cholesterol in large LDL | IVW | 14 | SD | 1.33 (1.04–1.70) | 0.025 | 10.83 | 0.625 | 0.979 | N/A | N/A |
| L.LDL.C—total cholesterol in large LDL | Weighted median | 14 | SD | 1.16 (0.85–1.59) | 0.343 | N/A | N/A | N/A | N/A | N/A | |
| L.LDL.C—total cholesterol in large LDL | MR Egger | 14 | SD | 1.24 (0.86–1.79) | 0.271 | 10.6 | 0.563 | 0.979 | 0.01 | 0.641 | |
| L.LDL.CE—cholesterol esters in large LDL | IVW | 14 | SD | 1.35 (1.05–1.74) | 0.021 | 14.06 | 0.369 | 0.942 | N/A | N/A | |
| L.LDL.CE—cholesterol esters in large LDL | Weighted median | 14 | SD | 1.17 (0.85–1.61) | 0.342 | N/A | N/A | N/A | N/A | N/A | |
| L.LDL.CE—cholesterol esters in large LDL | MR Egger | 14 | SD | 1.19 (0.81–1.75) | 0.396 | 13.25 | 0.351 | 0.942 | 0.03 | 0.406 | |
| L.LDL.FC—free cholesterol in large LDL | IVW | 11 | SD | 1.33 (1.04–1.71) | 0.022 | 9.94 | 0.445 | 0.919 | N/A | N/A | |
| L.LDL.FC—free cholesterol in large LDL | Weighted median | 11 | SD | 1.16 (0.86–1.57) | 0.336 | N/A | N/A | N/A | N/A | N/A | |
| L.LDL.FC—free cholesterol in large LDL | MR Egger | 11 | SD | 1.18 (0.81–1.70) | 0.409 | 9.13 | 0.426 | 0.919 | 0.03 | 0.393 | |
| L.LDL.L—total lipids in large LDL | IVW | 15 | SD | 1.37 (1.07–1.75) | 0.012 | 14.48 | 0.415 | 0.925 | N/A | N/A | |
| L.LDL.L—Total lipids in large LDL | Weighted median | 15 | SD | 1.17 (0.87–1.58) | 0.305 | N/A | N/A | N/A | N/A | N/A | |
| L.LDL.L—total lipids in large LDL | MR Egger | 15 | SD | 1.18 (0.82–1.71) | 0.388 | 13.39 | 0.418 | 0.925 | 0.03 | 0.323 | |
| L.LDL.P—concentration of large LDL particles | IVW | 14 | SD | 1.40 (1.09–1.80) | 0.008 | 13.41 | 0.417 | 0.873 | N/A | N/A | |
| L.LDL.P—concentration of large LDL particles | Weighted median | 14 | SD | 1.18 (0.85–1.62) | 0.326 | N/A | N/A | N/A | N/A | N/A | |
| L.LDL.P—concentration of large LDL particles | MR Egger | 14 | SD | 1.15 (0.79–1.69) | 0.472 | 11.71 | 0.469 | 0.873 | 0.04 | 0.217 | |
| L.LDL.PL—phospholipids in large LDL | IVW | 13 | SD | 1.35 (1.05–1.75) | 0.020 | 10.19 | 0.599 | 0.954 | N/A | N/A | |
| L.LDL.PL—phospholipids in large LDL | Weighted median | 13 | SD | 1.17 (0.85–1.62) | 0.341 | N/A | N/A | N/A | N/A | N/A | |
| L.LDL.PL—phospholipids in large LDL | MR Egger | 13 | SD | 1.22 (0.82–1.81) | 0.341 | 9.72 | 0.556 | 0.954 | 0.02 | 0.507 | |
| IDL | IDL.C—total cholesterol in IDL | IVW | 13 | SD | 1.47 (1.10–1.95) | 0.008 | 15.37 | 0.222 | 0.936 | N/A | N/A |
| IDL.C—total cholesterol in IDL | Weighted median | 13 | SD | 1.19 (0.86–1.64) | 0.30 | N/A | N/A | N/A | N/A | N/A | |
| IDL.C—total cholesterol in IDL | MR Egger | 13 | SD | 1.26 (0.80–1.98) | 0.333 | 14.38 | 0.213 | 0.936 | 0.03 | 0.403 | |
| IDL.FC—free cholesterol in IDL | IVW | 12 | SD | 1.53 (1.18–1.99) | 0.001 | 11.08 | 0.436 | 0.738 | N/A | N/A | |
| IDL.FC—free cholesterol in IDL | Weighted median | 12 | SD | 1.20 (0.85–1.69) | 0.296 | N/A | N/A | N/A | N/A | N/A | |
| IDL.FC—free cholesterol in IDL | MR Egger | 12 | SD | 1.17 (0.78–1.75) | 0.47 | 8.18 | 0.611 | 0.738 | 0.06 | 0.119 | |
| IDL.L—total lipids in IDL | IVW | 14 | SD | 1.48 (1.13–1.95) | 0.005 | 15.14 | 0.298 | 0.969 | N/A | N/A | |
| IDL.L—total lipids in IDL | Weighted median | 14 | SD | 1.21 (0.85–1.73) | 0.285 | N/A | N/A | N/A | N/A | N/A | |
| IDL.L—total lipids in IDL | MR Egger | 14 | SD | 1.32 (0.84–2.09) | 0.254 | 14.67 | 0.26 | 0.969 | 0.02 | 0.547 | |
| IDL.P—concentration of IDL particles | IVW | 13 | SD | 1.56 (1.20–2.03) | 0.001 | 11.2 | 0.511 | 0.817 | N/A | N/A | |
| IDL.P—concentration of IDL particles | Weighted median | 13 | SD | 1.23 (0.85–1.77) | 0.276 | N/A | N/A | N/A | N/A | N/A | |
| IDL.P—concentration of IDL particles | MR Egger | 13 | SD | 1.20 (0.76–1.87) | 0.448 | 9.15 | 0.608 | 0.817 | 0.05 | 0.179 | |
| IDL.PL—phospholipids in IDL | IVW | 12 | SD | 1.49 (1.14–1.94) | 0.004 | 8.62 | 0.657 | 0.887 | N/A | N/A | |
| IDL.PL—phospholipids in IDL | Weighted median | 12 | SD | 1.21 (0.86–1.7) | 0.264 | N/A | N/A | N/A | N/A | N/A | |
| IDL.PL—phospholipids in IDL | MR Egger | 12 | SD | 1.25 (0.81–1.93) | 0.327 | 7.65 | 0.663 | 0.887 | 0.04 | 0.347 | |
| IDL.TG—triglycerides in IDL | IVW | 11 | SD | 1.70 (1.13–2.56) | 0.010 | 11.92 | 0.29 | 0.935 | N/A | N/A | |
| IDL.TG—triglycerides in IDL | Weighted median | 11 | SD | 1.45 (0.84–2.48) | 0.180 | N/A | N/A | N/A | N/A | N/A | |
| IDL.TG—triglycerides in IDL | MR Egger | 11 | SD | 2.54 (0.87–7.35) | 0.121 | 11.14 | 0.266 | 0.935 | −0.05 | 0.446 | |
| XS.VLDL | XS.VLDL.L—total lipids in very small VLDL | IVW | 12 | SD | 1.80 (1.23–2.61) | 0.002 | 13.04 | 0.291 | 0.993 | N/A | N/A |
| XS.VLDL.L—total lipids in very small VLDL | Weighted median | 12 | SD | 1.72 (1.06–2.81) | 0.029 | N/A | N/A | N/A | N/A | N/A | |
| XS.VLDL.L—total lipids in very small VLDL | MR Egger | 12 | SD | 2.09 (0.62–7.11) | 0.263 | 12.95 | 0.226 | 0.993 | −0.02 | 0.8 | |
| XS.VLDL.P—concentration of very small VLDL particles | IVW | 12 | SD | 1.75 (1.17–2.61) | 0.007 | 14.44 | 0.209 | 0.990 | N/A | N/A | |
| XS.VLDL.P—concentration of very small VLDL particles | Weighted median | 12 | SD | 1.48 (0.88–2.49) | 0.141 | N/A | N/A | N/A | N/A | N/A | |
| XS.VLDL.P—concentration of very small VLDL particles | MR Egger | 12 | SD | 2.11 (0.61–7.32) | 0.267 | 14.3 | 0.16 | 0.990 | −0.02 | 0.758 | |
| XS.VLDL.PL—phospholipids in very small VLDL | IVW | 13 | SD | 1.59 (1.19–2.11) | 0.001 | 9.72 | 0.64 | 0.985 | N/A | N/A | |
| XS.VLDL.PL—phospholipids in very small VLDL | Weighted median | 13 | SD | 1.26 (0.85–1.84) | 0.247 | N/A | N/A | N/A | N/A | N/A | |
| XS.VLDL.PL—phospholipids in very small VLDL | MR Egger | 13 | SD | 1.44 (0.82–2.52) | 0.227 | 9.57 | 0.57 | 0.985 | 0.02 | 0.701 | |
| S.VLDL | S.VLDL.C—total cholesterol in small VLDL | IVW | 10 | SD | 1.70 (1.10–2.62) | 0.017 | 11.27 | 0.258 | 0.910 | N/A | N/A |
| S.VLDL.C—total cholesterol in small VLDL | Weighted median | 10 | SD | 1.29 (0.73–2.28) | 0.376 | N/A | N/A | N/A | N/A | N/A | |
| S.VLDL.C—total cholesterol in small VLDL | MR Egger | 10 | SD | 1.04 (0.32–3.34) | 0.948 | 10.26 | 0.247 | 0.910 | 0.06 | 0.401 |
EI indicates Egger intercept; IDL, intermediate‐density lipoprotein; IVW, inverse variance weighted; L.LDL, large low‐density lipoprotein; LDL, low‐density lipoprotein; MR, Mendelian randomization; N/A, Not applicable;OR, odds ratio; S.VLDL, small very low‐density lipoprotein; SNVs, single‐nucleotide variants; VLDL, very low‐density lipoprotein; and XS.VLDL, very small very low‐density lipoprotein.
Cochran Q statistic for heterogeneity.
QR: ratio of the statistical heterogeneity around the MR–Egger fitted slope, divided by the statistical heterogeneity around the IVW slope.
DISCUSSION
In this study, we employed an MR framework to elucidate the causal relationship between traits representing conventional risk factors (ie, hypertension, diabetes mellitus, smoking, and dyslipidemias) and polyvascular disease in individuals with PAD. We identified genetically determined levels of certain subclasses of lipoproteins and BP traits to be associated with polyvascular disease, suggesting pathways that could be targeted to limit diffuse atherosclerosis in patients with PAD.
Genetically elevated levels of large LDL and triglyceride‐rich lipoproteins such as IDL and smaller size VLDL resulted in increased risk of polyvascular involvement; very small VLDL had the strongest association: a 1 SD genetic increment in the circulating particle levels resulted in a 1.8‐fold increased risk of polyvascular disease. Although LDL cholesterol is a known causal factor for ASCVD, lipoprotein particles aside from LDL also contribute to ASCVD risk. 6 LDL enters the arterial intima through passive "molecular sieving," which is positively correlated with higher particle concentration, lower particle size, elevated BP, and overall accumulation of arterial wall injury. 6 This process also occurs for medium‐sized triglyceride‐rich lipoproteins, albeit at a slower rate than LDL. Lack of association with larger VLDL in our study could be attributed to lower cholesterol content of these lipoproteins and inefficient penetration of the arterial intima as a result of particle size. Increase in genetically predicted levels of small to medium size LDL and lipoprotein(a) had smaller effect size estimates and were not associated with polyvascular disease. Although these particles are highly atherogenic and strongly associated with PAD at baseline, 1 , 2 remnant cholesterol and triglyceride‐rich lipoproteins may have a more important role in progression of atherosclerosis in patients with PAD.
Genetically predicted diastolic and systolic BP were associated with polyvascular disease, with diastolic BP more strongly associated. This is of interest since isolated systolic hypertension has been considered a better predictor of adverse cardiovascular outcomes than isolated diastolic hypertension. 16 Genetically predicted hemoglobin A1c levels and genetic liability to smoking were not associated with increased risk of polyvascular disease. In a previous report 4 that studied the risk of major adverse cardiovascular events in patients with PAD, the proportions of individuals with a history of hypertension and dyslipidemia were 17% and 19% higher, respectively, among participants with polyvascular PAD compared with PAD alone, whereas these differences were 8% for history of diabetes mellitus and 5% for ever being a smoker. Consistent with this observation, in our cohort, hypertension and dyslipidemia were more prevalent in patients with polyvascular disease at baseline in comparison with history of diabetes mellitus and smoking. Although diabetes mellitus and smoking are the strongest risk factors for PAD, 1 , 2 these factors may have a lesser impact on progression of atherosclerosis in PAD.
One of the main goals of management in patients with PAD is to reduce the risk of adverse cardiovascular outcomes; coexistent disease in other vascular beds present further challenges in treatment. 2 There is significant reduction of major adverse cardiovascular events (cardiovascular death, myocardial infarction, and stroke) by evolucumab, a PCSK9 (proprotein convertase subtilisin/kexin 9) inhibitor, in patients with PAD who are receiving medium to high intensity statin therapy at baseline. 17 Our results suggest that there may be benefit in targeting non–high‐density lipoprotein cholesterol levels, which include not only LDL cholesterol but also all circulating atherogenic cholesterols, as well as targeting triglyceride‐rich lipoproteins.
Limitations
Our study should be interpreted in light of certain limitations. First, although robust genetic instruments were obtained using the largest available genome‐wide association studies, the relatively modest size of the study cohort may have limited the statistical power to detect weaker associations. A multivariable MR analysis using significant lipid exposure associations was not pursued because of multicollinearity and weak instrument bias after conditioning in the multivariable setting. We defined polyvascular disease based on electronic health record–derived diagnostic or procedure codes and may have missed "subclinical" polyvascular disease in patients with PAD. Although we used previously validated electronic phenotyping algorithms to ascertain ASCVD phenotypes, some degree of misclassification may persist. Our study included participants of European‐ancestry, which limits the generalizability of our findings to diverse ancestral/ethnic groups. Lastly, these findings need replication in additional studies.
CONCLUSIONS
Lifetime exposure to increased lipid content and levels of very small and small VLDL, IDL, large LDL particles, and elevated BP are associated with polyvascular involvement in patients with PAD. Lowering triglyceride‐rich lipoproteins and optimal BP control may limit progression of atherosclerosis in patients with PAD.
Sources of Funding
Dr. Kullo was funded by National Institutes of Health Grants U01 HG006379 and RO1 HL137010. Dr. Satterfield was supported by the Clinician‐Investigator Training Program at Mayo Clinic.
Disclosures
None.
Supporting information
Data S1
Tables S1–S2
Acknowledgments
We thank the investigators of the studies we used to obtain genetic instruments for making their genome‐wide association summary statistics publicly available.
(J Am Heart Assoc. 2020;9:e017740. DOI: 10.1161/JAHA.120.017740.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017740
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Fowkes FGR, Aboyans V, Fowkes FJI, McDermott MM, Sampson UKA, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14:156–170. [DOI] [PubMed] [Google Scholar]
- 2. Kullo IJ, Rooke TW. Clinical practice. Peripheral artery disease. N Engl J Med. 2016;374:861–871. [DOI] [PubMed] [Google Scholar]
- 3. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas J‐L, Goto S, Liau C‐S, Richard AJ, Röther J, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
- 4. Gutierrez JA, Mulder H, Jones WS, Rockhold FW, Baumgartner I, Berger JS, Blomster JI, Fowkes FGR, Held P, Katona BG, et al. Polyvascular disease and risk of major adverse cardiovascular events in peripheral artery disease: a secondary analysis of the EUCLID Trial. JAMA Netw Open. 2018;1:e185239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. VanderLaan Paul A, Reardon Catherine A, Getz GS. Site specificity of atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:12–22. [DOI] [PubMed] [Google Scholar]
- 6. Nordestgaard BG. Triglyceride‐rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547–563. [DOI] [PubMed] [Google Scholar]
- 7. Evangelou E, Warren HR, Mosen‐Ansorena D, Mifsud B, Pazoki R, Gao H, Ntritsos G, Dimou N, Cabrera CP, Karaman I, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018;50:1412–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kettunen J, Demirkan A, Würtz P, Draisma HHM, Haller T, Rawal R, Vaarhorst A, Kangas AJ, Lyytikäinen L‐P, Pirinen M, et al. Genome‐wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. 2016;7:11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, Datta G, Davila‐Velderrain J, McGuire D, Tian C, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wheeler E, Leong A, Liu C‐T, Hivert M‐F, Strawbridge RJ, Podmore C, Li M, Yao J, Sim X, Hong J, et al. Impact of common genetic determinants of Hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: a transethnic genome‐wide meta‐analysis. PLoS Med. 2017;14:e1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zekavat SM, Ruotsalainen S, Handsaker RE, Alver M, Bloom J, Poterba T, Seed C, Ernst J, Chaffin M, Engreitz J, et al. Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat Commun. 2018;9:2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan J, Arruda‐Olson AM, Leibson CL, Smith C, Liu G, Bailey KR, Kullo IJ. Billing code algorithms to identify cases of peripheral artery disease from administrative data. J Am Med Inform Assoc. 2013;20:e349–e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Safarova MS, Liu H, Kullo IJ. Rapid identification of familial hypercholesterolemia from electronic health records: the SEARCH study. J Clin Lipidol. 2016;10:1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye Z, Kalloo FS, Dalenberg AK, Kullo IJ. An electronic medical record‐linked biorepository to identify novel biomarkers for atherosclerotic cardiovascular disease. Glob Cardiol Sci Pract. 2013;2013:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et al. The MR‐Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strandberg TE, Pitkala K. What is the most important component of blood pressure: systolic, diastolic or pulse pressure? Curr Opin Nephrol Hypertens. 2003;12:293–297. [DOI] [PubMed] [Google Scholar]
- 17. Bonaca Marc P, Nault P, Giugliano Robert P, Keech Anthony C, Pineda Armando L, Kanevsky E, Kuder J, Murphy Sabina A, Jukema JW, Lewis Basil S, et al. Low‐density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease. Circulation. 2018;137:338–350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S2


