Abstract
Background
Although exercise training reduces office blood pressure (BP), scarcer evidence is available on whether these benefits also apply to ambulatory blood pressure (ABP), which is a stronger predictor of cardiovascular disease and mortality. The present study aims to assess the effects of exercise training on ABP in patients with hypertension based on evidence from randomized controlled trials.
Methods and Results
A systematic search of randomized controlled trials on the aforementioned topic was conducted in PubMed and Scopus (since inception to April 1, 2020). The mean difference between interventions (along with 95% CI) for systolic BP and diastolic BP was assessed using a random‐effects model. Sub‐analyses were performed attending to (1) whether participants were taking antihypertensive drugs and (2) exercise modalities. Fifteen studies (including 910 participants with hypertension) met the inclusion criteria. Interventions lasted 8 to 24 weeks (3–5 sessions/week). Exercise significantly reduced 24‐hour (systolic BP, −5.4 mm Hg; [95% CI, −9.2 to −1.6]; diastolic BP, −3.0 mm Hg [−5.4 to −0.6]), daytime (systolic BP, −4.5 mm Hg [−6.6 to −2.3]; diastolic BP, −3.2 mm Hg [−4.8 to −1.5]), and nighttime ABP (systolic BP, −4.7 mm Hg [−8.4 to −1.0]; diastolic BP, −3.1 mm Hg [−5.3 to −0.9]). In separate analyses, exercise benefits on all ABP measures were significant for patients taking medication (all P<0.05) but not for untreated patients (although differences between medicated and non‐medicated patients were not significant), and only aerobic exercise provided significant benefits (P<0.05).
Conclusions
Aerobic exercise is an effective coadjuvant treatment for reducing ABP in medicated patients with hypertension.
Keywords: blood pressure, cardiovascular risk, hypertension, physical activity
Subject Categories: Hypertension
Nonstandard Abbreviations and Acronyms
- AIT
aerobic interval training
- MICT
moderate‐intensity continuous training
- RT
resistance training
Clinical Perspective
What Is New?
Exercise interventions significantly reduce 24‐hour ambulatory blood pressure in patients with hypertension, with decreases in both daytime and nighttime ambulatory blood pressure.
What Are the Clinical Implications?
Aerobic exercise is an effective coadjuvant treatment for reducing ambulatory blood pressure in patients with hypertension.
Hypertension is the major cause of premature death worldwide, which is associated with an estimated global direct medical cost of $370 billion/year. 1 This condition has been traditionally identified by assessing blood pressure (BP) in a clinical setting (ie, office [or "clinic"] BP) and medical treatment adjusted accordingly. The 2017 American College of Cardiology/American Heart Association proposed office BP of ≥130/80 mm Hg as a new threshold for diagnosis of hypertension, 2 whereas the 2018 European Society of Cardiology/European Society of Hypertension maintained an office BP threshold of ≥140/90 mm Hg to define hypertension, similar to previous guidelines. 3 Yet, monitoring of BP at regular intervals during normal day life (ie, ambulatory BP [ABP]) has emerged as a stronger predictor of cardiovascular disease and mortality, 4 , 5 , 6 , 7 , 8 with threshold criteria to define hypertension based on 24‐hour ABP set at 125/75 and 130/80 mm Hg in the United States 2 and European guidelines, 3 respectively. Particularly, an increased 24‐hour and nighttime ABP is associated with a high cardiovascular disease risk 9 —even if office BP is apparently well controlled (ie, systolic BP [SBP]/diastolic BP [DBP] <130/80 mm Hg), leading to a prevalent and especially unfavorable hypertension phenotype, the so‐called "masked uncontrolled hypertension". 10 For this reason, assessment of ABP rather than—or at least together with—office BP is currently proposed for the diagnosis and control of hypertension. 11 , 12
Given the high prevalence and negative consequences of hypertension, strategies other than drug treatment are needed for the management of this condition. In this context, a main lifestyle intervention is physical exercise, 13 although unfortunately physical inactivity is reaching pandemic proportions. 14 Tailored exercise has been shown not only to reduce office BP in individuals with hypertension, but also to be as effective as most antihypertensive drugs for office BP reduction. 15 , 16 Furthermore, exercise has minimal side effects compared with drugs. 13 , 17 However, scarcer evidence is available on the effects of exercise on ABP. To the best of our knowledge, the largest meta‐analysis to date on this topic (including 37 studies published until 2015) 18 assessed the pre‐post effects of exercise training. Yet, there was no comparison with a control group, individuals with hypertension and normotension were assessed together, and some of the included studies combined an exercise intervention with a weight‐loss diet. Moreover, although meta‐analytical evidence supports the effectiveness of different exercise modalities (endurance ["aerobic"], resistance training [RT], or a combination thereof) to reduce office BP, 15 , 16 the evidence is also scarcer on their effects on ABP.
A recent meta‐analysis including only 2 studies reported that aerobic training significantly reduces ABP. 19 However, other studies not included in the aforementioned meta‐analysis 19 have assessed the effects on ABP of aerobic, 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 RT, 21 , 23 , 26 , 32 or multi‐component exercise training 24 , 28 , 33 , 34 and there is no meta‐analytical evidence pooling the effects of these different exercise modalities based on evidence from randomized controlled trials (RCTs).
It was therefore the aim of this study to assess the effects of different modalities of exercise training on ABP in individuals with hypertension pooling evidence from RCTs.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. The conduct and reporting of the current systematic review and meta‐analysis conform to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (Figure S1). 35
Data Sources and Search Strategies
Two authors (G.S.L. and P.L.V.) independently conducted a systematic search (first by title and abstract, and then by full‐text) in the electronic databases PubMed and Scopus (with no restriction on initial date to April 1, 2020) using the following search strategy: (exercise OR "physical activity" OR training) AND ("ambulatory blood pressure" OR "ambulatory BP" OR "ambulatory SBP" OR "ambulatory DBP" OR "24‐hour blood pressure" OR "24‐hour BP" OR "24‐h blood pressure" OR "24‐h BP" OR "daytime blood pressure" OR "day‐time blood pressure" OR "daytime BP"). The search was supplemented by a manual review of reference lists from relevant publications to find additional studies on the subject. 18 , 42
Study Selection
Studies were eligible for inclusion if they met each of the following criteria: (1) RCT design; (2) participants aged ≥18 years; (3) included a physical exercise intervention; (4) all participants reported to be hypertensive and/or to be on antihypertensive medication; and (5) assessed ABP before and upon completion of the intervention. Studies were excluded if: (1) they assessed the acute—but not the chronic—effects of physical exercise on ABP; (2) had a cross‐over design; and (3) the exercise intervention was combined with a hypocaloric diet. The latter exclusion criterion was meant to avoid the confounding—and well‐documented—BP‐lowering effect of diet‐induced weight loss per se. 13 , 43 No inclusion/exclusion criteria were set on the intensity or duration of exercise training sessions.
Data Extraction
Two reviewers (G.S.L. and P.L.V.) independently extracted the following data from each study: number of participants within each group, participants' and exercise intervention characteristics, end points, and results. Data were extracted as mean and SD. A specific software (WebPlotDigitizer 4.2, San Francisco, CA) was used to extract data when provided as a figure 22 , 23 , 24 , 33 and we contacted the authors of 1 study because the values could not be extracted from figures. 44
Quality Assessment
Two authors (G.S.L. and P.L.V.) independently assessed the methodological quality of the included studies with the PEDro scale. 45 A 0 to 10 total score was determined by counting the number of criteria satisfied by each study. Study quality was rated as poor (PEDro score ≤3), fair (4–5), or high (>5). All studies were used for data synthesis independently of their methodological quality.
Statistical Analysis
A meta‐analysis was performed to assess the mean difference in the change (post‐ minus pre‐intervention data, in mm Hg) between the control and intervention groups along with 95% CI. Given the existing differences between studies in terms of participants' characteristics and exercise interventions (modality, intensity, or duration), as well as our intent to generalize the results beyond the included studies, a random effects model was used. 46 No information was available from any of the meta‐analyzed studies for the correlation between pre‐ and post‐intervention data. We therefore decided to use a conservative correlation Pearson coefficient (r) value of 0.7 between pre‐ and post‐intervention data, which is lower than most average correlation coefficients reported for ABP reliability measures 47 , 48 (eg, 0.79 for 24‐hour DBP and 0.82 for 24‐hour SBP for both sexes in repeated‐days measurements 48 ). Sensitivity analyses with an r‐value of 0.2 and 0.5 were then performed when a significant result was found to estimate the worst‐case scenario. Egger test was used to determine the presence of publication bias, and the I2 statistic was used to assess heterogeneity across studies. Sub‐analyses were performed attending to (1) whether participants were on anti‐hypertensive medication or not and (2) exercise modality. Meta‐regression analyses were conducted using the random‐effects model (method of moments) to assess the association between the magnitude of the effect (mm Hg) and the duration of studies (weeks). All statistical analyses were performed using the statistical software package Comprehensive Meta‐analysis 2.0 (Biostat; Englewood, NJ) setting the level of significance at 0.05.
RESULTS
Study Characteristics
From the retrieved studies, 15 (including 910 participants) were included in the systematic review (Figure 1). All studies were conducted in patients with hypertension aged 45 to 70 years and with a weighted average 24‐hour ABP of 132±4 (SBP) and 79±2 mm Hg (DBP) at baseline. In 11 studies 20 , 44 participants were taking antihypertensive drugs during the intervention, whereas in the other 4 studies 22 , 23 , 34 , 49 they had refrained from taking their usual medication before the start of the intervention (usual "washout" period before enrolling in the intervention of 2–6 weeks). The characteristics of the included studies are summarized in Table 1.
Figure 1. Flowchart of literature search.
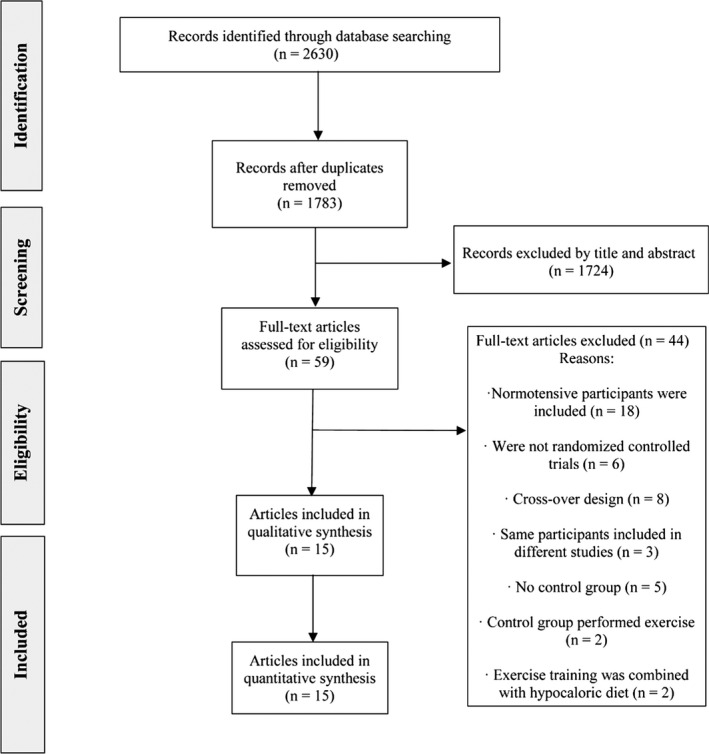
Table 1.
Main Characteristics of the Included Studies
| Study | Participants (Sample Size and Mean Age) | Exercise Intervention | Criteria to Define Hypertension | Antihypertensive Treatment | Main Effects on ABP |
|---|---|---|---|---|---|
|
Barroso et al 34 |
|
|
Office SBP/DBP ≥140/90 mm Hg after no drug treatment for ≥2 wk | No (drug washout before the study of 2 wk) |
|
| Bertani et al 21 |
|
AIT, alternating high and low‐intensity each 2 min for 20 min RT, 2 sets of 6–10 repetitions for 9 exercises
AIT: 60%–80% of MHR RT: 75% of 1RM |
"Hypertensives taking medication" (no other specification) |
Yes |
|
|
Blumenthal et al 22 |
ET: n=54 (≈46 y) CG: n=24 (≈47 y) |
|
"Unmedicated high normal BP" or stage 1–2 hypertension (mean office SBP 130–180 mm Hg and/or mean office DBP 85–110 mm Hg) |
No (drug washout before the study for at least 6 wk) |
|
| Blumenthal et al 23 |
ET: n=41 (≈54 y) RT+flexibility: n=35 (≈46 y) CG: n=23 (≈45 y) |
|
Office SBP 140–180 mm Hg or office DBP 90–105 mm Hg |
No (drug washout before the study of 4 wk) |
|
| Brito et al 44 |
ET: n=15 (≈51 y) MT: n=15 (≈49 y) CG: n=20 (≈50 y) |
|
Office SBP <160 mm Hg and office DBP <105 mm Hg while receiving anti‐hypertensive drugs for ≥4 mo |
Yes |
|
|
Dimeo et al 30 |
|
|
RH (ie, defined as office SBP/DBP ≥140/90 mm Hg in spite of concurrent use of 3 anti‐hypertensive drugs of different classes or a BP that is controlled with ≥4 anti‐hypertensive drugs) | Yes |
|
|
Farah et al 26 |
Age range, ≈58–61 y |
|
"Use of anti‐hypertensive medications" | Yes |
|
| Guimaraes et al 33 |
|
|
RH for >5 y with unchanged or regular use of 3 anti‐hypertensive drugs in the past 3 mo, with an office SBP/DBP ≥140/90 mm Hg | Yes |
|
|
Guimaraes et al 28 |
Age range, ≈45–50 y |
AIT: alternating 2 min at 50% and 1 min at 80% of HRR RT: submaximal strength training |
Hypertensive subjects on anti‐hypertensive medication with "controlled" office BP (SBP <140 mm Hg and DBP <90 mm Hg) | Yes |
|
| Lima et al 24 |
Age range, ≈67–69 y |
|
People regularly using anti‐hypertensive medication (hydrochlorothiazide, ACE inhibitors or ARB), with office SBP <160 mm Hg and office DBP <105 mm Hg) | Yes |
|
|
Molmen ‐Hansen et al 49 |
|
AIT 4×4 min intervals 85%–90% of MHR, with 3 min at 60%–70% of MHR |
Essential hypertension stage 1–2, defined as office SBP 140–179 mm Hg and/or office DBP 90–109 mm Hg |
No (drug washout before the study of 4 wk) |
|
| Motlagh et al 20 |
|
|
Diagnosed with primary hypertension, with an office SBP <170 mm Hg and taking ≥1 anti‐hypertensive medication | Yes |
|
|
Pagonas et al 29 |
|
|
Patients under anti‐hypertension treatment with ≥1 anti‐hypertensive drug and/or office SBP/DBP ≥140/90 mm Hg |
Yes |
|
| Stiller‐Moldovan et al 32 |
Age range, ≈60–62 y |
|
Individuals medicated for hypertension for ≥4 mo | Yes |
|
|
Westhoff et al 31 |
|
|
Current anti‐hypertension treatment, ambulatory DBP ≤90 mm Hg | Yes |
|
1RM indicates one maximum repetition; ABP, ambulatory blood pressure; ACE, angiotensin‐converting enzyme; AIT, aerobic interval training; ARB, angiotensin receptor blockers; BP, blood pressure; CG, control group; CT, combined training; DBP, diastolic blood pressure; ET, evening training; HIIT, high‐intensity interval training; HRR, heart rate reserve; IT, isometric handgrip training; MT, morning training; MHR, maximum heart rate; MICT, moderate‐intensity continuous training; MVC, maximal voluntary contraction; RCP, respiratory compensation point; RH, resistant hypertension; RHR, reserve heart rate; RT, resistance training; SBP, systolic blood pressure; and VO2max, maximal oxygen uptake.
Exercise interventions lasted between 8 and 24 weeks and included 3 to 5 sessions per week (≈24–60 minutes per session). Exercise sessions were supervised in 13 studies, 22 , 23 , 24 , 44 , 49 3 studies 26 , 28 , 32 included both supervised and non‐supervised exercise, and 2 included only non‐supervised exercise. 20 , 24 Different modalities of exercise were used, notably moderate‐intensity continuous training 20 , 21 , 22 , 23 , 24 , 44 , 49 or aerobic interval training for aerobic exercise, 29 , 30 , 31 , 49 RT 21 , 23 , 26 , 32 (consisting of only isometric handgrip training in 2 studies), 26 , 32 or a combination of both aerobic and RT 24 , 28 , 33 , 34 (ie, multicomponent exercise training, which was performed on a heated [30°C–32°C] swimming pool in 1 study). 33 On the other hand, 7 studies 22 , 23 , 26 , 28 , 29 , 33 , 44 reported the adherence rate to the exercise interventions, which ranged from 61% to 100% (weighted average 81%).
No study reported any type of adverse event related to the exercise sessions (eg, no musculoskeletal injury or excessive hypertensive/hypotensive response).
Quality Assessment and Publication Bias
The quality of the included studies was overall fair (median PEDro score=4.8 [range, 4–6]; Table 2). Thirteen studies showed fair methodological quality, 20 , 21 , 22 , 23 , 24 , 44 , 49 and 2 were deemed to have a high quality. 20 , 26
Table 2.
Methodological Quality of the Included Studies
| Items | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total Score* |
| Barroso et al 34 | + | + | − | + | − | − | − | + | − | + | + | 5 |
| Bertani et al 21 | + | + | − | − | − | − | − | + | − | + | + | 4 |
| Blumenthal et al 22 | + | + | − | + | − | − | − | − | + | + | + | 5 |
| Blumenthal et al 23 | + | + | − | + | − | − | − | + | − | + | + | 5 |
| Brito et al 44 | + | + | − | + | − | − | − | + | − | + | + | 5 |
| Dimeo et al 30 | + | + | − | + | − | − | − | + | − | + | + | 5 |
| Farah et al 26 | + | + | + | + | − | − | + | − | − | + | + | 6 |
| Guimaraes et al 33 | + | + | − | + | − | − | + | − | − | + | + | 5 |
| Guimaraes et al 28 | + | + | + | − | − | + | − | − | + | + | 5 | |
| Lima et al 24 | + | + | − | + | − | − | − | + | − | + | + | 5 |
| Molmen‐Hansen et al 49 | + | + | − | + | − | − | − | − | − | + | + | 4 |
| Motlagh et al 20 | + | + | + | + | − | − | − | − | + | + | + | 6 |
| Pagonas et al 29 | + | + | − | + | − | − | − | + | − | + | + | 5 |
| Stiller‐Moldovan et al 32 | + | + | + | + | − | − | − | − | − | − | + | 4 |
| Westhoff et al 31 | + | + | − | − | − | − | − | + | − | + | + | 4 |
Column numbers correspond to the following criteria on the PEDro scale: (1) eligibility criteria were specified; (2) subjects were randomly allocated to groups; (3) allocation was concealed; (4) groups were similar at baseline; (5) subjects were blinded; (6) therapists who administered the treatment were blinded; (7) assessors were blinded; (8) measures of key outcomes were obtained from >85% of subjects; (9) data were analyzed by intention to treat; (10) statistical comparisons between groups were conducted; (11) point measures and measures of variability were provided.
Total score from item 2.
Synthesis
The pooled effects of exercise interventions on ABP are summarized in Table 3. The pooled analysis of the 12 studies (n=582 participants) that assessed the effects of exercise on 24‐hour ABP showed a significant reduction in both SBP and DBP (Figure 2).† No heterogeneity (I2=0% for both) and no signs of publication bias (P=0.231 and 0.319 for SBP and DBP, respectively) were observed, and the effect remained significant in sensitivity analyses (P<0.05).
Table 3.
Summary of Pooled Results
| Condition | Studies (Participants) | Outcome |
Mean Difference (mm Hg , 95% CI) |
P Value |
|---|---|---|---|---|
| 24‐h ABP | ||||
| Overall | 12 (n=582) | SBP | −5.4 (−9.3 to −1.5) | 0.006* |
| DBP | −3.0 (−5.4 to −0.6) | 0.015* | ||
| Medicated patients | 10 (n=474) | SBP | −4.9 (−9.1 to −0.7) | 0.022* |
| DBP | −2.8 (−5.5 to −0.1) | 0.039* | ||
| Non‐medicated patients | 2 (n=108) | SBP | −7.9 (−17.8 to 2.0) | 0.117 |
| DBP | −3.8 (−10.0 to 2.4) | 0.230 | ||
| Daytime ABP | ||||
| Overall | 13 (n=711) | SBP | −4.5 (−6.6 to −2.3) | <0.001* |
| DBP | −3.2 (−4.8 to −1.5) | <0.001* | ||
| Medicated patients | 10 (n=468) | SBP | −4.7 (−7.3 to −2.1) | <0.001* |
| DBP | −3.3 (−5.3 to −1.3) | 0.001* | ||
| Non‐medicated patients | 3 (n=243) | SBP | −3.9 (−8.2 to 0.4) | 0.075 |
| DBP | −2.8 (−6.0 to 0.5) | 0.094 | ||
| Nighttime ABP | ||||
| Overall | 11 (n=587) | SBP | −4.7 (−8.4 to −1.0) | 0.013* |
| DBP | −3.1 (−5.3 to −0.9) | 0.007* | ||
| Medicated patients | 10 (n=514) | SBP | −5.2 (−9.2 to −1.3) | 0.009* |
| DBP | −3.4 (−5.8 to −1.0) | 0.005* | ||
| Non‐medicated patients | 1 (n=73) | SBP | 0.0 (−11.3 to 11.3) | 0.997 |
| DBP | 0.8 (−7.6 to 6.0) | 0.818 | ||
Mean difference is expressed in mm Hg. ABP indicates ambulatory blood pressure; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
Significant difference for the comparison between control and exercise groups (P < 0.05).
Figure 2. Effects of exercise interventions on 24‐hour ambulatory systolic (A) and diastolic ambulatory blood pressure (B) in individuals with hypertension.
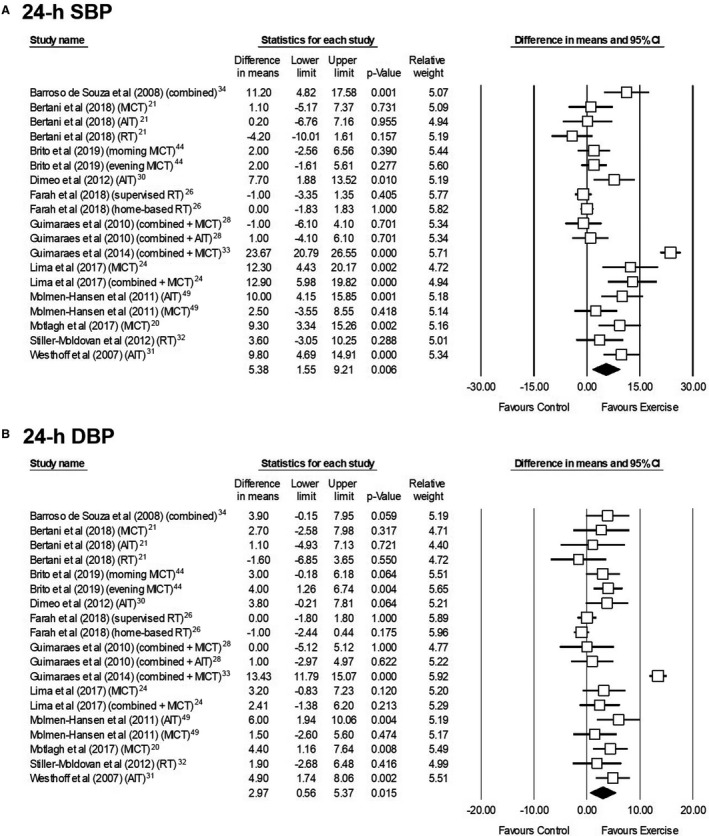
AIT indicates aerobic interval training; DBP, diastolic blood pressure; MICT, moderate‐intensity continuous training; RT, resistance training; and SBP, systolic blood pressure.
Thirteen studies (n=711 participants) assessed the effects of exercise on daytime ABP, with pooled analysis showing a significant reduction in SBP and DBP (Figure 3). 21 , 44 , 49 A moderate heterogeneity was found for the effects on SBP (I2=53.0%) but not on DBP (I2=13.5%), and no sign of publication bias was observed for any of these 2 measures (P=0.072 and 0.156, respectively). The effect remained significant in sensitivity analyses (P<0.05).
Figure 3. Effects of exercise interventions on daytime ambulatory systolic (A) and diastolic blood pressure (B) in individuals with hypertension.
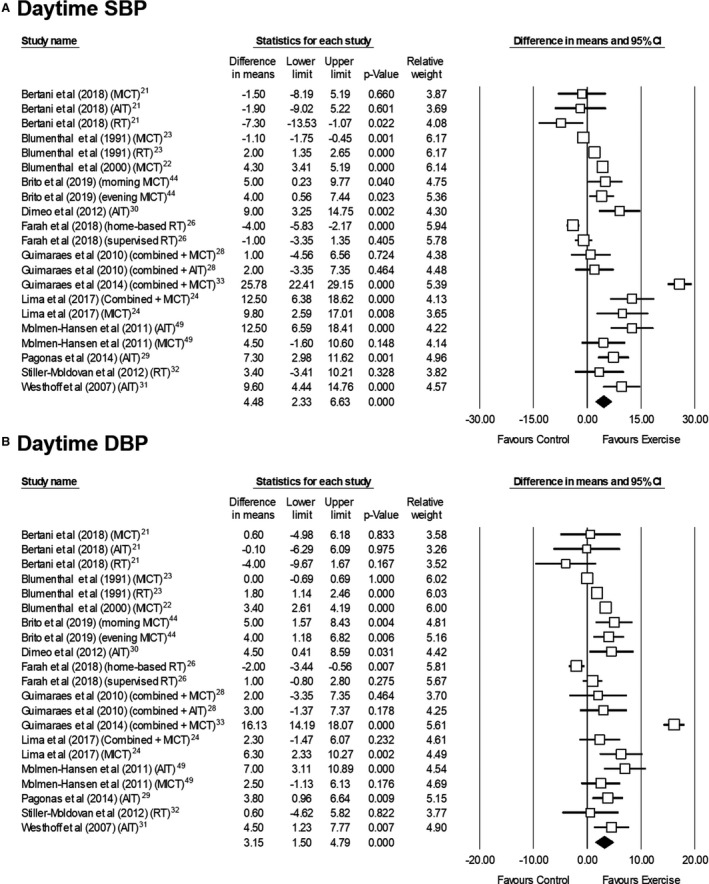
AIT indicates aerobic interval training; DBP, diastolic blood pressure; MICT, moderate‐intensity continuous training; RT, resistance training; and SBP, systolic blood pressure.
Eleven studies (n=587 participants) assessed exercise training effects on nighttime ABP, with pooled analysis indicating a significant reduction in SBP and DBP (Figure 4). 21 , 44 , 49 There was no sign of heterogeneity (I2=0% for both measures) or publication bias (P=0.221 and 0.110 for SBP and DBP, respectively), and effects remained significant in sensitivity analyses (P<0.05).
Figure 4. Effects of exercise interventions on nighttime ambulatory systolic (A) and diastolic blood pressure (B) in individuals with hypertension.
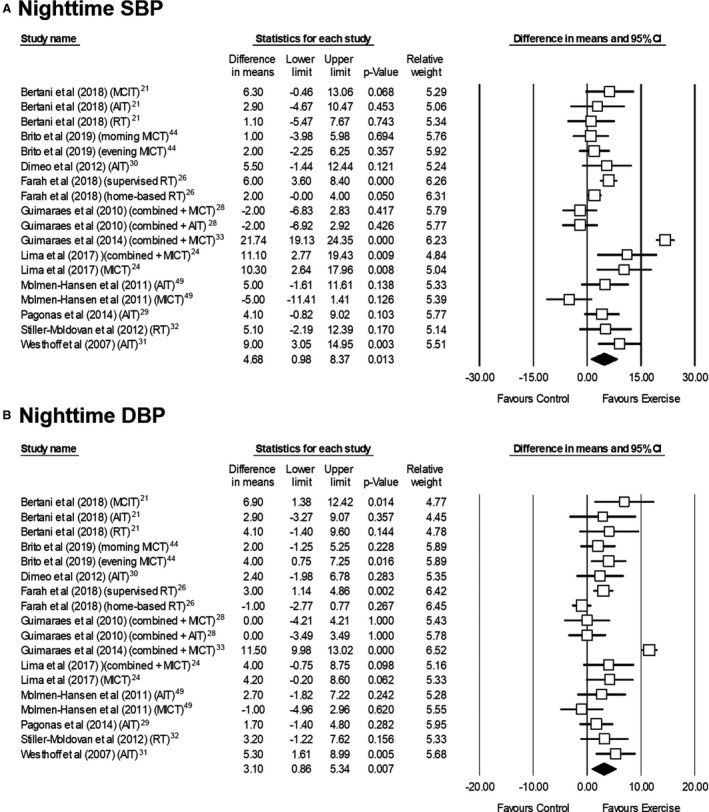
AIT indicates aerobic interval training; DBP, diastolic blood pressure; MICT, moderate‐intensity continuous training; RT, resistance training; and SBP, systolic blood pressure.
Exercise benefits on 24‐hour, daytime and nighttime ABP were significant in separate analyses of patients on medication during the study, 20 , 44 but not of those who were untreated (Table 3). 22 , 23 , 34 , 49 However, differences between medicated and non‐medicated patients did not reach statistical significance for any of the ABP measures (24 hour SBP: 2.97 mm Hg, 95% CI −7.75 to 13.69, P=0.587; 24 hour DBP: 0.99 mm Hg, 95% CI −5.77 to 7.75, P=0.774; daytime SBP: −0.83 mm Hg, 95% CI −5.82 to 4.16, P=0.746; daytime DBP: −0.54 mm Hg, 95% CI −4.31 to 3.23, P=0.779; nighttime SBP: −5.27 mm Hg, 95% CI −17.21 to 6.67, P=0.387; nighttime DBP: −0.53 mm Hg, 95% CI −1.48 to 0.42, P=0.484). Regarding the studies in untreated patients, 2 studies 22 , 49 found significant benefits on daytime ABP, of which one 49 also reported significant benefits for both nighttime and 24‐hour ABP. However, the remaining 2 studies found no benefits on any ABP marker. 23 , 34
Exercise benefits on all ABP measures could be separately confirmed for aerobic exercise, 20 , 21 , 22 , 23 , 24 , 49 whereas no significant benefits were observed for RT interventions combining both handgrip strength and whole‐body (or "large muscle mass") exercises, 21 , 23 , 26 , 32 or multicomponent training 24 , 28 , 33 , 34 on any ABP measure (Table 4). When separately analyzing the 2 studies 26 , 32 (3 interventions in total) that included only isometric handgrip exercise, no differences were found for any ABP measure (all P>0.05, Table 4). The same result was found when separately analyzing the 2 studies 21 , 23 that assessed the effects of whole‐body RT on daytime ABP (P>0.05). Two of the four studies that included a multicomponent training intervention reported benefits on at least one ABP measure. 24 , 33
Table 4.
Summary of Pooled Results for Each Exercise Modality
| Exercise Type | Outcome | Studies (Participants) | Mean Difference (mm Hg, 95% CI) | P Value |
|---|---|---|---|---|
| 24‐h ABP | ||||
| Aerobic | SBP | 7 (n=373) | −5.5 (−8.1 to −2.8) | <0.001* |
| DBP | −3.8 (−4.9 to −2.6) | <0.001* | ||
| Resistance | SBP | 3 (n=99) | 0.5 (−1.1 to 2.1) | 0.573 |
| DBP | 0.5 (−0.6 to 1.6) | 0.349 | ||
| Handgrip | SBP | 2 (n=68) | 0.2 (−1.2 to 1.6) | 0.784 |
| DBP | 0.5 (−0.7 to 1.6) | 0.421 | ||
| Combined | SBP | 4 (n=139) | −9.6 (−20.7 to 1.5) | 0.091 |
| DBP | −4.3 (−10.8 to 2.2) | 0.194 | ||
| Daytime ABP | ||||
| Aerobic | SBP | 9 (n=507) | −5.0 (−7.6 to −2.3) | <0.001* |
| DBP | −3.5 (−5.1 to −1.9) | <0.001* | ||
| Resistance | SBP | 4 (n=152) | 1.3 (−2.2 to 4.8) | 0.471 |
| DBP | 0.1 (−2.1 to 2.2) | 0.935 | ||
| Handgrip | SBP | 2 (n=68) | 1.7 (−1.5 to 4.8) | 0.309 |
| DBP | 0.4 (−2.0 to 2.8) | 0.754 | ||
| Whole‐body | SBP | 2 (n=84) | 2.1 (−6.9 to 11.2) | 0.644 |
| DBP | 0.4 (−5.1 to 5.9) | 0.882 | ||
| Combined | SBP | 3 (n=104) | −10.4 (−23.7 to 2.9) | 0.125 |
| DBP | −6.0 (−14.7 to 2.7) | 0.178 | ||
| Nighttime ABP | ||||
| Aerobic | SBP | 7 (n=367) | −3.8 (−6.4 to −1.3) | 0.003* |
| DBP | −2.9 (−4.1 to −1.6) | <0.001* | ||
| Resistance | SBP | 3 (n=99) | −3.7 (−6.4 to −0.9) | 0.009* |
| DBP | −1.9 (−4.6 to 0.8) | 0.175 | ||
| Handgrip | SBP | 2 (n=68) | −2.8 (−9.2 to 3.6) | 0.398 |
| DBP | −1.5 (−4.6 to 1.6) | 0.345 | ||
| Combined | SBP | 3 (n=104) | −7.3 (−21.6 to 7.1) | 0.321 |
| DBP | −4.0 (−11.0 to 2.9) | 0.257 | ||
Mean difference is expressed in mm Hg. ABP indicates ambulatory blood pressure; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
Significant difference for the comparison between control and exercise groups (P < 0.05).
Meta‐regression analyses showed no consistent association between the magnitude of the effect and the duration of exercise intervention. Thus, a direct association was found between intervention duration and magnitude of the reduction in daytime SBP (−0.2 mm Hg per each additional week of exercise, 95% CI, −0.3 to −0.1; P<0.001) but an inverse association was found for the reduction of nighttime SBP (+1.2 mm Hg per week, 95% CI, 0.4–1.9; P=0.002) and DBP (+0.6 mm Hg per week, 95% CI, 0.0–1.1; P=0.047). No association was found for the remainder of ABP measures.
DISCUSSION
This systematic review and meta‐analysis of RCTs found that exercise training interventions result in significant reductions in 24‐hour (−5.4 and −3.0 mm Hg for SBP and DBP, respectively), daytime (−4.5 and −3.2 mm Hg), and nighttime ABP (−4.7 and −3.1 mm Hg) among individuals with hypertension. In turn, aerobic exercise appeared as an effective training modality for reducing ABP, whereas RT and multicomponent training showed no overall benefits.
Previous evidence has indicated an overall beneficial effect of exercise training on ABP. 18 , 19 , 25 , 36 , 37 , 38 , 40 , 41 , 42 For instance, a meta‐analysis including both individuals with hypertension and normotension found that physical exercise reduces daytime (≈ −3.3 mm Hg) but not nighttime ABP. 40 Another meta‐analysis reported a significant reduction of daytime (≈ −3.2 mm Hg) but not in nighttime ABP when analyzing both individuals with normotension and hypertension, and this effect remained significant in separate analyses for individuals with hypertension only (≈ −3.8 mm Hg). 25 In turn, and in agreement with other studies, 18 the present results suggest that exercise training reduces both daytime and nighttime ABP.
To our knowledge, this is the first meta‐analysis that assesses the effects of exercise interventions separately in individuals with hypertension and pooling the results of RCTs, with the latter considered the greatest level of evidence. The present findings are clinically important, particularly given the role of ABP—beyond office BP—as a predictor of cardiovascular disease and mortality. 4 , 5 , 6 , 7 , 8 Specially relevant are the effects of exercise training on nighttime ABP, with the latter being a better predictor of adverse events in patients with hypertension than daytime ABP. 50 It must also be highlighted that office BP reductions of lower magnitude (−4.9 and −2.8 mm Hg for SBP and DBP, respectively) than those observed here for 24‐hour ABP have proven to reduce the risk of stroke and coronary heart disease. 51 In this regard, office BP usually tends to be higher than ABP, and thus the reductions we observed for ABP might correspond with larger reductions of office BP—for instance, in SPRINT (Systolic Blood Pressure Intervention Trial) an intensive medical treatment induced larger reductions in office (−16.0 mm Hg) than in 24‐hour SBP (−11.2 mm Hg). 52
Some controversy exists on whether lifestyle interventions, notably exercise, could be used as a surrogate of pharmacological treatment in patients with hypertension. The European guidelines 3 recommend an optimal lifestyle (including regular exercise) as the only treatment needed for people with grade 1 (mild)—but not for grade 2 or 3—hypertension during the first 3 to 6 months after diagnosis, with pharmacological treatment added after this period if hypertension is not well controlled. Supporting this recommendation, a recent network meta‐analysis concluded that exercise interventions might induce the same lowering effect on office BP—ABP was not assessed—as most anti‐hypertensive drugs 15 in patients with hypertension, although the included studies did not directly compare the effects of exercise versus drugs. On the other hand, the reason why in our separate analyses exercise appeared to be effective to decrease ABP in patients on medication but not in their untreated peers might be explained, at least partly, by the relative short duration of most interventions in the latter (ie, consistently ≤6 months) 22 , 23 , 34 , 49 as well as the moderate intensity of the aerobic exercise sessions (ie, usually moderate‐intensity continuous training, except for one study 49 using more intense workouts [aerobic interval training]). Further research is thus needed to determine whether longer or more intense aerobic exercise interventions can have a stronger anti‐hypertensive effect in the absence of medication. In any case, no significant differences were found between medicated and non‐medicated patients for each of the different ABP measures. Studies comparing exercise effects on the 2 types of patients might allow to draw more definite conclusions based on medication status.
Another major novelty of the present study is the analysis of the effects on ABP of different exercise modalities. Although aerobic exercise is commonly recommended as a first‐line antihypertensive lifestyle therapy, dynamic RT or the combination of both aerobic and RT exercise have been reported to elicit similar or even greater reductions in office BP. 16 , 18 , 53 In the present meta‐analysis, however, only aerobic training showed benefits on all ABP measures, with no significance reached for RT or multicomponent training. In this regard, the numerous biological underpinnings of the exercise benefits on BP at the multisystemic level—loss of adiposity (especially visceral adiposity), increased insulin sensitivity, attenuated oxidative stress and inflammation with subsequent improvements in vascular endothelial function, vascular remodeling with increase in the luminal diameter of conduit and resistance arteries, and improved arterial baroreflex control and thus autonomic balance—have been documented mainly with aerobic training, with scarcer evidence available for other exercise modalities. 13 Interestingly, no other lifestyle intervention (including weight loss) has proven to act on so many potential BP‐reducing mechanisms at the multisystemic level as aerobic exercise. 13
It must be noted that a limited number of studies 21 , 23 , 24 , 26 , 28 , 32 , 33 , 34 was available on the effects of exercise modalities other than aerobic training. Moreover, RT interventions included whole‐body exercises in some studies, 21 , 23 whereas in others they consisted solely of isometric handgrip exercise. 26 , 32 In this context, although some evidence from research on both individuals who were healthy or hypertensive suggests that isometric RT might be as effective as other exercise modalities to reduce office BP, 54 , 55 a recent meta‐analysis found that the ABP‐lowering effect of isometric RT among individuals with hypertension did not reach statistical significance. 15 Based on our results, regular aerobic exercise appears as an effective lifestyle intervention for reducing ABP in medicated patients with hypertension, with a minimal dose difficult to establish but possibly corresponding to ≥3 sessions/week, ≥30 min/session, and an intensity of ≈60% to 70% maximum heart rate or peak oxygen uptake for ≥3 months. Thus, these recommendations would be approximately in line with those of the World Health Organization‐determined minimum recommendations (ie, ≥150 min/week of moderate‐intensity physical activity [eg, walking/brisk walking] or ≥75 min/week of vigorous‐intensity physical activity [eg, very brisk walking], or a combination thereof). 56 Because no benefits were observed in separate analyses for interventions combining both RT and aerobic training, future studies should determine whether RT actually nullifies the beneficial effects of aerobic exercise on ABP. In this regard, the low number of studies available and the heterogeneity among studies in interventions' characteristics can be viewed as a potentially confounding factor. Further research is therefore needed to confirm the effects of exercise modalities other than aerobic exercise (notably whole‐body or isometric RT, and combined training)—as well as of different exercise intensities and/or intervention durations.
An important question that remains to be solved is the sustainability of exercise benefits on ABP since the longer exercise intervention lasted 6 months, 22 , 34 and none of the included studies performed a follow‐up. Moreover, our meta‐regression analysis yielded inconsistent results, with a positive association between BP reduction and intervention length observed for daytime ABP but the opposite trend observed for nighttime ABP—which might be due to the low number of studies available, potential methodological differences between studies, and lack of long‐term interventions. In this regard, some research suggests that exercise benefits on office BP might still be observed with long‐term interventions (≥12 months). 57 , 58 However, a meta‐analysis concluded that exercise interventions reduce office SBP in the short‐middle term (3–6 months) in young adults with prehypertension/hypertension but these benefits are lost at ≥12‐month follow‐up. 59 Future studies should also consider the levels of physical activity performed by both intervention arms (control and exercise) outside the exercise intervention per se (for instance, by means of accelerometers). Another important question is how exercise compares with antihypertensive medication in terms of patients' adherence. In this context, the average weighted value of 81% found in our meta‐analysis for exercise might suggest that adherence to this lifestyle intervention is not necessarily lower compared with drugs. For instance, a retrospective analysis of dosing histories of patients prescribed once a day antihypertensive drugs showed that half of the patients stopped treatment within a year 60 and a non‐adherence rate of 28.4% has been reported for newly prescribed medications against hypertension. 61
Some limitations must be acknowledged, notably the relatively low number of studies included—particularly for those conducted with non‐medicated patients with hypertension and for some exercise modalities such as RT or multicomponent training. Moreover, the paucity of studies and the lack of information provided for some variables (eg, exercise intensity relative to well‐accepted markers such as maximum oxygen consumption or maximum heart rate) also hindered performing sub‐analyses attending to exercise intensity. In addition, we analyzed studies implementing exercise interventions in individuals with different grades of hypertension (including resistant hypertension) and authors used different BP or medication criteria for patient inclusion. However, many studies had to be excluded due to the strict inclusion criteria we applied (ie, RCTs including only patients with hypertension who were not undergoing a weight‐loss diet), which increases in turn the validity of our findings.
CONCLUSIONS
The present findings suggest that exercise training results in significant reductions of all ABP measures (ie, 24‐hour, daytime, and nighttime ABP) in individuals with hypertension. Although further evidence is needed to elucidate whether it can replace antihypertensive drugs, exercise training (particularly with aerobic modalities) appears as an effective coadjuvant treatment in hypertension.
Sources of Funding
The work of Valenzuela is supported by University of Alcalá (FPI2016). Research by Lucia is funded by Instituto de Salud Carlos III (Ministerio de Ciencia, Innovación y Universidades, Spain) (PI18/00139) and FEDER funds from the European Union.
Disclosures
None.
Supporting information
Figure S1
Acknowledgments
We would like to express our appreciation to Dr Leandro Campos de Brito (Exercise Hemodynamic Laboratory, School of Physical Education and Sport, University of São Paulo ‐ Brazil) and Dr Claudia Forjaz (School of Physical Education and Sport, University of São Paulo ‐ Brazil) for providing the required data.
Author contributions: Study concept and design: Saco‐Ledo, Valenzuela and Lucia; Methodology and supervision: Valenzuela, Saco‐Ledo and Lucia; Interpretation of data: Valenzuela, Saco‐Ledo, and Lucia; Drafting of the manuscript: Saco‐Ledo, Valenzuela and Lucia; Statistical analysis: Valenzuela; Critical revision of the manuscript for important intellectual content: All authors; Approval of the final version of the manuscript: All authors.
(J Am Heart Assoc. 2020;9:e018487. DOI: 10.1161/JAHA.120.018487.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018487
For Sources of Funding and Disclosures, see page 13.
Footnotes
REFERENCES
- 1. Frieden TR, Jaffe MG. Saving 100 million lives by improving global treatment of hypertension and reducing cardiovascular disease risk factors. J Clin Hypertens. 2018;20:208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 3. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 4. Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O'Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. [DOI] [PubMed] [Google Scholar]
- 5. Kikuya M, Ohkubo T, Asayama K, Metoki H, Obara T, Saito S, Hashimoto J, Totsune K, Hoshi H, Satoh H, et al. Ambulatory blood pressure and 10‐year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension. 2005;45:240–245. [DOI] [PubMed] [Google Scholar]
- 6. Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp‐Pedersen C. Ambulatory blood pressure and mortality: a population‐based study. Hypertension. 2005;45:499–504. [DOI] [PubMed] [Google Scholar]
- 7. Fagard RH, Van Den Broeke C, De Cort P. Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens. 2005;19:801–807. [DOI] [PubMed] [Google Scholar]
- 8. Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow‐up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. [DOI] [PubMed] [Google Scholar]
- 9. Yang WY, Melgarejo JD, Thijs L, Zhang ZY, Boggia J, Wei FF, Hansen TW, Asayama K, Ohkubo T, Jeppesen J, et al.; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) Investigators . Association of Office and Ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruilope LM, Ruiz‐Hurtado G, Barderas MG, de la Cruz JJ, Lucia A, de la Sierra A, Gorostidi M, Vinyoles E, Segura J, Solís J, et al. Frequency and prognosis of treated hypertensive patients according to prior and new blood pressure goals: post hoc analysis of the Spanish ambulatory blood pressure monitoring registry. Hypertension. 2019;74:130–136. [DOI] [PubMed] [Google Scholar]
- 11. Segura J, Banegas JR, Ruilope LM. Usefulness of ambulatory blood pressure monitoring (ABPM) in daily clinical practice: data from the Spanish ABPM registry. Clin Exp Pharmacol Physiol. 2014;41:30–36. [DOI] [PubMed] [Google Scholar]
- 12. Anstey ED, Muntner P, Bello NA, Pugliese DN, Yano Y, Kronish IM, Reynolds K, Schwartz JE, Shimbo D. Diagnosing masked hypertension using ambulatory blood pressure monitoring, home blood pressure monitoring, or both? Hypertension. 2018;72:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Valenzuela PL, Carrera‐Bastos P, Gálvez BG, Ruiz‐Hurtado G, Ordovás JM, Ruilope LM, Lucia A. Lifestyle interventions for the prevention and treatment of hypertension. Nat Rev Cardiol. 2020. Oct 9 [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population‐based surveys with 1·9 million participants. Lancet Glob Health. 2018;6:e1077–e1086. [DOI] [PubMed] [Google Scholar]
- 15. Naci H, Salcher‐Konrad M, Dias S, Blum MR, Sahoo SA, Nunan D, Ioannidis JPA. How does exercise treatment compare with antihypertensive medications? A network meta‐analysis of 391 randomised controlled trials assessing exercise and medication effects on systolic blood pressure. Br J Sports Med. 2019;53:859–869. [DOI] [PubMed] [Google Scholar]
- 16. Pescatello LS, Buchner DM, Jakicic JM, Powell KE, Kraus WE, Bloodgood B, Campbell WW, Dietz S, Dipietro L, George SM, et al.; 2018 PHYSICAL ACTIVITY GUIDELINES ADVISORY COMMITTEE . Physical activity to prevent and treat hypertension: a systematic review. Med Sci Sports Exerc. 2019;51:1314–1323. [DOI] [PubMed] [Google Scholar]
- 17. Fiuza‐Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology. 2013;28:330–358. [DOI] [PubMed] [Google Scholar]
- 18. Sosner P, Guiraud T, Gremeaux V, Arvisais D, Herpin D, Bosquet L. The ambulatory hypotensive effect of aerobic training: a reappraisal through a meta‐analysis of selected moderators. Scand J Med Sci Sport. 2017;27:327–341. [DOI] [PubMed] [Google Scholar]
- 19. Cao L, Li X, Yan P, Wang X, Li M, Li R, Shi X, Liu X, Yang K. The effectiveness of aerobic exercise for hypertensive population: a systematic review and meta‐analysis. J Clin Hypertens. 2019;21:868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Motlagh Z, Hidarnia A, Kaveh MH, Kojuri J. Effect of theory‐based training intervention on physical activity and blood pressure in hypertensive patients: a randomized control trial. Iran Red Crescent Med J. 2017;19:e55610. [Google Scholar]
- 21. Bertani RF, Campos GO, Perseguin DM, Bonardi JMT, Ferriolli E, Moriguti JC, Lima NKC. Resistance exercise training is more effective than interval aerobic training in reducing blood pressure during sleep in hypertensive elderly patients. J Strength Cond Res. 2018;32:2085–2090. [DOI] [PubMed] [Google Scholar]
- 22. Blumenthal JA, Sherwood A, Gullette EC, Babyak M, Waugh R, Georgiades A, Craighead LW, Tweedy D, Feinglos M, Appelbaum M, et al. Exercise and weight loss reduce blood pressure in men and women with mild hypertension: effects on cardiovascular, metabolic, and hemodynamic functioning. Arch Intern Med. 2000;160:1947–1958. [DOI] [PubMed] [Google Scholar]
- 23. Blumenthal JA, Siegel WC, Appelbaum M. Failure of exercise to reduce blood pressure in patients with mild hypertension: results of a randomized controlled trial. JAMA. 1991;266:2098–2104. [PubMed] [Google Scholar]
- 24. Lima LG, Bonardi JTM, Campos GO, Bertani RF, Scher LML, Moriguti JC, Ferriolli E, Lima NKC. Combined aerobic and resistance training: are there additional benefits for older hypertensive adults? Clinics. 2017;72:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta‐analysis. J Hypertens. 2013;31:639–648. [DOI] [PubMed] [Google Scholar]
- 26. Farah BQ, Rodrigues SLC, Silva GO, Pedrosa RP, Correia MA, Barros MVG, Deminice R, Marinello PC, Smart NA, Vianna LC, et al. Supervised, but not home‐based, isometric training improves brachial and central blood pressure in medicated hypertensive patients: a randomized controlled trial. Front Physiol. 2018;9:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beard JR, Officer A, De Carvalho IA, Sadana R, Pot AM, Michel JP, Lloyd‐Sherlock P, Epping‐Jordan JE, Peeters GMEEG, Mahanani WR, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387:2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guimarães GV, Ciolac EG, Carvalho VO, D’Avila VM, Bortolotto LA, Bocchi EA. Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens Res. 2010;33:627–632. [DOI] [PubMed] [Google Scholar]
- 29. Pagonas N, Dimeo F, Bauer F, Seibert F, Kiziler F, Zidek W, Westhoff TH. The impact of aerobic exercise on blood pressure variability. J Hum Hypertens. 2014;28:367–371. [DOI] [PubMed] [Google Scholar]
- 30. Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W, Westhoff TH. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension. 2012;60:653–658. [DOI] [PubMed] [Google Scholar]
- 31. Westhoff TH, Franke N, Schmidt S, Vallbracht‐Israng K, Meissner R, Yildirim H, Schlattmann P, Zidek W, Dimeo F, van der Giet M. Too old to benefit from sports? The cardiovascular effects of exercise training in elderly subjects treated for isolated systolic hypertension. Kidney Blood Press Res. 2007;30:240–247. [DOI] [PubMed] [Google Scholar]
- 32. Stiller‐Moldovan C, Kenno K, McGowan CL. Effects of isometric handgrip training on blood pressure (resting and 24 h ambulatory) and heart rate variability in medicated hypertensive patients. Blood Press Monit. 2012;17:55–61. [DOI] [PubMed] [Google Scholar]
- 33. Guimaraes GV, De Barros Cruz LG, Fernandes‐Silva MM, Dorea EL, Bocchi EA. Heated water‐based exercise training reduces 24‐hour ambulatory blood pressure levels in resistant hypertensive patients: a randomized controlled trial (HEx trial). Int J Cardiol. 2014;172:434–441. [DOI] [PubMed] [Google Scholar]
- 34. Barroso WKS, Jardim PCBV, Vitorino PV, Bittencourt A, Miquetichuc F. Influência da atividade física programada na pressão arterial de idosos hipertensos sob tratamento não‐farmacológico. Rev Assoc Med Bras. 2008;54:328–333. [DOI] [PubMed] [Google Scholar]
- 35. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Way KL, Sultana RN, Sabag A, Baker MK, Johnson NA. The effect of high Intensity interval training versus moderate intensity continuous training on arterial stiffness and 24 h blood pressure responses: a systematic review and meta‐analysis. J Sci Med Sport. 2019;22:385–391. [DOI] [PubMed] [Google Scholar]
- 37. Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure‐regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46:667–675. [DOI] [PubMed] [Google Scholar]
- 38. Fagard RH. Exercise is good for your blood pressure: effects of endurance training and resistance training. Clin Exp Pharmacol Physiol. 2006;33:853–856. [DOI] [PubMed] [Google Scholar]
- 39. Pescatello LS, Kulikowich JM. The aftereffects of dynamic exercise on ambulatory blood pressure. Med Sci Sports Exerc. 2001;33:1855–1861. [DOI] [PubMed] [Google Scholar]
- 40. Fagard RH, Cornelissen VA. Effect of exercise on blood pressure control in hypertensive patients. Eur J Prev Cardiol. 2007;14:12–17. [DOI] [PubMed] [Google Scholar]
- 41. Thompson S, Wiebe N, Padwal RS, Gyenes G, Headley SAE, Radhakrishnan J, Graham M. The effect of exercise on blood pressure in chronic kidney disease: a systematic review and meta‐analysis of randomized controlled trials. PLoS One. 2019;14:e0211032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Costa EC, Hay JL, Kehler DS, Boreskie KF, Arora RC, Umpierre D, Szwajcer A, Duhamel TA. Effects of high‐intensity interval training versus moderate‐intensity continuous training on blood pressure in adults with pre‐ to established hypertension: a systematic review and meta‐analysis of randomized trials. Sport Med. 2018;48:2127–2142. [DOI] [PubMed] [Google Scholar]
- 43. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta‐analysis of randomized controlled trials. Hypertension. 2003;42:878–884. [DOI] [PubMed] [Google Scholar]
- 44. Brito LC, Peçanha T, Fecchio RY, Rezende RA, Sousa P, Da Silva‐Júnior ND, Abreu A, Silva G, Mion‐Junior D, Halliwill JR, et al. Morning versus evening aerobic training effects on blood pressure in treated hypertension. Med Sci Sports Exerc. 2019;51:653–662. [DOI] [PubMed] [Google Scholar]
- 45. Verhagen AP, De Vet HCW, De Bie RA, Kessels AG, Boers M, Bouter LM, Knipschild PG. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235–1241. [DOI] [PubMed] [Google Scholar]
- 46. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Res Synth Methods. 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- 47. Fujiwara T, Hoshide S, Kanegae H, Nishizawa M, Kario K. Reliability of morning, before‐dinner, and at‐bedtime home blood pressure measurements in patients with hypertension. J Clin Hypertens (Greenwich). 2018;20:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ash GI, Walker TJ, Olson KM, Stratton JH, Gómez AL, Kraemer WJ, Volek JS, Pescatello LS. Reproducibility of ambulatory blood pressure changes from the initial values on two different days. Clinics (Sao Paulo). 2013;68:1509–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Molmen‐Hansen HE, Stolen T, Tjonna AE, Aamot IL, Ekeberg IS, Tyldum GA, Wisloff U, Ingul CB, Stoylen A. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol. 2012;19:151–160. [DOI] [PubMed] [Google Scholar]
- 50. Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and nighttime blood pressure as predictors of death and cause‐specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61. [DOI] [PubMed] [Google Scholar]
- 51. Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–709. [PubMed] [Google Scholar]
- 52. Drawz PE, Pajewski NM, Bates JT, Bello NA, Cushman WC, Dwyer JP, Fine LJ, Goff DC Jr, Haley WE, Krousel‐Wood M, et al. Effect of intensive versus standard clinic‐based hypertension management on ambulatory blood pressure. Hypertension. 2017;69:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. MacDonald HV, Johnson BT, Huedo‐Medina TB, Livingston J, Forsyth KC, Kraemer WJ, Farinatti PT, Pescatello LS. Dynamic resistance training as stand‐alone antihypertensive lifestyle therapy: a meta‐analysis. J Am Heart Assoc. 2016;5:e003231. DOI: 10.1161/JAHA.116.003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smart NA, Way D, Carlson D, Millar P, McGowan C, Swaine I, Baross A, Howden R, Ritti‐Dias R, Wiles J, et al. Effects of isometric resistance training on resting blood pressure: individual participant data meta‐analysis. J Hypertens. 2019;37:1927–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jin YZ, Yan S, Yuan WX. Effect of isometric handgrip training on resting blood pressure in adults: a meta‐analysis of randomized controlled trials. J Sports Med Phys Fitness. 2017;57:154–160. [DOI] [PubMed] [Google Scholar]
- 56. World Health Organization . WHO Global Recommendations on Physical Activity for Health. Geneva; 2010. Available at: https://www.who.int/publications/i/item/9789241599979. Accessed July 10, 2020. [PubMed] [Google Scholar]
- 57. Cox KL, Burke V, Morton AR, Gillam HF, Beilin LJ, Puddey IB. Long‐term effects of exercise on blood pressure and lipids in healthy women aged 40–65 years: the Sedentary Women Exercise Adherence Trial (SWEAT). J Hypertens. 2001;19:1733–1743. [DOI] [PubMed] [Google Scholar]
- 58. Seals DR, Reiling MJ. Effect of regular exercise on 24‐hour arterial pressure in older hypertensive humans. Hypertension. 1991;18:583–592. [DOI] [PubMed] [Google Scholar]
- 59. Williamson W, Foster C, Reid H, Kelly P, Lewandowski AJ, Boardman H, Roberts N, McCartney D, Huckstep O, Newton J, et al. Will exercise advice be sufficient for treatment of young adults with prehypertension and hypertension? A systematic review and meta‐analysis. Hypertension. 2016;68:78–87. [DOI] [PubMed] [Google Scholar]
- 60. Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fischer MA, Stedman MR, Lii J, Vogeli C, Shrank WH, Brookhart MA, Weissman JS. Primary medication non‐adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


