Publisher's Note: There is a Blood Commentary on this article in this issue.
Key Points
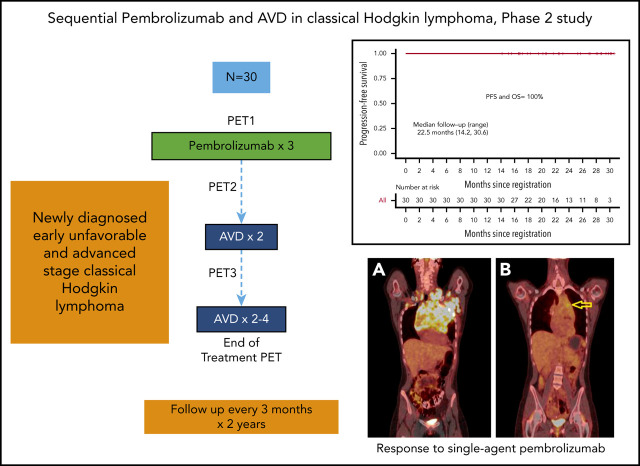
Sequential pembrolizumab and AVD are highly active in untreated cHL, including cases of bulky disease.
Sequential pembrolizumab and AVD is safe in previously untreated cHL with limited immune-related toxicity.
Abstract
Pembrolizumab, a humanized IgG4 monoclonal antibody targeting programmed death-1 protein, has demonstrated efficacy in relapsed/refractory classical Hodgkin lymphoma (cHL). To assess the complete metabolic response (CMR) rate and safety of pembrolizumab monotherapy in newly diagnosed cHL, we conducted a multicenter, single-arm, phase 2 investigator-initiated trial of sequential pembrolizumab and doxorubicin, vinblastine, and dacarbazine (AVD) chemotherapy. Patients ≥18 years of age with untreated, early, unfavorable, or advanced-stage disease were eligible for treatment. Thirty patients (early unfavorable stage, n = 12; advanced stage, n = 18) were treated with 3 cycles of pembrolizumab monotherapy followed by AVD for 4 to 6 cycles, depending on stage and bulk. Twelve had either large mediastinal masses or bulky disease (>10 cm). After pembrolizumab monotherapy, 11 patients (37%) demonstrated CMRs, and an additional 7 of 28 (25%) patients with quantifiable positron emission tomography computed tomography scans had >90% reduction in metabolic tumor volume. All patients achieved CMR after 2 cycles of AVD and maintained their responses at the end of treatment. With a median follow-up of 22.5 months (range, 14.2-30.6) there were no changes in therapy, progressions, or deaths. No patients received consolidation radiotherapy, including those with bulky disease. Therapy was well tolerated. The most common immune-related adverse events were grade 1 rash (n = 6) and grade 2 infusion reactions (n = 4). One patient had reversible grade 4 transaminitis and a second had reversible Bell’s palsy. Brief pembrolizumab monotherapy followed by AVD was both highly effective and safe in patients with newly diagnosed cHL, including those with bulky disease. This trial was registered at www.clinicaltrials.gov as #NCT03226249.
Visual Abstract
Introduction
Doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) chemotherapy is the standard of care for frontline treatment of classical Hodgkin lymphoma (cHL) in much of the world today. Unfortunately, ABVD alone fails to cure 15% to 30% of patients with early unfavorable and advanced-stage disease and is associated with acute and chronic toxicities, including bleomycin lung injury in ∼10% of patients.1-7 Bleomycin-free strategies that improve long-term remission rates without incorporating radiotherapy are needed. cHL is an immunologic tumor with unique biology characterized by rare malignant Hodgkin Reed Sternberg cells within an abundant mixed inflammatory infiltrate. The programmed death (PD-1) pathway represents a key mechanism for immune evasion in this disease.8,9 Anti-PD-1 antibodies that block the inhibitory signal between PD ligands on malignant cells and PD-1 expressed on inflammatory cells in the tumor microenvironment are active in relapsed and refractory disease. Evidence of the genetic reliance on this pathway includes frequent genomic alterations of chromosome 9p24.1, which harbor the coding regions for PD ligands and lead to increased expression of PD ligand 1 (PD-L1) and -L2 on the Hodgkin Reed Sternberg cell surface.9,10 cHL is uniquely poised to respond to PD-1 blockade.
Pembrolizumab is a humanized immunoglobulin G4 anti-PD-1 monoclonal antibody that is approved for a flat dose of 200 mg every 3 weeks for the treatment of relapsed cHL based on early-phase clinical trials demonstrating efficacy and safety in this population.11,12 In the phase II study of relapsed or refractory cHL, the overall response rate to single-agent pembrolizumab was 69%, with a complete response rate of 22.4%, with most responses achieved by 12 weeks.13 The impressive response rates after relapse provide a strong clinical and biologic rationale to study pembrolizumab as a frontline treatment for cHL. Herein, we report results from NU16H08, a phase II investigator-initiated trial of positron emission tomography (PET)–directed frontline treatment with pembrolizumab monotherapy followed by doxorubicin, vinblastine, and dacarbazine (AVD) chemotherapy. It represents the first reported experience with pembrolizumab in previously untreated patients with cHL. The primary study objective was to assess the complete metabolic response (CMR) rate for a brief course of single-agent pembrolizumab. Determination of the safety of sequential immunotherapy and AVD chemotherapy was a secondary objective.
Methods
NU16H08 is an investigator-initiated multicenter, single-arm phase II study of sequential pembrolizumab and AVD chemotherapy. Patients aged 18 years or older with untreated, advanced, or early unfavorable stage cHL by National Comprehensive Cancer Network (NCCN) criteria with an Eastern Cooperative Oncology Group Performance Status of 0 to 1 were eligible for enrollment.14 Patients with autoimmune disease, HIV, interstitial pulmonary disease (not including those with extranodal lung involvement), or central nervous system (CNS) involvement were excluded. Disease was staged per Ann Arbor staging with Cotswold modifications. A baseline diagnostic quality computed tomography (CT) and a PET-CT (PET1) were performed within 4 weeks of study initiation. NCCN criteria for patients with early unfavorable stage disease included the presence of at least 1 of the following risk factors: erythrocyte sedimentation rate ≥50 mm per hour, B symptoms, >3 nodal sites, mediastinal mass ratio >1:3 (maximum width of mass/maximum intrathoracic diameter), or mass >10 cm in any dimension. Notation was made of risk factors including bulk (either by size criteria >7 cm for those with early-stage disease or >10 cm for all patients or by mediastinal mass ratio).15 This study was performed in accordance with the Declaration of Helsinki. Institutional review board and independent ethics committee approval was received for the protocol, amendments, and consent forms before initiating the study at each site. Patients provided written informed consent before enrollment. The study protocol is described in the supplemental Data, available on the Blood Web site.
Treatment
Patients received pembrolizumab monotherapy in a flat dose of 200 mg by IV infusion over 30 minutes every 3 weeks for 3 doses, followed by interim PET-CT (PET2) for primary end point assessment. AVD chemotherapy (doxorubicin 25 mg/m2, vinblastine 6 mg/m2, dacarbazine 375 mg/m2, days 1 and 15 of a 28-day cycle, IV) was then administered for 4 to 6 cycles (study schema, supplemental Figure 1). Patients received pretreatment with acetaminophen and diphenhydramine. Growth factors (G-CSF) were permitted but not required for prophylactic use in all patients. Corticosteroids were prohibited during pembrolizumab monotherapy, except as supportive care measures for the management of adverse events (AEs) with potential immunologic etiology or as antiemetics during AVD chemotherapy. Patients with advanced-stage disease received a total of 6 cycles of AVD. Patients with early unfavorable-stage disease received 4, with the option for 6 cycles in patients with bulky disease at baseline. Patients had repeat PET-CT scans after 2 cycles of AVD (PET3). The protocol recommended but did not require that patients with positive interim PET-CTs after 2 cycles of AVD chemotherapy transition to escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone). Select older patients (age, >60 years) were eligible for 1 to 2 years of pembrolizumab consolidation if they had either a positive PET-CT after 2 cycles of AVD (PET3) or were unable to tolerate the full prescribed course of chemotherapy. End of treatment (EOT) imaging consisted of PET-CTs (PET4) for all patients except those with early-stage disease and a negative PET3, for whom a diagnostic CT scan was allowed. After completion of protocol therapy, patients were followed up every 3 months for 2 years, with surveillance CT scans performed every 6 months for 2 years, according to our institutional policies and consistent with NCCN guidelines.
End points and assessments
The primary study end point was the CMR rate after 3 doses of single-agent pembrolizumab, per independent radiology review according to 2014 Lugano criteria, defined as Deauville 5-point score (D5PS) of 1 to 3.16 Secondary end points included safety and tolerability of sequential therapy, progression-free survival (PFS), overall survival (OS), CMR after 2 cycles of AVD and EOT, and a decline in metabolic tumor volume (MTV) after pembrolizumab monotherapy. Toxicity was assessed by the incidence of grade 3 to 5 treatment-related AEs from the initiation of treatment through 30 days after cessation of treatment. The frequency and severity of AEs by type, severity (grade), timing, and attribution to pembrolizumab were assessed once per cycle, according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 4.03; http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf). All patients who received at least 1 dose of pembrolizumab were evaluable for toxicity end points.
Reponses were assessed by PET-CT and diagnostic quality computed tomography (CT) at baseline (PET1). PET-CT was repeated at the end of monotherapy (PET2), after 2 cycles of AVD (PET3), and at EOT (PET4), except in patients with early-stage disease and a negative PET3, for whom diagnostic CT were permitted at the EOT in lieu of PET4. PET-CTs were read locally and then reviewed centrally by our study radiologist (H.S.). Any discordance between the local assessment (positive, D5PS 4-5 vs negative, D5PS 1-3) and central review was adjudicated by a third nuclear medicine radiologist (G.D.).
PFS was defined as the length of time from study enrollment until the first occurrence of relapse, progression, reinitiation of cytotoxic chemotherapy, death caused by disease, or last contact, if the patient did not experience any of these, from the EOT through 2 years of follow-up. OS was defined as time from study enrollment until death or until last contact, if the patient had not died between the EOT and 2 years of follow-up. Exploratory end points included response assessment by measurement of decline in MTV to pembrolizumab monotherapy, 2 cycles of AVD, and EOT and tissue and blood biomarkers as predictors of response to pembrolizumab. MTV represents the total volume of the metabolically active tumor in a volume of interest. It was calculated with an automatic software tool applying a local standardized uptake value (SUV) maximum threshold of 41% (SyngoVia software; multifoci segmentation tool; Siemens).17 The decline in MTV was calculated to represent the quantitative response to pembrolizumab.
Statistical analysis
Patients who received at least 1 dose of pembrolizumab and had a PET-CT (PET2) scan before cycle 1 of chemotherapy were evaluable for the primary end point. A target sample size of 30 patients provided 84% power at a 1-sided α level of 10%, to detect an improvement in CMR rate in response to pembrolizumab monotherapy from the baseline level of ∼20% reported previously in relapsed/refractory patients to the hypothesized alternative CMR rate of 40% at PET2 (defined as D5PS 1-3, per Lugano 2014 criteria) in previously untreated patients in the current study.16 Follow-up was calculated both from study entry and from completion of therapy. To assess PFS and OS, Kaplan-Meier estimates for time-to-progression and overall survival time were calculated. MTV measurements at study entry were compared for patients who attained a CMR vs those who did not and for those with more than a 90% decline in MTV vs the others, using 2-sided, independent 2-sample Student t tests.
Results
Baseline patient characteristics
A total of 30 patients were enrolled (October 2017 through March 2019) at 4 academic medical centers where a diagnosis of cHL was confirmed by expert hematopathologists. No patient was excluded by screening. Baseline patient characteristics are shown in Table 1. Twelve patients (40%) had early-stage, unfavorable disease, and 18 (60%) had advanced-stage disease. Early-stage patients were all unfavorable by both NCCN and German Hodgkin Study Group (GHSG) criteria. Twelve patients (40%) had bulky disease by size criteria >10 cm (n = 10) or mediastinal mass more than one-third thoracic diameter (n = 9), and 3 additional patients with early unfavorable disease had tumor mass that measured >7 cm but <10 cm. At the time of this publication, the median follow-up was 22.5 months (range, 14.2-30.6).
Table 1.
Patients’ characteristics
| Characteristic | Patients (N = 30) | |
|---|---|---|
| n | % | |
| Median age, y (range) | 29 (21-77) | |
| Age 45-60 | 4 | 13.3 |
| Age >60 | 4 (67-77) | 13.3 |
| Sex | ||
| Male | 11 | 36.7 |
| Female | 19 | 63.3 |
| Disease stage | ||
| IIA | 6 | 20.0 |
| IIB | 6 | 20.0 |
| IIB with >10 cm mass | 5 | 16.7 |
| IIIA | 4 | 13.3 |
| IIIB | 1 | 3.3 |
| IVA | 6 | 20.0 |
| IVB | 7 | 23.3 |
| IPS Score* | ||
| 0-1 | 4 | 13.3 |
| 2 | 6 | 20.0 |
| 3 | 6 | 20.0 |
| ≥4 | 2 | 6.7 |
| ESR >50† | 6 | 50 |
| B symptoms | 14 | 46.7 |
| Extranodal disease | 16 | 53.3 |
| Bone‡ | 14 | 46.7 |
| Lung‡ | 3 | 10.0 |
| Bulky | ||
| >7 cm† | 11 | 91.7 |
| >10 cm | 10 | 33.3 |
| MMR >1/3 | 9 | 30.0 |
| >10 cm or MMR >1/3 | 12 | 40.0 |
IPS, International Prognostic Score; ESR, erythrocyte sedimentation rate, MMR, maximum mediastinal ratio.
Advanced stage patients only (n = 18).
Early-stage patients only (n = 12).
Direct extension to bone in 2 cases (stages IIAE and IIIAE) and to lung in 1 case (stage IIBE).
All patients completed prescribed therapy including pembrolizumab monotherapy and AVD chemotherapy and entered follow-up. Eight patients with early-stage disease received 4 cycles of AVD; 4 of these had bulky disease at baseline. The remaining 22 patients received 6 cycles of AVD (18 advanced-stage and 4 early unfavorable with bulky disease). Given the excellent tolerability and safety of the sequential therapy, no patients received pembrolizumab maintenance or transitioned to escalated BEACOPP therapy.
Safety
There were 126 pembrolizumab-related AEs; most were grade 1 to 2 (93.7%; n = 118), with 8 grade 3-4 AEs, and no grade 5 AEs (Table 2). Grade 3-4 pembrolizumab-related events included 1 patient with immune-related elevated liver enzymes after the first dose of AVD chemotherapy, 3 patients with grade 4 neutropenia, 1 with grade 3 lymphopenia, 1 with grade 3 diarrhea, and 1 patient with grade 3 Bell’s palsy.
Table 2.
Treatment-related toxicities potentially related to pembrolizumab
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total (%) | |
|---|---|---|---|---|---|
| Immune related | |||||
| Rash | 6 | 0 | 0 | 0 | 6 (20.0) |
| Infusion related reaction | 1 | 4 | 0 | 0 | 5 (16.7) |
| Thyroid disorders | 1 | 2 | 0 | 0 | 3 (10) |
| Pericarditis | 0 | 1 | 0 | 0 | 1 (3.3) |
| ALT increased | 0 | 0 | 0 | 1 | 1 (3.3) |
| AST increased | 0 | 0 | 0 | 1 | 1 (3.3) |
| Bell’s palsy* | 0 | 0 | 1 | 0 | 1 (3.3) |
| Hematologic >1 patient | |||||
| Leukopenia | 5 | 1 | 0 | 0 | 6 (20.0) |
| Anemia | 7 | 2 | 0 | 0 | 9 (30.0) |
| Neutropenia | 0 | 1 | 0 | 3 | 4 (13.3) |
| Lymphopenia | 3 | 0 | 1 | 0 | 4 (13.3) |
| Other >1 patient | |||||
| Hypertension | 4 | 4 | 0 | 0 | 8 (26.7) |
| ALT increased† | 5 | 1 | 0 | 0 | 6 (20.0) |
| ALK increased | 3 | 0 | 0 | 0 | 3 (10.0) |
| AST increased† | 5 | 0 | 0 | 0 | 5 (16.7) |
| Hyperglycemia | 2 | 2 | 0 | 0 | 4 (13.3) |
| Hyponatremia | 3 | 0 | 0 | 0 | 3 (10.0) |
| Hypoalbuminemia | 1 | 1 | 0 | 0 | 2 (6.7) |
| Other metabolic‡ | 3 | 1 | 0 | 0 | 4 (13.3) |
| Nausea | 4 | 1 | 0 | 0 | 5 (16.7) |
| Constipation | 2 | 0 | 0 | 0 | 2 (6.7) |
| Diarrhea | 0 | 1 | 1 | 0 | 2 (6.7) |
| Anorexia | 2 | 0 | 0 | 0 | 2 (6.7) |
| Other gastrointestinal§ | 1 | 3 | 0 | 0 | 4 (13.3) |
| Fatigue | 4 | 0 | 0 | 0 | 4 (13.3) |
| Arthralgia | 4 | 0 | 0 | 0 | 4 (13.3) |
| Headache | 2 | 1 | 0 | 0 | 3 (10.0) |
| Myalgia | 2 | 0 | 0 | 0 | 2 (6.7) |
| Alopecia | 2 | 0 | 0 | 0 | 2 (6.7) |
| Other | 16 | 4 | 0 | 0 | 20 (66.7) |
| Total | 88 | 30 | 3 | 5 | 126 |
ALT, alanine aminotransferase; ALK, alkaline phosphatase; AST, aspartate aminotransferase.
Possibly immune-related.
Nonimmune related.
Includes 1 hypernatremia (grade 1), 1 hypocalcemia (grade 2), 1 hypoglycemia (grade 1), and 1 hypokalemia (grade 1).
Includes 1 abdominal pain (grade 1), 1 gastroesophageal reflux (grade 2), 1 mucositis (grade 2), 1 vomiting (grade 2).
Potentially immune-mediated AEs included grade 1-2 infusion reactions in 5 patients, which consisted of fevers, headache, and nodal pain with the first dose of pembrolizumab which did not recur; 1 grade 4 elevation of liver enzymes, which was treated with steroids and a 2-week delay in AVD chemotherapy and which subsequently resolved; 6 episodes of grade 1 rash (3 acneiform, 3 maculopapular), which resolved with topical emollients; 3 thyroid disorders (2 hypothyroid, 1 hyperthyroid); 1 patient with pericarditis who had a history of pericarditis and was treated with nonsteroidal anti-inflammatory drugs alone; and 1 patient with Bell’s palsy, which occurred on cycle 3 day 2 of pembrolizumab monotherapy in the setting of a viral illness and completely resolved with steroid treatment. Four patients (13%) received systemic steroids for immune-related events. These included 1 patient with grade 4 transaminase elevations, 1 patient with Bell’s palsy, and 2 patients with grade 2 infusion reactions who received single doses of steroids acutely. Importantly, there were no AEs leading to treatment discontinuation or death.
Efficacy
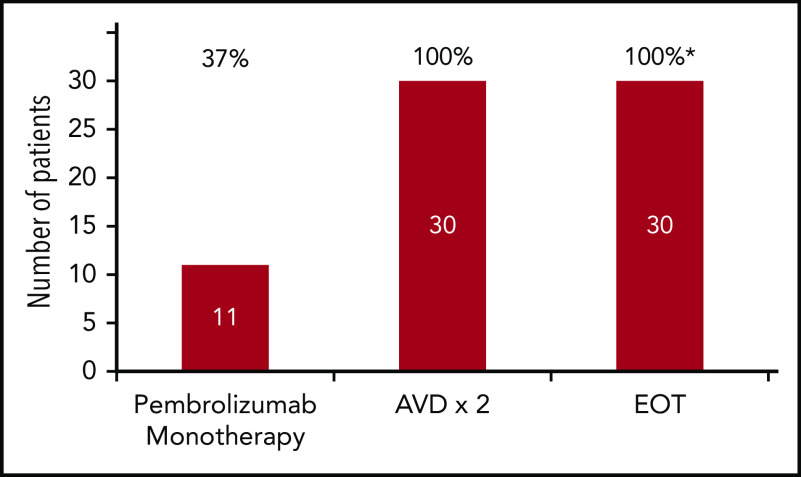
At the end of pembrolizumab monotherapy, the CMR per central review was 37%, with 11 of 30 patients achieving a D5PS of 1 (n = 2), 2 (n = 2), or 3 (n = 7) (Figure 1). All other patients had reduction in disease with the exception of 1 elderly patient with an atypical response categorized as progressive disease. Table 3 shows response rates to pembrolizumab monotherapy by stage and presence of bulky disease. Among the 11 patients with a CMR to pembrolizumab monotherapy, 6 had advanced-stage disease, 5 had early-stage disease, 4 had B-symptoms, and 3 had large mediastinal masses and/or bulky disease. The patient with apparent progressive disease had resolution of some sites after pembrolizumab monotherapy but appearance of new PET-avid lymph nodes and bone lesions. This patient had no associated symptoms, physical findings, or laboratory result abnormalities, and response was classified as indeterminate according to the Lymphoma Response to Immunomodulatory therapy criteria (LYRIC).18 All patients (n = 30; 100%) achieved CMRs by PET-CT after 2 cycles of AVD chemotherapy, including the patient with an indeterminate response, and these responses were maintained at EOT (n = 30), for a CMR rate of 100%. Radiologic assessments were adjudicated in 2 cases by a third nuclear medicine radiologist. In 1 case, a new axillary lymph node (Deauville 5) was detected 3 days after the patient received flu vaccine in the same arm, whereas all other sites resolved (Deauville 3) and in the second case, the central review resulted in a final reading of Deauville 4 rather than 2.
Figure 1.
Complete metabolic response rates by Lugano 2014 criteria. Response rates to pembrolizumab monotherapy, after 2 cycles of AVD and at EOT are shown (n = 30). *In 2 patients with early unfavorable stage cHL who received 4 cycles of AVD chemotherapy, diagnostic CT-scans substituted for PET4, as permitted by protocol at EOT.
Table 3.
Response rates to pembrolizumab monotherapy by stage and presence of bulky disease
| Response | All patients (n = 30) | Advanced stage (n = 18) | Early unfavorable (n = 12) | Bulky (n = 12)* | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| CMR (n = 30) | 11/30 | 37 | 6/18 | 33 | 5/12 | 42 | 3/12 | 25 |
| >90% but < 100% reduction in MTV | 7/28† | 25† | 4/18 | 22 | 3/10† | 30† | 5/10† | 50† |
| CMR or >90% but <100% reduction in MTV | 18/30 | 60 | 10/18 | 56 | 8/12 | 67 | 8/12 | 67 |
Any mass >10 cm in any dimension or a maximum mediastinal ratio >1:3.
Quantifiable cases; MTV could not be measured in 2 cases of bulky disease. In 1 case, areas of disease could not be distinguished from the PET-avid myocardium and in a second case, the computer program generated a 100% decline in MTV that was inconsistent with the qualitative assessment of Deauville 4.
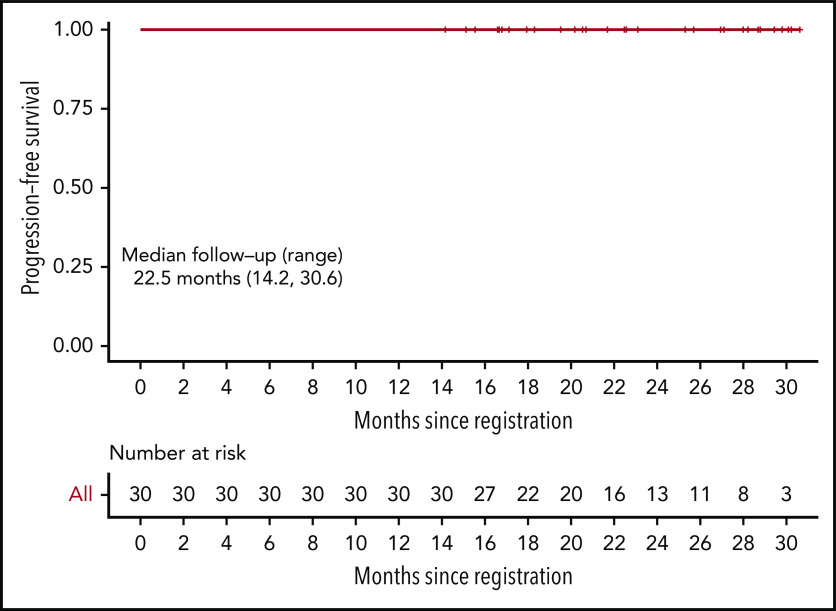
With a median follow-up of 22.5 months (range, 14.2-30.6) months from study entry and 15.4 (range, 6.6-24.9) months from last treatment, there have been no progressions, deaths, or subsequent therapy, and therefore median PFS and OS have not been reached. At the time of this analysis, PFS and OS are 100%. Figure 2 shows the Kaplan-Meier curve for PFS. The corresponding figure for OS is identical to that of PFS and is not shown.
Figure 2.
Kaplan-Meier estimate of PFS. Median follow-up 22.5 months (range, 14.2-30.6). OS is identical and not shown.
Exploratory end points
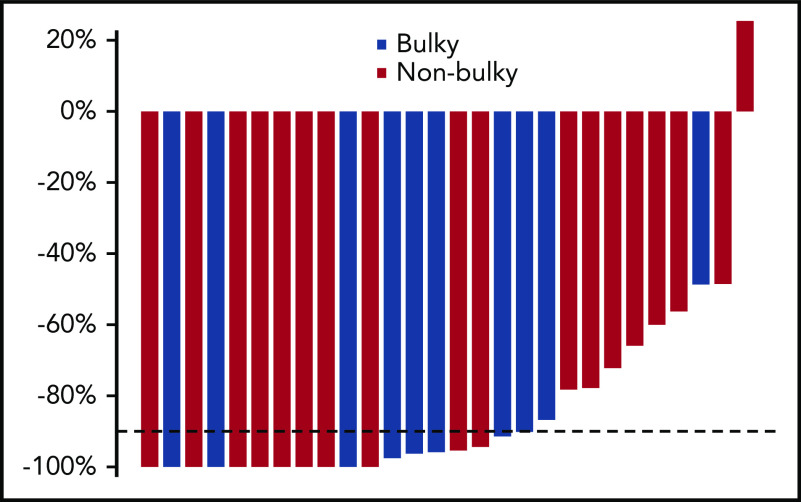
Change in MTV was assessed after pembrolizumab monotherapy (Figure 3). Eighteen of 28 assessable (64%) patients had >90% decline in MTV. Two patients with very bulky disease were not assessable for technical reasons; 1 patient had uptake that could not be distinguished from PET-avid myocardium because of close approximation, and another had a computer-generated decline in MTV of 100% that was inconsistent with clinical and qualitative assessment demonstrating a small focus of residual disease and a Deauville score of 4. The percentage change in MTV for the responding patients ranged from 47% to 97%, demonstrating the significant heterogeneity in response in this group. MTV at study entry was not associated with achieving a CMR after pembrolizumab monotherapy (P = .88) or >90% reduction in MTV (P = .30). Among the 7 patients with less than a CMR but >90% reduction in MTV, 4 had advanced-stage, 3 had early-stage disease, and 4 had large mediastinal masses or bulky (>10 cm) disease (Table 3). Notably, among patients with bulky disease by conventional criteria, 3 of 12 (25%) achieved CMR with 3 cycles of pembrolizumab and 4 of 10 who were assessable for MTV (40%) had >90% reduction in MTV. Overall, 7 of 12 patients with bulky disease had either a CMR or >90% decline in MTV, with 1 additional patient showing a near CMR that was not quantifiable with MTV.
Figure 3.
Decline in MTV. Percentage decline in MTV after pembrolizumab monotherapy in the 28 patients with quantifiable disease was calculated with an automatic software tool applying a local SUV maximum threshold of 41% (SyngoVia software, multifoci segmentation tool; Siemens).17 Each bar corresponds to an individual patient. The decline in MTV could not be measured in 2 of 30 cases; 1 patient had uptake that could not be distinguished from PET-avid myocardium, because of the close approximation, and another had a computer-generated decline in MTV of 100% that was inconsistent with clinical and qualitative assessment demonstrating a small focus of residual disease and a Deauville score of 4.
Discussion
A brief course of pembrolizumab monotherapy, previously not studied in patients with newly diagnosed cHL, was a powerful induction strategy in early unfavorable and advanced-stage disease. These findings were consistent with expectations that checkpoint inhibition with pembrolizumab would be highly effective in previously untreated individuals. Whereas 37% (11 of 30) of patients achieved complete metabolic responses with only 3 doses of pembrolizumab, 7 additional patients had quantifiable near complete responses resulting in a total of 60% of patients with >90% reduction in MTV with anti-PD-1 antibody therapy alone. Moreover, 100% of patients achieved a CMR after 2 cycles of chemotherapy, and all patients maintained their responses through the EOT. No patient was treated with radiation therapy, which is particularly notable, given the inclusion of 12 patients with bulky disease. At this writing, no patient has relapsed, had a change in therapy, or died. Therapy was safe with no discontinuations for toxicity. Only 2 patients experienced grade 3-4 immune-related AEs, which resolved rapidly with corticosteroid therapy. All patients completed the planned course of treatment.
Administration of a brief course of pembrolizumab monotherapy before conventional chemotherapy, afforded us the opportunity to assess the single-agent efficacy and toxicity of pembrolizumab when used as a frontline treatment. We chose a short course of only 3 doses to limit the risk of delaying chemotherapy in patients who may not achieve an early response. Complete metabolic responses and near complete responses occurred in young and old patients and did not appear to be related to stage or disease bulk in our study (Table 3). Notably, there was no apparent relationship between the bulk of disease and depth of response to single-agent pembrolizumab whether measured by conventional measures or MTV, as 7 of 12 patients with bulky disease achieved a CMR or >90% reduction in MTV. These results suggest that initial therapy with pembrolizumab is a safe and effective strategy for reducing disease bulk before chemotherapy in cHL (Table 3; Figure 4). One patient appeared to have progressive disease during pembrolizumab monotherapy with new sites including bone, despite resolution or shrinkage of most of the sites involved at baseline. This response would be classified as indeterminate, according to the new LYRIC response criteria.18 After 2 cycles of AVD, however, the patient achieved a CMR. Given that there was no opportunity for a confirmatory scan before the initiation of AVD chemotherapy, we are unable to conclude whether the new sites of uptake at PET2 represented true progression or tumor flare. No patient in our study received radiotherapy or required additional salvage treatment. Although median follow-up was short at 22.5 months, no patient has had local or distant recurrence thus far.
Figure 4.
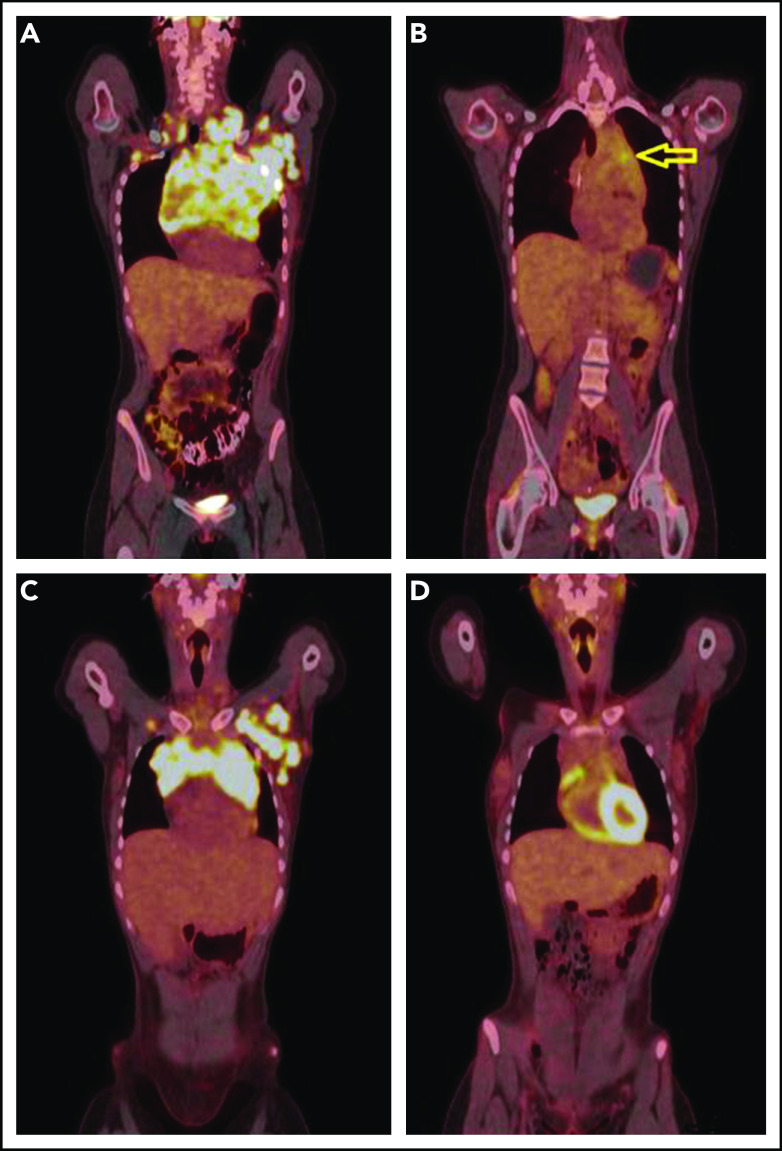
PET-CT’s before and after single agent pembrolizumab. (A) A coronal fused PET-CT image of a 23-year-old woman with cHL shows intensely hypermetabolic bulky lymphadenopathy involving the entire anterior mediastinum, left axillary, bilateral supraclavicular, and subpectoral nodal stations before therapy with pembrolizumab. (B) After 3 cycles of pembrolizumab monotherapy, there was marked anatomic and metabolic improvement of the disease. The residual mass has fluorodeoxyglucose (FDG) activity similar to liver background uptake, with the exception of mild FDG hypermetabolism in the anterior mediastinum (arrow), Deauville 4. (C) A coronal fused PET-CT image of another 23-year-old woman with cHL shows intensely hypermetabolic bulky lymphadenopathy involving the anterior mediastinum and the left axillary and left subpectoral nodal stations with a small right subpectoral lymph node before therapy. (D) After 3 cycles of pembrolizumab monotherapy with CMR, Deauville 3.
MTV assessed by PET-CT provides an indirect assessment of both total tumor volume and tumor biology and therefore may be a more powerful predictor of clinical outcome than measurement of tumor bulk by conventional imaging techniques.19 In addition to its potential prognostic significance, MTV provides a quantitative assessment of response that may better represent the impact of therapy than the 2014 Lugano response criteria, especially in the setting of immune checkpoint inhibition. There are various suggested techniques to measure metabolic volume in solid tumors and lymphomas. We chose a commonly used approach to calculate MTV using a fixed 41% SUV maximum threshold.20 In our study, excellent near CMR to pembrolizumab monotherapy that would be considered either partial metabolic response or stable disease by Lugano 2014 criteria were better characterized. The small volumes of residual uptake seen after pembrolizumab monotherapy may in fact represent inflammatory responses to pembrolizumab and not residual disease. These sites may have resolved with continued pembrolizumab or observation alone. Although MTV has been shown to predict the outcome of early-stage disease in the H10 trial of conventional chemotherapy and radiotherapy, it was not associated with response to pembrolizumab monotherapy in our study or to nivolumab monotherapy in the recently reported GHSG NIVAHL trial.19,21,22 These observations underscore the importance of reevaluating prognostic markers with each new therapy. Tumor characteristics may determine sensitivity to checkpoint inhibition rather than tumor bulk or the clinical features traditionally used to assess risk in cHL. In ongoing correlative studies, we seek to identify additional biomarkers for response to checkpoint inhibition in cHL and to provide a deeper understanding of the pathophysiology and effectiveness of the sequential strategy.
Frontline checkpoint blockade in cHL with the anti-PD-1 antibody nivolumab has recently been reported in 2 studies: cohort D of Checkmate 205 in advanced-stage cHL23 and the GHSG phase II NIVAHL trial in early unfavorable cHL.22 In cohort D, nivolumab was administered as monotherapy for 4 doses followed by 6 cycles of concurrent nivolumab and AVD. The response rate to single-agent nivolumab in this trial was identical with that reported previously for relapsed disease (18% CR). With the addition of AVD chemotherapy, the CR rate increased to 51% and 67% at interim and EOT time points. Although these response rates are lower than the CR rate of >80% reported with ABVD alone in modern studies, the PFS of 83% at 21 months in cohort D, is similar to the 80% to 90% 2-year PFS reported with ABVD and brentuximab vedotin+AVD (A+AVD) in ECHELON-1, and may be an indication of the limitations of response assessment by Lugano criteria in patients receiving concurrent PD-1 blockade and chemotherapy.1,2,16,24 Differences in patient populations make it difficult to make cross-trial comparisons. Although both early unfavorable and advanced-stage patients were enrolled in our study, 77% would have met eligibility criteria for Checkmate 205. Other characteristics were similar in age (>60; 12% in cohort D, and 13.3% in our study) and bulky disease (31% and 40%, respectively). In contrast to Checkmate 205, which assessed responses according to the 2007 International Working Group Criteria by a central review panel, we used Lugano 2014 and a local “central” reviewer.25 Nevertheless, our study with 100% CMR during the interim and EOT compares favorably, although follow-up was limited in both studies.
The GHSG randomized phase II NIVAHL trial for early unfavorable disease compared concurrent nivolumab and AVD to a sequential strategy.22 In contrast to our trial, all patients received consolidation with radiotherapy. The investigators found no significant differences in outcomes with concurrent or sequential approaches. Notably, this study demonstrated a CR rate of 53% to frontline single-agent nivolumab and a reduction in MTV of more than 80% in the subgroup of patients evaluated quantitatively. Consistent with our findings, the decline in MTV after nivolumab monotherapy in the GHSG NIVAHL was independent of baseline MTV.
Whether concurrent or sequential checkpoint blockade is superior remains unknown. The single trial in cHL comparing sequential and concurrent approaches (NIVAHL trial), failed to demonstrate any differences in outcome. Additional studies are needed to determine whether our excellent results are attributable to the sequential design, an approach that is also being explored for relapsed/refractory disease by others.22,26 In the nivolumab ICE (NICE) trial, patients with relapsed/refractory Hodgkin lymphoma were treated with up to 6 cycles of nivolumab before ICE chemotherapy (ifosfamide, carboplatin, etoposide) as the first salvage.26 After 3 cycles of single-agent nivolumab the overall response rate was 89%, with a CR rate of 59%, which increased to 90% and 77%, respectively, after 6 cycles, lending further support to a sequential strategy for checkpoint inhibition in cHL. Differences in response criteria and the absence of independent central review of imaging may underlie differences between these results and those previously reported for relapsed/refractory disease.
Limitations of our study include its small sample size and limited duration of follow-up. Our statistical design was ambitious with a sample size calculated to detect a doubling in the previously reported CMR rate to single-agent pembrolizumab from ∼20% in the relapsed patients to 40% in those previously untreated. Although we were 1 response short of achieving the trial's primary end point, the high rate of near CMR, along with data from the NICE clinical trial26 (which included up to 6 doses of pembrolizumab), suggests that additional pembrolizumab or time alone may result in even higher rates of CMR. These findings underscore the exquisite sensitivity of cHL to checkpoint inhibition possibly related to the more robust immune system in previously untreated individuals. Continued follow-up is necessary for determining long-term outcomes in this curable disease.
In summary, we demonstrated that sequencing a short course of pembrolizumab monotherapy and AVD chemotherapy is safe and highly effective in newly diagnosed, early unfavorable and advanced-stage cHL, including many patients with bulky disease, with all patients completing the prescribed course of therapy. The results were excellent with a CMR rate of 100% at interim and EOT. At a median follow-up of 22.5 months, the PFS and OS were 100%, and no patient has required salvage therapy or radiotherapy. Our results support further evaluation of this very promising sequential strategy.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the participating patients and their families and the nursing and clinical research staff who assisted with the study and particularly thank Merck & Co, Inc for its support.
This work was supported by Merck & Co, Inc, which supplied funding for our investigator-initiated clinical trial and investigational product, and by National Institutes of Health, National Cancer Institute Cancer Center Support Grant P30 CA060553 to the Robert H. Lurie Comprehensive Cancer Center.
Footnotes
Presented at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 5-8 December 2019.
Original data may be obtained by e-mail request to the corresponding author Jane N. Winter (j-winter@northwestern.edu). Data on individual participants will not be shared.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.M.E., P.B.A., L.I.G., and J.N.W. designed the clinical trial; P.B.A. and J.N.W. wrote the manuscript; P.B.A., A.M.E., R.H.A., B.P., R.K., E.M., R.A.B., R.M.E., B.P., J.B., and J.N.W. contributed to patient recruitment, data collection and data analysis; J.S.C. and K.O. assisted with data analysis; and H.S. and G.D. performed radiologic review, data collection, and data analysis.
Conflict-of-interest disclosure: P.B.A. has received honoraria from the advisory boards of Imbrium and Bayer and fees from the speakers bureaus of Curio Science and Purdue Pharma LP, outside the submitted work. A.M.E. has received grants from ORIEN and Tesaro and other support from Seattle Genetics, MorphoSys, Mylteni, Epizyme, Novartis, Pharmacyclics, Research to Practice, Physician Education Resource, Cota, and OncLive, outside the submitted work. R.H.A. reports consulting or advisory roles with Genentech/Roche, Portola Pharmaceuticals, Sanofi, Seattle Genetics, Takeda; grants from Celgene, Forty Seven, Inc, Genentech/Roche, Janssen Pharmaceutical, Kura, Merck, Millennium Pharmacyclics, Regeneron, and Seattle Genetics, outside the submitted work. B.P. has received grants and personal fees from Takeda and grants and personal fees from Seattle Genetics, outside the submitted work. R.K. has received rants and personal fees from Gilead and Kite; grants and personal fees from BMS, Celgene, and Juno; grants from Takeda; and personal fees from BeiGene, AstraZeneca, and Karyopharm, outside the submitted work; J.N.W. reports an honorarium for serving on an advisory board for Merck and for Karyopharm, and that her spouse serves on data and safety monitoring boards for Novartis, Ariad/Takeda, and Epizyme and as a consultant to Novartis, CVS/Caremark, Fly Pharma, Astra Zeneca, and Amgen. The remaining authors declare no competing financial interests.
Correspondence: Jane N. Winter, Robert H. Lurie Comprehensive Cancer Center, Suite 850, 676 North St Clair St, Chicago, IL 60611; e-mail: j-winter@northwestern.edu.
REFERENCES
- 1.Stephens DM, Li H, Schöder H, et al. Five-year follow-up of SWOG S0816: limitations and values of a PET-adapted approach with stage III/IV Hodgkin lymphoma. Blood. 2019;134(15):1238-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson P, Federico M, Kirkwood A, et al. Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin’s Lymphoma. N Engl J Med. 2016;374(25):2419-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer RM, Gospodarowicz MK, Connors JM, et al. ; Eastern Cooperative Oncology Group . ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;366(5):399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.André MPE, Girinsky T, Federico M, et al. Early Positron Emission Tomography Response-Adapted Treatment in Stage I and II Hodgkin Lymphoma: Final Results of the Randomized EORTC/LYSA/FIL H10 Trial. J Clin Oncol. 2017;35(16):1786-1794. [DOI] [PubMed] [Google Scholar]
- 5.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31(6):684-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin WG, Ristow KM, Habermann TM, Colgan JP, Witzig TE, Ansell SM. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin’s lymphoma. J Clin Oncol. 2005;23(30):7614-7620. [DOI] [PubMed] [Google Scholar]
- 7.Andersen MD, Kamper P, d’Amore A, Clausen M, Bentzen H, d’Amore F. The incidence of bleomycin induced lung toxicity is increased in Hodgkin lymphoma patients over 45 years exposed to granulocyte-colony stimulating growth factor. Leuk Lymphoma. 2019;60(4):927-933. [DOI] [PubMed] [Google Scholar]
- 8.Green MR, Aya-Bonilla C, Gandhi MK, et al. Integrative genomic profiling reveals conserved genetic mechanisms for tumorigenesis in common entities of non-Hodgkin’s lymphoma. Genes Chromosomes Cancer. 2011;50(5):313-326. [DOI] [PubMed] [Google Scholar]
- 9.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J Clin Oncol. 2016;34(23):2690-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armand P, Shipp MA, Ribrag V, et al. Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. J Clin Oncol. 2016;34(31):3733-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen R, Zinzani PL, Fanale MA, et al. ; KEYNOTE-087 . Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol. 2017;35(19):2125-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen R, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134(14):1144-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoppe RT, Advani RH, Ai WZ. Hodgkin Lymphoma (Version 2.2020). NCCN Clinical Practice Guidelines in Oncology. JNCCN. 2020;18(6):755-781. Available at: https://jnccn.org/view/journals/jnccn/18/6/article-p755.xml#d5965173e696/. Accessed 7 October 2020. [DOI] [PubMed]
- 15.Kumar A, Burger IA, Zhang Z, et al. Definition of bulky disease in early stage Hodgkin lymphoma in computed tomography era: prognostic significance of measurements in the coronal and transverse planes. Haematologica. 2016;101(10):1237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute . Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boellaard R, O’Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37(1):181-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson BD, Ansell S, Schwartz L, et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128(21):2489-2496. [DOI] [PubMed] [Google Scholar]
- 19.Cottereau AS, Versari A, Loft A, et al. Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood. 2018;131(13):1456-1463. [DOI] [PubMed] [Google Scholar]
- 20.Kostakoglu L, Chauvie S. Metabolic Tumor Volume Metrics in Lymphoma. Semin Nucl Med. 2018;48(1):50-66. [DOI] [PubMed] [Google Scholar]
- 21.Voltin C-A, Mettler J, Mueller H, et al. Metabolic Tumor Volume for Early Response Assessment in Early-Stage Unfavorable Hodgkin Lymphoma Treated with Nivolumab in the GHSG Nivahl Phase II Trial [abstract]. Blood. 2019;134(suppl 1). Abstract 4020. [Google Scholar]
- 22.Bröckelmann PJ, Goergen H, Keller U, et al. Efficacy of Nivolumab and AVD in Early-Stage Unfavorable Classic Hodgkin Lymphoma: The Randomized Phase 2 German Hodgkin Study Group NIVAHL Trial. JAMA Oncol. 2020;6(6):872-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramchandren R, Domingo-Domènech E, Rueda A, et al. Nivolumab for Newly Diagnosed Advanced-Stage Classic Hodgkin Lymphoma: Safety and Efficacy in the Phase II CheckMate 205 Study. J Clin Oncol. 2019;37(23):1997-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connors JM, Jurczak W, Straus DJ, et al. ; ECHELON-1 Study Group . Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N Engl J Med. 2018;378(4):331-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheson BD, Pfistner B, Juweid ME, et al. ; International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. [DOI] [PubMed] [Google Scholar]
- 26.Herrera AF, Chen RW, Palmer J, et al. PET-Adapted Nivolumab or Nivolumab Plus ICE As First Salvage Therapy in Relapsed or Refractory Hodgkin Lymphoma [abstract]. Blood. 2019;134(suppl_1). Abstract 239.31076442 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.