Abstract
Background
When acute aortic syndromes (AASs) are suspected, pretest clinical probability assessment and d‐dimer (DD) testing are diagnostic options allowing standardized care. Guidelines suggest use of a 12‐item/3‐category score (aortic dissection detection) and a DD cutoff of 500 ng/mL. However, a simplified assessment tool and a more specific DD cutoff could be advantageous.
Methods and Results
In a prospective derivation cohort (n=1848), 6 items identified by logistic regression (thoracic aortic aneurysm, severe pain, sudden pain, pulse deficit, neurologic deficit, hypotension), composed a simplified score (AORTAs) assigning 2 points to hypotension and 1 to the other items. AORTAs≤1 and ≥2 defined low and high clinical probability, respectively. Age‐adjusted DD was calculated as years/age × 10 ng/mL (minimum 500). The AORTAs score and AORTAs≤1/age‐adjusted DD rule were validated in 2 patient cohorts: a high‐prevalence retrospective cohort (n=1035; 22% AASs) and a low‐prevalence prospective cohort (n=447; 11% AASs) subjected to 30‐day follow‐up. The AUC of the AORTAs score was 0.729 versus 0.697 of the aortic dissection detection score (P=0.005). AORTAs score assessment reclassified 16.6% to 25.1% of patients, with significant net reclassification improvement of 10.3% to 32.7% for AASs and −8.6 to −17% for alternative diagnoses. In both cohorts, AORTAs≥2 had superior sensitivity and slightly lower specificity than aortic dissection detection ≥2. In the prospective validation cohort, AORTAs≤1/age‐adjusted DD had a sensitivity of 100%, a specificity of 48.6%, and an efficiency of 43.3%.
Conclusions
AORTAs is a simplified score with increased sensitivity, improved AAS classification, and minor trade‐off in specificity, amenable to integration with age‐adjusted DD for diagnostic rule‐out.
Keywords: age, aorta, d‐dimer, diagnosis, dissection, syndrome
Subject Categories: Diagnostic Testing, Aortic Dissection
Nonstandard Abbreviations and Acronyms
- AAD
acute aortic dissection
- AAS
acute aortic syndrome
- ADD
aortic dissection detection
- AltD
alternative diagnosis
- CTA
computed tomography angiography
- DD
d‐dimer
- DD500
d‐dimer cutoff of 500 ng/mL
- DDage‐adj
age‐adjusted d‐dimer cutoff
- NRI
net reclassification improvement
Clinical Perspective
What Is New?
The AORTAs is a simple noncategorical 6‐item score estimating the pretest clinical probability of acute aortic syndromes (AASs).
The AORTAs score was developed from a prospective multicenter cohort of patients with suspected AASs, aiming at integration with an age‐adjusted d‐dimer cutoff, already applied for rule‐out of pulmonary embolism; preliminary validation of the AORTAs score was obtained in 2 independent emergency department cohorts, showing higher sensitivity, better AAS classification, and lower specificity compared with the aortic dissection detection score.
For rule‐out of AASs, the performance of AORTAs score plus age‐adjusted d‐dimer cutoff was similar to aortic dissection detection score plus d‐dimer<500 ng/mL.
What Are the Clinical Implications?
The AORTAs score can be applied at the bedside and ab initio to all patients with suspected AAS, independent of hemodynamic status.
With AORTAs, hypotension or any combination of items defines a high clinical probability of AAS, thus reducing the risk of initial AAS misclassification and inappropriate use of d‐dimer.
Integration of AORTAs with age‐adjusted d‐dimer cutoff is practical and clinically meaningful, allowing improved single‐cutoff rule‐out at maximized specificity of both AASs and pulmonary embolism; further trial is needed for external multicenter validation.
Acute aortic syndromes (AASs) are deadly conditions involving the thoracic aorta and include acute aortic dissection (AAD), intramural hematoma, penetrating aortic ulcer, and spontaneous aortic rupture. AASs are rare in the general population (5–15 cases/100 000 individuals/y), present with unspecific symptoms, and lead to high morbidity and mortality. 1 Currently, highly accurate aortic biomarkers are not available, and conclusive diagnostic assessment requires contrast medium–enhanced computed tomography angiography (CTA). However, CTA uses radiation, may cause anaphylaxis and kidney injury, and is resource limited. Therefore, optimal patient selection for urgent CTA is challenging. Misdiagnosis may affect 1 in 3 to 7 AASs, leading to worse outcomes. 2 , 3 In the meantime, CTA overtesting and high variability in ordering are major issues, with diagnostic yields as low as 2% to 3%. 4 , 5
To assist and standardize diagnostic decisions, pretest clinical probability assessment has been recommended, as for pulmonary embolism (PE). Guidelines have adopted the aortic dissection detection (ADD) score as a tool partitioning their diagnostic algorithms. 6 , 7 The ADD score was initially developed from a large international registry of AADs and has obtained external validation. 8 , 9 However, this tool has limitations. 10 First, categorizing and assignment of points with a 12‐risk‐factor/3‐risk‐category score may be difficult/impractical in busy emergency departments (EDs). Second, several risk factors are rarely encountered in everyday practice, and others have vague definitions. Third, hypotension and shock, representing key alerts, do not define per se a high pretest probability. Finally, presence of ≥2 risk factors in a single category does not affect ADD score defined probability.
d‐dimer (DD), a plasma fibrin degradation product, is widely used as a rule‐out biomarker of PE. DD levels almost invariably increase in AASs. Using a cutoff of 500 ng/mL (DD500), DD has a sensitivity of 95% to 98% and low to moderate specificity for AASs. 11 Accordingly, low probability plus a negative DD can be used to safely avoid CTA in 15% to 50% of patients. 12 As the specificity of DD diminishes with aging, an age‐adjusted DD cutoff (DDage‐adj) is suggested for PE rule‐out as an alternative to DD500, providing similar sensitivity but higher specificity. 13 Use of a single DD cutoff optimizing specificity to rule out both PEs and AASs may be practical and efficient. However, only few studies have evaluated DDage‐adj for AASs so far. 12 , 14 , 15
Herein, we developed a simplified clinical probability assessment tool amenable to dichotomic rule‐out of AASs in combination with DDage‐adj. External validation was pursued in 2 independent patient cohorts, by comparison with current standard (ADD score and DD500).
Methods
The data, analytical methods and study materials will be made available to other researchers by contacting the corresponding author. For expanded methods, see Data S1. The study complies with the Declaration of Helsinki, the locally appointed ethics committees have approved the research protocol, and informed consent has been obtained from the participating subjects (or their legally authorized representative).
ADD Risk Score
The standard tool used to assess the pretest probability of AASs was the ADD score, based on 12 risk markers classified in 3 categories (Table S1): high‐risk conditions (Marfan syndrome, family history of aortic disease, known aortic valve disease, recent aortic manipulation, known thoracic aortic aneurysm), high‐risk pain features (sudden, severe, ripping/tearing), and high‐risk physical examination features (pulse deficit or systolic blood pressure differential, focal neurologic deficit, new aortic insufficiency murmur, hypotension/shock).
Derivation Cohort
The overall study design is shown in Figure 1. The derivation cohort was obtained from a previous multicenter, multinational, prospective diagnostic study. 16 Briefly, consecutive ED outpatients aged >18 years were enrolled in the presence of red‐flag symptoms (chest/abdominal/back pain, syncope, perfusion deficit) lasting ≤14 days and a physician‐defined clinical suspicion of AAS. Case adjudication was based on advanced imaging, surgery, autopsy, or 14‐day follow‐up data.
Figure 1. Overall study design.
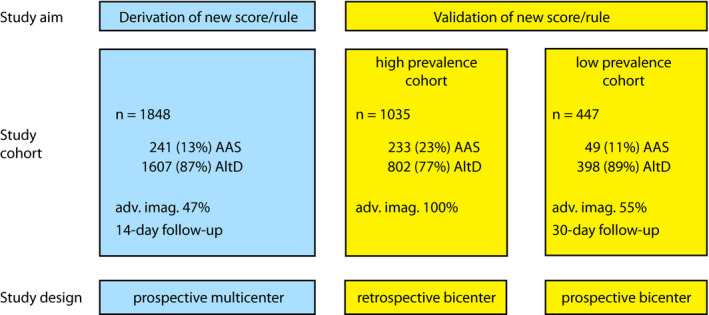
AAS indicates acute aortic syndrome; adv. imag., advanced imaging; and AltD, alternative diagnosis.
Validation Cohorts
Validation of the simplified score and rule was sought in 2 independent study cohorts: a retrospective high‐prevalence cohort and a prospective lower‐prevalence cohort.
Retrospective Validation Cohort
As a high‐prevalence population, we used a retrospective cohort, detailed elsewhere. 17 Briefly, this study enrolled outpatients presenting to 2 EDs of urban teaching hospitals from 2008 to 2013. Inclusion criteria were chest pain, back pain, abdominal pain, syncope, or symptoms of perfusion deficit, in conjunction with the absence of an obvious alternative diagnosis after the first medical evaluation. Clinical suspicion of AAS was high enough to have all patients undergo a CTA for final diagnosis. ADD score and DD test results were available for all patients.
Prospective Validation Cohort
As a cohort representative of Western ED practice at lower prevalence of AASs, outpatients with suspected AAS were prospectively enrolled in 2 urban teaching hospitals in a new study, from January 2018 to November 2019. Inclusion criteria were (1) presence of at least 1 red‐flag symptom among truncal (chest, abdominal, or back pain) pain, syncope, neurological deficit, and limb ischemia; (2) symptom(s) lasting for ≤14 days; and (3) AASs considered as meaningful differential diagnoses by the attending physician. Exclusion criteria were age <18 years, primary trauma, and an established diagnosis of AAS.
Patient Management
During the ED visit, a case report form including probability assessment was filled out by the attending physician or resident, before availability of blood test results and imaging exams. The recommended standard of care was represented by the European Society of Cardiology 2014 guidelines. 7 However, physicians were free to derogate from guideline indications, and clinical decisions were independent from patient’s participation in the study. Advanced imaging exams (CTA, transesophageal echocardiography, or magnetic resonance angiography) were performed and interpreted by specialized physicians not involved in the study. Discharged patients were instructed to return to the ED in case of new, worsening, or recurrent symptoms.
d‐dimer
Samples obtained during the visit were sent to the local laboratory for urgent processing. DD assays were the STA‐Liatest D‐Di assay (Stago, Asnières sur Seine, France) and HemosIL d‐Dimer HS (Instrumentation Laboratory, Bedford, MA, USA). We planned to analyze the DD test results on the basis of 2 different cutoffs: 500 ng/mL fibrinogen equivalent units and an age‐adjusted cutoff (DDadj). DDadj, already applicable for the rule‐out of PE, was calculated as follows: age (years) × 10 ng/mL (with a minimum of 500 ng/mL for patients aged ≤50 years; e.g., 500 ng/mL for a patient aged 40 years, 600 ng/mL for a patient aged 60 years, 750 ng/mL for a patient aged 75 years). 13 , 14 The attending physicians were not blinded to the DD test results.
Case Adjudication
Case adjudication was performed by 2 expert physicians who independently assessed ED chart, blood test results (excluding DD), imaging data, and 30‐day follow‐up data. The latter included results of a structured telephone interview evaluating subsequent diagnosis of any aortic disease, ED visits and admissions to hospital, and hospital database search for additional ED visits and hospital admissions within 30 days. For ED visits and hospital admissions, medical charts, surgical reports, autopsy reports (if applicable), imaging, and blood test results were obtained and reviewed. For patients lost to follow‐up, vital status was checked in the local public registries. In case of discordant adjudication, discussion between the 2 reviewers was planned for final decision.
Statistical Analysis
Age was assessed with mean and SD, and tested with Student’s t test. The variable “hours from symptom onset,” which was not normally distributed, was assessed with median and interquartile range, and tested with the Mann–Whitney U test. Categorical variables were assessed with proportion and 95% CI, and tested using the χ 2 or Fisher’s exact test.
Multivariate logistic regression analysis was used to identify independent predictors among ADD score items plus DD, and the natural logarithm of their odds ratios was used to weight each predictor. Contingency tables were built, including the number of true‐positive, false‐positive, false‐negative, and true‐negative patients. Standard diagnostic performance measures were sensitivity, specificity, and positive/negative likelihood ratio. The failure rate was calculated as false negative/(false negative + true negative), which corresponds to (1 – negative predictive value), as previously. 18 This measure indicates the number of AAS cases mistakenly ruled out with the diagnostic rule‐out protocol. The rule‐out efficiency was calculated as (true negative + false negative)/(true positive + false positive+true negative + false negative). For contingency tables containing cells with a 0 value, CIs were calculated using a bootstrap method. 19 Sensitivities and specificities were compared using an exact binomial method. 20 Likelihood ratios were compared using a regression model approach. 21
The diagnostic performance of different strategies was assessed using receiver operating characteristic (ROC) curve analysis, McNemar, test and net reclassification improvement (NRI). In ROC analysis, the areas under the curve (AUCs) were compared using DeLong’s test for paired AUCs. Improvement in risk prediction was assessed with NRI, which was split for patients with AASs and alternative diagnoses (AltDs). 22 A positive NRI value indicates improvement in risk prediction. The Pauker and Kassirer decision threshold model was applied to calculate 2 theoretical thresholds: a testing threshold and a test‐treatment threshold. 23
The prospective validation study was powered for comparison between the sensitivity of a high‐probability definition obtained with the new score and the sensitivity of the standard high‐probability definition (ADD score≥2), for diagnosis of AASs. Using a type I error of 0.05 (2 sided) and a power rate of 80% and assuming a prevalence of 10% of AASs, we estimated that at least 430 patients needed to be included.
P values were considered significant if <0.05. Statistical analysis was carried out using SPSS software version 25.0 (IBM Corp, Armonk, NY), except for ROC curve analysis, bootstrap CI, and diagnostic accuracy measure comparison (R version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Development of a New Simplified Risk Score and Dichotomic Rule
The derivation cohort included 1848 ED patients with suspected AASs (Figure 1). Demographic and clinical characteristics are described elsewhere. 16 To create a parsimonious model, ADD items with a prevalence <5% (Marfan syndrome, family history, new diastolic murmur, respectively, 0.3%, 1.7%, and 2.7%) were removed. In multivariable logistic regression analysis (Table 1), 6 items were found as independent predictors of AAS diagnosis: thoracic aortic aneurysm, severe pain, sudden‐onset pain, pulse deficit, neurologic deficit, and hypotension/shock. These variables were used to develop a simplified noncategorical assessment tool, amenable to integration with DDage‐adj. Based on the odds of AAS diagnosis weighted on a logarithmic scale, we assigned a score of 2 to hypotension/shock and a score of 1 to the other variables. The sum was called the aorta simplified (AORTAs) score. AORTAs ≤1 (associated with a disease probability of 4.6%; 95% CI, 3.6%–6%), defined low pretest clinical probability and AORTAs ≥2 defined high probability (Figure S1).
Table 1.
Logistic Regression Analysis for Simplified Score Development
| Clinical Item | OR (95% CI) | P | Ln (OR) | AORTAs Score Points |
|---|---|---|---|---|
| Known thoracic aortic aneurysm | 3.52 (2.18–5.66) | <0.001 | 1.26 | 1 |
| Severe pain | 2.72 (1.86–3.98) | <0.001 | 1.00 | 1 |
| Sudden‐onset pain | 2.98 (2.07–4.29) | <0.001 | 1.09 | 1 |
| Pulse deficit | 3.77 (2.24–6.33) | <0.001 | 1.33 | 1 |
| Neurologic deficit | 2.77 (1.41–5.42) | 0.003 | 1.02 | 1 |
| Hypotension/shock | 5.79 (3.38–9.93) | <0.001 | 1.76 | 2 |
| Known aortic valve disease | 0.89 (0.44–1.79) | 0.743 | — | — |
| Ripping/tearing pain | 1.02 (0.66–1.56) | 0.936 | — | — |
| d‐dimer>500 ng/mL | 37.67 (18.23–77.82) | <0.001 | — | — |
OR indicates odds ratio.
Compared with the ADD score, the AORTAs score had superior AUC (P<0.001; Figure S2a) and reclassified 23.7% of patients (Table S2), with significant NRI for both AASs (22.4%; P<0.001) and AltDs (−16.2%, P<0.001). AORTAs ≥2 was more sensitive and less specific than ADD ≥2 (Table 2). In the derivation cohort, the AORTAs ≤1/DDage‐adj rule reclassified 16.9% of patients (Table S3), with a significant NRI for AltDs (−5.4%; P<0.001), and was less specific than the ADD ≤1/DD500 rule (Table 3).
Table 2.
Diagnostic Performance of the AORTAs Score in the Study Cohorts
| Diagnostic Variable | Study Cohorts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Derivation Cohort | Validation Cohorts | ||||||||
| (n=1848) | High Prevalence Cohort (n=1035) | Low Prevalence Cohort (n=447) | |||||||
| AORTAs≥2 | ADD≥2 | P Value | AORTAs≥2 | ADD≥2 | P Value | AORTAs≥2 | ADD≥2 | P Value | |
| Sensitivity | 77.6% (71.8%–82.7%) | 55.2% (48.7%–61.6%) | <0.001 | 54.1% (48.1%–60.5%) | 34.8% (29.2%–40.8%) | <0.001 | 71.4% (56.7%–83.4%) | 38.8% (25.2%–53.8%) | <0.001 |
| Specificity | 70.8% (68.5%–73%) | 87.1% (85.3%–88.7%) | <0.001 | 75.9% (72.8%–78.8%) | 84.5% (81.9%–87%) | <0.001 | 72.1% (67.4%–76.5%) | 89.1% (85.6%–92%) | <0.001 |
| LR+ | 2.66 (2.40–2.94) | 4.26 (3.60–5.06) | <0.001 | 2.25 (1.89–2.67) | 2.25 (1.77–2.86) | 1.0 | 2.56 (2.02–3.25) | 3.55 (2.26–5.58) | 0.13 |
| LR‐ | 0.32 (0.25–0.40) | 0.52 (0.45–0.59) | <0.001 | 0.61 (0.52–0.70) | 0.77 (0.70–0.85) | <0.001 | 0.40 (0.25–0.62) | 0.69 (0.12–0.86) | 0.009 |
95% CI in parentheses. ADD indicates aortic dissection detection; and LR, likelihood ratio.
Table 3.
Diagnostic Performance of the Integrated AORTAs ≤1/DDage‐adj Rule in the Study Cohorts
| Diagnostic Variable | Study Cohorts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Derivation Cohort | Validation Cohorts | ||||||||
| (n=1848) | High Prevalence Cohort (n=1035) | Low Prevalence Cohort (n=447) | |||||||
| AORTAs≤1/DDage‐adj | ADD≤1/DD500 | P Value | AORTAs≤1/DDage‐adj | ADD≤1/DD500 | P Value | AORTAs≤1/DDage‐adj | ADD≤1/DD500 | P Value | |
| Sensitivity | 99.2% (97%–99.9%) | 98.8% (96.4%–99.7%) | 1.0 | 98.3% (95.7%–99.5%) | 99.1% (96.9%–99.9%) | 0.63 | 100% (92.7%–100%) | 98% (89.3%–99.6%) | 1.0 |
| Specificity | 51.9% (49.4%–54.4%) | 57.3% (54.9%–59.8%) | <0.001 | 30% (26.9%–33.4%) | 30.2% (27%–33.5%) | 1.0 | 48.7% (43.8%–53.7%) | 52.8% (47.9%–57.7%) | 0.08 |
| LR+ | 2.06 (1.96–2.17) | 2.31 (2.18–2.45) | <0.001 | 1.41 (1.34–1.47) | 1.42 (1.35–1.49) | 0.58 | 1.95 (1.74–2.14) | 2.08 (1.86–2.33) | 0.12 |
| LR‐ | 0.02 (0–0.06) | 0.02 (0.01–0.07) | 0.67 | 0.06 (0.02–0.15) | 0.03 (0.01–0.11) | 0.33 | 0 (0–0.12)* | 0.04 (0–0.14) | 0.95 |
95% CI in parentheses. ADD indicates aortic dissection detection; DDage‐adj, age‐adjusted d‐dimer cutoff; and LR, likelihood ratio.
To allow LR comparison, a false‐negative unit was added in the corresponding cell.
External Validation of the Simplified Score
As summarized in Figure 1, external validation of the new score and integrated rule was conducted in 2 independent cohorts of patients with suspected AAS from 2 EDs: a high‐prevalence retrospective cohort (applying advanced imaging adjudication for all patients) and a low‐prevalence prospective cohort (applying clinical follow‐up adjudication). The clinical and demographic characteristics of the former cohort (n=1035) are detailed elsewhere. 17 The latter cohort (Figure 2) included 447 patients, whose characteristics and diagnostic data are summarized in Table S4 and Figure S3. Advanced aortic imaging was performed in 245 (54.8%) patients; 161 (36.3%) patients were hospitalized, and 4 (0.9%) were lost at follow‐up (clinical details in Table S5). An AAS was adjudicated in 49 (11.1%) patients: type‐A AAD (n=31), type‐B AAD (n=7), and intramural aortic hematoma (n=11). AltDs were muscle‐skeletal pain (n=119), gastrointestinal disease (60), acute coronary syndrome (39), non–AAS‐related syncope (30), uncomplicated aortic aneurysm (30), pneumonia (23), pericarditis (21), PE (10), and other diagnoses (62). AORTAs ≤1 was associated with a disease prevalence of 4.7% (95% CI, 2.8%–7.7%; Figure 3).
Figure 2. Flow diagram of the prospective low‐prevalence validation cohort study.
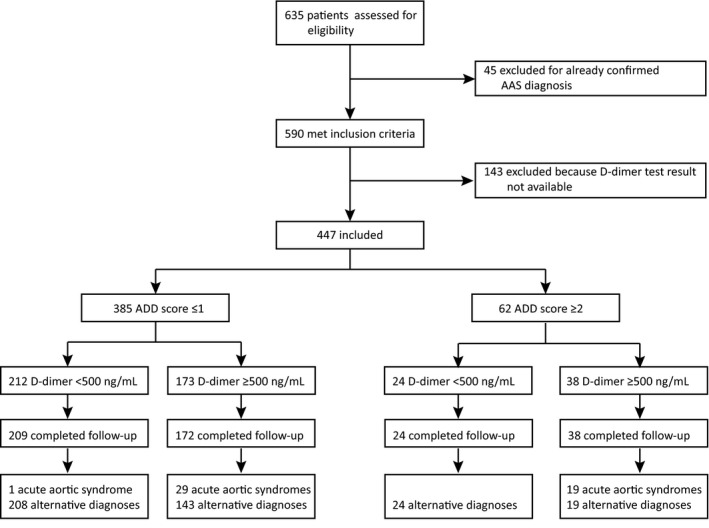
Figure 3. Prevalence of acute aortic syndromes associated with (A) AORTAs score and (B) ADD score values, in the prospective low‐prevalence validation cohort.
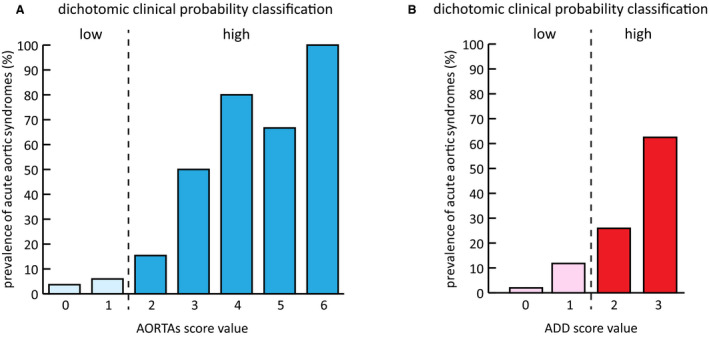
ADD indicates aortic dissection detection.
In composite ROC analysis of the validation cohorts, the AORTAs score had superior AUC versus the ADD score (P=0.005; Figure 4A, Figure S2B–S2C). Compared with the ADD score, the AORTAs score reclassified 16.6% (P<0.001) and 25.1% (P<0.001) of patients in the high‐ and low‐probability cohort, respectively. In the high‐prevalence cohort (Table S6), the AORTAs score reclassified 172 patients, including 55 with AASs (n=50 low to high P, n=5 high to low P; NRI, 19.3%; P<0.001) and 117 with AltDs (n=93 low to high P, and n=24 high to low P; NRI −8.6%; P<0.001). In the low‐prevalence cohort (Table S7), the AORTAs score reclassified 111 patients, including 18 with AASs (n=17 low to high P, n=1 high to low P; NRI 32.7%; P<0.001) and 93 with AltDs (n=80 low to high P, and n=13 high to low P; NRI −17%; P<0.001). AORTAs ≥2 was more sensitive and less specific than ADD ≥2 for diagnosis of AASs in both validation cohorts (Table 2).
Figure 4. ROC curves of (A) AORTAs versus ADD score, and (B) AORTAs ≤1/DDage‐adj vs ADD≤1/DD500 rule, in the validation cohorts.
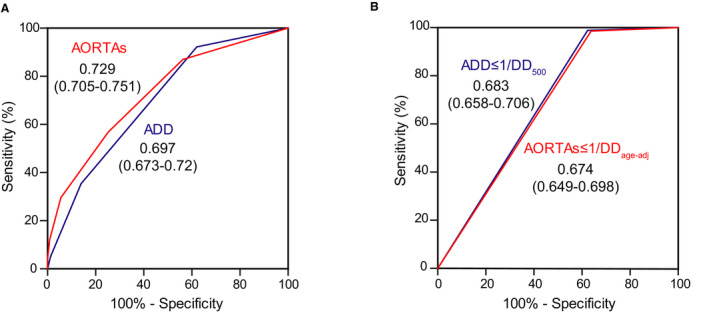
AUC values are presented in insets. N=1478 (282 with acute aortic syndromes, 1196 with alternative diagnoses). ADD indicates aortic dissection detection; DDage‐adj, age‐adjusted d‐dimer cutoff; and DD500, d‐dimer cutoff of 500 ng/mL.
External Validation of the Integrated Rule
In composite ROC analysis of the validation cohorts, the AORTAs ≤1/DDage‐adj and the ADD ≤1/DD500 rule had similar AUCs (P=0.18; Figure 4B). Compared with the ADD ≤1/DD500 rule, the AORTAs ≤1/DDage‐adj rule reclassified 9% (P=0.91) and 17.4% (P=0.53) of patients in the high‐ and low‐prevalence cohort, respectively. However, the NRI of the AORTAs≤1/DDage‐adj rule was not significant for both AASs and AltDs and in both cohorts (Tables S8–S9).
The diagnostic performance of the AORTAs ≤1/DDage‐adj and the ADD ≤1/DD500 rules was similar in both validation cohorts (Table 3). Integration of AORTAs ≤1 with DD500, instead, was less specific than both the AORTAs ≤1/DDage‐adj and the ADD ≤1/DD500 rule (Table S10). In the prospective validation cohort, there was 1 false‐negative case with the ADD≤1/DD500 rule. This was a 54‐year‐old woman with a type B intramural aortic hematoma presenting with severe and sudden anteroposterior thoracic pain radiating to the abdomen. The DD level was 493 ng/mL. With the AORTAs score, this patient was classified as high probability and was ruled in. In the prospective cohort, the failure rate was 0% (0%–2%) for the AORTAs ≤1/DDage‐adj rule and 0.5% (0.01%–2.7%) for the ADD ≤1/DD500 rule. Potential computed tomography scans avoided per 100 patients were 43 (95% CI, 39–48) for AORTAs ≤1/DDage‐adj and 47 (95% CI, 42–52) for ADD ≤1/DD500 (P=0.53).
According to the Pauker‐Kassirer decision threshold model, 23 , 24 , 25 , 26 the AORTAs ≤1/DDage‐adj rule could be applied if the probability of AAS is 1.1% to 46.3%, while the ADD≤1/DD500 rule could be applied if the probability is 1% to 32.6% (Figure 5).
Figure 5. Test‐treatment threshold analysis based on the prospective validation cohort study data.
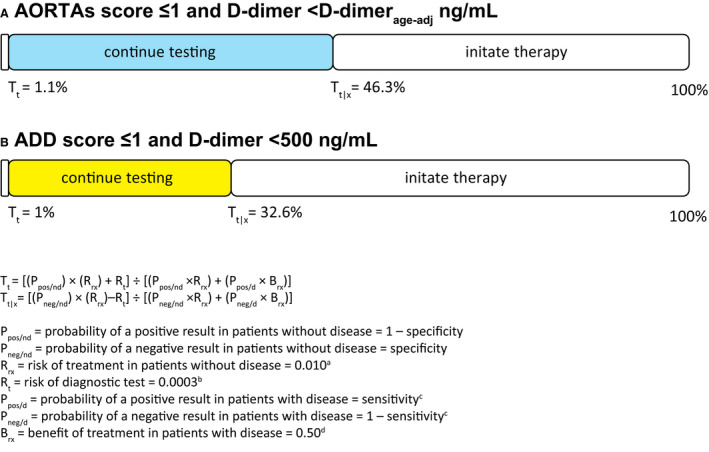
(A) Based on Taylor and Iyer 25 ; (B) based on Cochran 26 ; (C) the sensitivity of AORTAs ≤1/DDage‐adj was computed as 99%; (D) estimated form mortality of treated and untreated acute aortic dissection. 25 ADD indicates aortic dissection detection; DDage‐adj, age‐adjusted d‐dimer cutoff; Tt, testing threshold; and Tt|x, test‐treatment threshold.
Discussion
We describe development and validation of a simplified score assessing the clinical probability of AASs and of a rule integrating this score with an age‐adjusted DD cutoff for diagnostic rule‐out. In the latest guidelines, pretest clinical probability has received a class I/level B recommendation, and integrated DD rule‐out a IIa/B recommendation. 7
Proposals to modify ADD score–based classification and diagnostic flowchart have clinical, methodological, and pragmatic motivations. In clinical terms, the ADD score contradicts clinical gestalt when approaching patients with hemodynamic instability, typically caused by cardiac tamponade, myocardial ischemia, or aortic rupture. Per ADD score, hypotension/shock in the absence of risk factors in the other 2 categories (eg, pain not severe, sudden, or tearing; unknown aortic aneurysm) defines low probability (European Society of Cardiology) or intermediate risk (American Heart Association/American College of Cardiology) of AAS. 6 , 7 Because of the categorical structure of the ADD score, such underestimation persists even with perfusion deficit or aortic regurgitation in conjunction with hypotension/shock. Similar patients, however, are clinically unsuitable for integrated rule‐out and require urgent advanced aortic imaging.
The AORTAs score overcomes this contradiction by defining hypotension as a major predictor (2 points) leading per se to high pretest probability. Thus, AORTAs can be applied ab initio to all patients with suspected AAS independent of hemodynamic status and in keeping with clinical reasoning. Second, the AORTAs score attributes a high‐probability tag to all patients presenting with >1 item. For instance, patients with severe and sudden pain, or patients with neurological deficit and pulse deficit, are defined with AORTAs at high probability of AAS, thus excluding DD rule‐out. Accordingly, the main advantage of the AORTAs score is represented by increased sensitivity (consistently shown in 3 study cohorts), ideally providing increased safety and earlier diagnosis of AASs. A minor trade‐off in specificity slightly increases the false‐positive rate within patients at high probability undergoing urgent CTA. However, integration with a higher DD cutoff (providing per se higher specificity 12 , 15 ) was not associated with a significant change in specificity and efficiency for rule‐out in the validation cohorts.
Under a methodological point of view, the ADD score was developed from the IRAD database, a large international case series of AADs with minor representation of intramural aortic hematomas and penetrating aortic ulcers. 8 , 24 Formal derivation methods of the ADD score are unknown, and several issues regarding its development, refinement, validation, implementation, and dissemination remain open. 10 Most importantly, the IRAD database does not allow any estimate of the diagnostic accuracy of the ADD score as a screening or diagnostic tool, and so far, external validation has been attempted in few retrospective and only 1 prospective study. 9 In methodological terms, the AORTAs score is developed instead through a bottom‐up approach, taking advantage of a diagnostic prospective multicenter trial. 16 Preliminary external validation is provided herein in a large retrospective cohort and in a novel prospective cohort of ED outpatients. The decision threshold analysis also indicates that the AORTAs‐based rule‐out strategy could be applied to a wider range of disease probabilities.
Pragmatic considerations indicate that the AORTAs score may provide additional advantages. First, the AORTAs score uses only half of the items of the ADD score, which may ease applicability to busy EDs. Similar simplification processes have been done for commonly used scores such as the simplified Wells and Geneva scores for PE. 13 Second, application of a single higher‐specificity cutoff for both AAS and PE rule‐out appears convenient and practical.
This study has limitations. First, validation was performed in 2 patient cohorts, which were indeed fully independent but recruited in the same participating centers, potentially limiting external validity. Second, the retrospective nature of the first validation cohort has limits in score assignment through chart review because of potential underreporting of risk markers. Third, in the derivation and prospective validation cohorts, case adjudication was based on follow‐up, which might lead to potential slight underestimation of patients affected by AAS (differential verification bias). Finally, the study was powered to evaluate the diagnostic accuracy of the AORTAs score, and not for statistical comparison of different scores/DD integrations. A larger multicenter trial is needed for this purpose.
As with other similar tools, the AORTAs score is meant to aid in diagnostic decisions and to standardize clinical practice but not to substitute for clinical reasoning. Since study results were obtained in patients with a clinical suspicion of AAS (leading to a relatively high prevalence of AASs), results may not be generalized to unselected patients presenting to the ED (eg, with chest pain). Therefore, clinical gestalt should be applied by treating physicians for proper selection of patients suitable for standardized probability assessment and rule‐in/out protocols. Even posttest, a case‐by‐case diagnostic decision is always warranted.
In conclusion, we provide bottom‐up development of a simplified 6‐item noncategorical score for standardized assessment of the pretest probability of AASs, amenable to integration with an age‐adjusted DD assay for rule‐out applications (Figure 6). This score is easily applicable in the framework of current guidelines and clinical practice. In 3 independent cohorts, this score consistently showed higher sensitivity, better AAS classification and lower specificity compared with the ADD score, representing the current standard. Results also indicate comparable rule‐out performance when integrated with DDage‐adj. Further external validation is needed to evaluate clinical applicability.
Figure 6. Summary of the aorta simplified score (AORTAs) and the proposed diagnostic algorithm based on study results.
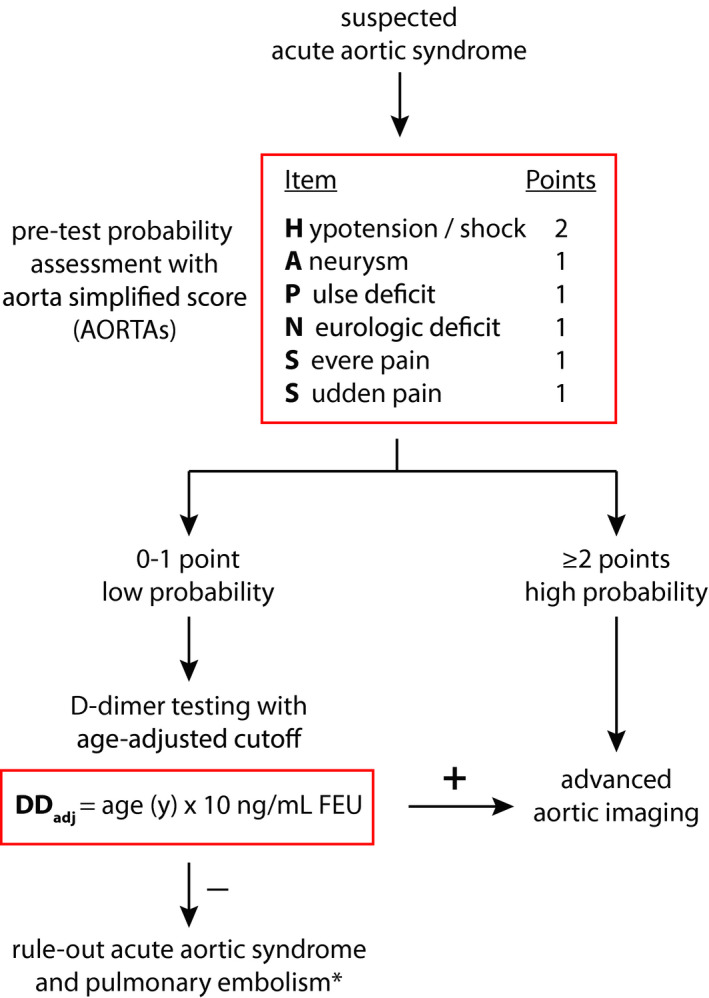
*If the probability of pulmonary embolism is nonhigh.
Sources of Funding
FM reports grant money from the Italian Ministry of Health (GR‐2013‐02355449, unrelated to the present work). PN reports grant money to conduct research from Università degli Studi di Firenze (16DPPN).
Disclosures
FM reports honoraria from Boehringer Ingelheim and Bayer for lectures and educational activities unrelated to the present work. The remaining authors have no disclosures to report.
Supporting information
Data S1‐Tables S1‐S10‐Figures S1‐S3
Acknowledgments
The authors thank all personnel in the medical, cardiothoracic, vascular, laboratory, and radiological divisions of the Molinette and Careggi hospitals for their valuable contributions.
(J Am Heart Assoc. 2021;10:e018425. DOI: 10.1161/JAHA.120.018425.)
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Bossone E, LaBounty TM, Eagle KA. Acute aortic syndromes: diagnosis and management, an update. Eur Heart J. 2018;39:739–749d. doi: 10.1093/eurheartj/ehx319. [DOI] [PubMed] [Google Scholar]
- 2. Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol. 2007;99:852–856. doi: 10.1016/j.amjcard.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 3. Zhan S, Hong S, Shan‐Shan L, Chen‐Ling Y, Lai W, Dong‐Wei S, Chao‐Yang T, Xian‐Hong S, Chun‐Sheng W. Misdiagnosis of aortic dissection: experience of 361 patients. J Clin Hypertens (Greenwich). 2012;14:256–260. doi: 10.1111/j.1751-7176.2012.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lovy AJ, Bellin E, Levsky JM, Esses D, Haramati LB. Preliminary development of a clinical decision rule for acute aortic syndromes. Am J Emerg Med. 2013;31:1546–1550. doi: 10.1016/j.ajem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohle R, Anjum O, Bleeker H, Wells G, Perry JJ. Variation in emergency department use of computed tomography for investigation of acute aortic dissection. Emerg Radiol. 2018;25:293–298. doi: 10.1007/s10140-018-1587-x. [DOI] [PubMed] [Google Scholar]
- 6. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Circulation. 2010;121:e266–e369. doi: 10.1161/cir.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 7. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 8. Rogers AM, Hermann LK, Booher AM, Nienaber CA, Williams DM, Kazerooni EA, Froehlich JB, O'Gara PT, Montgomery DG, Cooper JV, et al. Sensitivity of the aortic dissection detection risk score, a novel guideline‐based tool for identification of acute aortic dissection at initial presentation: results from the international registry of acute aortic dissection. Circulation. 2011;123:2213–2218. doi: 10.1161/CIRCULATIONAHA.110.988568. [DOI] [PubMed] [Google Scholar]
- 9. Tsutsumi Y, Tsujimoto Y, Takahashi S, Tsuchiya A, Fukuma S, Yamamoto Y, Fukuhara S. Accuracy of aortic dissection detection risk score alone or with d‐dimer: a systematic review and meta‐analysis. Eur Heart J Acute Cardiovasc Care. 2020:2048872620901831. DOI: 10.1177/2048872620901831. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10. Hill JM, Murphy TG, Fermann GJ. Aortic dissection detection risk score: a clinical decision rule that needs some parenting. Acad Emerg Med. 2019;26:695–697. doi: 10.1111/acem.13636. [DOI] [PubMed] [Google Scholar]
- 11. Asha SE, Miers JW. A systematic review and meta‐analysis of d‐dimer as a rule‐out test for suspected acute aortic dissection. Ann Emerg Med. 2015;66:368–378. doi: 10.1016/j.annemergmed.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 12. Bima P, Pivetta E, Nazerian P, Toyofuku M, Gorla R, Bossone E, Erbel R, Lupia E, Morello F. Systematic review of aortic dissection detection risk score plus d‐dimer for diagnostic rule‐out of suspected acute aortic syndromes. Acad Emerg Med. 2020;27:1013–1027. doi: 10.1111/acem.13969. [DOI] [PubMed] [Google Scholar]
- 13. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G‐J, Harjola V‐P, Huisman MV, Humbert M, Jennings CS, Jiménez D, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 14. Kotani Y, Toyofuku M, Tamura T, Shimada K, Matsuura Y, Tawa H, Uchikawa M, Higashi S, Fujimoto J, Yagita K, et al. Validation of the diagnostic utility of d‐dimer measurement in patients with acute aortic syndrome. Eur Heart J Acute Cardiovasc Care. 2017;6:223–231. doi: 10.1177/2048872616652261. [DOI] [PubMed] [Google Scholar]
- 15. Morello F, Mueller C, Soeiro AM, Leidel BA, Salvadeo SAT, Nazerian P; ADvISED Investigators . Response by Morello et al. to letters regarding article, “Diagnostic accuracy of the aortic dissection detection risk score plus d‐dimer for acute aortic syndromes: The ADvISED prospective multicenter study.” Circulation. 2018;138:448–449. doi: 10.1161/CIRCULATIONAHA.118.034861. [DOI] [PubMed] [Google Scholar]
- 16. Nazerian P, Mueller C, Soeiro AM, Leidel BA, Salvadeo SAT, Giachino F, Vanni S, Grimm K, Oliveira MT, Pivetta E, et al. Diagnostic accuracy of the aortic dissection detection risk score plus d‐dimer for acute aortic syndromes. Circulation. 2018;137:250–258. doi: 10.1161/circulationaha.117.029457. [DOI] [PubMed] [Google Scholar]
- 17. Nazerian P, Morello F, Vanni S, Bono A, Castelli M, Forno D, Gigli C, Soardo F, Carbone F, Lupia E, et al. Combined use of aortic dissection detection risk score and d‐dimer in the diagnostic workup of suspected acute aortic dissection. Int J Cardiol. 2014;175:78–82. doi: 10.1016/j.ijcard.2014.04.257. [DOI] [PubMed] [Google Scholar]
- 18. Righini M, Van Es J, Den Exter PL, Roy P‐M, Verschuren F, Ghuysen A, Rutschmann OT, Sanchez O, Jaffrelot M, Trinh‐Duc A, et al. Age‐adjusted d‐dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117. doi: 10.1001/jama.2014.2135. [DOI] [PubMed] [Google Scholar]
- 19. Marill KA, Chang Y, Wong KF, Friedman AB. Estimating negative likelihood ratio confidence when test sensitivity is 100%: a bootstrapping approach. Stat Methods Med Res. 2017;26:1936–1948. doi: 10.1177/0962280215592907. [DOI] [PubMed] [Google Scholar]
- 20. Zhou X‐H, Obuchowski N, McClish D. Statistical Methods in Diagnostic Medicine. 2nd ed. Hoboken, NJ: Wiley; 2011. doi: 10.1002/9780470906514. [DOI] [Google Scholar]
- 21. Gu W, Pepe MS. Estimating the capacity for improvement in risk prediction with a marker. Biostatistics. 2009;10:172–186. doi: 10.1093/biostatistics/kxn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 23. Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med. 1980;302:1109–1117. doi: 10.1056/NEJM198005153022003. [DOI] [PubMed] [Google Scholar]
- 24. Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, Ehrlich MP, Trimarchi S, Braverman AC, Myrmel T, et al. Insights from the international registry of acute aortic dissection. Circulation. 2018;137:1846–1860. doi: 10.1161/circulationaha.117.031264. [DOI] [PubMed] [Google Scholar]
- 25. Taylor RA, Iyer NS. A decision analysis to determine a testing threshold for computed tomographic angiography and d‐dimer in the evaluation of aortic dissection. Am J Emerg Med. 2013;31:1047–1055. doi: 10.1016/j.ajem.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 26. Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep. 2005;5:28–31. doi: 10.1007/s11882-005-0051-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1‐Tables S1‐S10‐Figures S1‐S3


