Abstract
Background
To determine if accelerometer measured sedentary behavior (SED), light‐intensity physical activity (LPA), and moderate‐to‐vigorous–intensity physical activity (MVPA) in midlife is prospectively associated with cognitive function.
Methods and Results
Participants were 1970 adults enrolled in the CARDIA (Coronary Artery Risk Development in Young Adults) study who wore an accelerometer in 2005 to 2006 (ages 38–50 years) and had cognitive function assessments completed 5 and/or 10 years later. SED, LPA, and MVPA were measured by an ActiGraph 7164 accelerometer. Cognitive function tests included the Digit Symbol Substitution Test, Rey Auditory Verbal Learning Test, and Stroop Test. Compositional isotemporal substitution analysis examined associations of SED, LPA, and MVPA with repeated measures of the cognitive function standardized scores. In men, statistical reallocation of 30 minutes of LPA with 30 minutes of MVPA resulted in an estimated difference of SD 0.07 (95% CI, 0.01–0.14), SD 0.09 (95% CI, 0.02–0.17), and SD −0.11 (95% CI, −0.19 to −0.04) in the Digit Symbol Substitution Test, Rey Auditory Verbal Learning Test, and Stroop scores, respectively, indicating better performance. Associations were similar when reallocating time in SED with MVPA, but results were less robust. Reallocation of time in SED with LPA resulted in an estimated difference of SD −0.05 (95% CI, −0.06 to −0.03), SD −0.03 (95% CI, −0.05 to −0.01), and SD 0.05 (95% CI, 0.03– 0.07) in the Digit Symbol Substitution Test, Rey Auditory Verbal Learning Test, and Stroop scores, respectively, indicating worse performance. Associations were largely nonsignificant among women.
Conclusions
Our findings support the idea that for men, higher‐intensity activities (MVPA) may be necessary in midlife to observe beneficial associations with cognition.
Keywords: cognition, compositional isotemporal substitution, epidemiology, physical activity, sedentary behavior
Subject Categories: Epidemiology, Exercise, Risk Factors, Mental Health
Nonstandard Abbreviations and Acronyms
- AICC
Corrected Akaike Information Criterion
- CARDIA
Coronary Artery Risk Development in Young Adults
- DSST
Digit Symbol Substitution Test
- ILR
isometric log‐ratio transformation
- LPA
light‐intensity physical activity
- MVPA
moderate‐to‐vigorous–intensity physical activity
- RAVLT
Rey Auditory Verbal Learning Test
- SED
sedentary behavior
Clinical Perspective
What Is New?
Increasing moderate‐to‐vigorous–intensity physical activity is one promising strategy to reduce dementia risk and preserve cognitive function; less is known about the role of lower‐intensity activities (sedentary behavior and light‐intensity physical activity) on cognitive function.
In this cohort of middle‐aged Black and White adults from the index CARDIA (Coronary Artery Risk Development in Young Adults) study, we found that statistical reallocation of time from lower‐intensity activities (sedentary behavior and/or light‐intensity physical activity) to moderate‐to‐vigorous–intensity physical activity was associated with better performance in the areas of processing speed, working memory, and executive function among men, but not women.
What Are the Clinical Implications?
Among men, higher‐intensity physical activity may be necessary in midlife to observe beneficial associations with cognition; additional research is needed to confirm observed sex differences.
The high and rising prevalence of dementia is a critical public health problem. An estimated 35.6 million adults were living with dementia in 2010, with this number expected to nearly double by 2030 due to the rapidly aging US population. 1 The World Health Organization concluded that among adults aged 60 years and older, dementia contributed 11.2% of years lived with disability, an amount larger than contributed by stroke, cardiovascular disease, or cancer. 2 Growing evidence suggests that the neuropathological changes leading to dementia begin decades before clinical features emerge. 3 Preventing or delaying the onset of dementia or cognitive impairment will lead to better survival, less disability, lower health care costs, and improved quality of life. Without effective pharmacological treatments for dementia, there is an immediate need to develop behavioral strategies to prevent or delay the onset and attenuate progression of the disease. To encourage additional research in this area, the National Institute on Aging released their guidelines “Aging Well in the 21st Century: Strategic Directions for Research on Aging in 2016,” describing the importance of identifying pathways by which behavioral, social, psychological, and economic factors affect aging‐related health in middle‐aged and older adults. 4
Increasing moderate‐to‐vigorous–intensity physical activity (MVPA) is one promising strategy to reduce dementia risk and preserve cognitive function. In 2018, the Physical Activity Guidelines Advisory Committee reviewed the existing literature and concluded there is a moderate to strong association between MVPA and various components of brain health, including processing speed, memory, and executive function, as well as a reduced risk of dementia, including Alzheimer disease. 5 However, despite the well‐established benefits of MVPA, <50% of US adults meet the aerobic physical activity guidelines, based on self‐reporting, 6 with substantially lower prevalence estimates obtained using accelerometry data (8.2%±0.8%), 6 and key differences observed by sex, race, age, and education, which may contribute to observed health disparities in Alzheimer disease and related dementias. 7
Given the low prevalence of the population meeting physical activity guidelines, alternate behavioral targets, such as reducing sedentary behavior (SED) with concurrent increases in light‐intensity physical activity (LPA) may be a more feasible public health approach within the US adult population, particularly among midlife and older adults, who are disproportionately inactive and at high risk for cognitive decline. Independent of MVPA, emerging evidence suggests that SED and LPA are predictors of cardiometabolic risk factors, including impaired lipid/glucose metabolism and hypertension, as well as cardiometabolic diseases, 8 , 9 , 10 , 11 which are also established risk factors for cognitive decline and dementia. 12 , 13 , 14 Therefore, it is biologically plausible for SED and LPA to play an important role in cognitive functioning by reducing inflammation and systemic peripheral risk factors that are also associated with cognitive decline. 15 However, due to our historical reliance on self‐reported physical activity questionnaires, which largely focus on leisure‐time MVPA, the associations of lower‐intensity activities (SED and LPA) with components of cognitive function remain poorly understood.
The objectives of this study were to determine if accelerometer‐measured SED, LPA, and MVPA in midlife (ages 38–50 years) is associated with measures of cognitive function assessed 5 and 10 years later using compositional isotemporal substitution. We hypothesized that replacing 30 daily minutes of SED with LPA or MVPA will be associated with better cognitive functioning assessed 5 and 10 years later. We also examined whether differences exist by sex, age, and race in the associations of accelerometer estimates and measures of cognitive function.
METHODS
Requests to access the data set, analytic methods, and study materials may be sent to the CARDIA (Coronary Artery Risk Development in Young Adults) Study Coordinating Center. Contact information can be found on the CARDIA website. 16
Study Population
The CARDIA study is an ongoing longitudinal cohort of 5115 Black and White men and women, aged 18 to 30 years, who took part in a clinical in‐person exam in 1985 to 1986 (year 0). Exams occurred at 1 of 4 field centers: Birmingham, AL; Minneapolis, MN; Chicago, IL; or Oakland, CA. Additional in‐person clinic examinations were held approximately every 2 to 5 years, including year 20 (2005–2006), year 25 (2010–2011), and year 30 (2015–2016), with retention rates of 72%, 72%, and 71% of surviving participants, respectively. Details on eligibility criteria, methods of participant selection, and follow‐up procedures have been reported previously. 17
For the current study, individuals were included if they took part in the CARDIA year 20 Fitness Ancillary Study (2005–2006), when accelerometry was first implemented, and had valid accelerometer wear data (N=2328). Individuals were excluded from these analyses if they were missing data on all cognitive function measures at both the CARDIA year 25 and 30 exams (n=163), or missing data on key covariates (n=193). Our final analytic sample consisted 1970 participants. The institutional review board at each center approved all study protocols for the primary CARDIA exam, as well as the CARDIA Fitness and Cognition Studies. Written informed consent was obtained at each exam, separately for the primary and ancillary studies.
Accelerometer‐Estimated SED, LPA, MVPA, and Sleep
The widely used and validated ActiGraph 7164 uniaxial accelerometer 18 was initialized to begin data collection at 12:00 am on the day of the in‐person CARDIA examination. Accelerometers were worn at the right hip on an elastic belt during all waking hours. The devices were initialized to record data in 60‐second epochs, and data collected were downloaded using ActiLife 6 software and processed using a modified version of the publicly available SAS algorithm developed for 2003 to 2004 National Health and Nutrition Examination Survey (NHANES) data. 19 Files were screened for wear time using the Troiano algorithm; valid wear was defined as ≥4 days with ≥10 hours per day. 20 Total and average accelerometer counts per day were calculated using summed counts recorded over wear periods and daily minutes per day spent in different physical activity intensity categories using standardized cut point threshold values. Freedson cut point threshold values were applied given their broad use in physical activity research and use in other CARDIA studies, 21 , 22 with SED defined as ≤100 counts per minute (cpm), LPA as 101 to 1951 cpm, and MVPA as ≥1952 cpm. 18 Summary estimates used in analyses were computed as summed daily estimates averaged across the number of valid wear days. Given that participants were instructed to wear the ActiGraph during all waking hours, we used nonwear time from the accelerometer to approximate sleep minutes if nonwear time was within 1 hour of self‐reported sleep time. If nonwear time and self‐reported sleep time differed by more than 1 hour, then we took the average of nonwear time and self‐reported sleep time as an estimate of sleep minutes. Although sleep duration is not our exposure variable of interest, it is a potential confounder of the activity‐cognition association, and therefore we chose to use a combination of accelerometer and self‐reported data to increase accuracy of our sleep estimate.
To calculate the 24‐hour activity cycle for compositional data analysis, we summed time spent in all 4 activity categories (SED, LPA, MVPA, and sleep). Participants were not asked to follow a 24‐hour wear protocol; therefore, because sleep time was estimated as described above, the total amount of time spent in the 4 categories did not sum to 24 hours (1440 minutes) in 85.9% of the analytic sample. To account for this, time recorded in each physical activity categories were rescaled by dividing the total observed time and multiplying by 1440 minutes (eg, ).
Outcome: Cognitive Function Measures
Cognitive measures were assessed using the Digit Symbol Substitution Test (DSST), Rey Auditory Verbal Learning Test (RAVLT), and Stroop Test, administered at both the CARDIA year 25 and year 30 exams. The DSST assesses visual motor speed, attention, and working memory. 23 Scores for the DSST range from 0 to 133, with higher scores indicating better cognitive performance. The RAVLT test assesses the ability to memorize and to retrieve words (verbal memory). 24 We used the delayed RAVLT score (trial 7; score range 0–15), with higher scores indicating better performance. The Stroop Test evaluates the ability to view complex visual stimuli and to respond to one stimulus dimension while suppressing the response to another dimension, an executive skill largely attributed to frontal lobe function. 25 , 26 We assessed the interference score of the Stroop Test with possible scores ranging from −160 to 160; a lower score indicates better performance. For analyses, all scores were standardized to z scores using the mean and standard deviation (separately at year 25 and year 30) to enhance comparability.
Covariates
Covariates from the CARDIA year 20 exam included age, sex, race (Black or White), years of education completed, self‐reported unemployment status (unemployed or employed), and health insurance status (health insurance over the last 2 years or not). Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale. Body mass index was calculated using measured height in meters and weight in kilograms (kilograms divided by height in meters squared). Diabetes mellitus status was determined using the following criteria: measured fasting glucose levels ≥126 mg/dL, self‐report of oral hypoglycemic medications or insulin, 2‐hour postload glucose ≥200 mg/dL, or glycated hemoglobin ≥6.5%. Blood pressure was measured 3 times on the right arm using an automated sphygmomanometer (Omron HEM907XL) in 1‐minute intervals after the participant was seated for 5 minutes. The average of the second and third blood pressure readings was used for analysis. Hypertension was defined as systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥80 mmHg as recommended by the 2017 American College of Cardiology/American Heart Association blood pressure guidelines, 27 or use of antihypertension medication. Lifestyle behaviors included smoking status (current, former, never), alcohol consumption (milliliters/day), self‐reported sleep quality (very good, fairly good, good, fairly bad, very bad), and self‐reported snoring status as a proxy for obstructive sleep apnea (most of the time or some of the time versus none).
Statistical Analysis
Initial univariate analyses were conducted for all cognitive function outcome variables. The distributions of each of these variables were visually assessed, and although most were integer valued, they were approximately normally distributed. Summary statistics were calculated for all potential covariates across accelerometer‐estimated MVPA quartiles.
After rescaling SED, LPA, MVPA, and sleep data to a 24‐hour period, compositional data analysis was used to generate a 4‐part composition, consisting of time spent in each behavior. Average daily time spent in SED, LPA, MVPA, and sleep were expressed as isometric log‐ratio coordinates using the default isometric log‐ratio (ILR) transformation that is included in the compositions R package. 28 The log‐ratio transformation allows us to use the traditional statistical methods on transformed data and translate the findings back to the original units of expression. 29 Mixed‐effects regression models with repeated‐measure outcomes were used to estimate the association of SED, LPA, and MVPA, represented as 3 ILR‐transformed variables, assessed at year 20 on cognitive function scores (RAVLT, DSST, and Stroop) assessed at years 25 and 30 (separate models for each outcome, 3 total models). Cognitive function data from year 25 and/or year 30 exams were used, thereby including in the analyses all participants who had cognitive function data at either exam to preserve a larger sample size with mixed model procedures and provide enhanced precision in our estimates. Preliminary analysis showed no significant differences between cognitive function scores between the year 25 and 30 exam; however, in exploratory analyses we estimated the associations of SED, LPA, and MVPA, represented as 3 ILR‐transformed variables, with change in cognition scores from the year 25 to 30 exams. A series of models were tested: Model 1 adjusted for demographic variables assessed at year 20, including race, age, sex, center, education and employment status; Model 2 additionally adjusted for chronic health conditions at year 20 known to influence cognitive performance, including depressive symptoms, diabetes mellitus, and hypertension; and Model 3 additionally adjusted for lifestyle factors at year 20, including body mass index, smoking, alcohol consumption, self‐reported sleep quality and snoring status. Corrected Akaike information criterion was used for model selection. Model 3 had the lowest Akaike information criterion (indicating best model) across all outcomes; therefore, we chose to present the final model across all outcomes for consistency.
Interactions of sex (male versus female), age (38–44 years versus 45–50 years), and race (White versus Black) with the accelerometer data were examined, as there are known differences between sex, age, and race in physical activity patterns and performance on these cognitive function measures. We also conducted several postreview sensitivity analyses. First, we additionally adjusted for an overall diet quality score assessed at the CARDIA year 20 exam as previously described, 30 and in separate analyses, presence of apolipoprotein E e4 (yes/no for 1 or 2 copies of the ε4 allele). 31 Results were unchanged, thus we report findings without adjustment for these potential confounders. In a second set of sensitivity analyses, we excluded individuals with preexisting cardiovascular or renal disease, including myocardial infarction, cardiac revascularization, acute coronary syndrome, congestive heart failure, stroke, carotid artery disease, peripheral artery disease, and end‐stage renal disease prior to the baseline assessment (n=75). Study findings remained consistent.
Compositional descriptive statistics, including compositional means and a variation matrix were calculated for physical activity variables, which were adjusted to a sum of 1440 minutes to determine the average minutes per 24 hours spent in each of the 4 behaviors, and expressed as percent time in each activity category. We used a compositional isotemporal substitution approach to assess the effect of reallocating 30 minutes of SED with an equal duration of time in LPA or MVPA, as well as reallocating 30 minutes of LPA with an equal duration of MVPA. A 30‐minute interval was selected for its common use in the isotemporal substitution literature. 21 , 32 , 33 To evaluate these differences, the geometric average amount of time spent in each category was calculated, and then 30 minutes was reallocated from SED to either LPA or MVPA, or 30 minutes was reallocated from LPA to MVPA. This gives 4 different hypothetical scenarios from the accelerometer data, which were transformed using the ILR transformation. Covariates included in the model were also averaged across age, race, and sex groups based on their individual observed values. Predicted values were then averaged based on model selection across race and sex. Confidence intervals for the difference between the estimated cognitive outcome at the mean ILR values and the 30‐minute exchange values were calculated using a Wald‐type confidence interval. SAS 9.4 and R 3.5.3 were used for analyses.
RESULTS
Descriptive information on characteristics of participants included and excluded from the current study can be found in Table S1. Compared with those who were excluded, participants included in the study were more likely to be White and women with more years of education, lower unemployment rate, and with health insurance coverage. In addition, participants included had fewer health conditions, were less likely to smoke, and had better performance on the cognitive function measures compared with those who were excluded. Descriptive information on characteristics of participants with and without cognitive function measures (ie, included versus subset of those excluded) are reported in Table S2.
Descriptive characteristics of the study population are listed in Table 1 by quartiles of MVPA. Sex, race, years of education, body mass index, diabetes mellitus status, hypertension, alcohol consumption, diet quality, all accelerometer measures, and several of the cognitive function measures were associated with physical activity. Women and Black participants were less likely to be in the highest quartile of MVPA. The higher MVPA quartiles were associated with more years of education, lower body mass index, lower incidence of diabetes mellitus and hypertension, and higher alcohol consumption. In addition, higher levels of MVPA were associated with less SED and sleep, and more LPA. Higher levels of MVPA were associated with better performance on the DSST (year 30) and Stroop Test (year 25 and 30). Participant characteristics by sex can be found in Table S3. Female participants had lower levels of SED and MVPA and more LPA and sleep compared with males. Overall, female participants had better cognitive performance across all measures at both the year 25 and year 30 exams.
Table 1.
Participant Characteristics by MVPA Quartile, the CARDIA Study (2005–2016)*
| Year 20 Participant Characteristics | Overall, min/d | Q1 (1.15, 18.72) | Q2 (18.72, 31.91) | Q3 (31.91, 49.96) | Q4 (49.96, 322.47) | P Value |
|---|---|---|---|---|---|---|
| Age, y ±SD | 45.27±3.56 | 45.16±3.75 | 45.13±3.63 | 45.54±3.47 | 45.24±3.38 | 0.247 |
| Female, n (%) | 1148 (58.27) | 373 (75.81) | 301 (61.05) | 250 (50.71) | 224 (45.53) | <0.001 |
| White, n (%) | 1179 (59.85) | 220 (44.72) | 283 (57.40) | 343 (69.57) | 333 (67.68) | <0.001 |
| Education, y ±SD | 15.32±2.53 | 14.80±2.44 | 15.18±2.46 | 15.58±2.54 | 15.71±2.59 | <0.001 |
| Unemployment, n (%) | 198 (10.05) | 58 (11.79) | 50 (10.14) | 47 (9.53) | 43 (8.74) | 0.435 |
| Health insurance, n (%) | 1766 (89.64) | 428 (86.99) | 437 (88.64) | 455 (92.29) | 446 (90.65) | 0.118 |
| CES‐D score ±SD | 8.48±7.21 | 8.70±7.59 | 8.75±7.34 | 8.38±7.35 | 8.08±6.51 | 0.678 |
| BMI, kg/m2 ±SD | 28.95±6.96 | 30.46±7.14 | 29.48±6.91 | 28.30±6.14 | 27.54±7.26 | <0.001 |
| Diabetes mellitus, n (%) | 154 (7.82) | 60 (12.20) | 36 (7.30) | 39 (7.91) | 19 (3.86) | <0.001 |
| Hypertension, n (%) | 579 (29.39) | 180 (36.59) | 174 (35.29) | 107 (21.70) | 118 (23.98) | <0.001 |
| Cardiovascular/renal disease, n (%) | 75 (3.86) | 23 (4.78) | 24 (4.92) | 13 (2.67) | 15 (3.07) | 0.153 |
| ApoE E4 allele, n (%), n=1794 | 508 (28.32) | 137 (31.21) | 140 (30.91) | 119 (26.15) | 112 (25.06) | 0.082 |
| Smoking status, n (%) | 0.378 | |||||
| Current | 293 (14.87) | 83 (16.87) | 71 (14.40) | 66 (13.39) | 73 (14.84) | |
| Former | 414 (21.02) | 92 (18.70) | 114 (23.12) | 113 (22.92) | 95 (19.31) | |
| Never | 1263 (64.11) | 317 (64.43) | 308 (62.47) | 314 (63.69) | 324 (65.85) | |
| Alcohol, mL/d ±SD | 11.01±22.85 | 7.34±21.24 | 10.53±24.61 | 12.18±24.30 | 13.98±20.48 | <0.001 |
| Diet‐quality score ±SD, n=1753 | 63.52±12.76 | 59.69±11.76 | 62.93±12.56 | 65.34±12.49 | 66.32±13.23 | <0.001 |
| Sleep quality, n (%) | 0.224 | |||||
| Very good | 353 (17.92) | 80 (16.26) | 94 (19.07) | 84 (17.04) | 95 (19.31) | |
| Fairly good | 720 (36.55) | 173 (35.16) | 188 (38.13) | 180 (36.51) | 179 (36.38) | |
| Good | 581 (29.49) | 150 (30.49) | 136 (27.59) | 148 (30.02) | 147 (29.88) | |
| Fairly bad | 292 (14.82) | 76 (15.45) | 71 (14.40) | 78 (15.82) | 67 (13.62) | |
| Very bad | 24 (1.22) | 13 (2.64) | 4 (0.81) | 3 (0.61) | 4 (0.81) | |
| Self‐reported snoring, n (%) | 1105 (56.1) | 281 (57.11) | 276 (55.98) | 289 (58.62) | 259 (52.64) | 0.277 |
| Self‐reported sleep, h ±SD | 6.70±1.30 | 6.60±1.33 | 6.74±1.35 | 6.76±1.41 | 6.71±1.07 | 0.182 |
| Accelerometer min/d ±SD | ||||||
| Sedentary | 490.574±101.48 | 516.23±93.03 | 506.99±102.71 | 486.45±96.61 | 452.61±101.62 | <0.001 |
| LPA | 360.61±85.44 | 340.27±83.81 | 358.24±81.44 | 365.75±84.08 | 378.18±86.13 | <0.001 |
| MVPA | 35.81±26.04 | 11.39±4.24 | 23.67±3.78 | 38.25±5.18 | 69.95±27.21 | <0.001 |
| Sleep † | 506.32±67.90 | 517.72±66.23 | 505.83±69.48 | 504.49±68.24 | 497.25±66.20 | 0.001 |
| Year 25 cognition scores ±SD | ||||||
| DSST ‡ | 72.98±15.20 | 71.98±15.28 | 73.12±14.65 | 73.28±15.08 | 73.55±15.78 | 0.406 |
| RAVLT § | 8.83±3.18 | 8.83±3.07 | 8.86±3.32 | 8.85±3.12 | 8.76±3.22 | 0.964 |
| Stroop ‖ | 21.40±9.82 | 22.23±10.72 | 21.85±9.77 | 20.99±9.18 | 20.53±9.50 | 0.033 |
| Year 30 cognition scores ±SD | ||||||
| DSST ‡ | 70.60±15.98 | 68.33±16.69 | 70.82±15.13 | 71.64±15.30 | 71.62±16.56 | 0.006 |
| RAVLT § | 8.99±3.32 | 8.75±3.37 | 9.01±3.32 | 9.06±3.31 | 9.15±3.26 | 0.303 |
| Stroop ‖ | 21.23±9.94 | 22.29±10.45 | 21.38±10.12 | 20.74±9.52 | 20.49±9.55 | 0.036 |
N=1970. ApoE E4, indicates apolipoprotein E e4; BMI, body mass index; CARDIA; Coronary Artery Risk Development in Young Adults; CES‐D, Center for Epidemiologic Studies Depression Scale; DSST, Digital Symbol Substitution Test; LPA, light‐intensity physical activity; MVPA, moderate‐to‐vigorous–intensity physical activity; and RAVLT, Rey Auditory Verbal Learning Test.
Data presented from the CARDIA year 20 exam (2005–2006, baseline for these analyses) unless otherwise specified.
Nonwear time from the accelerometer was used to approximate sleep minutes if accelerometer nonwear time was within 1 hour of self‐reported sleep time. If nonwear time and self‐reported sleep time differed by more than 1 hour, the average of nonwear time and self‐reported sleep were used to estimate sleep minutes.
DSST score range from 0 to 133, higher score indicates better performance.
RAVLT score range from 0 to 15, higher score indicates better performance.
Stroop score range from −160 to 160, higher score indicates worse performance.
Compositional descriptive statistics examining the proportion of time spent in the each of the 4 activity groups were similar between the arithmetic and compositional means, with ≈35.5% of time spent in sleep, 36% in SED, 26% in LPA, and 2.5% in MVPA (Table 2). The largest difference in our primary exposure variables (SED, LPA, and MVPA) was observed in the amount of time spent in MVPA, which was 0.52% (7.5 minutes) higher in arithmetic estimation compared with the compositional alternative measure. The variation matrix (Table 3), which contains all pair‐wise log‐ratio variances, summarizes the variability of the data. Values close to 0 suggested the time spent in the 2 corresponding behaviors are highly proportional. For example, the variance of log (Sleep/SED)=0.09, which suggested the highest proportional relationship or codependence between the 2 behaviors, whereas the variance of log (MVPA/SED)=0.68, suggested the lowest proportional relationship. MVPA had the highest log‐ratio variances with all other behaviors, indicating the time spent in MVPA was the least codependent on the other behaviors.
Table 2.
Standard and Compositional Descriptive Measures of the Percent Time Spent in Sleep, SED, LPA, and MVPA (2005–2006)
| Sleep | SED | LPA | MVPA | |
|---|---|---|---|---|
| Arithmetic mean | 35.16 | 35.85 | 26.38 | 2.61 |
| Compositional mean | 35.67 | 35.95 | 26.28 | 2.09 |
N=1970. The arithmetic means were calculated for each movement behavior separately. Compositional means were calculated by normalizing the geometric means of all movement behaviors. LPA indicates light‐intensity physical activity; MVPA, moderate‐to‐vigorous–intensity physical activity; and SED, sedentary behavior.
Table 3.
Compositional Variation Matrix Indicating the Dispersion of Sleep, SED, LPA, and MVPA Relative to Other Movement Behaviors (2005–2006)
| Sleep | SED | LPA | MVPA | |
|---|---|---|---|---|
| Sleep | 0 | 0.09 | 0.09 | 0.59 |
| SED | 0.09 | 0 | 0.17 | 0.68 |
| LPA | 0.09 | 0.17 | 0 | 0.55 |
| MVPA | 0.59 | 0.68 | 0.55 | 0 |
N=1970. Tabulated numbers are variances of log (A/B), where A and B are a pair of sleep, SED, LPA, MVPA. Values close to 0 indicate the 2 behaviors involved are consistently proportional (ie, highly correlated with each other). LPA indicates light‐intensity physical activity; MVPA, moderate‐to‐vigorous–intensity physical activity; and SED, sedentary behavior.
The estimated effect of reallocating 30 minutes from one behavior to another around the average composition on our main cognitive outcomes are shown in Table 4. Results are shown from the mixed‐effects linear regression models with each cognitive outcome as a separate model. Each row shows the estimates for a change in cognitive test score if an average subject in the study reallocated 30 minutes of time from one behavior to another. There was a significant sex interaction; thus, results are presented stratified by sex group. The race interaction was not statistically significant; however, estimated standardized scores are presented separately for White and Black participants to illustrate the disparities in scores between the 2 groups. There was no statistically significant interaction by age group.
Table 4.
Compositional Isotemporal Substitution, Estimated Difference in Mean Repeated Measures of Standardized Cognitive Function Variables Following 30‐Minute Time Reallocation of Sedentary Behavior, and Physical Activity by Sex and Race (2005–2016)
| Cognitive Test | Sex | Change Made | Estimated Score White | Estimated Score Black | Estimated Difference to Mean Values | 95% CI | P Value |
|---|---|---|---|---|---|---|---|
| DSST* | |||||||
| Female | Reference | 0.27 | −0.28 | … | … | … | |
| SED to LPA | 0.27 | −0.29 | −0.01 | −0.03 to 0.01 | 0.328 | ||
| SED to MVPA | 0.28 | −0.27 | 0.01 | −0.04 to 0.06 | 0.701 | ||
| LPA to MVPA | 0.29 | −0.27 | 0.02 | −0.04 to 0.07 | 0.509 | ||
| Male | Reference | −0.20 | −0.77 | … | … | ||
| SED to LPA | −0.24 | −0.81 | −0.05 | −0.06 to −0.03 | <0.001 | ||
| SED to MVPA | −0.17 | −0.74 | 0.03 | −0.03 to 0.08 | 0.364 | ||
| LPA to MVPA | −0.12 | −0.69 | 0.07 | 0.01 to 0.14 | 0.019 | ||
| RAVLT* | |||||||
| Female | Reference | 0.61 | −0.08 | … | … | … | |
| SED to LPA | 0.61 | −0.09 | −0.01 | −0.02 to 0.01 | 0.353 | ||
| SED to MVPA | 0.60 | −0.09 | −0.01 | −0.06 to 0.03 | 0.583 | ||
| LPA to MVPA | 0.61 | −0.09 | −0.01 | −0.06 to 0.04 | 0.833 | ||
| Male | Reference | 0.05 | −0.62 | … | … | … | |
| SED to LPA | 0.03 | −0.65 | −0.03 | −0.05 to −0.01 | 0.014 | ||
| SED to MVPA | 0.12 | −0.56 | 0.07 | 0.01 to 0.13 | 0.037 | ||
| LPA to MVPA | 0.14 | −0.53 | 0.09 | 0.02 to 0.17 | 0.009 | ||
| Stroop ‡ | |||||||
| Female | Reference | −0.18 | 0.52 | … | … | … | |
| SED to LPA | −0.17 | 0.53 | 0.01 | −0.01 to 0.03 | 0.239 | ||
| SED to MVPA | −0.12 | 0.58 | 0.06 | 0.01 to 0.11 | 0.023 | ||
| LPA to MVPA | −0.13 | 0.57 | 0.05 | −0.01 to 0.10 | 0.087 | ||
| Male | Reference | −0.11 | 0.55 | … | … | … | |
| SED to LPA | −0.06 | 0.60 | 0.05 | 0.03 to 0.07 | <0.001 | ||
| SED to MVPA | −0.17 | 0.48 | −0.06 | −0.14 to 0.01 | 0.089 | ||
| LPA to MVPA | −0.22 | 0.43 | −0.11 | −0.19 to −0.04 | 0.005 | ||
N=1970. Models adjusted for year 20 demographics (race, age, sex, center, education, employment status), chronic health conditions (depressive symptoms, diabetes, hypertension), and lifestyle factors (body mass index, smoking, alcohol consumption, sleep quality, snoring). DSST indicates Digit Symbol Substitution Test; LPA, light‐intensity physical activity; MVPA, moderate‐to‐vigorous–intensity physical activity; RAVLT, Rey Auditory Verbal Learning Test; and SED, sedentary behavior.
Standardized scores, higher score indicates better performance.
Standardized scores, higher score indicates worse performance.
In men, replacing 30 minutes of LPA with 30 minutes of MVPA resulted in an estimated difference of SD 0.07 (95% CI, 0.01–0.14, P=0.019) in the DSST score, indicating better performance. However, replacing 30 minutes of SED with 30 minutes of LPA was associated with a difference of SD −0.05 (95% CI, −0.06 to −0.03, P<0.001) in the DSST score, indicating worse performance. For the RAVLT test, significant associations were observed in men when replacing 30 minutes of SED with MVPA (SD 0.07, 95% CI, 0.01–0.13, P=0.037) or 30 minutes of LPA with MVPA (SD 0.09, 95% CI, 0.02–0.17, P=0.009), indicating better performance, whereas replacing 30 minutes of SED with 30 minutes of LPA resulted in a difference of SD −0.03 (95% CI, −0.05 to −0.01, P=0.014), indicating worse performance. Also, in men, the Stroop score was associated with a difference of SD −0.11 (95% CI, −0.19 to −0.04, P=0.005) when replacing 30 minutes of LPA with MVPA, indicating better performance, whereas replacing 30 minutes of SED with LPA resulted in a difference of SD 0.05 (95% CI, 0.03–0.07, P<0.001), indicating worse performance. Study findings in women were not significant, with the exception of the Stroop test, where replacement of 30 minutes of SED with MVPA resulted in a difference of SD 0.06 (95% CI, 0.01–0.11, P=0.023), indicating worse performance (see Figure for a graphical representation of study findings).
Figure 1. Estimated difference in mean repeated measures of standardized cognitive function variables following 30‐minute time reallocation of sedentary behavior and physical activity (2005–2016), N=1970.
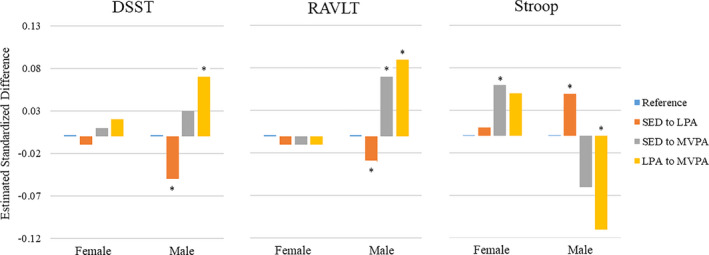
Higher standardized scores indicate better performance for the DSST and RAVLT and worse performance for the Stroop test. Models are adjusted for year 20 demographics (race, age, sex, center, education, employment status), chronic health conditions (depressive symptoms, diabetes mellitus, hypertension), and lifestyle factors (body mass index, smoking, alcohol consumption, sleep quality, snoring). Asterisk (*) indicate differences are statistically significant. DSST indicates Digit Symbol Substitution Test; LPA, light‐intensity physical activity; MVPA, moderate‐to‐vigorous–intensity physical activity; RAVLT, Rey Auditory Verbal Learning Test; and SED, sedentary behavior.
Study findings for the DSST, RAVLT, and Stroop test did not differ when examining associations of year 20 accelerometer estimates with year 25 cognitive function measures alone, or year 20 accelerometer estimates with year 30 cognitive function measures alone. No significant associations were observed when examining the year 20 accelerometer estimates with change in the cognitive function measures from year 25 to year 30 (Table S4).
DISCUSSION
This study examined the associations of accelerometer‐estimated SED, LPA, and MVPA with prospective assessments of cognitive function using a compositional isotemporal analysis approach in a large cohort of middle‐aged Black and White adults. We found that among men, statistical reallocation of time from lower‐intensity activities (SED and/or LPA) with MVPA resulted in better performance in the areas of processing speed (DSST), working memory (RAVLT), and executive function (Stroop). However, replacement of SED with LPA was associated with worse cognitive performance in the same domains in men. These findings were robust after accounting for sleep duration and after adjustment for potential confounders, including demographics, chronic health conditions associated with cognitive performance, and lifestyle factors. Findings in women were largely null.
This is one of the first studies to use a compositional isotemporal analysis approach to examine the prospective effects of replacing lower‐intensity activities with higher‐intensity activities on various domains of cognitive function. Our findings add to those of Fanning and colleagues, 34 who examined the cross‐sectional effects of replacing accelerometer‐measured SED with LPA and MVPA on cognitive function domains in a sample of 247 low‐active healthy older adults using a traditional (not compositional) isotemporal substitution approach. 35 Similar to our study, they found that substituting SED with MVPA, but not SED with LPA, resulted in better cognitive function scores, specifically better performance in the areas of working memory and executive function. Despite some evidence that LPA is associated with improvements in cardiometabolic health, 8 , 9 the results of the current study and those of Fanning et al, 34 indicate that LPA may not reach the required intensity threshold to see an association with cognitive function. Notably, the study by Fanning et al, 34 used different activity cut point threshold values than the present study, with SED defined as ≤50 cpm, LPA as 51 to 1040 cpm, and MVPA as ≥1041 cpm. 36 Although these cut point thresholds are lower than used in the current study, the age of participants in the Fanning et al, 34 study averaged 65.4 years, whereas the age of participants in the present study averaged 45.3 years at physical activity assessment (ie, 20‐year difference). Thus, the differences in cut point thresholds between studies (both used to estimate time spent in absolute intensity categories) may enhance comparability given the 20‐year average age difference between participants.
Our findings are also in line with others that illustrate a beneficial effect of MVPA on working memory, executive function, and processing speed. 5 , 37 , 38 , 39 The effect sizes observed in the current study when replacing 30 daily minutes of SED or LPA with 30 minutes of MVPA ranged from 0.03 to 0.11 SD units, which is the inverse of the typical age‐related decline observed in cognitive function among older adults (−0.04 SD units/year). 40 The relatively large effect sizes compared with the typical rate of cognitive decline indicate that consistent replacement of lower‐intensity activities with MVPA may be able to delay or prevent the decline in cognitive function that occurs with age, particularly in men.
Measurement error in the accelerometer estimates could partially explain our findings that replacing SED with LPA resulted in worse cognitive performance among men, given that a waist‐worn protocol was used that is unable to detect differences in posture. SED is defined as time spent awake and in a seated, reclining, or lying posture at low intensity. 41 It is likely that some time in LPA was misclassified as SED if participants were standing but stationary, which decreases the precision of our activity estimates and may bias results. In addition, we used standardized count thresholds values to estimate time spent in various intensity levels, which relies on absolute, rather than relative intensity levels. It is also possible that these substitution effects are accurate, and a reflection of the types of cognitive tasks individuals engage in while in SED compared with LPA. For example, individuals who sit for prolonged periods may be participating in cognitively demanding tasks, such as reading, writing, or artistic pursuits. Common tasks associated with LPA include food preparation, washing dishes, folding laundry, shopping, or cleaning at a low‐intensity level (<3.0 metabolic equivalents), which may not be as cognitively demanding. It is important for future studies to assess the types of activities individuals typically engage in while in SED or LPA to more accurately account for these contextual differences.
Surprisingly, we found no associations between accelerometer‐estimated physical activity and cognitive function measures in women, with 1 exception, which was in the opposite direction as hypothesized. Other studies have also identified sex differences in the associations of exercise and cognitive function in older adults; however, many show greater cognitive benefits of aerobic exercise training in women compared with men. 42 , 43 It is plausible that these differences in findings were in part due to ceiling effects, as women had higher cognitive function scores overall compared with men, or due to women being active at lower‐intensity levels compared with men. In addition, researchers have identified sex differences in genetic, cardiovascular, inflammatory, hormonal, and social and psychological risk factors associated with cognitive decline, which may contribute to the observed differences in associations of physical activity and cognition. 44 Sex may also moderate the efficacy of physical activity on cognition, through sex differences in neuroplasticity, brain‐derived neurotrophic factor, and physiological adaptations to exercise. 44 For example, a 12‐year longitudinal population‐based sample of older adults investigated whether the benefits of physical activity on cognitive preservation differed by brain‐derived neurotrophic factor and sex across multiple cognitive domains (including working memory and processing speed). 45 This study found that physical activity was not statistically significantly related to cognition in female participants regardless of genotype. Physical activity was dependent on brain‐derived neurotrophic factor carrier status in males, with cognition benefits from physical activity observed in male brain‐derived neurotrophic factor Val66Met noncarriers but not carriers. There is a clear need for additional research to better understand how biological sex may moderate the relation of exercise and cognition.
Strengths of this study include the use of a diverse cohort of Black and White adults and prospective study design with physical activity assessed prior to cognitive function, thus reducing the likelihood of reverse causation; repeated assessments of a variety of cognitive function domains; objective assessment of SED, LPA, and MVPA; and our analytic approach using compositional isotemporal substitution. However, several limitations must be noted. First, the CARDIA cohort was relatively young at the year 25 (ages 43–55 years) and year 30 (ages 48–60 years) exams, which may be too early to detect differences that are pertinent to future dementia risk. The CARDIA study will continue to assess cognitive function longitudinally, and this will provide a rich data source as the population moves into older adulthood. Second, measurement error may have occurred when estimating SED and LPA given that the waist‐worn accelerometer used in this study is unable to differentiate between a seated and standing posture. Furthermore, accelerometers worn at the waist are limited in their ability to detect certain activities such as weight training, swimming, and cycling. Use of anatomical placement sites that optimize the estimation of posture, specifically sitting versus standing (ie, thigh), and combining accelerometers with other physiological measures would provide enhanced estimates of time spent in physical activity intensity categories. 46 Third, Freedson cut point threshold values were used in the current study to define activity categories, 18 which limits our ability to directly compare our findings with others using alternate cut point thresholds. Fourth, although this study included assessments of many key domains of cognitive function, alternate assessments such as social cognition were not included. Fifth, participants included in this study differed in several ways from those excluded from analysis (eg, fewer health conditions, less likely to smoke, and better performance on cognitive tests), which may limit generalizability of study findings. Sixth, we did not include survival data in our analysis, and given the differential survival rates between men and women, survival bias may in part explain the lack of significant findings in women. However, there was a small number of deaths between the 2 cognitive function assessments (n=28) due to the relatively young age of the cohort, and therefore inclusion of survival data would have minimal effects on study findings. Seventh, we did not account for multiple comparisons in our analyses (eg, Bonferroni). Although this form of adjustment would decrease the type 1 error, it would also increase the risk of type 2 error for associations that are not null. 47 It is thus important to interpret study findings with caution. Finally, although we adjusted for many important potential confounders, the possibility of residual confounding remains.
In conclusion, we found that replacement of lower‐intensity activities (SED and/or LPA) with MVPA were associated with greater performance on tests of processing speed, working memory, and executive function over a 10‐year follow‐up period among men but not women. Contrary to our hypothesis, replacement of SED with LPA was associated with worse performance on the same cognitive domains in men. Continued follow‐up of the CARDIA cohort with future assessments of cognitive function will provide additional opportunities to further explore the associations of activity and cognition as the cohort ages and is expected to see more rapid declines in cognitive function. Our existing data support the idea that for men, higher‐intensity activities (MVPA) may be necessary in middle age to observe beneficial associations with cognition. Additional research is needed to confirm observed sex differences. As a next step, we will also examine whether these findings are supported by examining accelerometer estimated activity with brain magnetic resonance imaging data in the CARDIA cohort.
Sources of Funding
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). Accelerometer data collection was supported by grant R01 HL078972 from the NHLBI. P.P. is supported by grant R00AG052830 from the National Institute of Aging.
Disclosures
None.
Supporting information
Tables S1–S4
Acknowledgments
This manuscript has been reviewed by CARDIA for scientific content.
(J Am Heart Assoc. 2021;10:e018350. DOI: 10.1161/JAHA.120.018350.)
This manuscript was sent to Viola Vaccarino, MD, PhD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75.e62.DOI: 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . The Global Burden of Disease: 2004 Update. Geneva, Switzerland: WHO Press; 2008. [Google Scholar]
- 3. Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804.DOI: 10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Institute on Aging . Aging well in the 21st century: strategic directions for research on aging. Published 2016. https://www.nia.nih.gov/sites/default/files/2017‐07/nia‐strategic‐directions‐2016.pdf. Accessed July 24, 2018.
- 5. 2018 Physical Activity Guidelines Advisory Committee . Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: Department of Health and Human Services; 2018. [Google Scholar]
- 6. Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: adults compliance with the physical activity guidelines for Americans. Am J Prev Med. 2011;40:454–461.DOI: 10.1016/j.amepre.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 7. Weuve J, Barnes LL, Mendes de Leon CF, Rajan KB, Beck T, Aggarwal NT, Hebert LE, Bennett DA, Wilson RS, Evans DA. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology. 2018;29:151–159.DOI: 10.1097/EDE.0000000000000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LaMonte MJ, Lewis CE, Buchner DM, Evenson KR, Rillamas‐Sun E, Di C, Lee I‐M, Bellettiere J, Stefanick ML, Eaton CB, et al. Both light intensity and moderate‐to‐vigorous physical activity measured by accelerometry are favorably associated with cardiometabolic risk factors in older women: the Objective Physical Activity and Cardiovascular Health (OPACH) Study. J Am Heart Assoc. 2017;6:e007064. DOI: 10.1161/JAHA.117.007064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fuzeki E, Engeroff T, Banzer W. Health benefits of light‐intensity physical activity: a systematic review of accelerometer data of the National Health and Nutrition Examination Survey (NHANES). Sports Med. 2017;47:1769–1793.DOI: 10.1007/s40279-017-0724-0 [DOI] [PubMed] [Google Scholar]
- 10. Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996–2011. Am J Prev Med. 2011;41:207–215.DOI: 10.1016/j.amepre.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 11. Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, Khunti K, Yates T, Biddle SJ. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta‐analysis. Diabetologia. 2012;55:2895–2905.DOI: 10.1007/s00125-012-2677-z [DOI] [PubMed] [Google Scholar]
- 12. Rawlings AM, Sharrett AR, Schneider ALC, Coresh J, Albert M, Couper D, Griswold M, Gottesman RF, Wagenknecht LE, Windham BG, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161:785–793.DOI: 10.7326/M14-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, McGovern P, Folsom AR; Atherosclerosis Risk in Communities Study I . Cardiovascular risk factors and cognitive decline in middle‐aged adults. Neurology. 2001;56:42–48.DOI: 10.1212/WNL.56.1.42 [DOI] [PubMed] [Google Scholar]
- 14. Knopman DS, Mosley TH, Catellier DJ, Coker LH; Atherosclerosis Risk in Communities Study Brain MRIS . Fourteen‐year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement. 2009;5:207–214. [DOI] [PubMed] [Google Scholar]
- 15. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472.DOI: 10.1016/j.tins.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 16. Coronary artery risk development in young adults: New Investigators. https://www.cardia.dopm.uab.edu/invitation‐to‐new‐investigators. Accessed August 31, 2020.
- 17. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116.DOI: 10.1016/0895-4356(88)90080-7 [DOI] [PubMed] [Google Scholar]
- 18. Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. acceleromete. Med Sci Sports Exerc. 1998;30:777–781.DOI: 10.1097/00005768-199805000-00021 [DOI] [PubMed] [Google Scholar]
- 19. National Cancer Institute . SAS programs for analyzing NHANES 2003–2004 accelerometer data. Published 2016. https://epi.grants.cancer.gov/nhanes_pam/. Accessed October 25, 2017.
- 20. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188.DOI: 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- 21. Whitaker KM, Pettee Gabriel K, Buman MP, Pereira MA, Jacobs DR Jr, Reis JP, Gibbs BB, Carnethon MR, Staudenmayer J, Sidney S, et al. Associations of accelerometer‐measured sedentary time and physical activity with prospectively assessed cardiometabolic risk factors: the CARDIA study. J Am Heart Assoc. 2019;8:e010212. DOI: 10.1161/JAHA.118.010212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pettee Gabriel K, Sidney S, Jacobs DR Jr, Whitaker KM, Carnethon MR, Lewis CE, Schreiner PJ, Malkani RI, Shikany JM, Reis JP, et al. Ten‐year changes in accelerometer‐based physical activity and sedentary time during midlife: the CARDIA study. Am J Epidemiol. 2018;187:2145–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wechsler D. Wechsler Adult Intelligence Scale‐III (WAIS‐III). San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 24. Rosenberg SJ, Ryan JJ, Prifitera A. Rey Auditory‐Verbal Learning Test performance of patients with and without memory impairment. J Clin Psychol. 1984;40:785–787. [DOI] [PubMed] [Google Scholar]
- 25. Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643. [Google Scholar]
- 26. MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. [DOI] [PubMed] [Google Scholar]
- 27. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 28. Comprehensive R Archive Network . Package 'compositions'. Published 2018. https://cran.r‐project.org/web/packages/compositions/compositions.pdf. Accessed June 9, 2019.
- 29. Chastin SF, Palarea‐Albaladejo J, Dontje ML, Skelton DA. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio‐metabolic health markers: a novel compositional data analysis approach. PLoS One. 2015;10:e0139984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sijtsma FP, Meyer KA, Steffen LM, Shikany JM, Van Horn L, Harnack L, Kromhout D, Jacobs DR Jr. Longitudinal trends in diet and effects of sex, race, and education on dietary quality score change: the Coronary Artery Risk Development in Young Adults study. Am J Clin Nutr. 2012;95:580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Howard BV, Gidding SS, Liu K. Association of apolipoprotein E phenotype with plasma lipoproteins in African‐American and white young adults. The CARDIA Study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 1998;148:859–868.DOI: 10.1093/oxfordjournals.aje.a009711 [DOI] [PubMed] [Google Scholar]
- 32. Knaeps S, De Baere S, Bourgois J, Mertens E, Charlier R, Lefevre J. Substituting sedentary time with light and moderate to vigorous physical activity is associated with better cardiometabolic health. J Phys Act Health. 2018;15:197–203. [DOI] [PubMed] [Google Scholar]
- 33. Fishman EI, Steeves JA, Zipunnikov V, Koster A, Berrigan D, Harris TA, Murphy R. Association between objectively measured physical activity and mortality in NHANES. Med Sci Sports Exerc. 2016;48:1303–1311.DOI: 10.1249/MSS.0000000000000885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fanning J, Porter G, Awick EA, Ehlers DK, Roberts SA, Cooke G, Burzynska AZ, Voss MW, Kramer AF, McAuley E. Replacing sedentary time with sleep, light, or moderate‐to‐vigorous physical activity: effects on self‐regulation and executive functioning. J Behav Med. 2017;40:332–342.DOI: 10.1007/s10865-016-9788-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170:519–527.DOI: 10.1093/aje/kwp163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Copeland JL, Esliger DW. Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Act. 2009;17:17–30.DOI: 10.1123/japa.17.1.17 [DOI] [PubMed] [Google Scholar]
- 37. Rathore A, Lom B. The effects of chronic and acute physical activity on working memory performance in healthy participants: a systematic review with meta‐analysis of randomized controlled trials. Syst Rev. 2017;6:124. DOI: 10.1186/s13643-017-0514-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kamijo K, Takeda Y. Regular physical activity improves executive function during task switching in young adults. Int J Psychophysiol. 2010;75:304–311.DOI: 10.1016/j.ijpsycho.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 39. Frederiksen KS, Verdelho A, Madureira S, Bäzner H, O'Brien JT, Fazekas F, Scheltens P, Schmidt R, Wallin A, Wahlund L‐O, et al. Physical activity in the elderly is associated with improved executive function and processing speed: the LADIS Study. Int J Geriatr Psychiatry. 2015;30:744–750.DOI: 10.1002/gps.4220 [DOI] [PubMed] [Google Scholar]
- 40. Hayden KM, Reed BR, Manly JJ, Tommet D, Pietrzak RH, Chelune GJ, Yang FM, Revell AJ, Bennett DA, Jones RN. Cognitive decline in the elderly: an analysis of population heterogeneity. Age Ageing. 2011;40:684–689.DOI: 10.1093/ageing/afr101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer‐Cheung AE, Chastin SFM, Altenburg TM, Chinapaw MJM; Participants STCP . Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act. 2017;14:75. DOI: 10.1186/s12966-017-0525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Colcombe S, Kramer A. Fitness effects on the cognitive function of older adults: a meta‐analytic study. Psychol Sci. 2003;14:125–130.DOI: 10.1111/1467-9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- 43. Barha CK, Davis JC, Falck RS, Nagamatsu LS, Liu‐Ambrose T. Sex differences in exercise efficacy to improve cognition: a systematic review and meta‐analysis of randomized controlled trials in older humans. Front Neuroendocrinol. 2017;46:71–85.DOI: 10.1016/j.yfrne.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 44. Barha CK, Liu‐Ambrose T. Exercise and the aging brain: considerations for sex differences. Brain Plast. 2018;4:53–63.DOI: 10.3233/BPL-180067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Watts A, Andrews SJ, Anstey KJ. Sex differences in the impact of BDNF genotype on the longitudinal relationship between physical activity and cognitive performance. Gerontology. 2018;64:361–372.DOI: 10.1159/000486369 [DOI] [PubMed] [Google Scholar]
- 46. Chen KY, Bassett DR Jr. The technology of accelerometry‐based activity monitors: current and future. Med Sci Sports Exerc. 2005;37:S490–500.DOI: 10.1249/01.mss.0000185571.49104.82 [DOI] [PubMed] [Google Scholar]
- 47. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46.DOI: 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


